Abstract
OBJECTIVE
Treatment of NOD mice with the dipeptidyl peptidase-IV (DPP-IV) inhibitor sitagliptin preserved islet transplants through a pathway involving modulation of splenic CD4+ T-cell migration. In the current study, effects of sitagliptin on migration of additional subsets of CD4+ T-cells were examined and underlying molecular mechanisms were further defined.
RESEARCH DESIGN AND METHODS
Effects of sitagliptin on migration of NOD mouse splenic, thymic, and lymph node CD4+ T-cells were determined. Signaling modules involved in DPP-IV-, Sitagliptin- and incretin-mediated modulation of CD4+ T-cell migration were studied using Western blot and Rac1 and nuclear factor-κB (NF-κB) activity assays.
RESULTS
Migration of splenic and lymph node CD4+ T-cells of diabetic NOD mice was reduced by sitagliptin treatment. In vitro treatment of splenic, but not thymic or lymph node CD4+ T-cells, from nondiabetic NOD mice with soluble (s) DPP-IV increased migration. Sitagliptin abolished sDPP-IV effects on splenic CD4+ T-cell migration, whereas incretins decreased migration of lymph node, but not splenic, CD4+ T-cells. Splenic CD4+ T-cells demonstrating increased in vitro migration in response to sDPP-IV and lymph node CD4+ T-cells that were nonresponsive to incretins selectively infiltrated islets of NOD mice, after injection. Sitagliptin decreases migration of splenic CD4+ T-cells through a pathway involving Rac1/vasodilator-stimulated phosphoprotein, whereas its inhibitory effects on the migration of lymph node CD4+ T-cells involve incretin-activation of the NF-κB pathway.
CONCLUSIONS
Benefits of sitagliptin treatment in diabetic NOD mice may be mediated through selective effects on subpopulations of T-cells that are related to autoimmunity.
The incretin hormones, glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide (GLP)-1, potentiate glucose-stimulated insulin secretion during a meal and exert additional actions, including promotion of β-cell survival and proliferation (1–6). GIP and GLP-1 are primarily metabolized by the endopeptidase dipeptidyl peptidase IV (DPP-IV) (CD26), and both inhibitors of DPP-IV activity and DPP-IV-resistant incretin analogs have been targeted as type 2 diabetes therapeutic drugs, with the incretin mimetic exenatide (Byetta) and the DPP-IV inhibitors sitagliptin (Januvia) and saxagliptin (Onglyza) receiving U.S. Food and Drug Administration approval. Although the actions of DPP-IV inhibitors have been extensively studied for treatment of type 2 diabetes, considerably less is known about their potential in type 1 diabetes. In earlier studies, the DPP-IV inhibitor isoleucine thiazolidide was shown to improve glucose tolerance in type 1 diabetic animal models by increasing β-cell survival and, possibly, neogenesis (7,8). Additionally, sitagliptin (MK0431) was demonstrated to prolong islet graft survival in streptozotocin-induced (9) and NOD (10) mice. In the latter study, sitagliptin protected the islet graft through a mechanism that included modulation of splenic CD4+ T-cell migration (10). This response appeared to involve inhibition of direct DPP-IV effects on CD4+ T-cells, rather than through increasing levels of active incretins by preventing their degradation. However, the GLP-1 receptor (GLP-1R) is expressed in lymphoid tissue, and exendin-4 treatment was shown to increase numbers of CD4+ and CD8+ T-cells in lymph nodes and reduce the number of CD4+CD25+Foxp3+ regulatory T-cells in the thymus, but not the spleen, suggesting specific effects on different subpopulations of cells (11). One objective of the current studies was to examine responses to sitagliptin in additional subsets of CD4+ T-cell, including those from the thymus and lymph nodes. Using a double-labeling technique, we also examined whether in vitro treatment of splenic CD4+ T-cells with soluble (s) DPP-IV, or treatment of those from the lymph node with incretins, altered their ability to infiltrate islets of diabetic NOD mice.
Previously sDPP-IV was shown to increase migration of splenic CD4+ T-cells via a pathway involving cAMP/protein kinase A (PKA)/Rac1 GTP binding activity, with DPP-IV inhibition abolishing these effects (10). Active, GTP-bound Rac1 plays an important role in regulating cell migration through modulation of actin-rich lamellipodial protrusions, critical components for generating the driving force of cell movement (12). In several systems, inhibition of Rac resulted in complete prevention of cell movement (13–15), thus demonstrating its critical role. In the current study, we examined whether a protein involved in actin reorganization, vasodilator-stimulated phosphoprotein (VASP), contributes to effects of sDPP-IV on CD4+ T-cell migration.
We demonstrate that administration of sitagliptin in vivo reduces lymph node and splenic CD4+ T-cell migration, measured in vitro, via incretin- and nonincretin-mediated effects, respectively, and splenic sDPP-IV-responsive CD4+ T-cells and lymph node incretin nonresponsive CD4+ T-cells selectively infiltrated islets of diabetic NOD mice, after tail vein injection. We also identified a downstream role for VASP in sDPP-IV-stimulated CD4+ T-cell migration and for nuclear factor-κB (NF-κB) in GIP and GLP-1 stimulation of lymph node CD4+ T-cell migration.
RESEARCH DESIGN AND METHODS
Mice.
NOD/LtJ mice (NOD, H2g7) were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice (8–10 weeks old) were fed either a normal chow diet (NCD) (Purina Rodent Chow 5015) or a diet containing sitagliptin (16) (Purina Rodent Chow 5015 plus 4 g MK0431/kg; Research Diets, New Brunswick, NJ). All animal experiments were conducted in accordance with guidelines put forth by the University of British Columbia Committee on Animal Care and Canadian Council on Animal Care.
CD4+ T-cell isolation.
T-cells were prepared from spleen, thymus, and lymph nodes of nondiabetic female NOD mice. For studies in Figs. 1G and 2C, lymph node cells represent a pool from multiple sites, whereas for those in Fig. 2D–G, T-cells were isolated from inguinal, cervical, auxiliary, and mesenteric regions, as described in (17). In all subsequent studies, mesenteric lymph node T-cells were studied. CD4+ T-cells were enriched using the Dynabeads Mouse CD4+ T-cell-positive magnetic isolation kit (Invitrogen). Greater than 90% purity was confirmed by flow cytometry (95.1 ± 2.2% of purity for splenic, 99.7 ± 0.1% for thymic, and 99.2 ± 0.2% for lymph node CD4+ T-cells). (Representative fluorescence-activated cell sorter (FACS) profiles are included in supplementary Fig. 1A–C, available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db09-1618/DC1.)
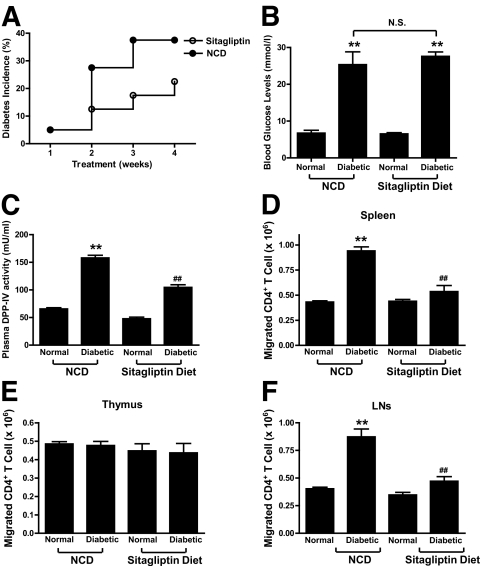
FIG. 1.
