Abstract
The effects of nociceptin/orphanin FQ on putative serotonin (5HT) neurons of the dorsal raphe nucleus (DRN), known to modulate the behavioral responses to stress, were investigated in vivo and in vitro. In DRN slices from unstressed rats, nociceptin/orphanin FQ concentration-dependently inhibited the firing rate of putative 5HT neurons (EC50 = 21.6 ± 1.21 nM) and the selective NOP receptor antagonist UFP-101 shifted the concentration response curve to the right (estimated pA2 6.86). Nociceptin/orphanin FQ potency was enhanced in slices prepared from rats previously subjected to a 15-min swim stress (EC50 = 1.98 ± 0.11 nM). Swim stress did not change the number or affinity of NOP receptors in DRN. Stress-elicited potentiation involved corticotropin-releasing factor (CRF)1 receptors, GABA signaling and protein synthesis, being attenuated by pretreatment with antalarmin (20 mg/kg, i.p.), diazepam (2.4 mg/kg, i.p.) and cycloheximide (2.5 mg/kg, i.p.), respectively. In anesthetized unstressed rats, locally applied nociceptin/orphanin FQ (0.03 and 0.1 ng/30 nl) inhibited the firing rate of DRN neurons (to 80 ± 7 and 54 ± 10% of baseline, respectively). Nociceptin/orphanin FQ inhibition was potentiated both 24 h after swim stress and 1 h after CRF (30 ng/30 nl intra-DRN). Stress-induced potentiation was prevented by the selective CRF1 receptor antagonist, NBI 30755 (20 mg/kg, i.p.). In contrast, the inhibitory response of DRN neurons to the 5HT1A agonist, 8OH-DPAT (1μg/1μl, intra-DRN) was not potentiated by swim stress, ruling out a nonspecific enhanced permeability of GIRK channel. Together, these findings suggest that CRF and the nociceptin/orphanin FQ/NOP system interact in the DRN during stress to control 5HT transmission; this may play a role in stress-related neuropsychopathologies.
Keywords: corticotropin releasing factor, dorsal raphe nucleus, electrophysiology, swim stress, 5-hydroxytryptamine, nociceptin/orphanin FQ
1. Introduction
The nociceptin/orphanin FQ (N/OFQ) - N/OFQ peptide (NOP) receptor system, the non-opioid branch of the opioid family, is widely distributed in the central nervous system (Neal et al., 1999; Calò et al., 2000; Civelli, 2008), where it restrains neuronal activity by activating inwardly rectifying K+ currents and by inhibiting Ca2+ influx and adenylyl cyclases (Vaughan and Christie, 1996; Moran et al., 2000). Consequently, synaptic efficiency is reduced in terms of neurosecretion and neuronal excitability (Siniscalchi et al., 1999; Meis and Pape, 2001; Bianchi et al., 2004, Tao et al., 2007).
Among its many central and peripheral actions (Meunier 1997; Civelli, 2008, Lambert, 2008), the N/OFQ-NOP receptor system plays a relevant role in anxiety and depression (Jenck et al., 1997; Gavioli and Calò, 2006). N/OFQ has been reported to be released during acute stress (Devine et al., 2003) and to interact with the main stress-related neuropeptide, the corticotropin releasing factor (CRF) (Ciccocioppo et al., 2001, 2003; Bale and Vale, 2004; Rodi et al., 2008; Nazzaro et al., 2009). Both peptide systems are well represented in areas crucial for the control of behavior, such as the amygdala, the bed nucleus of the stria terminalis, the hippocampus and the dorsal raphe nucleus (DRN), which is the major source of serotonin (5HT) inputs to forebrain areas (Millan, 2003; Le Maître et al., 2005; Valentino and Commons, 2005; Orozco-Cabal et al., 2006). Evidence suggests that either a single moderate stressful stimulus or low CRF doses reduce the overall firing activity of DRN-5HT neurons through CRF1 receptors (Price et al., 1998; Kirby et al., 2000; Roche et al., 2003; Lukkes et al., 2008), whereas intense and repeated stressful stimuli or high CRF doses increase it through CRF2 receptors (Price et al., 1998; Kirby et al., 2000; Pernar et al., 2004; Valentino and Commons, 2005). Stress affects the functional availability and the ratio of CRF1 and CRF2 receptors in certain regions including DRN neurons, the activation of the latter prevailing after intense and/or repeated stimulation (Korosi et al., 2006; Waselus et al., 2009). It is not known whether previous stress concomitantly changes DRN responsiveness to the functionally correlated peptide N/OFQ.
Taking into account the demonstrated opposite effects of N/OFQ and CRF in anxiety and on feeding behavior (Jenck et al., 1997; Ciccocioppo et al. 2001, 2003; Millan, 2003, Rodi et al., 2008) as well as the possible therapeutic value of NOP ligands in the treatment of anxiety and depression (Gavioli and Calò, 2006; Reinscheid, 2006), we decided to investigate the response of putative 5HT neurons to N/OFQ both in unstressed rats and in stressed rats. The stressor used was a 15 min swim stress that has been shown to release sufficient endogenous CRF to affect the DRN (Price et al., 2002; Waselus et al., 2009). Single unit extracellular recording was used both in vitro, in brainstem DRN-containing slices, and in anesthetized rats to monitor DRN neuronal activity. Part of the present results have previously been presented in abstract form (Nazzaro et al., 2008).
2. Materials and Methods
2.1 Animals
Animal manipulations were carried out according to the EC Directive 86/609/EEC and the Stokes Institute of the Children's Hospital of Philadelphia Institutional Animal Care and Use Committee directives and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. For in vitro experiments young adult male Sprague-Dawley rats (Morini S.p.A., Reggio Emilia, Italy), of 80/120 g weight, were used. In vivo experiments utilized adult male Sprague-Dawley rats (Taconic Farms, Germantown, NY, U.S.A.), of 250/275 g weight. Rats were housed three per cage on a 12 hr light/dark schedule in a temperature controlled (22°C) colony room and were given ad libitum access to standard rat chow and water.
2.2 Swim stress trial
For swim stress, rats were placed for 15 min in a cylindrical glass tank (46 cm high × 20 cm diameter) filled with water (25 ± 1 °C) to a depth of 30 cm. Immediately after the swim, rats were removed from the tank, towel dried, and put in a warming cage (37°C) for 5 min. For the in vitro experiments, the rats submitted to swim stress (here forward indicated as “stressed” rats) were immediately sacrificed, unless otherwise stated. The rats for in vivo experiments were returned to their home cage and were sacrificed 24 h later. In the experiments requiring pretreatment with diazepam (2.4 mg/kg), antalarmin (20 mg/kg) or cycloheximide (3 mg/kg) the rats were i.p. injected 1 h before swim stress, whereas for NBI 30775 pretreatment, i.p. injection occurred 30 min before swim stress. Soon after the injection the animals were returned to their home cage in order to minimize additional stress. Control unstressed rats were handled as for the stressed rats, but without swim stress.
2.3 In vitro experiments
Preparation of DRN slices
Rats were decapitated under light ether anesthesia. Blood samples were collected to assay corticosterone levels. The brains were rapidly removed and placed in ice-cold oxygenated (95% O2; 5% CO2) artificial cerebrospinal fluid (ACSF) of the following composition (mM): NaCl 120, KCl 3.5, NaH2PO4 1.2, MgCl2 1.3, CaCl2 2, NaHCO3 25, D-glucose 11 (pH 7.3). Brain slices were prepared as previously reported (Mlinar et al., 2005) with minor modifications: a block of tissue containing the DRN was cut out, glued on a teflon stage, immersed in ice-cold ACSF and sliced into three “DRN slices” of 350 μm thickness (approximately corresponding to plates 50 - 52; Paxinos and Watson, 1998) using a vibratome (DSK 3000, Dosaka, Japan). Then they were left to recover for at least 2 h at room temperature. A single slice was placed in a chamber (2 ml volume) submerged on a nylon mesh, superfused at a constant flow rate (2.5 ml/min, 34 °C) and allowed to equilibrate for at least 30 min before the beginning of the experiment. Drugs were administered through the perfusion line and a complete exchange of the chamber solution occurred in about 1 min.
