Abstract
Endosperm and embryo tissues from the seeds of Euonymus alatus (Burning Bush) accumulate high levels of 3-acetyl-1,2-diacyl-sn-glycerols (acTAGs) as their major storage lipids. In contrast, the aril tissue surrounding the seed produces long-chain triacylglycerols (lcTAGs) typical of most other organisms. The presence of the sn-3 acetyl group imparts acTAGs with different physical and chemical properties, such as a 30% reduction in viscosity, compared to lcTAGs. Comparative transcriptome analysis of developing endosperm and aril tissues using pyrosequencing technology was performed to isolate the enzyme necessary for the synthesis of acTAGs. An uncharacterized membrane-bound O-acyltransferase (MBOAT) family member was the most abundant acyltransferase in the endosperm but was absent from the aril. Expression of this MBOAT in yeast resulted in the accumulation of acTAGs but not lcTAG; hence, the enzyme was named EaDAcT (Euonymus alatus diacylglycerol acetyltransferase). Yeast microsomes expressing EaDAcT possessed acetyl-CoA diacylglycerol acetyltransferase activity but lacked long-chain acyl-CoA diacylglycerol acyltransferase activity. Expression of EaDAcT under the control of a strong, seed-specific promoter in Arabidopsis resulted in the accumulation of acTAGs, up to 40 mol % of total TAG in the seed oil. These results demonstrate the utility of deep transcriptional profiling with multiple tissues as a gene discovery strategy for low-abundance proteins. They also show that EaDAcT is the acetyltransferase necessary and sufficient for the production of acTAGs in Euonymus seeds, and that this activity can be introduced into the seeds of other plants, allowing the evaluation of these unusual TAGs for biofuel and other applications.
Keywords: 3-acetyl-1,2-diacyl-sn-glycerols; biodiesel; diacylglycerol acyl-CoA acyltransferase; membrane-bound O-acyltransferase; triacylglycerols
The seed oil of Euonymus alatus (Burning Bush) is comprised almost exclusively of 3-acetyl-1,2-diacyl-sn-glycerols (acTAGs), unusual triacylglycerols where the sn-3 position is esterified with acetate instead of the long-chain fatty acid found in the triacylglycerols (lcTAGs) common to most organisms. AcTAGs have also been identified in seeds of other members of the Celastraceae, as well as other plant families (1, 2), and also in milk fat (3).
Previous work has demonstrated that the formation of acTAGs in developing Euonymus seed is the result of an sn-1,2-diacylglycerol:acyl-CoA acyltransferase (DGAT) (E.C. 2.3.1.20) reaction (4). At least four different types of DGAT enzymes have been identified in various species. Two of these enzymes, DGAT1 and DGAT2, are responsible for the bulk of TAG synthesis in most organisms. DGAT1 proteins are members of the MBOAT protein superfamily and differ structurally from DGAT2 proteins. For example, DGAT1 proteins are larger than DGAT2 and possess six transmembrane domains compared to the two predicted in DGAT2 (5). Therefore, it is not surprising that these two enzymes play nonredundant roles in TAG synthesis in plants and other organisms. In the model plant Arabidopsis thaliana, only mutations in DGAT1, and not DGAT2, have been reported that affect seed oil levels (6–8). However, in some plants, DGAT2 orthologs appear to incorporate unusual fatty acids in the seed storage oils (9–11). The bifunctional DGAT/wax ester synthase ADP1 from Acinetobacter calcoaceticus, another member of the MBOAT superfamily, represents a third class of DGAT enzyme (12). Homologs of ADP1 have been characterized in petunia and Arabidopsis, but for these proteins the DGAT activity is either absent or much lower compared to the wax synthase activity (13, 14). Lastly, a soluble DGAT enzyme has been identified in peanut cotyledons (15), but functional orthologs have yet to be identified in other species. In addition to the DGATs, phospholipid:diacylglycerol acyltransferases (PDAT) (EC 2.3.1.43) also synthesize TAG, using phosphatidylcholine as the acyl donor (16, 17). PDAT contributes notably to TAG deposition in Arabidopsis seeds (18).
The substitution of a long acyl chain typically 16–18 carbons in length with the two-carbon acetate group confers acTAGs with different and potentially useful properties compared to lcTAGs. Here, we demonstrate that acTAGs possess a lower viscosity than lcTAGs, raising the possibility for direct use of TAG as a biofuel source. This potential application provided additional impetus for the identification of the enzyme responsible for the synthesis of acTAGs. A DGAT1 gene has been cloned from Euonymus and the enzyme shown to use both acetyl-CoA and long-chain acyl-CoA substrates for the synthesis of TAG in vitro (4). We show, however, that the Euonymus DGAT1 or DGAT2 orthologs are unlikely to synthesize acTAGs in the endosperm. We therefore used a transcript profiling approach using pyrosequencing to identify candidate acyltransferase enzymes responsible for the synthesis of acTAGs in Euonymus seed. In the past, the Sanger sequencing of cDNA clones has resulted in the identification of many enzymes responsible for the production of unusual lipids (19, 20), but none of these have been acyltransferases. Furthermore, no DGAT transcripts were present in 10,000 EST sequences of developing Arabidopsis seeds (21). A pyrosequencing-based approach offered the advantage of generating 10- to 100-fold more EST sequences, therefore allowing us to detect lower-abundance transcripts, including most acyltransferases. We demonstrate that one of these acyltransferase genes, named EaDAcT, encodes the enzyme responsible for the synthesis of acTAGs in Euonymus seed. Furthermore, EaDAcT is efficient in producing acTAGs in transgenic Arabidopsis plants, resulting in highly modified seed oil.
Results
Euonymus Fruit Accumulate Both lcTAGs and acTAGs in a Tissue-Specific Manner.
