Abstract
During the last decade, research focusing primarily on alterations in the peripheral and central nervous system has improved our understanding of the pathophysiological mechanisms of chronic visceral pain. These studies have demonstrated significant physiological changes following injury to the viscera in the firing patterns of both primary afferent neurons that transmit nociceptive information from the viscera and in central neurons that process the nociceptive information. A number of receptors, neurotransmitters, cytokines, and second messenger systems in these neurons have been implicated in the enhancement of visceral nociception. N-methyl-d-aspartic acid (NMDA) receptors play an important role in chronic visceral pain and hypersensitivity that is present in the setting of colonic inflammation. NMDA receptors are found in the peripheral nervous system as well as the central terminal of primary afferent neurons and have been shown to play an important role in regulating the release of nociceptive neurotransmitters. Recent work has demonstrated the presence of NMDA receptors in the enteric nervous system. In this article, we will discuss more recent evidence of the role of NMDA receptors in visceral pain associated with colitis.
Keywords: NMDA receptor, Colitis, Visceral pain, Visceral hypersensitivity
Introduction
N-methyl-d-aspartic acid (NMDA) receptors are ligand-gated ion channels that have an important role in long-term potentiation and are involved in memory in the central nervous system. The NMDA receptors are also found in the peripheral nervous system as well as the central terminal of primary afferent neurons and have been shown to play an important role in regulating the release of substance P (SP) and calcitonin gene related peptide (CGRP) at both terminals (64). Inflammation-induced changes in the activity of NMDA receptors may enhance release of SP and CGRP from both peripheral and central nerve terminals and lead to the development and maintenance of central sensitization. The NMDA receptors also mediate peripheral sensitization and visceral hypersensitivity. Research data have demonstrated the presence of NMDA receptors in the enteric nervous system (8,49,53,72,77). In this article, we will discuss more recent evidence of the role of NMDA receptors in visceral pain associated with colitis.
During the last decade, research focusing primarily on alterations in the peripheral and central nervous system has improved our understanding of the pathophysiological mechanisms of chronic visceral pain. These studies have demonstrated significant physiological changes following injury to the viscera in the firing patterns of both primary afferent neurons that transmit nociceptive information from the viscera and in central neurons that process the nociceptive information (2,11,19,68,69,86,101). Furthermore, a number of receptors, neurotransmitters, cytokines, and second messenger systems in these neurons have been implicated in the enhancement of visceral nociception (12,26, 42,67,73). Animal models of colitis have implicated the potential role of NMDA receptors in altered enteric nervous system function on visceral nociception (72,115-117). In the spinal cord, NMDA receptors have been found to play a pivotal role in the development and maintenance of allodynia and hyperalgesia in both visceral and somatic tissues (54,58,61,84,94,109). NMDA receptors integrate the activity of groups of neurons and provide a mechanism to amplify nociceptive signals. This process leads to central sensitization, which is characterized by enlarged neuronal receptive fields, allodynia, and hyperalgesia (4,28,66,76,84,89, 101,102,109,110). Recent studies have demonstrated that animal models of colitis modulate the functional properties of NMDA receptors in the spinal cord and dorsal root ganglia neurons (16,62,101). Understanding the implications of these studies requires examining the concept of the role of NMDA receptors in colitis related visceral pain and central sensitization.
NMDA Receptors
The properties of NMDA receptors contribute to neuronal plasticity (27,112) because NMDA receptors are ionotropic glutamate receptors that require glycine as a coagonist, display voltage-dependent inhibition by extracellular Mg2+, and have high permeability to Ca2+. NMDA receptors are composed of at least two subfamilies: NR1 and NR2 (56,75,76). A third subfamily of the NMDA receptor, NR3, has also been described, but it is not required for a functional receptor and its role is currently unclear (22,97). The NR1 forms eight functional splice variants based on the presence or absence of three alternatively spliced exons: exon 5 (N1), exon 21 (C1), and exon 22 (C2) (29,41,78,96,121). The presence of N1 enhances the current flow through the NMDA receptors and prevents glycine-independent stimulation of the receptors by spermine (52,114). The C1 cassette contains four serines that are known phosphorylation sites and an ER retention signal. Phosphorylation of the serines blocks the ER retention signal and allows transport of the receptors to the plasma membrane (9,91,111). The presence of the C2 cassette alters the C-terminus of the protein and changes the targeting of the protein for different cell structures (30,121). Thus, the various splice variants of NR1 have distinct properties that significantly influence the function of the fully formed receptor. There are four NR2 subunits designated NR2A, 2B, 2C, and 2D. The NR2A or NR2B subunits generate high-conductance channel openings with high sensitivity to voltage-dependent inhibition by Mg2+; however, NR2C or NR2D receptors generate low-conductance channel. Recombinations of these receptors, NR1 and NR2, have shown that single channel conductivity, glutamate, and glycine affinity are influenced by the composition of the NR2 subunits (21,74).
Spinal NMDA Receptors and Colonic Inflammation
Patients with colonic inflammation often have visceral hypersensitivity and visceral pain. This visceral pain is evoked by inflammatory mediators, such as bradykinin, histamine, serotonin, catecholamines, prostanoids, hydrogen and potassium ions, adenosine, substance P, cytokines, and other neuropeptides. These mediators may potentially sensitize nociceptors and affect nociceptive processing pathways and lead to chronic abdominal pain (Fig. 1). Alterations in ascending pathways to the spinal cord may also affect motor function of the enteric nervous system, leading to diarrhea or accelerated gastrointestinal transit time (Fig. 1).
Figure 1.
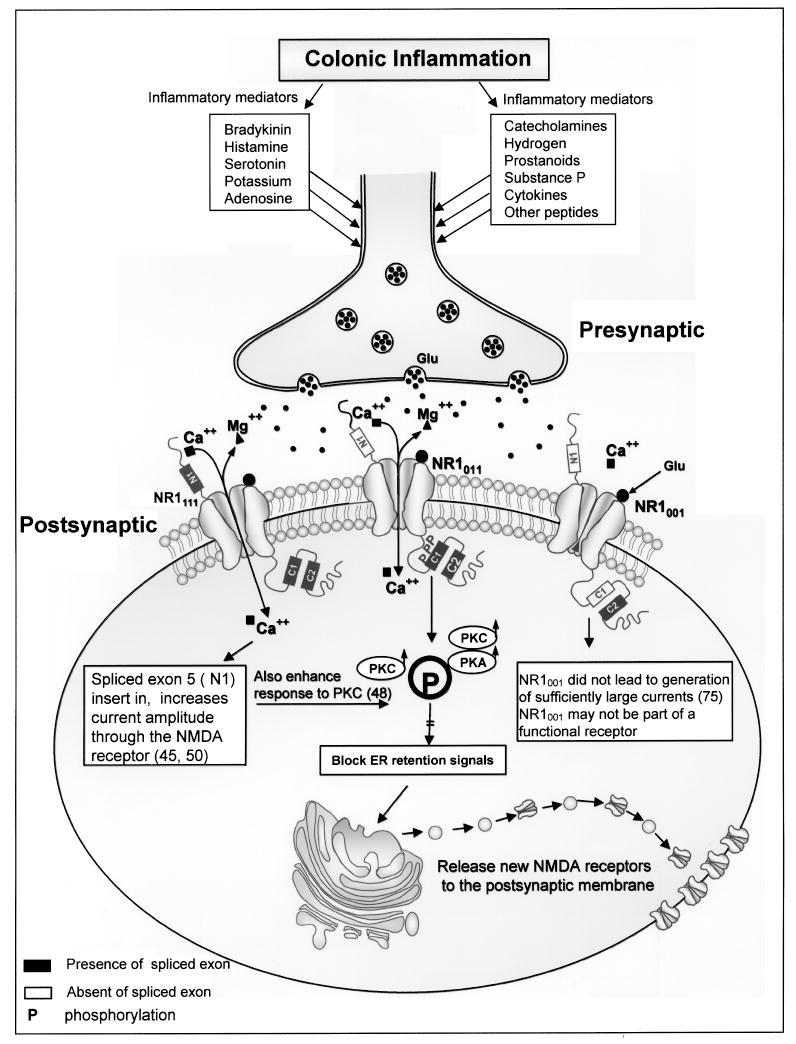
The figure depicts inflammatory mediators in colonic inflammation that lead to visceral pain. These mediators sensitize nociceptors, activate NMDA receptors, and affect nociceptive processing pathways that lead to visceral hypersensitivity and abdominal pain.
