Abstract
OBJECTIVE
Analysis of energy expenditure (EE) in mice is essential to obesity research. Since EE varies with body mass, comparisons between lean and obese mice are confounded unless EE is normalized to account for body mass differences. We 1) assessed the validity of ratio-based EE normalization involving division of EE by either total body mass (TBM) or lean body mass (LBM), 2) compared the independent contributions of LBM and fat mass (FM) to EE, and 3) investigated whether leptin contributes to the link between FM and EE.
RESEARCH DESIGN AND METHODS
We used regression modeling of calorimetry and body composition data in 137 mice to estimate the independent contributions of LBM and FM to EE. Subcutaneous administration of leptin or vehicle to 28 obese ob/ob mice and 32 fasting wild-type mice was used to determine if FM affects EE via a leptin-dependent mechanism.
RESULTS
Division of EE by either TBM or LBM is confounded by body mass variation. The contribution of FM to EE is comparable to that of LBM in normal mice (expressed per gram of tissue) but is absent in leptin-deficient ob/ob mice. When leptin is administered at physiological doses, the plasma leptin concentration supplants FM as an independent determinant of EE in both ob/ob mice and normal mice rendered leptin-deficient by fasting.
CONCLUSIONS
The contribution of FM to EE is substantially greater than predicted from the metabolic cost of adipose tissue per se, and the mechanism underlying this effect is leptin dependent. Regression-based approaches that account for variation in both FM and LBM are recommended for normalization of EE in mice.
The maintenance of stable body weight is achieved through a process termed “energy homeostasis” that matches energy intake to energy expenditure (EE) over long time intervals (1). Accordingly, when animals experience a sustained increase of energy intake (e.g., during consumption of an energy-rich highly palatable diet), an adaptive increase of metabolic rate can help to limit the associated weight gain (2). However, the ability to quantify adaptive changes of EE is confounded in that larger animals tend to have a higher metabolic rate than smaller ones. Therefore, to reliably detect changes in EE that are not due simply to differences in body size per se, EE must be normalized to body mass using a method that eliminates this confounding effect. To date, most rodent studies of obesity use ratio-based normalization methods whereby EE is divided by either total body mass (TBM) or lean body mass (LBM) (3–6). However, these two methods can give widely divergent results when applied to the same data (3,4,6,7).
A recent Diabetes Perspectives article (7) cogently reviewed the confounding effect of normalizing EE via division by TBM in mice, particularly when groups being compared differ in fat mass (FM). Accompanying this caution was the recommendation that EE be normalized via division by LBM instead (7) on grounds that FM consumes much less energy than LBM. Despite its intuitive appeal, dividing EE by LBM is theoretically problematic as a means to remove the influence of body size variation from group comparisons. The linear relationship between EE and either TBM or LBM is typically characterized by a positive y (EE) intercept term (8–13) due to heterogeneity inherent in the EE of various tissues comprising LBM (14). Consequently, dividing resting or average EE by either TBM or LBM mathematically forces heavier individuals to have a lower normalized EE than smaller ones (8–13), a concept first articulated >60 years ago (8). One approach that has been forwarded to obviate this mathematical bias is to use allometric scaling (15) wherein a TBM scaling exponent b and scaling coefficient a must be identified based on the data (15,16) such that EE divided by TBMb assumes the constant expected value a. This approach, however, is limited by interpretational and other difficulties (17), and the notion that a predetermined fixed TBM scaling exponent can be applied universally has been challenged (16,18).
Normalizing EE in human studies is now accomplished using multiple regression methods that adjust group comparisons of EE for differences in body mass so as to eliminate the influence of body size variation per se from evaluations of key independent variables such as ethnicity, sex, genotype, or nutritional status (9,19–28). Although multiple regression has been used in animal studies (11,29–35), the relative importance of FM and LBM as determinants of metabolic rate in mice remains an open question. Indeed, both human and animal investigations suggest that the energy cost of FM in vivo is greater than expected on the basis of its intrinsic metabolic rate (20,28,31,36). This possibility is consistent with evidence that changes in FM can influence metabolic rate at least in part through homeostatic adjustments of EE that promote body weight stability (2,37–42). Testing this hypothesis, however, requires the application of valid strategies for normalizing EE to body size.
In the current work, we demonstrate in a large sample of mice that ratio-based normalization of EE is problematic, even when LBM is used in lieu of TBM. Moreover, multiple regression analysis indicates that variation in FM makes a surprisingly large contribution to EE. These findings lead us to support recommendations for the broad use of regression-based approaches to normalizing EE in mice that take both FM and LBM into account (9–12,19,43,44). Based on the hypothesis that the effect of FM on EE reflects adaptive responses involving the adipocyte hormone leptin, we asked 1) whether the effect of FM on EE is absent in ob/ob mice that lack a leptin signal, 2) if the plasma leptin level supplants FM as an independent determinant of EE when leptin is administered to ob/ob mice at physiological doses, and 3) whether in wild-type (WT) mice rendered leptin-deficient by fasting, the plasma leptin level emerges as a determinant of EE when physiological replacement is achieved by exogenous leptin administration. Our results confirm each of these predictions and therefore implicate circulating leptin in the mechanism whereby FM variation affects EE.
RESEARCH DESIGN AND METHODS
Animal use and care.
The mouse sample used in the regression analysis to identify the independent roles of LBM and FM as determinants of EE (the main regression analysis) was aggregated from four separate projects involving separate mouse cohorts evaluated over the 2-year period from 2006 to 2008 at the Mouse Metabolic Phenotyping Center (MMPC, funded by the National Institutes of Health) located at the University of Washington. Per the policies of this program, the nature of the specific mutations affecting mice used in portions of this analysis (a total of four mutations affecting a subset of 71 mice) cannot be disclosed until the investigators who generated these mice have independently published their findings. Studies examining the effect leptin replacement has on EE used ob/ob mice and WT C57BL/6J mice obtained from Jackson Laboratories (Jackson Laboratories, Bar Harbor, ME). All animals were housed singly in a specific pathogen-free AAALAC-accredited facility (25–26°C; 12:12-h light-dark cycle) with free access to food and water unless otherwise indicated. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Washington.
Main regression analysis.
Each of the four mouse cohorts was arbitrarily assigned a project number. The total sample size was 137. All metabolic and body composition evaluations were conducted by the same investigator (K.O.) at the University of Washington MMPC facility. Additional inclusion criteria included body composition analysis via high-precision magnetic resonance methodology (described below) and being fully backcrossed onto the C57/BL6 background (minimum of 10 generations) (to avoid confounding by differences in background strain). Mice with monogenic obesity (e.g., ob/ob, db/db, Ay, etc.) were excluded based on the hypothesis that such mutations disrupt the normal relationship between body composition and metabolic rate. Mice were tested after periods of ad libitum feeding of either standard mouse chow (#5015; PMI Nutrition International, Brentwood, MO; 21% of calories from fat) or a high-fat diet (HFD) providing 42–60% of calories from fat depending on the project (P) as follows: P1: Bio-Serv (Frenchtown, NJ) #S3282 (58% of calories from fat); P2: Harlan-Teklad (Madison, WI) #TD88137 (42%); P3: Research Diets (New Brunswick, NJ) #D12492 (60%); P4: Research Diets #D12451 (45%).
