Abstract
Excessive mechanical loading of cartilage producing hydrostatic stress, tensile strain and fluid flow leads to chondrocyte apoptosis and osteoarthritis. High fluid flow induces cyclooxygenase-2 (COX-2) expression in sheared chondrocytes, which suppresses their antioxidant capacity and contributes to apoptosis. The pivotal role of COX-2 in shear-induced chondrocyte apoptosis and the conflicting literature data on the roles of prostaglandin (PG)E2, PGD2 and its metabolite 15-deoxy-Δ12,14-PGJ2 (15d-PGJ2) in chondrocyte apoptosis prompted us to investigate which COX-2-derived PG is involved in this process. We show that exogenously added PGD2 and 15d-PGJ2, but not PGE2, diminish the viability of human T/C-28a2 chondrocytes under static conditions. In agreement with these observations, knockdown of L-PGD synthase (L-PGDS) abolishes shear-induced chondrocyte apoptosis. Using cDNA microarrays in conjunction with clustering algorithms, we propose a novel signaling pathway by which high fluid shear mediates COX-2/L-PGDS-dependent chondrocyte apoptosis, which is validated by molecular interventions. We demonstrate that L-PGDS controls the downregulation of Protein Kinase A (PKA), which in turn regulates Polo-like kinase1 (Plk1) and Plk3. Plks target p53, which controls the transcription of p53 effectors (TP53INPs, FAS and Bax) involved in chondrocyte apoptosis. Reconstructing the signaling network regulating chondrocyte apoptosis may provide insights to optimize conditions for culturing artificial cartilage in bioreactors and for developing therapeutic strategies for arthritic disorders.
Keywords: chondrocyte, shear, apoptosis, cell-cycle, cyclooxygenase-2, prostaglandin (PG)D2, 15d-PGJ2, PGE2
INTRODUCTION
Excessive mechanical loading of cartilage producing hydrostatic stress, tensile strain and fluid flow1 results in irreversible cartilage erosion, chondrocyte apoptosis and osteoarthritic (OA) disease.2 Numerous in vitro studies support the notion that low fluid shear (<10 dyn/cm2) is chondroprotective3, whereas high shear (>10 dyn/cm2) promotes matrix degradation3 and chondrocyte apoptosis.4,5 We have shown that high fluid shear induces cyclooxygenase-2 (COX-2) expression4,6,7, which suppresses the antioxidant capacity of sheared chondrocytes and contributes to their apoptosis.4 Indeed, aberrant expression of COX-2 protein in articular cartilage is an earmark of arthritis8 associated with increased numbers of apoptotic chondrocytes.9,10
COX-2 catalyzes the rate-limiting step of prostaglandin (PG) synthesis. PGE2 and PGD2 are the major PGs synthesized by chondrocytes. PGD2 readily undergoes dehydration to yield the bioactive cyclopentenone-type PGs of the J2-series, such as 15-deoxy-Δ12,14-PGJ2 (15d-PGJ2). However, their role in the metabolism of articular cartilage is still a matter of debate. Although some studies have suggested possible anabolic effects associated with low concentrations of PGE211,12, several others suggest that PGE2 plays a major role in cartilage erosion and inflammation associated with arthritic disorders.8,13 Miwa et al.14 reported that PGE2 directly induces apoptosis in bovine articular chondrocytes via a cAMP-dependent pathway. In contrast, PGE2 by itself cannot elicit apoptosis in human chondrocytes, even though a COX-2 specific inhibitor, NS398, represses nitric oxide-induced chondrocyte apoptosis9, suggesting the potential involvement of other COX-2-derived PGs in this process. Prostaglandin D synthase (PGDS), responsible for the biosynthesis of PGD2 and J2 series, exists in two isoforms: hematopoietic (H)-and lipocalin (L)- type PGDS. L-PGDS is the predominant isoform in human cartilage and is markedly upregulated in OA relative to healthy cartilage.15 PGD2 is released by cytokine-activated human chondrocytes.15 Its metabolite 15d-PGJ2 is also secreted by human articular chondrocytes and detected in joint synovial fluids obtained from OA or rheumatoid arthritis patients.16 15d-PGJ2 has been reported to induce chondrocyte apoptosis in a dose- and time-dependent manner via a peroxisome proliferator-activated receptor γ (PPARγ)-dependent pathway.16 Although 15d-PGJ2 has also been shown to have a pro-apoptotic effect on other cell types, such as endothelial cells17, tumor cells18 and neurons19, several lines of evidence suggest that it may have chondroprotective effects. For instance, 15d-PGJ2 and PGD2 counteract the induction of matrix metalloproteinases in cytokine-activated chondrocytes20,21, which play a key role in cartilage degradation. 15d-PGJ2 also blocks apoptosis of human primary chondrocytes induced by the NF-kB inhibitor Bay 11-7085.17 Taken together, the contributions of PGE2, PGD2 and its metabolite 15d-PGJ2 to chondrocyte apoptosis remain controversial.
The signaling pathway by which COX-2 elicits chondrocyte apoptosis in response to high fluid shear4 has yet to be delineated. Moreover, the contradictory data in the literature regarding the potential roles of PGE2 and PGD2/15d-PGJ2 in chondrocyte apoptosis prompted us to investigate which COX-2-derived PG is involved in this process. In this study, we show that COX-2-derived PGD2 and its metabolite 15d-PGJ2, but not PGE2, mediate shear-induced chondrocyte apoptosis. Using cDNA microarrays in conjunction with clustering algorithms, we propose a novel signaling pathway by which high fluid shear mediates COX-2/L-PGDS-dependent chondrocyte apoptosis, which was validated by genetic interventions. We demonstrate that L-PGDS controls the downregulation of Protein Kinase A (PKA), which in turn regulate Polo-like kinase 1 (Plk1) and Plk3. Plks target p53, and control the transcription of p53 effectors (TP53INPs, FAS and Bax), which are involved in chondrocyte apoptosis.
