Abstract
The hair cells of the vertebrate inner ear posses active mechanical processes to amplify their inputs. The stereocilia bundle of various vertebrate animals can produce active movements. Though standard stereocilia-based mechanisms to promote amplification persist in mammals, an additional radically different mechanism evolved: the so called somatic electromotility which refers to the elongation/contraction of the outer hair cells’ (OHC) cylindrical cell body in response to membrane voltage changes. Somatic electromotility in OHCs, as the basis for cochlear amplification, is a mammalian novelty and it is largely dependent upon the properties of the unique motor protein prestin. We review recent literature which has demonstrated that although the gene encoding prestin is present in all vertebrate species, mammalian prestin has been under positive selective pressure to acquire motor properties, probably rendering it fit to serve somatic motility in outer hair cells. Moreover, we discuss data which indicates that a modified α10 nicotinic cholinergic receptor subunit has coevolved in mammals, most likely to give the auditory feedback system the capability to control somatic electromotility.
Keywords: prestin, electromotility, alpha9alpha10 nicotinic receptor, ligand-gated ion channel, positive selection, molecular evolution, outer hair cells
Introduction
The receptor cells of most sensory organs must amplify their signals to increase sensitivity in order to separate them from noise. In the case of the vertebrate auditory inner ear, sensitivity has been increased by a number of mechanisms, including using the external ear to funnel sounds to the eardrum, all the way to active mechanical processes within the inner ear to amplify their inputs (Ashmore, 2008; Dallos, 2008; Hudspeth, 2008; Manley, 2001). Thus in the ears of many species, including humans, the threshold of normal hearing lies at sound-pressure levels around zero decibels (0 dB). This level of sensitivity implies that we can hear stimuli down to a limit imposed by thermal vibrations in the ear, indicating the active process’s profound capacity for amplification.
The mechanism by which the mammalian inner ear achieved its high sensitivity has been the subject of numerous studies over a long period of time and is still today a field of intense research (Ashmore, 2008; Dallos, 2008; Hudspeth, 2008; Manley, 2001). Through the measurement of the motion of inner ear components at unphysiologically high sound pressure levels, von Békésy concluded that at threshold and using only passive mechanisms, inner ear sensory cells respond to motions equivalent to the diameter of hydrogen atoms (von Békésy, 1960). In 1948 Gold had proposed that the inner ear must contain an amplifying mechanism that released mechanical energy into the cellular system to increase sensitivity at low sound levels (Gold, 1948). It was Kemp in 1978 who demonstrated Gold’s prediction when he discovered that sounds were actively emitted by the inner ear when stimulated with brief sound stimuli (Kemp, 1978). This discovery over the years came to be known as otoacoustic emissions and provided a way to analyze the hearing organ non-invasively both in animals and humans. Davis (1983) suggested that such an active process underlies sensitive hearing and coined the term “cochlear amplifier” as a general designation for the mechanisms that feed mechanical energy into hair-cell motions to strongly increase the cells’ sensitivity. In fact, hair cell-possessing vertebrates express an ancient universal mechanism for amplification, based on mechanical feedback by stereocilia (Martin & Hudspeth, 1999). In addition, mammals posses a second amplifier based on somatic motility of outer hair cells (OHCs) in response to changes in membrane voltage, as originally described by Brownell et al. (1985). This somatic electromotility is brought about by molecular rearrangements in the motor protein prestin (Zheng et al., 2000). The present work reviews the evolutionary history of prestin and data which supports the notion that a positively-selected mammalian prestin serves as the motor protein only in mammals. In addition, it reviews work which indicates that molecular components of the medial olivocochlear (MOC) efferent innervation to hair cells were shaped by positive selection in mammals.
Two mechanisms for amplification
The hair cells of vertebrates have a similar structure and transduce mechanical stimuli in the same way. Each of these epithelial cells is surmounted by a hair bundle, an erect cluster of 20–300 cylindrical processes called stereocilia. When sound reaches the cochlea, it elicits mechanical vibrations that are sensed and transduced into an electrical response by motion of the hair bundles of hair cells which contain the mechanically-gated ion channels of unknown primary sequence. At the same time, however, the hair cells perform work by increasing the magnitude of their mechanical input. This amplification of the stimulus constitutes a positive feedback that enhances the sensitivity of hearing by countering the loss of energy through the viscous dissipation that accompanies the motion of hair bundles and other structures through the fluids of the inner ear (Hudspeth, 2008). It is not the aim of the present work to provide an extensive review of the data regarding cochlear amplification. For a comprehensive overview of this matter see (Ashmore, 2008; Dallos, 2008; Hudspeth, 2008).
Crawford and Fettiplace (1985) showed that stereocilia bundles of turtle hair cells are capable of producing active mechanical reactions to incoming mechanical stimuli as well as spontaneous movements. Numerous follow-up experiments amply demonstrated that the stereocilia bundle of various vertebrate animals can produce active movements (Assad et al., 1992; Howard et al., 1988; Manley et al., 2001; Martin et al., 1999; Martin et al., 2001; Martin et al., 2003; Ricci et al., 2000). Though standard stereocilia-based mechanisms to promote amplification persist in mammals (Chan et al., 2005; Jia et al., 2005; Kennedy et al., 2005), an additional radically different mechanism evolved: the so called somatic electromotility which refers to the elongation/contraction of the OHC’s cylindrical cell body in response to membrane hyperpolarization/depolarization cycles (Brownell et al., 1985). Somatic electromotility in OHCs, as the basis for cochlear amplification, is a mammalian novelty and it is largely dependent upon the properties of the unique motor protein prestin (Zheng et al., 2000). Although we are still left without a clear picture of how these two mechanisms interact, stereocilia and somatic motility could provide alternative or complementary ways for cochlear amplification in mammalian hair cells. Since stereocilia based motility is ubiquitous among vertebrate hair cells, it has the potential to produce amplification in any hair cell. By contrast, somatic motility, is exclusive of OHCs and thus of mammals, and therefore can amplify only in this restricted group of vertebrates. Hence, as proposed by Dallos (2008), it might be the case that stereocilia motility is the general amplifier and that in mammals somatic processes have evolved to control/adjust the amplifier.
Prestin: the OHC motor protein
Comprehensive reviews concerning the properties of prestin have already been published (Ashmore, 2008; Dallos, 2008; Dallos et al., 2006; Geleoc et al., 2003). Only some concepts concerning prestin’s operation are discussed here.
Based upon the knowledge that only OHCs express the putative gene that codes for the motor protein involved in amplification, prestin was identified using suppression subtractive hybridization PCR (Zheng et al., 2000). Prestin, encoded by the gene PRES, is the fifth member of the solute carrier anion-transport family 26 (SLC26A5), membrane antiporters that transfer anionic molecules across the cell membrane (Mount et al., 2004). Although prestin is best modeled as an anion transporter (Muallem et al., 2006), mammalian prestin does not undergo a full transport cycle (Schaechinger et al., 2007). It is an incomplete transporter, failing to unload a bound anion at the extracellular face of the protein (Oliver et al., 2001).
Prestin is an integral membrane protein, but its structure is not fully agreed upon. The hydropathy plot indicates a protein with a hydrophobic region extending over ~450 amino acids, similar to many transporters of the superfamily (Figure 1). It has a relatively short NH2-terminal region and an extended COOH-terminal end. Prestin has a hydropathy plot similar to that of pendrin, another member of the SLC26 family also expressed in cochlear tissue (Lohi et al., 2000). It is agreed that an even number of helices span the membrane as both NH2 terminal and COOH terminal ends lie within the cytoplasm, established experimentally by tagging the ends and expressing the protein in heterologous systems (Ludwig et al., 2001; Zheng et al., 2001). Subsequent reports have been more definite and identified 12 transmembrane α-helices (Oliver et al., 2001), 10 alpha-helices inserting across the membrane with 2 nonspanning helices present in the set of helices (Deak et al., 2005) or 10 transmembrane helices alone (Navaratnam et al., 2005). Transmembrane region 2 contains the sulfate motif defining the family. The long COOH terminal region from amino acids 496 to 744 contains runs of both positive and negative charges as well as a sequence defined as a STAS domain. When OHC membranes are examined in freeze fracture, densely packed ~11 nm diameter particles are revealed (Gulley et al., 1977). It is assumed that the particles consist of some multimer of the motor protein inasmuch as the 744 amino acid prestin molecule is too small to produce an 11 nm monomer. Both dimeric (Navaratnam et al., 2005) and tetrameric (Zheng et al., 2006) stoichiometries of prestin have been proposed.
Figure 1.
