Abstract
Most prior work on the biological basis of aging has focused on describing differences between young and old individuals but provided only limited insight into the mechanisms controlling the rate of aging. Natural selection has produced a goldmine of experimental material, in the form of species of differing aging rate, whose longevity can vary by 10-fold or more within mammalian orders, but these resources remain largely unexplored at the cellular level. In this review article we focus on one approach to comparative biogerontology: the strategy of evaluating the properties of cultured cells from organisms of varying lifespan and aging rate. In addition, we discuss problems associated with the analysis and interpretations of interspecific variation of cellular trait data among species with disparate longevity. Given the impressive array of ‘natural experiments’ in aging rate, overcoming the technical and conceptual obstacles confronting research in comparative cellular gerontology will be well worth the effort.
Keywords: longevity, evolution, fibroblast, stress, analysis, genetics
From 1960 to the mid 90’s, investigators of the biological basis of aging devoted most of their effort to describing differences between young and old individuals, whether human or rodent. These investigators used whatever new sets of methods seemed most powerful at the time: first enzyme assays and tests for hormone concentrations, then tests for mRNA levels, and, most recently, similar evaluations of proteome and metabolome status. These surveys did a fine job of documenting and anatomizing the outcome of the aging process, but did not go far enough towards explaining the process that generated old individuals from healthy young ones, or the factors that caused this process to go slowly in people and elephants, faster in bats, dogs and horses, and very rapidly in mice and rats.
The 1990’s produced a sea change in how biogerontologists attack problems in aging. The discovery of single gene mutants that extend lifespan of worms, flies, and (in 1996) mice has gradually produced a fundamentally new approach to biogerontology. This new paradigm focuses not on comparing old with young, but instead to evaluating, or changing, in young adults, those factors hypothesized to underlie differences in rate of aging between the long-lived mutant and the corresponding control. The demonstration that nematode lifespan was increased by mutations that block responses to hormones related to insulin led to studies of similar mutations in other taxa, revealing the deep evolutionary roots of these hormone-dependent control circuits. In addition, evidence that C. elegans mutants with increased life-span are resistant to many forms of lethal stress, such as heat, irradiation, oxidants, and heavy metals, triggered experiments designed to assess stress resistance in mice carrying anti-aging mutations and in mice that had been exposed to caloric restriction. The recognition that aging can also be slowed by low calorie and low methionine diets (Miller et al., 2005b; Orentreich et al., 1993; Weindruch and Walford, 1988) and by pharmaceutical inhibition of a critical protein kinase (mTOR) (Harrison et al., 2009), justified work to look for similarities and differences among the genetic, nutritional, and pharmacological approaches to anti-aging intervention, with studies of genetically flexible models (C. elegans, D. melanogaster) blazing promising trails for slower, more expensive studies in rodents. In each case the focus is on young adults that differ not in their chronological age, but in their expected pace of future aging.
These new paradigms have generated a flood of recent discoveries and renewed enthusiasm to biological aging research, but have also focused their experiments on one species at a time, and thus ignored a potential goldmine of experimental material: variation in the rates of aging among the rich collection of species generated by natural selection. Within a species, there seems to be limited flexibility for alteration of aging rate and longevity. For example, anti-aging diets and mutations in mice seem to be able to produce at most an increase of about 40% in maximal and average lifespan (Miller, 2002). Evolution can do a great deal better than this: longevity among related species varies 10-fold or more within some mammalian orders, for example from about 3 to about 30 years in rodents, and from about 8 to 120 years in primates. Genetic differences among species are the product of millions of years of evolution, in each instance to maximize reproductive fitness under specific environmental conditions. It has long been known that species separate in ecological space according to their life-history traits. Some species live a short time, have high reproductive rates, fast developmental rates, and often high rates of mass-specific metabolism, whereas others tend to live a long time, have low rates of reproduction, long development times, and have low rates of mass-specific metabolism. In niches that allow a higher probability of long-term survival, mutations that postpone the signs and symptoms of aging will improve reproductive fitness, and thus be retained in the genome. Most of the symptoms of aging – cataracts, loss of hearing, diminished cardiovascular capacity, immune senescence, loss of muscle strength and endurance, arthritis, neoplasia, and many others – are seen in mice, dogs, horses, and people. But these pathological conditions, and the changes in cell and tissue function that underlie them, materialize at different rates in different species. It is difficult if not impossible to find a three year old mouse that has escaped all of these problems, but rhesus monkeys typically evade all of these problems for a decade or more.
The goal of comparative studies in the biology of aging is to delineate the mechanistic basis for species-specific differences in the aging process. Differences in aging rate among species are so familiar to us, to scientists or laypersons alike, that the power of the comparative method is often overlooked. But the contrast in aging rate between long-lived and short-lived species is perhaps the central mystery of biological gerontology. Mice, dogs, horses, chimpanzees, and humans are organized in very much the same way: the lens, thymus, kidney, skin, liver, and joints of each of these species contain the same cell types, in the same layers, making very nearly the same proteins and responding to the same set of nutrients and endocrine signals, as in each of the other species on the list. Why, then, will a human lens resist cataracts for 50 years, and a mouse lens for only 2 years? Why do dog joints show arthritic changes after a decade, but some species of bats, primates, and elephants resist arthritis for 30 years or more? When a long-lived species evolves from a shorter-lived ancestor, a feat that has occurred many times during the radiation of vertebrates, are the same cellular and molecular attributes changed? We think this is the essential question to be addressed in comparative biogerontology.
Comparative cellular biogerontology
This review article focuses on a specific approach to comparative biogerontology: the strategy of evaluating the properties of cultured cells from organisms of varying lifespan and aging rate. We start by confronting an apparently fatal flaw in this strategy: few cell types grow well in culture, and those that do grow well, such as skin-derived fibroblasts, are considered to contribute little to aging in intact animals, and are rarely responsible for fatal late-life diseases.
It is easy to imagine how analyses, in cell cultures, of cardiomyocytes, brain neurons, chondrocytes, pre-neoplastic hematopoietic stem cells, and hepatocytes might give provocative insights into differences that distinguish short-lived from long-lived species, potentially including measurements of specific genes or proteins, before or after exposure to a cytotoxic agent. But attempts to produce growing, or even healthy and stable, primary cell cultures from these differentiated cell types is exceptionally difficult. Although specialist laboratories can develop methods to maintain such cells in relatively stable conditions for a day or a few weeks, the development of standardized methods that can produce large numbers of differentiated cells from multiple species, with their inherent differences in metabolism and biochemistry seems at best a distant goal.
In contrast, it is usually straightforward to create healthy and proliferating cultures of fibroblast cells from embryos or adult skin biopsies of multiple species of birds and mammals (Harper et al., 2007; Hart and Setlow, 1974; Salmon et al., 2008a). The dermal layer of skin contains small numbers of non-dividing cells (fibrocytes) that respond to wounding by differentiation into a dividing cell type (“fibroblast”) that participate in wound healing and regeneration of the protective skin barrier before subsiding again into near quiescence. The transition of fibrocyte into fibroblast, and the proliferation of fibroblasts in situ, are stimulated by a partly-characterized collection of growth factors produced in the wounded area, and can also be triggered in vitro by culture in the presence of serum. Cultured fibroblasts typically proliferate until they exhaust key nutrients in the growth medium, or until they reach a density in which each cell is in contact with other cells (“confluence”); adding fresh medium, or dispersing the cells at a lower density, leads to additional rounds of mitotic proliferation.
At first glance it seems unlikely that the study of these highly specialized cells, in such an artificial milieu, could cast any useful light onto the question of why, for example, human lens, thymus, liver, kidney, brain, and arteries continue to function for many decades after the corresponding cells of rats, lemurs, and dogs have long since succumbed to changes that lead to degenerative and neoplastic illnesses. Despite this intuition, evidence has begun to accumulate that properties of fibroblasts in culture may be at least partly representative of key characteristics of the species from which they arise. We note that fibroblasts themselves can now be transformed into pluripotent stem cells from which all other cell types can be derived (Yamanaka, 2009). Thus in the not-too-distant future, it is likely that fibroblasts can be an ultimate resource from which neurons, cardiomyocytes, and other cell types of central interest to aging researchers can be created. This article reviews the current evidence relating cellular properties of fibroblasts to differences among species in aging rate. We discuss methodological and analytical problems with this approach, some immediate and future goals, and the possible links to the development of anti-aging interventions.
Stress resistance in fibroblasts from long-lived mutant mice
The idea that the properties of short-term fibroblast cultures might yield clues to mechanisms of delayed aging is supported by a series of reports on cells from long-lived mice with mutations that lower GH and/or IGF-I levels. Primary cell lines derived from young adult Snell dwarf, Ames dwarf, and growth hormone receptor knockout (GHRKO) mice are significantly different in their ability to resist death induced by cytotoxic agents relative to cell lines from their normal littermates (Leiser et al., 2006; Murakami et al., 2003; Salmon et al., 2005). In particular, cell lines from these long-lived mice are (with a few exceptions) more resistant to the lethal effects of ultraviolet radiation (UV-C), the oxidative stressors hydrogen peroxide (H2O2) and paraquat, the heavy metal cadmium, the DNA alkylating agent methyl methanesulfonate (MMS), and heat stress (42° C) in culture.