Sitagliptin modulates the migration of splenic and lymph node CD4+ T-cells. Female NOD mice (8–10 weeks old) were fed a NCD or sitagliptin diet for 1 month, before isolation of lymphocytes. A–C: Effects of sitagliptin on incidence of diabetes (A), blood glucose levels (B), and plasma DPP-IV activity (C). d–F: Effect of sitagliptin on the migration of CD4+ T-cells. CD4+ T-cells were isolated from spleen (D), thymus (E), and lymph nodes (F) from the NCD and sitagliptin groups. The migration of CD4+ T-cells was determined using Transwell chamber (Corning) as described in research design and methods. All data are means ± SEM, and significance was tested using ANOVA with a Newman-Keuls post hoc test. **P < 0.05 vs. normal NCD group; ##P < 0.05 vs. diabetic NCD group. N.S., not significant.
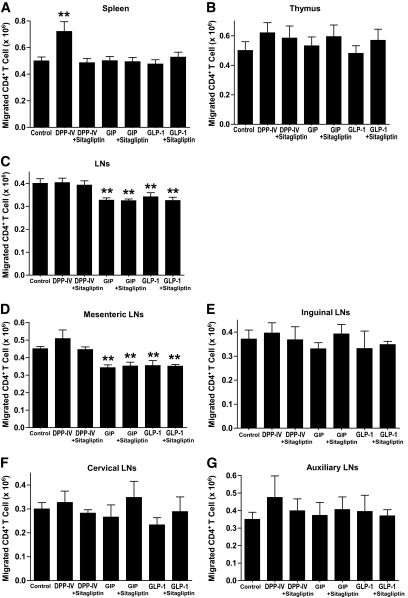
FIG. 2.
Effects of sDPP-IV and incretins on the migration of CD4+ T-cells. For these and all subsequent studies, CD4+ T-cells were isolated from nondiabetic female NOD mice receiving NCD. Responses of CD4+ T-cells from the spleen (A), thymus (B), and lymph nodes (LNs) (C) stimulated with sDPP-IV (100 mU/ml), GIP, or GLP-1 (100 nmol/l) in the presence or absence of the DPP-IV inhibitor sitagliptin (100 μmol/l) for 1 h. “Control” migration is CD4+ T-cell migration in the absence of sDPP-IV, GIP, or GLP-1. To further delineate the subset of responsive lymph node CD4+ T-cells, cells were isolated from the mesenteric (D), inguinal (E), cervical (F), and auxiliary (G) lymph nodes and treated in an identical fashion to those in (A–C). All data are means ± SEM, and significance was tested using ANOVA with a Newman-Keuls post hoc test. **P < 0.05 vs. control.
In vitro migration assay.
CD4+ T-cells (1 × 106 cells) were plated on membrane inserts (8-μm pore size) in serum-free RPMI 1640 medium. Cell migration was assayed using Transwell chambers (Corning) in media ± purified porcine kidney DPP-IV (18) (32.1 units/mg; 100 mU/ml final concentration) ± sitagliptin (100 μmol/l) or human GIP (100 nmol/l) or human GLP-1 (100 nmol/l), all kindly provided by Dr. Hans-Ulrich Demuth (Probiodrug, Halle/Saale, Germany). After 1 h, cells on the upper surface were removed mechanically and migrated cells in the lower compartment were counted.
In vivo migration assay.
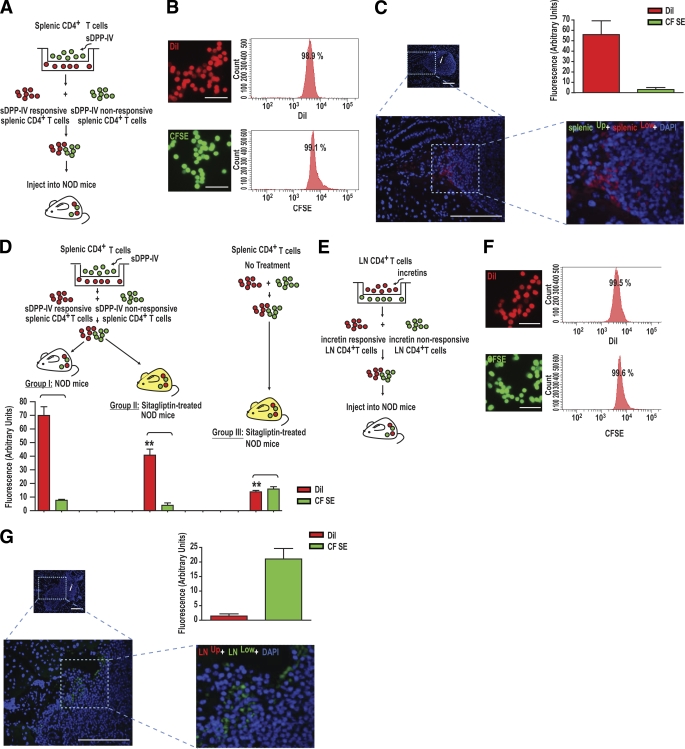
Splenic and lymph node CD4+ T-cells were plated on membrane inserts of Transwell chambers and stimulated in vitro for 1 h with sDPP-IV (100 mU/ml), GIP, or GLP-1 (100 nmol/l) (Fig. 3). With sDPP-IV treatment, cells in the lower compartment were considered “responsive,” whereas those in the upper chamber, not migrating, were considered “unresponsive.” With incretin-stimulated cells, the responsive cells remained in the upper chamber. Cells were differentially labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine iodide (DiI) or 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE) as indicated in Fig. 3A and D (DiI, red; CFSE, green). Separately labeled cells were then recombined and injected intravenously via the tail vein into diabetic recipient NOD mice (107 cells per labeling, a total of 2 × 107 cells per mouse). Forty-eight hours after injection, pancreata were snap frozen, cryostat sectioned, and examined by confocal fluorescent microscopy to detect CFSE- or DiI-labeled CD4+ T-cells in infiltrated islets. To determine total levels of CD4+ T-cell-associated fluorescent dye, pancreata were surgically removed, homogenized, and extracted in lysis buffer. Fluorescence was measured at excitation/emission = 470 nm/520 nm for CFSE and 560 nm/600 nm for DiI [standard: CFSE or DiI extracted from labeled CD4+ T-cells].
FIG. 3.
In vivo migration of splenic and lymph node CD4+ T-cells after in vitro treatment with sDPP-IV or incretins. A: Experimental designs for treatment of splenic CD4+ T-cells with sDPP-IV and administration to diabetic NOD mice. Splenic CD4+ T-cells (A) were stimulated for 1 h with sDPP-IV (100 mU/ml). Cells that migrated into the lower compartment were labeled with DiI, and the remaining cells on the upper surface were labeled with CFSE. For studies on incretins, lymph node (LN) CD4+ T-cells (E) were stimulated for 1 h with GIP and GLP-1 (100 nmol/l). Cells that migrated into the lower compartment were labeled with CFSE, and those remaining on the upper surface were labeled with DiI. In both cases, labeled cells were combined and intravenously injected into recipient mice, and pancreata were examined by fluorescent microscopy to detect DiI- or CFSE-labeled CD4+ T-cells in infiltrated islets. B and F: Similar labeling efficiencies of splenic (B) and lymph nodes (F) CD4+ T-cells with DiI or CFSE. Splenic (B) and lymph node (F) CD4+ T-cells were labeled with DiI or CFSE. The labeling was confirmed by fluorescent microscopy, and labeling efficiency was determined by flow cytometry. Shown are representative profiles from n = 3. Scale bar: 10 μm. C and G: Islet localization and quantification of labeled splenic (C) and lymph node (G) CD4+ T-cells after sDPP-IV or incretin stimulation. splenicUp and splenicLow (C) and LNUp and LNLow (G) represent splenic or lymph node lymphocytes from upper and lower chambers, respectively. Mixtures of DiI- and CFSE- labeled splenic (C) and lymph node (G) CD4+ T-cells were intravenously injected into diabetic recipient mice, and pancreatic homing of labeled lymphocytes was determined by confocal fluorescent microscopy. Number of recipient mice: n = 4/group. Infiltrated islets are represented with an i. Scale bar: 50 μm. Upper right: Quantification of recovered fluorescent label from the pancreata. Dye was extracted from pancreata and fluorescence was measured as described in research design and methods. D: Experimental designs for treatment of splenic CD4+ T-cells with sDPP-IV and administration into diabetic NOD mice ± treatment with sitagliptin. The experimental details are as in A, apart from treatment of mice with sitagliptin in groups II and II. Number of recipient mice: n = 6–10/group. Significance was tested using ANOVA with a Newman-Keuls post hoc test. **P < 0.05 vs. group I, DiI. A high-quality digital representation of this figure is available in the online issue.
