Abstract
CD4+CD25+FoxP3+ regulatory T cells (Tregs) possess the capacity to modulate both adaptive and innate immune responses. We hypothesized that Tregs could regulate hematopoiesis based on cytokine effector molecules they can produce. The studies here demonstrate that Tregs can affect the differentiation of myeloid progenitor cells. In vitro findings demonstrated the ability of Tregs to inhibit the differentiation of interleukin-3 (IL-3)/stem cell factor (colony-forming unit [CFU]-IL3)–driven progenitor cells. Inhibitory effects were mediated by a pathway requiring cell-cell contact, major histocompatibility complex class II expression on marrow cells, and transforming growth factor-β. Importantly, depletion of Tregs in situ resulted in enhanced CFU-IL3 levels after bone marrow transplantation. Cotransplantation of CD4+FoxP3+gfp Tregs together with bone marrow was found to diminish CFU-IL3 responses after transplantation. To address the consequence of transplanted Tregs on differentiated progeny from these CFU 2 weeks after hematopoietic stem cell transplantation, peripheral blood complete blood counts were performed and examined for polymorphonuclear leukocyte content. Recipients of cotransplanted Tregs exhibited diminished neutrophil counts. Together, these findings illustrate that both recipient and donor Tregs can influence hematopoietic progenitor cell activity after transplantation and that these cells can alter responses outside the adaptive and innate immune systems.
Introduction
CD4+CD25+ T lymphocytes (regulatory T cells [Tregs]) comprise a relatively small population within the normal peripheral CD4 T-cell compartment whose function is dependent on expression of the transcription factor FoxP3.1–4 Deletion of these cells in both neonatal and adult mice results in the rapid onset of peripheral T-cell activation and autoimmunity, indicating that the presence of functionally active FoxP3+ Tregs is requisite for maintaining the dominant tolerant state.2 Studies following the identification of this Treg population have demonstrated their capacity to modulate transplantation5–11 and antitumor12–14 responses. Importantly, cotransplantation of CD4+CD25+ T cells has been found to exert regulatory activity on responses mediated by multiple types of immune cells including CD4, CD8, and natural killer (NK) populations.10,15 Such studies have demonstrated the capacity of Tregs to suppress both adaptive and innate immune responses. Although model dependent, cytokines including transforming growth factor-β (TGF-β), interleukin-10 (IL-10), and IL-9 have been reported as effector molecules in both in vitro and in vivo settings.14,16–19 Hence, the presence of Tregs in hematolymphoid compartments led us to hypothesize that, after activation, CD4+CD25+FoxP3+ T cells may modulate hematopoietic function. Such regulatory activity could generate novel perspectives regarding the expanding applications of these cells in the field of hematopoietic stem cell transplantation (HSCT).6,8–10,20,21
Methods
Mice
BALB/c wild-type (WT) and C57BL/6 wild-type (B6 WT; from Charles River Laboratories or Animal Production Area at the National Cancer Institute–Frederick), B6.129S2-CD8atm1Mak (B6 CD8−/−), and B6.129-H2 (dlABl-Ea)/J (MHC II−/−; from The Jackson Laboratory or obtained from the Animal Production Area at the National Cancer Institute–Frederick) mice were used in this study. Some mice were subsequently bred and maintained at the University of Miami animal facilities. B6 cytotoxic double (perforin and Fas ligand [FasL])–deficient mice (B6-cdd) were generated from B6 Pfp+/− Faslgld/gld pairings or B6 Pfp−/− Faslgld/gld × B6 Pfp+/− Faslgld/gld pairings, as previously described.22 Mice were screened as previously described.23 Foxp3gfp breeder mice were obtained from Dr A. Y. Rudensky (University of Washington). Animal studies were carried out under a protocol approved by the University of Miami Animal Care and Use Committee.
MACS and flow-activated cell-sorting isolation of CD4+CD25+ Tregs
MACS.