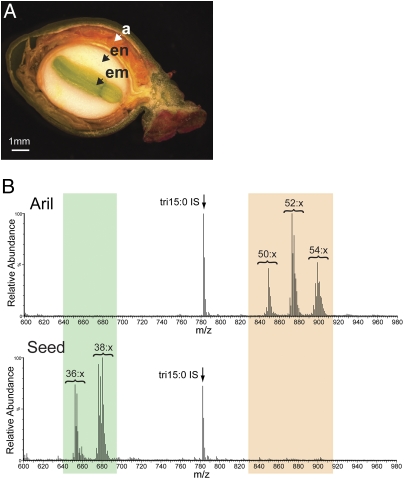
Although the storage lipids found in Euonymus endosperm are comprised almost entirely of acTAGs (Fig. 1 and Table 1), other tissues present in the Euonymus fruit also accumulate lcTAGs. For example, up to 30% of the TAG in the embryo is lcTAG (Table 1). Additionally, within the fruit capsule, Euonymus seeds are surrounded by an aril: a fleshy and often highly pigmented tissue derived from the funiculus (Fig. 1A). Significantly, Euonymus aril tissue is oil-rich and in contrast to the seed synthesizes lcTAGs and not acTAGs (Fig. 1B and Table 1).
Fig. 1.
Euonymus alatus fruit synthesize different types of TAGs in a tissue specific manner. (A) Cross-section of a developing fruit from Euonymus alatus illustrating the different TAG producing tissues. a, aril; em, embryo; en, endosperm. Bar represents 1 mm. (B) Positive-ion ESI mass spectra of lipid extracts from Euonymus alatus aril and seed tissues, demonstrating the accumulation of lcTAGs (highlighted in orange) in the aril and acTAGs (highlighted in green) in the seed. Peaks correspond to m/z values of the [M + NH4]+ adduct. Tripentadecanoin (tri15:0) was added as an internal standard. The number of acyl carbons in each series of TAG molecules is indicated; the number of double bonds (x) is not defined.
Table 1.
Lipid compositions of Euonymus seed tissues at mid-maturation
| Percent distribution* |
||||
| Lipid class | Embryo | Endosperm | Whole seed | Aril |
| Triacylglycerol | 26.1 | 1.6 | 3.3 | 92.2 |
| 3-Acetyl-1,2-diacylglycerol | 65.6 | 94.5 | 91.7 | 0.5 |
| 1,2-Diacylglycerol | 1.7 | 1.3 | 1.9 | 2.0 |
| Polar lipids | 6.6 | 2.6 | 3.1 | 4.9 |
Values are for total lipids extracted from a 60-seed sample.
*Measured as a percentage of total long-chain acyl groups.
EaDGAT1 and EaDGAT2 Do Not Synthesize acTAGs in Vivo.
The Euonymus DGAT1 ortholog has been cloned and demonstrated to possess both long-chain acyl- and acetyl-transferase activity in vitro (4). However, expression of EaDGAT1 in yeast (4) and in Arabidopsis (Fig. S1) resulted in detectable but very low levels of acTAGs, <0.05 mol %, suggesting that other factors are necessary for the production of acTAGs. Given the apparent role of various plant DGAT2 enzymes in the accumulation of unusual fatty acids in seed oil, we cloned the Euonymus DGAT2 ortholog (GenBank entry GU594062). When expressed in yeast, EaDGAT2 synthesized lcTAGs but not acTAGs (Fig. S2A). Similarly, in vitro analyses suggested that this enzyme possessed only long-chain acyltransferase activity and not acetyltransferase activity (Fig. S2B). Therefore, EaDGAT2 lacks the biochemical activity necessary for the accumulation of acTAGs in Euonymus endosperm.
Comparative Transcriptional Profiling of Euonymus TAG-Producing Tissues.
Because EaDGAT1 and EaDGAT2 were not sufficient to synthesize acTAGs at useful levels in transgenic plants, we used comparative deep transcriptional profiling to identify candidate proteins necessary for the production of these unusual storage lipids. We took advantage of the tissue-specific nature of acTAG synthesis in Euonymus endosperm and lcTAG synthesis in the aril to identify candidate enzymes that synthesized acTAGs. RNA was extracted from endosperm tissue at different time points during seed development, as well as from aril and embryo tissue at a point during maximal TAG production. cDNA was synthesized and then sequenced using 454 pyrosequencing technology (22) to achieve deep coverage of low-abundance transcripts. A total of 3.1 million sequences were obtained with at least 338,000 for each library (Table 2). Contigs were assembled from the compilation of all sequences and then annotated based on their homology to Arabidopsis proteins. Importantly, contigs corresponding to the previously cloned Euonymus genes EaDGAT1 and EaDGAT2 were identified, indicating that the EST deep sequencing and assembly methods functioned as expected. The transcript levels of these and other genes encoding enzymes important for TAG synthesis in other species were of obvious interest to us. Surprisingly, no EaDGAT1 transcripts were present in any of the endosperm libraries, including a normalized library enriched for less abundant sequences (Table 2), arguing that EaDGAT1 does not play a role in the formation of acTAGs. Additionally, endosperm expression levels of other TAG synthetic genes such as DGAT2 and PDAT were also low, comprising <0.001% of transcripts in each library. In contrast, transcripts from the DGAT1, PDAT, and PDAT-like genes were present in the aril at 10-fold higher levels, consistent with a role in the formation of the lcTAGs in that tissue. These results suggest that Euonymus endosperm contains a novel enzyme necessary for the production of acTAGs. To identify this enzyme, we selected genes annotated as acyltransferases with high transcript levels in the endosperm relative to the aril. One such gene, a member of the MBOAT gene family, and subsequently named EaDAcT (Euonymus alatus diacyl-glycerol acetyl transferase), is the highest expressed acyltransferase in the endosperm (0.03–0.06% of transcripts) and is absent from the aril (Table 2), consistent with a role in the synthesis of acTAGs but not lcTAGs.
Table 2.
