Abstract
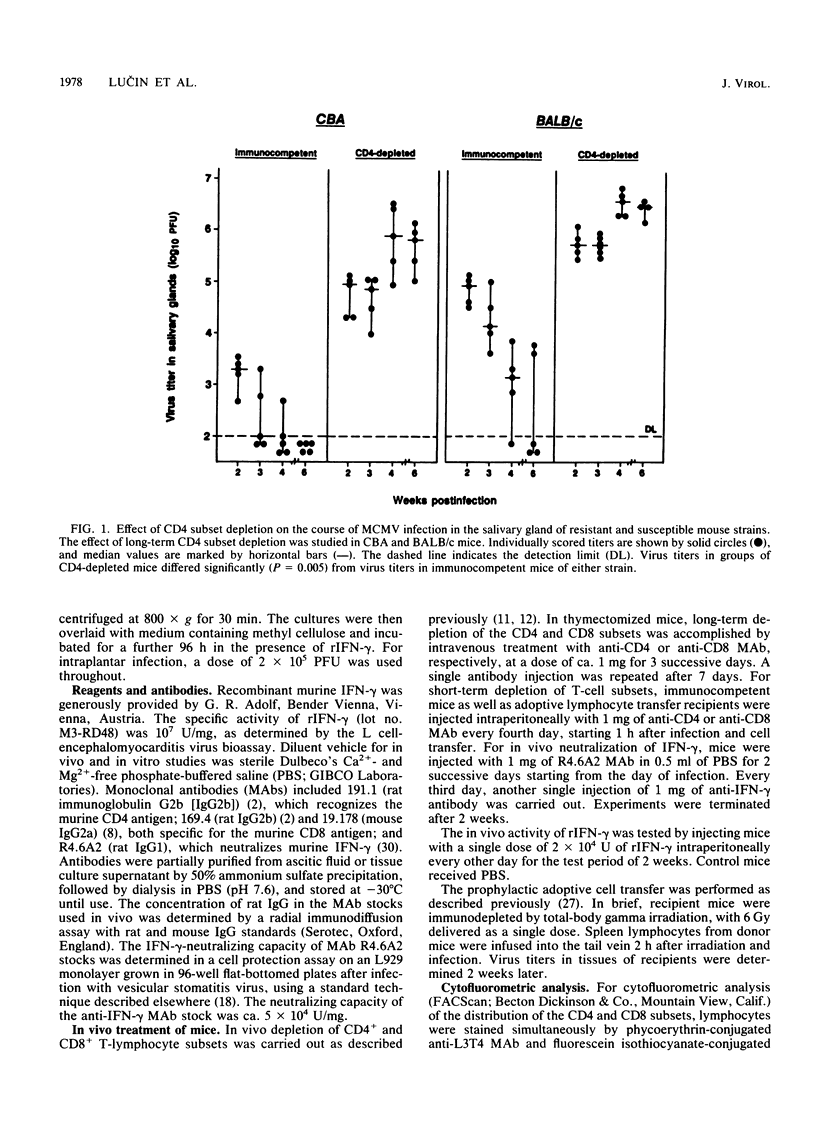
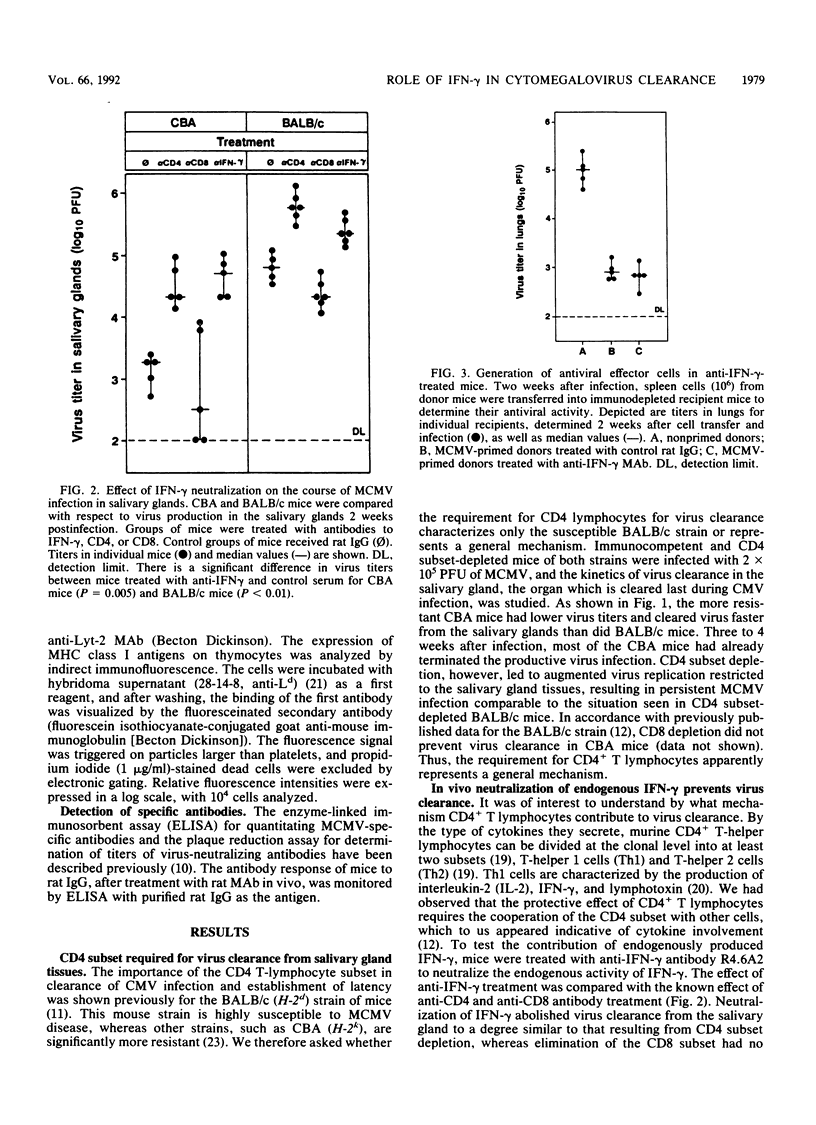
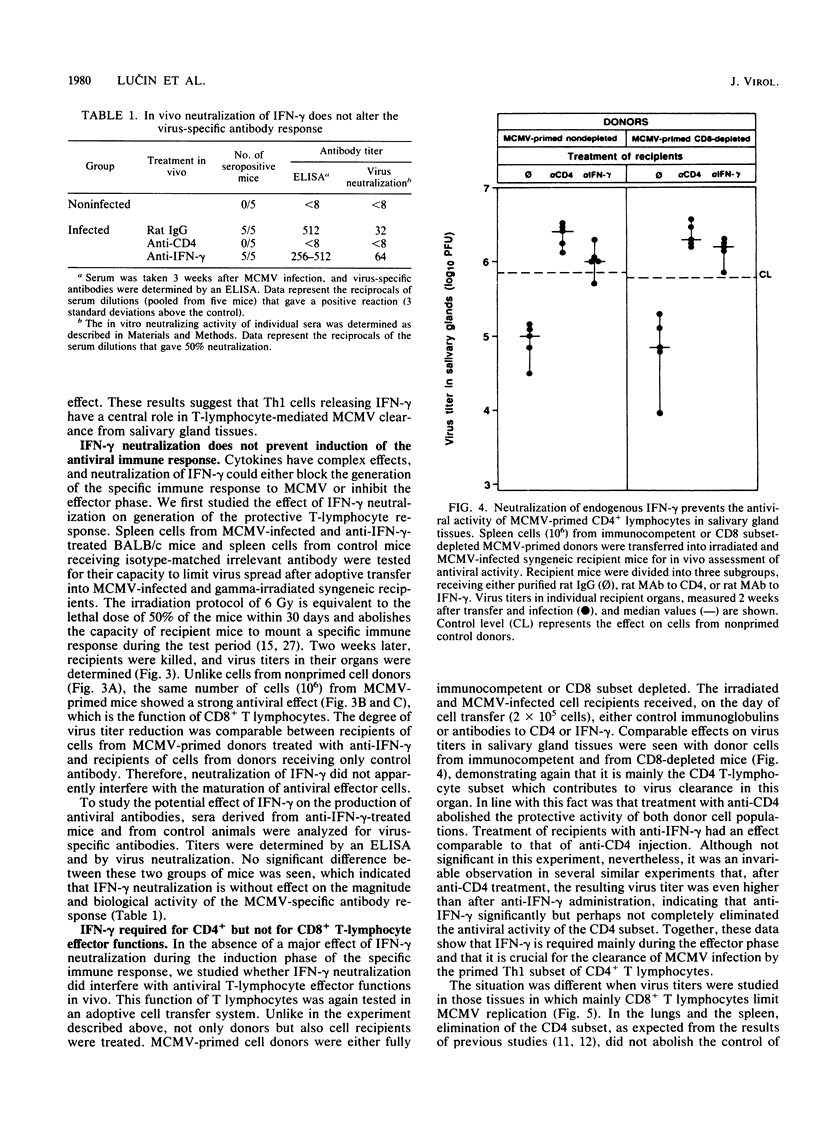
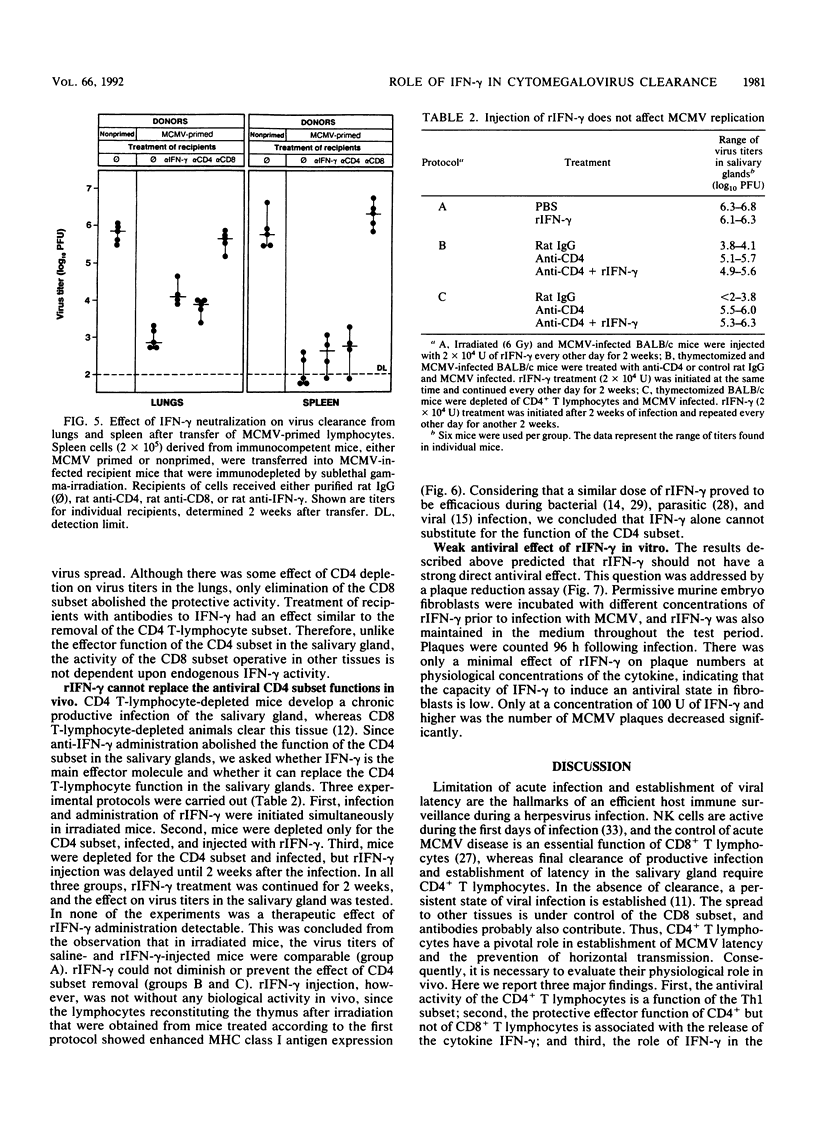
Cytomegalovirus (CMV), similar to other members of the Herpesviridae family, can establish both persistent and latent infections. Each of the CMVs that are found in many animal species replicates in the salivary gland, and oral secretion represents a source of horizontal transmission. Locally restricted replication characterizes the immunocompetent individual, whereas in the immunocompromised host, protean disease manifestations occur due to virus dissemination. The virus is cleared by immune surveillance, and CD8+ T lymphocytes play a major role. Remarkably, certain cell types of salivary gland tissues are exempt from CD8+ T-lymphocyte control of murine CMV infection and require the activity of CD4+ T lymphocytes. The results presented here suggest that this activity is a function of Th1 cells. Neutralization of endogenous gamma interferon abrogated the antiviral activity of Th1 cells but not that of CD8+ T lymphocytes in other tissues. Neutralization of endogenous gamma interferon did not interfere with the induction of the cellular and humoral immune response but acted during the effector phase. Recombinant gamma interferon could not replace the function of Th1 cells in vivo and had limited direct antiviral activity in vitro. The results therefore suggest that gamma interferon represents one, but not the only, essential factor involved in salivary gland clearance, establishment of CMV latency, and, eventually, the control of horizontal transmission.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bukowski J. F., Warner J. F., Dennert G., Welsh R. M. Adoptive transfer studies demonstrating the antiviral effect of natural killer cells in vivo. J Exp Med. 1985 Jan 1;161(1):40–52. doi: 10.1084/jem.161.