Spleen and lymph node cell (LNC) homogenates were depleted of B cells and CD8+ cells after incubation with anti-CD8 monoclonal antibody (mAb; clone 2.43) and panning on plates coated with goat anti–mouse immunoglobulin G (IgG)/IgM antibody (Millipore) for 30 minutes at room temperature. Enriched CD4+ T-cell preparations were labeled with anti-CD25 phycoerythrin (PE; clone PC61; BD Biosciences) and positively selected using anti-PE magnetic-activated cell sorting (MACS) microbeads (Miltenyi Biotec). CD4+CD25+ purity was routinely more than 93% (80%-90% Foxp3+) of total cells and more than 98% of CD4+ T cells.
Enrichment of Treg preparations was determined by antibody labeling before and after enrichment followed by flow cytometric analysis. Spleens and LNCs were stained with PE anti-CD25 (PC61) and PE-Cyanin5 anti-CD4 (RM4-5; BD Biosciences). Tregs were permeabilized and stained for Foxp3 using the Foxp3 Staining Kit, fluorescein isothiocyanate (FITC)–anti-FoxP3 (FJK-16s), or FITC-isotype control (eBioscience). Samples were acquired and analyzed using a FACScan, FACSCalibur, or LSR-I (Becton Dickinson).
FACS-purified Tregs.
Spleen and LNC homogenates were B cell– and CD8+ T cell–depleted as described. Enriched CD4+ T-cell preparations were labeled with Cy5–anti-CD4 and PE–anti-CD25 (Ab dilution 1:10 in sterile 1× 5% fetal bovine serum flow-activated cell sorting [FACS] buffer), incubated for 15 minutes at 4°C to 8°C. Excess Ab was washed and cell pellet resuspended in 1× 5% FACS buffer. Samples were separated into CD4+CD25+ (Tregs) and CD4+CD25− (conventional T cells) by positive and negative selection via a FACSAria.
MACS and FACS isolation of BMCs for in vitro cultures
T cell–depleted bone marrow preparations.
Bone marrow cells (BMCs) were primarily harvested from the long bones by flushing with a 261/2-gauge needle. Single-cell suspensions were T-cell depleted using anti-Thy1.2 (HO-13.4 supernatant) antibody at 1:5 vol/vol for 30 minutes on ice; followed by addition of 12% Low-Tox M rabbit complement (Cedarlane Labs) plus Ab/complement medium, incubated at 37°C for 45 minutes. Depletion efficiency was routinely more than 95%. The procedure was performed with all strains of mice from which TCD BM was cocultured with activated Tregs.
Lin−c-kit+–enriched BMCs.
BMCs were harvested as described and subsequently subjected to depletion of lineage-expressing cells by the Lineage Depletion Kit from Miltenyi Biotec based on MACS separation. Briefly, BMCs were incubated with a cocktail of biotin-conjugated monoclonal Abs (CD5, CD45R [B220], CD11b, Gr-1 [Ly6G/C], 7D4, and Ter119) for 10 minutes at 4°C to 8°C, followed by incubation with antibiotin microbeads for 15 minutes at 4°C to 8°C. Cells were passed through a single mass spectrometry column; the negative fraction was collected (average Lin− enrichment, ∼ 75%-80%) and labeled with PE–anti-CD117 (BD Biosciences) and subjected to a MACS-positive selection (mass spectrometry column). Preparations were more than 93% Lin−c-kit+. Some Lin− BM preparations used for in vitro assays were not further enriched for c-kit+ cells.
FACS-purified Lin−c-kit+ BMCs.
BMCs were subjected to depletion of lineage-expressing cells as described. Lin−-enriched samples were subsequently labeled with streptavidin-FITC and PE-CD117 to target residual lineage-expressing cells.
Ex vivo Treg cell activation and Treg/BMC coculture
CD4+CD25+ regulatory cells.
