Abstract
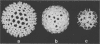
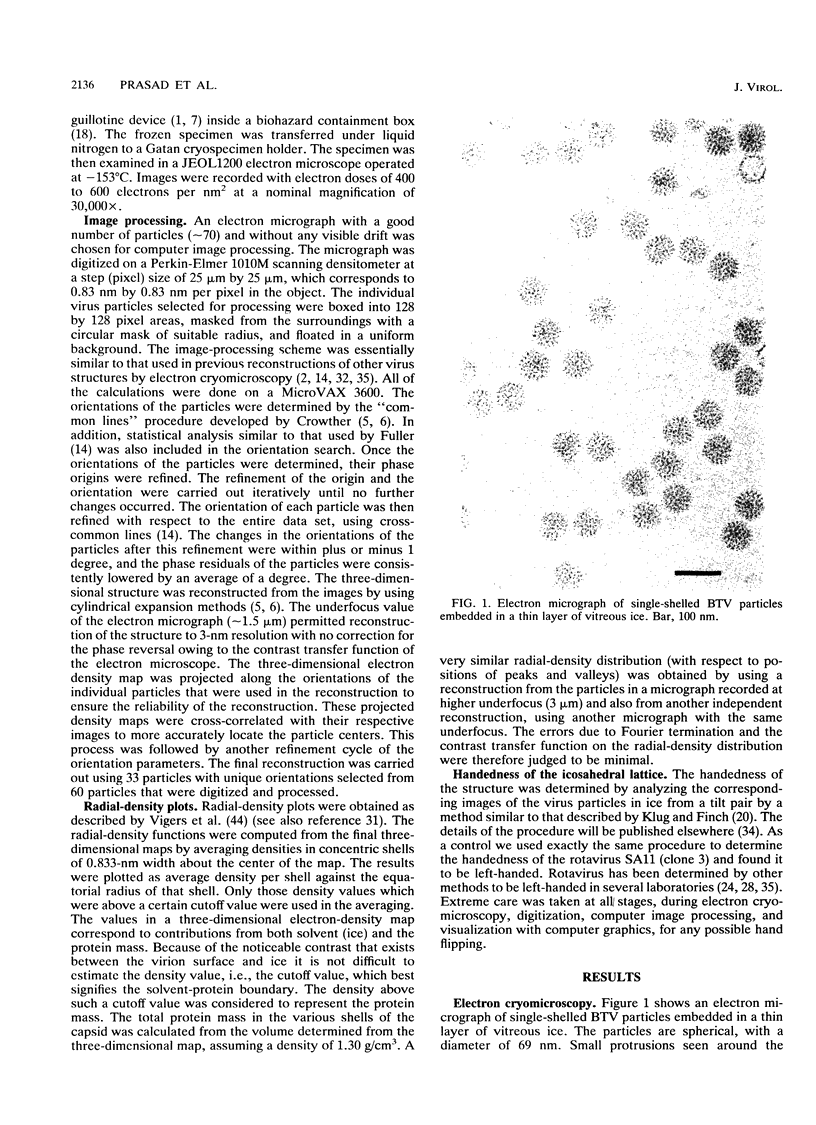
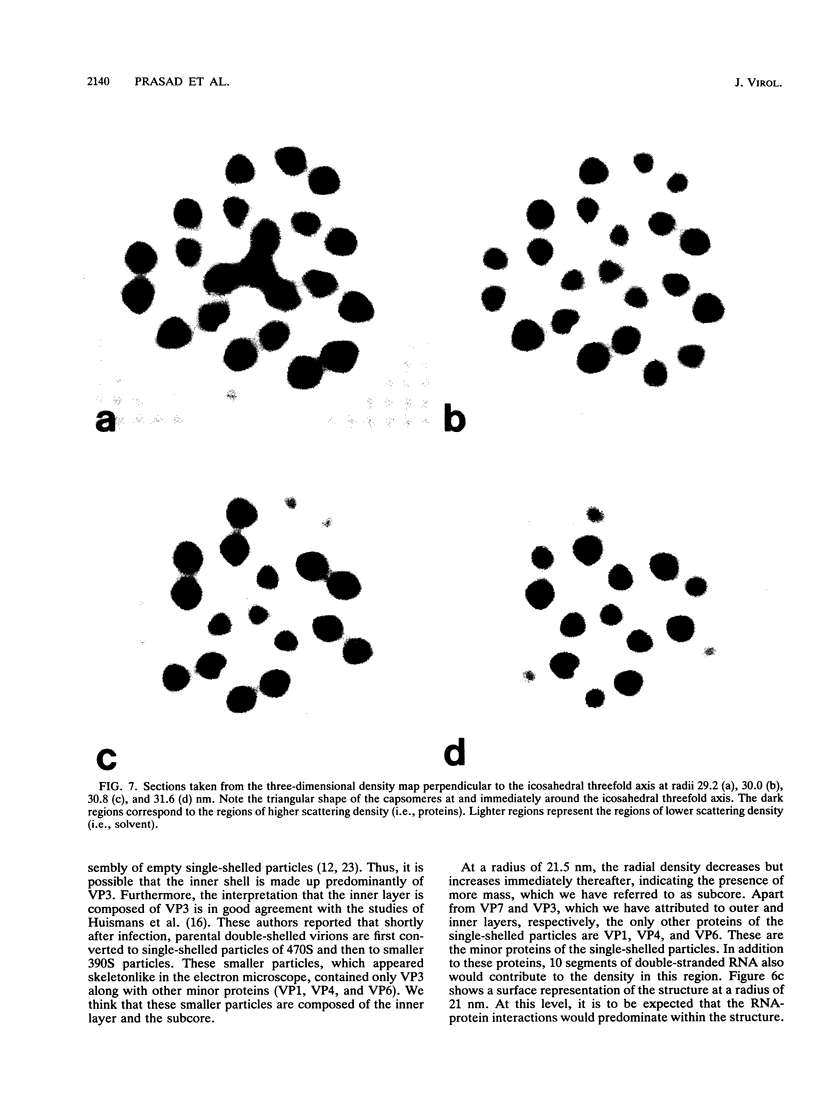
The three-dimensional structure of single-shelled bluetongue virus has been determined to a resolution of 3 nm by using electron cryomicroscopy and image-processing techniques. The single-shelled virion has a diameter of 69 nm. The three-dimensional structure of the virion has icosahedral symmetry with a triangulation number of 13 in a left-handed configuration. The three-dimensional structure can be described in terms of two concentric layers of density surrounding a central core density. Two distinctive features of the outer layer are the 260 knobby capsomeres located at all the local and strict threefold axes and the aqueous channels located at all the five- and six-coordinated positions. These protrusions extend outward from an inner radius of 28 nm. They are interconnected out to a radius of 30 nm by saddle-shaped densities across the local and strict twofold axes. The aqueous channels surrounded by these capsomeres are about 8 nm wide at the outer surface and 8 nm deep. Some of these channels extend inward, penetrating the inner layer. These channels may provide pathways for transporting the metabolites and mRNA during the transcriptase activity of the particles. The inner layer is a featureless smooth bed of density except for the indentations in register with the channels of the outer layer. We propose that the 260 capsomeres in the outer layer are made up of trimers of the major protein, VP7, and that the inner layer is composed of the second major protein, VP3. The density in the central portion of the structure at a radius of less than 21 nm is likely due to the minor proteins and the genomic RNA.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian M., Dubochet J., Lepault J., McDowall A. W. Cryo-electron microscopy of viruses. Nature. 1984 Mar 1;308(5954):32–36. doi: 10.1038/308032a0. [DOI] [PubMed] [Google Scholar]
- Baker T. S., Drak J., Bina M. Reconstruction of the three-dimensional structure of simian virus 40 and visualization of the chromatin core. Proc Natl Acad Sci U S A. 1988 Jan;85(2):422–426. doi: 10.1073/pnas.85.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T. S., Newcomb W. W., Booy F. P., Brown J. C., Steven A. C. Three-dimensional structures of maturable and abortive capsids of equine herpesvirus 1 from cryoelectron microscopy. J Virol. 1990 Feb;64(2):563–573. doi: 10.1128/jvi.64.2.563-573.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASPAR D. L., KLUG A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- Crowther R. A. Procedures for three-dimensional reconstruction of spherical viruses by Fourier synthesis from electron micrographs. Philos Trans R Soc Lond B Biol Sci. 1971 May 27;261(837):221–230. doi: 10.1098/rstb.1971.0054. [DOI] [PubMed] [Google Scholar]