Statistical analysis.
Data are expressed as means ± SEM with numbers of individual experiments presented in figure legends. Significance was tested using ANOVA with Newman-Keuls hoc test (P < 0.05).
An expanded version of research design and methods is available in an online appendix.
RESULTS
Treatment of NOD mice with sitagliptin results in reduced splenic and lymph node CD4+ T-cell migration.
By 12–14 weeks of age, incidence of diabetes was decreased in sitagliptin-treated mice compared with the NCD group; 22.5% (9 of 40) of mice developing diabetes in the sitagliptin group and 37.5% (15 of 40) in the NCD group (Fig. 1A). Animals with glucose <15 mmol/l were grouped as “normal” and those with glucose ≥15 mmol/l were grouped as “diabetic.” There were no significant differences in blood glucose levels between diabetic mice that had been fed NCD (25.3 ± 3.4 mmol/l; n = 15) and those receiving sitagliptin (27.6 ± 1.2 mmol/l; n = 9) (Fig. 1B). Plasma DPP-IV activities, measured ∼8 h after mice last ingested food, were as follows: 65.5 ± 2.1 (nondiabetic NCD group), 47.8 ± 2.9 (nondiabetic sitagliptin group), 157.9 ± 4.7 (diabetic NCD group), and 104.7 ± 4.8 mU/ml (diabetic sitagliptin group). Plasma DPP-IV activity was significantly greater (P < 0.05) in diabetic compared with nondiabetic mice, and diabetic sitagliptin mice showed significantly reduced DPP-IV activity compared with the diabetic NCD group (Fig. 1C). Consistent with previous results (10), in vitro migration of splenic CD4+ T-cells isolated from diabetic NCD mice was significantly increased, and sitagliptin restored levels toward those in the normal NCD group (Fig. 1D). In vitro migration of thymic CD4+ T-cells isolated from diabetic NCD mice did not differ significantly among groups (Fig. 1E). By contrast, in vitro migration of lymph node CD4+ T-cells isolated from the diabetic NCD group was significantly increased, compared with the normal NCD group, and sitagliptin treatment significantly reduced levels toward normal (Fig. 1F).
In vitro studies were next performed on CD4+ T-cells isolated from nondiabetic female NOD mice receiving NCD to determine whether elevated circulating incretins or DPP-IV were responsible for the altered migration. As previously reported (10), treatment of splenic CD4+ T-cells with sDPP-IV increased T-cell migration, compared with control (Fig. 2A), whereas there were no significant effects of GIP or GLP-1 ± sitagliptin on migration of splenic CD4+ T-cells. There were also no significant effects of sDPP-IV, GIP, or GLP-1 ± sitagliptin on migration of thymic CD4+ T-cells (Fig. 2B). However, GIP and GLP-1 significantly reduced migration of mixed inguinal, cervical, auxiliary, and mesenteric lymph node CD4+ T-cells (Fig. 2C). Concentration-dependent responses to sDPP-IV, GIP, or GLP-1 were observed in splenic or lymph node CD4+ T-cells (supplementary Fig. 2, available in an online appendix). Among the different sources of lymph node-derived CD4+ T-cells, only those harvested from the mesentery demonstrated reduced migration in response to GIP and GLP-1 (Fig. 2D). CD4+ T-cells from inguinal, cervical, and auxiliary lymph nodes showed no significant responses to treatment with GIP, GLP-1, or DPP-IV (Fig. 2E–G). These results strongly suggest that direct inhibition of DPP-IV by sitagliptin and resulting increases in active forms of GIP and GLP-1 impact on different subsets of CD4+ T-cells.
Differential in vivo migration patterns of splenic and lymph node CD4+ T-cells in response to sDPP-IV and incretins.
We developed an imaging method to determine whether sDPP-IV and incretin treatment of subsets of CD4+ T-cells has an impact on their in vivo pancreatic homing ability. As detailed in research design and methods, sDPP-IV-responsive CD4+ T-cells migrating into the lower compartment of Transwell chambers after sDPP-IV treatment were labeled with DiI, and nonresponsive splenic CD4+ T-cells remaining on the upper surface were labeled with CFSE (Fig. 3A). There were no significant differences in efficiencies of labeling splenic CD4+ T-cells with DiI or CFSE (96.7 ± 1.9% for DiI and 98.4 ± 0.9% for CFSE; representative FACS profiles in Fig. 3B). After injection of the mixed DiI- and CFSE-labeled splenic CD4+ T-cells into diabetic NOD mice, sDPP-IV-responsive splenic CD4+ T-cells (red) were detected in the infiltrated islets of recipient mice (Fig. 3C; additional images in supplementary Fig. 3A–D, available in an online appendix). Staining for sDPP-IV-nonresponsive splenic CD4+ T-cells (green) was only rarely detected.
Unfortunately, fractions of administered lymphocytes detected in the islets after tail vein administration were small and diffusely distributed, and it was not possible to perform cell quantification. However, quantity of dye extracted correlated with staining observed in tissue sections (Fig. 3C). To confirm this finding and evaluate the effects of sitagliptin on in vivo migration, a further experiment was performed, as outlined in Fig. 3D. Mixtures of sDPP-IV-responsive DiI-labeled and sDPP-IV-unresponsive CFSE-labeled splenic CD4+ T-cells were injected into diabetic NOD (group I) or sitagliptin-treated diabetic NOD mice (group II). In a third group, untreated splenic CD4+ T-cells were randomly divided into two groups, respectively, labeled with CFSE and DiI, and the mixed DiI- and CFSE-labeled splenic CD4+ T-cells were injected into sitagliptin-treated diabetic NOD mice (group III). The majority of dye extracted from pancreata of group I mice correlated with staining previously observed in tissue sections (Fig. 3C). Intriguingly, less DiI was extracted from sitagliptin-treated diabetic NOD mice (group II) compared with group I, reflecting reduced CD4+ cell uptake. With CD4+ T-cells not receiving in vitro sDPP-IV treatment, similar levels of both DiI and CFSE were extracted from diabetic NOD mice (group III), but much less DiI was extracted compared with groups I or II, in agreement with sDPP-IV exerting promigratory effects on CD4+ T-cells. In a similar experiment on lymph node CD4+ T-cells treated with incretins, responsive cells, remaining on the upper surface of the Transwell chambers, were labeled with DiI, and nonresponsive cells, migrating into the lower compartment, were labeled with CFSE (Fig. 3E). No significant differences in labeling efficiency of lymph node CD4+ T-cells between DiI and CFSE were observed (98.3 ± 1.2% for DiI and 98.4 ± 1.1% for CFSE; representative FACS profiles are shown in Fig. 3F). After injection of the mixed DiI- and CFSE-labeled lymph node CD4+ T-cells into diabetic NOD mice, incretin-nonresponsive lymph node CD4+ T-cells were detected in the infiltrated islets of recipient mice (Fig. 3G and H; additional images in supplementary Fig. 3E–H). Staining for incretin-responsive lymph node CD4+ T-cells (red) was rarely seen. Taken together, these results suggest that subpopulations of splenic CD4+ T-cells responding to sDPP-IV and of lymph node CD4+ T-cells that are unresponsive to incretins, exhibited pancreatic islet homing. A component of the reduction in severity of insulitis observed in mice treated with sitagliptin was therefore probably due to reduced migration of these populations of CD4+ T-cells into the pancreas.