Extracellular recording of DRN neurons
Extracellular recordings were made with glass microelectrodes (8-10 MΩ tip resistance) filled with 150 mM NaCl. In order to reproduce in slices the noradrenergic drive normally present during wakefulness, the spontaneous firing was facilitated by adding the α1 adrenoceptor agonist phenylephrine (10 μM) to the ACSF (Vandermaelen and Aghajanian, 1983). Neurons were identified as serotonergic according to established electrophysiological criteria (Mlinar et al., 2005). Only neurons with a 10 min stable baseline and firing rate within 0.6 and 3 Hz were used. Since in most experiments, pharmacological treatments caused reduction or cessation of the spiking, at the end of each experiment slices were perfused with the G-protein regulated inward rectifier (GIRK) channel blocker, BaCl2 (150 μM), which promptly reversed this spiking cessation if related to pharmacological treatment. Experiments in which BaCl2 failed to recover the spiking activity (to at least 90% of control firing rate) were discarded. Single unit potentials were amplified and filtered by a high input-impedance amplifier (A-M Systems 3000, Carlsborg, WA) and the signal was relayed to an oscilloscope and a PC via A/D converter National Instruments PCI-6221, controlled by custom Labview software for gap-free acquisition and on-line analysis. The firing rate was sampled on-line in 10 s bins. All the drugs were bath applied for 10 to 15 min or till a stable plateau was established. No more than one neuron was recorded from each slice.
Saturation Binding Experiments
DRN slices from unstressed and stressed rats were disrupted in a Polytron homogenizer in 20 volumes of 50 mM Tris HCl, 2 mM EDTA, 100 μM phenylmethylsulphonylfluoride (PMSF) at pH 7.4. The homogenate was centrifuged at 40,000 × g for 10 min and the pellet was resuspended in the same buffer. An aliquot of the homogenate was removed for protein assay according to a Bio Rad method, using bovine albumin as reference standard. In saturation binding experiments, membranes (100 μg of protein per assay) were incubated with 8 to 10 different concentrations of [3H]-Nociceptin, ranging from 0.2 to 20 nM for 120 min at 25°C (Varani et al., 1998). Non specific binding was defined as the binding observed in the presence of 10 μM N/OFQ and was about 20% of the total binding. Bound and free radioactivity were separated by filtering the assay mixture through Whatman GF/C glass-fibre filters using a Brandel cell harvester. The filter-bound radioactivity was counted in a Packard TR 2500 Spectrometer. A weighted non linear least-square curve fitting program LIGAND was used for computer analysis of saturation experiments.
2.4 In vivo experiments
For the in vivo single unit extracellular recordings, the previously described procedure (Pernar et al, 2004) was adopted. Rats were anesthetized continuously with isoflurane (1-2 %) in air mixture and body temperature was monitored and kept at 37°C with a homeothermic blanket control unit (Harvard Instrument, Cambridge, MA). The rats were positioned in a stereotaxic instrument with blunt ear bars with the head oriented at a 10° angle to the horizontal plane (nose down). The skull was exposed and a hole was drilled on the midline (5 mm posterior to bregma to 3-4 mm posterior to lambda). The dura mater and the sagittal sinus were ligated, transected and reflected to allow for a midline approach to the DRN with minimal blood loss. For i.c.v. administration, a hole was drilled 1.2 mm lateral to midline, and 1.0 mm caudal to bregma and a 26 gauge stainless steel cannula lowered to the lateral ventricle to a depth of 5.6 mm ventral to the skull surface. Double barrel glass micropipettes were used for extracellular single-unit recording of DRN discharge and simultaneous peptide microinfusion. The recording pipette tip (6-10 MΩ) was filled with 2% Pontamine Sky Blue (PSB) dye in 0.5 M sodium acetate. The infusion pipette (tip diameter 50-100 μm) was angled at 30-45° with its tip adjacent to the tip of the recording pipette but 100-125 μm dorsal. In the experiments where drugs were applied via i.c.v. injection, a single barrel electrode was used for the recording. The micropipette was positioned 8.7 to 9.4 mm posterior to bregma and midline, and advanced using an electronic microdrive (Kopf Instrument, CA); cells were isolated at a 4.5 to 6.7 mm depth from dura mater. Recordings were amplified and filtered (Axoclamp 1A, Axon Instruments, Foster City, CA), and fed to a window discriminator for calculating the frequency of the spike occurrence. Resulting levels were digitalized via CED interface controlled by Spike 2 software (Cambridge Electronic Design, Cambridge UK) for on-line visualization, storage and off-line analysis on PC. Once a single neuron was identified, basal discharge rate was recorded until stable steady state (almost 4 min). Peptides were microinfused by positive pressure pulses applied by a Picospritzer II (Parker Hannifin Corp., Fairfield, NJ) connected to the infusion pipette (delivered volume = 30 nl). The discharge rate was recorded for at least 5 min after complete peptide infusion. The recording site was marked by ionophoresis of PSB from the recording pipette (-19 μA, 15 min) at recording completion. No more than one cell per rat was recorded.
2.5 Drugs
Antalarmin, BaCl2, cycloheximide, diazepam, 8-hydroxy-D-propylaminotetralin (8OH-DPAT) and phenylephrine were purchased from Sigma Aldrich; Nociceptin/Orphanin FQ (N/OFQ) and UFP-101 ([Nphe1,Arg14,Lys15]N/OFQ-NH2) were prepared and purified as previously described (Guerrini et al., 1997). [3H]-Nociceptin was from Perkin Elmer Italia, Monza, Italy. All drugs were prepared at at least one thousand times the highest experimental concentration as stock solutions in distilled water, aliquoted and stored at -20°C until use. Ovine Corticotropin Releasing Factor (oCRF, Jean Rivier, The Salk Institute, La Jolla, CA), was diluted in water (1 mg/1 ml). Aliquots (10 μl) were concentrated using a Savant-Speed Vac concentrator, stored at -70°C, and dissolved in the appropriate volume of ACSF on the day of experiment. The selective CRF1 antagonist, NBI 30775 (3-[6-(dimethylamino)-4-methyl-pyrid-3-yl]-2,5-dimethyl-N, N-dipropyl-pyrazolo[2,3-a]pyrimidin-7-amine, R121919, Neurocrine Bioscience, San Diego, CA) was dissolved in a solution containing 5% ethanol and 5% cremaphor and i.p. administered.
2.6 Statistics
Unless otherwise stated, all values are expressed as mean ± s.e.m. Statistical significance of the differences has been assessed using GraphPad Prism Software package. Concentration-response curves for inhibition of firing for in vitro experiments were obtained by fitting experimental data to the logistic equation, b + (1 - b) / (1 + (EC50 / [drug]) nH), where EC50 is the half-maximally effective concentration, nH is the Hill coefficient, and b is the fraction of firing rate remaining at the maximal drug effect. For assessment of statistical significance of change in EC50 values, 95% confidence limits (95% C.I.) obtained from curve fittings were used. For DRN in vivo recordings, discharge rates were determined in 1 min intervals and were expressed as a percentage of the mean basal discharge rate determined 180 s before drug infusion. One way repeated measures or two-way analysis of variance (ANOVA) followed by Fisher post hoc test were performed. Student's t tests for paired and unpaired data were applied when appropriate. P values lower than 0.05 were considered to be statistically significant.
Results
3.1 In Vitro Single Unit Extracellular Recordings in Rat Dorsal Raphe Nucleus
Putative serotonergic neurons in DRN slices had a characteristic, high regularity in the firing of action potentials, driven by activation of α1-adrenoceptor by phenylephrine 10 μM, as previously described (Vandermaelen and Aghajanian, 1983), with a mean firing rate of 2.09 ± 0.25 Hz in DRN slices from unstressed rats (n=22) and 2.58 ± 0.3 Hz from stressed rats (n=19).