Regression modeling and statistical analysis.
Regression modeling was performed using R (www.r-project.org) and SPSS (v. 17; IBM, Chicago, IL) using robust variance estimation. Significance was established at P ≤ 0.05 (two-tailed). Correlations are reported as Pearson r values.
The 24-h average and minimum light cycle EE values, measures of average and resting metabolic rate, respectively, were the dependent variables in the main regression analysis. Minimum light cycle EE was the lowest EE recorded in association with no activity, and all values were compared to the 5th percentile value of EE to preclude the use of erroneous outlier values in the analysis. The independent variables included LBM, FM, diet, activity, and sex as well as the “project by genotype” (P×G) interaction. The latter enabled us to estimate the unique influence on EE due to the genotype (WT versus mutant) within each of the four projects (see the supplemental online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db09-1582/DC1). Age was also examined but proved inconsequential after adjustment for the other factors. We also conducted two sub-analyses restricted to each of the two largest projects: project 1 (n = 50) and project 2 (n = 41).
Concern regarding the validity of normalizing EE via division by TBM or LBM is based on evidence (8–13) that resting and average EE typically scale as linear functions of TBM or LBM characterized by positive y-intercepts [EE = b1 (TBM or LBM) + b0, where b1 is a positive slope parameter and b0 is a positive intercept term]. Consequently, ratio-normalized EE (RNEE) calculated by dividing EE by either TBM or LBM is predicted to scale as a rational function for which value decreases with increasing body size [RNEE = EE/(TBM or LBM) = b1 + b0/(TBM or LBM)] (8). Accordingly, we used nonlinear regression (KaleidaGraph; Synergy Software, Reading, PA) to fit a rational function of this form to ratio-normalized EE outcomes to assess the validity of ratio-based normalization methods.
Considerations relating to the use of allometric scaling to normalize EE data are addressed in the online appendix.
Respiratory gas exchange analysis and EE quantification.
VO2 and VCO2 were quantified using an Oxymax System (CLAMS; Columbus Instruments, Columbus, OH). After habituation to the respiratory chambers, mice were tested over a continuous duration of ≥36 h, encompassing a minimum of two dark cycles and one light cycle. The second light and dark cycles were selected as the 24-h period of metabolic data analysis. Ambient temperature during testing was 25.6 ± 0.5°C. Measurements of respiratory gas exchange were made at 27-min intervals, as were external air reference values to permit baselining (10). VO2 and VCO2 were calculated as described by Lighton (10). The respiratory exchange ratio (RER) was calculated as VCO2/VO2. EE data are expressed in terms of calories per minute using the Lusk equation (45): EE in cal/min = (3.815 + 1.232 × RER) × VO2 in ml/min.
Body composition analysis.
Body composition was evaluated by quantitative nuclear magnetic resonance spectroscopy using an EchoMRI 3-in-1 Animal Tissue Composition Analyzer (ET #103) and EchoMRI software (version 2004_1.54). Calibration standards were as follows: chicken breast for LBM, canola oil at 37°C for FM, and tap water at 37°C for free body water. LBM and FM were quantified based on the averages of triplicate measures in each of the 137 tests performed. The coefficients of variation for the triplicate measures were 1.97% for FM and 0.61% for LBM.
Leptin replacement studies.
The dependent variable in the leptin replacement studies was the 24-h average EE encompassing one complete light and dark cycle. Independent variables were LBM, FM, and plasma leptin levels.
Leptin replacement in ob/ob mice.
Adult male ob/ob mice (∼10 weeks of age) were individually housed and habituated to calorimeter cages before study. After baseline body composition analysis, mice were placed in the calorimeter just before dark cycle onset for 64 h with ad libitum access to standard chow and water. Baseline (pre–leptin/vehicle infusion) EE was based on average EE between 36 and 60 h. Animals were removed from the calorimeter, separated into weight-matched groups, and subcutaneously implanted with an osmotic minipump (Alzet Model 1007D; DURECT Corporation, Cupertino, CA) containing either vehicle (sodium bicarbonate; pH 7.4) or leptin at doses of 50, 100, or 200 ng/h (Dr. A.F. Parlow; National Hormone & Peptide Program, CA) (n = 7 per group), designed to achieve leptin levels in the low-, medium-, and high-physiological range. A Lynch coil was attached to each osmotic minipump to permit a 24-h period of saline vehicle infusion before drug delivery. Animals were then returned to the calorimeter, just before dark cycle onset, for an additional 64 h of monitoring. After a final body composition analysis, mice were killed, and blood was collected with plasma removed for measurement of leptin levels by ELISA (Crystal Chem, Chicago, IL).
Leptin replacement in fasted wild-type mice.
Age- and weight-matched male C57Bl/6J mice were implanted with an osmotic minipump containing either vehicle or leptin as described above. Thirty hours later, animals were placed in the calorimeter just before a dark cycle for 36 h with ad libitum access to water only. The reported EE was the average EE across a 24-h period between 12 and 36 h during the fasting studies. After calorimetry, body composition was performed and blood was obtained for plasma leptin measurements.
RESULTS
FM as an independent predictor of EE: subject characteristics and variability of fat and lean mass.
Subject characteristics stratified by diet are summarized in Table 1. Within diet groups, variability in LBM was modest (coefficient of variation [CV] 12–20%) relative to FM (CV 53%), with the latter being the predominant source of variability in TBM (CV 20–26%). As expected, percent body fat was substantially increased in HFD versus the standard chow groups (36.8 ± 12.3 vs. 15.8 ± 5.8% fat, respectively).
TABLE 1.
Characteristics of the mice used to estimate contributions of FM and LBM to EE
| Chow diet | HFD | |
|---|---|---|
| n | 89 | 48 |
| Body mass (g) | 21.51 ± 4.22 | 36.90 ± 9.47 |
| LBM (g) | 17.10 ± 3.33 | 21.16 ± 2.50 |
| FM (g) | 3.48 ± 1.85 | 14.60 ± 7.79 |
| % Body fat | 15.83 ± 5.78 | 36.81 ± 12.33 |
| Age (weeks) | 7.80 ± 3.77 | 16.17 ± 2.40 |
| Activity (counts/min) | 22.31 ± 8.81 | 12.06 ± 6.58 |
| 24-h mean RER | 0.928 ± 0.030 | 0.833 ± 0.050 |
| Minimum light cycle RER | 0.772 ± 0.058 | 0.752 ± 0.037 |
| 24-h mean EE (cal/min) | 6.64 ± 0.76 | 10.27 ± 2.08 |
| Minimum light cycle EE (cal/min) | 4.14 ± 0.62 | 7.44 ± 1.76 |
Data are means ± SD. RER, respiratory exchange ratio.
Analysis of ratio-based normalization methods.