It has been shown in experimental studies that chemical stimuli/irritation of the viscera leads to an alteration of normal reflexes (7,60,80,90) and an increased sensitization of the afferent innervations of these visceral structures (17,39,93). This leads to excitation of spinal neurons accompanied by a change in their receptive field properties (33,34). It has also been established that NMDA receptors contribute to colonic inflammation-evoked hyperalgesia and dorsal horn neuron hyperexcitability (2,16,54,55,100).
Ide and colleagues reported that colonic administration of turpentine in rats induced a significantly decreased visceromotor response (VMR) threshold for colorectal distension (CRD). However, intrathecal (T12-L1) NMDA antagonists (AP5) have been shown to block this decreased threshold produced by colonic inflammation (44). Rice and McMahon also reported that inflammation induced visceral pain by turpentine was prevented by preemptive intrathecal administration of AP5 (90). Kolhekar and Gebhart have reported that spinal NMDA receptors produced a dose-dependent facilitation effect of visceromotor response to noxious intensities of stimulation and that these effects were partially blocked by the NMDA receptor antagonist D-2-amino-5-phosphonvaleric acid (APV) (54,55). Activation of the NMDA receptor can also facilitate responses of spinal neurons receiving noxious visceral input and NMDA receptor activation leading to enlargement of convergent cutaneous receptive field size of viscerosomatic neurons (55).
Another study demonstrated that intracolonic zymosan produces an enhancement of the behavioral response to noxious and nonnoxious CRD. This enhancement is initiated by a local inflammatory process and can be attenuated in a dose-dependent and reversible manner by the noncompetitive NMDA receptor channel blocker MK-801 intrathecally (16). Traub and his colleagues demonstrated that repetitive noxious (80 mmHg) CRD induced Fos expression in the lumbosacral spinal cord and Fos expression was partially attenuated by a intrathecally administered NMDA receptor antagonist (100). Other studies have shown that intracolonic instillation of zymosan results in a significant enhancement of responses to colorectal distension stimuli and can be attenuated significantly by intrathecal administration of antagonists of NMDA receptor (18).
Taken together, theses results suggested an important role of spinal NMDA receptors in colonic inflammation and visceral pain: i) administration of NMDA receptor agonist has been shown to expand the convergent cutaneous receptive fields of viscerosomatic convergent neurons (55); ii) it is widely accepted that NMDA receptors contribution to colonic inflammation-evoked hyperalgesia and dorsal horn neuron hyperexcitability (16,44,55); iii) attenuation of the response of acutely sensitized spinal neurons was evident after administration of NMDA receptor antagonists (83).
The spinal sensitization may give insight to the pathophysiology of overlapping chronic abdominal pain. These data suggest that spinal NMDA receptors are involved directly in local mechanisms that lead to enhanced spinal transfer of visceral nociceptive information and thus may participate in mechanisms leading to central hyperexcitability and visceral hyperalgesia. These results suggest that experimental inflammatory sensitization of viscera involve NMDA mechanisms. These data suggest that analgesics act through NMDA receptors may be an appropriate agent as visceral pain medication.
Colitis-Induced Peripheral NMDA Receptor Expression
Several studies have shown the presence of NMDA receptors in the enteric nervous system (8,49,53,72, 77). They also found increased NMDA receptor expression following colonic inflammation. A study by our group examined expression of the NR1 splice variants in the colon of rats following trinitrobenzene sulfonic acid (TNBS)-induced colitis. The data demonstrated that NMDA receptors NR1-C1 and NR1-N1 subunits appeared at 14–28 days following TNBS-induced colitis using RT-PCR technique. We also found that protein expression of NR1001, NR1011, and NR1111 appeared in TNBS-treated rats with active colitis. Untreated control rats only expressed NR1001. In addition, NR1-N1 and NR1-C1 protein expression was present in the colonic myenteric plexus in TNBS-treated rats (115).
The NR1 subunit forms eight functional splice variants based on the presence or absence of three alternatively spliced exons: exon 5 (N1), exon 21 (C1), and exon 22 (C2). The NR1001 has exon 5 and exon 21 spliced out, while exon 22 is spliced in; NR1011 has exon 5 (N-terminal) spliced out, while exon 21 and exon 22 (C-terminal) are spliced in; NR1111 has all three exons (41,56). The functional properties of NMDA receptors depend on the NR1 splice variant combination. NR1 receptors, lacking the N-terminal exon, exhibited a high affinity for NMDA and marked potentiation by spermine (30). Presence of the N1 insert reduced the apparent affinity of homomeric NR1 receptors for NMDA and almost abolished potentiation by spermine at saturating glycine (30), while splicing in the N1 insert increased current amplitude (41,114). The NR1001 did not lead to generation of sufficiently large currents for analysis, even if the amount of RNA injected was increased from 10 to 50 ng per oocyte (30). Therefore, in our study we found NR1001 in normal colon and explained the NR1001 may not be part of a functional receptor. In this study NR1011 appeared only in rats following colonic inflammation. This has very important implications for visceral pain in that NR1011 may play an important role in the plasticity that occurs following transient inflammation. NR1111, which has the N1 insert, is also present in inflamed colon. Splicing in the N1 insert increased current amplitude (41,114). Therefore, NR1111 may increase the NMDA receptor activity in inflamed colon. Immunocytochemistry analysis revealed that the NR1-N1 was also expressed and presented on dendrites and cell membranes in the myenteric plexus following TNBS treatment (115). Expression of the N1 is associated with large current amplitudes and an enhanced responsiveness to PKC phosphorylation (121). More importantly, NR1011 and NR1111 could modulate increased visceral hypersensitivity and alter colonic motility present in patients following transient inflammatory injury to the colon (115) (Fig. 1).