Total library EST counts and transcript levels for genes involved in TAG synthesis in different Euonymus cDNA libraries
| Endosperm |
|||||||
| Collection date: | Aril 8/29 | Embryo 8/29 | 8/22 | 8/29 | 9/6 | 9/19 | Normalized |
| ESTs | 342,813 | 338,868 | 342,232 | 430,701 | 522,242 | 423,690 | 705,875 |
| Gene* | |||||||
| DGAT1 | 3.6 | 0 | 0 | 0 | 0 | 0 | 0 |
| DGAT2 | 0.9 | 0.3 | 0.9 | 0.4 | 0.4 | 1.2 | 2.8 |
| PDAT | 3.6 | 1.8 | 0.3 | 0 | 0 | 0.2 | 0.5 |
| PDAT-like | 10.3 | 3.0 | 0.6 | 0 | 0.4 | 0.5 | 0 |
| EaDAcT | 0 | 22.2 | 62.2 | 30.7 | 48.7 | 42.4 | 39.7 |
*Gene expression levels are represented as the number of transcripts per 100,000 transcripts.
EaDAcT encodes a predicted protein of 42 kDa (GenBank entry GU594061) and contains sequence domains present in the MBOAT family, including conserved asparagine and histidine residues important for catalysis in other family members (Fig. S3B). A hydropathy plot suggests at least five transmembrane domains (Fig. S3C); thus, EaDAcT is similar to other MBOAT proteins. The EaDAcT protein phylogenetically clusters with a group of mostly uncharacterized plant MBOAT proteins (Fig. S3D). These proteins have been annotated as putative wax synthases based on their similarity to the Jojoba wax synthase (23). Also included in this group is the Arabidopsis sterol transferase, AtSAT1 (24).
Yeast Transformed with EaDAcT Accumulates acTAGs but Not lcTAGs.
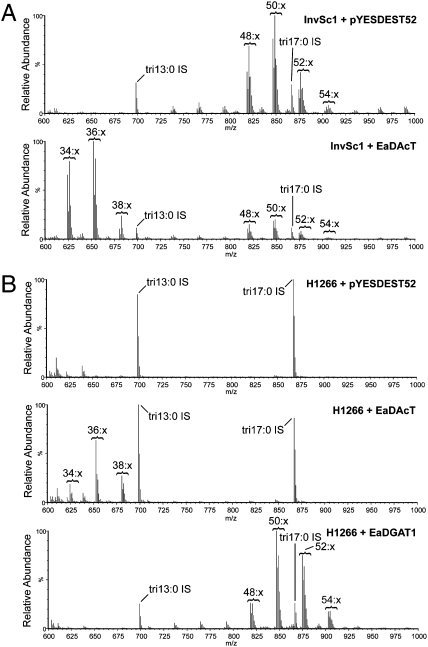
We expressed EaDAcT in wild-type yeast cells (strain InvSc1) and analyzed neutral lipid extracts using ESI-MS. The resultant mass spectrum revealed a number of novel peaks with masses corresponding to the [M+NH4]+ adduct of different acTAG molecular species (Fig. 2A). When subjected to ESI-MS2, these novel peaks produced daughter fragments consistent with acTAGs (Fig. S4). Interestingly, these ESI-MS2 analyses suggest that in contrast to the fatty acid profile of acTAGs produced by Euonymus, the acTAGs synthesized in yeast contain high levels of the four fatty acids, namely palmitate, stearate, palmitoleate, and oleate, that dominate yeast lipids. Quantification of the ESI-MS analyses revealed that InvSc1 yeast expressing EaDAcT produced 17.5 nmol of acTAG/mg DW and 12.3 nmol of lcTAG/mg DW. This compared to undetectable acTAGs and 32.9 nmol of lcTAG/mg DW in the empty vector control. These results demonstrate that EaDAcT is sufficient to produce acTAGs when expressed in yeast, suggesting it is the enzyme necessary for acTAG production in Euonymus endosperm.
Fig. 2.
Yeast expressing EaDAcT accumulate acTAGs but not lcTAGs. Positive-ion ESI mass spectra of neutral lipid extracts from yeast strains InvSc1 (A) or H1266 (B) expressing the empty vector pYES-DEST52, EaDAcT, or EaDGAT1. Tritridecanoin (tri13:0) and triheptadecanoin (tri17:0) were added as internal standards.
To determine whether EaDAcT was also capable of synthesizing lcTAGs, we expressed the gene in the yeast strain H1266. Three genes important for TAG synthesis have been ablated in H1266, rendering this strain unable to accumulate TAG (25). As expected, neutral lipid extracts from H1266 containing the empty vector (pYES-DEST52) did not contain any TAGs when analyzed using ESI-MS (Fig. 2B). When expressing EaDGAT1, H1266 yeast accumulated up to 13.6 nmol of lcTAG/mg DW, but no acTAGs (Fig. 2B). In contrast, H1266 yeast expressing EaDAcT accumulated 0.5 nmol of acTAG/mg DW but no lcTAGs (Fig. 2B), demonstrating that EaDAcT cannot synthesize lcTAGs when expressed in yeast.
EaDAcT Possesses Acetyltransferase but Not Long-Chain Acyltransferase Activity in Vitro.
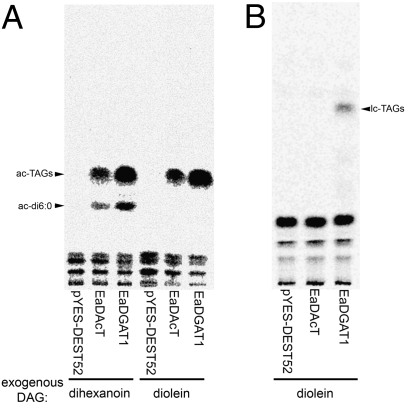
Previous work has demonstrated that acTAGs are synthesized in Euonymus endosperm by an 1,2-diacyl-sn-glycerol:acyl CoA acyltransferase reaction (4). To determine whether EaDAcT possesses this activity in vitro, we isolated microsomes from InvSc1 yeast expressing EaDAcT, EaDGAT1, and the empty vector. These microsomes were incubated with [14C]acetyl-CoA, resultant lipids extracted and then separated by TLC. Both EaDAcT and EaDGAT1 microsomes produced a labeled product that comigrates with acTAG (Fig. 3A). Using both reverse phase and silver nitrate TLC, this product from EaDGAT1 was previously shown to be [14C]acTAG (4). When quantified, EaDAcT microsomes possessed a specific acetyltransferase activity of 21 pmol/min/mg protein under these conditions, compared to 63 pmol/min/mg protein for EaDGAT1 microsomes. Thus, EaDAcT possesses the necessary biochemical activity in vitro for the production of acTAGs. To prove that EaDAcT was acetylating DAG to form acTAGs, in vitro acetyltransferase reactions were performed with the addition of dihexanoin. The presence of this exogenous, low carbon number DAG resulted in an additional labeled band corresponding to [14C]acetyl-dihexanoin from both the EaDAcT and EaDGAT1 microsomes (Fig. 3A), thus demonstrating that EaDAcT functions as a 1,2-diacylglycerol:acyl-CoA acyltransferase.
