Abstract
Cutaneous lymphomas (CLs) are a heterogeneous group of lymphoproliferative disorders that are manageable by immunotherapy. Twenty-one patients were enrolled in a prospective open-label, dose-escalation multicenter study evaluating the effects of repeated TG1042 [adenovirus-interferon (IFN)-γ] intralesional injections in patients with primary CLs, of which 18 were of T-cell and 3 of B-cell type. Repeated intralesional therapy using TG1042 consistently results in local tumor regressions in about half of treated patients and one-third of patients also in regressions in noninjected distant lesions, likely reflecting the systemic immune activation after intralesional therapy. Treatment was well tolerated with few adverse events including injection site reactions, chills, lymphopenia, and fever. Immune monitoring in the peripheral blood demonstrated systemic immune activation and the induction of antibodies against tumor antigens in some patients without clear association with clinical responses. CLs, in particular B-cell lymphomas with high objective response rates, seem to be excellent targets for this type of immunotherapy.
Introduction
Progress in immunology and molecular biology has improved insight into the nature of cutaneous lymphomas (CLs).1,2
CLs are treated preferentially with skin-directed therapies.3,4 In case of resistance, systemic therapies are used. Systemic cytokine treatment using interferons (IFNs), preferentially IFN-α, is effective in many patients.5 More than two decades ago, IFN-γ was administered systemically in patients with advanced cutaneous T-cell lymphoma (CTCL) leading to variable clinical remissions.6,7 Due to the short half-life, IFNs must be injected several times per week.5 Moreover, systemic administration of IFNs is associated with systemic toxicities, particularly in case of IFN-γ.6 Because cytokines are designed by nature to orchestrate short-distance immune responses, local secretion appears more attractive than systemic administration.
CLs are suitable targets for intralesional injection with genetically engineered4 or live viruses.8 Indeed, the use of a nonreplicating adenoviral vector encoding the IFN-γ (termed TG1042) was successfully tested in a previous phase I trial.9 Subsequent gene expression analysis revealed that intralesional IFN-γ expression together with the induction of a type I IFN response underlies the clinical response to TG1042 (ref. 10). Moreover, adenovirus with the IFN-γ insert (TG1042) was shown to have superior immunomodulatory properties to the adenoviral backbone without IFN-γ gene insert in inducing and polarizing immune response toward the Th-1 arm in vitro, which is considered to be the key event in the initiation of antitumor immunity.10 Herein, we report data from the consecutive multicenter phase II trial investigating further the efficacy and safety of intralesional application of TG1042 in CL.
Results
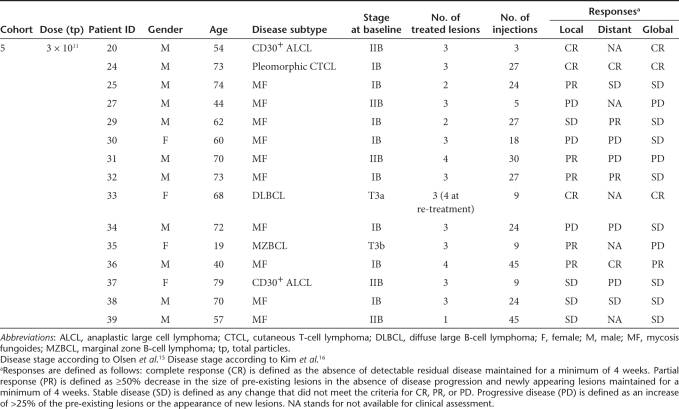
This clinical trial investigated the safety and efficacy of TG1042 applied intratumorally. Out of 21 enrolled patients, 20 were actually treated with TG1042, whereas 15 were evaluable for response analysis (i.e., without study protocol violations). Our therapeutic approach induced local in 8 out of 15 evaluable patients (53%) and also global responses of distant noninjected lesions in 4 out of 15 patients (27%) (for details, see Table 1). With respect to tumor type, local responses were observed in 6 out of 13 CTCL patients (46%) and 2 out of 2 cutaneous B-cell lymphoma (CBCL) patients (100%). Local complete responses were observed in three patients (two CTCL and one CBCL), and local partial responses were observed in five patients (four CTCL and one CBCL). Four patients exhibited a global response with disappearance of noninjected distant lesions, three of which had CTCL (CD30+ anaplastic large cell lymphoma, pleomorphic CTCL, and mycosis fungoides) and one with CBCL (diffuse large B-cell lymphoma of leg type).
Table 1.