McRoberts and his collogues reported that dorsal root ganglia (DRG) neurons innervating the rat colon express NMDA both on their cell bodies and their peripheral terminal innervating the gut wall (72). Moreover, they also found that retrograde labeled DRG neurons gave a greater Ca2+ response than randomly selected neurons (72). These data suggested that neurons innervating visceral tissues may express greater level of NMDA receptors on their cell surface or different NR2 subunits with higher permeability to Ca2+ (37).
As is known, CGRP is an important neuropeptide involved in the inflammatory process and particularly distributed in sensory neurons. Generally, CGRP is colocalized with SP in the primary afferent nociceptors (65). Studies have shown that the majority of the primary afferent fibers innervating visceral tissues include CGRP and SP (95). These neuropeptides play an important role in neurogenic inflammation and hyperalgesia (38,43,98,106). Interestedly, NMDA receptors are also located on peripheral and central terminals of primary afferent neurons and are known to play a key role in regulating the release of the CGRP and SP in these terminals (20,63). Therefore, NMDA receptor activation enhances the course of inflammation and leads to the development and maintenance of central sensitization.
A study has been done to measure NMDA-mediated CGRP release from spinal cord receiving afferent input from the colon. This study found that NMDA-induced CGRP release from the spinal cord slices receiving input from DRG neurons innervating the colon was significantly increased in colitis (62). Consistent with this, research data also demonstrated that the activity of NMDA receptors increased threefold in DRG neurons that had innervated the inflamed colon and in neurons within the same ganglia. An upregulation of NR2B subunit expression and persistent tyrosine phosphorylation of NR2B was the most likely mechanism underlying the increase in NMDA current identified by using the electrophysiological and molecular techniques (62). Furthermore, they found a significantly increased expression of NR2B in lumbosacral DRGs following TNBS induced colitis (62). Another study showed that the colonic NR2B was downregulated during the TNBS colitis at 7, 14, and 21 days, but it returned to control levels at 28 days after TNBS injection. However, NR2A expression appeared during TNBS colitis at 7, 14, and 21 after TNBS injection compared to controls, ant it was no longer present at 28 days after TNBS injection (117). These selective changes in the expression of the NR2 of the NMDA receptor occur during colonic inflammation and suggest a role for NMDA NR2 receptors in the development of neuronal plasticity and resulting visceral hyperalgesia.
Colitis-Evoked Phosphorylation of NMDA Receptors
Protein phosphorylation is a major mechanism for the regulation of NMDA receptor function (40,88). Recently, data have supported that phosphorylation of multiple sites in the cytoplasmic C terminal of the NR1 and NR2 subunits is known to modulate NMDA receptor activity and affect synaptic transmission (99,120). The NR1 subunit is phosphorylated by protein kinase A (PKA) and by protein kinase C (PKC) on serine in the NR1-C1 cassette (78,82). Phosphorylation of NR1 was demonstrated in the spinal cord following inflammation or tissue injury (84,88). Phosphorylation of the subunit was attenuated by NMDA receptor antagonists (6). These data suggest that the NMDA receptor is involved in a feed-forward mechanism for its own phosphorylation. Recent work has shown the presence of NMDA receptors in the enteric nervous system (49,53, 77). The NMDA NR1 subunits also demonstrated changes in subunit expression following colonic inflammation in which NR1-C1 is present in the myenteric plexus (115) but not in noninflamed control.
Our group has shown that TNBS-induced colitis induces serine phosphorylation of the NMDA receptor NR1 subunit in the rat colonic myenteric plexus (116). This study is consistent with our previous finding that the NMDA NR1-C1 subunit was present in the colonic myenteric plexus 14 days following TNBS treatment (115), but was not present in normal control rats. This finding parallels many other studies in the spinal cord. A study demonstrated that capsaicin administrated into the hind paw of rats resulted in an ipsilateral accumulation of phosphorylated NR1 subunits in spinothalamic tract neurons (120). Similarly, Caudle and his colleagues also demonstrated that spinal cord NR1 subunits were phosphorylated on serine residues following hind paw inflammation with carrageenan (10).
Phosphorylation of serine blocks the ER retention signal and promotes plasma membrane delivery of NMDA receptors (9,91,111). The serine phosphorylation occurs in the ER and suppresses the NMDA receptor ER retention signal that can initiate NMDA receptor trafficking (70,91,92,111) to the postsynaptic membrane opposite glutamanergic terminal (32) and present at nascent synapses initial contact by an active presynaptic terminal (82). The TNBS-induced colitis induces NR1 phosphorylation that leads to the insertion of more active receptors in the NMDA membrane; the serine phosphorylation may also make the NMDA receptor channel more conductive, leading to visceral pain and/or irregular bowel movements.
Evidence of Central Sensitization in Colitis
Hyperalgesia/hypersensitivity may be initiated and maintained in both the central and peripheral nervous system. Our early studies showed that hypersensitivity in IBS patients is not only limited to the colon but also wide-spreading alterations in central pain processing related to somatic hypersensitivity (103,104). Patients with visceral pain often exhibit a wide variety of extra-intestinal symptoms, including back pain, migraine headaches, and muscle pain. These symptoms may be consistent with central sensitization: referral of visceral pain to somatic tissues outside the area of immediate referral (13,14,35,59,81) or neural cross-talk in which afferent activation of one visceral structure influences efferent output in other structures and organs and is mediated by convergence of sensory pathways in the spinal cord (23-25,48,85).
Animal models have been developed to evaluate referred hypersensitivity following a nociceptive visceral stimulus. Uterine inflammation in rats has been shown to increase sensitivity to stimulation of flank muscles (107). Others have used bladder inflammation or urethral calculus to evaluate hypersensitivity of the paws and tail (36,45,71). Our study used an animal model of visceral pain to evaluate if TNBS-induced colitis leads to visceral and somatic hypersensitivity (118). We monitored the behavioral development in rats at multiple time points following the inflammatory colonic stimulus. We tested visceral and somatic hypersensitivity at 2, 7, 14, 21, and 28 days following TNBS administration in rats. The results of this study suggest that TNBS colitis in rats produces both visceral and somatic hypersensitivity. From a mechanistic perspective it is interesting that hypersensitivities to different stimuli developed at different time points. Thus, visceral hypersensitivity developed at 2 days, mechanical and thermal hypersensitivity to radiant heat developed at 14 days, and hypersensitivity on the tail reflex test developed at 21 days (118). Interestingly, these time points of development are the same as the development of increased expression of different types of NMDA receptor subunits between 14 to 28 days following TNBS administration (115). In contrast to visceral hypersensitivity that is based on immediately inflamed tissues, delays in somatic hypersensitivity may be due to gradual spatial spread of spinal cord neuron sensitization and/or delayed upregulation of different types of glutamate receptors, including NMDA and non-NMDA receptors. The somatic hypersensitivity present is likely to be a result of central sensitization of spinal dorsal horn neurons that receive viscerosomatic convergence ((23-25,48,85,118). An additional factor could be related to neural cross-talk in the pelvis (85). The persistent colitis may result in the peripheral and/or central release of some excitatory mediator that produces and maintains central sensitization, eventually leading to somatic hypersensitivity. There have been several previous studies that have used other visceral cavities (i.e., bladder, uterus) other than the colon to evaluate somatic hypersensitivity (5,31,45-47,51,57,59,71).
