Abstract
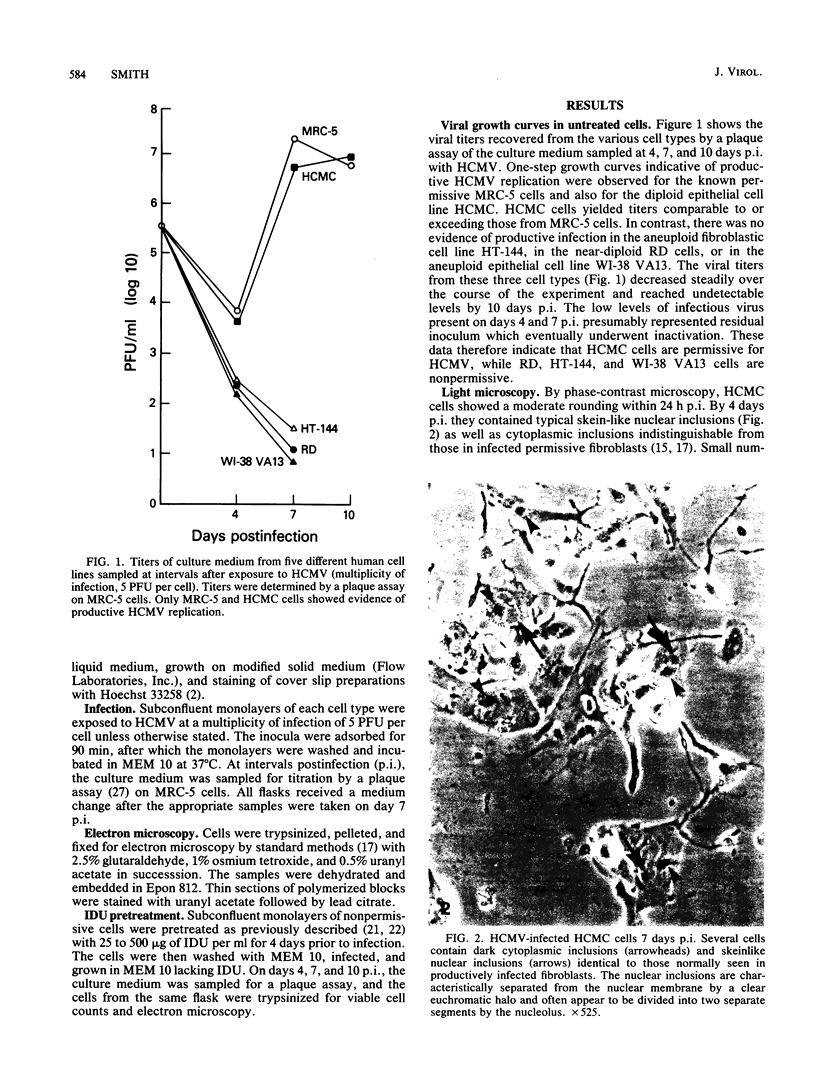
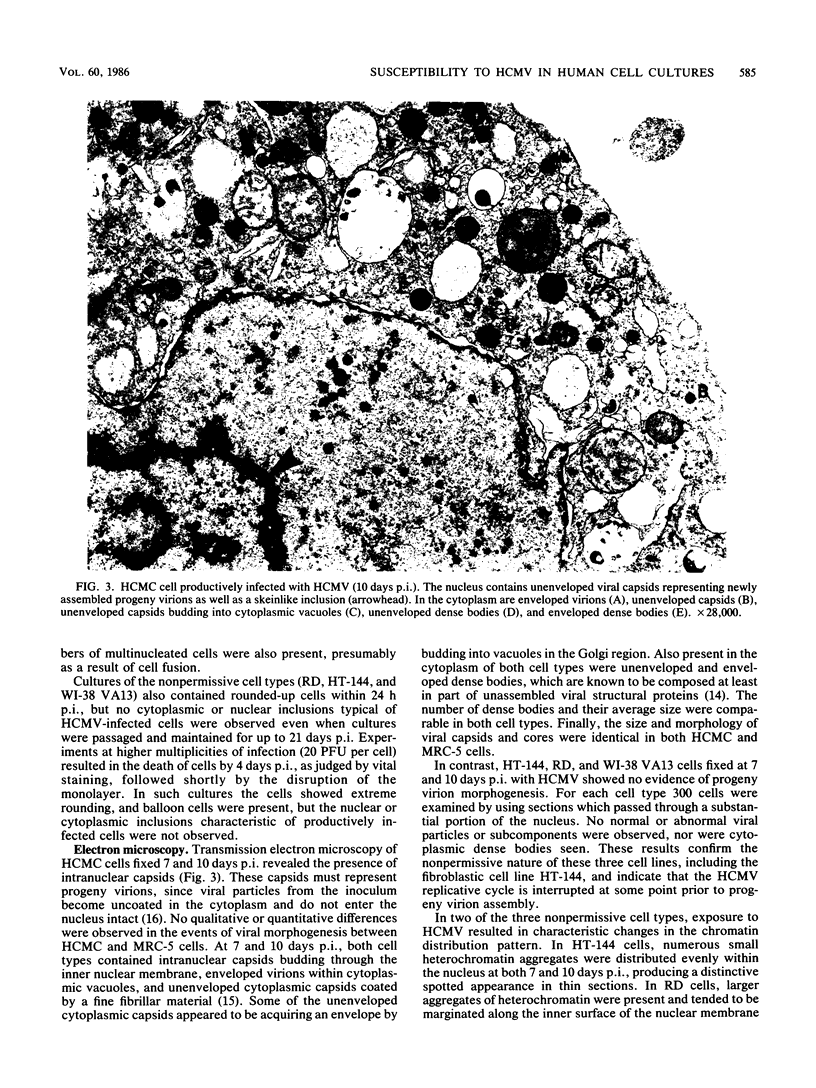
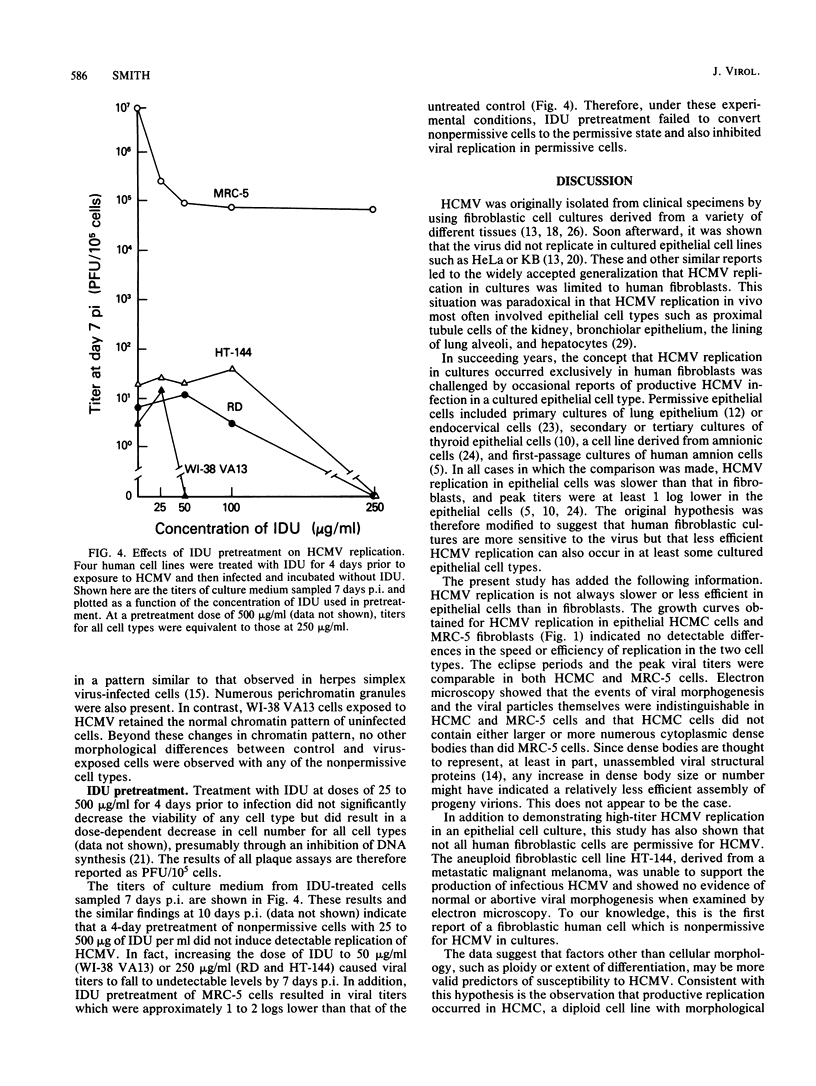
To more clearly define the characteristics which render a cell permissive for human cytomegalovirus (HCMV), we screened a panel of human cell lines differing in morphology, ploidy, and extent of differentiation for the ability to sustain productive HCMV replication. Cells were exposed to HCMV at 5 to 20 PFU per cell and examined at 4 to 14 days postinfection to detect the production of infectious virus by a plaque assay and the assembly of progeny virions by electron microscopy. By these criteria, high-titer HCMV replication (10(6) to 10(7) PFU/ml) occurred in a well-differentiated, diploid, epithelial cell line, HCMC, which had been derived from normal human colonic mucosa. In contrast, all aneuploid human cell types proved to be nonpermissive, including a fibroblastic cell line designated HT-144. These results indicate that HCMV replication in cultures is not strictly limited to fibroblasts and conversely that not all human fibroblastic cells are permissive for HCMV. Nonpermissive cell types were further investigated by attempts to chemically induce HCMV replication. Treatment of nonpermissive cell types with 25 to 500 micrograms of 5-iodo-2'-deoxyuridine per ml prior to infection did not convert them to the permissive state. The implications of these findings for the possible mechanisms maintaining the nonpermissive state are discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boldogh I., Gönczöl E., Gärtner L., Váczi G. Expression of the human cytomegalovirus genome in mouse cells and in human-mouse heterokaryons. Arch Virol. 1977;53(1-2):101–108. doi: 10.1007/BF01314851. [DOI] [PubMed] [Google Scholar]