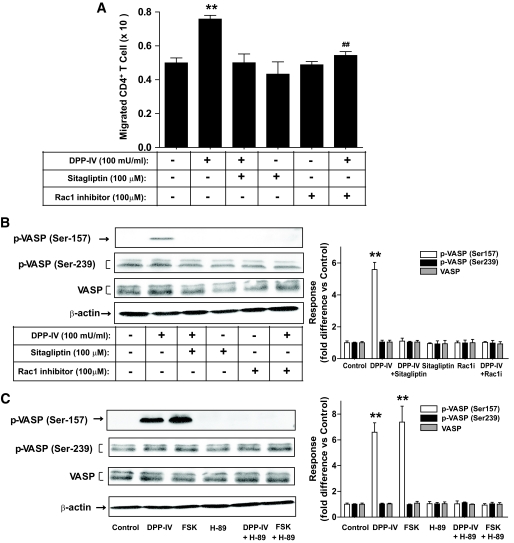
Mechanisms underlying sDPP-IV-mediated increases in splenic CD4+ T-cell migration.
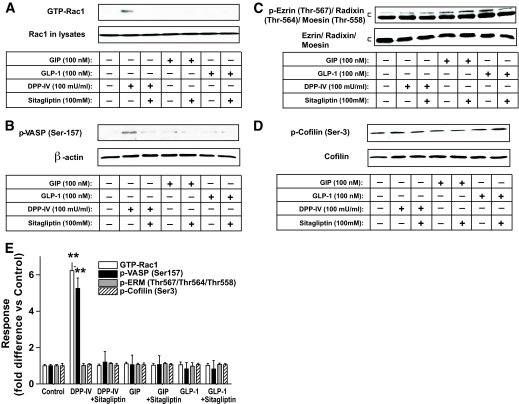
Because sDPP-IV activated a cAMP/PKA/Rac1 pathway in splenic CD4+ T-cells (10) and Rac1 is involved in regulation of the actin cytoskeleton (19), potential roles for adaptor proteins were investigated. VASP is a PKA substrate that links upstream signaling pathways to actin reorganization and cell movement (20). As shown in Fig. 4A and B, sDPP-IV activated Rac1 in splenic CD4+ T-cells and increased phosphorylation of VASP on serine 157, a critical site for its activation by PKA. DPP-IV inhibitor treatment attenuated this response. There were, however, no significant effects of DPP-IV on phosphorylation of VASP serine 239, a preferred site for cGMP-dependent protein kinase. Major bands for phosphorylated forms of other proteins involved in cytoskeletal organization were also unchanged by sDPP-IV treatment, including Ezrin (Thr567)/Radixin (Thr564)/Moesin (Thr558), and cofilin (Ser3) (Fig. 4C and D). There were also no significant effects of DPP-IV, GIP, or GLP-1 ± sitagliptin on Rac1 activity or phosphorylation of these signaling proteins in thymic (supplementary Fig. 4A–D, available in an online appendix) or mesenteric lymph node CD4+ T-cells (supplementary Fig. 4E–H), indicating differences in upstream activation systems or downstream signaling modules in these subsets of T-cells. As shown in Fig. 5A and B, a Rac1 inhibitor (NSC23766; 100 μmol/l) (21) abolished the stimulatory effects of sDPP-IV on splenic CD4+ T-cell migration and the phosphorylation of VASP (Ser157). Forskolin, a direct activator of adenylyl cyclase, mimicked the effect of DPP-IV on VASP, and its action was greatly reduced by treatment with the PKA inhibitor, H-89 (Fig. 5C). There were no significant effects of treatment with DPP-IV, forskolin or the Rac1 inhibitor on phosphorylation of Ezrin (Thr567)/Radixin (Thr564)/Moesin (Thr558), or cofilin (Ser3) (supplementary Fig. 5A–D, available in an online appendix). These results therefore suggest that sDPP-IV directly regulates the migration of splenic CD4+ lymphocytes via activation of cAMP/PKA/Rac1 and VASP phosphorylation, whereas an alternative pathway is involved in lymph node responses.
FIG. 4.
Signaling modules potentially involved in the effect of sitagliptin on splenic CD4+ T-cells. Splenic CD4+ T-cells were stimulated in vitro for 1 h with sDPP-IV (100 mU/ml), GIP, or GLP-1 (100 nmol/l) in the presence or absence of DPP-IV inhibitor (100 μmol/l), and total cellular extracts were isolated. A: Rac1 activation. Rac1 activity was determined by Rac1 pulldown assays as described in research design and methods. Briefly, cell lysates were incubated with the agarose-immobilized GST-PAK1, and the coprecipitates were subjected to Western blot hybridization using an anti-Rac1 antibody. Western blot analyses were performed with antibodies against phospho (p)-VASP (Ser157) (B), phospho-Ezrin (Thr567)/Radixin (Thr564)/Moesin (Thr558), Ezrin/Radixin/Moesin (C), phospho-cofilin (Ser3), cofilin (D), and β-actin. E: Densitometric analysis of A–D. All data are means ± SEM, and significance was tested using ANOVA with a Newman-Keuls post hoc test. **P < 0.05 vs. control. Western blots are representative of n = 3.
FIG. 5.
Involvement of a Rac1/cAMP/PKA module in sDPP-IV mediated splenic CD4+ T-cell migration and phosphorylation of the cytoskeletal organizing protein VASP. A: Effects of treatment with Rac1 inhibitor on splenic CD4+ T-cell migration. Splenic CD4+ T-cells were stimulated for 1 h with DPP-IV (100 mU/ml) in the presence or absence of Rac1 inhibitor (100 μmol/l), and the migration of CD4+ T-cells was determined. B: Effects of treatment with Rac1 inhibitor on the level of sDPP-IV-mediated phosphorylation of VASP (Ser157) and VASP (Ser239). Splenic CD4+ T-cells were treated as described above, and total cellular extracts were isolated. Western blots were quantified using densitometric analysis and are representative of n = 3. C: Effect of treatment with H-89 on the level of sDPP-IV- or forskolin-mediated phosphorylation of VASP (Ser157) and VASP (Ser239). Splenic CD4+ T-cells were stimulated for 1 h with sDPP-IV (100 mU/ml) or forskolin (FSK; 10 μmol/l) in the presence or absence of H-89 (10 μmol/l). Western blots were quantified using densitometric analysis and are representative of n = 3. All data are means ± SEM, and significance was tested using ANOVA with a Newman-Keuls post hoc test. **P < 0.05 vs. control; ##P < 0.05 vs. DPP-IV.
Mechanisms underlying incretin-mediated decreases in lymph node CD4+ T-cell migration.