In fact recent investigations suggest that in both animal models and patients with chronic visceral pain there is evidence of referred somatic hypersensitivity (2,79,103,105). These findings are different from earlier studies that have revealed somatic hyposensitivity in patients with chronic visceral pain disorders such as inflammatory bowel disease and IBS (1,14,15,35,108, 119). One very plausible explanation for these different findings in humans may be due to the specific type of nociceptive stimuli used. It is possible that previous studies failed to reveal somatic hypersensitivity in outside referral areas because they did not use nociceptive stimuli that were intense or long enough to stimulate NMDA receptor mechanisms associated with sensitization and somatic hypersensitivity.
Summary
NMDA receptors play an important role in chronic visceral pain and hypersensitivity that is present in the setting of colonic inflammation. NMDA receptors are found in the peripheral nervous system as well as the central terminal of primary afferent neurons and have been shown to play an important role in regulating the release of nociceptive neurotransmitters. Recent work has demonstrated the presence of NMDA receptors in the enteric nervous system. Alterations in the NMDA receptor as a result of colonic inflammation may be an underlying mechanism of visceral hypersensitivity and visceral pain in the setting of inflammation. These chronic changes in the NMDA receptor in the peripheral and central nervous system may be sites for the development of new pharmacologic agents to treat chronic abdominal pain.
Acknowledgments
G. N. Verne is supported by a Merit Review Award (PI: G. N. Verne) from the Medical Research Service of the Department of Veteran Affairs and a NIH Grant 1-R01-NS053090-01 (PI: G. N. Verne).
References
- 1.Accarino AM, Azpiroz F, Malagelada JR. Selective dysfunction of mechanosensitive intestinal afferents in irritable bowel syndrome. Gastroenterology. 1995;108:636–643. doi: 10.1016/0016-5085(95)90434-4. [DOI] [PubMed] [Google Scholar]
- 2.Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- 3.Al-Chaer ED, Lawand NB, Westlund KN, Willis WD. Pelvic visceral input into the nucleus gracilis is largely mediated by the postsynaptic dorsal column pathway. J Neurophysiol. 1996;76(4):2675–2690. doi: 10.1152/jn.1996.76.4.2675. [DOI] [PubMed] [Google Scholar]
- 4.Baranauskas G, Nistri A. Sensitization of pain pathways in the spinal cord: Cellular mechanisms. Prog Neurobiol. 1998;54:349–365. doi: 10.1016/s0301-0082(97)00067-1. [DOI] [PubMed] [Google Scholar]
- 5.Bon K, Lichtensteiger CA, Wilson SG, Mogil JS. Characterization of cyclophosphamide cystitis, a model of visceral and referred pain, in the mouse: Species and strain differences. J Urol. 2003;170:1008–1012. doi: 10.1097/01.ju.0000079766.49550.94. [DOI] [PubMed] [Google Scholar]
- 6.Brenner GJ, Ji RR, Shaffer S, Woolf CJ. Peripheral noxious stimulation induces phosphorylation of the NMDA receptor NR1 subunit at the PKC-dependent site, serine-896, in spinal cord dorsal horn neurons. Eur J Neurosci. 2004;20:375–384. doi: 10.1111/j.1460-9568.2004.03506.x. [DOI] [PubMed] [Google Scholar]
- 7.Bunton MB, Gebhart GF. Effects of intracolonic acetic acid on responses to colorectal distension in the rat. Brain Res. 1995;672:77–82. doi: 10.1016/0006-8993(94)01382-r. [DOI] [PubMed] [Google Scholar]
- 8.Burns GA, Ulibarri C, Stephens KE. Transiently catecholaminergic cells in the fetal rat express mRNA for the glutamate NMDAR1 receptor. Brain Res. 1996;718:117–123. doi: 10.1016/0006-8993(96)00082-0. [DOI] [PubMed] [Google Scholar]
- 9.Carroll RC, Zukin RS. NMDA-receptor trafficking and targeting: Implications for synaptic transmission and plasticity. Trends Neurosci. 2002;25:571–577. doi: 10.1016/s0166-2236(02)02272-5. [DOI] [PubMed] [Google Scholar]
- 10.Caudle RM, Perez FM, Valle-Pinero AY, Iadarola MJ. Spinal cord NR1 serine phosphorylation and NR2B subunit suppression following peripheral inflammation. Mol Pain. 2005;1:25. doi: 10.1186/1744-8069-1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cervero F. Visceral nociception: Peripheral and central aspects of visceral nociceptive systems. Phil Trans R Soc Lond B Biol Sci. 1985;308:325–337. doi: 10.1098/rstb.1985.0033. [DOI] [PubMed] [Google Scholar]
- 12.Cervero F, Laird JM. Understanding the signaling and transmission of visceral nociceptive events. J Neurobiol. 2004;61:45–54. doi: 10.1002/neu.20084. [DOI] [PubMed] [Google Scholar]
- 13.Cervero F, Laird JM. Visceral pain. Lancet. 1999;353:2145–2148. doi: 10.1016/S0140-6736(99)01306-9. [DOI] [PubMed] [Google Scholar]
- 14.Chang L, Munakata J, Mayer EA, Schmulson MJ, Johnson TD, Bernstein CN, Saba L, Naliboff B, Anton PA, Matin K. Perceptual responses in patients with inflammatory and functional bowel disease. Gut. 2000;47:497–505. doi: 10.1136/gut.47.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang L, Mayer EA, Johnson T, FitzGerald LZ, Naliboff B. Differences in somatic perception in female patients with irritable bowel syndrome with and without fibromyalgia. Pain. 2000;84:297–307. doi: 10.1016/s0304-3959(99)00215-8. [DOI] [PubMed] [Google Scholar]
- 16.Coutinho SV, Meller ST, Gebhart GF. Intracolonid zymosan produces visceral hyeralgesia in the rat that is mediated by spinal NMDA and non-NMDA receptors. Brain Res. 1996;736(1–2):7–15. doi: 10.1016/0006-8993(96)00661-0. [DOI] [PubMed] [Google Scholar]
- 17.Coutinho SV, Su X, Sengupta JN, Gebhart GF. Role of sensitized pelvic nerve afferents from the inflamed rat colon in the maintenance of visceral hyperalgesia. Prog Brain Res. 2000;129:375–387. doi: 10.1016/S0079-6123(00)29029-8. [DOI] [PubMed] [Google Scholar]
- 18.Coutinho SV, Urban MO, Gebhart GF. The role of NMDA receptor and nitric oxide in visceral hyperalgesia. Eur J Pharmacol. 2001;429:319–325. doi: 10.1016/s0014-2999(01)01331-0. [DOI] [PubMed] [Google Scholar]
- 19.Cross SA. Pathophysiology of pain. Mayo Clin Proc. 1994;69:375–383. doi: 10.1016/s0025-6196(12)62225-3. [DOI] [PubMed] [Google Scholar]
