Abstract
OBJECTIVE
This study aimed to estimate the current cumulative risk of end-stage renal disease (ESRD) due to diabetic nephropathy in a large, nationwide, population-based prospective type 1 diabetes cohort and specifically study the effects of sex and age at onset.
RESEARCH DESIGN AND METHODS
In Sweden, all incident cases of type 1 diabetes aged 0–14 years and 15–34 years are recorded in validated research registers since 1977 and 1983, respectively. These registers were linked to the Swedish Renal Registry, which, since 1991, collects data on patients who receive active uremia treatment. Patients with ≥13 years duration of type 1 diabetes were included (n = 11,681).
RESULTS
During a median time of follow-up of 20 years, 127 patients had developed ESRD due to diabetic nephropathy. The cumulative incidence at 30 years of type 1 diabetes duration was low, with a male predominance (4.1% [95% CI 3.1–5.3] vs. 2.5% [1.7–3.5]). In both male and female subjects, onset of type 1 diabetes before 10 years of age was associated with the lowest risk of developing ESRD. The highest risk of ESRD was found in male subjects diagnosed at age 20–34 years (hazard ratio 3.0 [95% CI 1.5–5.7]). In female subjects with onset at age 20–34 years, the risk was similar to patients' diagnosed before age 10 years.
CONCLUSIONS
The cumulative incidence of ESRD is exceptionally low in young type 1 diabetic patients in Sweden. There is a striking difference in risk for male compared with female patients. The different patterns of risk by age at onset and sex suggest a role for puberty and sex hormones.
Diabetic nephropathy is one of the most severe complications in patients with type 1 diabetes, leading to end-stage renal disease (ESRD) and the need for renal replacement therapy (dialysis and transplantation). Diabetic nephropathy is also a major predictor of cardiovascular morbidity and mortality in patients with type 1 diabetes (1). Although the incidence of type 1 diabetes has increased in children, and onset of disease occurs at younger age (2,3), a decrease in incidence of diabetic nephropathy and a longer duration from onset of diabetes to diabetic nephropathy and ESRD has been reported from dedicated centers (4). Recently, a follow-up of the Diabetes Control and Complications Trial–intensive treated type 1 diabetes case subjects showed a cumulative incidence of nephropathy of 9% at 30 years of diabetes duration compared with 25% in the conventionally treated group (5). A Finnish population-based study showed a cumulative incidence of ESRD of 7.8% after 30 years of diabetes duration (6). Next to Finland, Sweden has the highest incidence of childhood-onset diabetes reported worldwide (7), and since the 1980s Sweden has a strict nationwide childhood diabetes care program that includes intensive insulin treatment and home blood glucose monitoring to counteract development of late complications.
Poor glycemic control and high blood pressure are the two most important risk factors in the initiation and development of diabetic nephropathy (8,9), but they are not sufficient for development of diabetic nephropathy and ESRD. Other factors, such as genetic susceptibility and growth and sex hormones, seem to contribute (10,11). Some studies (12–14) suggest that male sex is a risk factor for development of diabetic nephropathy and ESRD. Several studies (6,15,16) indicate that young age at onset of diabetes can prolong the time until development of microalbuminuria, diabetic nephropathy, ESRD, and other vascular complications. It has thus been suggested that puberty could promote the development of chronic diabetes complications due to deterioration of glycemic control, rapid growth, and hormonal changes (17,18). An increased risk for both hospitalization due to severe vascular complications and a higher mortality rate have also been found in patients with pubertal onset of diabetes compared with those with younger age at onset (19,20). If puberty is associated with increased risk of diabetic nephropathy, diabetes onset after that age would decrease diabetic nephropathy risk and approach the risk of prepubertal-onset cases.
In the present study, we used data from two large, nationwide, population-based cohorts of young patients with type 1 diabetes for the following reasons:
to estimate the cumulative incidence and long-term risk of ESRD after 30 years of type 1 diabetes with recommended intensive insulin treatment; and
to study the effects of age at onset of diabetes and sex on these risks.
RESEARCH DESIGN AND METHODS
Since 1 July 1977, all incident cases of type 1 diabetes in those aged 0–14 years are recorded in the Swedish Childhood Diabetes Registry (SCDR). Only those who are insulin treated from diagnosis (∼99% of cases) are registered. Comparisons with external sources have shown that the level of ascertainment in the SCDR is 96–99% (21,22). The Diabetes Incidence Study in Sweden (DISS) records incident cases of diabetes in the age-group 15–34 years since 1 January 1983. The completeness of the DISS register has varied between 82 and 91%, depending on the source of ascertainment (23), with no significant sex difference. The classification into type 1, type 2, and unclassified diabetes is based on the treating doctors' clinical classification. During 1983–1991, the World Health Organization classification was used, and since 1992 the American Diabetes Association classification criteria were used. This change in diagnostic criteria would probably little affect the results, since only clinically overt cases of type 1 diabetes were included. Of all patients, <10% who were classified by clinical criteria as having type 1 diabetes at diagnosis are misclassified when the diagnosis was checked using autoantibodies and C-peptide (24).