Cells from these mice are not resistant to all cytotoxic agents, however: there is no difference between Snell and control mice to the lethal effects of TNF-α (Yasumura and Miller, unpublished), and Snell dwarf cells are significantly more sensitive than control cells to apoptotic cell death induced by their exposure to two agents, thapsigargin and tunicamycin, that induce the unfolded protein response (Salmon et al., 2008a). In addition, Snell dwarf cells differ from control cells in their relative resistance to conditions (lowered glucose levels, or the metabolic inhibitor rotenone) that inhibit the activity of the plasma membrane redox system (PMRS), a group of electron-transfer reactions that passes intracellular reducing equivalents through the plasma membrane to extracellular acceptors (Leiser and Miller, 2009; Leiser et al., 2006).
The distinction between Snell and control fibroblasts can be demonstrated for many weeks in continuous culture (at least through six passages, about 10 – 15 doublings; Salmon and Miller, unpublished), and must therefore represent epigenetic changes that are maintained through multiple mitotic cycles in a constant culture environment. Moreover, a study of genetically normal (i.e. non-mutant) mice found that those individual mice whose cells were most resistant to peroxide-induced death were also significantly more resistant to death induced by UV, cadmium, and paraquat (Leiser et al., 2006), and that those cell lines that were most resistant to the metabolic effects of low glucose were also significantly more resistant to the lethal effects of cadmium and hydrogen peroxide (Leiser et al., 2006).
Fibroblasts from Snell dwarf mice also have altered growth kinetics reflecting resistance to the toxic effects of oxygen in standard culture conditions, which traditionally expose the cells to atmospheric, i.e. non-physiologic, levels (20%) of O2. Previously, it was shown that mouse embryonic fibroblasts grown at 20% O2 develop genetic mutations and chromosomal aberrations which lead to a growth crisis characterized by high levels of cell death, low proliferative indices, and the over-growth of transformed, aneuploid, pre-neoplastic cells (Busuttil et al., 2003; Parrinello et al., 2003). Growing the cells at 3% O2, which corresponds more closely to the level seen in postcapillary parenchymal tissue beds, prevents this growth crisis entirely in cells from embryos, and delays it significantly in cells derived from adult skin of normal mice (Busuttil et al., 2003; Maynard and Miller, 2006). In contrast, cells from Snell dwarf mice postpone this growth crisis even when cultured in 20% O2 (Maynard and Miller, 2006). Thus the growth characteristics of dwarf cells at 20% O2 are similar to those seen in normal mouse cells only at 3% O2; hence dwarf cells are apparently resistant to the oxygen-mediated toxicity seen in standard culture conditions. If a similar resistance to oxidant-mediated mutation and chromosomal aberrations also affects cancer-prone cell types in the intact mouse, the resistance shown by Snell-derived cells could contribute to the resistance of these mice to cancer and perhaps other forms of late-life illness.
Tests for cellular stress resistance across species of mammals
The work reviewed above, showing the resistance of fibroblasts from long-lived mutant mice to multiple forms of stress, suggested that the evolution of long-lived mammalian species might also require augmentation of cellular defense systems, and that this hypothesis could be tested in cultures of primary cells from adult skin biopsies. Kapahi et al. (Kapahi et al., 1999), for example, had previously demonstrated that the resistance of fibroblasts to oxidative stressors was positively correlated with mammalian longevity across several taxonomic orders. Using cells from hamster, rat, marmoset, rabbit, sheep, pig, cow and human, these investigators found significant associations with species maximal life span for H2O2, paraquat, sodium arsenite, tert-butyl hydroperoxide, and sodium hydroxide.
In our own study, primary skin-derived cell lines from young adult mammals of 8 species of rodent and 1 species of bat also exhibited a relationship between species maximum life span and the degree of stress resistance, even after controlling for the potentially confounding effects of phylogeny or body mass (Harper et al., 2007). In general, we found that cell lines from long-lived rodents were significantly more resistant to cadmium and hydrogen peroxide toxicity relative to shorter-lived species. The data also suggested an association between resistance to heat stress and to the toxicity of the DNA alkylating agent MMS (methyl methanesulfonate). In contrast to cells from mutant mice, however, cell lines from long-lived rodents did not show a pattern of resistance to UV or paraquat. This may reflect variation in evolutionary pressures on specific defense systems involved in modulating UV and paraquat induced damage; its molecular basis needs further study.
Like cells from Snell dwarf mice, cell lines from the long-lived rodents were also more resistant to the metabolic effect of low glucose culture conditions and resistant to rotenone-induced inhibition of the PMRS. Cell lines from the Little Brown Bat were exceptionally resistant to each of these stressors, with the exception of UV radiation and low glucose, relative to short-lived mice and rats.
We also compared stress resistance in cell lines derived from laboratory mice with those from wild-caught mice. We had shown previously that wild-derived mice were longer lived than those from laboratory-adapted stocks (Harper et al., 2006; Miller et al., 2002a). Because laboratory stocks were derived from wild mice less than 100–200 years ago (Silver, 1995), this comparison could provide insight into the speed of evolutionary change that affects both longevity and cellular stress resistance.
We found that cell lines from wild-caught mice were significantly more resistant than those of the laboratory stock to death induced by the DNA damaging agents MMS and UV radiation, as well as to the effects of heat and low glucose media, with a similar trend (p = 0.07) to resistance to the effects of rotenone on the PMRS. There was, however, no significant difference in resistance to H2O2 toxicity. Surprisingly, cells from laboratory mice were significantly more resistant to the lethal effects of cadmium. This might reflect selection, in laboratory mouse colonies, for resistance to heavy metals seldom encountered in the environment of wild mice. Other groups have shown that fibroblast cell lines generated from captive wild-derived mice differ markedly from cell lines from laboratory mice in proliferative capacity, and in resistance to both spontaneous and oxidative stress induced cellular-senescence like changes. (Yuan et al., 2006).
We used a similar approach to evaluate stress resistance in skin-derived fibroblasts from the naked mole rat (NMR), an exceptionally long-lived rodent with a lifespan approaching 30 years (Buffenstein, 2005). In cultures maintained at 33° C, NMR cells were more resistant than cells from lab mice to cadmium, paraquat, heat, and MMS. Moreover, like cells from Snell dwarf mice, cells from NMR were vulnerable to death induced by the ER stress agents, thapsigargin and tunicamycin. Contrary to our working hypothesis, however, NMR cell lines were found to be significantly more sensitive than mouse cells to UV and H2O2. These findings are generally consistent with our expectation that cellular stress resistance, as measured in skin-derived fibroblasts, varies between organisms of different aging rates and maximal longevity, but also suggest that some long-lived species may exhibit idiosyncratic patterns of resistance and vulnerability.
The literature contains several studies comparing diverse taxa for DNA repair capability and resistance to oxidant stress, including data obtained both in vivo (Cortopassi and Wang, 1996; Francis et al., 1981; Hart and Setlow, 1974; Sohal et al., 1990; Tolmasoff et al., 1980) and in vitro using cell culture (Brown and Stuart, 2007; Hall et al., 1984; Hart and Setlow, 1974; Kato et al., 1980; Treton and Courtois, 1980) models. In many cases, however, the association does not survive correction for effects in body mass and its covariate, metabolic rate, making it difficult to decide whether the relationships seen are merely secondary to inter-species differences in metabolic rate (Speakman, 2005a; Speakman, 2005b). These early papers also tend to omit consideration of phylogenetic relationships, and thus may or may not exaggerate the statistical significance of any observed associations (Speakman, 2005b); for example there are well-known differences in p53-modulated DNA repair capacity in primates versus rodents (Tang and Chu, 2002) presumed to reflect each lineage’s evolutionary history from the most recent common ancestor. Lastly, the in vitro studies were conducted using cell lines grown and tested in the presence of 20% O2, i.e. under the toxic condition of hyperoxia (see above) that could select for oxidant-resistant cells, modify the responses to stress, or lead to differential effects in long-lived versus short-lived species (Brown and Stuart, 2007). It is now considered essential that the stress resistance properties of cell lines from long versus short-lived species be evaluated under low (3%) O2 tension, similar to post-capillary oxygen concentrations in vivo.
Telomere biology in cultured cells: mammals
The relationships among telomere length, telomerase function, in vitro senescence, and species-specific lifespan have proven to be complex. The evidence for a direct relationship between the propensity for cells to enter replicative senescence and organismal life span remains tenuous, and it is now clear that senescent changes can be produced in cells by many influences unrelated to telomere length.