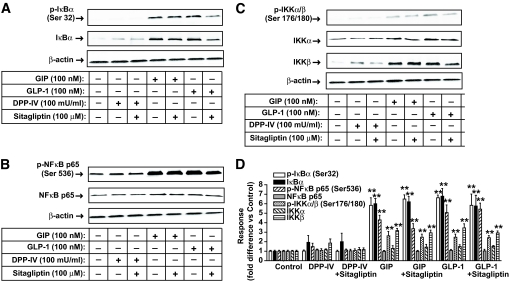
The involvement of immune responses in the development of type 1 diabetes (22), and the central role played by the NF-κB/Rel transcription factor family in regulating the maturation, survival, and activation of T-cells (23,24), led us to consider a possible role for NF-κB in incretin-mediated lymph node CD4+ T-cell migration. Treatment with GIP or GLP-1 increased phosphorylation of inhibitor of nuclear factor-κB (IκB) (Ser32), NF-κB p65 (Ser536), and IκB kinase complex (IKK) α/β (Ser176/180) in lymph node CD4+ T-cells, whereas neither DPP-IV nor sitagliptin was effective (Fig. 6A–C). Additionally, there were no significant effects of GIP, GLP-1, or sitagliptin on phosphorylation of IκB (Ser32), NF-κB p65 (Ser536), or IKK α/β (Ser176/180) in splenic or thymic CD4+ T-cells (supplementary Fig. 6A–F, available in an online appendix).
FIG. 6.
Signaling modules potentially involved in the effect of sitagliptin on lymph node CD4+ T-cells. Total cellular extracts were isolated from lymph nodes CD4+ T-cells of nondiabetic female NOD mice placed on NCD and treated as described in the legend to Fig. 3. Western blot analyses were performed with antibodies against phospho (p)-IκB (Ser32), IκB (A), phospho-NF-κB p65 (Ser536), NF-κB p65 (B), phospho-IKKα/β (Ser176/180), IKKα, IKKβ (C), and β-actin. D: Densitometric analysis of A–C. All data represent means ± SEM, and significance was tested using ANOVA with a Newman-Keuls post hoc test. **P < 0.05 vs. control. Western blots are representative of n = 3.
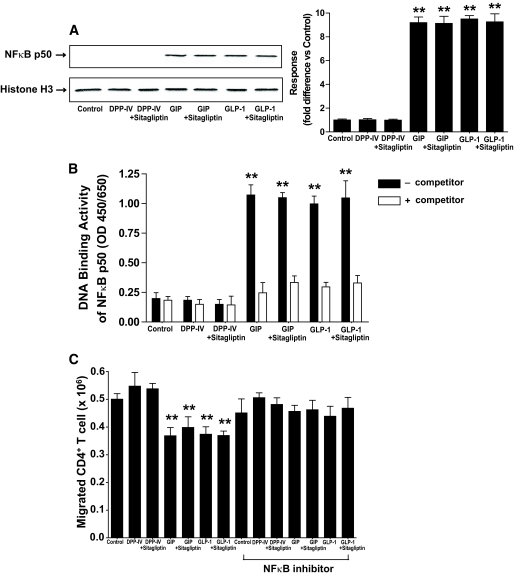
Phosphorylation of lymphocyte IκB, NF-κB p65, and IKK α/β results in release of the NF-κB complex, its cleavage, and nuclear translocation of the active p50 transcription factor. Both GIP and GLP-1 increased the nuclear localization and DNA binding of NF-κB p50 in lymph node CD4+ T-cells (Fig. 7A and B). The functional significance of incretin-mediated activation of NF-κB signaling modules for regulating lymph node CD4+ T-cell migration was next determined. Treatment of lymph node CD4+ T-cells with GIP or GLP-1 resulted in ∼25% reduction in cell migration, and decreased cell migration, induced by the incretins, was restored by NF-κB inhibitor treatment (Fig. 7C). Together, these results strongly suggest that incretins activate a pathway involving NF-κB signaling modules, which is involved in reduced lymph node CD4+ T-cell migration.
FIG. 7.
Involvement of NF-κB activation in the effect of incretins on lymph node CD4+ T-cells. A: Nuclear localization of NF-κB p50. Lymph node CD4+ T-cells were stimulated for 1 h with sDPP-IV (100 mU/ml), GIP, or GLP-1 (100 nmol/l) in the presence or absence of Sitagliptin (100 μmol/l), nuclear extracts were prepared, and Western blot analyses were performed with antibodies against NF-κB p50 and histone H3. Western blots were quantified using densitometric analysis and are representative of n = 3. B: NF-κB p50 transcription factor activation. Nuclear extracts were isolated from lymph node CD4+ T-cells and treated as described above. DNA binding activity of NF-κB p50 transcription factor was determined as described in research design and methods. C: Effect of treatment with NF-κB inhibitor on incretin-mediated lymph node CD4+ T-cell migration. Lymph node CD4+ T-cells were stimulated for 1 h with sDPP-IV (100 mU/ml), GIP, or GLP-1 (100 nmol/l) in the presence or absence of sitagliptin (100 μmol/l) and/or NF-κB inhibitor (7.5 μmol/l). The migration of lymph node CD4+ T-cells was determined using Transwell chambers as described in research design and methods. All data represent means ± SEM, and significance was tested using ANOVA with a Newman-Keuls post hoc test. **P < 0.05 vs. control.
DISCUSSION
We demonstrated previously that survival of islets transplanted in NOD mice was preserved by treatment with sitagliptin and that modulation of splenic CD4+ T-cell migration was involved (10). The objectives of the current study were to determine whether additional subsets of CD4+ T-cells contributed to the beneficial effects of sitagliptin treatment and to define further underlying molecular mechanisms responsible.
Lymphocytes are highly mobile cells that travel throughout the body in response to various stimuli. Naive lymphocytes recirculate through secondary lymphoid organs where priming occurs, followed by homing to effecter sites. Active migration of antigen-specific lymphocytes increases their chances of encountering specific antigens, and their migration involves a multitude of proteins responsible for formation and continual reorganization of the actin cytoskeleton (25). A number of actin-binding proteins have been identified and classified according to their effects on actin filaments. Cofilin promotes actin filament regeneration by severing preexisting filaments, and its severing activity is inhibited by phosphorylation (Ser3) (26,27). Ezrin, Radixin, and Moesin act as signal transducers between the plasma membrane and actin cytoskeleton and are involved in cell adhesion, membrane ruffling, and microvilli formation (28). VASP is an adaptor protein linking the cytoskeleton with signal transduction pathways. Three phosphorylation sites in VASP have been identified: Ser239 and Thr278 are phosphorylated by PKA and cGMP-dependent protein kinase (29), whereas Ser157 is phosphorylated by PKA and PKC (22,30,31). VASP promotes actin polymerization by restricting actin filament capping, with PKA phosphorylation inhibiting this anticapping activity, resulting in dynamic changes in lamellipodia and formation of filopodial protrusions (32). In the present study, sDPP-IV was found to increase phosphorylation of VASP on Ser157, in splenic CD4+ T-cells, a critical site for activation (Fig. 4B). However, there were no significant effects of sDPP-IV on phosphorylation levels of VASP (Ser157) in thymic (supplementary Fig. 4B) or lymph node CD4+ T-cells (supplementary Fig. 4F), suggesting that DPP-IV selectively regulates migration of splenic CD4+ lymphocytes via direct actions involving VASP Ser157 phosphorylation.