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold S. P., Jayasuriya A., Nash A., Prospero T. D., Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984 Dec 6;312(5994):548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- Del Val M., Münch K., Reddehase M. J., Koszinowski U. H. Presentation of CMV immediate-early antigen to cytolytic T lymphocytes is selectively prevented by viral genes expressed in the early phase. Cell. 1989 Jul 28;58(2):305–315. doi: 10.1016/0092-8674(89)90845-3. [DOI] [PubMed] [Google Scholar]
- Del Val M., Schlicht H. J., Ruppert T., Reddehase M. J., Koszinowski U. H. Efficient processing of an antigenic sequence for presentation by MHC class I molecules depends on its neighboring residues in the protein. Cell. 1991 Sep 20;66(6):1145–1153. doi: 10.1016/0092-8674(91)90037-y. [DOI] [PubMed] [Google Scholar]
- Del Val M., Schlicht H. J., Volkmer H., Messerle M., Reddehase M. J., Koszinowski U. H. Protection against lethal cytomegalovirus infection by a recombinant vaccine containing a single nonameric T-cell epitope. J Virol. 1991 Jul;65(7):3641–3646. doi: 10.1128/jvi.65.7.3641-3646.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew W. L. Cytomegalovirus infection in patients with AIDS. J Infect Dis. 1988 Aug;158(2):449–456. doi: 10.1093/infdis/158.2.449. [DOI] [PubMed] [Google Scholar]
- Goronzy J. J., Weyand C. M. Persistent suppression of virus-specific cytotoxic T cell responses after transient depletion of CD4+ T cells in vivo. J Immunol. 1989 Jun 15;142(12):4435–4440. [PubMed] [Google Scholar]
- Henson D., Strano A. J. Mouse cytomegalovirus. Necrosis of infected and morphologically normal submaxillary gland acinar cells during termination of chronic infection. Am J Pathol. 1972 Jul;68(1):183–202. [PMC free article] [PubMed] [Google Scholar]
- Hämmerling G. J., Hämmerling U., Flaherty L. Qat-4 and Qat-5, new murine T-cell antigens governed by the Tla region and identified by monoclonal antibodies. J Exp Med. 1979 Jul 1;150(1):108–116. doi: 10.1084/jem.150.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonjić S., Mutter W., Weiland F., Reddehase M. J., Koszinowski U. H. Site-restricted persistent cytomegalovirus infection after selective long-term depletion of CD4+ T lymphocytes. J Exp Med. 1989 Apr 1;169(4):1199–1212. doi: 10.1084/jem.169.