RESULTS
Shear-induced PGD2 and its metabolite 15d-PGJ2, but not PGE2, mediate chondrocyte apoptosis
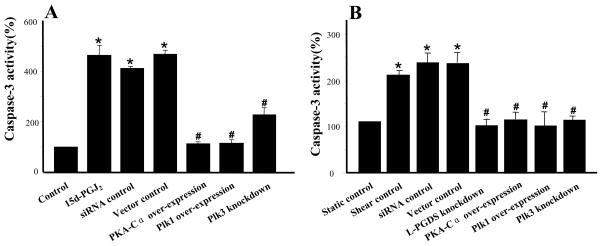
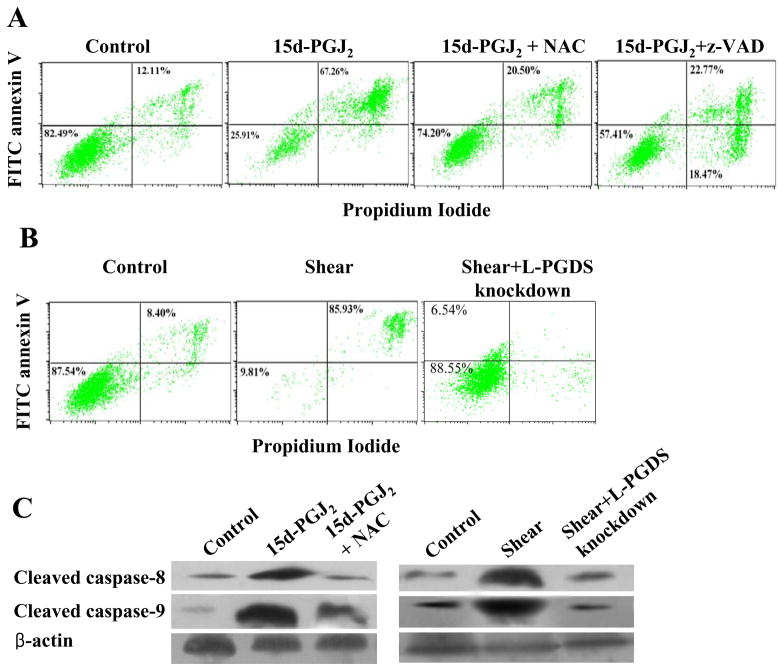
High fluid shear (>10 dyn/cm2) induces chondrocyte apoptosis4,5, which is markedly suppressed by COX-2 specific inhibitors.4 In this study, we aimed to delineate the mechanism(s) by which shear-induced COX-2 mediates chondrocyte death. The human T/C-28a2 chondrocyte cell line was chosen as a model system, since T/C-28a2 cells have been shown to behave much like primary human chondrocytes when cultured under appropriate conditions.22,23 As a first step, we evaluated the effect of exogenously added COX-2-derived prostaglandins on T/C-28a2 chondrocytic cell viability using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay. Our data reveal that PGD2 and its metabolite 15d-PGJ2 significantly reduce cell viability at concentrations of ≥20 μM and ≥3 μM, respectively (Suppl. Fig. 1A, 1B). In distinct contrast, the extent of T/C-28a2 cell viability was not suppressed by PGE2 even at 40 μM (Suppl. Fig. 1C). We next established that exogenously added 15d-PGJ2 (5 μM) induces T/C-28a2 chondrocyte apoptosis, as monitored by a caspase-3 colorimetric assay (Fig. 1A) and annexin V-propidium iodide (PI) double staining (Fig. 2A). Taken together, these data suggest that COX-2-derived PGD2 and its non enzymatic dehydration end product 15d-PGJ2, but not PGE2, might be involved in shear-mediated chondrocytic cell death. To validate this hypothesis, we first demonstrated that exposure of T/C-28a2 chondrocytes to high fluid shear (20 dyn/cm2) induces sustained PGD2 and 15d-PGJ2 production relative to static (untreated) controls (Suppl. Figs. 2A, 2B). Since PGDS is responsible for the biosynthesis of PGD2 and 15d-PGJ2, and L-PGDS is predominantly expressed in human cartilage15, we aimed to delineate the role of this enzyme in shear-induced chondrocyte apoptosis. Our data reveal that high shear stress (20 dyn/cm2) induces L-PGDS mRNA (Table 1) and protein (Fig. 3) expression in human T/C-28a2 chondrocytes. Knockdown of L-PGDS via RNA interference was demonstrated at both the transcriptional (Table 1) and translational (Fig. 3) levels, and was shown to block shear-induced chondrocyte apoptosis, as assessed by a caspase-3 colorimetric assay (Fig. 1B). In contrast, transfection of T/C-28a2 chondrocytes with control siRNA failed to impair shear-induced L-PGDS upregulation (Fig. 3) and the extent of shear-mediated apoptosis (Fig. 1B). Shear-induced chondrocyte apoptosis and its marked inhibition by L-PGDS knockdown were verified by annexin V-PI staining followed by flow cytometry (Fig. 2B). To further validate our observations that high fluid shear induces chondrocyte apoptosis, we examined the protein levels of cleaved caspase-8 and -9 in sheared and static control T/C-28a2 chondrocytes. Fig. 2C reveals that prolonged application of high shear stress (20 dyn/cm2; 48 h) induces a marked upregulation of caspase-8 and -9, which is reversed by L-PGDS knockdown.
Figure 1.
Effects of L-PGDS knockdown, PKA-Cα overexpression, Plk1 overexpression and Plk3 knockdown on (A) 15d-PGJ2- and (B) shear- induced chondrocyte apoptosis, as assessed by a caspase-3 colorimetric assay. T/C-28a2 chondrocytes were transfected with an siRNA oligonucleotide sequence specific for either L-PGDS or Plk3, or a plasmid containing the cDNA of PKA-Ca or Plk1, before their exposure to 15d-PGJ2 (5 μM for 48 h) or shear stress (20 dyn/cm2 for 48 h). In select experiments, T/C-28a2 chondrocytes remained either untransfected (control) or were transfected with an siRNA control oligonucleotide or the empty vector before being exposed to 15d-PGJ2 or fluid shear. Data represent the mean±S.D. of the absorption ratios at 405 nm of sheared chondrocytes to paired static controls (n=3, * p < 0.05 with respect to untreated or static control; # p < 0.05 with respect to 15d-PGJ2 or shear control).
Figure 2.
Effects of (A) 15d-PGJ2 and (B) shear stress on T/C-28a2 chondrocyte death. T/C-28a2 cells were exposed to 15d-PGJ2 stimulation (5 μM for 48 h) or high shear stress (20 dyn/cm2 for 48) or appropriate control conditions. In select experiments, T/C-28a2 cells were pre-treated with the pan caspase inhibitor z VAD-FMK (25 μM) for 2h or the antioxidant N-acetyl cycteine (NAC; 5 mM) for 1 s before the stimulation with 15d-PGJ2. In others, T/C-28a2 chondrocytes were transfected with an siRNA oligonucleotide sequence specific L-PGDS before being subjected to fluid shear (20 dyn/cm2, 48 h). Cells were then stained with FITC-conjugated Annexin V and PI and analyzed in a FACSCalibur flow cytometer (A, B). Alternatively, whole cell lysates were prepared and probed with antibodies specific for cleaved caspase-8 or -9 (C). Representative Western hybridization experiments for select genes are shown in the panel C. β-actin was probed as a loading control.
Table 1.
Effects of COX-2 specific inhibitor NS398 and L-PGDS knockdown on mRNA transcript ratios in sheared chondrocytes, as assessed by real-time PCR.
| Transcript ratio (shear/static) | |||
|---|---|---|---|
| Molecules of interest | Shear control† | NS398‡ | L-PGDS Knockdown§ |
| L-PGDS | 1.5±0.1 | 1.3±0.1 | 0.7±0.1 |
| PKA-Cα | 0.5±0.1 | 1.3±0.1 | 0.9±0.1 |
| Plk1 | 0.3±0.1 | 0.9±0.1 | 0.9±0.1 |
| Plk3 | 1.9±0.1 | 1.3±0.1 | 0.6±0.1 |
| Cdc25c | 0.3±0.1 | 0.9±0.2 | 0.8±0.1 |
| p53 | 0.8±0.1 | 0.9±0.1 | 1.1±0.1 |
| TP53INP1 | 1.5±0.2 | 0.6±0.1 | 0.6±0.1 |
| TP53INP11 | 1.6±0.1 | 0.5±0.1 | 0.7±0.1 |
| FAS | 7.6±0.1 | 4.5±0.1 | 0.6±0.1 |
| Bax | 2.0±0.1 | 1.2±0.1 | 0.5±0.1 |
All values represent transcript ratios for sheared (20 dyn/cm2, 48 h) to paired static controls (0 dyn/cm2, 48 h) of T/C28a2 chondrocytes. Paired treatments consisted of no treatment
shear treated with 50 μM NS398 and static treated with 0.1% DMSO
shear transfected with L-PGDS siRNA (shear) and static transfected with control siRNA (static)
Except for p53, all other genes tested were significantly regulated upon shear stress exposure (p < 0.05). Data represent mean ± S.D. (n ≥ 3).
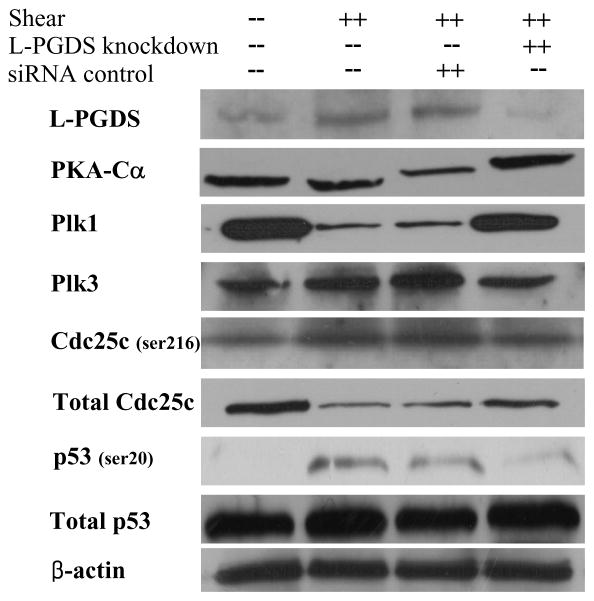
Figure 3.