There is good evidence that telomere-dependent replicative senescence has evolved as a defense mechanism against lethal tumors and contributes to extended lifespan in humans and other long-lived primates, and perhaps in other long-lived species as well (Campisi, 2001; Sedivy, 2007). A study of growth dynamics and telomere biology of cell lines, maintained under 3% O2, derived from 15 different rodent species covering multiple families (Seluanov et al., 2008) has begun to provide some insights. In this study, Seluanov et al., found that rodent cell lines generally fell into one of three groups: (A) Cells from rodent species of large body mass typically lack telomerase activity and tend to undergo replicative senescence; (B) Cells from small, short-lived rodent species that maintain telomerase activity do not undergo replicative senescence and show rapid, continuous growth; Lastly, (C) cells from small, long-lived rodents that possess telomerase activity in vitro and do not exhibit replicative senescence, but tend to grow slowly in culture, perhaps reflecting some alternative tumor-suppressor mechanism that is yet to be fully elucidated. These authors found no relationship between the presence or absence of cellular senescence and the life span of the species, although there was a significant association of senescence and body mass of the donor species. In contrast, rate of cell growth in vitro was associated with species life span rather than with body mass. The authors also noted that the combination of longevity and slow in vitro cellular growth rate had evolved independently in multiple rodent lineages, suggesting that these two changes could reflect common pathways of cell biology susceptible to evolutionary pressures. A good deal of additional work will be needed to see if similar sets of cellular traits are associated with, and might contribute to, longevity in other mammalian classes, including primates.
Comparative analyses of bird species
Despite the fact that birds have higher mass-specific metabolism and maintain a higher body temperature than mammals of the same body mass, they also typically live three times longer (Holmes and Martin, 2009). The comparison in longevity between bird and mammal species has called into question several attractive hypotheses about the control of lifespan since birds have higher mass-specific metabolic rates and higher blood glucose levels than mammals, but nonetheless show superior longevity for a given body size. Moreover there is a huge degree of variation in aging and life span among bird species, although much less is known about age-related changes in organ and tissue function, and about causes of death. The exceptional lifespan of many bird species is consistent with the idea that natural selection will favor postponement of late-life diseases in species that experience low rates of extrinsic mortality, including adult predation (Austad and Fischer, 1991). Physiological comparisons have begun to shed light on the ecological and physiological factors associated with longevity among species of birds (Cohen et al., 2008; Ricklefs, 1998; Wiersma et al., 2007), and have shown that body size (Holmes et al., 2001), mitochondrial mutation rate (Samuels, 2005), and the lipid composition of cellular membranes (Hulbert, 2008) are associated with longevity among bird species as they are among species of mammals (Austad and Fischer, 1991; Barja and Herrero, 2000; Hulbert et al., 2007).
Although there is some evidence for a relationship between life span and telomere length (in nucleated erythrocytes) among bird species (Bize et al., 2009; Haussmann and Mauck, 2008; Haussmann et al., 2005), developmental and environmental factors complicate the interpretation of these data (Hall et al., 2004). In long-term culture, avian fibroblast cell lines readily undergo replicative senescence (Michailidis et al., 2005; Nielsen and Ryan, 1981; Venkatesan and Price, 1998) as a result of regulatory pathways that more closely resemble those used by human, but not mouse, cell lines (Kim et al., 2002). Somatic tissues of birds contain ample levels of telomerase (Haussmann et al., 2004; Haussmann et al., 2007), which is rapidly lost in cultured cells (Venkatesan and Price, 1998), making it difficult to draw conclusions about bird aging from studies of cellular senescence in culture.
Early studies of oxidation resistance of cultured bird cells tested the hypothesis that the longer lifespan of birds, compared with mammals of similar size, would be accompanied by (and thus perhaps caused by) resistance to oxidative damage. Indeed, a study of cultured renal cells from 3 bird species (budgerigars, starlings, canaries) showed that avian cell lines were more resistant to oxidative stress and DNA damaging agents than was a mouse cell line tested in parallel (Ogburn et al., 1998). A second report suggested that cells from a short-lived bird (Japanese quail) were less stress resistant than cells derived from a longer-lived species (budgerigar) (Ogburn et al., 2001), but this work has proven difficult to confirm, and must be considered tentative (S. Austad, unpublished results).
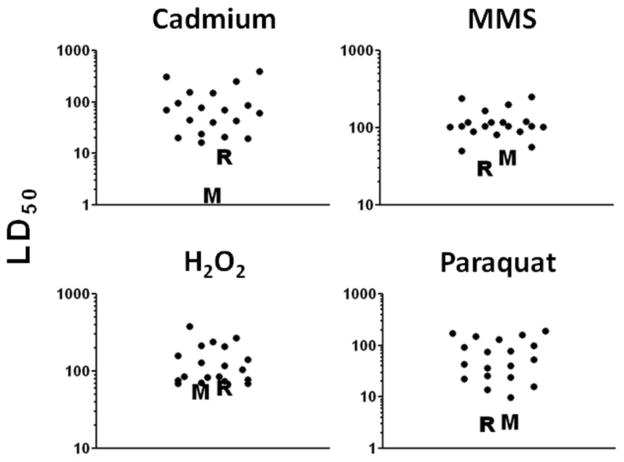
We have recently developed primary fibroblast cell lines from skin biopsies of adult birds from more than 40 temperate species collected in Ohio or tropical species collected in Panama. These studies were motivated by the idea that tropical species tend to display low rates of metabolism, low fecundity, long developmental periods, and high rates of survival, whereas temperate species tend to have high rates of metabolism and lower rates of survival (Wiersma et al., 2007). A series of pilot studies, using cell lines established from individual donors of 19 species, showed that the avian fibroblasts were more resistant to the lethal effects of cadmium, MMS, hydrogen peroxide and paraquat compared with fibroblasts from mice or rats. Figure 1 illustrates these data, showing that there was little or no overlap in the estimated LD50 in cell lines derived from birds and rodents; however bird cell lines were similar in their stress resistance profile to cells from the longest-lived species of rodents and cells from a bat species (Harper et al., 2007). Although bird core body temperature is typically somewhat higher than that of mammals (40° vs 37°, respectively), we found no effect of culture temperature on the relative resistance of bird fibroblasts compared with cells from mice (data not shown). These initial studies suggest that using dermal fibroblasts from wild-caught, birds should prove to be a useful tool in investigating the genetic and biochemical mechanisms behind the exceptional life span of this group, and it will be interesting to see whether cell lines from long-lived birds are or are not more resistant to these agents than cells from shorter-lived avian species. It will also be of great interest to see if the cellular traits that predict species longevity within rodents are associated with differences in the rate of aging among species of birds. If common factors are identified, this would provide a strong impetus for a more detailed examination of these pathways as predictors of lifespan and disease risk in human populations.
Figure 1.
Bird fibroblasts are more resistant to lethal stress than mouse or rat fibroblasts derived from wild-caught animals. Note the lack of overlap between values for bird cells and mean levels for mouse and rat cells. Values shown are LD50’s, corresponding to the dose of the toxic agent that results in death of 50% of the cells; note that the scale is logarithmic. Each symbol represents a different individual bird with one bird from each of 19 different species. All cell lines were derived using 20% O2, cryopreserved, and then expanded for two passages at 3% O2 before testing in 3% O2. (M) and (R) symbols indicate mean LD50 values detected in a previous set of experiments (Harper et al., 2007) using the same methods. MMS = methyl methanesulfonate. Units for LD50 are μM (cadmium and H2O2) and mM (paraquat and MMS).
Initial studies of cell lines from long- and short-lived breeds of dogs
Dogs provide some distinct advantages for analysis of cellular factors related to longevity, including a range of breed-specific maximum lifespan of about 2-fold. Large breeds are in general shorter-lived than small breeds, and body size accounts for over 50% of inter-breed differences in lifespan in some surveys of pet dogs (Miller and Austad, 1999). Small size is associated with increased longevity in mixed-breed dogs as well (Galis et al., 2007; Miller et al., 2005a; Patronek et al., 1997), as it is in genetically heterogeneous mice (Miller et al., 2002b). Small size (and thus higher mass-specific metabolic rate) are associated with increased longevity in dogs, as within other species (Speakman et al., 2003), and small dogs show a reduced level of serum insulin-like growth factor (IGF)-I (Eigenmann et al., 1988; Eigenmann et al., 1984) consistent with the growing body of evidence linking insulin/IGF axis activity to life span across multiple eukaryotic taxa. These differences in size and life span are almost certainly the product of centuries of directed selection for size to meet specific goals of multiple breeding programs (Galis et al., 2007). We know that differences in lifespan among breeds, in general, reflect delay or deceleration in risks of multiple aspects of aging, because longer-lived breeds show lower age-adjusted risks of death from multiple causes, including cancer, trauma, cardiac, locomotor, and neurological diseases (Bonnett et al., 2005; Egenvall et al., 2005). The association, among dog breeds, of small body size with low cancer risk (Michell, 1999) is similar to the association seen in multiple studies of human population variance (Albanes et al., 1988; Bjorge et al., 2005; Bjorge et al., 2006; Engeland et al., 2005; Engeland et al., 2003a, b, 2004; Petrelli et al., 2002; Tretli, 1989; Tretli and Magnus, 1990). The canine genome has already been sequenced, providing important resources for gene localization and identification (Austad, 1993), with evidence for multiple distinct lineages within the set of well-characterized breeds (Wayne and Ostrander, 2007). In addition, because most dogs are companion animals, there is a wealth of veterinary data and pathology records to serve as indices of functional changes of high relevance to human health.