ESRD is defined as need to start active uremia treatment due to renal failure (glomerular filtration rate <10–15 ml/min). The Swedish Renal Registry (SRR) collects data on all patients with chronic renal failure who start dialysis treatment or receive a kidney transplant. A validation study showed that >95% of the patients who started treatment for chronic renal failure had been reported to the SRR (25). The SRR started in 1991, and at that time none of the patients in the SCDR had diabetes duration longer than 13 years.
When type of diabetes differed between the two diabetes registries and the SRR, the classification reported to the SRR was used since this was made after a long clinical follow-up. Five patients were classified as having ESRD due to type 2 diabetes in the SRR and were therefore excluded from the analyses. Patients with ESRD due to other diagnoses than diabetes (n = 11) were also excluded.
The present study covers the majority of all cases of ESRD due to type 1 diabetes at 13 years duration or longer, during 1991–2007, and should hereby represent the Swedish type 1 diabetic population at large. Patients with 13 years duration (i.e., diabetes onset 1 July 1977 to 31 December 1995 for the SCDR and 1 January 1983 to 31 December 1995 for the DISS) would have equal chance of entering the SRR, starting in 1991. Thus, 6,789 patients with onset before age 15 years and 4,892 patients with onset of diabetes between age 15 and 34 years were included. Dates of death were obtained by linking the diabetes registers to the Swedish Cause of Death Register.
This study was approved by the regional research ethics committee in Umeå, according to the Swedish law on research ethics and in line with the principles of the Helsinki Declaration and the European convention on human rights and biomedicine. The study and the statistical analysis were designed and interpreted by the authors. The funding bodies had no role in the design and conduct of the study; in collection, management, analysis, or interpretation of the data; or in preparation and approval of the manuscript.
Statistical analyses.
The age at onset was divided into three groups, aged 0–9, 10–19, and 20–34 years; hence, the 10–19 age at onset group includes the pubertal years for the vast majority of the cohort. Incidence rates of ESRD were calculated as number of cases divided by number of years at risk in 6-year intervals (13–18, 19–24, and 25–30 years of diabetes). Kaplan-Meier analyses were used to calculate the cumulative incidences. Cox regression analyses were performed to estimate the hazard ratio (HR) of developing ESRD, to compare the HR by age-at-onset groups and sex, and to adjust for the potential confounding variables age at follow-up and sex. In these analyses, the time at risk was calculated from onset of diabetes until ESRD (i.e., date of first treatment with renal replacement therapy), death, or 31 December 2007.
Kaplan-Meier analyses may overestimate the cumulative incidence when the event of death is censored in the same way as when censoring for other reasons. Therefore, we also computed the cumulative incidence when taking into account death as a competing risk event. This method accounts for death as an event that removes the risk of ESRD and hence provides a more accurate estimate of the risk (26). SPSS 16.0 for Windows was used for the Kaplan-Meier and Cox regression analyses, while the R statistical software version 2.5.1 (The R foundation for statistical computing available at http://www.r-project.org/index.html), with the function “cuminc” from the “cmprsk” package, was used for calculations with death as a competing risk event.
RESULTS
Long-term incidence rate and cumulative incidence of ESRD.
The study included patients with at least 13 years duration of type 1 diabetes. In total, 127 patients had developed ESRD due to type 1 diabetes, 79 in the SCDR and 48 in the DISS (Table 1). No patient had developed ESRD before 13 years duration of diabetes. Maximum follow-up was 30.0 years for the SCDR and 24.9 years for the DISS. The median follow-up time was 21.2 years for patients in the SCDR and 18.9 for patients in the DISS. The median time from onset of diabetes to ESRD was 21.7 (range 14.7–28.2) and 18.5 (13.7–24.8), respectively. The overall incidence rate of ESRD during 237,592 person-years of follow-up was 0.53 per 1,000 person-years.
TABLE 1.