Predicted secondary structure and topology of human prestin based on the model previously described by Zheng et al. (2001, 2005, 2006). The location of the 12 transmembrane domains is based on Olivier et al. (2001) and Rajogopalan et al. (2006). Transmembrane domain 2 in blue contains the sulfate motif defining the family. The properties of the amino acid side chains are indicated by different colors: polar (white), non-polar (blue), acidic (red), basic (green), and cysteine residues (yellow). Mutations utilized to generate knockin mouse models with modified prestin function (Dallos et al., 2008; Gao et al., 2007) are indicated with black arrows. Note that in the model by Gao et al (2007) residues K233, K235 and R236 are intracellular. Positive-selected amino acids in mammals detected by the evolutionary analysis performed by Franchini and Elgoyhen (2006) are highlighted with red circles and indicated with red arrows. Positive selected amino acids in echolocating bats detected by Li et al. (2008) are highlighted by violet circles and indicated with violet arrows. Positive selected amino acids in bats that co-evolved in dolphins display an additional green circle.
The generation of a prestin knock-out mouse showed that prestin is necessary for electromotility in OHCs and for cochlear amplification (Liberman et al., 2002). However, this mouse model had some limitations, since OHCs were ~40% of their normal length, inner hair cells (IHCs) and OHCs were absent in the basal turn of the cochlea and mice had age-related hearing loss. These caveats were overcome by generating a knock-in mouse where only two amino acids were mutated in the protein sequence and obtaining the same lack of electromotility and amplification as in the knock-out model (Dallos et al., 2008), thus again demonstrating the importance of prestin in cochlear amplification mechanisms. It was hoped that the generation of these mouse models would settle the issue of whether stereocilia-based mechanisms or somatic electromotility underlie amplification in the mammalian cochlea. However, as amplification involves some cochlear feedback loop, modifications at any stage will affect the loop’s output. Thus if stereocilia processes were the amplifier and somatic motility were to crucially adjust the amplifier’s properties, eliminating electromotility could simulate lack of amplification (Fettiplace, 2006). A limited examination of prestin’s role as an operating-point adjustor of the stereocilia amplifier, performed with the aid of another knock-in mouse, suggests that OHC somatic motility does not adjust the operating point of stereocilia-based amplificatory mechanisms (Gao et al., 2007). Although further experiments are needed in order to elucidate the contribution of sterocilia- vs somatic-based mechanisms for amplification (or the interaction of both processes), experimental evidence clearly demonstrates that the latter plays an important role and that it is based on the motor protein prestin.
Mammalian prestin: a positively-selected protein
Intriguing questions are how this novel mechanism for amplification evolved in the lineage leading to mammals and how the appearance of somatic electromotility is related with the evolution of proteins underlying the functioning of the system. If prestin-driven electromotility was a key step for the evolution of sound amplification (and tuning) in the mammalian ear, prestin should either be a mammalian novelty or mammalian prestin should have acquired new functional features to serve as the OHC motor. In order to begin answering these questions, in a recent study we performed a phylogenetic and evolutionary analysis of the SLC26 super-family in order to establish the context in which prestin evolved (Franchini et al., 2006).
The analysis showed that PRES orthologs are present in all vertebrate species analyzed, indicating that prestin is not a mammalian exclusive protein. This is in accordance with previous data describing the cloning of zebrafish PRES (Weber et al., 2003). However, in comparison to other members of the SLC26 family, the high degree of conservation of placental mammal prestin genes is noteworthy. Comparisons of the percentage of protein identity (% ID), defined as the percentage of identical amino acids, among Homo Sapiens prestin with placental mammal and non-mammalian vertebrate prestin orthologs are shown in Table 1. Whereas human prestin sequence shares a very high % ID (95% and higher) with Mus musculus, Canis familiaris, Eqqus callus and Bos Taurus, there is a significant drop in identity (< 59%) when it is compared to those of non-mammalian vertebrates. In addition, the analysis indicated that mammalian prestin % ID values are the highest among all members of the SLC26 super-family (Franchini et al., 2006). Moreover, when compared to pendrin, a close related member of the SLC26 superfamily and a protein also involved in proper ear function (Mount et al., 2004), it is striking that the % ID among placental mammal pendrin sequences are not as high as those obtained for prestin (Table 1), suggesting that mammalian prestin has higher selective constraints acting on it. In addition, identity values between mammals/chicken and mammals/frog comparisons are much higher for pendrin than for prestin (Table 1), indicating that more molecular changes accumulated in prestin than in pendrin during the same period of time in the mammalian lineage (Franchini et al., 2006). Taken together, these results indicate that mammalian prestin had a somewhat different evolutionary history when compared to other members of the super-family, it accumulated more amino acid changes in its primary sequence, most likely to accommodate a novel function.
Table 1.
Identity values (% identical amino acids) among selected vertebrate PRES and PDS sequences.
| PRES | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PDS | human | mouse | dog | horse | cow | opossum | chicken | lizard | frog | zebrafish | |
| human | 95 | 96 | 95 | 96 | 81 | 59 | 59 | 56 | 52 | ||
| mouse | 87 | 96 | 95 | 95 | 82 | 60 | 60 | 56 | 52 | ||
| dog | 90 | 87 | 96 | 96 | 82 | 60 | 60 | 56 | 52 | ||
| horse | 90 | 87 | 92 | 96 | 82 | 60 | 59 | 57 | 52 | ||
| cow | 88 | 86 | 91 | 90 | 82 | 60 | 59 | 57 | 52 | ||
| opossum | 83 | 79 | 83 | 82 | 81 | 61 | 60 | 57 | 52 | ||
| chicken | 74 | 74 | 74 | 75 | 74 | 78 | 76 | 71 | 53 | ||
| lizard | 76 | 74 | 75 | 76 | 76 | 77 | 82 | 69 | 58 | ||
| frog | 65 | 66 | 66 | 66 | 66 | 68 | 68 | 70 | 60 | ||
| zebrafish | 56 | 56 | 56 | 57 | 56 | 57 | 56 | 57 | 54 | ||
Numbers in the table represent the percentage of identical residues among all ungapped positions between the pairs. PRES: gene encoding prestin; PDS: gene encoding pendrin. Sequences were obtained from Ensembl (www.ensembl.org). Human: Homo sapiens; mouse: Mus musculus; dog: Canis familiaris; horse: Equus caballus; cow: Bos Taurus; opossum: Monodelphis domestica; chicken: Gallus gallus; lizard: Anolis carolinensis; frog: Xenopus tropicalis; zebrafish: Danio rerio.
Making use of the nonsynonymous to synonymous substitution rate ratio Ka/Ks sliding window analysis (Nei et al., 1986), in conjunction with Codon-based Likelihood Models (Yang, 1997; Yang, 1998; Yang et al., 2002a; Yang et al., 2002b; Yang et al., 2000), we established that mammalian prestin underwent Darwinian selection and we predicted sites in its primary sequence that have been under positive selective pressure (Franchini et al., 2006 and Figure 1). Sites identified are located in some key areas of the prestin protein like the sulfate transport signature, the transmembrane regions (TM1, TM4, TM5) and in the sulfate transporter and antisigma (STAS) domain (Franchini et al., 2006 and Figure 1). Moreover, PRES has continued to undergo adaptive evolution since mammals diversified, and this appears to be related to the origin of the specialized form of constant-frequency echolocation which is characterized by sharp auditory tuning and high frequency selectivity in echolocating bats (Li et al., 2008). Evidence of positive-selected amino acids in echolocating bats was found in functionally important regions of prestin including the extacellular loops, the transmembrane domain and the STAS domain (Li et al., 2008 and Figure 1). It is interesting to note that some sites co-evolved in echolocating bats and dolphins, suggesting that they could serve an important common function in these two groups of mammals (Franchini and Elgoyhen, unpublished data, and Figure 1). In an independent study, using evolutionary trace analysis to identify candidate functional (trace) residues in prestin, followed by mutagenesis analysis (Rajagopalan et al., 2006), several amino acid residues in the sulfate transport domain were identified and implicated in non-lineal capacitance (accepted as the electrical signature of electromotility). In addition, making use of comparative genomic, evolution, and structural diversification approaches, a putative minimal essential motif for the electromotility motor spanning all transmembrane regions was proposed (Okoruwa et al., 2008).
