Abstract
TIM-4, a member of the TIM family expressed on antigen-presenting cells, binds to phosphatidylserine exposed on the surface of apoptotic bodies. However, the significance of this interaction in vivo remains unknown because other receptors have been implicated in the clearance of apoptotic bodies and could compensate for the TIM-4 deficiency in vivo. In this study, we describe the generation of TIM-4-deficient mice and address whether TIM-4 serves a unique function in vivo. We show that TIM-4−/− peritoneal macrophages and B-1 cells do not efficiently engulf apoptotic bodies in vitro, or clear apoptotic bodies in vivo. TIM-4-deficient mice have hyperactive T and B cells, elevated levels of serum Ig, and develop antibodies to double-stranded DNA. Taken together, we show that TIM-4 is critical for the clearance of apoptotic bodies in vivo, and that lack of TIM-4 results in aberrant persistence of apoptotic bodies leading to dysregulated lymphocyte activation and signs of systemic autoimmunity.
Keywords: phosphatidylserine receptor, phagocytosis, autoantibody, apoptosis
The T cell, Ig, mucin domain (TIM) family includes four expressed Tim genes (Tim-1 through Tim-4) and four predicted genes (Tim-5 through Tim-8) on mouse chromosome 11B1.1, whereas a syntenic region on human chromosome 5q33.2 contains three TIM genes (TIM-1, TIM-3, and TIM-4) (1–3). The syntenic genetic intervals containing the TIM loci in both mice and man have been linked to susceptibility to autoimmune and allergic disorders (2–4).
TIM-4, the only TIM protein not expressed on T cells, is found on antigen-presenting cells (APC), including dendritic cells and macrophages (5, 6). First described as a ligand for TIM-1 (7), which is expressed on activated T cells, we showed that TIM-4 costimulates T cell activation by promoting cell survival (5). We now know that TIM-4 exerts a bimodal effect on T cell immunity by inhibiting naïve T cells during the initiation phase of an immune response, and later enhancing T cell responses during the elicitation phase of T cell immunity (6). It is unclear, though, whether activating or inhibitory functions of TIM-4 predominate in vivo. With the generation of TIM-4-deficient mice, we are able to directly address the in vivo role of TIM-4.
Two independent groups found that TIM-4 binds to phosphatidylserine (PtdSer) and that blocking TIM-4 with anti-TIM-4 mAb inhibits the engulfment of apoptotic bodies (AB) by TIM-4-expressing macrophages (8, 9). Although these studies clearly showed that TIM-4 binds to AB through PtdSer, the physiologic role of TIM-4 in vivo has not been addressed owing to lack of TIM-4-deficient mice. It remains unknown whether TIM-4 is necessary for clearing AB in vivo or whether other receptors that bind AB, such as BAI1 (10) and stabilin-2 (11), compensate for TIM-4 deficiency.
Clearance of AB is a critical mechanism for maintaining normal tissue homeostasis in multicellular organisms. AB that are not rapidly cleared progress to secondary necrosis, during which auto- and neoantigens are exposed in an inflammatory milieu, resulting in the induction of immune responses to these antigens (12). We generated TIM-4-deficient mice and show that TIM-4 deficiency results in a cell-specific and compartment-specific defect in clearance of AB. Despite this compartment-specific defect, loss of TIM-4 leads to systemic hyperactivity of T and B cells and the development of lupus-like autoimmunity.
Results
TIM-4 Is Expressed on Peritoneal Macrophages and B-1 Cells, and Anti-TIM-4 Abrogates Phagocytosis of AB by These Populations In Vitro.
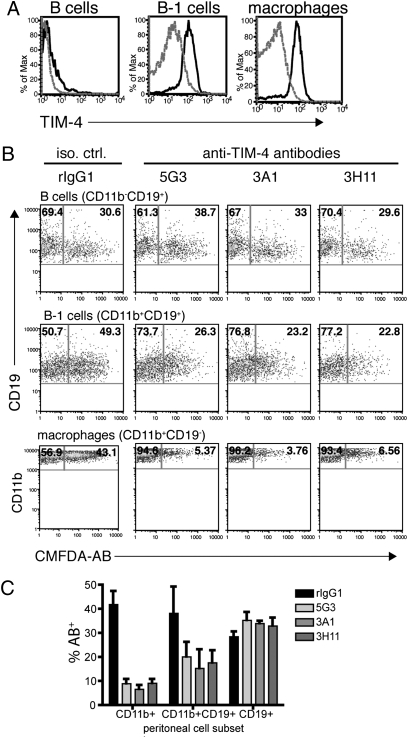
TIM-4 is expressed by splenic APC, including dendritic cells and macrophages, but is not found on splenic B cells (5, 6). It was recently found that resident peritoneal macrophages also express TIM-4, and treating macrophages with anti-TIM-4 mAb can inhibit their engulfment of AB (8, 9). To determine whether other peritoneal cell populations express TIM-4, we examined resident peritoneal cells for TIM-4 expression by flow cytometry. We found that in addition to CD19-CD11b+ peritoneal macrophages, CD19+CD11b+ B cells, or B-1 cells, also express TIM-4 (Fig. 1A). No TIM-4 was detected on CD19+CD11b− conventional B cells in the peritoneum (Fig. 1A). We have previously described three antibodies against the extracellular domain of TIM-4 (5G3, 3A1, and 3H11) (5). To functionally characterize these anti-TIM-4 antibodies, we tested their ability to block the binding or phagocytosis of AB by TIM-4-expressing cells. Resident peritoneal cells were harvested by lavage and treated with anti-TIM-4 mAb. AB labeled with the membrane-permeable dye 5-chloromethylfluorescein diacetate (CMFDA) were added to the antibody-treated peritoneal cells, and flow cytometry was used to analyze uptake of CMFDA-labeled AB. As expected, we did not observe TIM-4-mediated uptake by CD19+CD11b− conventional B cells, which do not express TIM-4. Interestingly, we observed that peritoneal B-1 cells were able to uptake AB, and this was partially inhibited by anti-TIM-4 mAb (Fig. 1B), suggesting that TIM-4 can mediate the engulfment of AB by B-1 B cells. As expected, all three anti-TIM-4 antibodies tested could block AB recognition by CD11b+ peritoneal macrophages (Fig. 1B). Results from multiple experiments demonstrate that all three anti-TIM-4 antibodies block uptake of AB (Fig. 1C).