Number of patients with and without ESRD
| Age at diagnosis (years) | Male subjects |
Female subjects |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
n (%) |
Median (range) time from diabetes onset to ESRD (years) |
n deaths (%) |
n (%) |
Median (range) time from diabetes onset to ESRD (years) |
n deaths (%) |
|||||
| Without ESRD | With ESRD | Without ESRD | With ESRD | Without ESRD | With ESRD | Without ESRD | With ESRD | |||
| 0–9 | 1,984 (99.2) | 15 (0.8) | 23.1 (18.2–26.2) | 23 (1.2) | 4 (0.2) | 1,860 (99.4) | 11 (0.6) | 21.7 (16.2–27.8) | 10 (0.5) | 4 (0.2) |
| 10–19 | 2,244 (98.2) | 41 (1.8) | 20.8 (15.3–28.2) | 30 (1.3) | 9 (0.4) | 1,796 (98.5) | 28 (1.5) | 18.9 (14.7–26.0) | 15 (0.8) | 5 (0.3) |
| 20–34 | 2,267 (98.9) | 25 (1.1) | 18.6 (13.7–24.8) | 86 (3.7) | 10 (0.4) | 1,403 (99.5) | 7 (0.5) | 17.9 (14.0–23.1) | 27 (1.9) | 1 (0.1) |
| 0–34 | 6,495 (98.8) | 81 (1.2) | 20.7 (13.7–28.2) | 139 (2.1) | 23 (0.3) | 5,059 (99.1) | 46 (0.9) | 19.5 (14.0–27.8) | 52 (1.0) | 10 (0.2) |
Median (range) time from diabetes onset to ESRD and number of deaths with and without ESRD, by age at diagnosis and sex, in patients with type 1 diabetes with at least 13 years duration.
Effect of age at onset and sex on development of ESRD.
Table 2 shows incidence rates of ESRD per 1,000 person-years of diabetes duration, at 13–18, 19–24, and 25–30 years after diabetes onset. The incidence increased with increasing diabetes duration. The sharpest increase in incidence was seen between 13–18 and 19–24 years, while the increase between 19–24 and 25–30 years was modest, partly due to the fact that the 20- to 34-year-onset group could not contribute as they had a maximum duration of 25 years.
TABLE 2.
Incidence rates per 1,000 person-years at 6-year intervals of diabetes duration (13–18, 19–24, and 25–30 years after diagnosis)
| Age at diagnosis (years) | Intervals of diabetes duration (years) |
||
|---|---|---|---|
| 13–18 years (male/female) | 19–24 years (male/female) | 25–30 years (male/female) | |
| 0–9 | 0.1 (−0.1 to 0.3)/0.2 (−0.1 to 0.5) | 1.9 (0.7–3.0)/1.0 (0.1–1.9) | 2.8 (0.1–5.6)/3.0 (0.1–5.9) |
| 10–19 | 1.0 (0.4–1.6)/1.7 (0.8–2.6) | 4.3 (2.6–6.0)/2.2 (0.8–3.6) | 4.6 (0.9–8.3)/2.8 (−0.4 to 6.0) |
| 20–34 | 1.3 (0.6–2.0)/0.8 (0.1–1.5) | 3.6 (1.6–5.6)/0.9 (−0.4 to 2.2) | — |
| 0–34 | 0.8 (0.5–1.1)/0.9 (0.5–1.3) | 3.2 (2.3–4.2)/1.5 (0.8–2.2) | 3.7 (1.4–5.9)/2.9 (0.8–5.1) |
Data are incidence rate (95% CI).
Tables 3 and 4 show the cumulative incidences in the different age-at-onset groups, by sex, with and without accounting for death as competing risk event, respectively. Only 224 of 11,681 patients (1.9%) had died, 33 of them after having developed ESRD (Table 1). Therefore, the analyses with death as competing risk did not change the results. The overall mortality in the study was 0.94 deaths per 1,000 person-years of diabetes duration. There was a 14 times higher risk of death among patients with ESRD (HR 14 [95% CI 9.7–21]), adjusted for sex and age at follow-up. Male patients had almost twice the risk of death due to any cause, compared with female patients (1.9 [1.4–2.6]), adjusted for ESRD and age at follow-up.
TABLE 3.
Cumulative incidences of ESRD with death as competing risk, by age at onset and sex, at different diabetes durations
| Age at diagnosis (years) | Duration of type 1 diabetes (years) |
||
|---|---|---|---|
| 20 years (male/female) | 25 years (male/female) | 30 years (male/female) | |
| 0–9 | 0.1 (0.0–0.4)/0.2 (0.1–0.7) | 1.3 (0.7–2.3)/0.7 (0.3–1.4) | 2.3 (1.3–3.7)/1.9 (0.9–3.5) |
| 10–19 | 1.0 (0.6–1.6)/1.3 (0.8–2.0) | 3.3 (2.3–4.6)/2.4 (1.5–3.5) | 5.2 (3.5–7.4)/3.2 (2.0–4.8) |
| 20–34 | 1.0 (0.6–1.7)/0.7 (0.3–1.5) | 5.7 (1.5–14.2)/1.1 (0.4–2.4) | — |
| 0–34 | 0.7 (0.5–1.0)/0.7 (0.5–1.0) | 2.6 (2.0–3.3)/1.4 (1.0–2.0) | 4.0 (3.0–5.2)/2.4 (1.6–3.5) |
Data are incidence rate (95% CI). The cumulative incidence with death as a competing risk takes into account that death is an event competing with the risk to develop ESRD.