The evolutionary analysis clearly indicated that PRES orthologues do exist in non-mammalian vertebrates (Franchini et al., 2006). Interesting questions are: do non-mammalian prestin properties differ from those of mammalian prestin (to account for the lack of somatic electromotility in non-mammalian vertebrate hair cells), and is PRES expressed in non-mammalian hair cells? Compared with mammalian prestin, charge movements mediated by zebrafish prestin display a weaker voltage dependence and slower kinetics; they occur at more positive membrane voltages, and are not associated with electromotile responses (Albert et al., 2007). Moreover, although zebrafish prestin is expressed in hair cells, it is distributed throughout the hair cells (Albert et al., 2007). This subcellular staining pattern contrasts with the exclusive basolateral membrane localization of prestin in mammalian OHCs, but resembles the somatic localization of prestin reported for mammalian vestibular hair cells (Adler et al., 2003). The lack of membrane localization of zebrafish prestin most likely precludes its function as a molecular motor. Even though nearly the full length protein is required for normal prestin expression and function, some amino acids in the C-terminus between positions 725 and 745 (corresponding to the human sequence) are key for the proper membrane targeting of prestin (Zheng et al., 2005). Our evolutionary analysis has shown that while the whole C-terminal region is highly conserved among placental mammals, some major changes are observed when compared to chicken, frog and fish orthologues (Franchini et al., 2006). In addition, within the STAS domain seven strongly positively-selected sites were detected (Figure 1). Thus, membrane localization of mammalian prestin most likely arose during evolution through the acquisition of positively-selected residues.
In his analysis of the cochlear amplifier Manley (2000) has stated that “evolution is a conservative process. Rather than developing functions or structures de novo, existing structures and processes tend to be modified, sometimes to accomplish new tasks”. Although evolutionarily conserved regions are functionally important, molecular innovations that provide adaptive changes are the mechanisms underlying the invention of new protein functions. This has certainly been the case for prestin. Thus, a protein common across vertebrates and expressed in hair cells acquired positively-selected sites in the mammalian lineage. These provided mammalian prestin with new functional capacities. It has been proposed that the voltage sensitivity of prestin may be brought about by the partial translocation of Cl− or HCO3−, which in turn may trigger the major conformational change that generates electromotility (Oliver et al., 2001). On the other hand, prestin orthologs from fish and birds have a full transport cycle (as other members of the SLC26 family), they mediate coupled divalent:Cl− exchange, but lack electromotility. The observation that divalent transport in chick and zebrafish prestin and electromotility in mammalian prestin are blocked by salycilate (Schaechinger et al., 2007), together with the fact that electromotility is dependent upon partial anion translocation (Oliver et al., 2001), has led to the suggestion that the mechanism by which prestin generates electromotility in outer hair cells is evolutionary derived from and mechanistically related to divalent:Cl− antiport (Schaechinger et al., 2007).
Medial olivocochlear efferent modulation of amplification
While OHC respond to auditory stimulation and modulate the micromechanics of the cochlear partition independent of CNS control, they are targets of efferent or centrifugal fibers which originate in the brain. The olivocochlear (OC) system is the final common path of descending activity destined for the cochlea, responsible for conveying efferent commands originating in the CNS to the organ of Corti and acetylcholine (ACh) the main neurotransmitter released at the efferent-OHC synapse (Eybalin, 1993; Rasmussen, 1955). The medial branch of the OC system projects to the OHC region, where MOC terminals synapse directly upon OHC; the lateral branch projects to the inner hair cell region and makes synaptic contacts with the dendrites of the afferent fibers (Guinan, 1996). Although the role(s) of the efferent innervation to the cochlea is still a matter of debate, the vast majority of efferent effects described to date can generally be ascribed to efferent synapses on the OHC. Efferent activation produces hyperpolarization of OHC (Fuchs et al., 1992a; Housley et al., 1991), controls the mechanical state of the cochlea, inhibits electromotility and reduces the sensitivity and the tuning of the auditory nerve fibers (Guinan, 1996). This action by ACh is thought to reflect a modulation of the contribution by OHCs to active cochlear mechanics (Mountain, 1980; Siegel et al., 1982). It is noteworthy that the developmental appearance of ACh-induced responses in OHCs coincides with the time period when OHCs develop prestin-driven motility (He et al., 1999; Katz et al., 2004).
Efferent innervation of hair cells is not exclusive to mammals. In fact, it is as old as hair cells themselves (Manley et al., 1998; Simmons, 2002). However, it is unclear when a population of dedicated auditory efferents first appeared. The primitive efferent innervation most likely synapsed on a range of end organs, both vestibular and, when present, lateral line. The very close association of efferent neurons with the brainstem branchial motor neurons across all vertebrates has led to the proposal that inner ear efferent neurons are phylogenetically derived from the motor column even though they innervate sensory neuroepithelium and not muscle (Meredith et al., 1987). Moreover, inner ear efferent neurons and motor neurons share a common embryological origin (Fritzsch, 1996; Fritzsch, 1999). In addition, in the absence of their normal target (after ear ablation), developing inner-ear efferents join the facial motor neurons, presumably reverting to their ancestral condition (Fritzsch, 1999).
Inner ear efferent neurons in fish and amphibians are organized into a single efferent nucleus, the octavolateralis efferent nucleus, that lies near the rostral end of the facial branchial motor nucleus and innervates auditory, vestibular, and lateral line hair cells (Roberts et al., 1992). The pattern seen in reptiles and birds also consists of a single, although more diffuse, nucleus near the facial branchial motor nucleus. In birds, inner ear efferent neurons exhibit a greater degree of topographic segregation according to the peripheral end organ innervated (Kaiser et al., 1994; Kaiser et al., 1996; Schwarz et al., 1981). In mammals, cochlear and vestibular efferent neurons are completely separate and cochlear efferents are divided into the two separate systems known as the lateral and medial olivocochlear systems (Warr, 1992). Thus, it has been proposed by Manley and Köppl (1998) that the evolution of a sophisticated efferent system exclusive for the modulation of cochlear function was triggered by the division of labor of motor and sensory functions between hair cells that occurred in birds and mammals.
The OHC cholinergic receptor: a peculiar member of the nicotinic cholinergic family
Acetylcholine is the principal neurotransmitter released by MOC terminals (Eybalin, 1993). While both muscarinic and nicotinic cholinergic receptors (nAChRs) have been proposed to mediate the effects of ACh in the cochlea, pharmacological and electrophysiological data suggest a key role for an unusual, nAChR located at the synapse between efferent fibers and OHCs (Blanchet et al., 1996; Chen et al., 1996; Doi et al., 1993; Dulon et al., 1996; Erostegui et al., 1994; Evans, 1996; Fuchs, 1996; Fuchs et al., 1992a; Fuchs et al., 1992b; Housley et al., 1991). Although muscle and neuronal nAChRs are excitatory, thus leading to depolarization of postsynaptic membranes (Karlin, 2002), the hair cell nAChR is unique in that it hyperpolarizes OHCs. Activation of the hair cell nAChR leads to an increase in intracellular Ca2+ and the subsequent opening of small conductance Ca2+-activated K+ (SK2) channels, thus leading to hyperpolarization of hair cells and reduction of electromotility (Dulon et al., 1998; Fuchs et al., 1992b; Housley et al., 1991; Oliver et al., 2000). Calcium-induced calcium-release from nearby synaptic cisterns is also thought to be involved (Lioudyno et al., 2004; Sridhar et al., 1997).
The cholinergic pharmacology of hair cells differs substantially from that of other cholinergic synapses. The hair cell’s cholinergic response can be elicited by ACh but not by muscarine or nicotine. Moreover, nicotine is a weak antagonist of ACh responses (Chen et al., 1996; Dulon et al., 1996; Erostegui et al., 1994; Fuchs et al., 1992a; McNiven et al., 1996). The reappearance of this unusual pharmacological profile in various end-organs and various species led to repeated proposals that a novel cholinergic receptor might mediate the efferent inhibition of hair cells. This prediction was validated when a novel subunit of the nicotinic receptor family, α9, was cloned (Elgoyhen et al., 1994). We now know that the nAChR of hair cells is composed of both α9 and α10 nicotinic subunits which assemble into a pentameric structure with a likely (α9)2(α10)3 stoichiometry (Elgoyhen et al., 1994; Elgoyhen et al., 2001; Lustig et al., 2001; Plazas et al., 2005; Sgard et al., 2002). The properties of recombinant receptors assembled from α9 and α10 subunits (Elgoyhen et al., 2009; Elgoyhen et al., 1994; Elgoyhen et al., 2001; Katz et al., 2000; Rothlin et al., 2000; Rothlin et al., 2003; Verbitsky et al., 2000; Weisstaub et al., 2002) recapitulate those of native receptors, precluding the need of additional subunits.