Effect of L-PGDS knockdown on protein expression or phosphorylation levels of select genes in shear-activated chondrocytes. T/C-28a2 chondrocytes were transfected with an siRNA oligonucleotide sequence specific L-PGDS, and then subjected to fluid shear (20 dyn/cm2, 48 h). In select experiments, T/C-28a chondrocytes were transfected with an siRNA control oligonucleotide before being exposed to fluid shear. Controls included T/C-28a2 chondrocytes subjected to static (no-flow) conditions for 48h. Representative Western hybridization experiments for select genes are shown in the figure. β-actin was probed as a loading control.
PGD2 exerts its effects primarily by binding and activating two plasma membrane receptors, the D prostanoid receptor (DP) 1 and DP2. In view of previous observations showing that PGD2 mediates neuronal protection via the DP1 receptor, whereas activation of DP2 receptor promotes neuronal loss24, we investigated the potential contribution of DP2 receptor to chondrocyte death. Use of the DP2 receptor antagonist BAY-u3405 (5 μM) failed to restore the diminished levels of 15d-PGJ2-mediated chondrocyte viability (Suppl. Fig. 3A) and to inhibit shear-induced chondrocyte apoptosis (data not shown), suggesting the lack of DP2 receptor involvement in chondrocyte death. The PGD2 metabolite 15d-PGJ2 is a natural ligand for PPARγ, and has been reported to induce apoptosis in diverse cell types16,25–27 through PPARγ-dependent or PPARγ-independent mechanisms. 15d-PGJ2 is also an activator of the DP2 receptor.28 Use of the PPARγ antagonist T0070907 (1 μM) either alone or in combination with BAY-u3405 (5 μM) failed to reverse the suppression of 15d-PGJ2-mediated chondrocyte viability (Suppl. Fig. 3A) and to reduce shear-induced chondrocyte apoptosis (data not shown). Together, these data suggest that chondrocyte death in response to high shear stress proceeds via a PPARγ-independent and DP2-independent pathway.
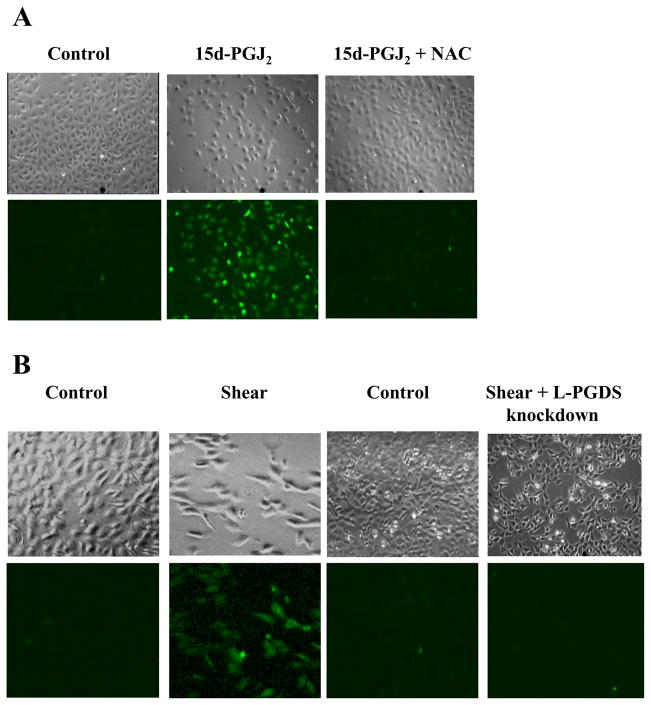
The cytotoxic potential of 15d-PGJ2 has been linked to its ability to generate reactive oxygen species (ROS)19, which are a group of highly reactive molecular forms of oxygen containing unpaired electrons. Using the Image-iT LIVE Green Reactive Oxygen Species Detection dye, we report that exogenously added 15d-PGJ2 (5 μM) induces ROS production in human T/C-28a2 chondrocytes, which is abrogated after T/C-28a2 cell treatment with the antioxidant N-acetyl cycteine (NAC; 5 mM) (Fig. 4A). Moreover, prolonged (48 h) exposure of T/C-28a2 cells to high shear stress (20 dyn/cm2) results in ROS generation, which is abolished by L-PGDS knockdown (Fig. 4B). Collectively, these data reveal the key role of PGD2 and its metabolite 15d-PGJ2 in shear-induced ROS production and chondrocyte death.
Figure 4.
Effects of (A) 15d-PGJ2 and (B) shear stress on ROS generation in chondrocytes. T/C-28a2 chondrocytes were exposed to 15d-PGJ2 stimulation (5 μM for 48 h) or high shear stress (20 dyn/cm2 or 48). Controls included/C-28a2 chondrocytes subjected to static (no-flow) conditions for 48h. In select experiments, T/C-28a2 cells were stimulated with 15d-PGJ2 in the presence of the antioxidant N-acetyl cycteine (NAC; 5 mM). In others, T/C-28a2 chondrocytes were transfected with an siRNA oligonucleotide sequence specific L-PGDS before being subjected to fluid shear (20 dyn/cm2, 48 h). Cells were then stained with the Image-iT LIVE Green Reactive Oxygen Species Detection dye and examined by fluorescent and phase-contrast microscopy. Micrographs are representative of three independent experiments.
PGD2 and 15d-PGJ2 mediate shear-induced chondrocyte apoptosis via Protein Kinase A-dependent regulation of Polo-like kinases
By coupling the DNA microarray technology with bioinformatics tools, we aimed to elucidate the signaling pathway of shear-induced COX-2-dependent chondrocyte apoptosis. Differentially regulated genes between control and shear-activated T/C-28a2 chondrocytes were identified by Significance Analysis of Microarray using the TMEV software.4,6 Using an average linkage hierarchical clustering algorithm with a Euclidean distance metric, in which genes are iteratively grouped together based on their distance metric, we determined that COX-2, Plk3, FAS, TP53INP1 and TP53INP11 are contained within the same sub-tree structure (Suppl. Fig. 4), thereby implicating co-regulation among these genes upon T/C-28a2 cell exposure to fluid shear. Plks are known to participate in the DNA damage response, and transduce the damage signals to specific effectors including p53 and Cdc2529. We therefore examined the roles of Plks, Cdc25, p53 and other apoptosis-related genes (FAS, TP53INPs, Bax) in shear-induced T/C-28a2 cell signaling. In view of our observations showing that high fluid shear (20 dyn/cm2) induces PGE24 and cAMP (Suppl. Fig. 2C) production, we also investigated the role of PKA, a cAMP-dependent ser/thr kinase, in shear-mediated chondrocyte apoptosis. As shown in Table 1, application of high shear stress to T/C-28a2 chondrocytes differentially regulates Plk1 and Plk3, downregulates Cdc25 and upregulates the mRNA synthesis of apoptosis-related genes (TP53INP1, TP53INP11, FAS and Bax). Even though application of high shear stress to T/C-28a2 chondrocytes induces pronounced and sustained cAMP production relative to static controls (Suppl. Fig. 2C), the expression of PKA catalytic subunit, PKA-Cα, is markedly suppressed at both transcriptional (Table 1) and translational (Fig. 3) levels at 48 h. Use of the COX-2 specific inhibitor, NS398 (50 μM), or selective knockdown of L-PGDS through siRNA technology reversed the shear-induced downregulation of PKA-Cα (Table 1 and Fig. 3), suggesting that the COX-2-derived PGD2 and its metabolite 15d-PGJ2 are responsible for the PKA-Cα regulation. Moreover, the aforementioned pharmacological and genetic interventions restored the shear-mediated changes in gene expression of Plk1, Plk3, Cdc25 and apoptosis-related genes (Table 1 and Fig. 3).