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonjić S., Pavić I., Lucin P., Rukavina D., Koszinowski U. H. Efficacious control of cytomegalovirus infection after long-term depletion of CD8+ T lymphocytes. J Virol. 1990 Nov;64(11):5457–5464. doi: 10.1128/jvi.64.11.5457-5464.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonjić S., del Val M., Keil G. M., Reddehase M. J., Koszinowski U. H. A nonstructural viral protein expressed by a recombinant vaccinia virus protects against lethal cytomegalovirus infection. J Virol. 1988 May;62(5):1653–1658. doi: 10.1128/jvi.62.5.1653-1658.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karupiah G., Blanden R. V., Ramshaw I. A. Interferon gamma is involved in the recovery of athymic nude mice from recombinant vaccinia virus/interleukin 2 infection. J Exp Med. 1990 Nov 1;172(5):1495–1503. doi: 10.1084/jem.172.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiderlen A. F., Kaufmann S. H., Lohmann-Matthes M. L. Protection of mice against the intracellular bacterium Listeria monocytogenes by recombinant immune interferon. Eur J Immunol. 1984 Oct;14(10):964–967. doi: 10.1002/eji.1830141019. [DOI] [PubMed] [Google Scholar]
- Klavinskis L. S., Geckeler R., Oldstone M. B. Cytotoxic T lymphocyte control of acute lymphocytic choriomeningitis virus infection: interferon gamma, but not tumour necrosis factor alpha, displays antiviral activity in vivo. J Gen Virol. 1989 Dec;70(Pt 12):3317–3325. doi: 10.1099/0022-1317-70-12-3317. [DOI] [PubMed] [Google Scholar]
- Koszinowski U. H., Del Val M., Reddehase M. J. Cellular and molecular basis of the protective immune response to cytomegalovirus infection. Curr Top Microbiol Immunol. 1990;154:189–220. doi: 10.1007/978-3-642-74980-3_8. [DOI] [PubMed] [Google Scholar]