Fig. 1.
TIM-4 expressed on peritoneal macrophages and B-1 cells mediates the uptake of apoptotic cells in vitro. (A) Peritoneal cells were harvested from WT C57BL/6 mice by lavage, stained with CD11b-Pacific blue, CD19-allophycocyanin, and TIM-4-PE, and analyzed by flow cytometry. B cells were gated on CD19+CD11b− cells, B-1 cells on CD19+CD11b+ cells, and macrophages on CD19−CD11b+ cells. Dashed gray lines represents isotype control staining, and blacks line are TIM-4 staining. (B) Peritoneal exudate cells were cultured with anti-TIM-4 mAb 5G3, 3A1, or 3H11 for 30 min before CMFDA-labeled AB were added to the cultures. Cells were harvested 30 min later, labeled with CD11b-Pacific blue and CD19-allophycocyanin, and analyzed by flow cytometry. Numbers in the upper right quadrants are the frequencies of AB+ peritoneal cells. (C) Results from three experiments performed as in B are summarized as percentage CMFDA-AB+ peritoneal subsets.
TIM-4-Deficient Mice Have Normal Splenic, Thymic, and Lymph Node Cellularity, but Hyperactive T and B Cells.
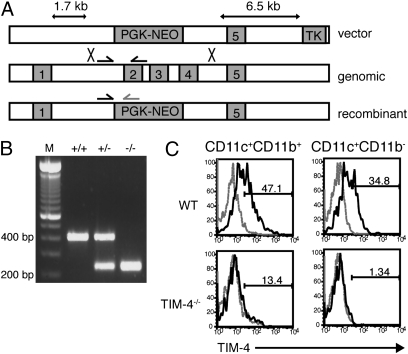
Although the effects of blocking TIM-4 on peritoneal cells suggest that TIM-4 mediates the engulfment of AB in vitro, we asked whether TIM-4 is required for clearing AB in vivo, or whether other pathways compensate for the TIM-4 deficiency. To address this, we generated TIM-4-deficient mice by replacing exons 2–4 in the Tim-4 gene with a PGK promoter-driven neomycin-resistance cassette (PGK-neo) (Fig. 2A). Chimeric mice generated from targeted ES cells were bred with C57BL/6 mice. The heterozygous progeny were interbred, and mice of all three expected genotypes were produced in normal Mendelian ratios (Fig. 2B). TIM-4 is normally expressed on splenic dendritic cells and macrophages, but no TIM-4 expression was detected in TIM-4−/− mice on these cells (Fig. 2C).
Fig. 2.
Targeting Tim-4 by homologous recombination to generate TIM-4−/− mice. (A) A 1.7-kb short arm upstream of Tim-4 exon 2 and a 6.5-kb long arm downstream of exon 4 were subcloned into the pKSTKneo targeting vector. Homologous recombination leading to replacement of exons 2–4 with PGK-neo would disrupt Tim-4 gene expression as depicted. (B) Confirmation of the TIM-4−/− genotype by PCR. Arrows in A represent primers for the PCR amplification strategy used to distinguish between WT, heterozygous, and homozygous TIM-4-mutant mice. WT band = 416 bp; KO band = 201 bp. Lane 1, 100-bp DNA ladder; lane 2, WT; lane 3, TIM-4+/−; lane 4, TIM-4−/−. (C) Splenic cells from WT and TIM-4−/− mice were stained with CD11c-allophycocyanin, CD11b-FITC, and TIM-4-PE. Dashed gray line represents isotype control staining, and black line is TIM-4 staining.
TIM-4 has been shown to regulate T cell responses both in vitro and in vivo (5–7), leading to either T cell expansion or inhibition, and these bimodal effects are thought to occur via the association of TIM-4 with different binding partners. On activated T cells, TIM-4 induces T cell expansion by binding to TIM-1 (5). However, on naïve T cells, TIM-4 inhibits T cell activation by binding to an unidentified receptor (6). To determine the predominant function of TIM-4 in vivo, we analyzed T cell responses in TIM-4−/− mice. The absolute cell numbers in spleen, lymph nodes, and thymus were not significantly different between TIM-4−/− mice and WT controls (Table S1), nor did the frequencies of immune cell subsets vary in these organs (Fig. S1).