Patients' characteristics and clinical responses
The treatment was well tolerated in treated patients. Most of the adverse drug reactions (ADRs) reported by the investigators were considered as mild or moderate. There were only 12 grade 3 ADRs, which occurred in seven patients. No grade 4 ADRs were reported during the study. Most commonly occurring ADRs were chills (65% of patients); pain at injection site (60%); lymphopenia (55%); fever (45%); headache, fatigue, and burning sensation at injection site (35%); swelling and erythema of injection site (25%); and gastrointestinal disorders (nausea and diarrhea) (20%). Reported grade 3 ADRs consisted of lymphopenia (seven episodes), chills (two episodes), high fever (one episode), pain (one episode), and pruritus (one episode) at the injection site. One treatment-related serious adverse event (a transient grade 3 fever with chills 7 hours after the 1st injection) was reported.
These side effects seem to reflect systemic immune activation. Indeed, elevated serum levels for IL-6, IL-10, IFN-γ, and neopterin were recorded. These immune parameters did not correlate with response or dose. No increase of antithyroperoxidase and antinuclear antibodies was noted.
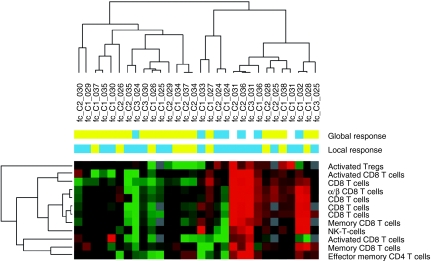
By immunophenotyping of T- and NK-cell subsets using 5-color flow cytometry, we identified a group of 12 markers that could be predictive for local objective response to TG1042 (Figure 1). Apart from cytotoxic T cells, activated regulatory T cells (CD4+CD25+CD69+) as well as effector memory T cells (CD4+CD45RA+CCR7−CD28−) were also assigned to the predictor list suggesting their important contribution to the clinical response.
Figure 1.
Classification of patient samples using immune cellular markers predicting clinical response to TG1042. The heat map was obtained by unsupervised hierarchical clustering of 30 conditions (fold-change differences in marker expression between the end of treatment cycle and baseline) and using 12 predictor immune cellular markers. The similarity of gene expression profiles among experimental samples is summarized in a dendrogram above the heat map, in which the pattern and the length of the branches reflect the relatedness of the conditions. Red in the heat map stands for high relative level of expression; green for low relative level of expression. Objective clinical response either local or global is represented by blue squares, whereas lack of objective response is represented by yellow squares.
Except for patient no. 031, who presented with increased baseline titers of neutralizing antiadenovirus antibodies, all treated patients had titers below or equal to the cutoff values. Neutralizing antiadenovirus antibody titers increased after one cycle of injections in all patients with titers ranging from 1:2,000 to 1:3,210,000. There was no additional increase later. No correlation between baseline or induced neutralizing antiadenovirus antibody levels, and clinical response was discerned.
We recorded significant, patient-specific increases in antibody titers against tumor antigens by fluorescent bead assay.11 By analyzing sera of 15 evaluable patients, we found in total 16 significant increases of specific antibodies to MAGE-A1 (one increase), MAGE-A3 (two), MAGE-A9 (two), se57-1 (one), c-TAGE5a (one for N- and C-terminus, respectively), p53 (two), and p16 (two) (for details, see Supplementary Figure S1). In contrast, only five decreases in specific antibody titers were observed, four of which (LAGE-1a, CAMEL, GAGE-7b, and p53) in the same patient (patient no. 031). The induction was often detected after the first cycle, but in some cases also later in the course of treatment. The most frequent antibody induction was found against p16 (in 6 out of 15 evaluable patients). No correlation between antibody reactivity and clinical response either local or distant was observed.
Discussion
In conjunction with the phase I trial,9,10 the data provide the proof of concept for further development of this innovative IFN-γ gene transfer in CL. Intralesional TG1042 application was well tolerated in our current study. Lack of significant changes in levels of other cytokines, inflammation, and autoimmune markers substantiates that no generalized pathologic perturbation of the immune system could be associated with frequent and repeated administrations of TG1042. The induction of cytotoxic T cells, activated regulatory T cells, and effector memory T cells shown by detailed immunophenotyping analysis is not surprising, given the critical role of cytotoxic T cells and their subsets in antitumor immune response. This was reflected in their predictive value for mounting of clinical objective response. We have recently shown that gene expression signatures in early biopsies from tumors treated with TG1042 can also be predictive of objective response further in the course of treatment.10 However, predictor markers from peripheral blood, as identified in this study, may be an elegant and less invasive method to assess the patients that may profit from the treatment with TG1042 in the future trials. Whereas our report reveals for the first time the kinetics of antibody responses to lymphoma- and cancer-associated antigens, no correlation with clinical responses could be made. Forthcoming investigations will be able to clarify the characteristics and importance of these responses in immunotherapy approaches in CL.