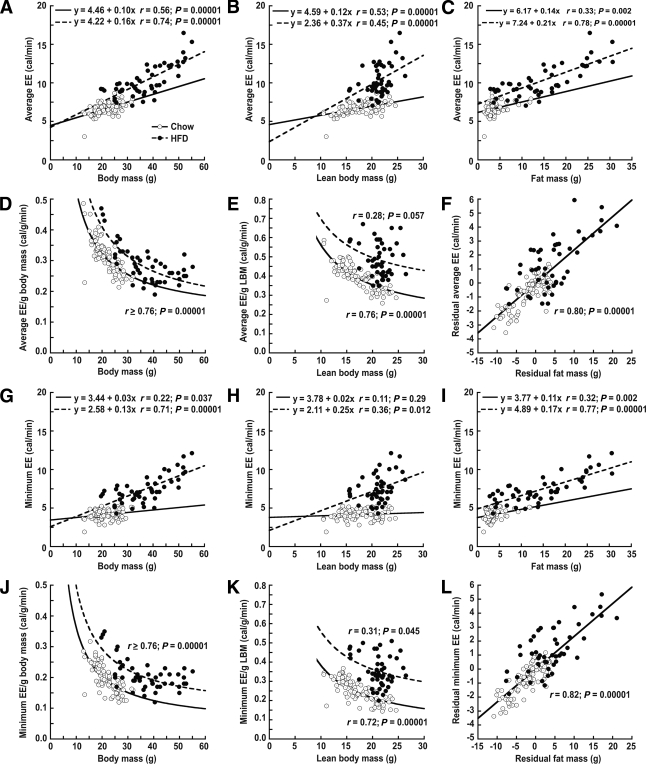
To determine whether dividing EE by TBM or LBM can effectively control for body size–related variation in EE, we performed the correlation analyses shown in Fig. 1. EE was linearly related to TBM (Fig. 1A and G) and LBM (Fig. 1B and H), and these linear relationships were characterized by positive y-intercepts, as expected (14). As a result of the positive intercepts, ratios formed from dividing EE by either TBM (Fig. 1D and J) or LBM (Fig. 1E and K) are mathematically forced to decrease with increasing values of body size. The mathematical basis for this effect is described in research design and methods. The negative slope of the relationships between normalized metabolic rate and either TBM or LBM demonstrate that ratio-based methods do not remove the effect of body size variation from metabolic rate. Consequently, within diet groups, heavier mice in Fig. 1 have a lower average ratio-normalized EE than do lighter animals, irrespective of whether or not the heavier mice actually have lower standardized EE values when EE is standardized to mass in a way that demonstrably removes the effect of mass variation from the group comparisons.
FIG. 1.
Limitations inherent in the use of traditional ratio methods for normalizing EE to TBM or LBM in mice fed either standard food (○, n = 89) or a HFD (●, n = 48). Both average (A and B) and minimum (G and H) EE vary as linear functions of TBM or LBM. Because the regression lines characterizing these relationships have positive intercepts, normalizing EE as a simple ratio of total or lean body mass (EE/TBM or EE/LBM) yield negative nonlinear associations between the normalized values and body mass compartments (D, E, J, and K). Consequently, the normalized EE values decrease with increasing body mass irrespective of whether or not the heavier mice actually have lower standardized EE values when EE is standardized to mass in a way that demonstrably controls for the influence of mass variation. (See research design and methods for the mathematical premise underlying this analysis). C and I depict the positive association between EE and FM across studies, while F and L demonstrate that the relationship between EE and FM remains highly significant even after accounting for the contribution of LBM to each trait, indicating that FM predicts EE independently of LBM in these mice. Interpretation: Within diet groups, commonly used ratio normalization methods spuriously assign a more efficient (lower) metabolic rate phenotype to larger animals. In addition, between diet groups, these ratio-based normalization methods favor assignment of an elevated metabolic rate phenotype to the HFD-fed heavier mice (and this bias is magnified when LBM is used in the ratio) because the increase of EE is disproportionate to the increase of LBM, as would be predicted if FM exerts an independent positive effect on EE.
In HFD-fed mice, non-normalized EE increased more steeply with increases of LBM than in chow-fed mice (Fig. 1B and H; comparisons of slope coefficients within diet groups are significant at P < 0.00001), and ratio-normalized EE values for the HFD group were displaced upward in comparison to those for chow-fed mice (Fig. 1D, E, J, and K). Thus, the average increase of EE per gram increase of LBM in HFD-fed mice exceeds that observed in chow-fed mice, implying that greater FM (or consuming an HFD per se) increases the metabolic rate of LBM. That diet composition influences the relationship between EE and LBM confounds LBM ratio–normalized EE values and suggests that variation in FM should also be taken into account.
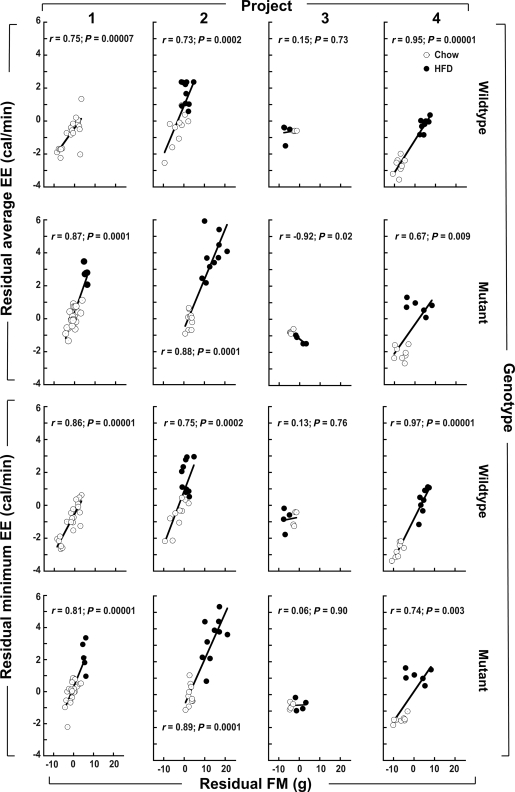
Surprisingly, we found that FM was more strongly correlated with both average and minimum EE (Fig. 1C and I; overall r ≥ 0.87 in both panels; P < 0.0001) than was LBM (Fig. 1B and H; overall r = 0.63 and r = 0.54, respectively; P < 0.0001). Notably, the overall relationship between FM and LBM was positive and highly significant (r = 0.59; P = 0.00001). As shown in Fig. 1F and L, when the components of EE that were uncorrelated with LBM (i.e., the EE residuals) were regressed on the components of FM that were uncorrelated with LBM (the FM residuals), the relationship between EE and FM remained strong and highly significant (r > 0.8; P = 0.00001), suggesting that FM and LBM each contribute to EE independently. Reinforcing this assessment, Fig. 2 shows the relationship between the EE residuals and the FM residuals depicted in Fig. 1F and L stratified by project and genotype. The residual EE versus residual FM associations in Fig. 2 are positive and significant in all subgroups except for those involving project 3, which entailed small sample sizes (n = 8 chow, n = 8 HFD). To further investigate the independent roles of LBM and FM as determinants of EE, we next performed multiple regression analysis.
FIG. 2.