Positively-selected mammalian α9α10 nicotinic cholinergic receptors
The nAChRs are well characterized transmembrane allosteric proteins involved in the physiological responses to ACh (Changeux et al., 1987). They are composed of five identical (homopentamers) or different (heteropentamers) polypeptide chains arranged symmetrically around an axis perpendicular to the membrane which line a channel pore (Figure 2A). The binding of ACh to the N-terminal extracellular portion of the receptor triggers a series of allosteric movements of the protein, with the consequent opening of the pore. Sequence analyses have revealed that the numerous nAChR subunits are homologous proteins that belong, together with the GABAA,C, glycine, 5-HT3, and some invertebrate glutamate receptors, to the “Cys-loop” superfamily of ligand-gated ion channels (Changeux et al., 1987; Galzi et al., 1991). Each member of this family possesses an N-terminal extracellular domain with the cys-loop: two disulfide bond-forming cysteines separated by 13 amino acid residues. The nAChR subunits genes fall into two main classes: the α subunits (α1–10) which possess two adjacent cysteines essential for ACh binding and the non-α referred to as β, γ, ε, or δ, which lack the double cysteines (Karlin, 2002). The nAChR vertebrate subunits include the following subfamilies, defined on the basis of protein sequence and gene structure (position of the introns in the coding sequence): subfamily I, epithelial α9 and α10; subfamily II, neuronal α7 and α8; subfamily III, neuronal α2–6 and β2–4; and subfamily IV, muscle α1, β1, γ, δ, and ε (Le Novere et al., 1995; Le Novere et al., 2002; Ortells et al., 1995, but see also Tsunoyama et al., 1998, who has suggested alternate subfamilies). These subfamilies of subunits, parallels the subgroups of receptors defined on the basis of biochemical, functional or pharmacological data. Thus, α9 and α10 form homopentamers by themselves and heteropentamers together. Similarly, α7 and α8 make up homopentamers by themselves and heteropentamers together. α2–α6 and β2–β4 are included in a range of complex heteropentamers, mostly present in neurons. Finally α1, β1, γ, δ, and ε form heteropentamers in muscle cells.
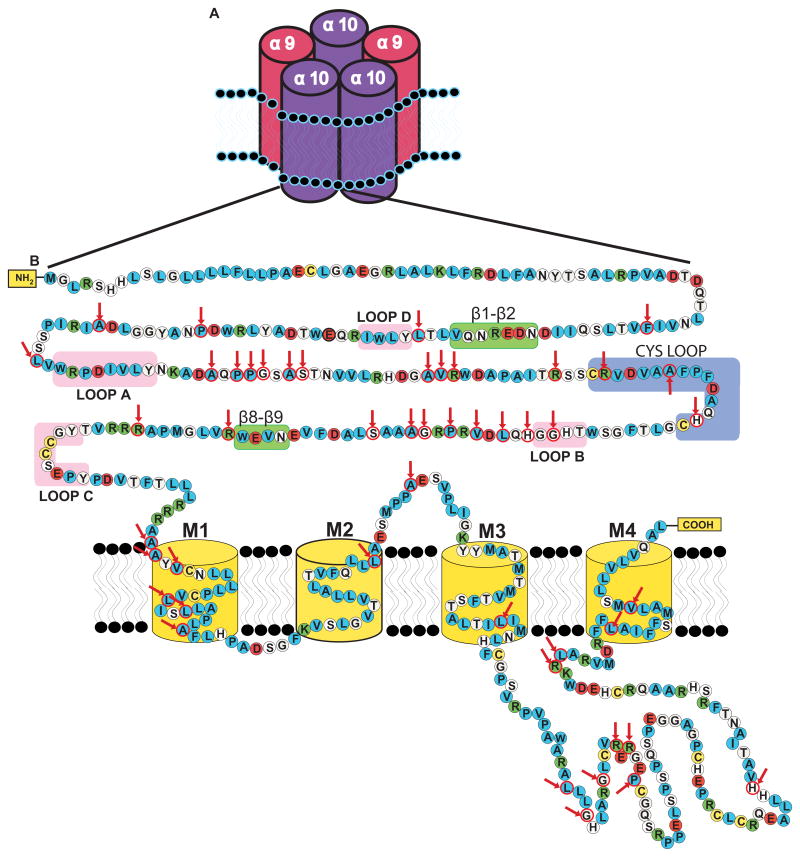
Figure 2.
(A) Schematic representation of the structure of the α9α10 nicotinic receptor, showing the arrangement of the subunits according to the predicted stoichiometry determined by Plazas et al. (2005). (B) Amino acid sequence and secondary structure of the human α10 receptor (the topology of the predicted α-helical structure of transmembrane regions are not reproduced). The properties of the amino acid side chains are indicated by different colors: polar (white), non-polar (blue), acidic (red), basic (green), and cysteine residues (yellow). Loops A, B, C, and D highlighted in pink correspond to regions involved in agonist binding. The Cys-loop (blue) and the β1–β2 and β8–β9 (green) correspond to regions involved in gating of the channel. Positive selected amino acids in mammals detected by the evolutionary analysis performed by Franchini and Elgoyhen (2006) are highlighted with red circles and indicated with red arrows.
This diversity of nAChR subunits is conserved through evolution, and thus it has been hypothesized that the presence of each individual subunit has probably been positively-selected. As suggested by Le Novere et al (2002), this is in contrast to the evolution of olfactory receptors where multiple paralogous genes systematically exist, but are often not orthologous between the various phyla (Young et al., 2002), revealing much higher rates of gene creation and inactivation in the course of evolution. The selective pressures for maintaining such a wide diversity of subunits remains an enigma (Dent, 2006; Le Novere et al., 2002). Phylogenetic analysis shows that α9 and α10 are direct descendants of the ancestral subunit (Le Novere et al., 1995; Le Novere et al., 2002; Ortells et al., 1995; Tsunoyama et al., 1998). When expressed in Xenopus laevis oocytes, α9 homomeric and α9α10 heteromeric receptors continue to conserve pharmacological properties typical of GABAA, glycine and 5-HT3 receptors (Elgoyhen et al., 1994; Elgoyhen et al., 2001; Rothlin et al., 1999; Rothlin et al., 2003), which suggests that they are primitive members of the Cys-loop family and that they had a very early evolutionary split.
Since cholinergic efferent feedback to hair cells is a common feature among all vertebrates, one would expect that the evolutionary history of the genes coding for the α9 and the α10 subunits would look similar along all vertebrate lineages. A phylogenetic analysis of α9 and α10 subunits across vertebrates has provided surprising results (Franchini et al., 2006). Thus, in mammals the genes coding for α10 subunits (CHRNA10) display a different evolutionary history. The analysis of the % ID among selected α9/α10 vertebrate orthologues (Table 2) indicates that both placental mammal α9 and α10 subunits show high % ID values. In contrast, the mammalian α9 compared to any non-mammalian α9 renders higher identity values than the equivalent comparison between α10 subunits; e.g., α9 mammals-chicken 74% (on average) vs. α10 mammals-chicken 66% (on average). In addition, it is worth mentioning that the identity between α9 and α10 is much higher for chicken (65%) than for mammals (54%) (Franchini et al., 2006). These data suggest a possible scenario for the evolution of these nicotinic receptor subunits: after a duplication event that created the CHRNA9 and CHRNA10 ancestors, these two genes co-existed without much functional differentiation. Then at some point, in the lineage leading to mammals, amino acid changes started to accumulate rapidly producing CHRNA10 to diverge from CHRNA9. A Ka/Ks sliding window analysis indicated that this divergence was shaped by positive selection acting only in mammalian CHRNA10 and purifying selection on CHRNA9 genes (Franchini et al., 2006). Moreover, making use of Codon-based Likelihood Models we predicted sites in the α10 primary sequence which have been under positive selection in the mammalian lineage. Positive selected sites were identified in key residues of the molecule involved in binding of the agonist, gating of the channel and the transmembrane regions (Franchini et al., 2006 and Figure 2B).
Table 2.
Identity values (% identical amino acids) among selected vertebrate CHRNA9 and CHRNA10 sequences.
| CHRNA10 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CHRNA9 | human | mouse | horse | cow | opossum | chicken | frog | zebrafish α9d | zebrafish α9c | |
| human | 91 | 91 | 92 | 78 | 66 | 59 | 60 | 59 | ||
| mouse | 91 | 89 | 89 | 76 | 66 | 59 | 60 | 59 | ||
| horse | 93 | 88 | 92 | 77 | 66 | 59 | 59 | 56 | ||
| cow | 93 | 90 | 95 | 77 | 66 | 60 | 60 | 59 | ||
| opossum | 84 | 81 | 84 | 85 | 66 | 59 | 58 | 60 | ||
| chicken | 74 | 73 | 74 | 74 | 76 | 73 | 69 | 68 | ||
| frog | 69 | 69 | 70 | 70 | 70 | 74 | 69 | 71 | ||
| zebrafish α9a | 65 | 64 | 65 | 65 | 66 | 68 | 68 | 69 | ||
| zebrafish α9b | 68 | 67 | 68 | 68 | 68 | 70 | 68 | 73 | ||
Numbers in the table represent the percentage of identical residues among all ungapped positions between the pairs. CHRNA10: gene encoding the cholinergic nicotinic α10 receptor subunit; CHRNA9: gene encoding the cholinergic nicotinic α9 receptor subunit. Sequences were obtained from Ensembl (www.ensembl.org). Human: Homo sapiens; mouse: Mus musculus; horse: Equus caballus; cow: Bos taurus; opossum: Monodelphis domestica; chicken: Gallus gallus; frog: Xenopus tropicalis; zebrafish: Danio rerio. Zebrafish Ensembl genes: α9d: ENSDARG00000044353; α9c: ENSDARG00000011113; α9a: ENSDARG00000054680; α9b: ENSDARG00000011029.