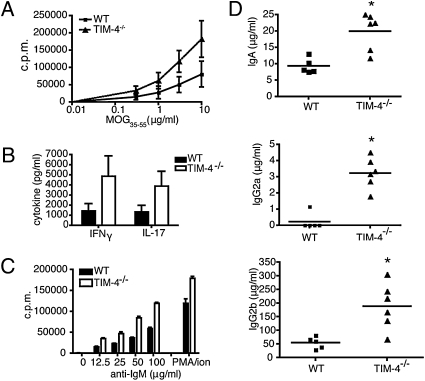
To test whether T cell responses were altered in the absence of TIM-4, WT littermates and TIM-4−/− mice were immunized with the myelin peptide MOG35–55. Spleens and lymph nodes were reactivated in vitro with MOG35–55 peptide. TIM-4−/− T cells had a higher proliferative response and also produced elevated levels of the inflammatory cytokines IFNγ and IL-17 when reactivated in vitro (Fig. 3 A and B, respectively).
Fig. 3.
TIM-4−/− T and B cells have a hyperactive phenotype. (A) WT and TIM-4−/− mice were immunized with MOG35–55/IFA plus M. tuberculosis s.c., and spleens and lymph nodes were harvested 8 days later. Cells were reactivated with MOG35–55 peptide, and proliferation was measured after 48 h by 3[H]-thymidine incorporation in triplicate wells. Results shown are the mean of three independent experiments (total n = 5 to 6 mice per group). (B) Supernatants were collected from the experiments described in A and analyzed by ELISA for IL-17 and IFNγ production. (C) CD19+ cells purified from peritoneal exudates and cultured in the presence of titrated anti-IgM or with PMA plus ionomycin. Proliferation was measured after 48h by 3[H]-thymidine incorporation in triplicate wells. Results shown are representative of 4 independent experiments. d) Ig isotype titers were measured by ELISA using serum from peripheral blood of naïve WT and TIM-4−/− mice. Data are representative of two independent experiments (total n = 10–11 mice per group).
Given our observation that peritoneal B-1 cells express TIM-4, we addressed whether TIM-4-deficient mice also had altered B cell responses. Peritoneal CD19+ cells were purified and activated with anti-IgM or with phorbol 12-myristate 13-acetate (PMA) plus ionomycin. TIM-4−/− peritoneal CD19+ cells proliferated more in response to both anti-IgM and PMA plus ionomycin stimulation when compared with WT controls (Fig. 3C). These effects were limited to peritoneal CD19+ B cells, because CD19+ B cells purified from the spleens of TIM-4-deficient mice did not proliferate more than WT splenic B cells in response to either stimulus (Fig. S2). Given that we could observe both hyperactive T cells and B cells in TIM-4−/− mice, we asked whether antibody production was also affected in the absence of TIM-4. We observed that TIM-4−/− mice also had elevated titers of serum Ig. Specifically, serum IgA, IgG2a, and IgG2b levels were significantly increased in unimmunized TIM-4-deficient mice when compared with WT controls (Fig. 3D).
TIM-4-Deficient Peritoneal Macrophages and B-1 Cells Cannot Efficiently Clear AB.
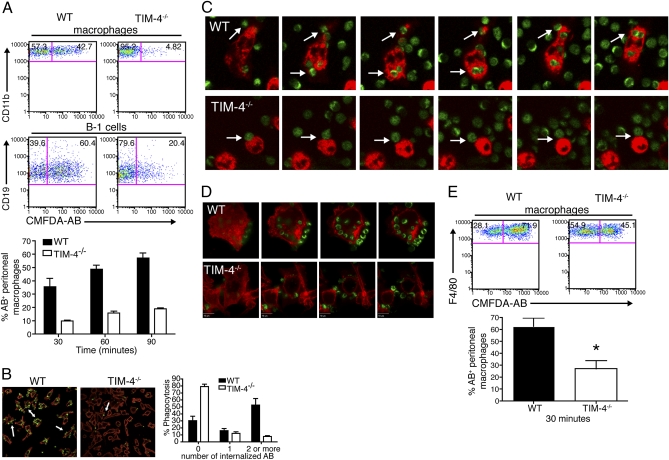
TIM-4 binds to PtdSer, and blocking TIM-4 inhibits the engulfment of AB in vitro (8, 9). We asked whether cells lacking TIM-4 expression had a defect in the clearance of AB in vitro. Briefly, resident peritoneal cells from naïve WT and TIM-4−/− mice were cultured with CMFDA-labeled AB. We observed that TIM-4−/− F4/80+CD11b+ peritoneal macrophages did not efficiently uptake AB in vitro (Fig. 4A), and this defect was not overcome even after 90 min of coculture (Fig. 4A, Bottom). CD19+CD11b+ peritoneal B-1 cells also showed a defect in uptake of AB (Fig. 4A), although this defect was not as striking as that observed with peritoneal macrophages. To further clarify the results obtained with flow cytometry and to test defects in engulfment vs. AB binding, we monitored resident peritoneal cell/AB cocultures using immunofluorescence microscopy. Peritoneal cells from WT and TIM-4−/− mice were cultured with CMFDA-labeled AB and then analyzed for AB uptake. The majority of WT CD11b+ cells (53.0%) had internalized AB after 3 h, but in contrast, less than 10% of the CD11b+ cells from TIM-4−/− mice had engulfed two or more AB during this time (Fig. 4B). Moreover, whereas WT macrophage membranes were decorated by external AB, very few AB were found associated with the cell surface of TIM-4−/− cells, suggesting that tethering of AB to the cell surface is impaired in the absence of TIM-4 (Fig. 4B). Accordingly, time-lapse images and Z-stack analyses of live cocultures of peritoneal cells and CMFDA-labeled AB showed that WT peritoneal CD11b+ cells internalized AB into phagosomal compartments, whereas TIM-4−/− cells did not uptake AB effectively (Fig. 4 C and D and Movies S1 and S2).