An analysis of cellular stress resistance, and other cellular properties, in fibroblasts from multiple dog breeds is likely to be particularly informative because the association of lifespan to body weight and metabolic parameters, within this species, is opposite in direction to that seen among groups of species. Small body size imposes high metabolic demands because these species have large surface to volume ratios which lead to high heat loss. Thus larger species, with smaller surface to volume ratios, typically exhibit lower metabolic rates, and putative associations between longevity and cellular traits across species are difficult to disentangle from the ways in which metabolic parameters might modulate the same cellular traits (Speakman, 2005a). Amongst breeds within a species, however, small body size remains associated with high metabolic rate, as expected from considerations of surface to volume ratios and heat loss (Speakman et al., 2003), but is now associated with longer lifespan. Thus relationships between a cellular trait and longevity that are seen both within dog breeds and across multiple species are not likely to be simply a consequence of metabolic rate and its associated consequences.
In a preliminary study, we have tested the resistance of primary dermal fibroblasts derived from the abdominal skin of dogs and found that they are highly resistant to cell death induced by cadmium, hydrogen peroxide or UV radiation when compared to our previous data generated by the same methods in cell lines from wild mice and rats (Harper et al., 2007). Figure 2 shows these results, for a collection of cell lines from 24 donors of 13 defined breeds, plus 12 other cell lines from mixed-breed dogs. The dog cell lines showed exceptional resistance to cadmium toxicity, ranging from 60 to more than 300-times the resistance of mouse cell lines, and invariably exceeding the values for birds (see Figure 1) and the longest-lived rodents (Harper et al., 2007). Almost all of the dog cell lines were also more resistant to H2O2, and to UV, than mouse and rat cells. Although these inferences reflect comparisons to published data from our lab, we made similar observations when rodent and dog cell lines were tested at the same time (not shown). More testing, with a wider range of breeds and additional forms of stress, will be needed to show which, if any, of the stress tests discriminate short-lived from long-lived breeds, and large from small dogs.
Figure 2.
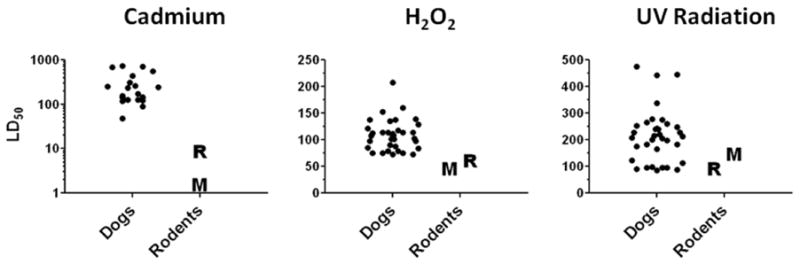
Legend: Dog fibroblasts are typically more resistant to lethal stress than mouse or rat fibroblasts from wild-caught individuals. There is little to no overlap in the observed values for dog cell lines with mean values of mouse and rat cells. Values shown are LD50’s, corresponding to the dose of the toxic agent that results in death of 50% of the cells; note that the scale is logarithmic only for the cadmium panel. Each symbol represents an individual dog, representing a mixture of purebred and mixed-breed animals (21 – 36 dogs per panel). The dog cell lines were derived using 3% O2, cryopreserved, and then expanded for two passages at 3% O2 before testing in 3% O2. (M) and (R) symbols indicate mean LD50 values detected in a previous set of experiments (Harper et al., 2007) using the same methods, except that the O2 tension was 20%. Pilot studies using mouse cell lines have indicated no consistent effect of initial O2 tension on LD50 values, regardless of test condition (not shown). Units for LD50 are μM (cadmium and H2O2) and J/m2 (UV).
Problems in analysis and interpretation
Evaluation of hypotheses about interspecific variation of cellular traits among species that have disparate longevity has encountered significant problems, and opinions vary regarding the correct approach in solving them. One problem is that large species of birds and mammals tend to live longer than smaller ones. Since body size and metabolic rate are coupled, cellular traits that evolve to adjust to alterations in metabolic needs will show the same, strong relationship to lifespan, across species of birds and mammals, which is seen in plots of species lifespan versus mass. The relationship is not likely to be causal, in that there are many situations (Austad and Fischer, 1991; Miller and Austad, 1999) in which smaller species, or those with high metabolic rate, are much longer-lived than would be predicted from their size alone. Both the general relationship, and many of the exceptions, can be better explained as a consequence of co-evolution of large body size and slow aging rate in response to ecological niche (Austad and Fischer, 1991). But the empirical association of large size (and low metabolic rate) with slower rates of aging certainly complicates interpretation of species distributions of cellular endpoints. A trivial example shows the key problem: larger species tend to have larger feet, but it would be misleading to conclude that foot size is a key regulator of aging rate and lifespan. Speakman (Speakman, 2005a) has compiled examples in which this objection has been ignored in interpreting comparative datasets as evidence for specific causative ideas about aging, and has recommended that all such analyses commence by calculating the residuals of life history variables and cellular or physiological traits against species body mass, and then testing hypotheses using the residuals in place of the original measurements. Austad has recommended a similar strategy, based on calculating the “longevity quotient,” for each species, as the ratio between the lifespan of the species and its weight-predicted lifespan using linear regression (Austad and Fischer, 1991). While each of these approaches will reduce the chances of reaching invalid conclusions relating aging to cellular traits of interest, this approach may also prevent detection of genuine cause-and-effect relationships. If, for example, resistance to DNA damage were a critical step in the evolution of a slow aging species, then larger animal species, which are slow aging, would be expected to have better DNA repair capacities than smaller, rapidly aging relatives. Statistical adjustment of DNA repair capabilities to “remove” the trend attributable to body weight effects would greatly reduce the strength of association between repair and aging rate – the very relationship we would wish to document and study further.
This is a serious problem that has no direct and obvious solution: how best to test relationships between aging rate and cellular traits without wasting time on traits that are merely surrogates for body size or metabolic rate. Associations that are strong enough to survive adjustment for body weight may deserve more attention than those that do not, but systems that help to disentangle size from longevity may deserve special attention. Some relief may come from studies of groups of animals (like dogs, or breeds of miniature horses) in which small size is associated with slow aging, and evaluation of groups of animals (like bats, gliding mammals, and many species of birds) that are long-lived despite small size.
The second controversial issue is whether or not to make an adjustment in statistical tests for phylogenetic relationships. In regressions of a particular cellular trait, such as stress resistance, against body mass, some argue that use of Phylogenetic Indendendent Constrasts (“PIC”) is the preferred or even obligatory method, because using contrast data adjusts for relatedness among species, “correcting” for phylogenetic history (Bennett and Owens, 2002; Garland et al., 2005; Harvey et al., 1995; Rezende et al., 2004). We see good reasons for incorporating phylogenies into comparative analyses when tests for phylogenetic signal (sensu (Abouheif, 1999) indicated that these methods are warranted, as long as there is sufficient statistical power to do so, but we do not think that they should be employed blindly, or as the only valid approach. Insistence on PIC can lead to errors of interpretation, and suggest caution in interpreting results obtained using PIC alone (Munoz-Garcia and Williams, 2005). New models currently being developed, based on the assumption that species mean values reflect adaptation to environment, emphasize the importance of evaluating the proper evolutionary model, and in some cases obviate the necessity of incorporating phylogenetic relationships among species (Kelly and Price, 2004). The primacy ascribed to PIC is based on the idea that this method solves a problem inherent in Conventional Least Squares Regression (CLSR), namely, that sister species are not independent (Felsenstein, 1985). But similarities among closely related species can result from similarities in current selection pressures and need not imply historical phylogenetic constraint (Ridley, 1996; Westoby et al., 1995; Williams, 1992). Uncertainties in the topology of the phylogenetic tree also complicate PIC analyses (Felsenstein, 1985, 1988), suggesting that this method should not be used alone (Lake and Moore, 1998). The evolutionary model implicit in PIC is that of stochastic evolution or Brownian motion (Diaz-Uriarte and Garland, 1996). If the evolution of physiological traits deviates significantly from a stochastic model, then PIC may provide inappropriate parameter estimates of allometric relationships across species (Kelly and Price, 2004; Price, 1997). Evidence suggests that natural selection and/or sexual selection, and not genetic drift, are the dominant forces promoting speciation (Coyne and Orr, 1998) invalidating the Brownian motion model.
These considerations suggest that when tests for phylogenetic signal are significant, both PIC and CLSR methods can be helpful in understanding the relation of cellular traits to species aging rate, and that when tests for signal are not significant, there is no reason to abandon the CLSR approach. Where investigators have used both regressions based on PIC and CLSR, they have generally found similar correlations (Price, 1997; Ricklefs and Starck, 1996), and a comparison of results from the two methods may provide insights that otherwise would not have come to light (Price, 1997).