Fig. 3.
EaDAcT possesses acetyltransferase but not oleoyltransferase activity in vitro. (A) TLC separation of lipid extracts from yeast microsomes (InvSc1 background) expressing EaDAcT, EaDGAT1, or empty vector (pYES-DEST52) incubated with 15 μM [14C]acetyl-CoA in the presence or absence of exogenous 230 μM dihexanoin. (B) TLC separation of lipid extracts from yeast microsomes (H1266 background) expressing EaDAcT, EaDGAT1, or empty vector (pYES-DEST52) incubated with 8 μM [14C]oleoyl-CoA.
Microsomes were also isolated from H1266 yeast expressing EaDAcT, EaDGAT1, and the empty vector and then incubated with [14C]oleoyl-CoA to determine whether EaDAcT possesses long-chain acyltransferase activity. Under these conditions, only EaDGAT1 produced a labeled product that comigrates with lcTAG (Fig. 3B). These results demonstrate that EaDAcT does not possess long-chain acyltransferase activity and are consistent with the in vivo accumulation of acTAGs, but not lcTAGs, by H1266 yeast expressing EaDAcT (Fig. 2B). When quantified, the oleoyltransferase activity of EaDGAT1 was 53 pmol/min/mg protein, similar to that seen previously (4).
Arabidopsis Seeds Expressing EaDAcT Accumulate acTAGs.
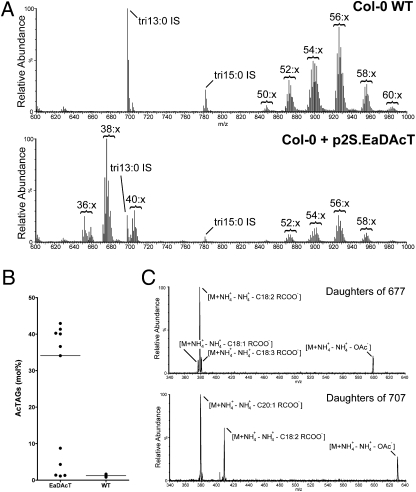
We expressed EaDAcT in Col-0 wild-type Arabidopsis under the control of the Arabidopsis 2S seed storage protein promoter. ESI-MS analysis of neutral lipid extracts from the seed of independent T3 transgenic lines revealed the presence of acTAGs in the seed oil (Fig. 4A). In over half of the transgenic lines, the proportion of acTAGs was high, with some lines accumulating up to 40 mol % acTAGs (Fig. 4B). We confirmed the identity of these acTAGs using ESI-MS2, which produced the expected daughter fragments (Fig. 4C), and by 1H and 13C NMR (Fig. S5). The acTAGs synthesized in Arabidopsis seed contained the long-chain fatty acid eicosenoate (20:1) characteristic of Arabidopsis seed oil (Fig. 4 A and C). This composition paralleled that of the acTAGs produced by the expression of EaDAcT in yeast, which also contained fatty acids characteristic of the host organism (Fig. 2 A and B). The lipid content of seed from transgenic lines accumulating high levels of acTAGs was not significantly different from that of wild-type seed (Table S1). Small, but significant changes in the fatty acid composition of seed lipids were observed in the acTAG-accumulating lines, the most notable being an increase in linoleate (18:2) content (Table S1).
Fig. 4.
Arabidopsis seeds expressing EaDAcT accumulate acTAGs. (A) Positive-ion ESI mass spectra of neutral lipid extracts from Col-0 wild-type seed or T3 seed from a representative Col-0 plant expressing EaDAcT. Tritridecanoin (tri13:0) and tripentadecanoin (tri15:0) were added as internal standards. (B) Scatter plot showing the distribution of the acTAG composition of T3 seed from different transgenic Col-0 plant lines expressing EaDAcT or of Col-0 wild-type seed. Horizontal lines represent the median value for each sample group. (C) ESI-MS2 analysis of neutral lipid extracts from the T3 seed of Col-0 plants expressing EaDAcT. Shown are the daughter fragment peaks from acTAGS with [M + NH4]+ adducts with m/z values of 653 and 677.
AcTAGs Possess Lower Viscosity than lcTAGs.
Because kinematic viscosity is foremost a function of molecular weight rather than molecular shape (26), acTAGs should possess a lower viscosity than lcTAG. We therefore measured the intrinsic viscosity and the density of acTAGs and lcTAGs purified from Euonymus seed oil, as well as of soybean oil and trioctanoin as standards (Table 3). Literature data for the intrinsic viscosity (at 25 °C) of sunflower oil (27) and trioctanoin (28), of 49 mPa s and 24.5 mPa s, respectively, are in good agreement with the measured values for the above controls. Soybean and sunflower oil have similar kinematic viscosities at 38 °C. Likewise, density measurements for soybean oil (29) and trioctanoin (28), of 0.917 g/cm3 and 0.95 g/cm3, respectively, are in good agreement with our measured values. In accordance with our expectations, the measured kinematic viscosity of acTAGs was 28.5 mm2·s−1, 39% lower than that of the lcTAGs (Table 3).
Table 3.
Measured viscosity values for acTAGs and lcTAGs
| Sample | Intrinsic viscosity, mPa s | Density, g/cm3 | Kinematic viscosity, mm2·s−1 |
| Soybean oil | 49.2 ± 2.4 | 0.921 | 53.5 |
| Trioctanoin | 23.3 ± 3.5 | 0.948 | 24.5 |
| LcTAG | 42.6 ± 2.90 | 0.916 | 46.5 |
| AcTAG | 26.3 ± 0.9 | 0.924 | 28.5* |
Data were obtained at ambient temperature (24 °C).