Relationship between EE and FM residuals (the components of EE and FM that are not explained by LBM) within eight subgroups defined by project number and genotype. ○, Chow-fed mice; ■, HFD-fed mice. The top two rows show the relationship between average EE and FM residuals depicted in Fig. 1F stratified by project and genotype, and the bottom two rows do likewise for the minimum EE and FM residuals depicted in Fig. 1L. The LBM-adjusted EE versus LBM-adjusted FM associations are positive and significant in all subgroups except for those involving project 3, which entailed small sample sizes (n = 8 chow, n = 8 HFD). Multiple regression models for EE as a function of LBM, FM, sex, diet, activity, and membership in each subgroup defined by project and genotype revealed that FM is a quantitatively important and highly significant determinant of murine EE (see text). Interpretation: FM is strongly associated with EE, even after controlling for the relationship between each of these traits and LBM.
Regression models for predicting EE.
In a multiple regression model predicting average EE as a function of LBM, FM, sex, diet, activity, and membership in each subgroup defined by project and genotype (Fig. 2), both LBM and FM were highly significant independent predictor variables (0.269 ± 0.039 and 0.144 ± 0.023 cal/g tissue mass/min, respectively; P = 0.00001 for both). Multiple regression modeling of minimum EE as a function of all these independent variables (except activity) revealed that both LBM and FM were highly significant independent predictor variables with similar magnitudes (0.144 + 0.030 and 0.143 + 0.021 cal/g tissue mass/min, respectively; P = 0.00001). The full models are shown in the online appendix. Note that the predicted change of 24-h average EE for each 1-g change of FM represents 53% of the estimated per gram impact of LBM, while the contributions of FM and LBM to minimum EE were essentially equal. Note also that the influence of FM is similar regardless of whether 24-h average or minimum EE is used in the regression models, whereas the LBM term is larger for 24-h average than for minimum EE. Thus, after controlling for the independent effects on EE of LBM, diet, sex, activity, and subgroup membership, FM remains a highly significant and quantitatively important determinant of murine EE. Importantly, the overall impact of FM varies in proportion to body fat content such that it would seem essential to include this compartment when EE comparisons are made between lean and obese mice to detect differences in EE that are not confounded by body size and composition.
Several of these regression-based findings are consistent with what is known about factors that influence EE. For example, the finding that the per gram values associated with LBM (but not FM) were higher for 24-h average than for minimum EE is consistent with the greater metabolic activity in LBM tissues (e.g., skeletal, cardiac, and respiratory muscles) needed to power activities such as locomotion and rearing that are reflected in average EE. Diet and sex were also significant independent predictors of EE (for coefficient estimates, see the online appendix).
The conclusion that FM and LBM are independent determinants of murine EE should not imply that the identified FM and LBM coefficients will generalize to any particular experimental setting. To the contrary, our results suggest that the relative impact of FM versus LBM on EE can vary substantially depending on common variables such as the genotype and diet intervention being studied. This point is illustrated by comparing project 1 (n = 50) and project 2 (n = 41) sub-analyses, which included LBM, FM, genotype, sex, and diet to predict average 24-h EE. For project 1, after adjustment for the other independent variables, the coefficient estimates were similar for FM and LBM (0.242 ± 0.046 cal/g/min [P = 0.00001] versus 0.221 ± 0.064 cal/g/min [P = 0.001]). By contrast, in project 2, the coefficient for FM was 0.091 ± 0.037 cal/g/min (P = 0.01), whereas for LBM it was 0.397 ± 0.118 cal/g/min (P = 0.001). Because the coefficients for FM and LBM can vary across studies, approaches to EE normalization based on a priori assumptions about the relative importance of FM versus LBM as predictors of EE are difficult to justify. By comparison, regression-based normalization provides unbiased estimates of the roles of FM and LBM as determinants of EE based on the dataset being analyzed and hence averts the need for such a priori assumptions. Unbiased analyses of the impact of FM and LBM on EE are essential to efforts both to identify the contribution of genetic or other factors in EE phenotypes and to clarify the molecular mechanisms involved.
Leptin replacement in ob/ob mice.
To investigate whether a leptin signal is required for variation in FM to affect EE, we performed multiple regression analysis of data obtained from ∼11-week-old leptin-deficient ob/ob mice at baseline and again during continuous subcutaneous infusion of either saline or leptin at a dose designed to achieve plasma leptin levels in the physiological range.
Subject characteristics and bivariate associations.
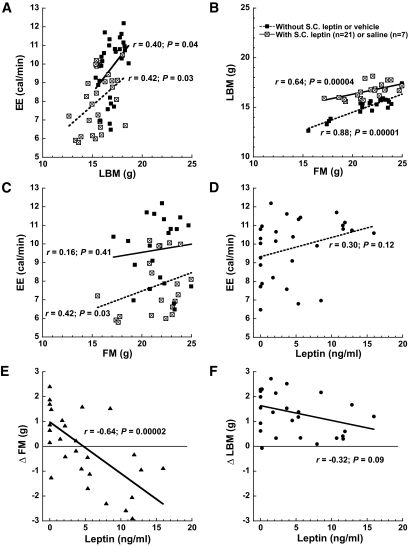
For these studies, EE was expressed as the average 24-h EE. Figure 3 illustrates key bivariate relationships involving EE, LBM, and FM and the consequences of leptin replacement.
FIG. 3.
Bivariate analyses indicate that caloric EE is positively related to LBM and to FM in ob/ob mice. Bivariate associations of average 24-h EE, LBM, FM, and plasma leptin levels in 28 ob/ob mice that were studied first at baseline and then again during continuous subcutaneous infusion of either saline (n = 7) or a dose of leptin intended to achieve physiological replacement (n = 21) (range of plasma leptin achieved is shown in D–F). A: EE was positively correlated with LBM in both the absence and presence of leptin administration, although the slope of this relationship was steeper in the latter setting, consistent with a leptin-mediated increase of metabolic cost per unit LBM. B: Highly significant co-variation between LBM and FM occurred in both the presence and absence of leptin, which confounds the analysis of relationships between EE and tissue compartments and hence illustrates the need for proper statistical control. C: EE was positively associated with FM before leptin replacement (but the effect was not significant after statistical adjustment; Table 2). D: EE varied directly with plasma leptin levels, but this association did not reach significance (this association became highly significant when multiple regression analysis was used to account for variation in tissue compartment masses; Table 2). E: As expected, leptin treatment was negatively related to the change from the baseline study in FM such that higher leptin concentrations were associated with greater FM loss. F: Although mean LBM increased among ob/ob mice receiving subcutaneous leptin, higher plasma leptin levels were associated with a tendency to limit LBM gain. Interpretation: Basic bivariate analyses indicate that the slope of EE on LBM was higher in animals receiving leptin, consistent with an effect of leptin to augment energy expenditure in this tissue (confirmed in Table 2). EE was positively related to plasma leptin levels, consistent with an effect of leptin to augment metabolic rate (confirmed in Table 2). S.C., subcutaneous.
Multiple regression models.
Table 2 shows coefficient estimates obtained in a multiple regression model for 24-h average EE in ob/ob mice in leptin-deficient and leptin-replaced states. In addition, to further investigate whether leptin administration affected the metabolic energy cost of LBM, we also used a repeated-measures model in which the LBM by state and FM by state interactions were evaluated together with the main effects of the tissue compartments. The LBM by state interaction was significant (P = 0.002), whereas the FM by state interaction was not (P = 0.37). The resultant coefficient for LBM in the leptin-replaced state (0.663 ± 0.206 cal/g/min) significantly exceeded (P = 0.002) by 1.8-fold that obtained for the leptin-deficient state (0.374 ± 0.273 cal/g/min). Thus, physiological leptin replacement in ob/ob mice increases the metabolic rate in a concentration-related fashion, and this effect involves an increase in metabolic energy expenditure occurring within LBM.