- 20.Cuesta MC, Arcaya JL, Cano G, Sanchez L, Maixner W, Suarez-Roca H. Opposite modulation of capsaicin-evoked substance P release by glutamate receptors. Neurochem Int. 1999;35:471–478. doi: 10.1016/s0197-0186(99)00081-9. [DOI] [PubMed] [Google Scholar]
- 21.Cull-Candy S, Brickley A, Farrant M. NMDA receptor subunits: Diversity development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- 22.Das S, Sasaki YF, Rothe T, Premkumar LS, Takasu M, Crandall JE, Dikkes P, Conner DA, Rayudu PV, Cheung W, Chen HS, Lipton SA, Nakanishi N. Increased NMDA current and spine density in mice lacking the NMDA receptor subunit NR3A. Nature. 1998;393:377–381. doi: 10.1038/30748. [DOI] [PubMed] [Google Scholar]
- 23.de Groat WC, Nadelhaft I, Milne RJ, Booth AM, Morgan C, Thor K. Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. J Auton Nerv Syst. 1981;3:135–160. doi: 10.1016/0165-1838(81)90059-x. [DOI] [PubMed] [Google Scholar]
- 24.de Groat WC, Booth AM, Yoshimura N. Neurophysiology of micturition and its modification in animal models of human disease. In: Maggi CA, editor. The autonomic nervous system. London: Harwood; 1993. pp. 227–290. [Google Scholar]
- 25.de Groat WC, Roppolo JR, Yoshimura N, Sugaya K. Neural control of the urinary bladder and colon. In: Tache Y, Wingate D, Burks T, editors. Proceedings of the second international symposium on brain-gut interactions. Boca Raton, FL: CRC Press; 1993. pp. 167–190. [Google Scholar]
- 26.Delvaux M. Role of visceral sensitivity in the pathophysiology of irritable bowel syndrome. Gut. 2002;51(Suppl. 1):i67–i71. doi: 10.1136/gut.51.suppl_1.i67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–62. [PubMed] [Google Scholar]
- 28.Dubner R, Ruda MA. Activity-dependent neuronal plasticity following tissue injury and inflammation. Trends Neurosci. 1992;15:96–103. doi: 10.1016/0166-2236(92)90019-5. [DOI] [PubMed] [Google Scholar]
- 29.Durand GM, Gregor P, Zheng X, Bennett MV, Uhl GR, Zukin RS. Cloning of an apparent splice variant of the rat N-methyl-D-aspartate receptor NMDAR1 with altered sensitivity to polyamines and activators of protein kinase C. Proc Natl Acad Sci USA. 1992;89:9359–9363. doi: 10.1073/pnas.89.19.9359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durand GM, Bennett MV, Zukin RS. Splice variants of the N-methyl-D-aspartate receptor NR1 identify domains involved in regulation by polyamines and protein kinase C. Proc Natl Acad Sci USA. 1993;90:6731–6735. doi: 10.1073/pnas.90.14.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farquhar-Smith WP, Rice AS. Administration of endocannabinoids prevents a referred hyperalgesia associated with inflammation of the urinary bladder. Anesthesiology. 2001;94:507–513. doi: 10.1097/00000542-200103000-00023. [DOI] [PubMed] [Google Scholar]
- 32.Friedman HV, Bresler T, Garner CC, Ziv NE. Assembly of new individual excitatory synapses: Time course and temporal order of synaptic molecule recruitment. Neuron. 2000;27:57–69. doi: 10.1016/s0896-6273(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 33.Gebhart GF, Nell TJ. Central mechanisms of visceral pain. Can J Physiol Pharmacol. 1991;69:627–634. doi: 10.1139/y91-093. [DOI] [PubMed] [Google Scholar]
- 34.Gebhart GF. Visceral pain mechanisms. In: Chapman CR, Foley KM, editors. Current and emerging issues in cancer pain: Research and practice. New York: Raven Press; 1993. pp. 99–111. [Google Scholar]
- 35.Giamberardino MA. Recent and forgotten aspects of visceral pain. Eur J Pain. 1999;3:77–92. doi: 10.1053/eujp.1999.0117. [DOI] [PubMed] [Google Scholar]
- 36.Giamberardino MA, Valente R, de Bigontina P, Vecchiet L. Artificial ureteral calculosis in rats: Behavioural characterization of visceral pain episodes and their relationship with referred lumbar muscle hyperalgesia. Pain. 1995;61:459–469. doi: 10.1016/0304-3959(94)00208-V. [DOI] [PubMed] [Google Scholar]
- 37.Grant ER, Bacskai BJ, Pleasure DE, Pritchett DB, Gallagher MJ, Kendrick SJ, Kricka LJ, Lynch DR. N-methyl-D-aspartate receptors expressed in a nonneuronal cell line mediate subunit-specific increases in free intracellular calcium. J Biol Chem. 1997;272:647–656. doi: 10.1074/jbc.272.1.647. [DOI] [PubMed] [Google Scholar]
- 38.Gschossmann JM, Coutinho SV, Miller JC, Huebel K, Naliboff B, Wong HC, Walsh JH, Mayer EA. Involvement of spinal calcitonin gene-related peptide in the development of acute visceral hyperalgesia in the rat. Neurogastroenterol Motil. 2001;13:299–236. doi: 10.1046/j.1365-2982.2001.00262.x. [DOI] [PubMed] [Google Scholar]
- 39.Habler H-J, Janig W, Koltzenburg M. Receptive properties of myelinated primary afferents innervating the inflamed urinary bladder of the cat. J Neurophysiol. 1993;69:395–405. doi: 10.1152/jn.1993.69.2.395. [DOI] [PubMed] [Google Scholar]
- 40.Hatt H. Modification of glutamate receptor channels: Molecular mechanisms and functional consequences. Naturwissenschaften. 1999;86:177–186. doi: 10.1007/s001140050593. [DOI] [PubMed] [Google Scholar]
- 41.Hollmann M, Boulter J, Maron C, Beasley L, Sullivan J, Pecht G, Heinemann S. Zinc potentiates agonist-induced currents at certain splice variants of the NMDA receptor. Neuron. 1993;10:943–954. doi: 10.1016/0896-6273(93)90209-a. [DOI] [PubMed] [Google Scholar]
- 42.Holzer P, Michl T, Danzer M, Jocic M, Schicho R, Lippe IT. Surveillance of the gastrointestinal mucosa by sensory neurons. J Physiol Pharmacol. 2001;52:505–521. [PubMed] [Google Scholar]
- 43.Holzer P, Lippe IT. Role of calcitonin gene-related peptide in gastrointestinal blood flow. Ann NY Acad Sci. 1992;657:228–239. doi: 10.1111/j.1749-6632.1992.tb22771.x. [DOI] [PubMed] [Google Scholar]
- 44.Ide Y, Maehara Y, Tsukahara S, Kitahata LM, Collins JG. The effects of an intrathecal NMDA antagonist (AP5) on the behavioral changes induced by colorectal inflammation with turpentine in rats. Life Sci. 1997;60(16):1359–1363. doi: 10.1016/s0024-3205(97)00081-7. [DOI] [PubMed] [Google Scholar]
- 45.Jaggar SI, Scott HC, Rice AS. Inflammation of the rat urinary bladder is associated with a referred thermal hyperalgesia which is nerve growth factor dependent. Br J Anaesth. 1999;83:442–448. doi: 10.1093/bja/83.3.442. [DOI] [PubMed] [Google Scholar]