- CRAIG J. M., MACAULEY J. C., WELLER T. H., WIRTH P. Isolation of intranuclear inclusion producing agents from infants with illnesses resembling cytomegalic inclusion disease. Proc Soc Exp Biol Med. 1957 Jan;94(1):4–12. doi: 10.3181/00379727-94-22841. [DOI] [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Danes B. S. Establishment of human colonic mucosal culture strains from colonoscopy specimens. Birth Defects Orig Artic Ser. 1980;16(2):275–281. [PubMed] [Google Scholar]
- Dutko F. J., Oldstone M. B. Cytomegalovirus causes a latent infection in undifferentiated cells and is activated by induction of cell differentiation. J Exp Med. 1981 Nov 1;154(5):1636–1651. doi: 10.1084/jem.154.5.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa M. E., Geder L., Rapp F. Infection of human amnion cells with cytomegalovirus. J Med Virol. 1978;2(4):369–375. doi: 10.1002/jmv.1890020410. [DOI] [PubMed] [Google Scholar]
- Glaser R., Rapp F. Rescue of Epstein-Barr virus from somatic cell hybrids of Burkitt lymphoblastoid cells. J Virol. 1972 Aug;10(2):288–296. doi: 10.1128/jvi.10.2.288-296.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gönczöl E., Andrews P. W., Plotkin S. A. Cytomegalovirus replicates in differentiated but not in undifferentiated human embryonal carcinoma cells. Science. 1984 Apr 13;224(4645):159–161. doi: 10.1126/science.6322309. [DOI] [PubMed] [Google Scholar]
- Jacobs J. P., Jones C. M., Baille J. P. Characteristics of a human diploid cell designated MRC-5. Nature. 1970 Jul 11;227(5254):168–170. doi: 10.1038/227168a0. [DOI] [PubMed] [Google Scholar]
- Knowles W. A. In-vitro cultivation of human cytomegalovirus in thyroid epithelial cells. Arch Virol. 1976;50(1-2):119–124. doi: 10.1007/BF01318006. [DOI] [PubMed] [Google Scholar]