Freshly isolated Tregs were plated in triplicates in 96-well round or V-bottom plates typically at 5.0 × 104 cells/well (for coculture with TCD-BM), at 1.0 × 104 cells/well (for coculture with Lin−-enriched BMCs) or at 7.0 × 103 cells/well (for coculture with FACS-purified Lin−c-kit+ BMCs) and activated for 3 days in the presence of anti-CD3/28 beads at a ratio of 3 beads to 1 cell or 1:1, and 200 ng/mL rIL-2 (Hoffman-LaRoche). Some cultures were allowed to remain in activation/expansion cultures and were reactivated at day 5 or 8 with fresh beads and IL-2 before coculture with BMCs. Medium (50 μL) was removed from each well containing day 3–activated CD4+CD25+ Tregs, and 50 μL of TCD-BM, Lin−c-kit+–enriched BMCs, or FACS-purified Lin−c-kit+ BMCs were added (25.0, 5.0, and 3.5 × 103/well, respectively), typically at 1:2 (BMCs/Tregs) in the presence of rmSCF 50 ng/mL (PeproTech) plus 6 U/mL rmIL-3 (R&D Systems) to provide growth/survival signals to progenitor populations during the coculture period of 1.5 to 3 days.
CFU assays
From Treg cocultures.
Contents from control and Treg/BM cocultures were harvested and pooled after the coculture period, placed in 3.5 mL of Base MethoCult medium (M3134; StemCell Technologies), which is routinely prepared with 10% fetal bovine serum, 1.0 mL of penicillin/streptomycin, 1.0 mL of l-glutamine, 30.0 mL of Iscove modified Dulbecco medium, and 520 μL of 2-mercaptoethanol (0.05 mM final concentration), and supplemented with 6.0 U/mL rmIL-3 (colony-forming unit [CFU]-IL3), a growth factor that drives predominantly granulocyte-macrophage CFU, granulocyte CFU, and megakaryocyte CFU colonies. Each tube was thoroughly vortexed, and 1.0-mL aliquots were plated in triplicates (12-well plates). Day-7 colonies (clusters ≥ 50 cells were scored as 1 colony) were counted. In some experiments granulocyte-macrophage colony-stimulating factor (GM-CSF; at 25-50 ng/mL; PeproTech) was used in combination with rIL-3. Results are presented as average number of CFU (± SD).
Other CFU assays.
CFU assays were also performed from spleen and BMC preparations obtained from mice that underwent transplantation. Single-cell suspensions were plated in Base MethoCult medium supplemented with the indicated cytokines in 12-well plates. Spleen CFU-IL3 cultures corresponding to day 7 after transplantation contained 7.5 × 104 to 1.0 × 105 cells per well. Bone marrow CFU-IL3 cultures corresponding to days 14 and 17 after transplantation contained 3.5 × 104 cells per well. Culture conditions and colony scores were as indicated. Total CFU-IL3 per compartment were generated as follows: spleen, total CFU-IL3 in spleen = average number of CFU-IL3 present in the triplicate cultures corresponding to 1 individual animal (ie, control or experimental) × total number of live mononuclear cells present in the spleen of the same animal at the indicated time after transplantation; bone marrow, total CFU-IL3 in BM = average number of CFU-IL3 present in the triplicate cultures corresponding to 1 individual animal × total number of BM mononuclear cells present in 2 femurs + 2 tibiae in the same animal at the indicated time after transplantation. A cytokine cocktail used to elicit granulocyte, erythrocyte, monocyte, macrophage colonies contained SCF (50 ng/mL), rmIL-6 (10 ng/mL), thrombopoietin (25 ng/mL; all from PeproTech), rmIL-3 (50 ng/mL), and rhEpo (8.0 U/mL; Akronbiotech).
Neutralization of cytokine bioactivity assays
TGF-β1 neutralization assay.