TABLE 4.
Cumulative incidences of ESRD, by age at onset and sex, at different diabetes durations
| Age at diagnosis (years) | Duration of type 1 diabetes (years) |
||
|---|---|---|---|
| 20 years (male/female) | 25 years (male/female) | 30 years (male/female) | |
| 0–9 | 0.1 (0.0–0.4)/0.2 (0.1–0.7) | 1.4 (0.7–2.4)/0.7 (0.3–1.4) | 2.3 (1.3–3.8)/1.9 (0.9–3.6) |
| 10–19 | 1.0 (0.6–1.7)/1.3 (0.8–2.0) | 3.3 (2.3–4.6)/2.4 (1.5–3.5) | 5.3 (3.5–7.5)/3.2 (2.1–4.8) |
| 20–34 | 1.0 (0.6–1.7)/0.7 (0.3–1.5) | 6.1 (1.5–15.5)/1.1 (0.4–2.5) | - |
| 0–34 | 0.7 (0.5–1.0)/0.7 (0.5–1.1) | 2.6 (2.0–3.3)/1.4 (1.0–2.0) | 4.1 (3.1–5.3)/2.5 (1.7–3.5) |
Data are incidence rate (95% CI). The cumulative incidence is estimated using the Kaplan-Meier method and given as percent.
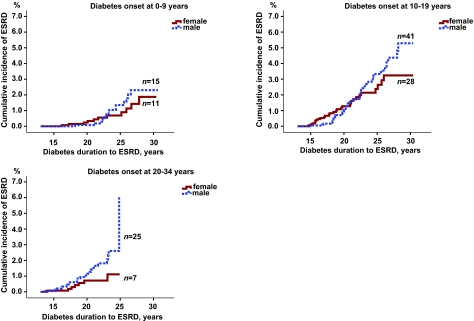
Among patients who developed type 1 diabetes before the age of 20 years, there was no difference between male and female subjects as can be seen in the cumulative incidence curves (Fig. 1). Male subjects who were aged 20–34 years when diagnosed with type 1 diabetes had twice as high risk of ESRD as female subjects (HR 2.3 [95% CI 0.99–5.3]). Taking death into account as competing risk reduced the male/female risk increase marginally (2.2 [0.97–5.2]).
FIG. 1.
Cumulative incidences of developing ESRD in male and female patients with type 1 diabetes onset at 0–9, 10–19, and 20–34 years. For patients with diabetes onset before 10 or 10–19 years of age, there is no significant difference between male and female subjects (P = 0.53 and P = 0.50), but with onset at 20–34 years of age there is a difference, although borderline significant, between male and female subjects in risk of developing ESRD (P = 0.05).
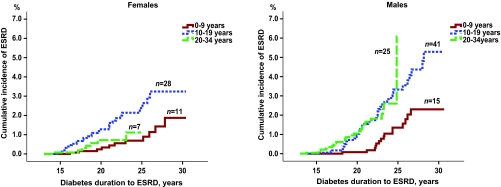
The cumulative incidences of ESRD for male and female subjects in the different age-at-onset groups (0–9, 10–19, and 20–34 years) are shown in Fig. 2. The lowest risk for ESRD was found in male and female subjects with onset of type 1 diabetes before 10 years of age (Fig. 2). Among male patients, the risk of developing ESRD was significantly increased in those developing diabetes at 20–34 years of age (HR 3.0 [95% CI 1.5–5.7]) as well as 10–19 years of age (2.6 [1.5–4.7]), compared with the youngest age-at-onset group (0–9 years). Taking death into account as a competing risk did not change the results.
FIG. 2.
Cumulative incidences of developing ESRD in male and female patients according to age at onset of type 1 diabetes. When using age at onset 0–9 years as reference, the risk of ESRD is significantly increased with age at onset 10–19 years and 20–34 years for male subjects. For female subjects, the risk is significantly increased with age at onset 10–19 years but not 20–34 years.
In female subjects, there was no difference in the risk of developing ESRD with diagnosis of type 1 diabetes at age 20–34 years compared with diagnosis at younger than 10 years of age, (HR 1.4 [95% CI 0.5–3.6]). The highest risk was observed for the 10–19 years age-group (2.8 [1.4–5.5]) (Fig. 2). Taking death into account as a competing risk did not change the results. Using a grouping of age at onset 0–9, 10–14, 15–24, and 25–34 years did not change the overall pattern of difference seen by sex.