Fig. 4.
TIM-4−/− peritoneal macrophages cannot efficiently engulf apoptotic cells in vitro or in vivo. (A) Peritoneal cells were cultured with CMFDA-labeled AB for various time points and analyzed by flow cytometry. Gates included CD11b+F4/80+ cells (Top) and CD11b+CD19+ cells (Middle). The average frequency of F4/80+CD11b+ that were CMFDA-AB+ after 30, 60, or 90 min is shown (Bottom). Data represent six independent experiments. (B) Peritoneal cells were cultured with CMFDA-labeled AB (green) for 3 h. Cells were stained with CD11b-PE (red) and analyzed by immunofluorescence microscopy. Arrows indicate nonengulfed AB associated with macrophage cell surface. Frequencies of CD11b+ cells that engulfed AB in two independent experiments are summarized (Right). (C) Images representing a 90-min time-lapse video of peritoneal cells cultured with CMFDA-labeled AB. Arrows indicate peritoneal cells associating with AB. (D) Representative images from a Z-stack analysis performed on peritoneal cells that were treated as in B. Images in C and D are representative of two independent experiments. (E) Mice were injected i.p. with CMFDA-labeled AB and killed 30 min later. Peritoneal cells were harvested and analyzed by flow cytometry (Top). Gate includes CD11b+F4/80+ cells. Data from three independent experiments are summarized (Bottom; *P < 0.05).
To test whether AB could be cleared in vivo, WT and TIM-4−/− mice were injected i.p. with CMFDA-labeled AB, and peritoneal cells were analyzed by flow cytometry 30 min later. We found that TIM-4−/− mice had a defect in clearing AB in vivo when compared with their WT counterparts. On average, TIM-4−/− peritoneal cells were half as effective in recognizing AB in vivo (Fig. 4E).
TIM-4-Mediated Clearance of AB Is Compartment Specific.
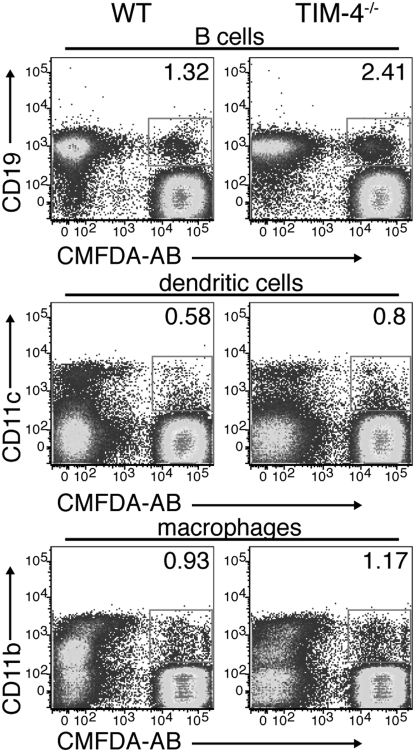
Given our observation that phagocytosis of AB was dependent on the expression of TIM-4 in subsets of peritoneal immune cells, we asked whether TIM-4-expressing splenic APC, such as CD11c+ dendritic cells and CD11b+ macrophages (5, 6), use TIM-4 to bind to and engulf AB. We cultured splenic macrophages, dendritic cells, and B cells with CMFDA-labeled AB and found that the frequency of TIM-4−/− CD19+, CD11c+, or CD11b+ cells that associated with and engulfed AB was comparable to the frequency observed with WT cells (Fig. 5A and Fig. S3).
Fig. 5.
Apoptotic body clearance by WT and TIM-4−/− splenic APC does not differ. Splenocytes from WT or TIM-4−/− mice were depleted of Thy1.2+ cells and then used for isolating CD19+, CD11c+, or CD11b+ cells. Isolated cell subsets were incubated with CMFDA-labeled AB for 30 min and then analyzed by flow cytometry. Data shown are representative of three independent experiments.
TIM-4 Deficiency Results in the Production of Anti-dsDNA Antibodies.