The third problem is more complex: the difficulty of developing numerical values that provide a robust estimate of inter-species differences in aging rate. The most commonly used value is the species’ “maximum recorded life span” (MRLS), i.e. the age of the oldest known member of the species. Extensive lists of MRLS data, with citations to the primary sources, can be found at several publicly accessible sites, including the Animal Ageing and Longevity Database (AnAge) (http://genomics.senescence.info/species/) and the extensive compilation, by James Carey and Deborah Judge, maintained by the Max Planck Institute for Demographic Research (see http://www.demogr.mpg.de/). MRLS values, however, have many flaws as measures of aging effects on species of animals. For one thing, the MRLS value is highly dependent on the number of individuals with a recorded lifespan: the longest-lived person in a group of 10,000 will almost certainly be older than the longest-lived person in a group of 10 or 100 or 1000. For many species, estimates of MRLS reflect longevity data on sample sizes of 10 – 100 individuals, and thus produce severe underestimates of life span potential. A second problem of relying on MRLS values is that they represent, by definition, an observation of a single individual; they cannot be used in statistical tests that depend on error estimates, and show great variation among subsamples of a larger population. When life tables for a species include thousands of well documented individuals (dogs, mice, humans, for example), differences in MRLS, though imprecise, nonetheless provide strong support for differences in aging rate. But when comparisons are made among species in which the number of documented individuals varies greatly, and is sometimes low, the value of MRLS as an estimate of biological aging rate is clearly circumscribed. Lastly, many species have no useful estimates of MRLS at all. Other estimators of species-specific aging rates have been proposed, for example based on age-dependent and age-independent parameters of the Gompertz and related survival models (Finch et al., 1990), but these pose their own problems: dependence on patterns of juvenile mortality that provide no information about aging rate, a requirement for detailed life tables with many deaths recorded at older ages, and a failure in some cases to account for nonlinearity in the relationship between log mortality risk and age. Two parameter models, like the Gompertz model, have an advantage in that they can distinguish species differences in time-dependent and time-independent effects on mortality risk, at least in principle, which may be under different sets of genetic and nutritional controls (see (Bartke et al., 2001) for an example).
Essentially, though, the absence of robust, well-studied, and widely accepted estimators of species differences in aging rate is a serious and unsolved problem for which the use of MRLS values is currently the best, but still a poor, solution. Ideally, assessment of inter-specific differences in aging rate would be based on the assessment of a wide range of physiological endpoints: hearing sensitivity, visual acuity and accommodation, joint mobility, immune responses, muscle strength, memory, cardiovascular reserves, etc., to see to what extent this collection of age-sensitive traits varies with time in each species. Rich datasets of this kind make it clear that aging proceeds more quickly in horses than in people, and more quickly still in mice; and have established beyond reasonable doubt the ability of calorie restricted diets to slow aging in rodents. It is clearly foolish to expect that a similar degree of confidence can emerge for data that consists entirely of survival records, but in practice, the only available data for most species will be from life tables, and often small or fragmentary life tables at that. Evaluation of multiple estimators from survival tables, and comparison of these various calculated parameters to one another, to MRLS, to what little is known of age-sensitive functional change in each species, and to cellular covariates is a complex project but one that deserves high priority.
Alternative endpoints for comparative cellular studies
Much of the published work in this area has relied on measures of cell growth and stress resistance, with a modicum of work on enzymology, including telomerase activity, and telomere length. It is time for comparative analyses to incorporate more ambitious programs to look at resistance of intracellular molecules and organelles to various forms of injury and stimulation. Studies of Snell dwarf cell lines, for example, have shown associations of stress resistance with augmentation of transcription of genes regulated by Nrf-2 (Leiser and Miller), higher levels of DNA repair after UV damage (Salmon et al., 2008b), lower levels of lipid peroxidation (Leiser and Miller)(Leiser and Miller, submitted), and altered activation for the stress kinase ERK (Sun et al., 2009). There is also a good deal of comparative data on how lipids in cellular membranes are associated with metabolism (Hulbert, 2005; Hulbert et al., 2006; Hulbert et al., 2007), and it will be of interest to see to what extent these variations are also seen in cultured fibroblasts from the same species. Cells from long-lived rodents resemble those of Snell dwarf mice in their resistance to inhibition of the plasma membrane redox system (PMRS) (Harper et al., 2007; Leiser et al., 2006), and it will be informative to see to what extent the ability of plasma membrane transport systems to maintain the redox potential of the membrane and extramembranous region is associated with differential longevity in tests of other species groups. The surprising vulnerability of Snell dwarf fibroblasts (and cells from naked mole rats) to agents that induce ER stress (Salmon et al., 2008a) justify more extensive testing of these and related pathways across a wider range of species. Studies done in a number of model organisms have suggested a role of autophagy, i.e. processes for removing damaged organelles and proteins, in the regulation of life span (Rajawat et al., 2009), and caloric restriction prevents the decline in autophagy normally seen with increasing age in mice (Cuervo, 2008). Both ER stress responses and autophagy can, depending on context, lead either to restoration of cellular homeostasis or to cell death, and the factors that regulate this decision are still only partly understood. It will be of interest to compare cells of multiple species, in baseline conditions or after exposure to nutritional or toxic stress, to evaluate both the restorative pathways and those that trigger apoptosis.
Comparisons among species in these and related cellular pathways are complicated by the need to devise tests that do not require species-specific reagents or sequence information. Evaluation of mRNA levels is difficult in comparisons of species in which mRNA sequences differ, and for which genomic sequences are (still) in many cases unknown; although the development of RNA-Seq techniques, also known as Whole Transcriptome Shotgun Sequencing, may soon preclude this limitation (Wang et al., 2009). Similarly, interspecific differences in protein sequence complicate quantification by gel electrophoresis, mass spectroscopic analysis, or immunoblotting methods, although antibodies to conserved amino acid sequences may provide some useful leverage for immunochemical approaches. Endpoints that exploit structures and processes shared by all eukaryotes, or at least all birds and mammals, are likely to be more immediately informative, such as tests of mitochondrial function, membrane lipid composition and resistance to damage, intracellular and secreted levels of metabolic products and precursors, DNA repair systems, chaperone levels, protein folding and misfolding rates, and other, similar approaches that do not require specific nucleic acid or protein sequence information.
Connections: implications of comparative data for biogerontology and for geriatrics
The approach we are advocating here is essentially descriptive, with all the strengths and weaknesses that adhere to unbiased searches. The studies are in no sense hypothesis-free – a decision to test DNA repair rates reflects a hypothesis about the role of DNA repair in the evolution of extended longevity – but the number of hypotheses to be tested in a systematic program of this kind is large enough that the overall program is, in a sense, a screening effort. Generalizations inferred from one set of data, such as the inference that resistance to cadmium toxicity is strongly associated with longevity in rodents, can be tested in other sets of rodents, other clades of mammals, groups of long-lived birds, and across dog breeds to see how robust they are as predictions, but are still only a first step towards definitive experiments, in which modification of the stress resistance pathway, in cells and tissues of a short-lived animal, can be shown to slow aging and postpone late-life diseases.
Still, the work in cells may provide key short-cuts towards the design of interventional experiments: if, for example, augmented resistance to cadmium toxicity is shown, in the fibroblast collection, to reflect alterations in one or more cadmium buffering proteins, this could serve as a strong rationale for studying mice with alterations in the proteins, drugs that induce expression of the proteins, and diets that modulate the levels of heavy metals, their competitors, chelators, etc. Tests for cellular stress resistance, and associated biophysical and biochemical traits, could be conducted fairly easily on fibroblast cultures derived from human volunteers, to support a search for polymorphic genes that modulate these traits in humans, and to explore the relationship of these endpoints to variations, among people, in the hormone levels that influence the traits in long-lived mutant mice (Pim et al., 2009; Suh et al., 2008). Correlation analyses among species will not, by themselves, provide definitive tests of causal hypotheses about control of aging, but may well focus attention and resources towards testing putative causal links that are most compatible with the cross-species inferences.
A concluding remark
Discussions with colleagues not themselves committed to work in comparative biology, and parallel interactions with peer reviewers of manuscripts and grant applications, have taught us that some, but not all, experts are skeptical of the merits of the line of experiments we are proposing here. About half of the skeptics seem convinced that there will be no interesting differences among cells from long- and short-lived species, on the grounds that the properties of skin-derived cells in culture will not provide useful information about the properties of tissues and organ systems that contribute to aging and disease in intact animals. The other half of the skeptics are convinced that any results will merely confirm the idea, which they consider to be obvious, that cells from long-lived species are more resistant to stress agents than are cells from short lived species, regardless of the stress agent used. In short, one group thinks there will be no signal; the other that the signal will carry no news. We are more optimistic: we suspect that there will be surprises and unanticipated patterns, such as specific forms of stress resistance or repair mechanisms that track lifespan in some groups of species but not in others; agents for which hair-trigger apoptosis, rather than resistance, is the hallmark of slower aging species; and forms of resistance that apply to primates only, or only to species with dramatically higher longevity than predicted from body weight, or in outlier species that are longer lived than their closest cousins. The available data, though limited, are at this point more often positive than skeptics have supposed, and less monotonous than anticipated by the other set of critics. The two evolutionary radiations of homeotherms have, over the last hundred million years, produced a bevy of creatures that are far longer lived than their ancestral species. Making long-lived mammals seems to be a fairly simple thing to do – birds, bats, humans, elephants, naked mole rats, and Sumatran crested porcupines arose independently, rather than from an especially long-lived tunicate or hagfish – and the amount of enhanced longevity in each of these and dozens of other instances far exceeds the best we can so far produce, at least in mammals, by laboratory interventions. Given such an impressive array of ‘natural experiments’ in aging rate, we argue that overcoming the technical and conceptual obstacles confronting research in comparative cellular gerontology will be well worth the effort.