3.1.1 Effects of N/OFQ in DRN slices from unstressed and stressed rats
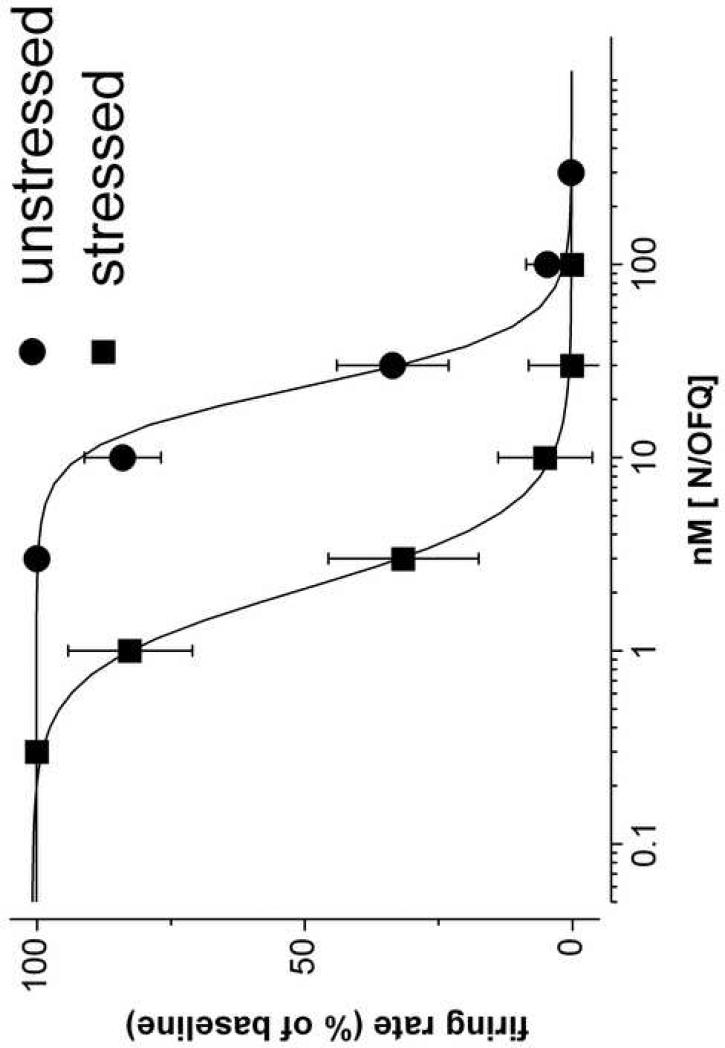
Bath application of N/OFQ (0.3 - 300 nM) reduced the firing rate of the recorded neurons from unstressed rats in a concentration dependent manner (Fig. 1). The effect was completely reversible, with a washout of about 30 min. UFP-101, a peptidic selective NOP receptor antagonist (Calò et al., 2002), added (1 μM) to the bath 15 min before N/OFQ and maintained throughout the whole experiment, did not affect the discharge rate of putative serotonergic DRN neurons, but shifted the N/OFQ concentration-response curve to the right (Table 1), with an estimated pA2 of 6.86. In DRN slices from stressed rats the inhibitory effect of N/OFQ on 5HT neuron firing rate was increased by about 10 times (as judged by the EC50, Table 1) and the concentration-response curve was shifted to the left (Fig. 1). Bath application of the antagonist UFP-101 (1 μM) 15 min before N/OFQ, increased the N/OFQ EC50 (Table 1) and shifted to the right the N/OFQ concentration-response curve, with an estimated pA2 of 6.71, similar to the one calculated for the unstressed rats group.These findings indicate that N/OFQ inhibits the firing rate of putative 5HT neurons via stimulation of NOP receptors; swim stress significantly increases its potency.
Figure 1.
Single unit extracellular recordings in rat dorsal raphe nucleus slices from unstressed rats and from rats submitted to 15 min of forced swim (stressed rats). Concentration-response curve to Nociceptin/Orphanin FQ (N/OFQ), bath applied for 10 to 15 min. The spontaneous firing, facilitated by adding 10 μM phenylephrine, was sampled on-line in 10 s bins. No more than one neuron was recorded from each slice. Basal firing rate was 1.76 ± 0.16 Hz in dorsal raphe nucleus slices from unstressed rats (n=8) and 2.08 ± 0.22 Hz from stressed rats (n=8).
Table 1.
Inhibition by nociceptin/orphanin FQ (N/OFQ) of dorsal raphe nucleus serotonergic neurons in vitro. Bath application of UFP-101.
| N/OFQ EC50 (nM) | ||
|---|---|---|
| Treatment | Unstressed rats [n] | Stressed rats [n] |
| Control | 21.6 ± 1.21 [8] | 1.98 ± 0.11** [8] |
| UFP 101 1 μM | 97.1 ± 28.4o [8] | 9.10 ± 3.9o [8] |
Values are means ± 95% C.I.
P<0.001, significantly different from unstressed rats
P<0.05 from the corresponding control, F-test for comparison of fits.
3.1.2 Effects of in vivo pretreatments on N/OFQ effects in DRN slices from stressed rats
In order to establish whether a classic anxiolytic drug affected the stress-induced increase in N/OFQ potency, diazepam was used. When administered 1 h before the stress, diazepam (2.4 mg/kg i.p.) attenuated the shift to the left of the N/OFQ concentration-response curve (Table 2). CRF released during swim stress targets the DRN to affect receptor localization and 5HT release (Price et al., 2002; Waselus et al., 2009). To detect whether or not CRF release during swim stress could somehow be related to the increase in N/OFQ potency, a selective CRF1 antagonist (antalarmin, 20 mg/kg, i.p.) was administered 1 h before swim stress. In DRN slices from antalarmin-pretreated stressed rats the N/OFQ concentration-response curve was shifted to the right and the EC50 value (Table 2) was significantly different from that determined in slices from control stressed rats.
Table 2.
Inhibition by N/OFQ of DRN serotonergic neurons in vitro from stressed rats. In vivo pretreatments.
| Treatment [n] | N/OFQ EC50 (nM) |
|---|---|
| Control (stressed) [8] | 1.98 ± 0.11 |
| Diazepam 2.4 mg/kg i. p. [5] | 14.6 ± 4.01* |
| Antalarmin 20 mg/kg i.p. [5] | 12.8 ± 3.27* |
| Cycloheximide 2.5 mg/kg i.p.[5] | 13.3 ± 2.31* |
Values are means ± 95% C.I.
P<0.01 significantly different from the stressed control, F-test for comparison of fits.
The increase in inhibitory effect of N/OFQ following swim stress could be ascribed to several mechanisms, such as up-regulation of NOP receptors or changes in the downstream signaling pathway. The protein synthesis inhibitor cycloheximide was administered (2.5 mg/kg i.p.) to the rats 1 h before swim stress in order to verify whether the synthesis of new protein molecules (either new receptors or proteins enrolled in the signaling pathway) was involved in the increase in N/OFQ potency. The visual inspection of rats at the time of sacrifice did not reveal any change in behavior. Cycloheximide attenuated the increase in inhibitory effect of N/OFQ induced by swim stress (Table 2): the EC50 calculated for N/OFQ in cycloheximide-pretreated stressed rats was statistically different from the EC50 measured in control stressed animals. These findings indicate that the stress-related potentiation of N/OFQ effect involves CRF1 receptors and protein synthesis.