Two concentrations of biotinylated chicken (IgY)–derived anti–TGF-β1 (AF-101-NA; high concentration = 6 ng/mL and low concentration = 0.6 ng/mL; R&D Systems) or biotinylated isotype control (R&D Systems) was added to Treg-activated cultures before coculture with BMCs (TCD- or Lin−c-kit+–enriched preparations as indicated) and incubated at room temperature for 30 minutes. BMCs were then added as described above and these Treg/BM cocultures incubated for the indicated time periods, and CFU activity was assessed after the coculture period as described.
Suppression of CD4+ T-cell proliferation
CD4+CD25+ cells were isolated by MACS as described and activated for 3 days with anti-CD3/28 microcoated beads (1:3) or used immediately (ie, “fresh”) after isolation. Medium (50 μL) was removed from microtiter (triplicate) wells of day-3 Treg-activated cultures before addition of CD4+CD25− responder cells. Responder cells were obtained from the negative fraction of B6 CD8−/− spleen cells after Treg isolation (purity of CD4+CD25− > 95%), added to the activated or freshly isolated Tregs, and cocultured for 3 days. Responder cells were stimulated by addition of anti-CD3/CD28 microbeads. Treg/responder cocultures were pulsed with 1.0 μCi (0.037 MBq) 3H-thymidine (Perkin Elmer) for the final 16 hours of culture and harvested using a Tomtec Autotrap 24 harvester and pump system. Counts were obtained using a Wallac 1205 Betaplate Liquid Scintillation Counter and the data are presented as average counts per minute/group (± SD).
T-cell depletion, bone marrow transplantation, and Treg coinfusion
T-cell depletion ex vivo.
BM was collected from femurs/tibias of BALB/c, C57BL/6, and MHC II–deficient donors. BMCs were T-cell depleted by treatment with anti-Thy1.2 mAb (2 μg per 1 × 106 of cells, clone 38-H-12, or 1:500 ascites clone HO-13.4) or anti-CD4 mAb (clone 72.4) and anti-CD8 mAb (clone H02.2) for 30 minutes at 4°C. Cells were washed 1 time in PBS (Cambrex) and centrifuged. Rabbit complement (Cedarlane Labs) was prepared as per the manufacturer's instructions and added to the cells for 45 minutes at 37°C. Cells were then washed 3 times in PBS and centrifuged, and cell concentration was adjusted for the transplantations.
BALB/c or B6 mice were given sulfomethoxazole-trimethoprim (Schein Pharmaceutical Inc) in drinking water 7 days before bone marrow transplantation (BMT). Before BMT, mice were injected intraperitoneally with the following antibodies: 1 mg of rat IgG (Jackson ImmunoResearch Laboratories) or anti-CD25 (PC61; produced at the National Cell Culture Center) at days −4 and −2 before BMT. On the day of transplantation (day 0), recipients were lethally irradiated with a 137Cs source (7.5 Gy, BALB/c; 9.5 Gy, B6), and later BALB/c mice were injected intravenously with 5.0 × 105 or 1.0 × 106 TCD-BMCs from BALB/c mice and B6 mice were injected with 2.0 × 105, 5.0 × 105, or 1.0 × 106 TCD-BMCs from C57BL/6 or MHC II–deficient mice.