The treatment with TG1042 seems to be especially promising in CBCL considering the efficacy results of both phase I and our current trial. There are limited treatment options for CBCL and currently, no registered drugs for this indication. The best established therapy is radiation therapy.12 However, radiotherapy has its limitations, especially in young patients. From the group of immunomodulatory agents, IFN-α was employed in indolent CBCL types on several occasions showing high complete remission rates (summarized in ref. 12). A recently completed open-label phase II trial of TG1042 in CBCL will provide more information on the effectiveness of this approach in this CL subtype. The risk/benefit ratio of TG1042 is encouraging and compares favorably with other immunomodulatory treatments (local and systemic) used for these rare diseases.
Materials and Methods
Study design and patients. The primary objective of this phase II study was to determine the local and/or distant efficacy of TG1042 intratumoral injections. The secondary objectives were determination of the immunobiological effects as well as collection of safety data.
The study was approved by the local institutional ethical committees and the national authorities for biosafety in Switzerland, Germany, and France, and conducted in accordance with the ethical principles of the Declaration of Helsinki, in compliance with the approved protocol, and its amendments, and good clinical practices. Twenty-one patients were enrolled by six study centers between March 2004 and December 2005. Eighteen patients presented with primary CTCL, of which 13 cases suffered from mycosis fungoides, 3 had CD30+ anaplastic large cell lymphoma, and 2 pleomorphic CTCL with 13 out of 18 patients evaluable per protocol. Three patients were diagnosed with primary CBCL with two out of three patients evaluable per protocol. Patients' characteristics and clinical responses of the evaluable patients are shown in Table 1. Patients had received a median number of four prior lines of therapy, including systemic ones. The most important inclusion criteria were as follows: histologically proven CTCL (except Sézary syndrome and lymphomatoid papulosis) or multilesional CBCL, clinical disease stage IB or higher for CTCL, and failure of local tumor control by at least two first-line treatments.
Study drug, administration procedure and immunomonitoring. TG1042 is a nonreplicating (E1 and E3 regions deleted) adenovirus type 5 (group C) vector containing a human IFN-γ cDNA insert under cytomegalovirus promoter control.9 TG1042 was manufactured under cGMP conditions.11 Adenovirus-mediated expression of the IFN-γ gene results in the prolonged local production of IFN-γ.9 Patients received intratumoral injections of TG1042 into up to three lesions during 3 weeks on days 1, 8, and 15. The total dose administered was 3 × 1011 total particles divided in up to three lesions. The length of each treatment cycle was 4 weeks. In the absence of progression, patients continued up to 12 cycles. Readministration of TG1042 was authorized in case of complete response and subsequent relapse occurring in the 12-month period after inclusion.
All patients but one completed at least the first treatment cycle (three injections). The median treatment duration was 27.5 weeks. The injected lesions had to be accurately measured at the baseline and at the end of each cycle8 using WHO (World Health Organization) tumor response assessment classification. The intensity of clinical adverse events was graded according to NCI-CTC (National Cancer Institute-Common Toxicity Criteria) version 2.0.
Apart from standard laboratory evaluations, autoantibodies, antibodies to adenovirus, IFN-γ and against tumor antigens,13,14 as well as serum cytokine levels, were determined. Peripheral blood mononuclear cells were drawn at baseline and day 29. Peripheral blood mononuclear cells were stained for groups of markers associated with an individual cell phenotype, using 5-color flow cytometry. The analyses were done at the ISO (International Organization for Standardization) accredited lab Thymed (Wendelsheim, Germany). Following groups of cellular markers were detected: lymphocyte/NK cell (CD3/CD4/CD8/TCRα a/β), T-cell differentiation/homing (CD4/CD8/CD28/CD45RA/CCR7), T-cell activation/suppressor (CD4/CD8/CD25/CD69/CD127), and NK/NK T-cell markers (CD16/CD56/CD69).
SUPPLEMENTARY MATERIALFigure S1. Antibody responses to tumor antigens in patients from phase I (no. 01-018) and phase II trial (no. 020-039).
Supplementary Material
Antibody responses to tumor antigens in patients from phase I (no. 01-018) and phase II trial (no. 020-039).