3.1.3 Effects of N/OFQ in DRN slices 24 h after swim stress
Exposure to swim stress induces various physiological and behavioral changes, some of which last up to one week in rodent brain (Davis et al., 1995). A set of experiments was performed on slices prepared from rats sacrificed 24 h after swim stress investigating whether or not the increase in inhibitory effect of N/OFQ detected in DRN slices prepared from rats sacrificed immediately after the swim stress is also maintained any longer. The mean firing rate of putative serotonergic neurons in DRN slices from stressed rats, sacrificed 24 h after swim stress, was 2.68 ± 0.44 Hz (n=6). Bath applied N/OFQ reduced neuronal activity in a concentration-dependent manner, with an EC50 of 18.01 ± 3.96 (n=6). This was significantly different (P<0.01) from the value obtained in DRN slices from rats sacrificed immediately after swim stress (1.98 ± 0.11, Table 1), and similar to the EC50 obtained in DRN slices from unstressed rats (21.6 ± 1.21, Table 1). Thus, the potentiation of N/OFQ effect observed immediately after swim stress is not maintained in slices prepared 24 h later.
3.2 Saturation Binding Experiments
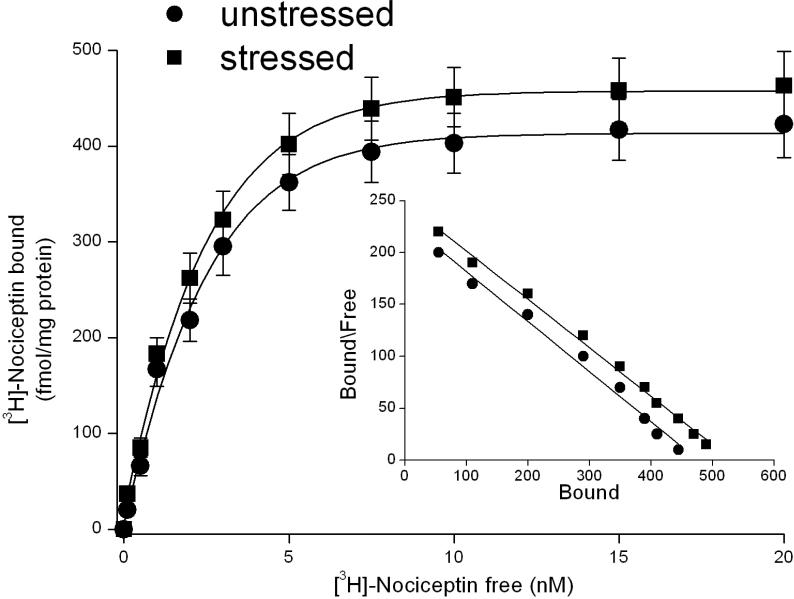
To verify whether an increase in NOP receptor number occurred following swim stress, the receptor binding technique was employed. In DRN slices from unstressed rats, saturation binding experiments revealed the presence of NOP receptors with a KD of 2.17 ± 0.15 nM and a Bmax of 488 ± 21 fmol/mg protein (n=5 independent experiments). In DRN slices from stressed rats, saturation binding experiments revealed the presence of NOP receptors with a KD of 2.01 ± 0.09 nM and a Bmax of 517 ± 21 fmol/mg protein (n=5 independent experiments, Fig. 2): the number and affinity of NOP receptors in DRN are not changed immediately after swim stress.
Figure 2.
Saturation curves and Scatchard plot of [3H]-Nociceptin binding in dorsal raphe nucleus slices from unstressed rats and from rats submitted to 15 min of forced swim (stressed rats). Saturation binding experiments were performed as described in Materials and Methods. The points represent the means ± s.e.m. of 5 experiments.
3.3 In Vivo Single Unit Extracellular Recordings in Rat Dorsal Raphe Nucleus
The DRN receives inputs from multiple brain areas involved in stress coping behavior. To study N/OFQ effects on the DRN, in a preparation in which these inputs are maintained, extracellular recordings in anesthetized animals were carried out. A total of 65 neurons were recorded from DRN. In putative serotonergic neurons from the DRN the mean firing rate was 1.33 ± 0.17 Hz (n = 33) in unstressed rats and 1.39 ± 0.2 Hz (n=32) in rats exposed to swim stress and sacrificed 24 h later (stressed rats).
3.3.1 Effects of N/OFQ locally applied to the DRN in unstressed and stressed rats
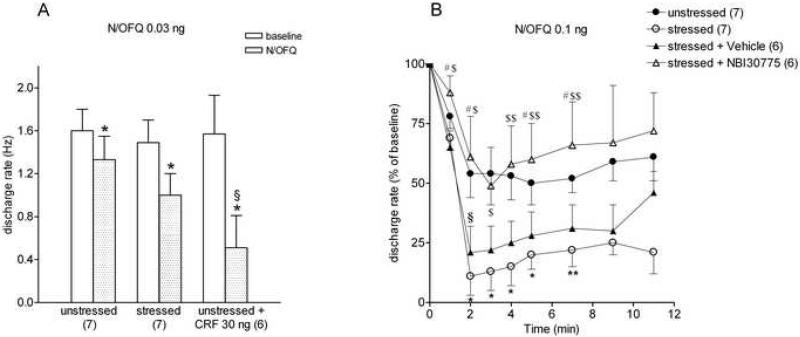
In unstressed rats, local infusion of N/OFQ reduced the firing activity of putative serotonergic neurons in the DRN. The effect was dose-dependent, in the range of 0.03 ng - 30 ng, and reached the maximum at 4-5 min after the injection. The lowest dose (0.03 ng/30 nl, n = 7, Fig. 3A) reduced firing rate to a maximum of 80 ± 7 % of baseline (one-way ANOVA repeated measures over the first 7 min (F(6,41)=2.57, P = 0.0474). A higher dose (0.1 ng/30 nl, n=7, Fig. 3B) reduced the firing activity of putative 5HT neurons to 54 ± 10%. A one-way ANOVA repeated measures over the first 7 min after drug infusion revealed a significant drug effect (F(6,41)=4.94, P=0.002). In some of the cells the same dose was able to fully abolish firing rate. The highest N/OFQ dose (30 ng/30 nl) totally inhibited the discharge activity of neurons, with a duration of over 15 min (n=11), (F(10, 65)=7.23; P< 0.0001 one-way ANOVA repeated measures over the first 7 min after drug infusion). Given that prior stress enhanced N/OFQ inhibition in vitro, only the two lower N/OFQ doses (0.03 ng/30 nl, 0.1 ng/30 nl) were tested in rats 24 hours after swim stress. The maximum inhibition induced by 0.03 ng/30 nl N/OFQ within 4-5 min of intra-DRN infusion, was 66 ± 14% of baseline in rats with a history of swim stress (n=7, Fig. 3A). A one-way ANOVA repeated measures over the first 7 min after drug infusion revealed a statistically significant effect of drug (F(6, 41)=3.24; P = 0.0186). Figure 3B shows that swim stress substantially enhanced the inhibitory effect of 0.1 ng/30 nl N/OFQ to a maximum inhibition of up to 15 ± 8% of the basal discharge rate (n=7). One-way ANOVA repeated measures over the first 7 min after drug infusion revealed a significant effect of drug (F(6, 41)=36.61; P< 0.001); a two-way ANOVA repeated measures revealed a significant effect of stress (F(1,12) = 8.36; P = 0.0136), time (F(1,5) = 23.734; P < 0.0001) and a stress-time interaction: F(12,60) = 3.376, P = 0.0094). These findings confirm, in anesthetized rats, the inhibitory effect of N/OFQ on DRN neurons and the stress-induced potentiation observed in slices.
Figure 3.
In vivo single unit extracellular recordings in rat dorsal raphe nucleus (DRN). A: inhibitory effect of intra-raphe infusion of 0.03 ng/30 nl Nociceptin/Orphanin FQ (N/OFQ) in unstressed rats, in rats exposed to swim stress 24 h prior to recording, in rats treated with 30 ng/30 nl oCRF 1 h prior to recording. The histograms represent the means ± s.e.m. of the discharge rates of DRN units (Hz); in parentheses the number of experiments. * P<0.05, ** P< 0.01, significantly different vs. baseline, Student's t test for paired data; § P<0.05 vs. unstressed rats, Student's t test for unpaired data. B: Time course of the effect of intra-raphe infusion of 0.1 ng/30 nl N/OFQ on DRN neuronal activity in unstressed, stressed, vehicle-injected and NBI 30755-injected stressed rats. Either the CRF1 antagonist NBI 30755 (20 mg/kg, i.p.) or its vehicle were administered 30 min prior to the swim stress. Abscissa: time after N/OFQ administration; ordinate: mean discharge rate, expressed as a percentage of the mean determined over 180 s prior to N/OFQ infusion (baseline); in parentheses the number of experiments.