- McAllister R. M., Melnyk J., Finkelstein J. Z., Adams E. C., Jr, Gardner M. B. Cultivation in vitro of cells derived from a human rhabdomyosarcoma. Cancer. 1969 Sep;24(3):520–526. doi: 10.1002/1097-0142(196909)24:3<520::aid-cncr2820240313>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Michelson-Fiske S., Arnoult J., Febvre H. Cytomegalovirus infection of human lung epithelial cells in vitro. Intervirology. 1975;5(6):354–363. doi: 10.1159/000149933. [DOI] [PubMed] [Google Scholar]
- ROWE W. P., HARTLEY J. W., WATERMAN S., TURNER H. C., HUEBNER R. J. Cytopathogenic agent resembling human salivary gland virus recovered from tissue cultures of human adenoids. Proc Soc Exp Biol Med. 1956 Jun;92(2):418–424. [PubMed] [Google Scholar]
- SMITH M. G. Propagation in tissue cultures of a cytopathogenic virus from human salivary gland virus (SGV) disease. Proc Soc Exp Biol Med. 1956 Jun;92(2):424–430. doi: 10.3181/00379727-92-22498. [DOI] [PubMed] [Google Scholar]
- STERN H., LAMBERT H. P., SHAKESPEARE W. G. ISOLATION OF CYTOMEGALOVIRUS IN AN INFANT WITH AN ANGIOSARCOMA. Arch Dis Child. 1963 Dec;38:626–631. doi: 10.1136/adc.38.202.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarov I., Abady I. The morphogenesis of human cytomegalovirus. Isolation and polypeptide characterization of cytomegalovirions and dense bodies. Virology. 1975 Aug;66(2):464–473. doi: 10.1016/0042-6822(75)90218-4. [DOI] [PubMed] [Google Scholar]
- Smith J. D., De Harven E. Herpes simplex virus and human cytomegalovirus replication in WI-38 cells. I. Sequence of viral replication. J Virol. 1973 Oct;12(4):919–930. doi: 10.1128/jvi.12.4.919-930.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. D., Moore D. M. Effects of canavanine treatment on herpesvirus morphogenesis in cultured cells. Intervirology. 1981;16(4):233–243. doi: 10.1159/000149272. [DOI] [PubMed] [Google Scholar]