TABLE 2.
Multiple regression models for 24-h average EE (cal/min) in ob/ob mice (n = 28) evaluated in the leptin-deficient state and subsequently evaluated again in the leptin-replaced state
| Model for leptin-deficient state | ||
| Independent variable | Coefficient* ± SE | P |
| Intercept | 1.989 ± 2.859 | 0.49 |
| Lean body mass (g) | 0.212 ± 0.281 | 0.46 |
| Fat mass (g) | 0.120 ± 0.106 | 0.27 |
| Model for leptin-replaced state | ||
| Intercept | −4.229 ± 4.499 | 0.36 |
| Leptin (ng/ml) | 0.115 ± 0.042 | 0.01 |
| Lean body mass (g) | 0.912 ± 0.373 | 0.02 |
| Fat mass (g) | −0.086 ± 0.122 | 0.49 |
*Estimated change in mean EE per unit change in the independent variable. Separate models were fit within each state. Interpretation: Plasma leptin level and LBM are significant independent determinants of EE in ob/ob mice receiving short-term leptin replacement in the physiological range. In addition to being significant in the leptin-replaced state, the estimated EE cost per gram increase in LBM is over fourfold larger than it was in the leptin-deficient state. By comparison, the contribution of FM to EE in ob/ob mice did not achieve significance in either the presence or absence of leptin administration.
Effect of physiological leptin replacement in fasted wild-type mice.
As an additional test of whether leptin contributes to the effect of variation in FM on EE, we performed multiple regression analysis of body composition and metabolic data obtained from WT mice rendered leptin-deficient by fasting. During a 36-h fast, eight mice received continuous subcutaneous infusion of saline and remained leptin-deficient, whereas another 24 animals received subcutaneous leptin at doses designed to maintain physiological plasma leptin levels (i.e., designed to block fasting-induced reduction of the plasma leptin level).
Subject characteristics and bivariate associations.
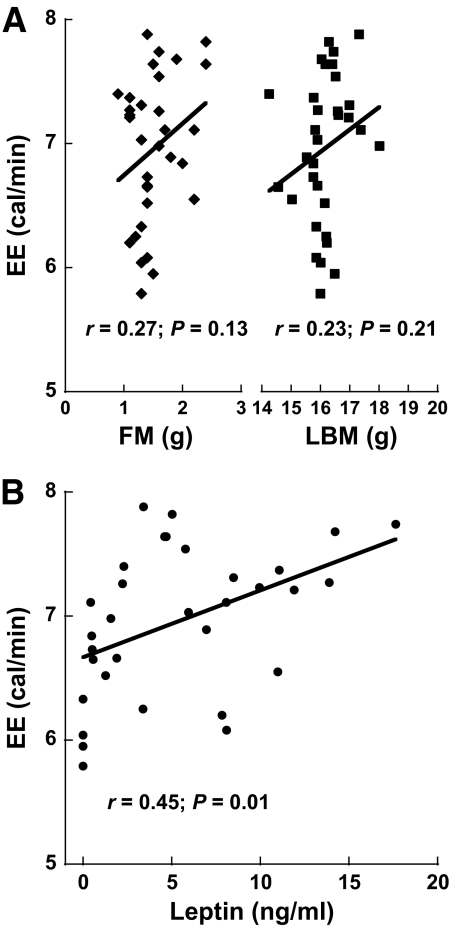
EE was evaluated as the 24-h average EE recorded during the final 24 h of a 36-h fast. Table 3 summarizes morphological and metabolic variables, whereas Fig. 4 shows scatterplots of 24-h average EE as functions of body mass compartments and leptin levels in fasted mice receiving either saline or leptin. Note the significant positive association between plasma leptin levels and EE (Fig. 4B).
TABLE 3.
Morphological and metabolic variable summaries by treatment category in ∼10-week-old fasted wild-type mice that received continuous subcutaneous infusion of either vehicle or leptin at a physiological dose
| Saline | Group |
Highest leptin† | |||
|---|---|---|---|---|---|
| All leptin* | |||||
| n = 8 | n = 24 | n = 8 | P§ | P‖ | |
| Body mass (g) | 19.3 ± 1.0 | 19.4 ± 0.8 | 19.4 ± 0.6 | 0.81 | 0.77 |
| LBM (g) | 16.0 ± 0.8 | 16.2 ± 0.7 | 16.3 ± 0.5 | 0.47 | 0.32 |
| FM (g) | 1.6 ± 0.4 | 1.5 ± 0.4 | 1.4 ± 0.3 | 0.78 | 0.29 |
| % Body fat | 8.0 ± 1.4 | 7.7 ± 1.9 | 6.9 ± 1.6 | 0.67 | 0.17 |
| Leptin (ng/ml) | 0.3 ± 0.3 | 7.1 ± 4.5 | 11.9 ± 3.2 | 0.0002 | 0.00001 |
| Activity (counts/min) | 55.4 ± 14.7 | 44.2 ± 16.2 | 40.4 ± 9.7 | 0.09 | 0.03 |
| RER | 0.73 ± 0.005 | 0.73 ± 0.007 | 0.73 ± 0.005 | 0.62 | 1.0 |
| 24-h mean EE (cal/min) | 6.43 ± 0.47 | 7.14 ± 0.53 | 7.37 ± 0.23 | 0.002 | 0.0002 |
Data are means ± SD. n = 32.
*Group includes animals receiving 50, 100, and 200 ng leptin/h.
†Group includes animals receiving only 200 ng leptin/h.
§Significance of comparison between saline and all leptin-treated mice.
‖Significance of comparison between saline and mice receiving 200 ng leptin/h.
FIG. 4.
Leptin replacement in the physiological range determines caloric EE in fasting mice. A: Scatterplots of average EE values recorded during the last 24-h period of a 36-h fast versus body mass compartments in wild-type mice receiving continuous subcutaneous infusion of either saline (n = 8) or a physiological dose of leptin (n = 24). B: Association of average EE values with leptin concentrations in plasma obtained from the same mice. Interpretation: Bivariate analyses indicates that EE in fasted WT mice receiving leptin replacement in the physiological range is more determined by the plasma leptin level than by LBM or FM (confirmed in Table 4).
Multiple regression model.
Multiple regression analysis (Table 4) showed that whereas plasma leptin levels were positively and significantly associated with EE after adjustment for FM and LBM, neither LBM nor FM were identified as significant predictors of EE. When the effect of fasting to lower plasma leptin levels is prevented by exogenous leptin infusion, therefore, the effect of leptin on metabolic rate exceeds that of either FM or LBM.
TABLE 4.