- Dubochet J., Adrian M., Chang J. J., Homo J. C., Lepault J., McDowall A. W., Schultz P. Cryo-electron microscopy of vitrified specimens. Q Rev Biophys. 1988 May;21(2):129–228. doi: 10.1017/s0033583500004297. [DOI] [PubMed] [Google Scholar]
- Els H. J., Verwoerd D. W. Morphology of bluetongue virus. Virology. 1969 Jun;38(2):213–219. doi: 10.1016/0042-6822(69)90362-6. [DOI] [PubMed] [Google Scholar]
- Emmons R. W. Colorado tick fever: prolonged viremia in hibernating Citellus lateralis. Am J Trop Med Hyg. 1966 May;15(3):428–433. doi: 10.4269/ajtmh.1966.15.428. [DOI] [PubMed] [Google Scholar]
- Fenner F. Classification and nomenclature of viruses. Second report of the International Committee on Taxonomy of Viruses. Intervirology. 1976;7(1-2):1–115. doi: 10.1159/000149938. [DOI] [PubMed] [Google Scholar]
- French T. J., Roy P. Synthesis of bluetongue virus (BTV) corelike particles by a recombinant baculovirus expressing the two major structural core proteins of BTV. J Virol. 1990 Apr;64(4):1530–1536. doi: 10.1128/jvi.64.4.1530-1536.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukusho A., Yu Y., Yamaguchi S., Roy P. Completion of the sequence of bluetongue virus serotype 10 by the characterization of a structural protein, VP6, and a non-structural protein, NS2. J Gen Virol. 1989 Jul;70(Pt 7):1677–1689. doi: 10.1099/0022-1317-70-7-1677. [DOI] [PubMed] [Google Scholar]
- Fuller S. D. The T=4 envelope of Sindbis virus is organized by interactions with a complementary T=3 capsid. Cell. 1987 Mar 27;48(6):923–934. doi: 10.1016/0092-8674(87)90701-x. [DOI] [PubMed] [Google Scholar]
- Gorman B. M., Taylor J., Walker P. J., Davidson W. L., Brown F. Comparison of bluetongue type 20 with certain viruses of the bluetongue and Eubenangee serological groups of orbiviruses. J Gen Virol. 1981 Dec;57(Pt 2):251–261. doi: 10.1099/0022-1317-57-2-251. [DOI] [PubMed] [Google Scholar]
- Huismans H., van Dijk A. A., Els H. J. Uncoating of parental bluetongue virus to core and subcore particles in infected L cells. Virology. 1987 Mar;157(1):180–188. doi: 10.1016/0042-6822(87)90327-8. [DOI] [PubMed] [Google Scholar]
- Hyatt A. D., Eaton B. T. Ultrastructural distribution of the major capsid proteins within bluetongue virus and infected cells. J Gen Virol. 1988 Apr;69(Pt 4):805–815. doi: 10.1099/0022-1317-69-4-805. [DOI] [PubMed] [Google Scholar]