DISCUSSION
In this nationwide population-based study of patients with type 1 diabetes and at least 13 years duration, the cumulative incidence of ESRD due to diabetic nephropathy is surprisingly low (3.3% at 30 years of duration). The Swedish Pediatric Association Working Group for Diabetes in Children in the nationwide Diabetes in Childhood Care Program already in 1982 (updated regularly) recommended intensive insulin treatment and home blood glucose monitoring. In adults, national guidelines for treatment of diabetes were issued by the Swedish Board of Health and Welfare in 1977 and since then regularly updated. These guidelines also involve intensive treatment of glucose and blood pressure, including ACE inhibition, for type 1 diabetic patients. These active treatment programs may clearly contribute to the low rate of ESRD in Sweden, and our findings are in accord with that of a decrease in incidence of albuminuria as reported from a dedicated center in Sweden (4).
Since the study is based on incidence registers, we have no access to individual A1C data, but according to the Swedish National Diabetes Register (NDR) since 1996 estimate markers of quality of care, the yearly mean A1C values, have decreased from 8.5% (Diabetes Control and Complications Trial standard) to 8.1% during the time period 1996–2005.
Previous studies, from different populations with different years of onset of diabetes, have reported a cumulative incidence of ESRD of 7–13% at 20 years of diabetes duration (27–29). The cumulative incidence seen in this study is also lower than reported in a recent Finnish nationwide population-based study, in which 2.2% at 20 years of follow-up and 7.8% at 30 years of follow-up were found (6). The Finnish study also showed a time-period effect, with a decline in cumulative incidence over time (1965–1999). In our cohort, however, there was no difference in risk of ESRD depending on year of diabetes onset, which may be explained by the later starting date of our study (1977) and more active treatment programs for both metabolic control and signs of incipient nephropathy. This difference between the cohorts may also contribute to the discrepancies in cumulative incidences. A decline in cumulative incidence of ESRD has been indicated by an unchanged reporting rate for type 1 diabetes in both the European Dialysis and Transplant Association registry, including the SRR, through the 1990–2000s, despite an increase in prevalence of type 1 diabetes and longer survival in patients with type 1 diabetes (30).
The peak incidence of diabetic nephropathy has been found to occur 15–25 years after the onset of type 1 diabetes (27,31), and the median duration from onset of diabetic nephropathy to ESRD is usually ∼10 years (27). The development of ESRD due to diabetic nephropathy within 15 years of diabetes duration is rare; in this study, only three patients had developed ESRD before this duration. The relatively constant incidence rates at 19–24 and 25–30 years of diabetes duration may indicate that the peak incidence of ESRD had been reached at 30 years of diabetes duration or that the peak incidence has been delayed beyond 30 years of diabetes duration. Both alternatives suggest a favorable change in the natural history of diabetic nephropathy also in susceptible patients. These findings of a favorable time trend in diabetes nephropathy is in correspondence with the results of Pittsburg Epidemiology of Diabetes Complications Study (32).
Our study confirms previous findings of a reduced risk, or a delay, in development of ESRD in patients diagnosed with type 1 diabetes before the age 5 (n = 2) and 10 years (6,14,15,33). A similar age dependency of risk has been found for severe retinopathy and blindness due to diabetes (33). The reasons for this age-at-onset effect could be, for example, genetic, endocrine, or health care related. It could be argued that children and families who become used to insulin treatment at an early age might adhere better to treatment and diet than those that are diagnosed with diabetes at an older age and especially during puberty. Previous studies (17,34) have indicated that prepubertal years with diabetes involve a reduced risk or a longer time to development of diabetic nephropathy and retinopathy. It also has long been speculated that puberty, characterized by rapid growth, hormonal changes (especially in growth hormone and sex hormones), and worsening in glycemic control, may accelerate the processes leading to chronic diabetes complications (35,36). We speculated that if puberty was a strong determinant for development of diabetic nephropathy, then diabetes onset after puberty would give a similar risk as prepubertal onset. In our study, this was found in female subjects only. In male subjects, the ESRD risk was increased also after puberty compared with onset of diabetes at 0–9 years of age. The group with age at onset at 10–19 years includes both prepubertal and postpubertal cases, which could dilute the actual effect of puberty; however, this group includes almost all with diabetes onset during the pubertal years.