Multiple regression model for EE as a function of body mass compartments and plasma leptin concentration in fasted WT mice (n = 32) that received continuous subcutaneous infusion of either saline vehicle or leptin at a physiological dose
| Independent variable | Coefficient* ± SE | P |
|---|---|---|
| Intercept | 3.703 ± 2.531 | 0.15 |
| LBM (g) | 0.145 ± 0.152 | 0.35 |
| FM (g) | 0.407 ± 0.243 | 0.10 |
| Leptin (ng/ml) | 0.055 ± 0.014 | 0.0005 |
*Estimated change in mean EE (cal/min) per unit change in the independent variable. Interpretation: When leptin is administered to mice at doses that prevent the effect of fasting to lower plasma levels, the circulating leptin concentration is identified as a significant independent positive predictor of EE. The roles of LBM and FM could not be reliably determined in this analysis.
DISCUSSION
Although the concept that EE must be adjusted to control for differences in body size is widely accepted (8,9,11,13,19–35), the optimal method for this normalization in mice remains to be established and is a focus of intense current interest (7). Here we addressed whether it matters how this normalization is accomplished and whether a more optimal normalization strategy can help to clarify the link between body composition and the control of EE. We demonstrated that normalizing metabolic rate by simply dividing EE by either TBM or by LBM does not effectively control for the influence of mass variation. Indeed, we confirm previous evidence in humans (8,9,13,43) that these ratio-based methods systematically introduce error into the relationship between EE and body mass. By comparison, regression-based methods analogous to those that are well established in human obesity research (9,19–28) provide valid control for the influence of body size variation in comparisons involving EE. Combined with our findings that FM is an unexpectedly important determinant of metabolic rate in mice and that the relative importance of FM versus LBM as a determinant of EE can vary substantially across different mouse cohorts (which challenges normalization strategies based on a priori assumptions about the contributions made by these body mass compartments), these observations support the use of regression-based rather than ratio-based approaches when analyzing EE in this species. We also report that the plasma leptin level (but not the level of FM) emerges as a key determinant of metabolic rate when leptin is administered to ob/ob mice at doses that achieve physiological plasma levels, implying a role for leptin in the link between variation in FM and metabolic rate.
The conclusion that FM is a quantitatively important predictor of murine metabolic rate is supported by multiple regression analyses using either of two measures of EE (mean daily or 24-h minimum values) and by showing that FM is associated with EE independently of LBM in normal lean mice as well as in animals with obesity induced by HF feeding. Although one might expect that FM would incur an inconsequential metabolic energy cost because it predominantly consists of metabolically inert triglyceride, this prediction is inconsistent both with our current results and with previous studies (20,28,31,36,40–42). Rather than reflecting variation in the metabolic activity of adipose tissue per se, we favor the hypothesis that the demonstrated link between FM and EE involves compensatory responses involved in energy homeostasis triggered by changes of body adiposity (2,37–42). Indeed, obesity induced by consumption of an HFD occurs despite an adaptive increase of metabolic rate that depends on the sympathetic nervous system and serves to limit weight gain (46,47). In rodents, adaptive thermogenesis involves activation of brown adipose tissue, which is specialized to generate heat in response to sympathetic nervous system stimulation, as occurs both during the consumption of a meal and after the switch to a highly palatable energy-rich diet (46–48). Similarly, the efficiency of skeletal muscle work during physical activity is also modulated in response to changes of energy balance and storage (49,50). Each of these factors could potentially contribute to the mechanism whereby changes of FM affect EE.
The hormone leptin regulates both energy intake and EE and contributes to sympathetic nervous system–mediated adaptive changes of both brown adipose tissue thermogenesis and skeletal muscle work efficiency (50). Leptin mediates these effects by binding to and activating leptin receptors in hypothalamic arcuate (51,52), ventromedial (51,52), and dorsomedial (53) nuclei and in other brain areas that integrate afferent signals pertinent to energy balance (51). Thus, the elevated EE of fatter mice could result, at least in part, from higher circulating leptin levels. To test this hypothesis, we investigated whether leptin is required for the effect of FM variation on EE.
In leptin-deficient ob/ob mice, FM was not a significant independent determinant of EE, in contrast to what was observed in the main regression analysis. While our modest ob/ob sample size precludes ruling out a role of FM as a determinant of EE in these mice, the data do suggest an obligate role for leptin in the link between FM variation and EE. In support of this hypothesis, we found that when leptin is administered to ob/ob mice at doses that achieve physiological replacement, the plasma leptin level emerges as a robust independent predictor of EE, even after adjusting for relevant covariates. Our multiple regression models further suggest that this leptin effect involves a marked increase in the metabolic energy cost of LBM, consistent with previous studies (6,50).
Our observation that during leptin replacement, leptin levels were positively associated with EE in leptin-treated ob/ob mice, despite an attendant loss of FM, contrasts sharply with the positive association between FM and EE in normal mice that have not received exogenous leptin. This finding supports the hypothesis that variation in FM affects EE via changes in leptin level, and hence that leptin plays a more direct role to determine EE than FM per se. Pertinent to these findings is the well-documented effect of leptin to increase sympathetic outflow to brown adipose tissue (54). The finding of reduced FM in leptin-treated ob/ob mice also supports the hypothesis that the leptin-stimulated increase of EE in these animals was fueled in part via a marked increase of lipid oxidation, consistent with previous findings (6) and the finding that RER was negatively associated with leptin levels in leptin-treated ob/ob mice (r = −0.62; P = 0.0004). In considering these observations, it is worth noting that loss of body fat in most other situations (e.g., during weight loss during caloric restriction) is characterized by a fall in EE that conserves fuel stores.
To determine if leptin can supplant FM as a determinant of EE in a genetically normal animal model, we performed an analogous physiological leptin replacement study in WT mice rendered leptin deficient by fasting. Our findings suggest that the effect of fasting to lower EE may depend on a low leptin level, since fasting markedly reduces both plasma leptin levels and EE and since EE increases in proportion to the plasma leptin concentration when leptin is administered to fasted mice. As in ob/ob mice, we found that physiological variation in the plasma leptin level was a highly significant positive predictor of EE in this setting, despite the fact that FM was reduced in leptin-treated animals. These observations support a model in which the potent effect of fasting to reduce EE is mediated, at least in part, via reduced leptin levels.
The notion that increased EE in heavier mice depends on hyperleptinemia seems at odds with the concept of obesity-associated leptin resistance, typically defined as a reduced ability of exogenous leptin either to suppress food intake (55) or to activate signal transduction in key hypothalamic areas such as the arcuate nucleus (54,56,57). Indeed, this resistance is implicated in both the pathogenesis of obesity and in the mechanism underlying hyperleptinemia in obese animals (1). Yet leptin exerts wide-ranging physiological effects via widely distributed neuronal populations (57) that appear to be differentially sensitive to obesity-induced leptin resistance. Thus, acquired resistance to the food intake suppressing action of leptin can coexist with the preservation of continued sensitivity to other leptin effects, including stimulation of renal and cardiovascular sympathetic nervous system outflow (56,58). Accordingly, obese hyperleptinemic animals that are relatively resistant to leptin's anorexic effect may nonetheless experience a heightened leptin stimulation of EE. Evaluating the mechanisms underlying and extent of resistance to leptin-stimulated increases in EE in obese animals is fertile ground for future study.