- Jeng T. W., Talmon Y., Chiu W. Containment system for the preparation of vitrified-hydrated virus specimens. J Electron Microsc Tech. 1988 Apr;8(4):343–348. doi: 10.1002/jemt.1060080402. [DOI] [PubMed] [Google Scholar]
- Klug A., Finch J. T. Structure of viruses of the papilloma-polyoma type. IV. Analysis of tilting experiments in the electron microscope. J Mol Biol. 1968 Jan 14;31(1):1–12. doi: 10.1016/0022-2836(68)90050-8. [DOI] [PubMed] [Google Scholar]
- Labbé M., Charpilienne A., Crawford S. E., Estes M. K., Cohen J. Expression of rotavirus VP2 produces empty corelike particles. J Virol. 1991 Jun;65(6):2946–2952. doi: 10.1128/jvi.65.6.2946-2952.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loudon P. T., Roy P. Assembly of five bluetongue virus proteins expressed by recombinant baculoviruses: inclusion of the largest protein VP1 in the core and virus-like proteins. Virology. 1991 Feb;180(2):798–802. doi: 10.1016/0042-6822(91)90094-r. [DOI] [PubMed] [Google Scholar]
- Ludert J. E., Gil F., Liprandi F., Esparza J. The structure of the rotavirus inner capsid studied by electron microscopy of chemically disrupted particles. J Gen Virol. 1986 Aug;67(Pt 8):1721–1725. doi: 10.1099/0022-1317-67-8-1721. [DOI] [PubMed] [Google Scholar]
- Martin S. A., Zweerink H. J. Isolation and characterization of two types of bluetongue virus particles. Virology. 1972 Nov;50(2):495–506. doi: 10.1016/0042-6822(72)90400-x. [DOI] [PubMed] [Google Scholar]
- Mertens P. P., Brown F., Sangar D. V. Assignment of the genome segments of bluetongue virus type 1 to the proteins which they encode. Virology. 1984 May;135(1):207–217. doi: 10.1016/0042-6822(84)90131-4. [DOI] [PubMed] [Google Scholar]
- Mertens P. P., Burroughs J. N., Anderson J. Purification and properties of virus particles, infectious subviral particles, and cores of bluetongue virus serotypes 1 and 4. Virology. 1987 Apr;157(2):375–386. doi: 10.1016/0042-6822(87)90280-7. [DOI] [PubMed] [Google Scholar]
- Metcalf P. The symmetry of the reovirus outer shell. J Ultrastruct Res. 1982 Mar;78(3):292–301. doi: 10.1016/s0022-5320(82)80004-x. [DOI] [PubMed] [Google Scholar]
- Murphy F. A., Borden E. C., Shope R. E., Harrison A. Physicochemical and morphological relationships of some arthropod-borne viruses to bluetongue virus--a new taxonomic group. Electron microscopic studies. J Gen Virol. 1971 Nov;13(2):273–288. doi: 10.1099/0022-1317-13-2-273. [DOI] [PubMed] [Google Scholar]
- Murphy F. A., Coleman P. H., Harrison A. K., Gary G. W., Jr Colorado tick fever virus: an electron microscopic study. Virology. 1968 May;35(1):28–40. doi: 10.1016/0042-6822(68)90302-4. [DOI] [PubMed] [Google Scholar]