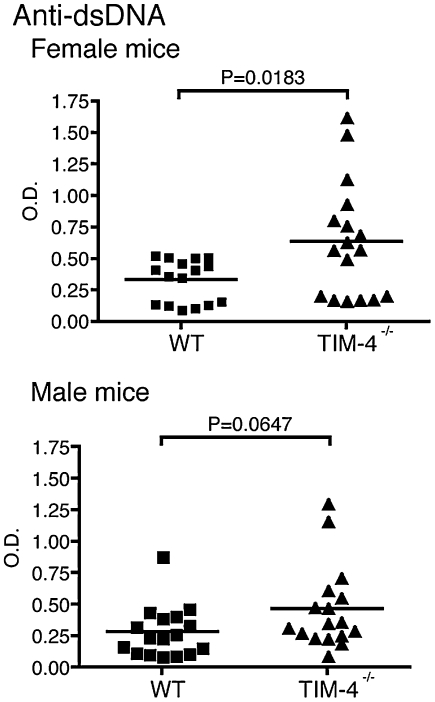
The inability to properly remove AB has been associated with the development of autoimmune responses to nuclear antigens, such as dsDNA (12–15). However, because TIM-4 deficiency only affected clearance of AB by cells in the peritoneal cavity and not in the spleen, it was not clear whether TIM-4 deficiency would result in the development of autoantibodies associated with defective AB clearance. Peripheral blood was collected from 6- to 8-week-old naïve TIM-4−/− and WT age-matched control mice and tested for the presence of anti-dsDNA Ab. Although the difference was not significant, TIM-4−/− male mice had higher titers of antibody against dsDNA than did WT littermates. Female TIM-4−/− mice, however, had significantly higher titers of antibody against dsDNA than did their WT control counterparts (Fig. 6), suggesting that tolerance to self-antigens is abrogated in TIM-4−/− mice.
Fig. 6.
TIM-4−/− mice produce antibodies to dsDNA. Serum from naïve WT and TIM-4−/− female and male mice was tested for the presence of anti-dsDNA antibodies by ELISA. Data shown are from four to five independent experiments (n = 16 to 17 mice per group).
Discussion
Both TIM-1 and TIM-4 have been implicated in the clearance of AB by binding directly to PtdSer exposed on the surface of AB (8, 9, 16). The relevance of these observations, however, remained largely untested in vivo. In this study, using TIM-4−/− mice, we show that TIM-4 is required for the clearance of AB both in vitro and in vivo, although this requirement is cell and tissue specific. TIM-4−/− mice have a defect in clearing AB in the peritoneal cavity but not in the spleen. Despite this compartment-specific defect in AB clearance, TIM-4−/− mice develop antibodies to dsDNA, an autoantigen associated with systemic autoimmune diseases, such as systemic lupus erythematosus (SLE).
Peritoneal macrophages express TIM-4, and blocking this expression can abrogate the engulfment of AB by this population in vitro (8, 9). We observed that a subset of B cells in the peritoneum also express TIM-4. This population expressed both CD19 and CD11b, thus representing peritoneal B-1 cells. Phagocytosis by B-1 cells has been observed previously and might be linked to a common precursor from which both cell types arise (17–20). However, TIM-4-dependent engulfment of AB by peritoneal B-1 cells had not been previously described and could represent a unique role for B-1 cells in the induction of tolerance.
Our present observations suggest that TIM-4-mediated clearance of AB is cell subset and compartment specific and that other phagocytic receptors present on TIM-4−/− splenic APC were sufficient to mediate normal AB clearance. Both stabilin-2 (11) and BAI1 (10) bind to PtdSer and are involved in engulfment of AB. Stabilin-2 is expressed in splenic, lymph node, and liver sinusoidal endothelial cells (21), whereas BAI1 has been detected in both human and mouse monocytes and macrophages (10). It is possible that multiple receptors mediate the engulfment of AB by phagocytes in the spleen. One possible explanation for the tissue-specific role of TIM-4 in the peritoneum vs. the spleen may be the levels at which TIM-4 protein is expressed in these two compartments. TIM-4 expression is higher on peritoneal APC subsets when compared with splenic APC subsets (Fig. S4). It is possible that the higher levels of TIM-4 protein found on peritoneal cell subsets are necessary to efficiently bind to and mediate the engulfment of AB. It is not known whether changes in the level of TIM-4 on splenic APC during different pathologic conditions promote engulfment of AB.
Binding of AB to the surface of phagocytes and subsequent engulfment results in clearance of the AB under noninflammatory conditions. However, AB that are not rapidly cleared persist, resulting in secondary necrosis, which leads to the release of autoantigens under inflammatory conditions (12). Exposure to these autoantigens is believed to cause activation of self-reactive lymphocytes and the development of autoimmunity in both mice and humans (15, 22). The accumulation of apoptotic debris in vivo from aberrant AB can serve as a survival signal for autoreactive B cells (23), and this is believed to be a signal for inducing hyperactive peripheral B cells in systemic diseases like SLE (24). This is consistent with our data showing that peritoneal B cells from TIM-4−/− mice hyperproliferated in response to activation, suggesting that peritoneal B cell responses are dysregulated in TIM-4−/− mice secondary to deficient clearance of AB. Dysregulation of B cell responses in TIM-4−/− mice is specific to cells in the peritoneum, because splenic CD19+ cells did not hyperproliferate in response to stimuli. In the absence of TIM-4, we found that T cell responses were also elevated, which could be due to enhanced antigen presentation or specific cytokine production by peripheral B cells in TIM-4−/− mice. It is also possible that in the absence of TIM-4, T cell immune responses are dysregulated owing to lack of a TIM-4-mediated inhibitory signal. Previous findings have shown that TIM-4 inhibits naïve T cell responses both in vitro and in vivo (6, 7). These observations would suggest that, although TIM-4 is capable of inducing bimodal signals to responding T cells, TIM-4-mediated inhibition is predominant in vivo.