- Leist T. P., Eppler M., Zinkernagel R. M. Enhanced virus replication and inhibition of lymphocytic choriomeningitis virus disease in anti-gamma interferon-treated mice. J Virol. 1989 Jun;63(6):2813–2819. doi: 10.1128/jvi.63.6.2813-2819.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. D. Cytomegalovirus infection following marrow transplantation: risk, treatment, and prevention. Birth Defects Orig Artic Ser. 1984;20(1):101–117. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Moore K. W. The role of IL-10 in crossregulation of TH1 and TH2 responses. Immunol Today. 1991 Mar;12(3):A49–A53. doi: 10.1016/S0167-5699(05)80015-5. [DOI] [PubMed] [Google Scholar]
- Ozato K., Hansen T. H., Sachs D. H. Monoclonal antibodies to mouse MHC antigens. II. Antibodies to the H-2Ld antigen, the products of a third polymorphic locus of the mouse major histocompatibility complex. J Immunol. 1980 Dec;125(6):2473–2477. [PubMed] [Google Scholar]
- Pearce E. J., Caspar P., Grzych J. M., Lewis F. A., Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991 Jan 1;173(1):159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinnan G. V., Jr, Manischewitz J. F. Genetically determined resistance to lethal murine cytomegalovirus infection is mediated by interferon-dependent and -independent restriction of virus replication. J Virol. 1987 Jun;61(6):1875–1881. doi: 10.1128/jvi.61.6.1875-1881.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddehase M. J., Koszinowski U. H. Significance of herpesvirus immediate early gene expression in cellular immunity to cytomegalovirus infection. Nature. 1984 Nov 22;312(5992):369–371. doi: 10.1038/312369a0. [DOI] [PubMed] [Google Scholar]
- Reddehase M. J., Mutter W., Münch K., Bühring H. J., Koszinowski U. H. CD8-positive T lymphocytes specific for murine cytomegalovirus immediate-early antigens mediate protective immunity. J Virol. 1987 Oct;61(10):3102–3108. doi: 10.1128/jvi.61.10.3102-3108.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddehase M. J., Rothbard J. B., Koszinowski U. H. A pentapeptide as minimal antigenic determinant for MHC class I-restricted T lymphocytes. Nature. 1989 Feb 16;337(6208):651–653. doi: 10.1038/337651a0. [DOI] [PubMed] [Google Scholar]
