Abstract
OBJECTIVE
Brown adipose tissue (BAT) regulates energy homeostasis and fat mass in mammals and newborns and, most likely, in adult humans. Because BAT activity and BAT mass decline with age in humans, the impact of BAT on adiposity may decrease with aging. In the present study we addressed this hypothesis and further investigated the effect of age on the sex differences in BAT activity and BAT mass.
RESEARCH DESIGN AND METHODS
Data from 260 subjects (98 with BAT and 162 study date–matched control subjects) who underwent 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) under thermoneutral conditions were analyzed. BAT activity and BAT mass were determined in the upper body.
RESULTS
BAT activity and BAT mass were higher in female (1.59 ± 0.10 and 32 ± 5 g vs. 1.02 ± 0.10 and 18 ± 4 g, both P ≤ 0.0006) than in male subjects. In multivariate analyses, sex (P < 0.0001), age (P < 0.0001), and BMI (P = 0.0018) were associated independently with BAT activity. Interestingly, only in male subjects was there an interaction between BMI and age in determining BAT activity (P = 0.008) and BAT mass (P = 0.0002); BMI decreased with increasing BAT activity and BAT mass in the lowest age tertile (Spearman rank correlation coefficient rs = −0.38, P = 0.015 and rs = −0.37, P = 0.017, respectively), not in the higher age tertiles. Furthermore, BAT activity and mass differed between female and male subjects only in the upper two age tertiles (all P ≤ 0.09).
CONCLUSIONS
Our data corroborate that, in general, BAT activity and BAT mass are elevated in female subjects and in younger people. Importantly, we provide novel evidence that the impact of BAT activity and BAT mass on adiposity appears to decline with aging only in male subjects. Furthermore, while BAT activity and BAT mass only moderately decline with increasing age in female subjects, a much stronger effect is found in male subjects.
Brown adipose tissue (BAT) is involved in the dissipation of chemical energy as heat, thereby maintaining core temperature in small mammals and in newborn humans, particularly during cold exposure without shivering (1). This cold- and also diet-induced heat production is considered as a target in the prevention and treatment of obesity (2–4). However, because BAT mass and BAT activity in humans, i.e., in contrast to rodents, is rapidly lost within the first years of life, it was thought for a long time that it has only a minor impact on energy homeostasis after infancy. In 2007, Nedergaard, Bengtsson, and Cannon (5) summarized and reviewed several studies showing that BAT is present in adult humans at a considerable amount, that human BAT activity is acutely cold-induced, and that human BAT is stimulated via the sympathetic nervous system. Importantly, in 2009, areas identified as possible BAT in human scans were found to truly represent BAT, as they were positive for the presence of BAT-unique UCP1 expression (6). In the same year, studies using the combined 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/CT) technology provided strong and convincing evidence for a major role of BAT in the regulation of body weight and energy homeostasis in adult humans (7–14). Thus, the presence of BAT may help to explain the large interindividual differences in energy efficiency in regard to weight gain in humans (15–17).
Several points have now emerged concerning the regulation of BAT mass and BAT activity and their relationships with age, sex, adiposity, and glucose and lipid metabolism in humans. Among them is the question of whether BAT mass and BAT activity constantly decline during childhood, adolescence, and adulthood. While BAT mass was found to strongly decline with age in some recent studies (7,12), as well as in our study (11), a weaker trend was found in another study (13), and no decline with increasing age was reported in a study by van Marken et al. (9). Furthermore, it is unclear whether such a decline in BAT mass and BAT activity may help to explain the observed overall increase in adiposity with aging. In addition, it is unknown which factors are responsible for the often observed higher BAT activity in female than in male subjects of similar age and whether age affects this relationship.
RESEARCH DESIGN AND METHODS
A total of 3,604 patients underwent 5,776 consecutive 18F-FDG-PET/CT examinations between January 2005 and July 2009 at the Department of Radiology of the University of Tübingen (Tübingen, Germany) for a variety of diagnostic reasons. BAT was documented in the radiologist's report for 110 scans. In a previous analysis a total of 198 scans from patients without documented BAT, who had a PET/CT examination on the same day, were matched to these scans (11). For analyses with continuous parameters, only one PET/CT scan from each patient was included in the present evaluation, resulting in a total of 260 scans. The study was approved by the ethics committee of the University of Tübingen.