Acknowledgments
The work reported here was supported by NIA grants AG023122, AG022873, and AG013283, NSF IBN 0212587 and a grant from the National Academies Keck Futures Initiative. We are grateful for technical assistance to Melissa Han, Bill Kohler for cell culture assistance, and Jennifer Ro, Jen Olson, and Liz Calhoon for helping collect species of birds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abouheif E. A method for testing the assumption of phylogenetic independence in comparative data. Evolutionary Ecology Research. 1999;1:895–909. [Google Scholar]
- Albanes D, Jones DY, Schatzkin A, Micozzi MS, Taylor PR. Adult stature and risk of cancer. Cancer Res. 1988;48:1658–1662. [PubMed] [Google Scholar]
- Austad SN. The comparative perspective and choice of animal models in aging research. Aging Clin Exp Res. 1993;5:259–267. doi: 10.1007/BF03324171. [DOI] [PubMed] [Google Scholar]
- Austad SN, Fischer KE. Mammalian aging, metabolism, and ecology: evidence from the bats and marsupials. J Gerontol. 1991;46:B47–B53. doi: 10.1093/geronj/46.2.b47. [DOI] [PubMed] [Google Scholar]
- Barja G, Herrero A. Oxidative damage to mitochondrial DNA is inversely related to maximum life span in the heart and brain of mammals. FASEB J. 2000;14:312–318. doi: 10.1096/fasebj.14.2.312. [DOI] [PubMed] [Google Scholar]
- Bartke A, Wright JC, Mattison JA, Ingram DK, Miller RA, Roth GS. Longevity: Extending the lifespan of long-lived mice. Nature. 2001;414:412–412. doi: 10.1038/35106646. [DOI] [PubMed] [Google Scholar]
- Bennett PM, Owens IPF. Evolutionary ecology of birds: life history, mating system and extinction. Oxford University Press; Oxford, UK: 2002. [Google Scholar]
- Bize P, Criscuolo Fo, Metcalfe NB, Nasir L, Monaghan P. Telomere dynamics rather than age predict life expectancy in the wild. Proc Royal Soc B Biol Sci. 2009;276:1679–1683. doi: 10.1098/rspb.2008.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorge T, Tretli S, Engeland A. Height and body mass index in relation to cancer of the small intestine in two million Norwegian men and women. Br J Cancer. 2005;93:807–810. doi: 10.1038/sj.bjc.6602789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorge T, Tretli S, Lie AK, Engeland A. The impact of height and body mass index on the risk of testicular cancer in 600,000 Norwegian men. Cancer Causes Control. 2006;17:983–987. doi: 10.1007/s10552-006-0032-8. [DOI] [PubMed] [Google Scholar]
- Bonnett BN, Egenvall A, Hedhammar A, Olson P. Mortality in over 350,000 insured Swedish dogs from 1995–2000: I. Breed-, gender-, age- and cause-specific rates. Acta Vet Scand. 2005;46:105–120. doi: 10.1186/1751-0147-46-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MF, Stuart JA. Correlation of mitochondrial superoxide dismutase and DNA polymerase β in mammalian dermal fibroblasts with species maximal lifespan. Mech Ageing Dev. 2007;128:696–705. doi: 10.1016/j.mad.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Buffenstein R. The Naked Mole-Rat: A New Long-Living Model for Human Aging Research. J Gerontol A Biol Sci Med Sci. 2005;60:1369–1377. doi: 10.1093/gerona/60.11.1369. [DOI] [PubMed] [Google Scholar]
- Busuttil RA, Rubio M, Dolle ME, Campisi J, Vijg J. Oxygen accelerates the accumulation of mutations during the senescence and immortalization of murine cells in culture. Aging Cell. 2003;2:287–294. doi: 10.1046/j.1474-9728.2003.00066.x. [DOI] [PubMed] [Google Scholar]
- Campisi J. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 2001;11:S27–31. doi: 10.1016/s0962-8924(01)02151-1. [DOI] [PubMed] [Google Scholar]
- Cohen AA, McGraw KJ, Wiersma P, Williams JB, Robinson WD, Robinson TR, Brawn JD, Ricklefs RE. Interspecific Associations between Circulating Antioxidant Levels and Life History Variation in Birds. Am Nat. 2008;172:178–193. doi: 10.1086/589456. [DOI] [PubMed] [Google Scholar]
- Cortopassi GA, Wang E. There is substantial agreement among interspecies estimates of DNA repair activity. Mech Ageing Dev. 1996;91:211–218. doi: 10.1016/s0047-6374(96)01788-5. [DOI] [PubMed] [Google Scholar]
- Coyne JA, Orr HA. The Evolutionary Genetics of Speciation. Philos Trans: Biol Sci. 1998;353:287–305. doi: 10.1098/rstb.1998.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo AM. Autophagy and aging: keeping that old broom working. Trends Genet. 2008;24:604–612. doi: 10.1016/j.tig.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Uriarte R, Garland T., Jr Testing Hypotheses of Correlated Evolution Using Phylogenetically Independent Contrasts: Sensitivity to Deviations from Brownian Motion. Syst Biol. 1996;45:27–47. [Google Scholar]
- Egenvall A, Bonnett BN, Hedhammar A, Olson P. Mortality in over 350,000 insured Swedish dogs from 1995–2000: II. Breed-specific age and survival patterns and relative risk for causes of death. Acta Vet Scand. 2005;46:121–136. doi: 10.1186/1751-0147-46-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigenmann JE, Amador A, Patterson DF. Insulin-like growth factor I levels in proportionate dogs, chondrodystrophic dogs, and in giant dogs. Acta Endocrinol (Copenh) 1988;118:105–108. doi: 10.1530/acta.0.1180105. [DOI] [PubMed] [Google Scholar]
- Eigenmann JE, Patterson DF, Froesch ER. Body size parallels insulin-like growth factor I levels but not growth hormone secretory capacity. Acta Endocrinol (Copenh) 1984;106:448–453. doi: 10.1530/acta.0.1060448. [DOI] [PubMed] [Google Scholar]
- Engeland A, Tretli S, Austad G, Bjorge T. Height and body mass index in relation to colorectal and gallbladder cancer in two million Norwegian men and women. Cancer Causes Control. 2005;16:987–996. doi: 10.1007/s10552-005-3638-3. [DOI] [PubMed] [Google Scholar]
- Engeland A, Tretli S, Bjorge T. Height, body mass index, and ovarian cancer: a follow-up of 1.1 million Norwegian women. J Natl Cancer Inst. 2003a;95:1244–1248. doi: 10.1093/jnci/djg010. [DOI] [PubMed] [Google Scholar]
- Engeland A, Tretli S, Bjorge T. Height, body mass index, and prostate cancer: a follow-up of 950000 Norwegian men. Br J Cancer. 2003b;89:1237–1242. doi: 10.1038/sj.bjc.6601206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeland A, Tretli S, Bjorge T. Height and body mass index in relation to esophageal cancer; 23-year follow-up of two million Norwegian men and women. Cancer Causes Control. 2004;15:837–843. doi: 10.1023/B:CACO.0000043434.21558.ea. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Phylogenies and the comparative method. Am Nat. 1985;125:15. doi: 10.1086/703055. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Phylogenies and Quantitative Characters. Ann Rev Ecol Syst. 1988;19:445–471. [Google Scholar]
- Finch CE, Pike MC, Witten M. Slow mortality rate accelerations during aging in some animals approximate that of humans. Science. 1990;249:902–904. doi: 10.1126/science.2392680. [DOI] [PubMed] [Google Scholar]
- Francis AA, Lee WH, Regan JD. The relationship of DNA excision repair of ultraviolet-induced lesions to the maximum life span of mammals. Mech Ageing Dev. 1981;16:181–189. doi: 10.1016/0047-6374(81)90094-4. [DOI] [PubMed] [Google Scholar]
- Galis F, Van der Sluijs I, Van Dooren TJ, Metz JA, Nussbaumer M. Do large dogs die young? J Exp Zoolog B Mol Dev Evol. 2007;308:119–126. doi: 10.1002/jez.b.21116. [DOI] [PubMed] [Google Scholar]
- Garland T, Jr, Bennett AF, Rezende EL. Phylogenetic approaches in comparative physiology. J Exp Biol. 2005;208:3015–3035. doi: 10.1242/jeb.01745. [DOI] [PubMed] [Google Scholar]
- Hall KY, Hart RW, Benirschke AK, Walford RL. Correlation between ultraviolet-induced DNA repair in primate lymphocytes and fibroblasts and species maximum achievable life span. Mech Ageing Dev. 1984;24:163–173. doi: 10.1016/0047-6374(84)90068-x. [DOI] [PubMed] [Google Scholar]