A positively-selected mammalian α10 subunit suggests differential functional properties of mammalian α9α10 receptors. If this is so, why would mammals need a modified α9α10 nAChR? Although there is no clearcut answer to this question at present, the fact that efferent innervation to OHCs has to modulate prestin-driven somatic electromotility, a phenomena not present in non-mammalian hair cells, suggests that the existence of differential properties of a positive selected α9α10 nAChR is not a surprising finding. Thus, mammalian α9α10 receptors would have to provide the necessary signaling cascade to subserve the effects derived from the activation of the efferent system in this group of vertebrates, such as hyperpolarization of the OHC and increase of axial stiffness (Dallos et al., 1997; Frolenkov et al., 2003) through phosphorylation of cytoskeletal proteins (Sziklai et al., 2001). These phenomena require the increase of intracellular Ca2+ concentration (Frolenkov et al., 2003). Mammalian α9α10 receptors have a very high calcium permeability (Weisstaub et al., 2002), similar to that of ligand-gated channels bearing the highest calcium permeability, such as the N-methyl-D-aspartate receptors and the cyclic nucleotide-gated channels from bovine retinal cones and olfactory sensory neurons (Frings et al., 1995; Mayer et al., 1987).
Preliminary data indicates that the calcium permeability of chick α9α10 receptors is low (Lipovsek, Franchini and Elgoyhen, unpublished observations). This result might indicate differential participation of calcium influx through the nAChR versus calcium release from intracellular stores (Housley et al., 1990; Kakehata et al., 1993; Shigemoto et al., 1990; Shigemoto et al., 1991; Yoshida et al., 1994), known to participate in the efferent signaling process. Further experiments are underway in order to understand the consequences of positive selected sites in the α10 nAChR subunit.
Conclusion
The specialization of the inner ear has lead to the division of labor in mammals: IHCs for sensory transduction and OHCs for amplification. In addition, a novel mechanism for amplification arose in mammals based on the activity of prestin. This protein acquired motor properties through the acquisition of non-synonymous substitutions in its primary sequence, only in the lineage leading to mammals. In addition, division of labor of motor and sensory functions between hair cells of species like birds and mammals triggered the evolution of a sophisticated efferent system for the modulation of cochlear function. Compared to birds, evolution of the mammalian efferent system included a positively-selected α10 nAChR subunit which most likely rendered an α9α10 receptor with selective advantages to modulate prestin-based somatic motility.
Acknowledgments
We want to thank Geoffrey Manley for his comments on the manuscript. ABE is supported by the National Institutes of Deafness and other Communication Disorders (NIDCD) Grant R01DC001508, an International Research Scholar Grant from the Howard Hughes Medical Institute, the Tinnitus Research Initiative, Research Grants from ANPCyT (Argentina) and the University of Buenos Aires (Argentina). LFF is supported by a Research Grant from ANPCyT and CONICET (Argentina).
Abbreviations
- ACh
acetylcholine
- CHRNA9
gene enconding de α9 nicotinic cholinergic subunit
- CHRNA10
gene enconding de α10 nicotinic cholinergic subunit
- IHCs
inner hair cells
- MOC
medial olivocochlear
- nAChR
nicotinic cholinergic receptor
- OHCs
outer hair cells
- PDS
gene encoding pendrin
- PRES
gene enconding prestin
- STAS
sulfate transporter and antisigma
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler HJ, Belyantseva IA, Merritt RC, Jr, Frolenkov GI, Dougherty GW, Kachar B. Expression of prestin, a membrane motor protein, in the mammalian auditory and vestibular periphery. Hear Res. 2003;184:27–40. doi: 10.1016/s0378-5955(03)00192-8. [DOI] [PubMed] [Google Scholar]
- Albert JT, Winter H, Schaechinger TJ, Weber T, Wang X, He DZ, Hendrich O, Geisler HS, Zimmermann U, Oelmann K, Knipper M, Gopfert MC, Oliver D. Voltage-sensitive prestin orthologue expressed in zebrafish hair cells. J Physiol. 2007;580:451–461. doi: 10.1113/jphysiol.2007.127993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore J. Cochlear outer hair cell motility. Physiol Rev. 2008;88:173–210. doi: 10.1152/physrev.00044.2006. [DOI] [PubMed] [Google Scholar]
- Assad JA, Corey DP. An active motor model for adaptation by vertebrate hair cells. J Neurosci. 1992;12:3291–3309. doi: 10.1523/JNEUROSCI.12-09-03291.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchet C, Erostegui C, Sugasawa M, Dulon D. Acetylcholine-induced potassium current of guinea pig outer hair cells: its dependence on a calcium influx through nicotinic-like receptors. J Neurosci. 1996;16:2574–2584. doi: 10.1523/JNEUROSCI.16-08-02574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell W, Bader C, Bertrand D, de Ribaupierre Y. Evoked mechanical responses of isolated cochlear hair cells. Science. 1985;227:194–196. doi: 10.1126/science.3966153. [DOI] [PubMed] [Google Scholar]
- Crawford AC, Fettiplace R. The mechanical properties of ciliary bundles of turtle cochlear hair cells. J Physiol. 1985;364:359–379. doi: 10.1113/jphysiol.1985.sp015750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DK, Hudspeth AJ. Ca2+ current-driven nonlinear amplification by the mammalian cochlea in vitro. Nat Neurosci. 2005;8:149–155. doi: 10.1038/nn1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J, Giraudat J, Dennis M. The nicotinic acetylcholine receptor: Molecular architecture of a ligand-regulated ion channel. TIPS. 1987;8:459–465. [Google Scholar]
- Chen C, LeBlanc C, Bobbin R. Differences in cholinergic responses from outer hair cells of rat and guinea pig. Hearing Research. 1996;98:9–17. doi: 10.1016/0378-5955(96)00049-4. [DOI] [PubMed] [Google Scholar]
- Dallos P. Cochlear amplification, outer hair cells and prestin. Curr Opin Neurobiol. 2008;18:370–376. doi: 10.1016/j.conb.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P, Zheng J, Cheatham MA. Prestin and the cochlear amplifier. J Physiol. 2006;576:37–42. doi: 10.1113/jphysiol.2006.114652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P, He DZ, Lin X, Sziklai I, Mehta S, Evans BN. Acetylcholine, outer hair cell electromotility, and the cochlear amplifier. J Neurosci. 1997;17:2212–2226. doi: 10.1523/JNEUROSCI.17-06-02212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P, Wu X, Cheatham MA, Gao J, Zheng J, Anderson CT, Jia S, Wang X, Cheng WH, Sengupta S, He DZ, Zuo J. Prestin-based outer hair cell motility is necessary for mammalian cochlear amplification. Neuron. 2008;58:333–339. doi: 10.1016/j.neuron.2008.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis H. An active process in cochlear mechanics. Hear Res. 1983;9:79–90. doi: 10.1016/0378-5955(83)90136-3. [DOI] [PubMed] [Google Scholar]
- Deak L, Zheng J, Orem A, Du GG, Aguinaga S, Matsuda K, Dallos P. Effects of cyclic nucleotides on the function of prestin. J Physiol. 2005;563:483–496. doi: 10.1113/jphysiol.2004.078857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent JA. Evidence for a diverse Cys-loop ligand-gated ion channel superfamily in early bilateria. J Mol Evol. 2006;62:523–535. doi: 10.1007/s00239-005-0018-2. [DOI] [PubMed] [Google Scholar]