Even though TIM-4-deficiency did not seem to affect the clearance of AB in the spleen, absence of TIM-4 was still sufficient to increase Ig production and the development of autoantibodies in TIM-4−/− mice. Gender bias has been previously described for multiple animal models of lupus in which females are more susceptible to disease (22). Sex hormones have been implicated in driving the maturation of high-affinity autoreactive B cells (25). Therefore, additional effects conferred by the female genetic background might be required to develop higher levels of systemic autoantibodies than those found in male TIM-4−/− mice.
Our findings suggest that although TIM-4 deficiency is sufficient to trigger spontaneous production of autoantibodies due to a lack of AB clearance by specific APC subsets, the clearance defect is not sufficient to drive further signs of spontaneous lupus-like disease. Nevertheless, our studies demonstrate that TIM-4 forms the first step in breaking tolerance to AB and that other genetic factors may be required for the progression of disease. This is consistent with the recent genetic dissection of SLE in mice, whereby different genetic loci affect T and B cell hyperactivity and disease progression (22).
In summary, we demonstrate that TIM-4 plays a critical role in clearing AB in the peritoneum but not in the spleen. Nevertheless, there is a niche-specific defect in clearance of AB concurrent with generalized T and B cell hyperactivity and induction of systemic anti-dsDNA antibodies in TIM-4-deficient mice. Thus TIM-4, a PtdSer receptor, may play a crucial first step in the development of many systemic autoimmune diseases.
Materials and Methods
Antibodies and Mice.
Anti-TIM-4 mAbs 5G3, 3A1, and 3H11 were previously described (5), and rIgG1 isotype control was from eBioscience. Antibodies used in this study were CD11b-Pacific blue (M1/70), CD62L-phycoerythrin (CD62L-PE) (MEL-14), CD44-PE (IM7), CD25-PE (PC61), TIM-4-PE (5G3), and CD4-allophycocyanin (RM4-5) from BioLegend; CD19-allophycocyanin/PE (ID3), CD11c-allophycocyanin (HL3), I-A/I-E-PE (M5/114.15.2), B7.1-PE (16-10A1), and CD3-FITC (145-2C11) from BD Biosciences; and CD11b-allophycocyanin/FITC (M1/70), F4/80-PE (BM8), B7.2-PE (GL1), and CD8-FITC (53-6.7) from eBioscience. Anti-IgM was from Jackson ImmunoResearch.
All animal experiments in this study were performed in compliance with the approval of the Harvard Medical Area Standing Committee on Animals (protocol 696; Harvard Medical School). Female and male C57BL/6 mice were purchased from Jackson Laboratories.
Generation of TIM-4-Deficient Mice.
To create a TIM-4-deficient mouse, a targeting vector was constructed using the RP23-43C21 BAC clone. A short arm (1.7 kb) upstream of exon 2 of Tim-4 was subcloned into a pKSTKneo targeting vector using ClaI sites. The long arm (6.5 kb), containing exon 5, was subcloned using SalI and NotI sites. Homologous recombination in electroporated Bruce4 ES cells was confirmed by Southern blotting and PCR and resulted in the replacement of Tim-4 exons 2–4 with the PGK-neo cassette from the targeting vector. PCR primers used for screening progeny were: WT Tim-4 sense, 5′ TAGCACAGGTTTTGCGTGAC 3′; WT Tim-4 antisense, 5′ CTCTGGGACCACGAGAGGTA 3′; and PGK-neo antisense, 5′ GCCAGAGGCCACTTGTGTAG 3′.
Splenocytes from WT or TIM-4−/− mice were treated with ACK lysis buffer (Lonza) and then stained with CD11b, CD11c, and TIM-4 antibodies. Frequencies and activation states of immune cell subsets were determined by flow cytometry using antibodies to cell surface molecules.
In Vitro Phagocytosis Assay.
Labeled AB were prepared by incubating C57BL/6 thymocytes with 0.2 μg/mL CellTracker Green CMFDA (Invitrogen) in serum-free media for 30 min at room temperature. Cells were washed and resuspended in 10% FBS DMEM and then treated with 1 μM dexamethasone (Sigma) for 6 h. AB were washed and resuspended in 10% FBS DMEM. Annexin V-allophycocyanin and propidium iodide (BD Biosciences) staining was used to confirm apoptosis of thymocytes.