Tregs were obtained by several strategies: (1) Treg preparations were isolated from B6 Foxp3gfp mice by FACS. Briefly, spleen and LN from 9 mice were depleted of B cells and CD8 T cells as previously described. The remaining cells were collected and labeled with allophycocyanin anti-CD4 mAb. Labeled cells were then subjected to negative (CD4+GFP−) and positive (CD4+GFP+) separation (FACSAria cell sorter). (2) Donor CD4+CD25+ cells were also expanded in vivo as previously described.11,24 Briefly, B6 wild-type or CD8−/− animals were administered infusions (intraperitoneal) of rmIL-2 plus anti-IL2 mAb immune complex (rmIL-2/JES6-1A12 mAb [eBioscience] in 500 μL of PBS immediately after preparation 3 times every other day). These Treg donor animals were killed 2 days after the last treatment (day 0 of transplantation) and cell homogenates from spleens and lymph nodes were obtained and subjected to CD4+CD25+ enrichment as described. In vivo–expanded CD4+CD25+ cells (1 × 106) together with 0.3 × 106 syngeneic TCD-BMCs (n = 3-4/group) were cotransplanted into each animal. Highly purified CD4+FoxP3GFP cells (> 99.8%) or CD4+CD25+ cells (> 97% of CD4+ T cells) were then coinfused together with syngeneic B6 TCD-BMCs via intravenous tail vein injection (0.3 × 106 TCD-BMCs + 1 × 106 CD4+FoxP3GFP+ or CD4+CD25+) into lethally irradiated B6 recipients (total body irradiation [TBI] = 9.5 Gy 24 hours before BMT). Hematopoietic CFU-IL3 activity in the spleen of control (BMCs alone) versus experimental (received Tregs + BMCs) was scored and analyzed at day 7 after BMT as described.
Statistical analyses
Statistical comparisons in experiments involving a control and single experimental group were performed using an unpaired t test (2 tailed). A P value less than .05 was considered significant. A 1-way ANOVA was performed together with the Bonferroni multiple comparison test to determine statistical significance in experiments involving 3 or more groups (P < .05).
Peripheral blood neutrophil counts
Animals were bled (40-50 μL) at noon every 4 to 7 days, starting at day 6 after transplantation and continuing until day 35. Complete blood counts plus differential were obtained using the automated veterinary hematology system Hemavet 950 (Drew Scientific). Total white blood cell (WBC) range for an adult C57BL/6 mouse is estimated to range between 4.5 and 9.1 × 103/μL of blood. Percentage of blood cell composition of neutrophils normally ranges from 21% to 57%. The total numbers of neutrophils per 1.0 μL of peripheral blood was calculated multiplying the percentage of the mature neutrophils by the total WBC/μL (ie, number of segmented neutrophils present in 1.0 μL of peripheral blood at a given time after transplantation = % segmented neutrophils × total WBCs/μL). Statistical significance (P < .05) was computed using the unpaired t test (2-tailed P value) from GraphPad Prism Software.
Results
Activated CD4+CD25+ T regulatory cells suppress IL-3–induced colony-forming units in vitro via a pathway involving cell contact and TGF-β
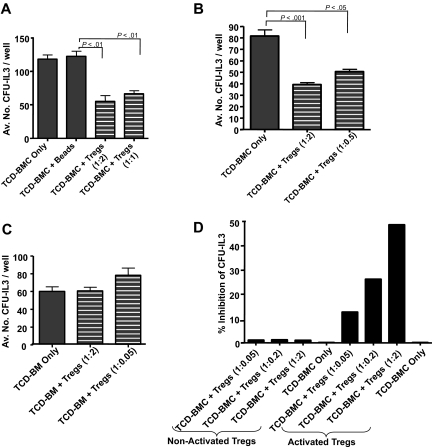
To begin addressing the possibility of Treg cell modulation of hematopoietic activity, highly enriched CD4+CD25+ T cells were isolated from BALB/c and B6-CD8−/− spleen and lymph node (> 97% of CD4+ T cells were CD25+), activated with anti-CD3/anti-CD28 beads and rIL2 (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) and plated together with syngeneic T cell–depleted bone marrow (TCD-BM). These activated B6-CD8−/− Tregs (as well as fresh CD4+CD25+ T cells; data not shown) were also tested in a CD4+ T-cell suppressor assay and exhibited inhibitory activity (supplemental Figure 1B). Analyses of hematopoietic progenitor cell (PC) function was assessed by subsequent culture with rIL-3 and SCF. Syngeneic TCD-BM cocultured with activated BALB/C (Figure 1A) Tregs contained diminished colony levels versus identical TCD-BM cultures not containing regulatory cells. Freshly isolated Tregs cultured with BMCs in the presence of anti-CD3/CD28 stimulation did not exhibit CFU-IL3 inhibition (Figure 1C), and inhibition was not observed without activating the Tregs (Figure 1D). Notably, increasing the numbers of added Tregs to the cultures only modestly increased the level of suppression. To exclude contribution of a minor population of contaminating CD8 T cells that may have accounted for these findings, experiments were performed using activated CD4+CD25+ Foxp3+ Tregs obtained from B6-CD8−/− donor mice and similar inhibition was observed (Figure 1B). Activated Tregs were capable of mediating inhibition against allogeneic as well as syngeneic TCD-BM populations (supplemental Figure 1C).