- Doi T, Ohmori H. Acetylcholine increases intracellular Ca2+ concetration and hyperpolarizes the guinea-pig outer hair cell. Hearing Res. 1993;67:179–188. doi: 10.1016/0378-5955(93)90245-v. [DOI] [PubMed] [Google Scholar]
- Dulon D, Lenoir M. Cholinergic responses in developing outer hair cells of the rat cochlea. European J Neurosci. 1996;8:1945–1952. doi: 10.1111/j.1460-9568.1996.tb01338.x. [DOI] [PubMed] [Google Scholar]
- Dulon D, Luo L, Zhang C, Ryan AF. Expression of small-conductance calcium-activated potassium channels (SK) in outer hair cells of the rat cochlea. Eur J Neurosci. 1998;10:907–915. doi: 10.1046/j.1460-9568.1998.00098.x. [DOI] [PubMed] [Google Scholar]
- Elgoyhen AB, Katz E, Fuchs PA. The nicotinic receptor of cochlear hair cells: a possible pharmacotherapeutic target? Biochem Pharmacol. 2009;78:712–719. doi: 10.1016/j.bcp.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgoyhen AB, Johnson DS, Boulter J, Vetter DE, Heinemann S. a9: an acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell. 1994;79:705–715. doi: 10.1016/0092-8674(94)90555-x. [DOI] [PubMed] [Google Scholar]
- Elgoyhen AB, Vetter D, Katz E, Rothlin C, Heinemann S, Boulter J. Alpha 10: A determinant of nicotinic cholinergic receptor function in mammalian vestibular and cochlear mechanosensory hair cells. Proc Natl Acad Sci USA. 2001;98:3501–3506. doi: 10.1073/pnas.051622798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erostegui C, Norris CH, Bobbin RP. In vitro characterization of a cholinergic receptor on outer hair cells. Hearing Res. 1994;74:135–147. doi: 10.1016/0378-5955(94)90182-1. [DOI] [PubMed] [Google Scholar]
- Evans M. Acetylcholine activates two currents in guinea-pig outer hair cells. J Physiol. 1996;491:563–578. doi: 10.1113/jphysiol.1996.sp021240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eybalin M. Neurotransmitters and neuromodulators of the mammalian cochlea. Physiol Rev. 1993;73:309–373. doi: 10.1152/physrev.1993.73.2.309. [DOI] [PubMed] [Google Scholar]
- Fettiplace R. Active hair bundle movements in auditory hair cells. J Physiol. 2006;576:29–36. doi: 10.1113/jphysiol.2006.115949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchini LF, Elgoyhen AB. Adaptive evolution in mammalian proteins involved in cochlear outer hair cell electromotility. Mol Phylogenet Evol. 2006;41:622–635. doi: 10.1016/j.ympev.2006.05.042. [DOI] [PubMed] [Google Scholar]
- Frings S, Seifert R, Godde M, Kaupp UB. Profoundly different calcium permeation and blockage determine the specific function of distinct cyclic nucleotide-gated channels. Neuron. 1995;15:169–179. doi: 10.1016/0896-6273(95)90074-8. [DOI] [PubMed] [Google Scholar]
- Fritzsch B. Development of the labyrinthine efferent system. Ann N Y Acad Sci. 1996;781:21–33. doi: 10.1111/j.1749-6632.1996.tb15690.x. [DOI] [PubMed] [Google Scholar]
- Fritzsch B. Ontogenetic and evolutionary evidence for motoneuronal nature of vestibular and cochlear efferents. In: CIB, editor. The efferent auditory system: basic science and clinical applications. Singular Publishing; San Diego, CA: 1999. pp. 31–60. [Google Scholar]
- Frolenkov GI, Mammano F, Kachar B. Regulation of outer hair cell cytoskeletal stiffness by intracellular Ca2+: underlying mechanism and implications for cochlear mechanics. Cell Calcium. 2003;33:185–195. doi: 10.1016/s0143-4160(02)00228-2. [DOI] [PubMed] [Google Scholar]
- Fuchs P. Synaptic transmission at vertebrate hair cells. Current Opinion in Neurobiol. 1996;6:514–519. doi: 10.1016/s0959-4388(96)80058-4. [DOI] [PubMed] [Google Scholar]
- Fuchs PA, Murrow BW. A novel cholinergic receptor mediates inhibition of chick cochlear hair cells. Proc R Soc Lond B. 1992a;248:35–40. doi: 10.1098/rspb.1992.0039. [DOI] [PubMed] [Google Scholar]
- Fuchs PA, Murrow BW. Cholinergic inhibition of short (outer) hair cells of the chick’s cochlea. J Neurosci. 1992b;12:800–809. doi: 10.1523/JNEUROSCI.12-03-00800.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galzi J-L, Revah F, Bessis A, Changuex JP. Functional architecture of the nicotinic acetylcholine receptor: from electric organ to brain. Annu Rev Pharmacol. 1991;31:37–72. doi: 10.1146/annurev.pa.31.040191.000345. [DOI] [PubMed] [Google Scholar]
- Gao J, Wang X, Wu X, Aguinaga S, Huynh K, Jia S, Matsuda K, Patel M, Zheng J, Cheatham M, He DZ, Dallos P, Zuo J. Prestin-based outer hair cell electromotility in knockin mice does not appear to adjust the operating point of a cilia-based amplifier. Proc Natl Acad Sci U S A. 2007;104:12542–12547. doi: 10.1073/pnas.0700356104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geleoc GS, Holt JR. Auditory amplification: outer hair cells pres the issue. Trends Neurosci. 2003;26:115–117. doi: 10.1016/S0166-2236(03)00030-4. [DOI] [PubMed] [Google Scholar]
- Gold T. Hearing II: the basis of the action of the cochlea. Proc R Soc B. 1948;135:492–498. [Google Scholar]
- Guinan JJ. Physiology of olivocochlear efferents. In: Dallos, Popper, Fay, editors. The Cochlea. Springer-Verlag; New York: 1996. pp. 435–502. [Google Scholar]
- Gulley RL, Reese TS. Regional specialization of the hair cell plasmalemma in the organ of corti. Anat Rec. 1977;189:109–123. doi: 10.1002/ar.1091890108. [DOI] [PubMed] [Google Scholar]
- He DZ, Dallos P. Development of acetylcholine-induced responses in neonatal gerbil outer hair cells. J Neurophysiol. 1999;81:1162–1170. doi: 10.1152/jn.1999.81.3.1162. [DOI] [PubMed] [Google Scholar]
- Housley GD, Ashmore JF. Direct measurement of the action of acetylcholine on isolated outer hair cells of the guinea pig cochlea. Proc R Soc Lond B. 1991;244:161–167. doi: 10.1098/rspb.1991.0065. [DOI] [PubMed] [Google Scholar]
- Housley GD, Norris CH, Guth PS. Cholinergically-induced changes in outward currents in hair cells isolated from the semicircular canal of the frog. Hear Res. 1990;43:121–133. doi: 10.1016/0378-5955(90)90221-a. [DOI] [PubMed] [Google Scholar]
- Howard J, Hudspeth AJ. Compliance of the hair bundle associated with gating of mechanoelectrical transduction channels in the bullfrog’s saccular hair cell. Neuron. 1988;1:189–199. doi: 10.1016/0896-6273(88)90139-0. [DOI] [PubMed] [Google Scholar]
- Hudspeth AJ. Making an effort to listen: mechanical amplification in the ear. Neuron. 2008;59:530–545. doi: 10.1016/j.neuron.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia S, He DZ. Motility-associated hair-bundle motion in mammalian outer hair cells. Nat Neurosci. 2005;8:1028–1034. doi: 10.1038/nn1509. [DOI] [PubMed] [Google Scholar]
- Kaiser A, Manley GA. Physiology of single putative cochlear efferents in the chicken. J Neurophysiol. 1994;72:2966–2979. doi: 10.1152/jn.1994.72.6.2966. [DOI] [PubMed] [Google Scholar]
- Kaiser A, Manley GA. Brainstem connections of the macula lagenae in the chicken. J Comp Neurol. 1996;374:108–117. doi: 10.1002/(SICI)1096-9861(19961007)374:1<108::AID-CNE8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Kakehata S, Nakagawa T, Takasaka T, Akaike N. Cellular mechanism of acetylcholine-induced response in dissociated outer hair cells of guinea-pig cochlea. J Physiol (Lond) 1993;463:227–244. doi: 10.1113/jphysiol.1993.sp019592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin A. Ion channel structure: emerging structure of the nicotinic acetylcholine receptors. Nature Reviews Neurosc. 2002;3:102–114. doi: 10.1038/nrn731. [DOI] [PubMed] [Google Scholar]
- Katz E, Verbitsky M, Rothlin C, Vetter D, Heinemann S, Elgoyhen A. High calcium permeability and calcium block of the α9 nicotinic acetylcholine receptor. Hearing Res. 2000;141:117–128. doi: 10.1016/s0378-5955(99)00214-2. [DOI] [PubMed] [Google Scholar]