Repeated-measures analysis of variance indicated a statistically significant effect of group [F(3,22) = 4.657, P= 0.0115] and time [F(5,110) = 28.740, P < 0.0001].
Symbols indicate differences determined by Fisher post hoc test:
* P < 0.05, **P <0 .01, stressed vs. unstressed;
§ P < 0.05, stressed + vehicle vs. unstressed;
$ P < 0.05, $$ P <0 .01, stressed + NBI 30775 vs. stressed;
# P < 0.05, stressed + NBI 30775 vs. stressed + vehicle.
3.3.2 Effects of 8OH-DPAT locally applied to DRN in unstressed and stressed rats
It is well known that the NOP receptor activates the same K+ channel regulated by 5HT1A receptors (Ikeda et al., 1997). To challenge the hypothesis of a nonspecific, stress-induced increase in channel permeability, the effect of the selective 5HT1A receptor agonist 8OH-DPAT was compared in unstressed and stressed rats. In order to choose an effective dose of 8OH-DPAT, two different i.c.v. doses, 1 μg/1 μl and 3 μg/3 μl, were consecutively injected in unstressed animals with a10 min interval or after a steady state effect was obtained. 8OH-DPAT 1 μg/1 μl induced a modest inhibitory effect on the discharge rate of putative serotonergic neurons of DRN, reducing the discharge rate to a maximum of 69 ± 14 % (n=7). A one-way ANOVA repeated measures over 7 min since drug infusion revealed a significant decrease (F(6, 27)= 3.18; P= 0.049), with the maximum effect 5 min after the injection. The higher dose (3 μg/3 μl) completely inhibited neuronal firing of the same neurons within 3-5 min after the injection in unstressed rats. Therefore, the 1 μg/1 μl 8OH-DPAT dose was chosen to determine whether prior stress enhanced the inhibitory effect of 8OH-DPAT. The firing activity of DRN neurons was reduced to 79 ± 4% in stressed rats (n=5; F(4,19)= 12 P = 0.0006 one-way ANOVA repeated measures over 7 min since drug infusion), with a time course almost identical to that seen for the unstressed group. Two-way ANOVA repeated measures confirmed no significant differences between the two groups (F(1,10) = 0.17; P = 0.898). The stress-induced potentiation of N/OFQ effect does not involve a non-specific enhanced permeability of GIRK channel.
3.3.3 Effect of N/OFQ locally applied to DRN in CRF-pretreated rats
To determine whether stress-induced sensitization of DRN neurons to N/OFQ could involve CRF released into DRN during swim stress, oCRF (30 ng/30 nl) was microinjected into the DRN in unstressed rats 1 h before local infusion of N/OFQ. Although it has been shown that oCRF inhibits the firing rate of DRN neurons for at least 10 min (Waselus et al., 2009), at the time of N/OFQ administration the inhibitory effect was no longer present, as the baseline activity of DRN neurons of oCRF-pretreated rats (1.57 ± 0. 36 Hz, n = 6) was not different from that of control animals. In rats pretreated with oCRF, N/OFQ (0.03 ng/30 nl) drastically reduced the firing activity of DRN neurons to 20 ± 11% of the basal discharge rate (n = 6, Fig. 3A), the effect being significantly higher than in control rats (P< 0.01, Student's t test for unpaired data). In some of the tested cells the injection of N/OFQ fully stopped the firing activity of the cell for longer than 15 min. The potentiation of N/OFQ inhibitory effect observed in stressed rats is displayed by oCRF-pretreated rats as well.
3.3.4 Effect of N/OFQ locally applied to DRN in stressed rats pretreated with a selective CRF1 antagonist
Evidence suggests that either a single moderate stressful stimulus or low CRF doses reduce the overall firing activity of DRN-5HT neurons through CRF1 receptors (Price et al., 1998; Kirby et al., 2000; Roche et al., 2003; Lukkes et al., 2008). The selective CRF1 antagonist NBI 30755 (also known as R 121919), which is known to prevent many of the autonomic and behavioral effects of stress (Heinrichs et al., 2002), was administered (20 mg/kg, i.p.) 30 min prior to the swim stress. Control rats received vehicle. The gross behavior of the rats treated with the antagonist did not differ from that of rats injected with vehicle. The mean basal DRN discharge rate of rats pretreated with vehicle (1.21 ± 0.25, n = 6) was not different from rats pretreated with NBI 30755 (1.52 ± 0.26 Hz, n = 6). In stressed vehicle-pretreated rats, N/OFQ (0.1 ng/30 nl) inhibited DRN neuronal discharge to a maximum of 26 ± 11% (n=6) of the basal activity. This was similar to its effects following swim stress in untreated rats (15 ± 8%, see Fig. 3B). In stressed rats pretreated with NBI 30775 the inhibitory effect of N/OFQ was greatly reduced (49 ± 16% of baseline, n=6) and not significantly different to its effect in unstressed rats (Fig. 3 B). Two-way ANOVA repeated measures over the first 7 min after drug infusion showed a statistical significance between unstressed vs. stressed + vehicle, but not unstressed vs. stressed + NBI 30775 group. (group: F(3,22) = 4.657; P = 0.0115, time: F(5,110) = 28.74; P < 0.0001). These results indicate that the stress-induced potentiation of N/OFQ inhibition involves CRF1 receptors.
4. Discussion
In this study the effects of N/OFQ on putative serotonergic neurons of rat DRN were investigated, both in vitro and in vivo, with the single unit extracellular recording technique. The most relevant findings were: (i) in rat DRN slices exogenous N/OFQ inhibited the firing rate of putative 5HT neurons via stimulation of NOP receptors; N/OFQ potency was significantly enhanced immediately, but not 24 h after swim stress; (ii) the potentiation involved CRF1 receptors, GABA signaling and protein synthesis; however, the number and affinity of NOP receptors in DRN were unchanged immediately after swim stress; (iii) in anesthetized rats, locally applied N/OFQ inhibited DRN neurons; the inhibitory effect was significantly increased 1 h after local infusion of oCRF and, unlike in DRN slices, it persisted 24 h after swim stress; (iv) the potentiation involved CRF1 receptors, and did not depend on non-specific enhanced permeability of GIRK channel.
4.1 Single Unit Extracellular Recordings in Rat Dorsal Raphe Nucleus Slices
The presence of N/OFQ and its receptors in DRN 5HT neurons have previously been demonstrated (Vaughan and Christie, 1996; Le Maître et al., 2005; Civelli, 2008). N/OFQ has been shown to decrease 5-HT efflux in the DRN, both in vivo (Tao et al., 2007) and in vitro (Nazzaro et al., 2009). The present findings provide electrophysiological evidence for functionality of NOP receptors, since the competitive antagonist UFP-101 shifted to the right the concentration-response curve to N/OFQ with a pA2 in line with data from the literature (Calò et al., 2005). The most interesting point of this part of the study is the rapid increase in responsiveness to N/OFQ in the DRN slices prepared soon after a single swim stress, i.e. when the influence of the stress on brain functions was already operative, as suggested by the enhanced corticosterone blood levels (Nazzaro et al., 2009). Despite no significant variation of the basal firing rate of DRN neurons detected immediately after a single 15-min session of swim stress, stress reduced by about 10 times the EC50 for the inhibition caused by N/OFQ. UFP-101 displayed similar values of pA2 in slices from stressed and unstressed rats, demonstrating the involvement of NOP receptors in N/OFQ effects under both experimental conditions.