Figure 1.
Activated CD4+CD25+ Tregs affect inhibition of ex vivo CFU-IL3 activity by BMCs. CD4+CD25+ T cells (Tregs) were prepared (supplemental Figure 1) from spleen and LNC and activated ex vivo with anti-CD3/CD28 beads and rIL2 for 3 days before coculture with syngeneic TCD-BM. After coculture, cells were harvested and plated for CFU activity with rmIL-3 as described. Data are presented as the mean number of CFU ± SD calculated from triplicate wells. P < .05 was considered statistically significant by analysis of variance (ANOVA). Activated syngeneic CD4+CD25+ T cells prepared from the spleen and LN of BALB/c (A) and CD8−/− (B) inhibited CFU-IL3 compared with TCD-BM cocultured alone or supplemented with Ab-coated beads and rmIL2. (C) Freshly isolated CD4+CD25+ T cells as described in panel A from CD8−/− spleen cells were cocultured at indicated ratios with 2.5 × 104 syngeneic TCD-BMCs in triplicate microwells in medium supplemented with appropriate growth factors. After 72 hours, cells were collected, washed, and plated for CFU-IL3 as described. No inhibition (P > .05) of CFU-IL3 levels was observed. (D) CD4+CD25+ T cells from B6-CD8−/− spleen and LN cells were cocultured with anti-CD3/CD28 beads and rIL-2 or rIL-2 only for 3 days. Cells were washed and cocultured in methylcellulose-based medium with syngeneic TCD-BM. Inhibition was observed only in cultures with stimulating beads plus rIL-2–activated Tregs.
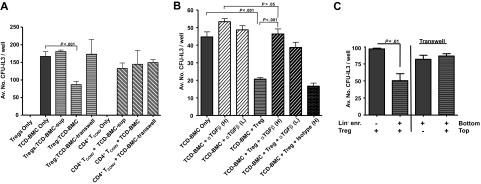
Inhibition of immune activity ex vivo by CD4+CD25+ T cells has generally been found to be contact dependent.16,25 BALB/c TCD-BM were therefore separated from activated BALB/c CD4+CD25+ T cells using transwell chambers (Figure 2A). Inhibition of CFU-IL3 was abolished under these conditions. In addition, supernatants from cultures of activated Tregs also failed to mediate CFU-IL3 suppression (Figure 2A) and identical results were obtained using a different (ie, BL/6) strain (data not shown). Finally, no suppression was detected in transwell cultures (or supernatants) using CD25− (CD4+ conventional T cells) T cells (Figure 2A). These results support the notion that similar to the observed Treg modulation of lymphoid cell responses ex vivo, contact dependency is also required for Treg-mediated regulation of IL-3–responsive progenitors. Because TGF-β1, both membrane bound and soluble, has been reported to be an effector molecule of Tregs, anti–TGF-β antibody was added during the coculture of B6 CD8−/− Tregs with TCD-BM.16 Antibodies used were reactive against both the active (25 kDa) form (ie, 1D11; data not shown) and the LAP of TGF-β1 (chicken IgY), which is reportedly associated with membrane-localized TGF-β1.16 Inhibition was abolished using both reagents involving B6-CD8−/− Tregs (Figure 2B) as well as BALB/c Tregs (data not shown). To ascertain whether ex vivo–activated CD4+FoxP3+ Tregs mediate contact-dependent regulation of CFU-IL3, knockin Tregs (B6-Foxp3gfp) were FACS purified to more than 99%. Activation of these cells using our in vitro protocol followed by their coculture with lineage-depleted BMCs (< 0.5% lineage marker–positive) also resulted in inhibition of CFU-IL3 levels indistinguishable from that observed using enriched CD4+CD25+ populations when cocultured but not separated in transwell culture (Figure 2C).