*Estimated kinematic viscosity at 40 °C is ≈18 mm2·s−1.
Discussion
Deep Transcriptional Profiling Reveals a Distinct Type of DGAT.
An earlier attempt to isolate the enzyme responsible for the synthesis of acTAGs in the developing seeds of Euonymus alatus led to the cloning of the Euonymus DGAT1 ortholog (4). The importance of DGAT1 enzymes in TAG synthesis and the in vitro acetyltransferase activity of EaDGAT1 were consistent with a hypothesis that this enzyme synthesized acTAGs in Euonymus seeds. However, expression of EaDGAT1 in yeast (4) and Arabidopsis (Fig. S1) produced mostly lcTAGs and only low levels of acTAGs, suggesting that additional factors were necessary for the production of acTAGs. Furthermore, characterization of a Euonymus DGAT2 ortholog revealed that it lacked any potential to be the necessary acetyltransferase (Fig. S2). To identify additional acetyltransferase and/or modifier genes, we used deep transcriptional profiling to compare gene expression levels in the acTAG producing endosperm with the lcTAG containing aril, leading to the discovery of EaDAcT, a previously uncharacterized MBOAT family member.
This study therefore demonstrates the power of comparative deep transcriptional profiling as a gene discovery strategy. Most conventional EST projects based on Sanger sequencing have been limited in scope, usually producing <10,000 sequences from a single tissue. Such approaches would have resulted in only 3–6 EaDAcT ESTs in 10,000, depending on the particular endosperm library sequenced. Because up to 4 ESTs is statistically not significantly different from zero ESTs (30), it is likely that EaDAcT could have been overlooked in light of many other acyltransferase candidates. Additionally, this study was greatly facilitated by the dissection, extraction, and characterization of Euonymus fruit lipids to reveal the distinctive compositions and high oil content of the aril and endosperm tissues, thereby prompting a multitissue transcriptome analysis.
The MBOAT family of acyltransferases possesses a wide range of substrate specificities. We are, however, unaware of any MBOATs that use acetyl-CoA as a substrate. A diverse array of acyl acceptors includes DAG, various phospholipids, fatty alcohols, sterols, and proteins (31). Even among the plant MBOATs closely related to EaDAcT, a wide range of substrate specificity exists, with known wax synthases and sterol acyltransferases clustering with EaDAcT (Fig. S3C). EaDAcT acetylates a wide range of DAG substrates, including those with very short acyl chains. For example, EaDAcT microsomes synthesized acetyl-dihexanoin when incubated with exogenous dihexanoin (Fig. 3A). Additionally, the variation in the molecular species composition between acTAGs isolated from Euonymus endosperm (Fig. 1B), yeast expressing EaDAcT [enriched in saturated and monounsaturated fatty acids (Fig. 2)], and Arabidopsis seed expressing EaDAcT [containing very long-chain fatty acids (Fig. 3A)] suggests that EaDAcT can accommodate a range of DAG substrates.
Collectively, compelling evidence demonstrates that EaDAcT synthesizes acTAGs in developing Euonymus seeds. First, EaDAcT is expressed at relatively high levels (0.02–0.06% of total transcripts) in the acTAG accumulating endosperm and embryo tissues, whereas no DGAT1 transcripts were present in the over 2.4 million ESTs derived from endosperm tissue (Table 1). The low levels of DGAT1 transcript present in the embryo might contribute to the synthesis of the lcTAG fraction in that tissue. Second, yeast and Arabidopsis expressing EaDAcT synthesize acTAGs (Figs. 2 and 4). In contrast, expression of DGAT1 in these species results in the accumulation of lcTAGs (Fig. 2B and Fig. S1). Third, consistent with the heterologous expression results, EaDAcT possesses in vitro diacylglycerol acetyltransferase activity necessary for the production of acTAGs and lacks long-chain acyltransferase activity (Fig. 3). The inability of EaDAcT to synthesize lcTAGs is thus also consistent with the very low level of lcTAGs in the endosperm; additionally, the absence of EaDAcT transcript in the aril (Table 2) provides further evidence that this enzyme does not play a role in the production of lcTAGs. In summary, the expression profile, the in vitro activities, and the effect of heterologous expression conclusively demonstrate that EaDAcT is responsible for the production of acTAGs in Euonymus seeds.
Physical Properties of acTAG Suggest Utility for a Variety of Applications.
Although it is not an agronomic oilseed, the best lines of wild-type Arabidopsis transformed with EaDAcT exhibited ∼40 mol % of acTAG in total TAG (Fig. 4). Previously, expression of a single biosynthetic gene has generally not resulted in such high accumulations of novel products in transgenic oilseeds (32). Only in the cases of long-chain liquid wax esters (23) and short- and medium-chain fatty acids (33, 34), where multiple biosynthetic genes have been coexpressed, have the accumulations of unusual lipid or fatty acid products reached 40–50 mol %. The synthesis of ∼40 mol % acTAGs was achieved with competition from endogenous enzymes of TAG synthesis, particularly DGAT1 and PDAT (18), suggesting that despite its diverse phylogenetic origin and predominantly endosperm expression, EaDAcT efficiently integrates with the TAG assembly mechanisms present in Arabidopsis cotyledon membranes.
In anticipation of further improved yields of acTAG it is worthwhile to consider the utility of a vegetable oil enriched in acTAG. The presence of an acetyl group results in acTAGs possessing substantially different physical and chemical properties compared to their longer chain counterparts, making these unusual TAGs potentially useful in diverse applications.
For example, the high viscosity of vegetable oils renders them unsuitable for direct use in diesel engines; biodiesel is therefore synthesized by transesterifying TAGs to form less viscous fatty acid esters (35). However, the lower viscosity of acTAGs (Table 3) raises the possibility that a vegetable oil rich in acTAGs could be directly combusted without the need for transesterification. Indeed, the kinematic viscosity of acTAGs is within the ASTM specifications for Diesel Grade No. 4-D (5–24 mm2·s−1 at 40 °C), a heavier fuel used mainly in low- and medium-speed engines (36). Additionally, the reduced number of unsaturated acyl groups/mole in acTAG compared with lcTAG may substantially reduce TAG polymerization and hence coking and gum formation problems caused by direct use of these vegetable oils.