Given the increase in blood corticosterone and evidence for CRF release in the DRN during swim stress (Price et al, 2002; Waselus et al., 2009), the increased neuronal responsiveness to N/OFQ in stressed rats could involve CRF release. CRF neurons, as well as CRF1 and CRF2 receptors, are present in DRN (Bale and Vale 2004; Valentino and Commons, 2005; Orozco-Cabal et al., 2006). Evidence suggests that CRF1 effects in the DRN are, at least in part, mediated by activation of GABA neurons (Roche et al, 2003; Waselus et al., 2005). Since both CRF receptors primarily couple to GS-type G-proteins (Grammatopoulos et al., 2001), post-synaptic stimulation of target neurons can be expected following CRF release (Kirby et al., 2008). Consequently, GABA-mediated 5HT neuron inhibition via CRF1 occurs. Accordingly, CRF applied to DRN slices consistently reduced electrically evoked 5HT release and this effect was prevented by both antalarmin and bicuculline (Nazzaro et al., 2009). In the in vitro experiments decribed in the present work, antalarmin effectively reduced the potentiation of N/OFQ effect induced by prior stress, further supporting the role played by CRF1 receptors. The experiments with diazepam and protein synthesis inhibitor cycloheximide were performed in the attempt to explain the mechanism of stress-induced potentiation of N/OFQ effect. Diazepam, given 1 h before sacrifice, increased the N/OFQ EC50 in DRN slices from stressed rats to a value similar to that of unstressed animals. This suggests that diazepam relieves the stress of the swim and inhibits the stress-mediated, phasic CRF release, by enhancing the GABA signaling of interneurons located upstream of CRF neurons. Consequently the neurochemical and functional effects of CRF on 5HT cells are reduced.
Accordingly, benzodiazepines have been reported to inhibit the CRF neuronal system and its gene transcription and biosynthesis (Imaki et al., 1995; Skelton et al., 2000; Swiergiel et al., 2008). The observation that cycloheximide, known to induce anxiolytic-like behavior (Mikics et al., 2006), was able to cancel the potentiation of N/OFQ after swim stress, underscores that rapid, metabolic intervention was responsible for the potentiation. This happens without appreciable changes in the number and affinity of NOP receptors evaluated with the classical binding technique. It cannot be excluded that such changes could be revealed with more sophisticated experimental approaches. Alternatively, the ultimate mechanism(s) of swim stress-induced potentiation could involve changes in other elements of the NOP receptor signaling pathway (e. g. G-protein, adenylylcyclase), detectable by means of molecular biology approaches.
It is noteworthy that the enhancement of N/OFQ inhibition was no longer evident in slices taken from rats submitted to swim stress 24 h before the sacrifice. Therefore the rapid adaptive changes detectable in vitro soon after swim stress (antalarmin-, diazepam- and cycloheximide sensitive) did not persist.
4.2 In Vivo Single Unit Extracellular Recordings in Rat Dorsal Raphe Nucleus
The basal firing rate of DRN neurons did not differ between unstressed and stressed rats in vivo, as found in vitro. On the other hand, the responsiveness to locally applied N/OFQ was increased in rats submitted to swim stress 24 h before. This effect did not depend on non-specific changes in GIRK channels sensitivity, but was selective to NOP receptor activation, as indicated by the finding that swim stress did not alter DRN responses to 8OH-DPAT.
The persistence of sensitization to N/OFQ after 24 h in the anesthetized animals deserves a brief comment. This observation, at variance with that found in the slices (in which the stress-induced potentiation of N/OFQ effect was present immediately after, but not 24 h after swim stress), suggests the existence of a long-lasting sensitization, perhaps due to functionally active reverberating circuits (intact in vivo, but cut in the slices) between DRN and forebrain structures (Celada et al., 2001; Jankowski et al., 2004), able to maintain the increased responsiveness. Accordingly, using an experimental approach very similar to that of the present work, Bambico et al. (2007) reported that glutamatergic efferences from prefrontal cortex to raphe neurons were active during anesthesia and were removed through surgical deafferentation.
The key role of CRF in the sensitization to N/OFQ is supported by convergent findings. First, 1 h after local application of oCRF 30 ng to DRN neurons, a dose of N/OFQ that was minimally effective (0.03 ng) was able to greatly reduce or even completely inhibit the firing activity of DRN neurons for more than 15 min. CRF has been suggested to interact with the N/OFQ-NOP system in other brain areas (Ciccocioppo et al., 2003; Rodi et al., 2008) and is known to be released during swim stress (Price et al., 2002; Reyes et al., 2008; Waselus et al., 2009). More importantly, as seen in DRN slices, sensitization of NOP produced by prior exposure to swim stress was blocked by pretreatment with a selective CRF1 antagonist, NBI 30775. Together, these findings confirm a physiologically relevant cooperation between CRF1 and NOP receptors, active during stress.
4.3. Functional implications
The present study demonstrated potent inhibitory effects of N/OFQ on DRN neuronal activity in vitro and in vivo. These effects were potentiated by a single swim stress trial in both cases. The findings that exposure of DRN neurons to CRF could mimic the effects of swim stress, and that pretreatment with selective CRF1 antagonists prevented stress-induced potentiation of N/OFQ, suggest that CRF released into the DRN during swim stress participates in the mechanism of stress-induced potentiation of N/OFQ. Thus, these findings highlight a cooperation between N/OFQ and CRF (via CRF1 receptors) following a single stress, to inhibit DRN-5HT activity.
Inhibition of DRN neurons by CRF1 receptors is engaged during an initial exposure to stress and has been linked to active coping behavior (Hammack et al., 2003 a). A more severe or prolonged stress induces the cellular redistribution of CRF receptor subtypes, so that CRF2 receptors are recruited and predominate on plasma membrane (Waselus et al., 2009). This cellular effect shifts the response of serotonergic neurons to CRF into activation, which has been linked to passive coping behavior (Hammack et al., 2003 b). Thus, CRF1 and CRF2 receptors regulate the DRN-5HT system in opposing manners (inhibition and excitation, respectively), with contrasting behavioral consequences.
In summary, we propose a dual interaction between N/OFQ and CRF, depending on the experimental conditions: CRF released by a single/moderate stress potentiates the inhibitory effects of N/OFQ on DRN neurons, via activation of CRF1 receptors, while the inversion of CRF1/CRF2 ratio observed in DRN after repeated/intense stress may provide an explanation for the reported functional antagonism between N/OFQ and CRF. The hypothesis that N/OFQ maintains its inhibitory effect on DRN neurons (as well as active coping behavior) in animals with a history of repeated stress deserves further investigations, also in view of possible therapeutic applications of NOP receptor ligands in stress related behavioral disorders.
Acknowledgments
This work was supported by grants from Italian Ministry of University and Research (PRIN 2004055475) and from University of Ferrara to A. S. and from USPHS (MH058250) to RJV. Nociceptin/OrphaninFQ and UFP-101 were kindly provided by Dr. Remo Guerrini, Dept. of Pharmaceutical Sciences, University of Ferrara.