PET/CT imaging.
Most patients used a car, and few used public transportation, to get to the Tübingen University Hospital. From the time of entry in the hospital until the PET/CT measurement, patients spent about 2 h in an air-conditioned environment at about 22°C. Patients fasted overnight for at least 6 h. All patients underwent PET/CT measurements on the Hi-Rez Biograph 16 (Siemens Medical Solutions, Knoxville, TN), consisting of a high-resolution three-dimensional LSO PET and a 16-row multidetector CT.
Image analysis.
FDG distribution was evaluated visually and semiquantitatively using average and maximum standard uptake values (SUVmean, SUVmax). BAT was suspected if 1) focal FDG uptake exceeded normal regional tracer accumulation and exceeded SUVmax values of 2.0 in 2) tissue with a density between −250 and −50 Hounsfield units (HU) and fat-like appearance on CT and 3) characteristic localization bilaterally in the neck and supra- and infraclavicular regions and paravertebrally (lobster sign), unrelated to muscle, joints, and pathological findings (18). For the assessment of BAT volume and activity, we used three-dimensional (3D) isocontour regions of interest (ROIs) with an SUV isocontour threshold of 2.0 (7,18). In patients without BAT (matched control subjects), ellipsoid 3D ROIs were manually placed over nuchal adipose tissue.
In the present study, mean SUVs were automatically derived from a large 3D ROI comprising the whole head and neck region as previously described (7,8,18) (supplemental Figures A and B, available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db10-0004/DC1) and are referred to as “single” ROI measurements (SUVsing). In addition, each manifestation of BAT was individually assessed, resulting in multiple 3D isocontour ROIs (SUVmult), without overlap (supplemental Figures C and D). Outdoor temperature of the study dates in the city of Tübingen was obtained from the local weather station.
Statistical analyses.
Differences between male and female subjects were determined using the nonparametric Wilcoxon rank-sum test. Data that were not normally distributed (Shapiro-Wilk W test) were logarithmically transformed to approximate a normal distribution. Patients with documented BAT activity in the radiologist's report were matched to patients without BAT. This resulted in skewed distributions for BAT activity and BAT mass that were apparent even after logarithmical transformation of the data. Therefore, for correlation analyses involving BAT mass and BAT activity, Spearman rank correlation coefficient (rs) was calculated. To adjust the effects of covariates and identify independent relationships, we performed multiple regression analyses.
RESULTS
Age, anthropometrics, and metabolic characteristics of the patients covered a wide range (Table 1). First we tested whether outdoor temperature was associated with BAT activity. No significant relationship was found with BAT activity determined as SUVsing (rs = −0.05, P = 0.43) or SUVmult (rs = −0.04, P = 0.49). Thus, no correction for outdoor temperature was necessary for the following analyses. Next we tested the relationships of BAT activity determined as SUVsing, or SUVmult with each other and with age, sex, and BMI. The BAT activity measures were closely correlated with each other (rs = 0.73, P < 0.0001). However, SUVmult consistently correlated stronger with age, sex, and BMI than SUVsingle (data not shown), suggesting that BAT activity individually assessed with multiple 3D isocontour ROIs may be superior to the single ROI measurements. Therefore, for the further analyses, SUVmult was used as an estimate of BAT activity.
TABLE 1.
Subject characteristics
| Characteristics | Mean ± SE | Range |
|---|---|---|
| Sex (female/male) | 136/124 | |
| Age (years) | 48 ± 1 | 11–82 |
| Height (cm) | 170 ± 1 | 112–200 |
| Weight (kg) | 71.3 ± 1.0 | 35.0–137.0 |
| BMI (kg/m2) | 24.5 ± 0.3 | 15.5–40.8 |
| Fasting glucose (mmol/l) | 5.17 ± 0.06 | 2.50–10.22 |
| BAT activity | 1.32 ± 0.07 | 0.16–3.84 |
| BAT mass (g) | 25.24 ± 3.20 | 0.02–287.90 |
Next we found that both BAT activity (1.59 ± 0.10 vs. 1.02 ± 0.10, P = 0.0003) and BAT mass (32 ± 5 vs. 18 ± 4 g, P = 0.0006) were higher in female than in male subjects. In univariate analyses, age and BMI correlated negatively with BAT activity (rs = −0.44, P < 0.0001 and rs = −0.33, P < 0.0001) and with BAT mass (rs = −0.34, P < 0.0001 and rs = −0.21, P = 0.0006). In multivariate analyses (Table 2), sex (P < 0.0001), age (P < 0.0001), and BMI (P = 0.0018) remained independently associated with BAT activity. Similar relationships, although weaker, were found for BAT mass with sex (P = 0.0002) and age (P < 0.0001), however, not for BAT mass with BMI (P = 0.37).