- Hall ME, Nasir L, Daunt F, Gault EA, Croxall JP, Wanless S, Monaghan P. Telomere loss in relation to age and early environment in long-lived birds. Proc R Soc Lond B. 2004;271:1571–1576. doi: 10.1098/rspb.2004.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JM, Durkee SJ, Dysko R, Austad SN, Miller RA. Genetic modulation of hormone levels and life span in hybrids between laboratory and wild-derived mice. J Gerontol Biol Med Sci. 2006;61:1019–1029. doi: 10.1093/gerona/61.10.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JM, Salmon AB, Leiser SF, Galecki AT, Miller RA. Skin-derived fibroblasts from long-lived species are resistant to some, but not all, lethal stresses and to the mitochondrial inhibitor rotenone. Aging Cell. 2007;6:1–13. doi: 10.1111/j.1474-9726.2006.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart RW, Setlow RB. Correlation between deoxyribonucleic acid excision-repair and life-span in a number of mammalian species. Proc Natl Acad Sci U S A. 1974;71:2169–2173. doi: 10.1073/pnas.71.6.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PH, Read AF, Nee S. Why ecologists need to be phylogenetically challenged. J Ecol. 1995;83:585–586. [Google Scholar]
- Haussmann MF, Mauck RA. Telomeres and Longevity: Testing an Evolutionary Hypothesis. Mol Biol Evol. 2008;25:220–228. doi: 10.1093/molbev/msm244. [DOI] [PubMed] [Google Scholar]
- Haussmann MF, Winkler DW, Huntington CE, Nisbet ICT, vleck CM. Telomerase Expression Is Differentially Regulated in Birds of Differing Life Span. Ann N Y Acad Sci. 2004;1019:186–190. doi: 10.1196/annals.1297.029. [DOI] [PubMed] [Google Scholar]
- Haussmann MF, Winkler DW, Huntington CE, Nisbet ICT, Vleck CM. Telomerase activity is maintained throughout the lifespan of long-lived birds. Exp Gerontol. 2007;42:610–618. doi: 10.1016/j.exger.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Haussmann MF, Winkler DW, Vleck CM. Longer telomeres associated with higher survival in birds. Biology Letters. 2005;1:212–214. doi: 10.1098/rsbl.2005.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D, Martin K. A bird’s-eye view of aging: What’s in it for the ornithologist? The Auk. 2009;126:24. [Google Scholar]
- Holmes DJ, Flückiger R, Austad SN. Comparative biology of aging in birds: an update. Exp Gerontol. 2001;36:869–883. doi: 10.1016/s0531-5565(00)00247-3. [DOI] [PubMed] [Google Scholar]
- Hulbert A. Explaining longevity of different animals: is membrane fatty acid composition the missing link? AGE. 2008;30:89–97. doi: 10.1007/s11357-008-9055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulbert AJ. On the importance of fatty acid composition of membranes for aging. J Theor Biol. 2005;234:277–288. doi: 10.1016/j.jtbi.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Hulbert AJ, Faulks SC, Harper JM, Miller RA, Buffenstein R. Extended longevity of wild-derived mice is associated with peroxidation-resistant membranes. Mech Ageing Dev. 2006;127:653–657. doi: 10.1016/j.mad.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulbert AJ, Pamplona R, Buffenstein R, Buttemer WA. Life and Death: Metabolic Rate, Membrane Composition, and Life Span of Animals. Physiol Rev. 2007;87:1175–1213. doi: 10.1152/physrev.00047.2006. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Boulton ME, Kirkwood TB. Positive correlation between mammalian life span and cellular resistance to stress. Free Radic Biol Med. 1999;26:495–500. doi: 10.1016/s0891-5849(98)00323-2. [DOI] [PubMed] [Google Scholar]
- Kato H, Harada M, Tsuchiya K, Moriwaki K. Absence of correlation between DNA repair in ultraviolet irradiated mammalian cells and life span of the donor species. Jpn J Genet. 1980;55:99–108. [Google Scholar]
- Kelly C, Price T. Comparative methods based on species mean values. Math Biosci. 2004;187:135–144. doi: 10.1016/j.mbs.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Kim H, You S, Farris J, Kong B-W, Christman SA, Foster LK, Foster DN. Expression Profiles of p53-, p16INK4a-, and Telomere-Regulating Genes in Replicative Senescent Primary Human, Mouse, and Chicken Fibroblast Cells. Exp Cell Res. 2002;272:199–208. doi: 10.1006/excr.2001.5420. [DOI] [PubMed] [Google Scholar]
- Lake J, Moore JE. Phylogenetic analysis and comparative genomics 1998 [Google Scholar]
- Leiser SF, Miller RA. Nrf2 Signaling, a Mechanism for Cellular Stress Resistance in Long-Lived Mice. Mol Cell Biol. 30:871–884. doi: 10.1128/MCB.01145-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiser SF, Miller RA. Nrf2 Signaling: a Mechanism for Cellular Stress Resistance in Long-lived Mice. Mol Cell Biol. 2009:MCB.01145–01109. doi: 10.1128/MCB.01145-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiser SF, Salmon AB, Miller RA. Correlated resistance to glucose deprivation and cytotoxic agents in fibroblast cell lines from long-lived pituitary dwarf mice. Mech Ageing Develop. 2006;127:821–829. doi: 10.1016/j.mad.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Maynard SP, Miller RA. Fibroblasts from long-lived Snell dwarf mice are resistant to oxygen-induced in vitro growth arrest. Aging Cell. 2006;5:89–96. doi: 10.1111/j.1474-9726.2006.00187.x. [DOI] [PubMed] [Google Scholar]
- Michailidis G, Saretzki G, Hall J. Endogenous and ectopic expression of telomere regulating genes in chicken embryonic fibroblasts. Biochem Biophys Res Comm. 2005;335:240–246. doi: 10.1016/j.bbrc.2005.07.058. [DOI] [PubMed] [Google Scholar]
- Michell AR. Longevity of British breeds of dog and its relationships with sex, size, cardiovascular variables and disease. Vet Rec. 1999;145:625–629. doi: 10.1136/vr.145.22.625. [DOI] [PubMed] [Google Scholar]
- Miller R, Austad S. Large animals in the fast lane. Science. 1999;285:199. doi: 10.1126/science.285.5425.199b. [DOI] [PubMed] [Google Scholar]
- Miller RA. Extending Life: Scientific Prospects and Political Obstacles. The Milbank Quarterly. 2002;80:155–174. doi: 10.1111/1468-0009.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Austad SN, Masoro EJ. Handbook of the Biology of Aging. Elsevier; 2005a. Growth and Aging: Why do Big Dogs Die young? [Google Scholar]
- Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, Smith-Wheelock M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell. 2005b;4:119–125. doi: 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Harper JM, Dysko R, Durkee SJ, Austad SN. Longer life spans and delayed maturation in wild-derived mice. Exp Biol Med. 2002a;227:500–508. doi: 10.1177/153537020222700715. [DOI] [PubMed] [Google Scholar]
- Miller RA, Harper JM, Galecki A, Burke D. Big mice die young: early life body weight predicts longevity in genetically heterogenous mice. Aging Cell. 2002b;1:22–29. doi: 10.1046/j.1474-9728.2002.00006.x. [DOI] [PubMed] [Google Scholar]
- Munoz-Garcia A, Williams JB. Basal Metabolic Rate in Carnivores Is Associated with Diet after Controlling for Phylogeny. Physiological and Biochemical Zoology. 2005;78:1039–1056. doi: 10.1086/432852. [DOI] [PubMed] [Google Scholar]
- Murakami S, Salmon AB, Miller RA. Multiplex stress resistance in cells from long-lived dwarf mice. FASEB J. 2003;17:1565–1566. doi: 10.1096/fj.02-1092fje. [DOI] [PubMed] [Google Scholar]
- Nielsen PJ, Ryan JM. Cumulative population doublings as the determinant of chick cell lifespan in vitro. J Cell Physiol. 1981;107:8. doi: 10.1002/jcp.1041070308. [DOI] [PubMed] [Google Scholar]
- Ogburn CE, Austad SN, Holmes DJ, Kiklevich JV, Gollahon K, Rabinovitch PS, Martin GM. Cultured renal epithelial cells from birds and mice: Enhanced resistance of avian cells to oxidative stress and DNA damage. J Gerontol Biol Med Sci. 1998;53:B287–B292. doi: 10.1093/gerona/53a.4.b287. [DOI] [PubMed] [Google Scholar]
- Ogburn CE, Carlberg K, Ottinger MA, Holmes DJ, Martin GM, Austad SN. Exceptional cellular resistance to oxidative damage in long-lived birds requires active gene expression. J Gerontol Biol Med Sci. 2001;56:B468–B474. doi: 10.1093/gerona/56.11.b468. [DOI] [PubMed] [Google Scholar]