- 46.Jaggar SI, Hasnie FS, Sellaturay S, Rice AS. The anti- hyperalgesic actions of the cannabinoid anandamide and the putative CB2 receptor agonist palmitoylethanolamide in visceral and somatic inflammatory pain. Pain. 1998;76:189–199. doi: 10.1016/s0304-3959(98)00041-4. [DOI] [PubMed] [Google Scholar]
- 47.Jaggar SI, Scott HC, James IF, Rice AS. The capsaicin analogue SDZ249-665 attenuates the hyper-reflexia and referred hyperalgesia associated with inflammation of the rat urinary bladder. Pain. 2001;89:229–235. doi: 10.1016/s0304-3959(00)00366-3. [DOI] [PubMed] [Google Scholar]
- 48.Janig W, Koltzenburg M. On the function of spinal primary afferent fibres supplying colon and urinary bladder. J Auton Nerv Syst. 1990;30(Suppl):S89–S96. doi: 10.1016/0165-1838(90)90108-u. [DOI] [PubMed] [Google Scholar]
- 49.Jankovic SM, Milovanovic D, Matovic M, Iric-Cupic V. The effects of excitatory amino acids on isolated gut segments of the rat. Pharmacol Res. 1999;39:143–148. doi: 10.1006/phrs.1998.0422. [DOI] [PubMed] [Google Scholar]
- 50.Ji Y, Traub RJ. Spinal NMDA receptors contribute to neuronal processing of acute noxious and nonnoxious colorectal stimulation in the rat. J Neurophysiol. 2001;86(4):1783–1791. doi: 10.1152/jn.2001.86.4.1783. [DOI] [PubMed] [Google Scholar]
- 51.Kalmari J, Pertovaara A. Colorectal distension-induced suppression of a nociceptive somatic reflex response in the rat: Modulation by tissue injury or inflammation. Brain Res. 2004;1018:106–110. doi: 10.1016/j.brainres.2004.05.064. [DOI] [PubMed] [Google Scholar]
- 52.Kashiwagi K, Pahk AJ, Masuko T, Igarashi K, Williams K. Block and modulation of N-methyl-D-aspartate receptors by polyamines and protons: Role of amino acid residues in the transmembrane and pore-forming regions of NR1 and NR2 subunits. Mol Pharmacol. 1997;52:701–713. doi: 10.1124/mol.52.4.701. [DOI] [PubMed] [Google Scholar]
- 53.Kirchgessner AL. Glutamate in the enteric nervous system. Curr Opin Pharmacol. 2001;1:591–596. doi: 10.1016/s1471-4892(01)00101-1. [DOI] [PubMed] [Google Scholar]
- 54.Kolhekar R, Gebhart GF. NMDA and quisqualate modulation of visceral nociception in the rat. Brain Res. 1994;651:215–226. doi: 10.1016/0006-8993(94)90700-5. [DOI] [PubMed] [Google Scholar]
- 55.Kolhekar R, Gebhar GF. Modulation of spinal visceral nociceptive transmission by NMDA receptor activation in the rat. J Neurophysiol. 1996;75:2344–2353. doi: 10.1152/jn.1996.75.6.2344. [DOI] [PubMed] [Google Scholar]
- 56.Kutsuwada T, Kashiwabuchi N, Mori H, Sakimura K, Kushiya E, Araki K, Meguro H, Masaki H, Kumanishi T, Arakawa M. Molecular diversity of the NMDA receptor channel. Nature. 1992;358:36–41. doi: 10.1038/358036a0. [DOI] [PubMed] [Google Scholar]
- 57.La JH, Kim TW, Sung TS, Kang JW, Kim HJ, Yang IS. Visceral hypersensitivity and altered colonic motility after subsidence of inflammation in a rat model of colitis. World J Gastroenterol. 2003;9:2791–2795. doi: 10.3748/wjg.v9.i12.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Laird JM, de la Rubia PP, Cervero F. Excitability changes of somatic and viscero-somatic nociceptive reflexes in the decerebrate-spinal rabbit: Role of NMDA receptors. J Physiol. 1995;489(Pt. 2):545–555. doi: 10.1113/jphysiol.1995.sp021071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laird JM, Martinez-Caro L, Garcia-Nicas E, Cervero F. A new model of visceral pain and referred hyperalgesia in the mouse. Pain. 2001;92:335–342. doi: 10.1016/S0304-3959(01)00275-5. [DOI] [PubMed] [Google Scholar]
- 60.Langlois A, Diop P, Riviere PJM, Pascaud X, Junien J-L. Effect of fedotozine on the cardiovascular pain reflex induced by distension of the irritated colon in the anesthetized rat. Eur J Pharmacol. 1994;271:245–251. doi: 10.1016/0014-2999(94)90780-3. [DOI] [PubMed] [Google Scholar]
- 61.Li J, McRoberts JA, Nie J, Ennes HS, Mayer EA. Electrophysiological characterization of N-methyl-D-aspartate receptors in rat dorsal root ganglia neurons. Pain. 2004;109:443–452. doi: 10.1016/j.pain.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 62.Li J, McRoberts JA, Ennes HS, Trevisani M, Nicletti P, Mittal Y, Mayer EA. Experimental colitis modulations the functional properties of NMDA receptors in dorsal root ganglia neurons. Am J Physiol Gastrointest Liver Physiol. 2006;29(2):G219–228. doi: 10.1152/ajpgi.00097.2006. [DOI] [PubMed] [Google Scholar]
- 63.Liu H, Mantyh PW, Basbaum AI. ANDA-receptor regulation of substance P release from primary afferent nociceptors. Nature. 1997;386:721–724. doi: 10.1038/386721a0. [DOI] [PubMed] [Google Scholar]
- 64.Lu H, Mantyh PW, Basbaum AI. NMDA-receptor regulation of substance P release from primary afferent nociceptors. Nature. 1997;386:721–724. doi: 10.1038/386721a0. [DOI] [PubMed] [Google Scholar]
- 65.Lu Y, Westlund KN. Effects of baclofen on colon inflammation-induced Fos, CGRP and SP expression in spinal cord and brainstem. Brain Res. 2001;889:118–130. doi: 10.1016/s0006-8993(00)03124-3. [DOI] [PubMed] [Google Scholar]
- 66.Ma QP, Woolf CJ. Noxious stimuli induce an N-methyl-D-aspartate receptor-dependent hypersensitivity of the flexion withdrawal reflex to touch: Implications for the treatment of mechanical allodynia. Pain. 1995;61:383–390. doi: 10.1016/0304-3959(94)00195-K. [DOI] [PubMed] [Google Scholar]
- 67.Mach T. The brain–gut axis in irritable bowel syndrome—clinical aspects. Med Sci Monit. 2004;10:RA125–RA131. [PubMed] [Google Scholar]
- 68.Mayer EA, Gebhart GF. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology. 1994;107:271–293. doi: 10.1016/0016-5085(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 69.Mayer EA, Lembo T, Chang L. Approaches to the modulation of abdominal pain. Can J Gastroenterol. 1999;13(Suppl. A):65A–70A. [PubMed] [Google Scholar]
- 70.McIlhinney RA, Le Bourdelles B, Molnar E, Tricaud N, Streit P, Whiting PJ. Assembly intracellular targeting and cell surface expression of the human N-methyl-D-aspartate receptor subunits NR1a and NR2A in transfected cells. Neuropharmacology. 1998;37:1355–1367. doi: 10.1016/s0028-3908(98)00121-x. [DOI] [PubMed] [Google Scholar]