- Katz E, Elgoyhen AB, Gomez-Casati ME, Knipper M, Vetter DE, Fuchs PA, Glowatzki E. Developmental regulation of nicotinic synapses on cochlear inner hair cells. J Neurosci. 2004;24:7814–7820. doi: 10.1523/JNEUROSCI.2102-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp DT. Stimulated acoustic emissions from within the human auditory system. J Acoust Soc Am. 1978;64:1386–13891. doi: 10.1121/1.382104. [DOI] [PubMed] [Google Scholar]
- Kennedy HJ, Crawford AC, Fettiplace R. Force generation by mammalian hair bundles supports a role in cochlear amplification. Nature. 2005;433:880–883. doi: 10.1038/nature03367. [DOI] [PubMed] [Google Scholar]
- Le Novere N, Changeux J. Molecular evolution of the nicotinic acetylcholine receptor: an example of multigene family in excitable cells. J Molec Evol. 1995;40:155–172. doi: 10.1007/BF00167110. [DOI] [PubMed] [Google Scholar]
- Le Novere N, Corringer PJ, Changeux JP. The diversity of subunit composition in nAChRs: evolutionary origins, physiologic and pharmacologic consequences. J Neurobiol. 2002;53:447–456. doi: 10.1002/neu.10153. [DOI] [PubMed] [Google Scholar]
- Li G, Wang J, Rossiter SJ, Jones G, Cotton JA, Zhang S. The hearing gene Prestin reunites echolocating bats. Proc Natl Acad Sci U S A. 2008;105:13959–13964. doi: 10.1073/pnas.0802097105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC, Gao J, He DZ, Wu X, Jia S, Zuo J. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature. 2002;419:300–304. doi: 10.1038/nature01059. [DOI] [PubMed] [Google Scholar]
- Lioudyno M, Hiel H, Kong JH, Katz E, Waldman E, Parameshwaran-Iyer S, Glowatzki E, Fuchs PA. A “synaptoplasmic cistern” mediates rapid inhibition of cochlear hair cells. J Neurosci. 2004;24:11160–11164. doi: 10.1523/JNEUROSCI.3674-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohi H, Kujala M, Kerkela E, Saarialho-Kere U, Kestila M, Kere J. Mapping of five new putative anion transporter genes in human and characterization of SLC26A6, a candidate gene for pancreatic anion exchanger. Genomics. 2000;70:102–712. doi: 10.1006/geno.2000.6355. [DOI] [PubMed] [Google Scholar]
- Ludwig J, Oliver D, Frank G, Klocker N, Gummer AW, Fakler B. Reciprocal electromechanical properties of rat prestin: the motor molecule from rat outer hair cells. Proc Natl Acad Sci U S A. 2001;98:4178–4183. doi: 10.1073/pnas.071613498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig LR, Peng H, Hiel H, Yamamoto T, Fuchs P. Molecular cloning and mapping of the human nicotinic acetylcholine receptor α10 (CHRNA10) Genomics. 2001;73:272–283. doi: 10.1006/geno.2000.6503. [DOI] [PubMed] [Google Scholar]
- Manley GA. Cochlear mechanisms from a phylogenetic viewpoint. Proc Natl Acad Sci U S A. 2000;97:11736–11743. doi: 10.1073/pnas.97.22.11736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley GA. Evidence for an active process and a cochlear amplifier in nonmammals. J Neurophysiol. 2001;86:541–549. doi: 10.1152/jn.2001.86.2.541. [DOI] [PubMed] [Google Scholar]
- Manley GA, Koppl C. Phylogenetic development of the cochlea and its innervation. Curr Opin Neurobiol. 1998;8:468–474. doi: 10.1016/s0959-4388(98)80033-0. [DOI] [PubMed] [Google Scholar]
- Manley GA, Kirk DL, Koppl C, Yates GK. In vivo evidence for a cochlear amplifier in the hair-cell bundle of lizards. Proc Natl Acad Sci U S A. 2001;98:2826–2831. doi: 10.1073/pnas.041604998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P, Hudspeth AJ. Active hair-bundle movements can amplify a hair cell’s response to oscillatory mechanical stimuli. Proc Natl Acad Sci U S A. 1999;96:14306–14311. doi: 10.1073/pnas.96.25.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P, Hudspeth AJ. Compressive nonlinearity in the hair bundle’s active response to mechanical stimulation. Proc Natl Acad Sci U S A. 2001;98:14386–14291. doi: 10.1073/pnas.251530498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P, Bozovic D, Choe Y, Hudspeth AJ. Spontaneous oscillation by hair bundles of the bullfrog’s sacculus. J Neurosci. 2003;23:4533–4548. doi: 10.1523/JNEUROSCI.23-11-04533.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M, Westbrook G. Permeation and block of N-methyl-D-aspartic acid receptor channles by divalent cations in mouse cultured central neurones. J Physiol (Lond) 1987;394:501–527. doi: 10.1113/jphysiol.1987.sp016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNiven AI, Yuhas WA, Fuchs PA. Ionic dependence and agonist preference of an acetylcholine receptor in hair cells. Auditory Neurosci. 1996;2:63–77. [Google Scholar]
- Meredith GE, Roberts BL. Distribution and morphological characteristics of efferent neurons innervating end organs in the ear and lateral line of the European eel. J Comp Neurol. 1987;265:494–506. doi: 10.1002/cne.902650404. [DOI] [PubMed] [Google Scholar]
- Mount DB, Romero MF. The SLC26 gene family of multifunctional anion exchangers. Pflugers Arch. 2004;447:710–721. doi: 10.1007/s00424-003-1090-3. [DOI] [PubMed] [Google Scholar]
- Mountain DC. Changes in endolymphatic potential and crossed olivocochlear bundle stimulation alter cochlear mechanics. Science. 1980;210:71–72. doi: 10.1126/science.7414321. [DOI] [PubMed] [Google Scholar]
- Muallem D, Ashmore J. An anion antiporter model of prestin, the outer hair cell motor protein. Biophys J. 2006;90:4035–4045. doi: 10.1529/biophysj.105.073254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navaratnam D, Bai JP, Samaranayake H, Santos-Sacchi J. N-terminal-mediated homomultimerization of prestin, the outer hair cell motor protein. Biophys J. 2005;89:3345–3352. doi: 10.1529/biophysj.105.068759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M, Gojobori T. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol Biol Evol. 1986;3:418–426. doi: 10.1093/oxfordjournals.molbev.a040410. [DOI] [PubMed] [Google Scholar]
- Okoruwa OE, Weston MD, Sanjeevi DC, Millemon AR, Fritzsch B, Hallworth R, Beisel KW. Evolutionary insights into the unique electromotility motor of mammalian outer hair cells. Evol Dev. 2008;10:300–315. doi: 10.1111/j.1525-142X.2008.00239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D, Klocker N, Schuck J, Baukrowitz T, Ruppersberg JP, Fakler B. Gating of Ca2+-activated K+ channels controls fast inhibitory synaptic transmission at auditory outer hair cells. Neuron. 2000;26:595–601. doi: 10.1016/s0896-6273(00)81197-6. [DOI] [PubMed] [Google Scholar]
- Oliver D, He DZ, Klocker N, Ludwig J, Schulte U, Waldegger S, Ruppersberg JP, Dallos P, Fakler B. Intracellular anions as the voltage sensor of prestin, the outer hair cell motor protein. Science. 2001;292:2340–2343. doi: 10.1126/science.1060939. [DOI] [PubMed] [Google Scholar]
- Ortells M, Lunt G. Evolutionary history of the ligand-gated ion-channel superfamily of receptors. TINS. 1995;18:121–127. doi: 10.1016/0166-2236(95)93887-4. [DOI] [PubMed] [Google Scholar]
- Plazas PV, Katz E, Gomez-Casati ME, Bouzat C, Elgoyhen AB. Stoichiometry of the alpha9alpha10 Nicotinic Cholinergic Receptor. J Neurosci. 2005;25:10905–10912. doi: 10.1523/JNEUROSCI.3805-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan L, Patel N, Madabushi S, Goddard JA, Anjan V, Lin F, Shope C, Farrell B, Lichtarge O, Davidson AL, Brownell WE, Pereira FA. Essential helix interactions in the anion transporter domain of prestin revealed by evolutionary trace analysis. J Neurosci. 2006;26:12727–12734. doi: 10.1523/JNEUROSCI.2734-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen GL. Descending, or “feedback-back” connections of the auditory system of the cat. Am J Physiol. 1955;183:653. [Google Scholar]