Additionally, the introduction of acTAG into edible oilseed crops may provide an opportunity to develop reduced-calorie fats and oils with a molecular structure similar to existing commercial products such as SALATRIM (short and long acyltriglyceride molecule) (37). The calculated calorific values of tristearin and acetyldistearin are 9.36 and 8.75 kcal/g, respectively. Thus, the absence of a third long acyl chain reduces the calorific content of acTAGs by ∼6.5%. The deposition of most dietary fats in animals requires lipolysis, absorption, and resynthesis of lcTAG from 2-monoacylglycerols and fatty acids in the intestinal mucosa. We estimate that lcTAG reassembled from acTAG should actually reduce the lcTAG calories available for deposition in the adipose tissues by at least 13%. Furthermore, the release of free acetic acid by the gastrointestinal lipases may further suppress body fat accumulation (38, 39).
In conclusion, we have identified the enzyme responsible for the synthesis of acTAGs in Euonymus endosperm; EaDAcT is a distinct type of DGAT within the MBOAT family of acyltransferases. In addition, the novel chemical and physical properties of acTAGs may make them useful for various applications, including direct-use biofuels. However, because Euonymus is not suitable for vegetable oil production, the expression of EaDAcT in current commercial oilseed crops could lead to the large-scale production of these unusual TAGs.
Materials and Methods
Plant Materials.
Developing Euonymus alatus fruit were collected from 27 August to 8 October 2008 on the grounds of Michigan State University, courtesy of the Grounds Department of Campus Parks. The aril, endosperm, and embryo tissues were dissected and then frozen and stored at −80 °C for RNA extraction or dried at 85 °C for lipid analysis. Transgenic Arabidopsis plants were selected by germinating surface sterilized T1 seeds on MS media containing 30 μg·ml−1 hygromycin. Lines homozygous for individual insertion events were generated by selection at the T2 and T3 generations.
Lipid Extractions and Analyses.
Lipids were extracted from dried, ground Euonymus tissues with 3:2 (vol/vol) hexane: isopropanol according to the protocol of Hara and Radin (40). Lipids were assayed gravimetrically, by analysis of fatty acid methyl esters released by transmethylation, or by ESI-MS. Cell pellets from 30ml yeast cultures grown to stationary phase were lysed in a vibration mill (Retsch) with 500-μL glass beads for 5 min and extracted with 3.8 mL of 2:1 (vol/vol) chloroform:methanol. 1.25 mL chloroform and 1.3 mL 0.15 M acetic acid were added, the organic phase removed and the aqueous phase re-extracted with 2 mL of chloroform. Neutral lipids were recovered by passage of the total lipid extracts over a small silica gel column with 99:1 (vol/vol) chloroform:methanol. Total Arabidopsis seed lipids were transmethylated in the seed, followed by GC-FID analysis of the fatty acid methyl esters according to Li et al. (41). Alternatively, total lipids were extracted from the seed (41) and neutral lipids then purified as for yeast. Lipid extracts were analyzed using ESI-MS as described in ref. 42.
cDNA Library Construction and 454 Pyrosequencing.
Extraction of RNA from Euonymus tissues, synthesis of cDNA and subsequent sequencing and assembly are described in SI Materials and Methods.
Isolation and Expression of cDNAs Encoding EaDAcT and Euonymus DGATs.
The isolation and expression of EaDAcT, EaDGAT1, and EaDGAT2 are described in SI Materials and Methods.
Microsome Isolation and Assays for DGAT Activity.
Cultures of InvSc1 and H1266 containing pYES-DEST52, pEaDAcT, or pEaDGAT1 were grown in SC medium lacking uracil and containing galactose for ≈48 h. Microsomes were isolated according to Milcamps et al. (4) except that the final pellet was resuspended in buffer consisting of 50 mM Tris·HCl (pH 7.9), 20% (vol/vol) glycerol, and 1 mM DTT. Protein concentrations were determined using a BCA protein assay kit (Pierce). DGAT assays contained 20 μg of microsomal protein, 100 nCi of [1-14C]acetyl-CoA (18 μM) or 50 nCi [1-14C]oleoyl-CoA (8.6 μM), 230 μM 1,2-dihexanoyl-sn-glycerol or 230 μM 1,2-dioleoyl-sn-glycerol, and reaction buffer [50 mM Hepes (pH 7.4), 10% glycerol, 5 mM MgCl2, 1mM DTT] in a total volume of 100 μL. Reactions were incubated at 30 °C for 30 min, quenched by adding hot isopropanol, and lipids extracted according to the method of Hara and Radin (40). Radioactivity in the lipid extracts was quantified by liquid scintillation counting. Lipid extracts were separated on K6 Silica TLC plates (Whatman) developed with hexane:diethyl ether:acetic acid, 70:30:1 (vol/vol/vol). Radioactive bands were visualized and quantified using an Instant Imager (Packard Instruments).
Viscosity Determination.
Oil was extracted from mature, dried, and ground Euonymus alatus seeds (seed plus aril) by Soxhlet extraction with hexane. Large-scale purification of lcTAG and acTAG from winterized Euonymus alatus seed oil was achieved by silica column chromatography using a gradient of 2–4% isopropyl ether in petroleum ether. Intrinsic viscosity measurements were made on purified lcTAG and acTAG fractions, each of >98.5% purity as measured by ESI-MS, using a TA Instruments ARES parallel plate rheometer at ambient temperature (24 °C). Oil samples were placed between 2-inch-diameter plates set 0.9 mm apart, and shear rates increased incrementally from 0.1 to 100 s−1. Viscosity measurements reported are averages over the 10- to 100-s−1 range (6 data points), when the viscosity was independent of shear, in accordance with the expectation that the fluids would exhibit Newtonian behavior. Density measurements were made at ambient temperature using a 25-mL graduated flask.