Strong evidence for involvement of cAMP/PKA/Rac1 activation in DPP-IV-mediated splenic CD4+ T-cell migration was previously obtained (10), although the protein activation sequence was unclear. In studies on neutrophils, PKA has been shown to increase Rac1 activity (33), whereas Rac1 activated PKA in endothelial cells (34). DPP-IV-mediated phosphorylation of VASP was ablated by treatment with inhibitors of both Rac1 (Fig. 5B) and PKA (H89; Fig. 5C), suggesting that VASP is downstream of both proteins, and the studies with forskolin support this proposal. No evidence of altered phosphorylation of other actin-binding proteins was obtained (supplementary Figs. 4D and 5A). One pathway by which DPP-IV interaction with lymphocytes could promote migration is, therefore, through cAMP-mediated activation of PKA, phosphorylation of Rac GDP/GTP exchange factor(s), such as β-PIX (34), and activation of Rac1. However, although Rac1 may lie downstream of PKA, serine 157 in VASP is within a PKA consensus sequence, suggesting that it could be directly phosphorylated. We therefore prefer an alternative scenario, in which lymphocyte binding of DPP-IV results in activation of a small pool of Rac1 that binds to and activates PKA. Activated PKA may then phosphorylate Rac GDP/GTP exchange factor(s), thus further activating Rac1 and PKA, resulting in VASP (Ser157) phosphorylation by PKA. Such changes in activation and spatial distribution of PKA during regulation of cell migration are critical components of its action (35). As mentioned, an additional possible pathway for VASP activation is through PKG (36), although details of the pathways involved remain to be defined. The mechanisms underlying sDPP-IV interaction with upstream signaling pathways are also uncertain. Lymphocytes contain significant amounts of membrane-bound DPP-IV in a dimeric form (37) that is believed to promote cell-cell interaction. It is therefore possible that sDPP-IV disrupts this interaction, with resulting soluble/membrane-bound tetrameric forms inducing conformational changes that result in activation of intracellular signaling modules. It is additionally conceivable that interaction with the phosphatase CD45 or fibronectin are involved. Alternatively, because sitagliptin decreases CD4+ T-cell migration, DPP-IV activation of a cytokine-mediated promigratory pathway may result in autocrine/paracrine-mediated responses.
In addition to its incretin action, GLP-1 has been shown to play an immunodulatory role in rodents (11,38,39), and expression of the GLP-1R was demonstrated in immune cells from spleen, thymus, and lymph nodes of both normoglycemic and diabetic NOD mice (11). However, although activation of GLP-1 receptors was shown to modulate the number of thymic CD4+CD25+Foxp3+ regulatory T-cells (11), we observed no effect of either GIP or GLP-1 on splenic or thymic CD4+ T-cell migration in vitro (Fig. 2A and B). The NOD mouse thymic CD4+ T-cell population consisted of 82.9 ± 1.2% CD4+CD8+ double-positive and 16.8 ± 1.1% CD4+CD8− single-positive cells (representative FACS profiles are shown in supplementary Fig. 1C). Therefore, lack of effect of DPP-IV or incretins on thymic CD4+ T-cell migration in vitro may be due to the presence of a high percentage of immature CD4+CD8+ cells. Indeed, differential GLP-1R expression in sorted immune cell populations and downregulation of GLP-1R in NOD mice immature thymic CD4+CD8+ cells were recently reported (40).
Nevertheless, GIP and GLP-1 significantly reduced migration of lymph node CD4+ T-cells (Fig. 2C), and this was associated with activation of the NF-κB signaling pathway, through phosphorylation of IκB (Ser32), NF-κB p65 (Ser536), and IKK α/β (Ser176/180), thus increasing DNA binding activity of active NFκB p50 (Fig. 7B). This pathway has previously been implicated in prosurvival effects of GLP-1 in INS832/13 β-cells (41). The finding that decreased CD4+ T-cell migration induced by GIP and GLP-1 was partially restored by an NF-κB inhibitor (Fig. 7C) provided additional support for NFκB involvement.
Activation of NF-κB can modulate the expression of a variety of genes associated with the immune response, inflammation, cellular stress, cell adhesion, and apoptosis. Notably, a group of genes related to cell migration possess consensus NF-κB binding sites in their promoter regions, including CD44, DC-SIGN, ELAM-1, endoglin, ICAM-1, P-selectin, tenascin-C, and VCAM-1 (GenBank and MatInspector databases). However, it is unknown whether any of these gene products are involved in incretin-mediated reduction in T-cell migration. The few functional studies performed on NF-κB signaling and cell migration have produced conflicting results, with NF-κB promoting breast cancer cell migration (42), whereas high glucose-induced activation of NF-κB inhibited endothelial cell migration (43). Such disparities may be due to the cell type studied, the intensity and duration of the signal (transient vs. long-lasting), and whether p50/p50 homodimers (trans-repression) or p50/p65 heterodimers (trans-activation) are involved.
We previously found that the majority of islets in sections from untreated diabetic NOD mice demonstrated severe insulitis, whereas islets from sitagliptin-treated mice exhibited more intact structure (10). Islet β-cell area was also significantly increased by inhibitor treatment (10). Additionally, in both the earlier and the current study, numbers of mice in the treated group developing diabetes were decreased (10) (Fig. 1A). However, sitagliptin treatment did not significantly reduce the level of hyperglycemia in the diabetic mice. A number of factors may be responsible for this lack of response. The in vitro treatment studies showed that overall effects of sDPP-IV or incretins on migration of splenic and lymph node CD4+ T-cells, respectively, were fairly modest (Fig. 2), probably reflecting changes in responsiveness of only a small fraction of the total CD4+ T-cell population. This conclusion is supported by the labeling studies, which showed that subpopulations of both sDPP-IV-responsive splenic and incretin-unresponsive lymph node CD4+ T-cells selectively infiltrated the islets (Fig. 3), with dye extraction showing a similar distribution. Of particular interest is the observation that prior treatment of diabetic NOD mice with sitagliptin resulted in lower pancreatic DiI levels, reflecting reduced CD4+ T-cell uptake. Therefore, although T-cells resident in islets may exhibit low responsiveness to sitagliptin, earlier treatment with DPP-IV inhibitor and/or combination therapy, for example, with a proton pump inhibitor (44), may reduce lymphocyte infiltration, thus attenuating progression to diabetes. DPP-IV also exhibits a number of other actions on immune function (45,46), and factors other than migration may be affected by DPP-IV inhibition, including expression of homing receptors and chemokine or cytokine production by CD4+ T-cells or antigen-presenting cells. Earlier sitagliptin treatment may also have an impact on these responses.
In addition to the beneficial effects of DPP-IV inhibitor treatment in the NOD mouse, a number of studies have demonstrated their potential in other immune-related disorders. Lymphocyte CD26 expression is increased by cytokines (47), and several autoimmune diseases have been linked to altered DPP-IV expression/function, including rheumatoid arthritis, multiple sclerosis, and autoimmune encephalomyelitis (46,48). In rodent studies, DPP-IV inhibitor treatment has been found to suppress arthritis (49) and to abrogate heart transplant rejection and prolong lung allograft function (50). Perhaps surprisingly, studies on DPP-IV mutant Fisher 344 rats and DPP-IV knockout mice have not revealed major defects in immune function (51,52), and neither chronic treatment of rodents with highly selective DPP-IV inhibitors nor their administration to humans with type 2 diabetes (53) have shown deleterious effects on immune function. It is therefore possible that benefits of DPP-IV inhibitor treatment are mediated via effects on subpopulations of T-cells related to autoimmunity.