REFERENCES
- Kim EJ, Hess S, Richardson SK, Newton S, Showe LC, Benoit BM, et al. Immunopathogenesis and therapy of cutaneous T cell lymphoma. J Clin Invest. 2005;115:798–812. doi: 10.1172/JCI24826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768–3785. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- Rajan, GP, Seifert, B, Prümmer, O, Joller-Jemelka, HI, Burg, G., and , Dummer, R. Incidence and in-vivo relevance of anti-interferon antibodies during treatment of low-grade cutaneous T-cell lymphomas with interferon alpha-2a combined with acitretin or PUVA. Arch Dermatol Res. 1996;288:543–548. doi: 10.1007/BF02505252. [DOI] [PubMed] [Google Scholar]
- Dummer R., and , Dreyling M.ESMO Guidelines Working Group (2008Primary cutaneous lymphoma: ESMO clinical recommendations for diagnosis, treatment and follow-up Ann Oncol 19suppl. 2): ii72–ii76. [DOI] [PubMed] [Google Scholar]
- Olsen EA. Interferon in the treatment of cutaneous T-cell lymphoma. Dermatol Ther. 2003;16:311–321. doi: 10.1111/j.1396-0296.2003.01643.x. [DOI] [PubMed] [Google Scholar]
- Kaplan EH, Rosen ST, Norris DB, Roenigk HH, Jr, Saks SR., and , Bunn PA., Jr Phase II study of recombinant human interferon gamma for treatment of cutaneous T-cell lymphoma. J Natl Cancer Inst. 1990;82:208–212. doi: 10.1093/jnci/82.3.208. [DOI] [PubMed] [Google Scholar]
- Jimbow K, Yamana K, Ishida O, Kawamura M, Ito Y., and , Maeda K. [Evaluation of rIFN-gamma in the treatment of lymphoma and melanoma of the skin by systemic and intralesional administration] Gan To Kagaku Ryoho. 1987;14:152–158. [PubMed] [Google Scholar]
- Heinzerling L, Künzi V, Oberholzer PA, Kündig T, Naim H., and , Dummer R. Oncolytic measles virus in cutaneous T-cell lymphomas mounts antitumor immune responses in vivo and targets interferon-resistant tumor cells. Blood. 2005;106:2287–2294. doi: 10.1182/blood-2004-11-4558. [DOI] [PubMed] [Google Scholar]
- Dummer R, Hassel JC, Fellenberg F, Eichmüller S, Maier T, Slos P, et al. Adenovirus-mediated intralesional interferon-gamma gene transfer induces tumor regressions in cutaneous lymphomas. Blood. 2004;104:1631–1638. doi: 10.1182/blood-2004-01-0360. [DOI] [PubMed] [Google Scholar]
- Urosevic M, Fujii K, Calmels B, Laine E, Kobert N, Acres B, et al. Type I IFN innate immune response to adenovirus-mediated IFN-gamma gene transfer contributes to the regression of cutaneous lymphomas. J Clin Invest. 2007;117:2834–2846. doi: 10.1172/JCI32077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusky M. Good manufacturing practice production of adenoviral vectors for clinical trials. Hum Gene Ther. 2005;16:281–291. doi: 10.1089/hum.2005.16.281. [DOI] [PubMed] [Google Scholar]
- Senff NJ, Noordijk EM, Kim YH, Bagot M, Berti E, Cerroni L, et al. European Organization for Research and Treatment of Cancer; International Society for Cutaneous Lymphoma. (2008European Organization for Research and Treatment of Cancer and International Society for Cutaneous Lymphoma consensus recommendations for the management of cutaneous B-cell lymphomas Blood 1121600–1609. [DOI] [PubMed] [Google Scholar]
- Eichmuller S, Usener D, Dummer R, Stein A, Thiel D., and , Schadendorf D. Serological detection of cutaneous T-cell lymphoma-associated antigens. Proc Natl Acad Sci USA. 2001;98:629–634. doi: 10.1073/pnas.021386498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann TB, Thiel D, Dummer R, Schadendorf D., and , Eichmüller S. SEREX identification of new tumour-associated antigens in cutaneous T-cell lymphoma. Br J Dermatol. 2004;150:252–258. doi: 10.1111/j.1365-2133.2004.05651.x. [DOI] [PubMed] [Google Scholar]
- Olsen E, Vonderheid E, Pimpinelli N, Willemze R, Kim Y, Knobler R, et al. ISCL/EORTC. (2007Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC) Blood 1101713–1722. [DOI] [PubMed] [Google Scholar]
- Kim YH, Willemze R, Pimpinelli N, Whittaker S, Olsen EA, Ranki A, et al. ISCL and the EORTC. (2007TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC) Blood 110479–484. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Antibody responses to tumor antigens in patients from phase I (no. 01-018) and phase II trial (no. 020-039).