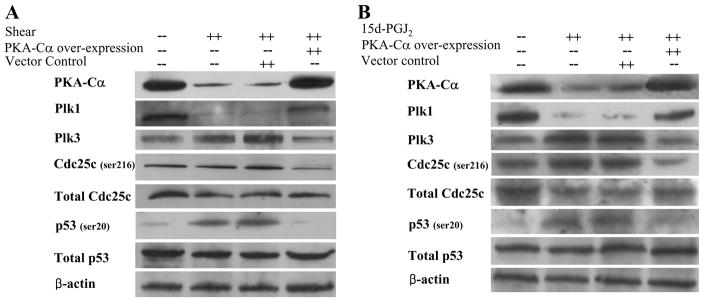
To delineate the role of PKA-Cα in the signaling cascade of shear-induced chondrocyte apoptosis, and in light of its marked downregulation in sheared chondrocytes, experiments were performed using T/C-28a2 chondrocytes transfected with a plasmid containing the cDNA of PKA-Cα. The efficacy of this genetic intervention was demonstrated at both transcriptional (Table 2) and translational levels (Fig. 5A). Ectopic expression of PKA-Cα abolished shear-induced chondrocyte apoptosis, as evidenced by a caspase-3 colorimetric assay (Fig. 1B) and the blockade of shear-induced mRNA upregulation of pro-apoptotic genes (FAS, TP53INP11) (Table 2). Interestingly, PKA-Cα overexpression reversed the shear-mediated changes in Plk1 and Plk3 expression (Table 2 and Fig. 5A) without affecting COX-2 mRNA upregulation (4.6±1.6 in control cells versus 4.2±0.8 in cells overexpressing PKA-Cα). Inhibition of PKA activity using H89 (2 μM) further exacerbated the shear-induced mRNA changes of Plks and pro-apoptotic genes (Table 2). Taken together, these data suggest that PKA-Cα is downstream of COX-2, controls the regulation of Plk1 and Plk3, and has an anti-apoptotic effect. This conclusion is corroborated by our observations showing that exogenous 15d-PGJ2 (5 μM) downregulates PKA-Cα expression in T/C-28a2 chondrocytes, whereas PKA-Cα overexpression reverses 15d-PGJ2-mediated changes in Plk1 and Plk3 expression at the mRNA (Suppl. Table 1) and protein (Fig. 5B) levels.
Table 2.
Effects of PKA-Cα overexpression, PKA inhibitor H89, Plk1 overexpression and Plk3 knockdown on mRNA transcript ratios in sheared chondrocytes, as assessed by real-time PCR.
| Transcript ratio (shear/static) | |||||
|---|---|---|---|---|---|
| Molecules of interest | Shear control† | PKA ectopic expression§ | H89‡ | Plk1 ectopic expression§ | Plk3 knockdown§ |
| PKA-Cα | 0.3±0.1 | 2.0±0.1 | 0.3±0.1 | 0.5±0.1 | 0.3±0.1 |
| Plk1 | 0.5±0.1 | 1.3±0.1 | 0.2±0.1 | 2.9±0.1 | 0.7±0.1 |
| Plk3 | 1.9±0.1 | 0.6±0.1 | 2.4±0.1 | 1.1±0.1 | 1.0±0.1 |
| Cdc25c | 0.3±0.1 | 0.7±0.1 | 0.2±0.1 | 0.7±0.1 | 0.5±0.1 |
| TP53INP1 | 1.5±0.1 | 1.4±0.1 | 2.9±0.1 | 1.0±0.1 | 1.0±0.1 |
| TP53INP11 | 3.5±0.1 | 0.5±0.1 | 1.4±0.1 | 1.2±0.1 | 1.5±0.1 |
| FAS | 7.6±0.1 | 0.6±0.1 | 26.7±0.1 | 2.5±0.1 | 1.5±0.1 |
All values represent transcript ratios for sheared (20 dyn/cm2, 48 h) to paired static controls (0 dyn/cm2, 48 h) of T/C28a2 chondrocytes. Paired treatments consisted of no treatment
shear transfected with PKA and Plk1 cDNA or Plk3 siRNA (shear) and static transfected with control vector or siRNA (static)
shear treated with H89 (5 μM) and static treated with 0.1% DMSO
Data are mean ± S.D (n ≥ 3). Gene expression of TP53INP1 was not significantly affected by PKA ectopic expression relative to shear control.
Figure 5.
Effect of PKA-Cα overexpression on protein expression or phosphorylation levels of select genes in (A) shear-activated and (B) 15d-PGJ2-stimulated chondrocytes. T/C-28a2 chondrocytes were transfected with a plasmid containing the cDNA of PKA-Ca, and then subjected either to fluid shear (20 dyn/cm2, 48 h) or 15d-PGJ2 stimulation (5 μM, 48 h). In select experiments, T/C-28a chondrocytes were transfected with the empty vector before being exposed to fluid shear or 15d-PGJ2 stimulation. Controls included T/C-28a2 chondrocytes subjected to static (no-flow) conditions for 48h. Representative Western hybridization experiments for select genes are shown in the figure. β-actin was probed as a loading control.
Plk1 overexpression and Plk3 knockdown reverse shear-induced chondrocyte apoptosis
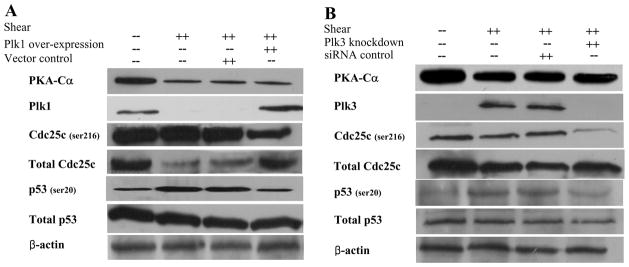
To elucidate the roles of Plk1 and Plk3 in the signaling pathway of shear-induced chondrocyte apoptosis, and in view of their differential regulation patterns in sheared chondrocytes, experiments were performed using T/C-28a2 chondrocytes transfected with either a plasmid containing the cDNA of Plk1 or an siRNA oligonucleotide sequence specific for Plk3. The efficacy of Plk1 overexpression and Plk3 knockdown was demonstrated at both transcriptional (Table 2) and translational levels (Fig. 6). These genetic interventions abrogated shear-induced chondrocyte apoptosis (Fig. 1B), but failed to alter PKA-Cα mRNA and protein expression (Table 2 and Fig. 6), thereby confirming that Plks are downstream of PKA-Cα.
Figure 6.
Effects of (A) Plk1 overexpression and (B) Plk3 knockdown on protein expression or phosphorylation levels of select genes in shear-activated chondrocytes. T/C-28a2 chondrocytes were transfected with either a plasmid containing the cDNA of Plk1 or an siRNA oligonucleotide sequence specific for Plk3, and then subjected to shear stress (20 dyn/cm2 for 48 h). In select experiments, T/C-28a chondrocytes were transfected with the empty vector or an siRNA control oligonucleotide before being exposed to fluid shear. Controls included T/C-28a2 chondrocytes subjected to static (no-flow) conditions for 48h. Representative Western hybridization experiments for select genes are shown in the figure. β-actin was probed as a loading control.
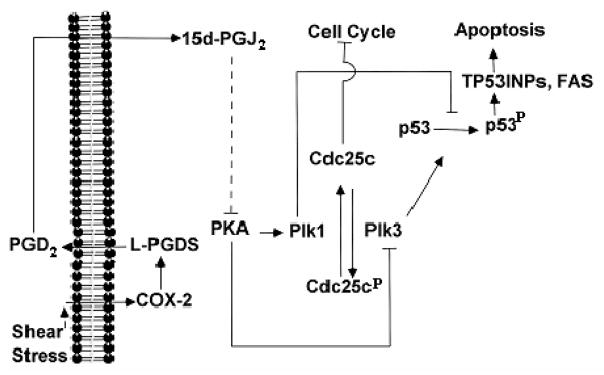
In light of previous observations showing that Cdc25c and p53 are directly targeted by Plks29, we investigated the effects of high fluid shear and Plk modulation on p53 and Cdc25c regulation. Our data reveal that p53 transcription (Table 1) and translation (Figs. 3, 5–6) are not regulated in sheared chondrocytes. However, high shear stress increases p53 phosphorylation at ser20, which is blocked by Plk1 overexpression (Fig. 6A) and Plk3 knockdown (Fig. 6B). Similarly, L-PGDS knockdown (Fig. 3) and PKA-Cα overexpression (Fig. 5A) restore shear-induced p53 phosphorylation to basal levels. All aforementioned genetic interventions were also effective in inhibiting the transcription of p53 effectors (TP53INPs and FAS), which are involved in the apoptosis pathway (Table 2). Exposure of T/C-28a2 chondrocytes to high fluid shear suppressed Cdc25c transcription (Table 1) and translation (Figs. 3, 5–6) whereas it increased Cdc25c phosphorylation (ser216) levels normalized to total Cdc25c protein (Figs. 3, 5–6). These data are consistent with DNA damage-induced cell cycle arrest, which precedes apoptosis.29 Genetic modulation of Plks as well as of genes acting upstream of Plk1 and Plk3 negated the shear-induced decrease in Cdc25c transcription and translation as well as the increase in Cdc25c phosphorylation (Figs. 3, 5–6). Taken together, our data reveal a novel signaling pathway by which high fluid shear mediates COX-2/L-PGDS-dependent chondrocytic cell cycle arrest and apoptosis (Fig. 7).