References
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu. Rev. Pharmacol. Toxicol. 2004;44:525–57. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Bambico FR, Katz N, Debonnel G, Gobbi G. Cannabinoids elicit antidepressant-like behavior and activate serotonergic neurons through the medial prefrontal cortex. J. Neurosci. 2007;27(43):11700–11. doi: 10.1523/JNEUROSCI.1636-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi C, Marani L, Barbieri M, Marino S, Beani L, Siniscalchi A. Effects of nociceptin/orphanin FQ and endomorphin-1 on glutamate and GABA release, intracellular [Ca2+] and cell excitability in primary cultures of rat cortical neurons. Neuropharmacology. 2004;47(6):873–83. doi: 10.1016/j.neuropharm.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Calò G, Guerrini R, Rizzi A, Salvadori S, Regoli D. Pharmacology of nociceptin and its receptor: a novel therapeutic target. Br. J. Pharmacol. 2000;129(7):1261–83. doi: 10.1038/sj.bjp.0703219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calò G, Rizzi A, Rizzi D, Bigoni R, Guerrini R, Marzola G, Marti M, McDonald J, Morari M, Lambert DG, Salvadori S, Regoli D. [Nphe1,Arg14,Lys15]nociceptin-NH2, a novel potent and selective antagonist of the nociceptin/orphanin FQ receptor. Br. J. Pharmacol. 2002;136(2):303–11. doi: 10.1038/sj.bjp.0704706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calò G, Guerrini R, Salvadori S, Burmeister M, Kapusta DR, Lambert DG, Regoli D. UFP-101, a peptide antagonist selective for the nociceptin/orphanin FQ receptor. CNS Drugs Rev. 2005;11(2):97–112. doi: 10.1111/j.1527-3458.2005.tb00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada P, Puig MV, Casanovas JM, Guillazo G, Artigas F. Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: Involvement of serotonin-1A, GABA(A), and glutamate receptors. J. Neurosci. 2001;21(24):9917–29. doi: 10.1523/JNEUROSCI.21-24-09917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Martin-Fardon R, Weiss F, Massi M. Nociceptin/orphanin FQ inhibits stress- and CRF-induced anorexia in rats. Neuroreport. 2001;12(6):1145–9. doi: 10.1097/00001756-200105080-00019. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Fedeli A, Economidou D, Policani F, Weiss F, Massi M. The bed nucleus is a neuroanatomical substrate for the anorectic effect of corticotropin-releasing factor and for its reversal by nociceptin/orphanin FQ. J. Neurosci. 2003;23(28):9445–51. doi: 10.1523/JNEUROSCI.23-28-09445.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civelli O. The orphanin FQ/nociceptin (OFQ/N) system. Results Probl. Cell Differ. 2008;46:1–25. doi: 10.1007/400_2007_057. [DOI] [PubMed] [Google Scholar]
- Davis S, Heal DJ, Stanford SC. Long-lasting effects of an acute stress on the neurochemistry and function of 5-hydroxytryptaminergic neurones in the mouse brain. Psychopharmacology (Berl.) 1995;118(3):267–72. doi: 10.1007/BF02245954. [DOI] [PubMed] [Google Scholar]
- Devine DP, Hoversten MT, Ueda Y, Akil H. Nociceptin/orphanin FQ content is decreased in forebrain neurones during acute stress. J Neuroendocrinol. 2003;15(1):69–74. doi: 10.1046/j.1365-2826.2003.00868.x. [DOI] [PubMed] [Google Scholar]
- Gavioli EC, Calò G. Antidepressant- and anxiolytic-like effects of nociceptin/orphanin FQ receptor ligands. Naunyn Schmiedebergs Arch. Pharmacol. 2006;372(5):319–30. doi: 10.1007/s00210-006-0035-8. [DOI] [PubMed] [Google Scholar]
- Grammatopoulos DK, Randeva HS, Levine MA, Kanellopoulou KA, Hillhouse EW. Rat cerebral cortex corticotropin-releasing hormone receptors: evidence for receptor coupling to multiple G-proteins. J. Neurochem. 2001;76(2):509–19. doi: 10.1046/j.1471-4159.2001.00067.x. [DOI] [PubMed] [Google Scholar]
- Guerrini R, Calo G, Rizzi A, Bianchi C, Lazarus LH, Salvadori S, Temussi PA, Regoli D. Address and message sequences for the nociceptin receptor: a structure-activity study of nociceptin-(1-13)-peptide amide. J. Med. Chem. 1997;40(12):1789–93. doi: 10.1021/jm970011b. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Pepin JL, DesMarteau JS, Watkins LR, Maier SF. Low doses of corticotropin-releasing hormone injected into the dorsal raphe nucleus block the behavioral consequences of uncontrollable stress. Behav. Brain Res. 2003a;147(1-2):55–64. doi: 10.1016/s0166-4328(03)00133-5. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Schmid MJ, LoPresti ML, Der-Avakian A, Pellymounter MA, Foster AC, Watkins LR, Maier SF. Corticotropin releasing hormone type 2 receptors in the dorsal raphe nucleus mediate the behavioral consequences of uncontrollable stress. J. Neurosci. 2003b;23(3):1019–25. doi: 10.1523/JNEUROSCI.23-03-01019.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs SC, De Souza EB, Schulteis G, Lapsansky JL, Grigoriadis DE. Brain penetrance, receptor occupancy and antistress in vivo efficacy of a small molecule corticotropin releasing factor type I receptor selective antagonist. Neuropsychopharmacology. 2002;27(2):194–202. doi: 10.1016/S0893-133X(02)00299-3. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Kobayashi K, Kobayashi T, Ichikawa T, Kumanishi T, Kishida H, Yano R, Manabe T. Functional coupling of the nociceptin/orphanin FQ receptor with the G-protein-activated K+ (GIRK) channel. Brain Res. Mol. Brain Res. 1997;45(1):117–26. doi: 10.1016/s0169-328x(96)00252-5. [DOI] [PubMed] [Google Scholar]
- Imaki T, Wang XQ, Shibasaki T, Harada S, Chikada N, Takahashi C, Naruse M, Demura H. Chlordiazepoxide attenuates stress-induced activation of neurons, corticotropin-releasing factor (CRF) gene transcription and CRF biosynthesis in the paraventricular nucleus (PVN). Brain Res. Mol. Brain Res. 1995;32(2):261–70. doi: 10.1016/0169-328x(95)00086-8. [DOI] [PubMed] [Google Scholar]
- Jankowski MP, Sesack SR. Prefrontal cortical projections to the rat dorsal raphe nucleus: ultrastructural features and associations with serotonin and gamma-aminobutyric acid neurons. J. Comp. Neurol. 2004;19468(4):518–29. doi: 10.1002/cne.10976. [DOI] [PubMed] [Google Scholar]
- Jenck F, Moreau JL, Martin JR, Kilpatrick GJ, Reinscheid RK, Monsma FJ, Jr., Nothacker HP, Civelli O. Orphanin FQ acts as an anxiolytic to attenuate behavioral responses to stress. Proc. Natl. Acad. Sci. U. S. A. 1997;94(26):14854–8. doi: 10.1073/pnas.94.26.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby LG, Freeman-Daniels E, Lemos JC, Nunan JD, Lamy C, Akanwa A, Beck SG. Corticotropin-releasing factor increases GABA synaptic activity and induces inward current in 5-hydroxytryptamine dorsal raphe neurons. J. Neurosci. 2008;28(48):12927–37. doi: 10.1523/JNEUROSCI.2887-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby LG, Rice KC, Valentino RJ. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology. 2000;22(2):148–62. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Korosi A, Veening JG, Kozicz T, Henckens M, Dederen J, Groenink L, van der Gugten J, Olivier B, Roubos EW. Distribution and expression of CRF receptor 1 and 2 mRNAs in the CRF over-expressing mouse brain. Brain Res. 2006;1072(1):46–54. doi: 10.1016/j.brainres.2005.12.034. [DOI] [PubMed] [Google Scholar]
- Lambert DG. The nociceptin/orphanin FQ receptor: a target with broad therapeutic potential. Nat. Rev. Drug Discov. 2008;7(8):694–710. doi: 10.1038/nrd2572. [DOI] [PubMed] [Google Scholar]