- Olson N. H., Baker T. S., Johnson J. E., Hendry D. A. The three-dimensional structure of frozen-hydrated Nudaurelia capensis beta virus, a T = 4 insect virus. J Struct Biol. 1990 Oct-Dec;105(1-3):111–122. doi: 10.1016/1047-8477(90)90105-l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad B. V., Burns J. W., Marietta E., Estes M. K., Chiu W. Localization of VP4 neutralization sites in rotavirus by three-dimensional cryo-electron microscopy. Nature. 1990 Feb 1;343(6257):476–479. doi: 10.1038/343476a0. [DOI] [PubMed] [Google Scholar]
- Prasad B. V., Wang G. J., Clerx J. P., Chiu W. Three-dimensional structure of rotavirus. J Mol Biol. 1988 Jan 20;199(2):269–275. doi: 10.1016/0022-2836(88)90313-0. [DOI] [PubMed] [Google Scholar]
- Schrag J. D., Prasad B. V., Rixon F. J., Chiu W. Three-dimensional structure of the HSV1 nucleocapsid. Cell. 1989 Feb 24;56(4):651–660. doi: 10.1016/0092-8674(89)90587-4. [DOI] [PubMed] [Google Scholar]
- Studdert M. J., Pangborn J., Addison R. B. Bluetongue virus structure. Virology. 1966 Jul;29(3):509–511. doi: 10.1016/0042-6822(66)90232-7. [DOI] [PubMed] [Google Scholar]
- Van Dijk A. A., Huismans H. The in vitro activation and further characterization of the bluetongue virus-associated transcriptase. Virology. 1980 Jul 30;104(2):347–356. doi: 10.1016/0042-6822(80)90339-6. [DOI] [PubMed] [Google Scholar]
- Verwoerd D. W., Els H. J., De Villiers E. M., Huismans H. Structure of the bluetongue virus capsid. J Virol. 1972 Oct;10(4):783–794. doi: 10.1128/jvi.10.4.783-794.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwoerd D. W., Huismans H. Studies on the in vitro and the in vivo transcription of the bluetongue virus genome. Onderstepoort J Vet Res. 1972 Dec;39(4):185–191. [PubMed] [Google Scholar]
- Vigers G. P., Crowther R. A., Pearse B. M. Location of the 100 kd-50 kd accessory proteins in clathrin coats. EMBO J. 1986 Sep;5(9):2079–2085. doi: 10.1002/j.1460-2075.1986.tb04469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel R. H., Provencher S. W., von Bonsdorff C. H., Adrian M., Dubochet J. Envelope structure of Semliki Forest virus reconstructed from cryo-electron micrographs. Nature. 1986 Apr 10;320(6062):533–535. doi: 10.1038/320533a0. [DOI] [PubMed] [Google Scholar]
- Yeager M., Dryden K. A., Olson N. H., Greenberg H. B., Baker T. S. Three-dimensional structure of rhesus rotavirus by cryoelectron microscopy and image reconstruction. J Cell Biol. 1990 Jun;110(6):2133–2144. doi: 10.1083/jcb.110.6.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]