Figure 7.
Proposed cascade of signaling events in human T/C-28a2 chondrocytic cells subjected to high shear stress. In chondrocytes, prolonged application of high fluid shear stress (20 dyn/cm2) mediates cyclooxygenase-2 (COX-2)/L-prostaglandin D synthase (L-PGDS)-dependent chondrocyte apoptosis. L-PGDS, through PGD2/15d-PGJ2 accumulation and generation of reactive oxygen species (ROS), controls the downregulation of Protein Kinase A (PKA), which in turn regulate Polo-like kinase 1 (Plk1) and Plk3. Plks directly target Cdc25c and p53, and control the transcription of p53 effectors (TP53INPs, FAS and Bax), which are involved in chondrocyte apoptosis.
DISCUSSION
Excessive mechanical loading can directly damage the articular cartilage, adversely affect chondrocyte function and precipitate OA.2,30 We have previously demonstrated that application of high shear stress (20 dyn/cm2) to human chondrocytes induces COX-2 expression4,6,7, which represses their antioxidant capacity and contributes to apoptosis.4 The pivotal role of COX-2 in shear-induced chondrocyte apoptosis and the conflicting data in the literature on the roles of PGE29,14 and PGD2/15d-PGJ215–17 in chondrocyte apoptosis prompted us to investigate which COX-2-derived PG is involved in this process. Here we show that exogenously added PGD2 and 15d-PGJ2, but not PGE2, markedly diminish the viability of human T/C-28a2 chondrocytes under static (no shear) conditions. Along these lines, exogenous 15d-PGJ2 induces T/C-28a2 chondrocyte apoptosis, as assessed by a caspase 3 colorimetric assay and annexin V/PI double staining. In agreement with these observations, knockdown of L-PGDS, which is predominantly responsible for biosynthesis of PGD2 and J2 series in human chondrocytes, abolishes shear-induced chondrocyte apoptosis. In contrast to a previous report showing that 15d-PGJ2 induces chondrocyte apoptosis via a PPARγ-dependent pathway16, our data disclose that apoptosis of human T/C-28a2 chondrocytes induced by either 15d-PGJ2 or high shear stress proceeds via a PPARγ-independent and DP2-independent mechanism. Our findings are in concert with apoptosis data obtained using diverse cell types stimulated with exogenously added 15d-PGJ2.27,31 We and others26 have observed that the applied concentration of exogenous PGD2 and 15d-PGJ2 to induce apoptosis in different cell types exceeds that measured in cell culture supernatants. This discrepancy may be attributed to the enhanced efficiency of the endogenously released 15d-PGJ2 in stimulating apoptosis via an autocrine mechanism versus that of the commercial exogenously added compound.
Prior work has suggested that the cytotoxic potential of 15d-PGJ2 is associated with its ability to generate ROS in diverse cell types19, presumably due to its unsaturated α,β carbonyl moieties in the cyclopentanone ring. These moieties are also capable of reacting with thiol groups by Michael addition, thereby modifying the function of target proteins. Indeed, exogenously added 15d-PGJ2 induces ROS production in human T/C-28a2 chondrocytes. Treatment of T/C-28a2 chondrocytes with NAC inhibited in a dose-dependent manner the reduction of cell viability induced by 15d-PGJ2, with maximal inhibition observed at 5 mM (Suppl. Fig. 3B). NAC (5 mM) was also effective in inhibiting ROS production in 15d-PGJ2-stimulated T/C-28a2 cells. We have also found that prolonged application of high shear stress induces ROS generation in human T/C-28a2 chondrocytes. Although we were not able to test the effect of NAC (5 mM) treatment on shear-induced chondrocyte apoptosis and ROS accumulation due to cell detachment from glass slides during the shearing assay, we have reported that the decreased shear-induced antioxidant capacity of chondrocytes contributes to their apoptosis.4 This and other studies have also shown that elevated mechanical stress, including shear stress, releases ROS from chondrocytes30,32, and that antioxidants repress stress-induced cell death.4,30 Taken together, these findings reveal that oxidative stress mediates the damaging effects of fluid and mechanical shear stress on chondrocytes.
Plks are known to play a pivotal role in the DNA damage response and apoptosis.29 By coupling the DNA microarray technology with clustering algorithms, we determined that COX-2, Plk3 and p53 effectors including TP53INP1, TP53INP11 and FAS are co-regulated in shear-activated T/C-28a2 chondrocytes. Using molecular interventions, we demonstrated for the first time that high fluid shear differentially regulates Plk1 and Plk3 expression in a COX-2/L-PGDS-dependent manner in sheared chondrocytes, which are in turn responsible for the transcriptional and translational downregulation of Cdc25c and the increased phosphorylation of p53. p53 regulation can be mediated via different mechanisms such as de novo transcription, increased translation of p53 mRNA, p53 protein stabilization or covalent protein modification. Prior work has shown that p53 protein expression is upregulated in shear-stimulated bovine aortic endothelial cells33, in smooth muscle cells stimulated with cyclic strain34 and in human chondrocyte explants subjected to elevated mechanical stress.30 However, our data reveal that high fluid shear does not alter p53 expression at the transcriptional or translational level. Instead, the accumulation of active p53 in response to high shear stress occurs through a post-translational mechanism, as evidenced by increased p53 phosphorylation (ser20) levels. p53 phosphorylation is blocked by overexpressing Plk1 or knocking down Plk3. These findings are in accord with previous studies showing that Plk3 interacts and phosphorylates p53 in hydrogen peroxide-stimulated human fibroblasts35, whereas Plk1 negatively affects the pro-apoptotic function of p53 in human tumor cell lines.29
We have previously shown that shear-induced chondrocyte apoptosis is characterized by cell shrinkage, membrane blebbing, DNA fragmentation, mitochondrial depolarization and caspase-9 activation4, thereby suggesting that it proceeds via the intrinsic apoptotic pathway. p53 is known to play a key role in mitochondrial membrane stability and regulation of apoptosis-related proteins.36 Our data, in conjunction with previously published results36, suggest that p53 activation, as evidenced by increased p53 phosphorylation levels in sheared chondrocytes, is responsible for the transactivation of Bax, disruption of mitochondrial integrity, and apoptosis via the intrinsic pathway. Apoptosis can also be initiated by death receptor ligation such as FAS-FAS ligand. Our results reveal that high shear stress also upregulates the mRNA synthesis of FAS in human T/C-28a2 chondrocytes. In view of prior observations identifying FAS as a primary target gene regulated by p5337, our data suggest that shear-induced apoptosis can also proceed via the p53-mediated death receptor pathway. This is corroborated by our observations showing that high shear stress activates caspase-8, which is blocked by PGDS knockdown. Exogenous 15d-PGJ2 activates caspase-9 and -8, thereby illustrating the involvement of both the intrinsic and extrinsic pathways in the apoptosis of human T/C-28a2 chondrocytes. Moreover, use of the pan caspase inhibitor zVAD-FMK (25 μM) partially inhibited the extent of 15d-PGJ2-mediated chondrocyte death.