Figure 2.
CFU-IL3 suppression by Tregs is contact-dependent and can be blocked with anti–TGF-β mAb. BALB/c Tregs were activated and cocultured with TCD-syngeneic BMCs. In some cultures, Tregs were physically separated from BMCs by use of transwell plates. In contrast to inhibition after direct coculture, suppression was abolished in the transwell cultures. (A) Activated conventional (CD25−) CD4 T cells failed to inhibit CFU-IL3 in this assay. (B) Activated Tregs derived from B6-CD8−/− mice were cocultured with TCD-B6 BMCs in the absence or presence of anti–TGF-β neutralizing antibody. Chicken anti–TGF-β1 mAb at 6-ng/mL (high [H]) and 3-ng/mL (low [L]) concentrations but not chicken IgY isotype control mAb (Iso H) added during the Treg + BMCs coculture period inhibited levels of CFU-IL3. Statistical analyses: BMCs + Treg versus BMCs + Treg + anti-TGF-β (L) = P <.001; BMCs + Treg versus BMCs + Treg + isotype (H) = P > .05. (C) CD4+FoxP3+ Tregs mediate regulation of CFU-IL3. B6-Foxp3gfp Tregs were FACS purified to > 99%. Activation of these cells followed by coculture with lineage-depleted (< 0.5% lineage marker–expressing) B6 BMCs resulted in contact-dependent inhibition of CFU-IL3 levels. Data are presented as the average CFU-IL3 number per 5 × 103 lineage-depleted BMCs either cocultured in contact with Tregs or separated in transwell cultures.
Highly enriched Tregs inhibit CFU-IL3 from lineage-depleted wild-type but not class II–deficient bone marrow
CD4+CD25+ T cells express TcRαβ receptors with diversity commensurate with conventional T cells and are presumed to recognize antigen presented by MHC class II.26 TCD-BMCs from B6-wt or B6-class II–deficient mice were therefore cocultured with activated B6-CD8−/− Tregs. As predicted, inhibition of CFU-IL3 was observed against cultures containing B6-wt TCD-BMCs; however, inhibition was not detected in BMCs deficient in MHC class II expression (supplemental Figure 2A). This pattern has been observed with different progenitor cell–containing populations including TCD-BM, as well as rigorously depleted marrow (supplemental Figure 2B-C). Together with the findings that cell contact is required for inhibition, these results suggest that a cognate interaction between Tregs and marrow cells that requires MHC class II may be important. Furthermore, the findings are consistent with the notion that direct Treg-PC interaction may be occurring.
To further examine this possibility, we next investigated the capacity of highly purified (99.7% CD4+CD25+) Tregs for their inhibitory activity. This population inhibited CFU-IL3 activity of B6-wt but not B6-MHC class II−/− TCD-BM (Figure 3A). Moreover, when this highly purified Treg population was cocultured with rigorously lineage-depleted BMCs from B6-wt or B6-MHC class II−/− mice, this same pattern of inhibition was observed (Figure 3B). In contrast to the likelihood that Tregs can “indirectly” modulate hematopoiesis via regulation of T- and NK cell function, these results strongly suggest that direct regulation of hematopoietic PC function can also be mediated by Tregs. To determine whether cytotoxicity by Tregs may be important in this hematopoietic regulation, Tregs from B6 mice deficient in functional perforin and FasL were examined and found to be capable of effecting inhibition of CFU-IL3 (supplemental Figure 2D). Thus, neither cytolytic-mediated apoptotic signaling pathway is necessary to affect hematopoietic regulation observed.