Resident peritoneal exudate cells were harvested by lavage using 10 mL ice-cold PBS containing 0.5% BSA and 2 mM EDTA. Splenocytes were depleted of Thy1.2+ cells and then used for the purification of CD19+ cells using magnetic-activated cell sorting (MACS) columns (Miltenyi Biotech). The remaining splenocytes, enriched for dendritic cells and macrophages, along with the purified splenic CD19+ cells and peritoneal exudate cells, were resuspended in complete medium and plated at 3 × 105 cells per well in a 24-well plate. After 2 h at 37 °C, 6 × 105 CMFDA-labeled AB were added to the wells. In some experiments, 25 μg/mL anti-TIM-4 mAb or rIgG1 isotype control were added during the last 30 min of incubation before the addition of AB. Cocultures were incubated at 37 °C for the specified times and then harvested with Versene (Lonza). Cells were washed once with PBS and then resuspended in FACS buffer (0.5% BSA/PBS) for cell surface staining.
In Vivo Phagocytosis Assay.
WT or TIM-4−/− mice were injected i.p. with 10 × 106 CMFDA-labeled AB in 500 μL PBS. The animals were killed after 30 min, and peritoneal exudates were collected by lavage. Cells were washed once in PBS, resuspended in FACS buffer, and stained for cell surface markers for subsequent FACS analysis.
Detection of Serum Ig and Anti-dsDNA Antibodies.
Peripheral blood from tail vein was collected from 6- to 8-week-old WT and TIM-4−/− mice. After incubating at room temperature for 20 min, blood samples were spun for 15 min at 3,500 × g, and serum was collected. Ig serum titers were assessed using the SBA Clonotyping System (Southern Biotech) according to the manufacturer's instructions. Anti-dsDNA ELISA (Alpha Diagnostic) was performed to measure the levels of anti-nuclear autoantibody in serum samples.
Immunofluorescence Microscopy and Live-Cell Imaging.
Peritoneal exudate cells from WT and TIM-4−/− mice were cultured with CMFDA-labeled AB for 3 h. The cultures were then fixed and washed to remove nonassociated AB. The remaining cells were labeled with CD11b-PE and analyzed by confocal microscopy. In another experiment, peritoneal exudate cells were labeled with CD11b-PE or F4/80-PE and cultured with CMFDA-labeled AB. AB clearance was monitored over 90 min by laser scanning confocal microscopy on live cells using a Nikon TE2000-E inverted microscope equipped with a C1Si confocal system (Nikon), an argon ion laser at 488 nm, and diode-pumped solid-state lasers at 404 nm and 561 nm (Melles Griot). Temperature was maintained at ≈37 °C and 5% CO2 using an environmental control chamber (InVivo Scientific). Images were acquired using EZ-C1 software using an oil-immersion Nikon Plan Fluor 40× 1.3 n.a. objective with phase contrast optics. The frequency of phagocytes that had internalized AB was quantified from random frames after incubating the cells with AB for 3 h, washing twice with PBS, and then fixing with 4% paraformaldehyde. Peritoneal macrophages were identified using CD11b-PE, and the number of internalized AB per cell (categorized as 0, 1, or 2 or more particles) was quantified.
Lymphocyte Proliferation Assays.
WT or TIM-4−/− mice were immunized s.c. in both flanks and above the sternum using a total of 100 μL of 100 μg MOG35–55 peptide emulsified in incomplete Freund's adjuvant supplemented with 0.5 μg/mL Mycobacterium tuberculosis. After 8 days, draining lymph nodes and splenocytes were harvested, and 3 × 105 cells were restimulated with MOG35–55 peptide. Proliferation was measured after 48 h by 3[H]-thymidine incorporation in triplicate wells. Supernatants were collected at 48 h, and cytokines were analyzed by ELISA.
CD19+ cells from naïve WT or TIM-4-deficient mice were purified from resident peritoneal exudates or spleens using MACS columns and resuspended at 1 × 106 cells/mL in 10% FBS DMEM. Cells (1 × 105 per well) were plated in a round-bottom 96-well plate to which goat, anti-IgM, or 50 ng/mL PMA plus 1 μg/mL ionomycin were added in 100 μL. Proliferation was measured after 48 h by 3[H]-thymidine incorporation in triplicate wells.
Supplementary Material
Acknowledgments
We thank D. H. Lee and R. Chandwaskar for technical assistance, and S. M. Liu for critical review of the manuscript. This work was supported by research grants from the National Multiple Sclerosis Society (RG3996 and RG2571D9) and the National Institutes of Health (NS045973, NS035685, NS030843, AI139671, AI045757, and NS038037). D.R.G. is supported by Grant AI044828 from the National Institutes of Health. R.R.-M. is supported by Grant NS056503 from the National Institute of Neurological Disorders and Stroke. V.K.K. is a recipient of the Javits Neuroscience Investigator Award from the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910359107/DCSupplemental.