- Reddehase M. J., Weiland F., Münch K., Jonjic S., Lüske A., Koszinowski U. H. Interstitial murine cytomegalovirus pneumonia after irradiation: characterization of cells that limit viral replication during established infection of the lungs. J Virol. 1985 Aug;55(2):264–273. doi: 10.1128/jvi.55.2.264-273.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S. G. In vivo administration of recombinant IFN-gamma induces macrophage activation, and prevents acute disease, immune suppression, and death in experimental Trypanosoma cruzi infections. J Immunol. 1988 Jun 15;140(12):4342–4347. [PubMed] [Google Scholar]
- Sasaki T., Mieno M., Udono H., Yamaguchi K., Usui T., Hara K., Shiku H., Nakayama E. Roles of CD4+ and CD8+ cells, and the effect of administration of recombinant murine interferon gamma in listerial infection. J Exp Med. 1990 Apr 1;171(4):1141–1154. doi: 10.1084/jem.171.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitalny G. L., Havell E. A. Monoclonal antibody to murine gamma interferon inhibits lymphokine-induced antiviral and macrophage tumoricidal activities. J Exp Med. 1984 May 1;159(5):1560–1565. doi: 10.1084/jem.159.5.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. M., Meager A., Askonas B. A. Influenza virus-specific T cells lead to early interferon gamma in lungs of infected hosts: development of a sensitive radioimmunoassay. J Gen Virol. 1989 Apr;70(Pt 4):975–978. doi: 10.1099/0022-1317-70-4-975. [DOI] [PubMed] [Google Scholar]
- Volkmer H., Bertholet C., Jonjić S., Wittek R., Koszinowski U. H. Cytolytic T lymphocyte recognition of the murine cytomegalovirus nonstructural immediate-early protein pp89 expressed by recombinant vaccinia virus. J Exp Med. 1987 Sep 1;166(3):668–677. doi: 10.1084/jem.166.3.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R. M., Brubaker J. O., Vargas-Cortes M., O'Donnell C. L. Natural killer (NK) cell response to virus infections in mice with severe combined immunodeficiency. The stimulation of NK cells and the NK cell-dependent control of virus infections occur independently of T and B cell function. J Exp Med. 1991 May 1;173(5):1053–1063. doi: 10.1084/jem.173.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]