In conclusion, sitagliptin decreased migration of a subpopulation of splenic CD4+ T-cells through a pathway involving Rac1/VASP, whereas its inhibitory effects on the migration of lymph node CD4+ T-cells involves incretin-mediated activation of the NF-κB pathway. In the setting of type 1 diabetes, clarification of the mechanisms underlying the effects of both DPP-IV inhibitor- and incretin-mediated reductions in splenic and lymph node CD4+ T-cell migration, respectively, could provide additional targets for therapeutic intervention.
Supplementary Material
ACKNOWLEDGMENTS
These studies were generously supported by funding to C.H.S.M. from Merck Frosst, Canada. We thank Dr. H.-U. Demuth (Probiodrug, Halle/Saale, Germany) for the GIP, GLP-1, and DPP-IV.
C.H.S.M. has performed basic research on dipeptidyl peptidase inhibitors since 1995 and has been associated with the following companies that have been or are involved in the development of inhibitors for diabetes therapy: Scientific Advisory Board for Probiodrug from 1998 until 2005; consultant for Merck Frosst, Canada, Merck, USA, Boehringer Ingleheim, and Takeda; and research funding from Probiodrug, OSI, U.K., and Merck Frosst. No other potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Brubaker PL, Drucker DJ: Minireview: Glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology 2004;145:2653–2659 [DOI] [PubMed] [Google Scholar]
- 2.Drucker DJ: The role of gut hormones in glucose homeostasis. J Clin Invest 2007;117:24–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yusta B, Baggio LL, Estall JL, Koehler JA, Holland DP, Li H, Pipeleers D, Ling Z, Drucker DJ: GLP-1 receptor activation improves beta cell function and survival following induction of endoplasmic reticulum stress. Cell Metab 2006;4:391–406 [DOI] [PubMed] [Google Scholar]
- 4.Ehses JA, Casilla VR, Doty T, Pospisilik JA, Winter KD, Demuth HU, Pederson RA, McIntosh CH: Glucose-dependent insulinotropic polypeptide promotes beta-(INS-1) cell survival via cyclic adenosine monophosphate-mediated caspase-3 inhibition and regulation of p38 mitogen-activated protein kinase. Endocrinology 2003;144:4433–4445 [DOI] [PubMed] [Google Scholar]
- 5.Kim SJ, Winter K, Nian C, Tsuneoka M, Koda Y, McIntosh CH: Glucose-dependent insulinotropic polypeptide (GIP) stimulation of pancreatic beta-cell survival is dependent upon phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB) signaling, inactivation of the forkhead transcription factor Foxo1, and down-regulation of bax expression. J Biol Chem 2005;280:22297–22307 [DOI] [PubMed] [Google Scholar]
- 6.Kim SJ, Nian C, Widenmaier S, McIntosh CH: Glucose-dependent insulinotropic polypeptide-mediated up-regulation of β-cell antiapoptotic Bcl-2 gene expression is coordinated by cyclic AMP (cAMP) response element binding protein (CREB) and cAMP-responsive CREB coactivator 2. Mol Cell Biol 2008;28:1644–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pospisilik JA, Martin J, Doty T, Ehses JA, Pamir N, Lynn FC, Piteau S, Demuth HU, McIntosh CH, Pederson RA: Dipeptidyl peptidase IV inhibitor treatment stimulates beta-cell survival and islet neogenesis in streptozotocin-induced diabetic rats. Diabetes 2003;52:741–750 [DOI] [PubMed] [Google Scholar]
- 8.Pospisilik JA, Ehses JA, Doty T, McIntosh CH, Demuth HU, Pederson RA: Dipeptidyl peptidase IV inhibition in animal models of diabetes. Adv Exp Med Biol 2003;524:281–291 [DOI] [PubMed] [Google Scholar]
- 9.Kim SJ, Nian C, Doudet DJ, McIntosh CH: Inhibition of dipeptidyl peptidase IV with sitagliptin (MK0431) prolongs islet graft survival in streptozotocin-induced diabetic mice. Diabetes 2008;57:1331–1339 [DOI] [PubMed] [Google Scholar]
- 10.Kim SJ, Nian C, Doudet DJ, McIntosh CH: Dipeptidyl peptidase IV inhibition with MK0431 improves islet graft survival in diabetic NOD mice partially via T-cell modulation. Diabetes 2009;58:641–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadjiyanni I, Baggio LL, Poussier P, Drucker DJ: Exendin-4 modulates diabetes onset in nonobese diabetic mice. Endocrinology 2008;149:1338–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A: The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 1992;70:401–410 [DOI] [PubMed] [Google Scholar]
- 13.Ridley AJ, Comoglio PM, Hall A: Regulation of scatter factor/hepatocyte growth factor responses by Ras, Rac, and Rho in MDCK cells. Mol Cell Biol 1995;15:1110–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen WE, Zicha D, Ridley AJ, Jones GE: A role for Cdc42 in macrophage chemotaxis. J Cell Biol 1998;141:1147–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nobes CD, Hall A: Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol 1999;144:1235–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim D, Wang L, Beconi M, Eiermann GJ, Fisher MH, He H, Hickey GJ, Kowalchick JE, Leiting B, Lyons K, Marsilio F, McCann ME, Patel RA, Petrov A, Scapin G, Patel SB, Roy RS, Wu JK, Wyvratt MJ, Zhang BB, Zhu L, Thornberry NA, Weber AE: (2R)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-amine: a potent, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J Med Chem 2005;48:141–151 [DOI] [PubMed] [Google Scholar]
- 17.Matheu MP, Parker I, Cahalan MD: Dissection and 2-photon imaging of peripheral lymph nodes in mice. J Vis Exp 2007;7:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bär J, Weber A, Hoffmann T, Stork J, Wermann M, Wagner L, Aust S, Gerhartz B, Demuth HU: Characterisation of human dipeptidyl peptidase IV expressed in Pichia pastoris. A structural and mechanistic comparison between the recombinant human and the purified porcine enzyme. Biol Chem 2003;384:1553–1563 [DOI] [PubMed] [Google Scholar]
- 19.Hall A: Rho GTPases and the actin cytoskeleton. Science 1998;279:509–514 [DOI] [PubMed] [Google Scholar]
- 20.Eckert RE, Jones SL: Regulation of VASP serine 157 phosphorylation in human neutrophils after stimulation by a chemoattractant. J Leukoc Biol 2007;82:1311–1321 [DOI] [PubMed] [Google Scholar]
- 21.Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y: Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci U S A 2004;101:7618–7623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaarala O, Atkinson MA, Neu J: The “perfect storm” for type 1 diabetes: the complex interplay between intestinal microbiota, gut permeability, and mucosal immunity. Diabetes 2008;57:2555–2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weih F, Caamaño J: Regulation of secondary lymphoid organ development by the nuclear factor-κB signal transduction pathway. Immunol Rev 2003;195:91–105 [DOI] [PubMed] [Google Scholar]
- 24.Zheng Y, Vig M, Lyons J, Van Parijs L, Beg AA: Combined deficiency of p50 and cRel in CD4+ T cells reveals an essential requirement for nuclear factor κB in regulating mature T cell survival and in vivo function. J Exp Med 2003;197:861–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchison TJ, Cramer LP: Actin-based cell motility and cell locomotion. Cell 1996;84:371–379 [DOI] [PubMed] [Google Scholar]