Male sex has been reported to be a risk factor for development of diabetic nephropathy, even though this relationship is not as strong as in nondiabetic renal diseases (12,14). In this study, male subjects had an increased overall cumulative incidence of ESRD compared with female subjects but only in patients with age at onset of diabetes at ≥20 years. The higher male-to-female ratio of ESRD found in our study is further supported by data generated from the NDR (personal communication) showing that the mean prevalence of both micro- and macroalbuminuria was higher in men than in women in 2009 (15.6 vs. 11.6% and 8.4 vs. 7.2%, respectively).
The factors involved in this sex-specific difference could possibly include lifestyle, diet, kidney and glomerular size, differences in glomerular hemodynamics, and direct effects of sex hormones (37,38). When accounting for death as a competing risk event, male subjects still had twice the risk of developing ESRD, however not statistically significant, which can be explained by the higher death rates in male subjects. Experimental evidence from animal studies suggests that both estrogens and testosterone play a role in the development of renal disease (39); estrogens slow progression rate (40,41), while testosterone exacerbates it, and the absence of testosterone attenuates the development of renal disease (42). The pattern of cumulative incidence by age and sex in the present study indicates that different combinations of factors play a role in postpubertal development of ESRD, and further studies are needed to confirm and understand these effects.
In conclusion, the cumulative incidence of ESRD in young patients with type 1 diabetes, with onset after 1977, is very low in Sweden. Prepubertal age at onset of diabetes seems to protect against, or prolong, the time to ESRD development, and the same may be true for postpubertal onset in female subjects (aged ≥20 years at onset of diabetes). The finding of a sex difference specifically in patients with diabetes onset after 20 years of age is of clear interest and needs further exploration.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grants from the Swedish Research Council (project no. 07531), the Faculty of Medicine at Umeå University, the Lundström Foundation, the Swedish Association for Patients With Kidney Disease, the Swedish Society for Medicine, and the Västerbotten County Council.
No potential conflicts of interest relevant to this article were reported.
A.M. and M.S. researched data and wrote the manuscript. I.W. researched data and contributed to the manuscript. Y.B. reviewed/edited the manuscript. S.S., L.N. and H.A. supervised data collection and reviewed/edited the manuscript. G.D. supervised data collection and wrote the manuscript.
We thank L. Mustonen (Department of Public Health and Clinical Medicine, Epidemiology, and Global Health, Umeå University) and S. Gabara (Department of Internal Medicine, Ryhov County Hospital) for valuable assistance in processing data from the SCDR and the SRR, respectively, and S. Gudbjörnsdottir, NDR, for personal communication regarding albuminuria and sex-differences.
Footnotes
*A full list of members of the Swedish Childhood Diabetes Study Group, the Diabetes Incidence Study in Sweden, and the Swedish Renal Registry is available in the online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db09-1744/DC1.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Borch-Johnsen K, Andersen PK, Deckert T: The effect of proteinuria on relative mortality in type 1 (insulin- dependent) diabetes mellitus. Diabetologia 1985;28:590–596 [DOI] [PubMed] [Google Scholar]
- 2.Patterson CC, Dahlquist GG, Gyurus E, Green A, Soltesz G: Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet 2009;373:2027–2033 [DOI] [PubMed] [Google Scholar]
- 3.Pundziute-Lyckå A, Dahlquist G, Nystrom L, Arnqvist H, Bjork E, Blohme G, Bolinder J, Eriksson JW, Sundkvist G, Ostman J: The incidence of Type I diabetes has not increased but shifted to a younger age at diagnosis in the 0–34 years group in Sweden 1983–1998. Diabetologia 2002;45:783–791 [DOI] [PubMed] [Google Scholar]
- 4.Nordwall M, Bojestig M, Arnqvist HJ, Ludvigsson J: Declining incidence of severe retinopathy and persisting decrease of nephropathy in an unselected population of type 1 diabetes-the Linkoping Diabetes Complications Study. Diabetologia 2004;47:1266–1272 [DOI] [PubMed] [Google Scholar]