In summary, our analysis supports the recommendation that both FM and LBM be taken into account when comparing EE among groups of mice with differing body mass. Moreover, simple normalization methods such as dividing EE by body weight or LBM do not adequately account for the effect of variation in body size, whereas multiple regression-based approaches achieve this goal effectively provided that sample sizes are appropriate (see the online appendix). Lastly, we conclude that FM contributes to EE in mice via mechanisms that are related less to the metabolic cost of adipose tissue per se than to leptin-dependent adaptive responses involved in the homeostasis of body energy stores.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health (NIH) Grants DK068384, DK052989, DK083042, and DA023484; by the NIH-supported Clinical Nutrition Research Unit (C.N.R.U., DK035816), the Diabetes Endocrine Research Center (D.E.R.C., DK017047), and the Mouse Metabolic Phenotyping Center (M.M.P.C; U24 DK076126; U24DK076169) at the University of Washington; and by an American Heart Association Scientist Development Grant (to G.J.M.). G.J.M. was also supported by a Naomi Berrie Investigator in Diabetes Research Award from Columbia University.
No potential conflicts of interest relevant to this article were reported.
The authors are indebted to Drs. Edward Boyko and John Lighton for their expert reviews of this manuscript and to Alex Cubelo and Iaela David for providing technical assistance.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW: Central nervous system control of food intake and body weight. Nature 2006;443:289–295 [DOI] [PubMed] [Google Scholar]
- 2.Keesey RE, Corbett SW: Metabolic defense of the body weight set-point. Res Publ Assoc Res Nerv Ment Dis 1984;62:87–96 [PubMed] [Google Scholar]
- 3.Himms-Hagen J: On raising energy expenditure in ob/ob mice. Science 1997;276:1132–1133 [DOI] [PubMed] [Google Scholar]
- 4.Erickson JC, Hollopeter G, Palmiter RD: Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y. Science 1996;274:1704–1707 [DOI] [PubMed] [Google Scholar]
- 5.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F: Effects of the obese gene product on body weight regulation in ob/ob mice. Science 1995;269:540–543 [DOI] [PubMed] [Google Scholar]
- 6.Breslow MJ, Min-Lee K, Brown DR, Chacko VP, Palmer D, Berkowitz DE: Effect of leptin deficiency on metabolic rate in ob/ob mice. Am J Physiol 1999;276:E443–E449 [DOI] [PubMed] [Google Scholar]
- 7.Butler AA, Kozak LP: A recurring problem with the analysis of energy expenditure in genetic models expressing lean and obese phenotypes. Diabetes 2010;59:323–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanner JM: Fallacy of per-weight and per-surface area standards, and their relation to spurious correlation. J Appl Physiol 1949;2:1–15 [DOI] [PubMed] [Google Scholar]
- 9.Poehlman ET, Toth MJ: Mathematical ratios lead to spurious conclusions regarding age- and sex-related differences in resting metabolic rate. Am J Clin Nutr 1995;61:482–485 [DOI] [PubMed] [Google Scholar]
- 10.Lighton JRB: Measuring Metabolic Rates: A Manual for Scientists New York, Oxford University Press, 2008 [Google Scholar]
- 11.Arch JR, Hislop D, Wang SJ, Speakman JR: Some mathematical and technical issues in the measurement and interpretation of open-circuit indirect calorimetry in small animals. Int J Obes (Lond) 2006;30:1322–1331 [DOI] [PubMed] [Google Scholar]
- 12.Allison DB, Paultre F, Goran MI, Poehlman ET, Heymsfield SB: Statistical considerations regarding the use of ratios to adjust data. Int J Obes Relat Metab Disord 1995;19:644–652 [PubMed] [Google Scholar]
- 13.Katch VL: Use of the oxygen-body weight ratio in correlational analyses: spurious correlations and statistical considerations. Med Sci Sports 1973;5:253–257 [PubMed] [Google Scholar]
- 14.Gallagher D, Belmonte D, Deurenberg P, Wang Z, Krasnow N, Pi-Sunyer FX, Heymsfield SB: Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am J Physiol 1998;275:E249–E258 [DOI] [PubMed] [Google Scholar]
- 15.White CR, Seymour RS: Allometric scaling of mammalian metabolism. J Exp Biol 2005;208:1611–1619 [DOI] [PubMed] [Google Scholar]
- 16.White CR, Cassey P, Blackburn TM: Allometric exponents do not support a universal metabolic allometry. Ecology 2007;88:315–323 [DOI] [PubMed] [Google Scholar]
- 17.Packard GC, Birchard GF: Traditional allometric analysis fails to provide a valid predictive model for mammalian metabolic rates. J Exp Biol 2008;211:3581–3587 [DOI] [PubMed] [Google Scholar]
- 18.Kolokotrones T, Van Savage, Deeds EJ, Fontana W: Curvature in metabolic scaling. Nature 2010;464:753–756 [DOI] [PubMed] [Google Scholar]
- 19.Toth MJ: Comparing energy expenditure data among individuals differing in body size and composition: statistical and physiological considerations. Curr Opin Clin Nutr Metab Care 2001;4:391–397 [DOI] [PubMed] [Google Scholar]
- 20.Nelson KM, Weinsier RL, Long CL, Schutz Y: Prediction of resting energy expenditure from fat-free mass and fat mass. Am J Clin Nutr 1992;56:848–856 [DOI] [PubMed] [Google Scholar]
- 21.Nelson KM, Weinsier RL, James LD, Darnell B, Hunter G, Long CL: Effect of weight reduction on resting energy expenditure, substrate utilization, and the thermic effect of food in moderately obese women. Am J Clin Nutr 1992;55:924–933 [DOI] [PubMed] [Google Scholar]
- 22.Weyer C, Pratley RE, Salbe AD, Bogardus C, Ravussin E, Tataranni PA: Energy expenditure, fat oxidation, and body weight regulation: a study of metabolic adaptation to long-term weight change. J Clin Endocrinol Metab 2000;85:1087–1094 [DOI] [PubMed] [Google Scholar]
- 23.Martin CK, Heilbronn LK, de Jonge L, DeLany JP, Volaufova J, Anton SD, Redman LM, Smith SR, Ravussin E: Effect of calorie restriction on resting metabolic rate and spontaneous physical activity. Obesity 2007;15:2964–2973 [DOI] [PubMed] [Google Scholar]
- 24.Wyatt HR, Grunwald GK, Seagle HM, Klem ML, McGuire MT, Wing RR, Hill JO: Resting energy expenditure in reduced-obese subjects in the National Weight Control Registry. Am J Clin Nutr 1999;69:1189–1193 [DOI] [PubMed] [Google Scholar]
- 25.Bandini LG, Must A, Spadano JL, Dietz WH: Relation of body composition, parental overweight, pubertal stage, and race-ethnicity to energy expenditure among premenarcheal girls. Am J Clin Nutr 2002;76:1040–1047 [DOI] [PubMed] [Google Scholar]
- 26.Larson DE, Ferraro RT, Robertson DS, Ravussin E: Energy metabolism in weight-stable postobese individuals. Am J Clin Nutr 1995;62:735–739 [DOI] [PubMed] [Google Scholar]