TABLE 2.
Relationships of BAT activity and BAT mass with sex, age, and BMI in multivariate linear regression analyses
| Parameter | Estimate | SE | P |
|---|---|---|---|
| BAT activity | |||
| Intercept | 3.66 | 0.81 | <0.0001 |
| Male sex | −0.20 | 0.05 | <0.0001 |
| Age | −0.02 | 0.05 | <0.0001 |
| BMI | −0.85 | 0.27 | 0.0018 |
| BAT mass | |||
| Intercept | 5.96 | 2.61 | 0.023 |
| Male sex | −0.56 | 0.15 | 0.0002 |
| Age | −0.06 | 0.01 | <0.0001 |
| BMI | −0.77 | 0.86 | 0.37 |
Intercept sets the “baseline” event rate when all covariate values are set equal to zero. Estimates are the coefficients of the linear model found by least squares. SE is the standard error, an estimate of the standard deviation of the distribution of the parameter estimate. P < 0.05 is often considered significant evidence that the parameter is not zero.
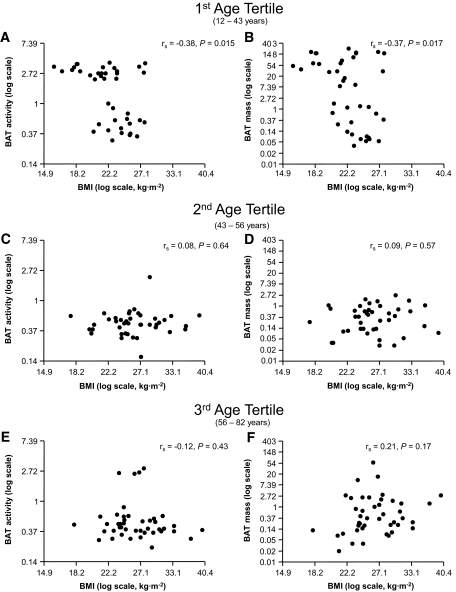
Because age turned out to be the strongest determinant of BAT activity and BAT mass, we studied whether the relationships between BAT activity and BAT mass with BMI and sex are similar among different age categories. Interestingly, there was an interaction between age and BMI in determining BAT activity (P = 0.008) and BAT mass (P = 0.0002) in male but not in female subjects (P = 0.41 and P = 0.61). When males were divided into tertiles, BMI decreased with increasing BAT activity and BAT mass only in the lower age tertile (Fig. 1A and B; rs = −0.38, P = 0.015 and r = −0.37, P = 0.017) but not in the upper two tertiles (Fig. 1C–F).
FIG. 1.
Relationships of BMI with BAT activity and BAT mass in male subjects divided into tertiles by age (Spearman rank correlation) (1st/2nd/3rd age tertiles, respectively: n = 83/90/87).
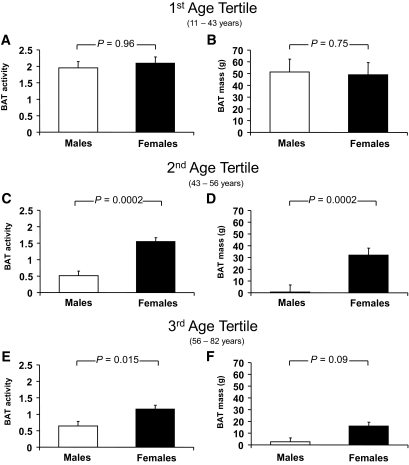
Finally, we tested whether age also impacts the observed differences in BAT activity and BAT mass between male and female subjects. Interestingly, BAT mass and BAT activity were not different in subjects in the lowest age tertile (Fig. 2A and B); however, BAT mass and BAT activity were higher in female than in male subjects in the 2nd and 3rd age tertiles (Fig. 2C–F).