- 5.Nathan DM, Zinman B, Cleary PA, Backlund JY, Genuth S, Miller R, Orchard TJ: Modern-day clinical course of type 1 diabetes mellitus after 30 years' duration: the diabetes control and complications trial/epidemiology of diabetes interventions and complications and Pittsburgh epidemiology of diabetes complications experience (1983–2005). Arch Intern Med 2009;169:1307–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finne P, Reunanen A, Stenman S, Groop PH, Gronhagen-Riska C: Incidence of end-stage renal disease in patients with type 1 diabetes. JAMA 2005;294:1782–1787 [DOI] [PubMed] [Google Scholar]
- 7.DIAMOND Project Group Incidence and trends of childhood type 1 diabetes worldwide 1990–1999. Diabet Med 2006;23:857–866 [DOI] [PubMed] [Google Scholar]
- 8.Parving HH: Initiation and progression of diabetic nephropathy. N Engl J Med 1996;335:1682–1683 [DOI] [PubMed] [Google Scholar]
- 9.Rossing P, Hougaard P, Parving HH: Risk factors for development of incipient and overt diabetic nephropathy in type 1 diabetic patients: a 10-year prospective observational study. Diabetes Care 2002;25:859–864 [DOI] [PubMed] [Google Scholar]
- 10.Vasylyeva TL, Ferry RJ, Jr: Novel roles of the IGF-IGFBP axis in etiopathophysiology of diabetic nephropathy. Diabetes Res Clin Pract 2007;76:177–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Q, Wells CC, Garman JH, Asico L, Escano CS, Maric C: Imbalance in sex hormone levels exacerbates diabetic renal disease. Hypertension 2008;51:1218–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orchard TJ, Dorman JS, Maser RE, Becker DJ, Drash AL, Ellis D, LaPorte RE, Kuller LH: Prevalence of complications in IDDM by sex and duration: Pittsburgh Epidemiology of Diabetes Complications Study II. Diabetes 1990;39:1116–1124 [DOI] [PubMed] [Google Scholar]
- 13.Hovind P, Tarnow L, Rossing P, Jensen BR, Graae M, Torp I, Binder C, Parving HH: Predictors for the development of microalbuminuria and macroalbuminuria in patients with type 1 diabetes: inception cohort study. BMJ 2004;328:1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raile K, Galler A, Hofer S, Herbst A, Dunstheimer D, Busch P, Holl RW: Diabetic nephropathy in 27,805 children, adolescents, and adults with type 1 diabetes: effect of diabetes duration, A1C, hypertension, dyslipidemia, diabetes onset, and sex. Diabetes Care 2007;30:2523–2528 [DOI] [PubMed] [Google Scholar]
- 15.Svensson M, Nystrom L, Schon S, Dahlquist G: Age at onset of childhood-onset type 1 diabetes and the development of end-stage renal disease: a nationwide population-based study. Diabetes Care 2006;29:538–542 [DOI] [PubMed] [Google Scholar]
- 16.Porta M, Sjoelie AK, Chaturvedi N, Stevens L, Rottiers R, Veglio M, Fuller JH: Risk factors for progression to proliferative diabetic retinopathy in the EURODIAB Prospective Complications Study. Diabetologia 2001;44:2203–2209 [DOI] [PubMed] [Google Scholar]
- 17.Dahlquist G, Rudberg S: The prevalence of microalbuminuria in diabetic children and adolescents and its relation to puberty. Acta Paediatr Scand 1987;76:795–800 [DOI] [PubMed] [Google Scholar]
- 18.Cummings EA, Sochett EB, Dekker MG, Lawson ML, Daneman D: Contribution of growth hormone and IGF-I to early diabetic nephropathy in type 1 diabetes. Diabetes 1998;47:1341–1346 [DOI] [PubMed] [Google Scholar]
- 19.Dahlquist G, Möllsten A, Kallen B: Hospitalization for vascular complications in childhood onset type 1 diabetes: effects of gender and age at onset. Acta Paediatr 2008;97:483–488 [DOI] [PubMed] [Google Scholar]
- 20.Nishimura R, Tajima N, Matsushima M, LaPorte RE: Puberty, IDDM, and death in Japan: Diabetes Epidemiology Research International Study Group. Diabetes Care 1998;21:1674–1679 [DOI] [PubMed] [Google Scholar]
- 21.Dahlquist G, Blom L, Holmgren G, Hagglof B, Larsson Y, Sterky G, Wall S: The epidemiology of diabetes in Swedish children 0–14 years: a six-year prospective study. Diabetologia 1985;28:802–808 [DOI] [PubMed] [Google Scholar]
- 22.Nyström L, Dahlquist G, Rewers M, Wall S: The Swedish childhood diabetes study: an analysis of the temporal variation in diabetes incidence 1978–1987 Int J Epidemiol 1990;19:141–146 [DOI] [PubMed] [Google Scholar]