In light of our results showing that high fluid shear induces PGE2 and cAMP production, we also investigated the contribution of PKA-Cα, a cAMP-dependent ser/thr kinase, to shear-mediated chondrocyte apoptosis. Previous studies have presented conflicting data regarding the involvement of cAMP/PKA in chondrocyte function. Specifically, Miwa et al.14 reported that PGE2 directly induces apoptosis in bovine articular chondrocytes via a cAMP-dependent pathway. In contrast, Karsdal et al.38 reported that increased cAMP levels in mouse chondrocytes inhibited cytokine-induced MMP expression and activity, and blocked cartilage degradation. Our data reveal that high shear stress induces a pronounced and sustained release of cAMP. Although PKA-Cα transcriptional activity is upregulated in sheared chondrocytes at short (1–2 h) shear exposure times (data not shown), prolonged (48h) exposure of T/C-28a2 cells to high fluid shear markedly downregulates PKA-Cα mRNA synthesis and protein expression. Since PKA-Cα downregulation is reversed by L-PGDS knockdown, we hypothesize that PGD2 and its non enzymatic dehydration end product 15d-PGJ2 are involved in this process, presumably through the generation of ROS. Our results disclose that ectopic expression of PKA-Cα effectively blocks shear-induced chondrocyte apoptosis, thereby suggesting that PKA-Cα confers protection to chondrocyte apoptosis in response to shear stress. In accord with these observations, inhibition of PKA-Cα using H89 or use of the cAMP inhibitor SQ22536 (data not shown) exacerbates the extent of shear-induced apoptosis.
Using molecular interventions, we demonstrated for the first time that PKA-Cα, which is downstream of L-PGDS, controls the differential regulation of Plk1 and Plk3 (Fig. 7). Plk1 and Plk3 regulate in turn p53 activation and Cdc25c transcription, translation and phosphorylation (Fig. 7). p53 may be directly responsible for shear-induced Cdc25c transcriptional and translational regulation, in light of previous findings revealing the presence of a p53 binding site in the Cdc25c promoter, which confers p53-dependent repression.39 Thus, p53 appears to promote shear-induced chondrocyte apoptosis via chondrocytic cell cycle arrest and activation of both the extrinsic and intrinsic apoptotic pathways. Reconstructing the signaling pathways regulating cartilage degradation and chondrocyte apoptosis in response to high shear stress may identify potential therapeutic targets for controlling arthritic pathogenesis and/or progression and may be useful in the design of bioreactors for cartilage tissue engineering applications.
MATERIALS and METHODS
Reagents
PGD2, 15d-PGJ2, PGE2, the COX-2 specific inhibitor NS398, the PPARγ antagonist T0070907 and Bayu3405 were obtained from Cayman Chemical (Ann Arbor, MI, USA). L-PGDS siRNA (SC-41640), Plk3 siRNA (SC-39150) and fluorescein-conjugated siRNA control (SC-44240) were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Plk1 cDNA (SC110978) and PKA-Cα cDNA (SC125607) were supplied from OriGene Technologies (Rockville, MD, USA). Antibodies specific for PKA-Cα (4782), Plk1 (4513), phospho-Cdc25c (ser216) (4901), cleaved caspase-8 (9496), and cleaved caspase-9 (9505) were purchased from Cell Signaling Technology (Danvers, MA, USA). Antibodies for p53 (SC6243), phospho-p53 (ser20) (SC-18079-R), Cdc25c (SC-5620), Plk3 (SC-25422), and L-PGDS (SC-14824) were Santa Cruz Biotechnology. H89 was purchased from Biomol international (Plymouth Meeting, PA, USA). The pan caspase inhibitor z-VAD-FMK (carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone) was from Promega (Madison, WI, USA). All other reagents were from Invitrogen (Carlsbad, California, USA), unless otherwise specified.
Cell Culture and Shear Stress Exposure
Human T/C-28a2 chondrocytic cells were grown (37°C in 5% CO2) on glass slides in 1:1 Ham’s F12/DMEM medium supplemented with 10% FBS.4,6,7 Before shear exposure, T/C-28a2 cells were incubated for 18 h in serum-free medium containing 1% Nutridoma (Sigma-Aldrich, St. Louis, MO, USA), a low protein serum replacement that maintains chondrocyte phenotype. T/C-28a2 cells were subjected to a laminar shear stress (20 dyn/cm2; for 48 h or 72 h) in media containing 1% Nutridoma by the use of a streamer gold flow device (Flexcell International, Hillsborough, NC, USA).
Microarray Hybridization and Analysis
Microarray experiments were performed as previously described.4 Briefly, total RNA was isolated from five independent, paired static and sheared T/C-28a2 chondrocyte specimens using TRIzol, and purified with the RNeasy Mini Kit combined with DNase treatment on column, according to the manufacturer’s protocol (Qiagen, Valencia, CA, USA). Total RNA (15 μg) was reversed transcribed in the presence of random primers and aminoallyl(aa)-dUTP with Superscript II Reverse Transcriptase. aa-dUTP-labeled targets from sheared and static control specimens were coupled to NHS-Cy-5 and NHS-Cy-3 (GE Healthcare, Piscataway, NJ, USA), respectively. Cy-5 and Cy-3-labeled targets were mixed, and co-hybridized onto microarray slides printed with a set of 39,936 human ESTs. Expression ratios were derived by using TIGR Spotfinder.4,6 Differentially expressed genes were identified by Significance Analysis of Microarrays and further analyzed with the software TMEV.4,6
Quantitative Real-Time PCR (qRT-PCR)
qRT-PCR assays were performed on the iCycler iQ detection system (Biorad, Hercules, CA, USA) using total RNA, the iScript one-step RT-PCR kit with SYBR green (Biorad) and primers. The GenBank accession numbers and forward (F-) and reverse (R-) primers are as follows: L-PGDS (NM_000954), F-GCCTCGCCTCCAACTCGAGC, R-TGCAGCAGCATGGTTCGGGT; PKA-Cα (NM_002730), F-GCGTGAAAGAATTCTTAGCCA, R-CCACCTTCTGTTTGTCGAGGA; Plk1 (NM_005030), F-TTGCTGACCCAGAAGATGG, R-CACAGTGTCAATGCCTCCAA; Plk3 (NM_004073), F-TTGTGCTGGTGGGATTGTAGTGCACAG, R-GTGGCCACAGTAGTGGAGTCAGCCC; CDC25C (NM_001790), F-GACACCCAGAAGAGAATAATCATC, R-CGACACCTCAGCAACTCAG; p53 (NM_000546), F-TGCGTGTGGAGTATTTGGATG, R-TGGTACAGTCAGAGCCAACCAG; FAS (NM_152876), F-CAGAACTTGGAAGGCCTGCATC, R-TCTGTTCTGCTGTGTCTTGGAC; TP53INP1 (NM_033285), F-GCATGTCTGTCTATGCTGTGC, R-TTCATTTTGAGCTTCCACTCTG; TP53INP11 (NM_006034), F-CACGGACAAAGGGACACACA, R-GTACATGCCAAGGGGCAAGA; Bax (NM_000181), F-TGGAGCTGCAGAGGATGATT; R-CAGTTGAAGTTGCCGTCAGA; GAPDH (NM_002046), F-CCACCCATGGCAAATTCCATGGCA, R-TCTAGACGGCAGGTCAGGTCCACC.
GAPDH was used as internal control. Reaction mixtures were incubated at 50 °C for 15 min followed by 95 °C for 5min, and then 35 PCR cycles were performed with the following temperature profile: 95 °C 15s, 58 °C 30s, 68 °C 1 min, 77 °C 20s. Data were collected at the (77 °C 20 s) step to remove possible fluorescent contribution from dimer-primers. 40
Transient Transfection
For ectopic expression of PKA-Cα and Plk1, T/C28a2 chondrocytes were transfected with 5 μg/ml of plasmid containing the cDNA of the gene of interest by using Lipofectamine 2000. In control experiments, cells were transfected with 5 μg/ml of the empty vector pCMV6-XL (OriGene Technologies). In RNA interference assays, T/C-28a2 cells were transfected with 100 nM of an siRNA oligonucleotide sequence specific for either L-PGDS or Plk3, or control siRNA. Transfected cells were allowed to recover for at least 12 h in growth medium, and then incubated overnight in medium containing 1% Nutridoma before their exposure to shear or static conditions.