- 27.Spadano JL, Bandini LG, Must A, Dallal GE, Dietz WH: Longitudinal changes in energy expenditure in girls from late childhood through midadolescence. Am J Clin Nutr 2005;81:1102–1109 [DOI] [PubMed] [Google Scholar]
- 28.Bitz C, Toubro S, Larsen TM, Harder H, Rennie KL, Jebb SA, Astrup A: Increased 24-h energy expenditure in type 2 diabetes. Diabetes Care 2004;27:2416–2421 [DOI] [PubMed] [Google Scholar]
- 29.Meyer CW, Klingenspor M, Rozman J, Heldmaier G: Gene or size: metabolic rate and body temperature in obese growth hormone-deficient dwarf mice. Obes Res 2004;12:1509–1518 [DOI] [PubMed] [Google Scholar]
- 30.Meyer CW, Neubronner J, Rozman J, Stumm G, Osanger A, Stoeger C, Augustin M, Grosse J, Klingenspor M, Heldmaier G: Expanding the body mass range: associations between BMR and tissue morphology in wild type and mutant dwarf mice (David mice). J Comp Physiol B 2007;177:183–192 [DOI] [PubMed] [Google Scholar]
- 31.Selman C, Lumsden S, Bünger L, Hill WG, Speakman JR: Resting metabolic rate and morphology in mice (Mus musculus) selected for high and low food intake. J Exp Biol 2001;204:777–784 [DOI] [PubMed] [Google Scholar]
- 32.Selman C, Phillips T, Staib JL, Duncan JS, Leeuwenburgh C, Speakman JR: Energy expenditure of calorically restricted rats is higher than predicted from their altered body composition. Mech Ageing Dev 2005;126:783–793 [DOI] [PubMed] [Google Scholar]
- 33.MacLean PS, Higgins JA, Johnson GC, Fleming-Elder BK, Donahoo WT, Melanson EL, Hill JO: Enhanced metabolic efficiency contributes to weight regain after weight loss in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol 2004;287:R1306–R1315 [DOI] [PubMed] [Google Scholar]
- 34.Pamir N, McMillen TS, Kaiyala KJ, Schwartz MW, LeBoeuf RC: Receptors for tumor necrosis factor-alpha play a protective role against obesity and alter adipose tissue macrophage status. Endocrinology 2009;150:4124–4134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blanc S, Schoeller D, Kemnitz J, Weindruch R, Colman R, Newton W, Wink K, Baum S, Ramsey J: Energy expenditure of rhesus monkeys subjected to 11 years of dietary restriction. J Clin Endocrinol Metab 2003;88:16–23 [DOI] [PubMed] [Google Scholar]
- 36.Johnstone AM, Murison SD, Duncan JS, Rance KA, Speakman JR: Factors influencing variation in basal metabolic rate include fat-free mass, fat mass, age, and circulating thyroxine but not sex, circulating leptin, or triiodothyronine. Am J Clin Nutr 2005;82:941–948 [DOI] [PubMed] [Google Scholar]
- 37.Corbett SW, Stern JS, Keesey RE: Energy expenditure in rats with diet-induced obesity. Am J Clin Nutr 1986;44:173–180 [DOI] [PubMed] [Google Scholar]
- 38.Keesey RE, Corbett SW: Adjustments in daily energy expenditure to caloric restriction and weight loss by adult obese and lean Zucker rats. Int J Obes 1990;14:1079–1084 [PubMed] [Google Scholar]
- 39.Keesey RE, Hirvonen MD: Body weight set-points: determination and adjustment. J Nutr 1997;127:1875S–1883S [DOI] [PubMed] [Google Scholar]
- 40.Dulloo AG, Jacquet J: Adaptive reduction in basal metabolic rate in response to food deprivation in humans: a role for feedback signals from fat stores. Am J Clin Nutr 1998;68:599–606 [DOI] [PubMed] [Google Scholar]
- 41.Dulloo AG, Jacquet J: An adipose-specific control of thermogenesis in body weight regulation. Int J Obes Relat Metab Disord 2001;25(Suppl. 5):S22–S29 [DOI] [PubMed] [Google Scholar]
- 42.Dulloo AG, Jacquet J, Girardier L: Poststarvation hyperphagia and body fat overshooting in humans: a role for feedback signals from lean and fat tissues. Am J Clin Nutr 1997;65:717–723 [DOI] [PubMed] [Google Scholar]
- 43.Toth MJ, Goran MI, Ades PA, Howard DB, Poehlman ET: Examination of data normalization procedures for expressing peak VO2 data. J Appl Physiol 1993;75:2288–2292 [DOI] [PubMed] [Google Scholar]
- 44.Goran MI, Allison DB, Poehlman ET: Issues relating to normalization of body fat content in men and women. Int J Obes Relat Metab Disord 1995;19:638–643 [PubMed] [Google Scholar]
- 45.Mclean J, Tobin G: Animal and Human Calorimetry Cambridge, U.K., Cambridge University Press, 1987 [Google Scholar]
- 46.Voss-Andreae A, Murphy JG, Ellacott KL, Stuart RC, Nillni EA, Cone RD, Fan W: Role of the central melanocortin circuitry in adaptive thermogenesis of brown adipose tissue. Endocrinology 2007;148:1550–1560 [DOI] [PubMed] [Google Scholar]
- 47.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J: UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab 2009;9:203–209 [DOI] [PubMed] [Google Scholar]
- 48.DeRuisseau LR, Parsons AD, Overton JM: Adaptive thermogenesis is intact in B6 and A/J mice studied at thermoneutrality. Metabolism 2004;53:1417–1423 [DOI] [PubMed] [Google Scholar]
- 49.Rosenbaum M, Vandenborne K, Goldsmith R, Simoneau JA, Heymsfield S, Joanisse DR, Hirsch J, Murphy E, Matthews D, Segal KR, Leibel RL: Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. Am J Physiol Regul Integr Comp Physiol 2003;285:R183–R192 [DOI] [PubMed] [Google Scholar]
- 50.Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, Gallagher D, Mayer L, Murphy E, Leibel RL: Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest 2005;115:3579–3586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams KW, Scott MM, Elmquist JK: From observation to experimentation: leptin action in the mediobasal hypothalamus. Am J Clin Nutr 2009;89:985S–990S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S, Jr, Elmquist JK, Lowell BB: Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 2006;49:191–203 [DOI] [PubMed] [Google Scholar]
- 53.Dimicco JA, Zaretsky DV: The dorsomedial hypothalamus: a new player in thermoregulation. Am J Physiol Regul Integr Comp Physiol 2007;292:R47–R63 [DOI] [PubMed] [Google Scholar]
- 54.Rahmouni K, Sigmund CD, Haynes WG, Mark AL: Hypothalamic ERK mediates the anorectic and thermogenic sympathetic effects of leptin. Diabetes 2009;58:536–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Widdowson PS, Upton R, Buckingham R, Arch J, Williams G: Inhibition of food response to intracerebroventricular injection of leptin is attenuated in rats with diet-induced obesity. Diabetes 1997;46:1782–1785 [DOI] [PubMed] [Google Scholar]
- 56.Munzberg H: Leptin-signaling pathways and leptin resistance. Forum Nutr 2010;63:123–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Myers MG, Cowley MA, Münzberg H: Mechanisms of leptin action and leptin resistance. Annu Rev Physiol 2008;70:537–556 [DOI] [PubMed] [Google Scholar]
- 58.Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG: Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes 2005;54:2012–2018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.