Supplementary Material
Acknowledgments
We thank Yan Wang for advice about RNA extractions and cDNA preparation, Curtis Wilkerson and Nicholas Thrower for sequence assembly and database construction, Mike Rich (Composite Materials and Structures Center, College of Engineering, Michigan State University) for viscosity measurements, Doug Allen for assistance with NMR, and Christa Pennacchio and Erika Lindquist (Joint Genome Institute) for 454 pyrosequencing. ESI-MS analyses were carried out in the Mass Spectrometry Facility at Michigan State University. This work was supported in part by US Department of Agriculture–Cooperative State Research, Education, and Extension Service Grant 2005-35504-16195 and Department of Energy–Great Lakes Bioenergy Research Center Cooperative Agreement DE-FC02-07ER64494. The work conducted by the US Department of Energy Joint Genome Institute is supported by the Office of Science of the US Department of Energy under Contract No. DE-AC02-05CH11231.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database [accession nos. GU594061 (EaDAcT) and GU594062 (EaDGAT2)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1001707107/-/DCSupplemental.
References
- 1.Bagby MO, Smith CR., Jr. Asymmetric triglycerides from Impatiens edgeworthii seed oil. Biochim Biophys Acta. 1967;137:475–477. doi: 10.1016/0005-2760(67)90128-2. [DOI] [PubMed] [Google Scholar]
- 2.Kleiman R, Miller RW, Earle FR, Wolff IA. (S)-1,2-diacyl-3-acetins: Optically active triglycerides from Euonymus verrucosus seed oil. Lipids. 1967;2:473–478. doi: 10.1007/BF02533174. [DOI] [PubMed] [Google Scholar]
- 3.Parodi PW. Detection of acetodiacylglycerols in milkfat lipids by thin-layer chromatography. J Chromatogr A. 1975;111:223–226. doi: 10.1016/s0021-9673(01)80168-0. [DOI] [PubMed] [Google Scholar]
- 4.Milcamps A, et al. Isolation of a gene encoding a 1,2-diacylglycerol-sn-acetyl-CoA acetyltransferase from developing seeds of Euonymus alatus. J Biol Chem. 2005;280:5370–5377. doi: 10.1074/jbc.M410276200. [DOI] [PubMed] [Google Scholar]
- 5.Yen CLE, Stone SJ, Koliwad S, Harris C, Farese RV., Jr. DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res. 2008;49:2283–2301. doi: 10.1194/jlr.R800018-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katavic V, et al. Alteration of seed fatty acid composition by an ethyl methanesulfonate-induced mutation in Arabidopsis thaliana affecting diacylglycerol acyltransferase activity. Plant Physiol. 1995;108:399–409. doi: 10.1104/pp.108.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Routaboul JM, Benning C, Bechtold N, Caboche M, Lepiniec L. The TAG1 locus of Arabidopsis encodes for a diacylglycerol acyltransferase. Plant Physiol Biochem. 1999;37:831–840. doi: 10.1016/s0981-9428(99)00115-1. [DOI] [PubMed] [Google Scholar]
- 8.Zou JT, et al. The Arabidopsis thaliana TAG1 mutant has a mutation in a diacylglycerol acyltransferase gene. Plant J. 1999;19:645–653. doi: 10.1046/j.1365-313x.1999.00555.x. [DOI] [PubMed] [Google Scholar]
- 9.Burgal J, et al. Metabolic engineering of hydroxy fatty acid production in plants: RcDGAT2 drives dramatic increases in ricinoleate levels in seed oil. Plant Biotechnol J. 2008;6:819–831. doi: 10.1111/j.1467-7652.2008.00361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kroon JTM, Wei W, Simon WJ, Slabas AR. Identification and functional expression of a type 2 acyl-CoA:diacylglycerol acyltransferase (DGAT2) in developing castor bean seeds which has high homology to the major triglyceride biosynthetic enzyme of fungi and animals. Phytochemistry. 2006;67:2541–2549. doi: 10.1016/j.phytochem.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 11.Shockey JM, et al. Tung tree DGAT1 and DGAT2 have nonredundant functions in triacylglycerol biosynthesis and are localized to different subdomains of the endoplasmic reticulum. Plant Cell. 2006;18:2294–2313. doi: 10.1105/tpc.106.043695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalscheuer R, Steinbüchel A. A novel bifunctional wax ester synthase/acyl-CoA:diacylglycerol acyltransferase mediates wax ester and triacylglycerol biosynthesis in Acinetobacter calcoaceticus ADP1. J Biol Chem. 2003;278:8075–8082. doi: 10.1074/jbc.M210533200. [DOI] [PubMed] [Google Scholar]
- 13.King A, Nam JW, Han J, Hilliard J, Jaworski JG. Cuticular wax biosynthesis in petunia petals: cloning and characterization of an alcohol-acyltransferase that synthesizes wax-esters. Planta. 2007;226:381–394. doi: 10.1007/s00425-007-0489-z. [DOI] [PubMed] [Google Scholar]
- 14.Li F, et al. Identification of the wax ester synthase/acyl-coenzyme A: diacylglycerol acyltransferase WSD1 required for stem wax ester biosynthesis in Arabidopsis. Plant Physiol. 2008;148:97–107. doi: 10.1104/pp.108.123471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saha S, Enugutti B, Rajakumari S, Rajasekharan R. Cytosolic triacylglycerol biosynthetic pathway in oilseeds. Molecular cloning and expression of peanut cytosolic diacylglycerol acyltransferase. Plant Physiol. 2006;141:1533–1543. doi: 10.1104/pp.106.082198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dahlqvist A, et al. Phospholipid:diacylglycerol acyltransferase: an enzyme that catalyzes the acyl-CoA-independent formation of triacylglycerol in yeast and plants. Proc Natl Acad Sci USA. 2000;97:6487–6492. doi: 10.1073/pnas.120067297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ståhl U, et al. Cloning and functional characterization of a phospholipid:diacylglycerol acyltransferase from Arabidopsis. Plant Physiol. 