Cell Viability, PGD2 and cAMP Production, caspase-3 activity
Cell viability was monitored with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay (TOX1, Sigma-Aldrich), according to the manufacturer’s protocol. 15d-PGJ2, PGD2 and cAMP levels were determined by the 15d-Δ12,14-PGJ2 EIA kit (Assay Designs, Ann Arbor, MI, USA), the prostaglandin D2 monoclonal EIA kit and cyclic AMP EIA kit (Cayman Chemical), respectively, following the manufacturer’s instructions. For PGD2 and 15d-PGJ2 measurements, the medium volume per slide was used as loading control, and the results were expressed as pg/ml. For cAMP measurements, the protein concentration of total cell lysate was used as loading control, and the results were expressed as pmol/ml. Caspase-3 activity was quantified by the caspase-3 colorimetric assay kit (Sigma-Alderich).
ROS Detection and Annexin V/PI Assay
ROS generation was detected by staining T/C-28a2 chondrocytes with the Image-iT ™ LIVE Green Reactive Oxygen Species Detection dye (136007) and examining them by fluorescent microscopy. T/C-28a2 cell death was monitored by FITC-conjugated annexin V and PI double staining (V13242), performed according to the manufacturer’s instructions, and analysis by flow cytometry (FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA).
Western blot analysis
T/C-28a2 cells, from sheared and matched static control specimens, were lysed in RIPA buffer (25 mM Tris·HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) containing a cocktail of proteinase inhibitors (Pierce, Rockford, IL, USA). The protein content of the cell lysates was determined using bicinchoninic acid (BCA) protein assay reagent (Pierce, Rockford, IL, USA). Total cell lysates (10 μg) were subjected to SDS-PAGE, transferred to a membrane, and probed with a panel of specific antibodies. Each membrane was only probed using two different antibodies. Membranes were stripped using the Restrore Plus Stripping Buffer (Pierce) prior to the use of the second probing antibody. Phospho-specific antibody detections preceded those of the non-phosphorylated (total) antibody. β-actin was used as loading control. All Western hybridizations were performed at least in triplicate using a different cell preparation each time.
Supplementary Material
Acknowledgments
This work was supported, in whole or in part, by the National Institutes of Health NIAMS Grants RO1 AR053358 (KK) and AR52768 (AKK) and the Masson-Agarwal Faculty Scholar award (KK).
Abbreviations
- COX
cyclooxygenase
- PG
prostaglandin
- 15d-PGJ2
15-deoxy-Δ12,14-PGJ2
- L-PGDS
Lipocalin type-prostaglandin D synthase
References
- 1.Carter DR, Beaupre GS, Wong M, Smith RL, Andriacchi TP, Schurman DJ, et al. The mechanobiology of articular cartilage development and degeneration. Clin Orthop. 2004:S69–77. doi: 10.1097/01.blo.0000144970.05107.7e. [DOI] [PubMed] [Google Scholar]
- 2.Buckwalter JA, Martin JA, Brown TD. Perspectives on chondrocyte mechanobiology and osteoarthritis. Biorheology. 2006;43:603–9. [PubMed] [Google Scholar]
- 3.Yokota H, Goldring MB, Sun HB. CITED2-mediated regulation of MMP-1 and MMP-13 in human chondrocytes under flow shear. J Biol Chem. 2003;278:47275–80. doi: 10.1074/jbc.M304652200. [DOI] [PubMed] [Google Scholar]
- 4.Healy ZR, Lee NH, Gao X, Goldring MB, Talalay P, Kensler TW, et al. Divergent responses of chondrocytes and endothelial cells to shear stress: cross-talk among COX-2, the phase 2 response, and apoptosis. Proc Natl Acad Sci U S A. 2005;102:14010–5. doi: 10.1073/pnas.0506620102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee MS, Trindade MCD, Ikenoue T, Goodman SB, Schurman DJ, Smith RL. Regulation of nitric oxide and bcl-2 expression by shear stress in human osteoarthritic chondrocytes in vitro. J Cell Biochem. 2003;90:80–86. doi: 10.1002/jcb.10611. [DOI] [PubMed] [Google Scholar]
- 6.Abulencia JP, Gaspard R, Healy ZR, Gaarde WA, Quackenbush J, Konstantopoulos K. Shear-induced cyclooxygenase-2 via a JNK2/c-Jun-dependent pathway regulates prostaglandin receptor expression in chondrocytic cells. J Biol Chem. 2003;278:28388–94. doi: 10.1074/jbc.M301378200. [DOI] [PubMed] [Google Scholar]
- 7.Healy ZR, Zhu F, Stull JD, Konstantopoulos K. Elucidation of the signaling network of COX-2 induction in sheared chondrocytes: COX-2 is induced via a Rac/MEKK1/MKK7/JNK2/c-Jun-C/EBPbeta-dependent pathway. Am J Physiol Cell Physiol. 2008;294:C1146–57. doi: 10.1152/ajpcell.00542.2007. [DOI] [PubMed] [Google Scholar]
- 8.Amin AR, Attur M, Patel RN, Thakker GD, Marshall PJ, Rediske J, et al. Superinduction of cyclooxygenase-2 activity in human osteoarthritis-affected cartilage. Influence of nitric oxide. J Clin Invest. 1997;99:1231–7. doi: 10.1172/JCI119280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Notoya K, Jovanovic DV, Reboul P, Martel-Pelletier J, Mineau F, Pelletier JP. The induction of cell death in human osteoarthritis chondrocytes by nitric oxide is related to the production of prostaglandin E2 via the induction of cyclooxygenase-2. J Immunol. 2000;165:3402–10. doi: 10.4049/jimmunol.165.6.3402. [DOI] [PubMed] [Google Scholar]
- 10.Pelletier JP, Fernandes JC, Jovanovic DV, Reboul P, Martel-Pelletier J. Chondrocyte death in experimental osteoarthritis is mediated by MEK 1/2 and p38 pathways: role of cyclooxygenase-2 and inducible nitric oxide synthase. J Rheumatol. 2001;28:2509–19. [PubMed] [Google Scholar]
- 11.Di Battista JA, Dore S, Morin N, He Y, Pelletier JP, Martel-Pelletier J. Prostaglandin E2 stimulates insulin-like growth factor binding protein-4 expression and synthesis in cultured human articular chondrocytes: possible mediation by Ca(++)-calmodulin regulated processes. J Cell Biochem. 1997;65:408–19. [PubMed] [Google Scholar]
- 12.Tchetina EV, Di Battista JA, Zukor DJ, Antoniou J, Poole AR. Prostaglandin PGE2 at very low concentrations suppresses collagen cleavage in cultured human osteoarthritic articular cartilage: this involves a decrease in expression of proinflammatory genes, collagenases and COL10A1, a gene linked to chondrocyte hypertrophy. Arthritis Res Ther. 2007;9:R75. doi: 10.1186/ar2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Portanova JP, Zhang Y, Anderson GD, Hauser SD, Masferrer JL, Seibert K, et al. Selective neutralization of prostaglandin E2 blocks inflammation, hyperalgesia, and interleukin 6 production in vivo. J Exp Med. 1996;184:883–91. doi: 10.1084/jem.184.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miwa M, Saura R, Hirata S, Hayashi Y, Mizuno K, Itoh H. Induction of apoptosis in bovine articular chondrocyte by prostaglandin E(2) through cAMP-dependent pathway. Osteoarthritis Cartilage. 2000;8:17–24. doi: 10.1053/joca.1999.0266. [DOI] [PubMed] [Google Scholar]