- 71.McMahon SB, Abel C. A model for the study of visceral pain states: Chronic inflammation of the chronic decerebrate rat urinary bladder by irritant chemicals. Pain. 1987;28:109–127. doi: 10.1016/0304-3959(87)91065-7. [DOI] [PubMed] [Google Scholar]
- 72.McRoberts JA, Coutinho SV, Marvizon JC, Grady EF, Tognetto M, Sengupta JN, Ennes HS, Chaban VV, Amadesi S, Creminon C, Lanthorn T, Geppetti P, Bunnett NW, Mayer EA. Role of peripheral n-methyl-d-aspartate (NMDA) receptors invisceral nociception in rats. Gastroenterology. 2001;120:1737–1748. doi: 10.1053/gast.2001.24848. [DOI] [PubMed] [Google Scholar]
- 73.Mertz H. Review article: Visceral hypersensitivity. Aliment Pharmacol Ther. 2003;17:623–633. doi: 10.1046/j.1365-2036.2003.01447.x. [DOI] [PubMed] [Google Scholar]
- 74.Michaelis EK. Molecular biology of glutamate recepuors in the central nervous system and their role in excitotoxicity, oxidative stress and aging. Prog Neurobiol. 1998;54:369–415. doi: 10.1016/s0301-0082(97)00055-5. [DOI] [PubMed] [Google Scholar]
- 75.Monaghan DT, Bridges RJ, Cotman CW. The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- 76.Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. Heteromeric NMDA receptors: Molecular and functional distinction of subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- 77.Nagahama M, Ma N, Semba R. L-Aspartate-immunoreactive neurons in the rat enteric nervous system. Cell Tissue Res. 2004;318:483–492. doi: 10.1007/s00441-004-0961-z. [DOI] [PubMed] [Google Scholar]
- 78.Nakanishi N, Axel R, Shneider NA. Alternative splicing generates functionally distinct N-methyl-D-aspartate receptors. Proc Natl Acad Sci USA. 1992;89:8552–8556. doi: 10.1073/pnas.89.18.8552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Naliboff BD, Munakata J, Fullerton S, Gracely RH, Kodner A, Harraf F, Mayer EA. Evidence for two distinct perceptual alterations in irritable bowel syndrome. Gut. 1997;41:505–512. doi: 10.1136/gut.41.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ness TJ, Randuch A, Gebhart GF. Further behavioral evidence that colorectal distension is a “noxious” visceral stimulus in rats. Neurosci Lett. 1991;131:113–116. doi: 10.1016/0304-3940(91)90349-x. [DOI] [PubMed] [Google Scholar]
- 81.Ness TJ, Gebhart GF. Visceral pain: A review of experimental studies. Pain. 1990;1:167–234. doi: 10.1016/0304-3959(90)90021-5. [DOI] [PubMed] [Google Scholar]
- 82.O’Brien RJ, Lau LF, Huganir RL. Molecular mechanisms of glutamate receptor clustering at excitatory synapses. Curr Opin Neurobiol. 1998;8:364–369. doi: 10.1016/s0959-4388(98)80062-7. [DOI] [PubMed] [Google Scholar]
- 83.Peles S, Miranda A, Shaker R, Sengupta JN. Acute nociceptive somatic stimulus sensitizes neurons in the spinal cord to colonic distension in the rat. J Physiol. 2004;560(1):291–302. doi: 10.1113/jphysiol.2004.069070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Petrenko AB, Yamakura T, Baba H, Shimoji K. The role of N-methyl-D-aspartate (NMDA) receptors in pain: A review. Anesth Analg. 2003;97:1108–1116. doi: 10.1213/01.ANE.0000081061.12235.55. [DOI] [PubMed] [Google Scholar]
- 85.Pezzone MA, Liang R, Fraser MO. A model of neural cross-talk and irritation in the pelvis: Implications for the overlap of chronic pelvic pain disorders. Gastroenterology. 2005;128:1953–1964. doi: 10.1053/j.gastro.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 86.Qin C, Chandler MJ, Foreman RD, Farber JP. Upper thoracic respiratory interneurons integrate noxious somatic and visceral information in rats. J Neurophysiol. 2002;88:2215–2223. doi: 10.1152/jn.00120.2002. [DOI] [PubMed] [Google Scholar]
- 87.Qin C, Malykhina AP, Akbarali HI, Foreman RD. Cross-organ sensitization of lumbosacral spinal neurons receiving urinary bladder input in rats with inflamed colon. Gastroenterology. 2005;129:1967–1978. doi: 10.1053/j.gastro.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 88.Raymond LA, Tingley WG, Blackstone CD, Roche KW, Huganir RL. Glutamate receptor modulation by protein phosphorylation. J Physiol Paris. 1994;88:181–192. doi: 10.1016/0928-4257(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 89.Ren K, Dubner R. Central nervous system plasticity and persistent pain. J Orofac Pain. 1999;13:155–163. [PubMed] [Google Scholar]
- 90.Rice AS, McMahon SB. Pre-emptive intrathecal administration of an NMDA receptor antagonist (AP5) prevents hyper-reflexia in a model of persistent visceral pain. Pain. 1994;57:335–340. doi: 10.1016/0304-3959(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 91.Scott DB, Blanpied TA, Swanson GT, Zhang C, Ehlers MD. An NMDA receptor ER retention signal regulated by phosphorylation and alternative splicing. J Neurosci. 2001;21:3063–3072. doi: 10.1523/JNEUROSCI.21-09-03063.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Scott DB, Blanpied TA, Ehlers MD. Coordinated PKA and PKC phosphorylation suppresses RXR-mediated ER retention and regulates the surface delivery of NMDA receptors. Neuropharmacology. 2003;45:755–767. doi: 10.1016/s0028-3908(03)00250-8. [DOI] [PubMed] [Google Scholar]
- 93.Sengupta JN, Gebhart GF. Mechanosensitive properties of pelvic nerve afferent fibers innervating the urinary bladder of the rat. J Neurophysiol. 1994;72:2420–2430. doi: 10.1152/jn.1994.72.5.2420. [DOI] [PubMed] [Google Scholar]
- 94.Sorkin LS, Wallace MS. Acute pain mechanisms. Surg Clin North Am. 1999;79:213–229. doi: 10.1016/s0039-6109(05)70380-7. [DOI] [PubMed] [Google Scholar]
- 95.Sternini C. Enteric and visceral afferent CGRP neurons. Targets of innervation and differential expression patterns. Ann NY Acad Sci. 1992;657:170–186. doi: 10.1111/j.1749-6632.1992.tb22766.x. [DOI] [PubMed] [Google Scholar]
- 96.Sugihara H, Moriyoshi K, Ishii T, Masu M, Nakanishi S. Structures and properties of seven isoforms of the NMDA receptor generated by alternative splicing. Biochem Biophys Res Commun. 1992;185:826–832. doi: 10.1016/0006-291x(92)91701-q. [DOI] [PubMed] [Google Scholar]