2004;135:1324–1335. doi: 10.1104/pp.104.044354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang M, Fan J, Taylor DC, Ohlrogge JB. DGAT1 and PDAT1 acyltransferases have overlapping functions in Arabidopsis triacylglycerol biosynthesis and are essential for normal pollen and seed development. Plant Cell. 2009 doi: 10.1105/tpc.109.071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bao X, Katz S, Pollard M, Ohlrogge J. Carbocyclic fatty acids in plants: biochemical and molecular genetic characterization of cyclopropane fatty acid synthesis of Sterculiafoetida. Proc Natl Acad Sci USA. 2002;99:7172–7177. doi: 10.1073/pnas.092152999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cahoon EB, Shah S, Shanklin J, Browse J. A determinant of substrate specificity predicted from the acyl-acyl carrier protein desaturase of developing cat's claw seed. Plant Physiol. 1998;117:593–598. doi: 10.1104/pp.117.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White JA, et al. A new set of Arabidopsis expressed sequence tags from developing seeds. The metabolic pathway from carbohydrates to seed oil. Plant Physiol. 2000;124:1582–1594. doi: 10.1104/pp.124.4.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Margulies M, et al. Genome sequencing in microfabricated high-density picolitre reactors. Nature. 2005;437:376–380. doi: 10.1038/nature03959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lardizabal KD, et al. Purification of a jojoba embryo wax synthase, cloning of its cDNA, and production of high levels of wax in seeds of transgenic arabidopsis. Plant Physiol. 2000;122:645–655. doi: 10.1104/pp.122.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Q, Steinhauer L, Hammerlindl J, Keller W, Zou J. Biosynthesis of phytosterol esters: identification of a sterol o-acyltransferase in Arabidopsis. Plant Physiol. 2007;145:974–984. doi: 10.1104/pp.107.106278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandager L, et al. Storage lipid synthesis is non-essential in yeast. J Biol Chem. 2002;277:6478–6482. doi: 10.1074/jbc.M109109200. [DOI] [PubMed] [Google Scholar]
- 26.Niedzielski EL. Neopentyl polyol ester lubricants—bulk property optimization. Ind Eng Chem Prod Res Dev. 1976;15:54–58. [Google Scholar]
- 27.Abramovic H, Klofutar C. The temperature dependence of dynamic viscosity for some vegetable oils. Acta Chim Slov. 1998;45:69–77. [Google Scholar]
- 28.Eiteman MA, Goodrum JW. Rheology of the triglycerides tricaproin, tricaprylin, and tricaprin and of diesel fuel. Trans ASAE. 1993;36:503–507. [Google Scholar]
- 29.Rice P, Hamm W. Densities of soybean oil/solvent mixtures. J Am Oil Chem Soc. 1988;65:1177–1179. [Google Scholar]
- 30.Audic S, Claverie JM. The significance of digital gene expression profiles. Genome Res. 1997;7:986–995. doi: 10.1101/gr.7.10.986. [DOI] [PubMed] [Google Scholar]
- 31.Hofmann K. A superfamily of membrane-bound O-acyltransferases with implications for wnt signaling. Trends Biochem Sci. 2000;25:111–112. doi: 10.1016/s0968-0004(99)01539-x. [DOI] [PubMed] [Google Scholar]
- 32.Cahoon EB, et al. Engineering oilseeds for sustainable production of industrial and nutritional feedstocks: solving bottlenecks in fatty acid flux. Curr Opin Plant Biol. 2007;10:236–244. doi: 10.1016/j.pbi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Knutzon DS, Hayes TR, Wyrick A, Xiong H, Voelker TA Maelor Davies H. Lysophosphatidic acid acyltransferase from coconut endosperm mediates the insertion of laurate at the sn-2 position of triacylglycerols in lauric rapeseed oil and can increase total laurate levels. Plant Physiol. 1999;120:739–746. doi: 10.1104/pp.120.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leonard JM, Knapp SJ, Slabaugh MB. A Cuphea beta-ketoacyl-ACP synthase shifts the synthesis of fatty acids towards shorter chains in Arabidopsis seeds expressing Cuphea FatB thioesterases. Plant J. 1998;13:621–628. doi: 10.1046/j.1365-313x.1998.00066.x. [DOI] [PubMed] [Google Scholar]
- 35.Durrett TP, Benning C, Ohlrogge J. Plant triacylglycerols as feedstocks for the production of biofuels. Plant J. 2008;54:593–607. doi: 10.1111/j.1365-313X.2008.03442.x. [DOI] [PubMed] [Google Scholar]
- 36.ASTM International. ASTM D975-2002: Standard Specifications for Diesel Fuel Oils. West Conshohocken, PA: American Society for Testing and Materials; 2002. [Google Scholar]
- 37.Smith RE, Finley JW, Leveille GA. Overview of SALATRIM: A family of low-calorie fats. J Agric Food Chem. 1994;42:432–434. [Google Scholar]
- 38.Kondo T, Kishi M, Fushimi T, Kaga T. Acetic acid upregulates the expression of genes for fatty acid oxidation enzymes in liver to suppress body fat accumulation. J Agric Food Chem. 2009;57:5982–5986. doi: 10.1021/jf900470c. [DOI] [PubMed] [Google Scholar]
- 39.Kondo T, Kishi M, Fushimi T, Ugajin S, Kaga T. Vinegar intake reduces body weight, body fat mass, and serum triglyceride levels in obese Japanese subjects. Biosci Biotechnol Biochem. 2009;73:1837–1843. doi: 10.1271/bbb.90231. [DOI] [PubMed] [Google Scholar]
- 40.Hara A, Radin NS. Lipid extraction of tissues with a low-toxicity solvent. Anal Biochem. 1978;90:420–426. doi: 10.1016/0003-2697(78)90046-5. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Beisson F, Pollard M, Ohlrogge J. Oil content of Arabidopsis seeds: The influence of seed anatomy, light and plant-to-plant variation. Phytochemistry. 2006;67:904–915. doi: 10.1016/j.phytochem.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 42.Bates PD, Durrett TP, Ohlrogge JB, Pollard M. Analysis of acyl fluxes through multiple pathways of triacylglycerol synthesis in developing soybean embryos. Plant Physiol. 2009;150:55–72. doi: 10.1104/pp.109.137737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.