FIG. 2.
Differences between sex in BAT activity and BAT mass (Wilcoxon rank-sum test) (1st/2nd/3rd age tertiles for male (and female) subjects, respectively: n = 40 (43)/41 (49)/43 (44).
DISCUSSION
In the present study we addressed the questions of whether BAT activity has similarly strong impacts on the development of adiposity in younger and older people and whether the observed sex differences in BAT activity and BAT mass are apparent among several age categories. First we determined BAT activity on 18F-FDG-PET/CT scans, either applying the widely used single ROI measurement or using multiple 3D isocontour ROIs to assess BAT activity at each manifestation, individually. We found that these measurements were highly correlated with each other. Furthermore, BAT activity estimated from the multiple ROIs turned out to be more strongly associated with age, sex, and BMI, indicating that this method may be the more accurate one.
Next we confirmed the findings of most studies that BAT activity is higher in female subjects. These findings indicate that yet unknown parameters in humans, possibly sex hormones, may be involved in the regulation of BAT function. A sexual dimorphism in BAT activity and in UCP-1 expression (higher levels in females at 22°C) was found in rats (19). However, in that study the steroid hormones β-estradiol, estrone, and progesterone did not increase but actually reduced norepinephrine-induced UCP-1 synthesis in brown adipocytes that were differentiated in primary culture. In another study in rodent brown adipocytes differentiated in culture, testosterone treatment dose-dependently inhibited norepinephrine-induced UCP-1 mRNA expression, while 17-β-estradiol did not have any remarkable effect (20). In addition, because sex hormones modulate 5′-iodothyronine deiodinase activity (21), which is responsible for enzymatic conversion of thyroxine into the bioactive form, triiodothyronine, which regulates the thermogenic function of BAT (22), indirect effects of sex steroids may be involved in the regulation of BAT activity. Because in our study a large decline in BAT activity was observed in 43- to 56-year-old male subjects, who have declining testosterone levels, it would be important to specifically study the effect of testosterone levels and/or testosterone supplementation on BAT activity.
We then found strong negative univariate correlations of BAT activity both with age and BMI. In multivariate analyses BAT activity was independently associated with age, sex, and BMI. This supports that, in general, BAT activity is lower in male subjects, declines with aging, and most likely has direct effects on the regulation of adiposity.
We next investigated whether the relationship of BAT activity with BMI is similarly strong among different age-groups. We can provide novel data that, only in younger male subjects, a significant negative relationship between BAT activity and BMI can be found. This suggests that in older male subjects BAT is not an important regulator of adiposity, anymore. In contrast, in female subjects similarly strong negative relationships of BAT activity with BMI were found among all studied age-groups. An interaction of BAT with age in determining BMI was found in the study by Cypess et al. (7). In contrast to our data, in that study the relationship between the presence of BAT and BMI became stronger with increasing age. Differences between both studies may be explained by the fact that male and female subjects were analyzed together in the study by Cypess et al. and there were differences in the age of the studied populations and differences in the methodology used for detection of BAT.
Finally we investigated whether age impacts the observed differences in BAT activity between male and female subjects. Interestingly, these differences were not apparent in the lowest age tertile. However, while in both male and female subjects BAT activity declined with aging, this process appears to be accelerated in male subjects. Thus, a significantly higher BAT activity in female subjects can be found, particularly in older women.
Limitations of our study are that our measurements of BAT activity were not done under cold exposure, which further increases BAT activity and thus may allow for more precise determination of BAT activity and BAT mass. Nevertheless, because such studies with a large number of subjects are not available yet, findings from our and other studies may help to stimulate and focus such research in this important field in the future.
In conclusion, we provide novel data that only in male subjects the impact of BAT activity on adiposity appears to decline with aging. In addition, while with increasing age BAT activity only moderately declines in female subjects, a much stronger effect is found in male subjects. If confirmed by other studies, these findings may considerably impact future research about the role of BAT in humans.
Supplementary Material
ACKNOWLEDGMENTS
Dr. Stefan is currently supported by a Heisenberg-Grant of the Deutsche Forschungsgemeinschaft (STE 1096/1-1).
No potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the 70th Scientific Sessions of the American Diabetes Association, Orlando, Florida, 25–29 June 2010.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Cannon B, Nedergaard J: Brown adipose tissue: function and physiological significance. Physiol Rev 2004;84:277–359 [DOI] [PubMed] [Google Scholar]
- 2.Lowell BB, Spiegelman BM: Towards a molecular understanding of adaptive thermogenesis. Nature 2000;404:652–660 [DOI] [PubMed] [Google Scholar]
- 3.Lowell BB, S-Susulic V, Hamann A, Lawitts JA, Himms-Hagen J, Boyer BB, Kozak LP, Flier JS: Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature 1993;366:740–742 [DOI] [PubMed] [Google Scholar]
- 4.Cannon B, Nedergaard J: Developmental biology: neither fat nor flesh. Nature 2008;454:947–948 [DOI] [PubMed] [Google Scholar]
- 5.Nedergaard J, Bengtsson T, Cannon B: Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 2007;293:E444–452 [DOI] [PubMed] [Google Scholar]
- 6.Zingaretti MC, Crosta F, Vitali A, Guerrieri M, Frontini A, Cannon B, Nedergaard J, Cinti S: The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J 2009;23:3113–3120 [DOI] [PubMed] [Google Scholar]
- 7.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR: Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009;360:1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerbäck S, Nuutila P: Functional brown adipose tissue in healthy adults. N Engl J Med 2009;360:1518–1525 [DOI] [PubMed] [Google Scholar]
- 9.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ: Cold-activated brown adipose tissue in healthy men. N Engl J Med 2009;360:1500–1508 [DOI] [PubMed] [Google Scholar]
- 10.Celi FS: Brown adipose tissue–when it pays to be inefficient. N Engl J Med 2009;360:1553–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stefan N, Pfannenberg C, Häring HU: The importance of brown adipose tissue. N Engl J Med 2009;361:416–417 [PubMed] [Google Scholar]
- 12.Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, Kawai Y, Tsujisaki M: High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 2009;58:1526–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Au-Yong IT, Thorn N, Ganatra R, Perkins AC, Symonds ME: Brown adipose tissue and seasonal variation in humans. Diabetes 2009;58:2583–2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seale P, Lazar MA: Brown fat in humans: turning up the heat on obesity. Diabetes 2009;58:1482–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouchard C, Tremblay A, Després JP, Nadeau A, Lupien PJ, Thériault G, Dussault J, Moorjani S, Pinault S, Fournier G: The response to long-term overfeeding in identical twins. N Engl J Med 1990;322:1477–1482 [DOI] [PubMed] [Google Scholar]
- 16.Leibel RL: Molecular physiology of weight regulation in mice and humans. Int J Obes (Lond) 32 Suppl2008;7:S98–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wijers SL, Saris WH, van Marken Lichtenbelt WD: Recent advances in adaptive thermogenesis: potential implications for the treatment of obesity. Obes Rev 2009;10:218–226 [DOI] [PubMed] [Google Scholar]
- 18.Williams G, Kolodny GM: Method for decreasing uptake of 18F-FDG by hypermetabolic brown adipose tissue on PET. AJR Am J Roentgenol 2008;190:1406–1409 [DOI] [PubMed] [Google Scholar]
- 19.Quevedo S, Roca P, Picó C, Palou A: Sex-associated differences in cold-induced UCP1 synthesis in rodent brown adipose tissue. Pflugers Arch 1998;436:689–695 [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez AM, Monjo M, Roca P, Palou A: Opposite actions of testosterone and progesterone on UCP1 mRNA expression in cultured brown adipocytes. Cell Mol Life Sci 2002;59:1714–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lisbôa PC, Curty FH, Moreira RM, Oliveira KJ, Pazos-Moura CC: Sex steroids modulate rat anterior pituitary and liver iodothyronine deiodinase activities. Horm Metab Res 2001;33:532–535 [DOI] [PubMed] [Google Scholar]
- 22.Bianco AC, Silva JE: Intracellular conversion of thyroxine to triiodothyronine is required for the optimal thermogenic function of brown adipose tissue. J Clin Invest 1987;79:295–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.