- Ricci AJ, Crawford AC, Fettiplace R. Active hair bundle motion linked to fast transducer adaptation in auditory hair cells. J Neurosci. 2000;20:7131–7142. doi: 10.1523/JNEUROSCI.20-19-07131.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B, Meredith GE. The efferent innervation of the ear: variations on an enigma. In: Webster D, Fay R, Popper A, editors. The evolutionary biology of hearing. Springer-Verlag; New York: 1992. pp. 185–210. [Google Scholar]
- Rothlin C, Verbitsky M, Katz E, Elgoyhen A. The α9 nicotinic acetylcholine receptor shares pharmacological properties with type A γ-aminobutyric acid, glycine and type 3 serotonin receptors. Molec Pharmacol. 1999;55:248–254. doi: 10.1124/mol.55.2.248. [DOI] [PubMed] [Google Scholar]
- Rothlin CV, Katz E, Verbitsky M, Vetter D, Heinemann S, Elgoyhen AB. Block of the α9 nicotinic receptor by ototoxic aminoglycosides. Neuropharmacology. 2000;39:2525–2532. doi: 10.1016/s0028-3908(00)00056-3. [DOI] [PubMed] [Google Scholar]
- Rothlin CV, Lioudyno MI, Silbering AF, Plazas PV, Casati ME, Katz E, Guth PS, Elgoyhen AB. Direct interaction of serotonin type 3 receptor ligands with recombinant and native alpha 9 alpha 10-containing nicotinic cholinergic receptors. Mol Pharmacol. 2003;63:1067–1074. doi: 10.1124/mol.63.5.1067. [DOI] [PubMed] [Google Scholar]
- Schaechinger TJ, Oliver D. Nonmammalian orthologs of prestin (SLC26A5) are electrogenic divalent/chloride anion exchangers. Proc Natl Acad Sci U S A. 2007;104:7693–7698. doi: 10.1073/pnas.0608583104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz IE, Schwarz DW, Fredrickson JM, Landolt JP. Efferent vestibular neurons: a study employing retrograde tracer methods in the pigeon (Columba livia) J Comp Neurol. 1981;196:1–12. doi: 10.1002/cne.901960102. [DOI] [PubMed] [Google Scholar]
- Sgard F, Charpentier E, Bertrand S, Walker N, Caput D, Graham D, Bertrand D, Besnard F. A novel human nicotinic receptor subunit, α10, that confers functionality to the α9-subunit. Molec Pharmacol. 2002;61:150–159. doi: 10.1124/mol.61.1.150. [DOI] [PubMed] [Google Scholar]
- Shigemoto T, Ohmori H. Muscarinic agonists and ATP increase the intracellular Ca2+ concentration in chick cochlear hair cells. J Physiol (Lond) 1990;420:127–148. doi: 10.1113/jphysiol.1990.sp017904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto T, Ohmori H. Muscarinic receptor hyperpolarizes cochlear hair cells of chick by activating Ca(2+)-activated K+ channels. J Physiol. 1991;442:669–690. doi: 10.1113/jphysiol.1991.sp018814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JH, Kim DO. Efferent neural control of cochlear mechanics? Olivocochlear bundle stimulation affects cochlear biomechanical nonlinearity. Hear Res. 1982;6:171–182. doi: 10.1016/0378-5955(82)90052-1. [DOI] [PubMed] [Google Scholar]
- Simmons DD. Development of the inner ear efferent system across vertebrate species. J Neurobiol. 2002;53:228–250. doi: 10.1002/neu.10130. [DOI] [PubMed] [Google Scholar]
- Sridhar TS, Brown MC, Sewell WF. Unique postsynaptic signaling at the hair cell efferent synapse permits calcium to evoke changes on two time scales. J Neurosci. 1997;17:428–437. doi: 10.1523/JNEUROSCI.17-01-00428.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sziklai I, Szonyi M, Dallos P. Phosphorylation mediates the influence of acetylcholine upon outer hair cell electromotility. Acta Otolaryngol. 2001;121:153–156. doi: 10.1080/000164801300043280. [DOI] [PubMed] [Google Scholar]
- Tsunoyama K, Gojobori T. Evolution of nicotinic acetylcholine receptor subunits. Mol Biol Evol. 1998;15:518–527. doi: 10.1093/oxfordjournals.molbev.a025951. [DOI] [PubMed] [Google Scholar]
- Verbitsky M, Rothlin C, Katz E, Elgoyhen AB. Mixed nicotinic-muscarinic properties of the a9 nicotinic cholinergic receptor. Neuropharmacology. 2000;39:2515–2524. doi: 10.1016/s0028-3908(00)00124-6. [DOI] [PubMed] [Google Scholar]
- von Bekesy G, editor; Wever EG, translator. Experiments in Hearing. McGraw-Hill; New York: 1960. [Google Scholar]
- Warr W. Organization of olivocochlear efferent sytems in mammals. In: Douglas W, Popper A, Fay R, editors. The mammalian auditory pathway: Neuroanatomy. Springler-Verlag; New York: 1992. pp. 410–448. [Google Scholar]
- Weber T, Gopfert MC, Winter H, Zimmermann U, Kohler H, Meier A, Hendrich O, Rohbock K, Robert D, Knipper M. Expression of prestin-homologous solute carrier (SLC26) in auditory organs of nonmammalian vertebrates and insects. Proc Natl Acad Sci U S A. 2003;100:7690–7695. doi: 10.1073/pnas.1330557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisstaub N, Vetter D, Elgoyhen A, Katz E. The alpha9/alpha10 nicotinic acetylcholine receptor is permeable to and is modulated by divalent cations. Hearing Res. 2002;167:122–135. doi: 10.1016/s0378-5955(02)00380-5. [DOI] [PubMed] [Google Scholar]
- Yang Z. PAML: a program package for phylogenetic analysis by maximum likelihood. Comput Appl Biosci. 1997;13:555–556. doi: 10.1093/bioinformatics/13.5.555. [DOI] [PubMed] [Google Scholar]
- Yang Z. Likelihood ratio tests for detecting positive selection and application to primate lysozyme evolution. Mol Biol Evol. 1998;15:568–573. doi: 10.1093/oxfordjournals.molbev.a025957. [DOI] [PubMed] [Google Scholar]
- Yang Z, Nielsen R. Codon-substitution models for detecting molecular adaptation at individual sites along specific lineages. Mol Biol Evol. 2002a;19:908–917. doi: 10.1093/oxfordjournals.molbev.a004148. [DOI] [PubMed] [Google Scholar]
- Yang Z, Swanson WJ. Codon-substitution models to detect adaptive evolution that account for heterogeneous selective pressures among site classes. Mol Biol Evol. 2002b;19:49–57. doi: 10.1093/oxfordjournals.molbev.a003981. [DOI] [PubMed] [Google Scholar]
- Yang Z, Nielsen R, Goldman N, Pedersen AM. Codon-substitution models for heterogeneous selection pressure at amino acid sites. Genetics. 2000;155:431–449. doi: 10.1093/genetics/155.1.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida N, Shigemoto T, Sugai T, Ohmori H. The role of inositol triphosphate on ACh-induced outward currents in bullfrog saccular hair cells. Brain Res. 1994;644:90–100. doi: 10.1016/0006-8993(94)90351-4. [DOI] [PubMed] [Google Scholar]
- Young JM, Trask BJ. The sense of smell: genomics of vertebrate odorant receptors. Hum Mol Genet. 2002;11:1153–1160. doi: 10.1093/hmg/11.10.1153. [DOI] [PubMed] [Google Scholar]
- Zheng J, Long KB, Shen W, Madison LD, Dallos P. Prestin topology: localization of protein epitopes in relation to the plasma membrane. Neuroreport. 2001;12:1929–1935. doi: 10.1097/00001756-200107030-00032. [DOI] [PubMed] [Google Scholar]
- Zheng J, Shen W, He DZ, Long KB, Madison LD, Dallos P. Prestin is the motor protein of cochlear outer hair cells. Nature. 2000;405:149–155. doi: 10.1038/35012009. [DOI] [PubMed] [Google Scholar]
- Zheng J, Du GG, Anderson CT, Keller JP, Orem A, Dallos P, Cheatham M. Analysis of the oligomeric structure of the motor protein prestin. J Biol Chem. 2006;281:19916–19924. doi: 10.1074/jbc.M513854200. [DOI] [PubMed] [Google Scholar]
- Zheng J, Du GG, Matsuda K, Orem A, Aguinaga S, Deak L, Navarrete E, Madison LD, Dallos P. The C-terminus of prestin influences nonlinear capacitance and plasma membrane targeting. J Cell Sci. 2005;118:2987–2996. doi: 10.1242/jcs.02431. [DOI] [PubMed] [Google Scholar]