- Orentreich N, Matias JR, DeFelice A, Zimmerman JA. Low methionine ingestion by rats extends lifespan. J Nutr. 1993;123:269–274. doi: 10.1093/jn/123.2.269. [DOI] [PubMed] [Google Scholar]
- Parrinello S, Samper E, Krtolica A, Goldstein J, Melov S, Campisi J. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat Cell Biol. 2003;5:741–747. doi: 10.1038/ncb1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patronek GJ, Waters DJ, Glickman LT. Comparative longevity of pet dogs and humans: implications for gerontology research. J Gerontol Biol Med Sci. 1997;52:B171–B178. doi: 10.1093/gerona/52a.3.b171. [DOI] [PubMed] [Google Scholar]
- Petrelli JM, Calle EE, Rodriguez C, Thun MJ. Body mass index, height, and postmenopausal breast cancer mortality in a prospective cohort of US women. Cancer Causes Control. 2002;13:325–332. doi: 10.1023/a:1015288615472. [DOI] [PubMed] [Google Scholar]
- Pim D, Andrea BM, Diana van H, Corine de K-T, Joke B, Roeland WD, Hans JT, Rudi GJW. Stress-induced responses of human skin fibroblasts in vitro reflect human longevity. Aging Cell. 2009;8:595–603. doi: 10.1111/j.1474-9726.2009.00506.x. [DOI] [PubMed] [Google Scholar]
- Price T. Correlated evolution and independent contrasts. Phil Transac R Soc Lond B. 1997;352:11. doi: 10.1098/rstb.1997.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajawat YS, Hilioti Z, Bossis I. Aging: Central role for autophagy and the lysosomal degradative system. Ageing Res Rev. 2009;8:199–213. doi: 10.1016/j.arr.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Rezende EL, Bozinovic F, Garland T, Merilä J. Climatic adaptation and the evolution of basal and maximum rates of metaolism in rodents. Evolution. 2004;58:1361–1374. doi: 10.1111/j.0014-3820.2004.tb01714.x. [DOI] [PubMed] [Google Scholar]
- Ricklefs RE. Evolutionary Theories of Aging: Confirmation of a Fundamental Prediction, with Implications for the Genetic Basis and Evolution of Life Span. Am Nat. 1998;152:24–44. doi: 10.1086/286147. [DOI] [PubMed] [Google Scholar]
- Ricklefs RE, Starck JM. Applications of Phylogenetically Independent Contrasts: A Mixed Progress Report. Oikos. 1996;77:167–172. [Google Scholar]
- Ridley M. Evolution. 2. Blackwell Science; Cambridge, MA: 1996. [Google Scholar]
- Salmon AB, Akha AAS, Buffenstein R, Miller RA. Fibroblasts From Naked Mole-Rats Are Resistant to Multiple Forms of Cell Injury, But Sensitive to Peroxide, Ultraviolet Light, and Endoplasmic Reticulum Stress. J Gerontol A Biol Sci Med Sci. 2008a;63:232–241. doi: 10.1093/gerona/63.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon AB, Ljungman M, Miller RA. Cells From Long-Lived Mutant Mice Exhibit Enhanced Repair of Ultraviolet Lesions. J Gerontol A Biol Sci Med Sci. 2008b;63:219–231. doi: 10.1093/gerona/63.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon AB, Murakami S, Bartke A, Kopchick J, Yasumura K, Miller RA. Fibroblast cell lines from young adult mice of long-lived mutant strains are resistant to multiple forms of stress. Am J Physiol Endocrinol Metab. 2005;289:E23–E29. doi: 10.1152/ajpendo.00575.2004. [DOI] [PubMed] [Google Scholar]
- Samuels DC. Life span is related to the free energy of mitochondrial DNA. Mech Ageing Dev. 2005;126:1123–1129. doi: 10.1016/j.mad.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Sedivy JM. Telomeres limit cancer growth by inducing senescence: long-sought in vivo evidence obtained. Cancer Cell. 2007;11:389–391. doi: 10.1016/j.ccr.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Seluanov A, Hine C, Bozzella M, Hall A, Sasahara TH, Ribeiro AA, Catania KC, Presgraves DC, Gorbunova V. Distinct tumor suppressor mechanisms evolve in rodent species that differ in size and lifespan. Aging Cell. 2008;7:813–823. doi: 10.1111/j.1474-9726.2008.00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver LM. Mouse Genetics. Oxford University Press; New York: 1995. [Google Scholar]
- Sohal RS, Sohal BH, Brunk UT. Relationship between antioxidant defenses and longevity in different mammalian species. Mech Ageing Dev. 1990;53:217–227. doi: 10.1016/0047-6374(90)90040-m. [DOI] [PubMed] [Google Scholar]
- Speakman JR. Body size, energy metabolism and lifespan. J Exp Biol. 2005a;208:1717–1730. doi: 10.1242/jeb.01556. [DOI] [PubMed] [Google Scholar]
- Speakman JR. Correlations between physiology and lifespan - two widely ignored problems with comparative studies. Aging Cell. 2005b;4:167–175. doi: 10.1111/j.1474-9726.2005.00162.x. [DOI] [PubMed] [Google Scholar]
- Speakman JR, van Acker A, Harper EJ. Age-related changes in the metabolism and body composition of three dog breeds and their relationship to life expectancy. Aging Cell. 2003;2:265–275. doi: 10.1046/j.1474-9728.2003.00061.x. [DOI] [PubMed] [Google Scholar]
- Suh Y, Atzmon G, Cho M-O, Hwang D, Liu B, Leahy DJ, Barzilai N, Cohen P. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proceedings of the National Academy of Sciences. 2008;105:3438–3442. doi: 10.1073/pnas.0705467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun LY, Steinbaugh MJ, Masternak MM, Bartke A, Miller RA. Fibroblasts from long-lived mutant mice show diminished ERK1/2 phosphorylation but exaggerated induction of immediate early genes. Free Radical Biology and Medicine. 2009;47:1753–1761. doi: 10.1016/j.freeradbiomed.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Chu G. Xeroderma pigmentosum complementation group E and UV-damaged DNA-binding protein. DNA Repair. 2002;1:601–616. doi: 10.1016/s1568-7864(02)00052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolmasoff JM, Ono T, Cutler RG. Superoxide dismutase: correlation with lifespan and specific metabolic rate in primate species. Proc Natl Acad Sci U S A. 1980;77:2777–2781. doi: 10.1073/pnas.77.5.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretli S. Height and weight in relation to breast cancer morbidity and mortality. A prospective study of 570,000 women in Norway. Int J Cancer. 1989;44:23–30. doi: 10.1002/ijc.2910440105. [DOI] [PubMed] [Google Scholar]
- Tretli S, Magnus K. Height and weight in relation to uterine corpus cancer morbidity and mortality. A follow-up study of 570,000 women in Norway. Int J Cancer. 1990;46:165–172. doi: 10.1002/ijc.2910460204. [DOI] [PubMed] [Google Scholar]
- Treton JA, Courtois Y. Correlation between DNA excision repair and mammalian lifespan in lens epithelial cells. Cell Bio Int Rep. 1980;6:253–260. doi: 10.1016/0309-1651(82)90077-7. [DOI] [PubMed] [Google Scholar]
- Venkatesan RN, Price C. Telomerase expression in chickens: Constitutive activity in somatic tissues and down-regulation in culture. Proc Natl Acad Sci USA. 1998;95:14763–14768. doi: 10.1073/pnas.95.25.14763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10:57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne RK, Ostrander EA. Lessons learned from the dog genome. Trends Genet. 2007;23:557–567. doi: 10.1016/j.tig.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Walford RL. The Retardation of Aging and Disease by Dietary Restriction. Charles C. Thomas; Springfield, IL: 1988. [Google Scholar]
- Westoby M, Leishman M, Lord J. Issues of interpretation after relating comparative datasets to phylogeny. J Ecol. 1995;83:12. [Google Scholar]
- Wiersma P, Munoz-Garcia A, Walker A, Williams JB. Tropical birds have a slow pace of life. Proc Natl Acad Sci USA. 2007;104:9340–9345. doi: 10.1073/pnas.0702212104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JB. Longevity on Dune larks in the Namib desert. Safring News. 1992;21:17–18. [Google Scholar]
- Yamanaka S. A Fresh Look at iPS Cells. Cell. 2009;137:13–17. doi: 10.1016/j.cell.2009.03.034. [DOI] [PubMed] [Google Scholar]
- Yuan R, Flurkey K, Van Aelst-Bouma R, Zhang W, King B, Austad S, Miller RA, Harrison DE. Altered growth characteristics of skin fibroblasts from wild-derived mice, and genetic loci regulating fibroblast clone size. Aging Cell. 2006;5:203–212. doi: 10.1111/j.1474-9726.2006.00208.x. [DOI] [PubMed] [Google Scholar]