- Le Maître E, Vilpoux C, Costentin J, Leroux-Nicollet I. Opioid receptor-like 1 (NOP) receptors in the rat dorsal raphe nucleus: evidence for localization on serotoninergic neurons and functional adaptation after 5,7-dihydroxytryptamine lesion. J. Neurosci. Res. 2005;81(4):488–96. doi: 10.1002/jnr.20571. [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Forster GL, Renner KJ, Summers CH. Corticotropin-releasing factor 1 and 2 receptors in the dorsal raphé differentially affect serotonin release in the nucleus accumbens. Eur. J. Pharmacol. 2008;578(2-3):185–93. doi: 10.1016/j.ejphar.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meis S, Pape HC. Control of glutamate and GABA release by nociceptin/orphanin FQ in the rat lateral amygdala. J. Physiol. 2001;532(Pt 3):701–12. doi: 10.1111/j.1469-7793.2001.0701e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier JC. Nociceptin/orphanin FQ and the opioid receptor-like ORL1 receptor. Eur. J. Pharmacol. 1997;340(1):1–15. doi: 10.1016/s0014-2999(97)01411-8. [DOI] [PubMed] [Google Scholar]
- Mikics E, Barsy B, Barsvári B, Haller J. Behavioral specificity of non-genomic glucocorticoid effects in rats: effects on risk assessment in the elevated plus-maze and the open-field. Horm. Behav. 2005;48(2):152–62. doi: 10.1016/j.yhbeh.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Millan MJ. The neurobiology and control of anxious states. Prog. Neurobiol. 2003;70(2):83–244. doi: 10.1016/s0301-0082(03)00087-x. [DOI] [PubMed] [Google Scholar]
- Mlinar B, Tatini F, Ballini C, Nencioni S, Della Corte L, Corradetti R. Differential autoinhibition of 5-hydroxytryptamine neurons by 5-hydroxytryptamine in the dorsal raphe nucleus. Neuroreport. 2005;16(12):1351–5. doi: 10.1097/01.wnr.0000175249.25535.bf. [DOI] [PubMed] [Google Scholar]
- Moran TD, Abdulla FA, Smith PA. Cellular neurophysiological actions of nociceptin/orphanin FQ. Peptides. 2000;21(7):969–76. doi: 10.1016/s0196-9781(00)00235-7. [DOI] [PubMed] [Google Scholar]
- Nazzaro C, Barbieri M, Valentino RJ, Siniscalchi A. Previous stress potentiates the inhibitory effects of nociceptin on dorsal raphe nucleus neurons: potential role of corticotropin-releasing factor. Society for Neuroscience Meeting; Washington, DC. 2008. Abstr. 282.3/OO10. [Google Scholar]
- Nazzaro C, Marino S, Barbieri M, Siniscalchi A. Inhibition of serotonin outflow by Nociceptin/OrphaninFQ in dorsal raphe nucleus slices from normal and stressed rats: role of Corticotropin Releasing Factor. Neurochem. Int. 2009;54:378–384. doi: 10.1016/j.neuint.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Neal CR, Jr., Mansour A, Reinscheid R, Nothacker HP, Civelli O, Watson SJ., Jr. Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J. Comp. Neurol. 1999;406(4):503–47. [PubMed] [Google Scholar]
- Orozco-Cabal L, Pollandt S, Liu J, Shinnick-Gallagher P, Gallagher JP. Regulation of synaptic transmission by CRF receptors. Rev. Neurosci. 2006;17(3):279–307. doi: 10.1515/revneuro.2006.17.3.279. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press Inc.; Oxford: 1998. [Google Scholar]
- Pernar L, Curtis AL, Vale WW, Rivier JE, Valentino RJ. Selective activation of corticotropin-releasing factor-2 receptors on neurochemically identified neurons in the rat dorsal raphe nucleus reveals dual actions. J. Neurosci. 2004;24(6):1305–11. doi: 10.1523/JNEUROSCI.2885-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price ML, Curtis AL, Kirby LG, Valentino RJ, Lucki I. Effects of corticotropin-releasing factor on brain serotonergic activity. Neuropsychopharmacology. 1998;18(6):492–502. doi: 10.1016/S0893-133X(97)00197-8. [DOI] [PubMed] [Google Scholar]
- Price ML, Kirby LG, Valentino RJ, Lucki I. Evidence for corticotropin-releasing factor regulation of serotonin in the lateral septum during acute swim stress: adaptation produced by repeated swimming. Psychopharmacology (Berl.) 2002;162(4):406–14. doi: 10.1007/s00213-002-1114-2. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK. The Orphanin FQ / Nociceptin receptor as a novel drug target in psychiatric disorders. CNS Neurol. Disord. Drug Targets. 2006;5(2):219–24. doi: 10.2174/187152706776359628. [DOI] [PubMed] [Google Scholar]
- Reyes BA, Valentino RJ, Van Bockstaele EJ. Stress-induced intracellular trafficking of corticotropin-releasing factor receptors in rat locus coeruleus neurons. Endocrinology. 2008;149(1):122–30. doi: 10.1210/en.2007-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche M, Commons KG, Peoples A, Valentino RJ. Circuitry underlying regulation of the serotonergic system by swim stress. J. Neurosci. 2003;23(3):970–7. doi: 10.1523/JNEUROSCI.23-03-00970.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodi D, Zucchini S, Simonato M, Cifani C, Massi M, Polidori C. Functional antagonism between nociceptin/orphanin FQ (N/OFQ) and corticotropin-releasing factor (CRF) in the rat brain: evidence for involvement of the bed nucleus of the stria terminalis. Psychopharmacology (Berl.) 2008;196(4):523–31. doi: 10.1007/s00213-007-0985-7. [DOI] [PubMed] [Google Scholar]
- Siniscalchi A, Rodi D, Beani L, Bianchi C. Inhibitory effect of nociceptin on [3H]-5-HT release from rat cerebral cortex slices. Br. J. Pharmacol. 1999;128(1):119–23. doi: 10.1038/sj.bjp.0702793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton KH, Nemeroff CB, Knight DL, Owens MJ. Chronic administration of the triazolobenzodiazepine alprazolam produces opposite effects on corticotropin-releasing factor and urocortin neuronal systems. J. Neurosci. 2000;20(3):1240–8. doi: 10.1523/JNEUROSCI.20-03-01240.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiergiel AH, Li Y, Wei ZY, Dunn AJ. Effects of chlordiazepoxide on footshock- and corticotropin-releasing factor-induced increases in cortical and hypothalamic norepinephrine secretion in rats. Neurochem. Int. 2008;52(6):1220–5. doi: 10.1016/j.neuint.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Tao R, Ma Z, Thakkar MM, McCarley RW, Auerbach SB. Nociceptin/orphanin FQ decreases serotonin efflux in the rat brain but in contrast to a kappa-opioid has no antagonistic effect on mu-opioid-induced increases in serotonin efflux. Neuroscience. 2007;147(1):106–16. doi: 10.1016/j.neuroscience.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Commons KG. Peptides that fine-tune the serotonin system. Neuropeptides. 2005;39(1):1–8. doi: 10.1016/j.npep.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Vandermaelen CP, Aghajanian GK. Electrophysiological and pharmacological characterization of serotonergic dorsal raphe neurons recorded extracellularly and intracellularly in rat brain slices. Brain Res. 1983;289(1-2):109–19. doi: 10.1016/0006-8993(83)90011-2. [DOI] [PubMed] [Google Scholar]
- Varani K, Calò G, Rizzi A, Merighi S, Toth G, Guerrini R, Salvadori S, Borea PA, Regoli D. Nociceptin receptor binding in mouse forebrain membranes: thermodynamic characteristics and structure activity relationships. Br. J. Pharmacol. 1998;125:1485–90. doi: 10.1038/sj.bjp.0702226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan CW, Christie MJ. Increase by the ORL1 receptor (opioid receptor-like1) ligand, nociceptin, of inwardly rectifying K conductance in dorsal raphe nucleus neurones. Br. J. Pharmacol. 1996;117(8):1609–11. doi: 10.1111/j.1476-5381.1996.tb15329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waselus M, Valentino RJ, Van Bockstaele EJ. Ultrastructural evidence for a role of gamma-aminobutyric acid in mediating the effects of corticotropin-releasing factor in the rat dorsal raphe serotonin system. J. Comp. Neurol. 2005;482:155–165. doi: 10.1002/cne.20360. [DOI] [PubMed] [Google Scholar]
- Waselus M, Valentino RJ, Van Bockstaele EJ. Differential trafficking of corticotropin-releasing factor receptor type 1 and 2 following exposure to swim stress. Biol. Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.02.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]