- 97.Sun L, Margolis FL, Shipley MT, Lidow MS. Identification of a long variant of mRNA encoding the NR3 subunit of the NMDA receptor: Its regional distribution and developmental expression in the rat brain. FEBS Lett. 1998;441:392–396. doi: 10.1016/s0014-5793(98)01590-7. [DOI] [PubMed] [Google Scholar]
- 98.Sun RQ, Tu YJ, Lawand NB, Yan JY, Lin Q, Willis WD. Calcitonin gene-related peptide receptor activation produces PKA- and PKC-dependent mechanical hyperalgesia and central sensitization. J Neurophysiol. 2004;92:2859–2899. doi: 10.1152/jn.00339.2004. [DOI] [PubMed] [Google Scholar]
- 99.Tingley WG, Ehlers MD, Kameyama K, Doherty C, Ptak JB, Riley CT, Huganir RL. Characterization of protein kinase A and protein kinase C phosphorylation of the N-methyl-D-aspartate receptor NR1 subunit using phosphorylation site-specific antibodies. J Biol Chem. 1997;272:5157–5166. doi: 10.1074/jbc.272.8.5157. [DOI] [PubMed] [Google Scholar]
- 100.Traub RJ, Pechman P, Iadarola MJ, Gebhart GF. Fos-like proteins in the lumbosacral spinal cord following noxious and non-noxious colorectal distention in the rat. Pain. 1992;94:393–403. doi: 10.1016/0304-3959(92)90247-9. [DOI] [PubMed] [Google Scholar]
- 101.Urban MO, Gebhart GF. Supraspinal contributions to hyperalgesia. Proc Natl Acad Sci USA. 1999;96:7687–7692. doi: 10.1073/pnas.96.14.7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Urban MO, Gebhart GF. Central mechanisms in pain. Med Clin North Am. 1999;83:585–596. doi: 10.1016/s0025-7125(05)70125-5. [DOI] [PubMed] [Google Scholar]
- 103.Verne GN, Robinson ME, Price DD. Hypersensitivity to visceral and cutaneous pain in the irritable bowel syndrome. Pain. 2001;93:7–14. doi: 10.1016/S0304-3959(01)00285-8. [DOI] [PubMed] [Google Scholar]
- 104.Verne GN, Price DD. Irritable bowel syndrome as a common precipitant of central sensitization. Curr Rheumatol Rep. 2002;4(4):322–328. doi: 10.1007/s11926-002-0041-x. [DOI] [PubMed] [Google Scholar]
- 105.Verne GN, Robinson ME, Vase L, Price DD. Reversal of visceral and cutaneous hyperalgesia by local rectal anesthesia in irritable bowel syndrome (IBS) patients. Pain. 2003;105:223–230. doi: 10.1016/s0304-3959(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 106.Weinstock JV. The role of substance P, hemokinin and their receptor in governing mucosal inflammation and granulomatous responses. Front Biosci. 2004;9:1936–1943. doi: 10.2741/1375. [DOI] [PubMed] [Google Scholar]
- 107.Wesselmann U, Czakanski PP, Affaitati G, Giamberardino MA. Uterine inflammation as a noxious visceral stimulus: Behavioral characterization in the rat. Neurosci Lett. 1998;246:73–76. doi: 10.1016/s0304-3940(98)00234-1. [DOI] [PubMed] [Google Scholar]
- 108.Whitehead WE, Holtkotter B, Enck P, Hoelzl R, Holmes KD, Anthony J, Shabsin HS, Schuster MM. Tolerance for rectosigmoid distention in irritable bowel syndrome. Gastroenterology. 1990;98:1187–1192. doi: 10.1016/0016-5085(90)90332-u. [DOI] [PubMed] [Google Scholar]
- 109.Willert RP, Woolf CJ, Hobson AR, Delaney C, Thompson DG, Aziz Q. The development and maintenance of human visceral pain hypersensitivity is dependent on the N-methyl-D-aspartate receptor. Gastroenterology. 2004;126:683–692. doi: 10.1053/j.gastro.2003.11.047. [DOI] [PubMed] [Google Scholar]
- 110.Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation: Implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- 111.Xia H, Hornby ZD, Malenka RC. An ER retention signal explains differences in surface expression of NMDA and AMPA receptor subunits. Neuropharmacology. 2001;41:714–723. doi: 10.1016/s0028-3908(01)00103-4. [DOI] [PubMed] [Google Scholar]
- 112.Yamakura T, Shimoji K. Subunit- and site-specific pharmacology of the NMDA receptor channel. Prog Neurobiol. 1999;59:279–298. doi: 10.1016/s0301-0082(99)00007-6. [DOI] [PubMed] [Google Scholar]
- 113.Zhai Q, Traub RJ. The NMDA receptor antagonist MK-801 attenates c-Fos expression in the lumbosacral spinal cord following repetitive noxious and non-noxious colorectal distention. Pain. 1999;83:321–329. doi: 10.1016/s0304-3959(99)00116-5. [DOI] [PubMed] [Google Scholar]
- 114.Zheng X, Zhang L, Durand GM, Bennett MV, Zukin RS. Mutagenesis rescues spermine and Zn2+ potentiation of recombinant NMDA receptors. Neuron. 1994;12:811–818. doi: 10.1016/0896-6273(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 115.Zhou Q, Caudle RM, Price DD, Del Valle-Pinero AY, Verne GN. Selective up-regulation of NMDA-NR1 receptor expression in myenteric plexus after TNBS induced colitis in rats. Mol Pain. 2006;17:2–3. doi: 10.1186/1744-8069-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhou Q, Caudle RM, Moshiree B, Price DD, Verne GN. Phosphorylation of NMDA NR1 subunits in the myenteric plexus during TNBS induced colitis. Neurosci Lett. 2006;406(3):250–255. doi: 10.1016/j.neulet.2006.07.059. [DOI] [PubMed] [Google Scholar]
- 117.Zhou Q, Caudle R, Price D, Stamm P, Verne GN. 11th World Congress on pain. Seattle, WA: IASP Press; 2005. Selective modulation of NMDA-NR2 receptors subunits after TNBS induced colitis in rats; pp. 1499–P2. [Google Scholar]
- 118.Zhou Q, Price DD, Caudle RM, Verne GN. Visceral and somatic hypersensitivity after TNBS-induced colitis in rats. Dig Dis Sci. 2007 doi: 10.1007/s10620-007-9881-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zighelboim J, Talley NJ, Phillips SF, Harmsen WS, Zinsmeister AR. Visceral perception in irritable bowel syndrome. Rectal and gastric responses to distension and serotonin type 3 antagonism. Dig Dis Sci. 1995;40:819–827. doi: 10.1007/BF02064986. [DOI] [PubMed] [Google Scholar]
- 120.Zou X, Lin Q, Willis WD. Enhanced phosphorylation of NMDA receptor 1 subunits in spinal cord dorsal horn and spinothalamic tract neurons after intradermal injection of capsaicin in rats. J Neurosci. 2000;20:6989–6997. doi: 10.1523/JNEUROSCI.20-18-06989.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zukin RS, Bennett MV. Alternatively spliced isoforms of the NMDARI receptor subunit. Trends Neurosci. 1995;18:306–313. doi: 10.1016/0166-2236(95)93920-s. [DOI] [PubMed] [Google Scholar]