- 26.Yang N, Higuchi O, Ohashi K, Nagata K, Wada A, Kangawa K, Nishida E, Mizuno K: Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature 1998;393:809–812 [DOI] [PubMed] [Google Scholar]
- 27.Toshima J, Toshima JY, Takeuchi K, Mori R, Mizuno K: Cofilin phosphorylation and actin reorganization activities of testicular protein kinase 2 and its predominant expression in testicular Sertoli cells. J Biol Chem 2001;276:31449–31458 [DOI] [PubMed] [Google Scholar]
- 28.Ivetic A, Ridley AJ: Ezrin/radixin/moesin proteins and Rho GTPase signalling in leucocytes. Immunology 2004;112:165–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ibarra-Alvarado C, Galle J, Melichar VO, Mameghani A, Schmidt HH: Phosphorylation of blood vessel vasodilator-stimulated phosphoprotein at serine 239 as a functional biochemical marker of endothelial nitric oxide/cyclic GMP signaling. Mol Pharmacol 2002;61:312–319 [DOI] [PubMed] [Google Scholar]
- 30.Smolenski A, Bachmann C, Reinhard K, Hönig-Liedl P, Jarchau T, Hoschuetzky H, Walter U: Analysis and regulation of vasodilator-stimulated phosphoprotein serine 239 phosphorylation in vitro and in intact cells using a phosphospecific monoclonal antibody. J Biol Chem 1998;273:20029–20035 [DOI] [PubMed] [Google Scholar]
- 31.Chitaley K, Chen L, Galler A, Walter U, Daum G, Clowes AW: Vasodilator-stimulated phosphoprotein is a substrate for protein kinase C. FEBS Lett 2004;556:211–215 [DOI] [PubMed] [Google Scholar]
- 32.Barzik M, Kotova TI, Higgs HN, Hazelwood L, Hanein D, Gertler FB, Schafer DA: Ena/VASP proteins enhance actin polymerization in the presence of barbed end capping proteins. J Biol Chem 2005;280:28653–28662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Connor KL, Mercurio AM: Protein kinase A regulates Rac and is required for the growth factor-stimulated migration of carcinoma cells. J Biol Chem 2001;276:47895–47900 [DOI] [PubMed] [Google Scholar]
- 34.Kou R, Michel T: Epinephrine regulation of the endothelial nitric-oxide synthase: roles of RAC1 and β3-adrenergic receptors in endothelial NO signaling. J Biol Chem 2007;282:32719–32729 [DOI] [PubMed] [Google Scholar]
- 35.Howe AK, Baldor LC, Hogan BP: Spatial regulation of the cAMP-dependent protein kinase during chemotactic cell migration. Proc Natl Acad Sci U S A 2005;102:14320–14325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo D, Tan YC, Wang D, Madhusoodanan KS, Zheng Y, Maack T, Zhang JJ, Huang XY: A Rac-cGMP signaling pathway. Cell 2007;128:341–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Engel M, Hoffmann T, Wagner L, Wermann M, Heiser U, Kiefersauer R, Huber R, Bode W, Demuth HU, Brandstetter H: The crystal structure of dipeptidyl peptidase IV (CD26) reveals its functional regulation and enzymatic mechanism. Proc Natl Acad Sci U S A 2003;100:5063–5068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sherry NA, Chen W, Kushner JA, Glandt M, Tang Q, Tsai S, Santamaria P, Bluestone JA, Brillantes AM, Herold KC: Exendin-4 improves reversal of diabetes in NOD Mice treated with anti-CD3 monoclonal antibody by enhancing recovery of β-cells. Endocrinology 2007;148:5136–5144 [DOI] [PubMed] [Google Scholar]
- 39.Zhang J, Tokui Y, Yamagata K, Kozawa J, Sayama K, Iwahashi H, Okita K, Miuchi M, Konya H, Hamaguchi T, Namba M, Shimomura I, Miyagawa JI: Continuous stimulation of human glucagon-like peptide-1 (7–36) amide in a mouse model (NOD) delays onset of autoimmune type 1 diabetes. Diabetologia 2007;50:1900–1909 [DOI] [PubMed] [Google Scholar]
- 40.Hadjiyanni I, Siminovitch KA, Danska JS, Drucker DJ: Glucagon-like peptide-1 receptor signalling selectively regulates murine lymphocyte proliferation and maintenance of peripheral regulatory T cells. Diabetologia 2010;53:730–740 [DOI] [PubMed] [Google Scholar]
- 41.Buteau J, El-Assaad W, Rhodes CJ, Rosenberg L, Joly E, Prentki M: Glucagon-like peptide-1 prevents beta cell glucolipotoxicity. Diabetologia 2004;47:806–815 [DOI] [PubMed] [Google Scholar]
- 42.Helbig G, Christopherson KW, 2nd, Bhat-Nakshatri P, Kumar S, Kishimoto H, Miller KD, Broxmeyer HE, Nakshatri H: NF-κB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J Biol Chem 2003;278:21631–21638 [DOI] [PubMed] [Google Scholar]
- 43.Hamuro M, Polan J, Natarajan M, Mohan S: High glucose induced nuclear factor κB mediated inhibition of endothelial cell migration. Atherosclerosis 2002;162:277–287 [DOI] [PubMed] [Google Scholar]
- 44.Suarez-Pinzon WL, Cembrowski GS, Rabinovitch A: Combination therapy with a dipeptidyl peptidase-4 inhibitor and a proton pump inhibitor restores normoglycaemia in non-obese diabetic mice. Diabetologia 2009;52:1680–1682 [DOI] [PubMed] [Google Scholar]
- 45.Dang NH, Torimoto Y, Deusch K, Schlossman SF, Morimoto C: Comitogenic effect of solid-phase immobilized anti-1F7 on human CD4 T cell activation via CD3 and CD2 pathways. J Immunol 1990;144:4092–4100 [PubMed] [Google Scholar]
- 46.Thompson MA, Ohnuma K, Abe M, Morimoto C, Dang NH: CD26/dipeptidyl peptidase IV as a novel therapeutic target for cancer and immune disorders. Mini Rev Med Chem 2007;7:253–273 [DOI] [PubMed] [Google Scholar]
- 47.Salgado FJ, Vela E, Martín M, Franco R, Nogueira M, Cordero OJ: Mechanisms of CD26/dipeptidyl peptidase IV cytokine-dependent regulation on human activated lymphocytes. Cytokine 2000;12:1136–1141 [DOI] [PubMed] [Google Scholar]
- 48.Ohnuma K, Takahashi N, Yamochi T, Hosono O, Dang NH, Morimoto C: Role of CD26/dipeptidyl peptidase IV in human T cell activation and function. Front Biosci 2008;13:2299–2310 [DOI] [PubMed] [Google Scholar]
- 49.Tanaka S, Murakami T, Horikawa H, Sugiura M, Kawashima K, Sugita T: Suppression of arthritis by the inhibitors of dipeptidyl peptidase IV. Int J Immunopharmacol 1997;19:15–25 [DOI] [PubMed] [Google Scholar]
- 50.Korom S, De Meester I, Stadlbauer TH, Chandraker A, Schaub M, Sayegh MH, Belyaev A, Haemers A, Scharpé S, Kupiec-Weglinski JW: Inhibition of CD26/dipeptidyl peptidase IV activity in vivo prolongs cardiac allograft survival in rat recipients. Transplantation 1997;63:1495–1500 [DOI] [PubMed] [Google Scholar]
- 51.Coburn MC, Hixson DC, Reichner JS: In vitro immune responsiveness of rats lacking active dipeptidylpeptidase IV. Cell Immunol 1994;158:269–280 [DOI] [PubMed] [Google Scholar]
- 52.Vora KA, Porter G, Peng R, Cui Y, Pryor K, Eiermann G, Zaller DM: Genetic ablation or pharmacological blockade of dipeptidyl peptidase IV does not impact T cell-dependent immune responses. BMC Immunol 2009;10:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amori RE, Lau J, Pittas AG: Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007;298:194–206 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.