- 23.Ostman J, Lonnberg G, Arnqvist HJ, Blohme G, Bolinder J, Ekbom Schnell A, Eriksson JW, Gudbjornsdottir S, Sundkvist G, Nystrom L: Gender differences and temporal variation in the incidence of type 1 diabetes: results of 8012 cases in the nationwide Diabetes Incidence Study in Sweden 1983–2002. J Intern Med 2008;263:386–394 [DOI] [PubMed] [Google Scholar]
- 24.Borg H, Arnqvist HJ, Bjork E, Bolinder J, Eriksson JW, Nystrom L, Jeppsson JO, Sundkvist G: Evaluation of the new ADA and WHO criteria for classification of diabetes mellitus in young adult people (15–34 yrs) in the Diabetes Incidence Study in Sweden (DISS). Diabetologia 2003;46:173–181 [DOI] [PubMed] [Google Scholar]
- 25.Schön S, Ekberg H, Wikstrom B, Oden A, Ahlmen J: Renal replacement therapy in Sweden. Scand J Urol Nephrol 2004;38:332–339 [DOI] [PubMed] [Google Scholar]
- 26.Satagopan JM, Ben-Porat L, Berwick M, Robson M, Kutler D, Auerbach AD: A note on competing risks in survival data analysis. Br J Cancer 2004;91:1229–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krolewski AS, Warram JH, Christlieb AR, Busick EJ, Kahn CR: The changing natural history of nephropathy in type I diabetes. Am J Med 1985;78:785–794 [DOI] [PubMed] [Google Scholar]
- 28.Matsushima M, Tajima N, LaPorte RE, Orchard TJ, Tull ES, Gower IF, Kitagawa T: Markedly increased renal disease mortality and incidence of renal replacement therapy among IDDM patients in Japan in contrast to Allegheny County, Pennsylvania, USA: Diabetes Epidemiology Research International (DERI) US-Japan Mortality Study Group. Diabetologia 1995;38:236–243 [DOI] [PubMed] [Google Scholar]
- 29.Krolewski M, Eggers PW, Warram JH: Magnitude of end-stage renal disease in IDDM: a 35 year follow-up study. Kidney Int 1996;50:2041–2046 [DOI] [PubMed] [Google Scholar]
- 30.Van Dijk PC, Jager KJ, Stengel B, Gronhagen-Riska C, Feest TG, Briggs JD: Renal replacement therapy for diabetic end-stage renal disease: data from 10 registries in Europe (1991–2000). Kidney Int 2005;67:1489–1499 [DOI] [PubMed] [Google Scholar]
- 31.Andersen AR, Christiansen JS, Andersen JK, Kreiner S, Deckert T: Diabetic nephropathy in Type 1 (insulin-dependent) diabetes: an epidemiological study. Diabetologia 1983;25:496–501 [DOI] [PubMed] [Google Scholar]
- 32.Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ: The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes 2006;55:1463–1469 [DOI] [PubMed] [Google Scholar]
- 33.Morimoto A, Nishimura R, Matsudaira T, Sano H, Tajima N: Is pubertal onset a risk factor for blindness and renal replacement therapy in childhood-onset type 1 diabetes in Japan? Diabetes Care 2007;30:2338–2340 [DOI] [PubMed] [Google Scholar]
- 34.Olsen BS, Sjolie AK, Hougaard P, Johannesen J, Marinelli K, Jacobsen BB, Mortensen HB: The significance of the prepubertal diabetes duration for the development of retinopathy and nephropathy in patients with type 1 diabetes. J Diabetes Complications 2004;18:160–164 [DOI] [PubMed] [Google Scholar]
- 35.Lundbaek K, Christensen NJ, Jensen VA, Johansen K, Olsen TS, Hansen AP, Orskov H, Osterby R: Diabetes, diabetic angiopathy, and growth hormone. Lancet 1970;2:131–133 [DOI] [PubMed] [Google Scholar]
- 36.Lane PH: Diabetic kidney disease: impact of puberty. Am J Physiol Renal Physiol 2002;283:F589–F600 [DOI] [PubMed] [Google Scholar]
- 37.Neugarten J: Gender and the progression of renal disease. J Am Soc Nephrol 2002;13:2807–2809 [DOI] [PubMed] [Google Scholar]
- 38.Miller JA, Anacta LA, Cattran DC: Impact of gender on the renal response to angiotensin II. Kidney Int 1999;55:278–285 [DOI] [PubMed] [Google Scholar]
- 39.Maric C: Sex, diabetes and the kidney. Am J Physiol Renal Physiol 2009;296:F680–F688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keck M, Romero-Aleshire MJ, Cai Q, Hoyer PB, Brooks HL: Hormonal status affects the progression of STZ-induced diabetes and diabetic renal damage in the VCD mouse model of menopause. Am J Physiol Renal Physiol 2007;293:F193–F199 [DOI] [PubMed] [Google Scholar]
- 41.Mankhey RW, Bhatti F, Maric C: 17beta-Estradiol replacement improves renal function and pathology associated with diabetic nephropathy. Am J Physiol Renal Physiol 2005;288:F399–F405 [DOI] [PubMed] [Google Scholar]
- 42.Reckelhoff JF, Yanes LL, Iliescu R, Fortepiani LA, Granger JP: Testosterone supplementation in aging men and women: possible impact on cardiovascular-renal disease. Am J Physiol Renal Physiol 2005;289:F941–F948 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.