References
- 1.Kuchroo VK, Dardalhon V, Xiao S, Anderson AC. New roles for TIM family members in immune regulation. Nat Rev Immunol. 2008;8:577–580. doi: 10.1038/nri2366. [DOI] [PubMed] [Google Scholar]
- 2.Kuchroo VK, Meyers JH, Umetsu DT, DeKruyff RH. TIM family of genes in immunity and tolerance. Adv Immunol. 2006;91:227–249. doi: 10.1016/S0065-2776(06)91006-2. [DOI] [PubMed] [Google Scholar]
- 3.Kuchroo VK, Umetsu DT, DeKruyff RH, Freeman GJ. The TIM gene family: Emerging roles in immunity and disease. Nat Rev Immunol. 2003;3:454–462. doi: 10.1038/nri1111. [DOI] [PubMed] [Google Scholar]
- 4.Meyers JH, Sabatos CA, Chakravarti S, Kuchroo VK. The TIM gene family regulates autoimmune and allergic diseases. Trends Mol Med. 2005;11:362–369. doi: 10.1016/j.molmed.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Manzanet R, et al. TIM-4 expressed on APCs induces T cell expansion and survival. J Immunol. 2008;180:4706–4713. doi: 10.4049/jimmunol.180.7.4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mizui M, et al. Bimodal regulation of T cell-mediated immune responses by TIM-4. Int Immunol. 2008;20:695–708. doi: 10.1093/intimm/dxn029. [DOI] [PubMed] [Google Scholar]
- 7.Meyers JH, et al. TIM-4 is the ligand for TIM-1, and the TIM-1-TIM-4 interaction regulates T cell proliferation. Nat Immunol. 2005;6:455–464. doi: 10.1038/ni1185. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi N, et al. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity. 2007;27:927–940. doi: 10.1016/j.immuni.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyanishi M, et al. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 10.Park D, et al. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature. 2007;450:430–434. doi: 10.1038/nature06329. [DOI] [PubMed] [Google Scholar]
- 11.Park SY, et al. Rapid cell corpse clearance by stabilin-2, a membrane phosphatidylserine receptor. Cell Death Differ. 2008;15:192–201. doi: 10.1038/sj.cdd.4402242. [DOI] [PubMed] [Google Scholar]
- 12.Schulze C, et al. Clearance deficiency—a potential link between infections and autoimmunity. Autoimmun Rev. 2008;8:5–8. doi: 10.1016/j.autrev.2008.07.049. [DOI] [PubMed] [Google Scholar]
- 13.Janko C, et al. Inflammatory clearance of apoptotic remnants in systemic lupus erythematosus (SLE) Autoimmun Rev. 2008;8:9–12. doi: 10.1016/j.autrev.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 14.Munoz LE, et al. Apoptosis in the pathogenesis of systemic lupus erythematosus. Lupus. 2008;17:371–375. doi: 10.1177/0961203308089990. [DOI] [PubMed] [Google Scholar]
- 15.Yasutomo K. Pathological lymphocyte activation by defective clearance of self-ligands in systemic lupus erythematosus. Rheumatology (Oxford) 2003;42:214–222. doi: 10.1093/rheumatology/keg081. [DOI] [PubMed] [Google Scholar]
- 16.Santiago C, et al. Structures of T cell immunoglobulin mucin protein 4 show a metal-Ion-dependent ligand binding site where phosphatidylserine binds. Immunity. 2007;27:941–951. doi: 10.1016/j.immuni.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cumano A, Paige CJ, Iscove NN, Brady G. Bipotential precursors of B cells and macrophages in murine fetal liver. Nature. 1992;356:612–615. doi: 10.1038/356612a0. [DOI] [PubMed] [Google Scholar]
- 18.Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat Immunol. 2006;7:293–301. doi: 10.1038/ni1301. [DOI] [PubMed] [Google Scholar]
- 19.Li J, et al. B lymphocytes from early vertebrates have potent phagocytic and microbicidal abilities. Nat Immunol. 2006;7:1116–1124. doi: 10.1038/ni1389. [DOI] [PubMed] [Google Scholar]
- 20.Popi AF, et al. Co-ordinated expression of lymphoid and myeloid specific transcription factors during B-1b cell differentiation into mononuclear phagocytes in vitro. Immunology. 2009;126:114–122. doi: 10.1111/j.1365-2567.2008.02883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falkowski M, Schledzewski K, Hansen B, Goerdt S. Expression of stabilin-2, a novel fasciclin-like hyaluronan receptor protein, in murine sinusoidal endothelia, avascular tissues, and at solid/liquid interfaces. Histochem Cell Biol. 2003;120:361–369. doi: 10.1007/s00418-003-0585-5. [DOI] [PubMed] [Google Scholar]
- 22.Fairhurst AM, Wandstrat AE, Wakeland EK. Systemic lupus erythematosus: Multiple immunological phenotypes in a complex genetic disease. Adv Immunol. 2006;92:1–69. doi: 10.1016/S0065-2776(06)92001-X. [DOI] [PubMed] [Google Scholar]
- 23.Gaipl US, et al. Clearance deficiency and systemic lupus erythematosus (SLE) J Autoimmun. 2007;28:114–121. doi: 10.1016/j.jaut.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Liossis SN, Kovacs B, Dennis G, Kammer GM, Tsokos GC. B cells from patients with systemic lupus erythematosus display abnormal antigen receptor-mediated early signal transduction events. J Clin Invest. 1996;98:2549–2557. doi: 10.1172/JCI119073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen-Solal JF, et al. Hormonal regulation of B-cell function and systemic lupus erythematosus. Lupus. 2008;17:528–532. doi: 10.1177/0961203308089402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.