- Smith J. D., de Harven E. Herpes simplex virus and human cytomegalovirus replication in WI-38 cells. II. An ultrastructural study of viral penetration. J Virol. 1974 Oct;14(4):945–956. doi: 10.1128/jvi.14.4.945-956.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jeor S., Rapp F. Cytomegalovirus replication in cells pretreated with 5-iodo-2'-deoxyuridine. J Virol. 1973 Jun;11(6):986–990. doi: 10.1128/jvi.11.6.986-990.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jeor S., Rapp F. Cytomegalovirus: conversion of nonpermissive cells to a permissive state for virus replication. Science. 1973 Sep 14;181(4104):1060–1061. doi: 10.1126/science.181.4104.1060. [DOI] [PubMed] [Google Scholar]
- Stagno S., Pass R. F., Dworsky M. E., Britt W. J., Alford C. A. Congenital and perinatal cytomegalovirus infections: clinical characteristics and pathogenic factors. Birth Defects Orig Artic Ser. 1984;20(1):65–85. [PubMed] [Google Scholar]
- Vesterinen E., Leinikki P., Saksela E. Cytopathogenicity of cytomegalovirus to human ecto- and endocervical epithelial cells in vitro. Acta Cytol. 1975 Sep-Oct;19(5):473–481. [PubMed] [Google Scholar]
- Vonka V., Anisimová E., Macek M. Replication of cytomegalovirus in human epitheloid diploid cell line. Arch Virol. 1976;52(4):283–296. doi: 10.1007/BF01315617. [DOI] [PubMed] [Google Scholar]
- WYATT J. P., SAXTON J. Generalized cytomegalic inclusion disease. J Pediatr. 1950 Mar;36(3):271-94, illust. doi: 10.1016/s0022-3476(50)80097-5. [DOI] [PubMed] [Google Scholar]
- Weller T. H. The cytomegaloviruses: ubiquitous agents with protean clinical manifestations. I. N Engl J Med. 1971 Jul 22;285(4):203–214. doi: 10.1056/NEJM197107222850406. [DOI] [PubMed] [Google Scholar]
- Wentworth B. B., French L. Plaque assay of cytomegalovirus strains of human origin. Proc Soc Exp Biol Med. 1970 Nov;135(2):253–258. doi: 10.3181/00379727-135-35031. [DOI] [PubMed] [Google Scholar]