- 15.Zayed N, Li X, Chabane N, Benderdour M, Martel-Pelletier J, Pelletier JP, et al. Increased expression of lipocalin-type prostaglandin D2 synthase in osteoarthritic cartilage. Arthritis Res Ther. 2008;10:R146. doi: 10.1186/ar2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shan ZZ, Masuko-Hongo K, Dai SM, Nakamura H, Kato T, Nishioka K. A potential role of 15-deoxy-delta(12,14)-prostaglandin J2 for induction of human articular chondrocyte apoptosis in arthritis. J Biol Chem. 2004;279:37939–50. doi: 10.1074/jbc.M402424200. [DOI] [PubMed] [Google Scholar]
- 17.Relic B, Benoit V, Franchimont N, Ribbens C, Kaiser MJ, Gillet P, et al. 15-deoxy-delta12,14-prostaglandin J2 inhibits Bay 11–7085-induced sustained extracellular signal-regulated kinase phosphorylation and apoptosis in human articular chondrocytes and synovial fibroblasts. J Biol Chem. 2004;279:22399–403. doi: 10.1074/jbc.M314118200. [DOI] [PubMed] [Google Scholar]
- 18.Tsubouchi Y, Sano H, Kawahito Y, Mukai S, Yamada R, Kohno M, et al. Inhibition of human lung cancer cell growth by the peroxisome proliferator-activated receptor-gamma agonists through induction of apoptosis. Biochem Biophys Res Commun. 2000;270:400–5. doi: 10.1006/bbrc.2000.2436. [DOI] [PubMed] [Google Scholar]
- 19.Kondo M, Shibata T, Kumagai T, Osawa T, Shibata N, Kobayashi M, et al. 15-Deoxy-Delta(12,14)-prostaglandin J(2): the endogenous electrophile that induces neuronal apoptosis. Proc Natl Acad Sci U S A. 2002;99:7367–72. doi: 10.1073/pnas.112212599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fahmi H, Di Battista JA, Pelletier JP, Mineau F, Ranger P, Martel-Pelletier J. Peroxisome proliferator--activated receptor gamma activators inhibit interleukin-1beta-induced nitric oxide and matrix metalloproteinase 13 production in human chondrocytes. Arthritis Rheum. 2001;44:595–607. doi: 10.1002/1529-0131(200103)44:3<595::AID-ANR108>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 21.Zayed N, Afif H, Chabane N, Mfuna-Endam L, Benderdour M, Martel-Pelletier J, et al. Inhibition of interleukin-1beta-induced matrix metalloproteinases 1 and 13 production in human osteoarthritic chondrocytes by prostaglandin D2. Arthritis Rheum. 2008;58:3530–40. doi: 10.1002/art.23958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kokenyesi R, Tan L, Robbins JR, Goldring MB. Proteoglycan production by immortalized human chondrocyte cell lines cultured under conditions that promote expression of the differentiated phenotype. Arch Biochem Biophys. 2000;383:79–90. doi: 10.1006/abbi.2000.2044. [DOI] [PubMed] [Google Scholar]
- 23.Goldring MB, Birkhead JR, Suen LF, Yamin R, Mizuno S, Glowacki J, et al. Interleukin-1 beta-modulated gene expression in immortalized human chondrocytes. J Clin Invest. 1994;94:2307–16. doi: 10.1172/JCI117595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang X, Wu L, Hand T, Andreasson K. Prostaglandin D2 mediates neuronal protection via the DP1 receptor. J Neurochem. 2005;92:477–86. doi: 10.1111/j.1471-4159.2004.02870.x. [DOI] [PubMed] [Google Scholar]
- 25.Bishop-Bailey D, Hla T. Endothelial cell apoptosis induced by the peroxisome proliferator-activated receptor (PPAR) ligand 15-deoxy-Delta12, 14-prostaglandin J2. J Biol Chem. 1999;274:17042–8. doi: 10.1074/jbc.274.24.17042. [DOI] [PubMed] [Google Scholar]
- 26.Eichele K, Ramer R, Hinz B. Decisive role of cyclooxygenase-2 and lipocalin-type prostaglandin D synthase in chemotherapeutics-induced apoptosis of human cervical carcinoma cells. Oncogene. 2008;27:3032–44. doi: 10.1038/sj.onc.1210962. [DOI] [PubMed] [Google Scholar]
- 27.Xiang Z, Lin T, Reeves SA. 15d-PGJ2 induces apoptosis of mouse oligodendrocyte precursor cells. J Neuroinflammation. 2007;4:18. doi: 10.1186/1742-2094-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monneret G, Li H, Vasilescu J, Rokach J, Powell WS. 15-Deoxy-delta 12,14-prostaglandins D2 and J2 are potent activators of human eosinophils. J Immunol. 2002;168:3563–9. doi: 10.4049/jimmunol.168.7.3563. [DOI] [PubMed] [Google Scholar]
- 29.Xie S, Xie B, Lee MY, Dai W. Regulation of cell cycle checkpoints by polo-like kinases. Oncogene. 2005;24:277–86. doi: 10.1038/sj.onc.1208218. [DOI] [PubMed] [Google Scholar]
- 30.Martin JA, Buckwalter JA. Post-traumatic osteoarthritis: the role of stress induced chondrocyte damage. Biorheology. 2006;43:517–21. [PubMed] [Google Scholar]
- 31.Li L, Tao J, Davaille J, Feral C, Mallat A, Rieusset J, et al. 15-deoxy-Delta 12,14-prostaglandin J2 induces apoptosis of human hepatic myofibroblasts. A pathway involving oxidative stress independently of peroxisome-proliferator-activated receptors. J Biol Chem. 2001;276:38152–8. doi: 10.1074/jbc.M101980200. [DOI] [PubMed] [Google Scholar]
- 32.Yamazaki K, Fukuda K, Matsukawa M, Hara F, Matsushita T, Yamamoto N, et al. Cyclic tensile stretch loaded on bovine chondrocytes causes depolymerization of hyaluronan: involvement of reactive oxygen species. Arthritis Rheum. 2003;48:3151–8. doi: 10.1002/art.11305. [DOI] [PubMed] [Google Scholar]
- 33.Lin K, Hsu PP, Chen BP, Yuan S, Usami S, Shyy JY, et al. Molecular mechanism of endothelial growth arrest by laminar shear stress. Proc Natl Acad Sci U S A. 2000;97:9385–9. doi: 10.1073/pnas.170282597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayr M, Hu Y, Hainaut H, Xu Q. Mechanical stress-induced DNA damage and rac-p38MAPK signal pathways mediate p53-dependent apoptosis in vascular smooth muscle cells. Faseb J. 2002;16:1423–5. doi: 10.1096/fj.02-0042fje. [DOI] [PubMed] [Google Scholar]
- 35.Xie S, Wang Q, Wu H, Cogswell J, Lu L, Jhanwar-Uniyal M, et al. Reactive oxygen species-induced phosphorylation of p53 on serine 20 is mediated in part by pololike kinase-3. J Biol Chem. 2001;276:36194–9. doi: 10.1074/jbc.M104157200. [DOI] [PubMed] [Google Scholar]
- 36.Green DR, Kroemer G. Cytoplasmic functions of the tumour suppressor p53. Nature. 2009;458:1127–30. doi: 10.1038/nature07986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kannan K, Amariglio N, Rechavi G, Jakob-Hirsch J, Kela I, Kaminski N, et al. DNA microarrays identification of primary and secondary target genes regulated by p53. Oncogene. 2001;20:2225–34. doi: 10.1038/sj.onc.1204319. [DOI] [PubMed] [Google Scholar]
- 38.Karsdal MA, Sumer EU, Wulf H, Madsen SH, Christiansen C, Fosang AJ, et al. Induction of increased cAMP levels in articular chondrocytes blocks matrix metalloproteinase-mediated cartilage degradation, but not aggrecanase-mediated cartilage degradation. Arthritis Rheum. 2007;56:1549–58. doi: 10.1002/art.22599. [DOI] [PubMed] [Google Scholar]
- 39.St Clair S, Giono L, Varmeh-Ziaie S, Resnick-Silverman L, Liu WJ, Padi A, et al. DNA damage-induced downregulation of Cdc25C is mediated by p53 via two independent mechanisms: one involves direct binding to the cdc25C promoter. Mol Cell. 2004;16:725–36. doi: 10.1016/j.molcel.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Zhu F, Massana R, Not F, Marie D, Vaulot D. Mapping of picoeucaryotes in marine ecosystems with quantitative PCR of the 18S rRNA gene. FEMS Microbiol Ecol. 2005;52:79–92. doi: 10.1016/j.femsec.2004.10.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.