Figure 3.
Highly enriched Treg cell preparations inhibit lineage-depleted bone marrow from syngeneic wild-type but not MHC class II−/− mice. (A) CD4+CD25+ T cells from B6-CD8−/− spleen and lymph node cells were prepared by initially depleting B cells with anti–mouse IgG and IgM Abs. Remaining cells were labeled with anti–CD4-CYC and anti–CD25-PE. Cells were separated on a FACSAria. Postsort analysis indicated enrichment at 99.7% (A inset). These cells were activated for 3 days as described. Bone marrow populations from B6-wt and B6-MHC class II−/− mice were depleted of T cells and cocultured with these activated Tregs. B6-wt (P < .05) but not B6-MHC class II−/− cultures containing Tregs showed diminished CFU-IL3 levels. After 1.5 days of coculture, cells were harvested and plated for CFU-IL3. (B) Tregs obtained from the same highly enriched preparation as in panel A were cocultured with lineage-depleted B6-wt and B6-MHC class II−/− BMCs as in panel A. To more stringently lineage deplete the marrow populations, the Miltenyi Biotec lineage depletion kit (biotinylated mAb cocktail) was initially used, and the remaining cells were labeled with streptavidin-FITC and anti–c-kit-PE mAb. After isolation by cell sorting (FACSAria), the FITC−PE+ fraction was > 99.6% for the 2 bone marrow populations (B insets). B6-wt (P < .001) but not B6-MHC class II−/− cultures with Tregs exhibited decreased CFU-IL3 numbers. CFU-IL3 were performed as described.
Inhibition of the recipient's CD4+CD25+Foxp3+ compartment alters hematopoietic progenitor cell activity after HSCT
Based on experimental findings, there is increasing consideration of application of CD4+CD25+ Treg populations to regulate graft-versus-host disease (GVHD) and PC resistance pathways after allogeneic BMT, and initial trials involving such therapy in clinical HSCT are under way.5–9 The present observations support the notion that activated Tregs may directly modulate the hematopoietic compartment during the immediate reconstitution period after BMT. BALB/c mice were therefore pretreated with anti-CD25 mAb before lethal conditioning and syngeneic TCD-BMCs (Figure 4A). The CD25+FoxP3+ compartment was diminished in anti-CD25–treated mice as assessed after this mAb administration (Figure 4A bottom right panel). Although anti-CD25 treatment markedly diminished the host FoxP3 compartment, up to 40% of CD4+ Tregs can be CD25−.27 Regardless, splenic CFU-IL3 levels were elevated in these depleted recipients versus controls 1 week after BMT (Figure 4B). We further tested the hypothesis that MHC class II is required for such regulation by transplanting B6-wt or MHC class II−/− BMCs into lethally conditioned B6-wt recipients (Figure 4C left panel). In contrast to enhanced CFU levels detected in the B6-wt recipients receiving B6-wt BMCs, recipients receiving BMCs lacking MHC class II did not contain augmented colony activity after anti-CD25 mAb administration (Figure 4C right panel). These findings are consistent with the in vitro observations supporting the notion that a cognate, contact-dependent interaction between CD4+CD25+FoxP3+ and targeted cells is required for the observed regulation and is dependent on MHC class II.
Figure 4.
In vivo depletion of host CD25+ cells enhances CFU-IL3 after transplantation of syngeneic BMCs. (A) Lymph node cells (3-4 mice/group) from control rat IgG– and anti-CD25–treated (PC61 mAb) BALB/c (H2d) mice (1 mg intraperitoneally at days −4 and −2) were stained on day 0 for CD4 (PerCp) and CD25 (PE, clone 7D4) followed by anti-Foxp3 (FITC) intracellular staining. In comparison with rat IgG–treated control mice (A top panels), the CD4+ cells of lymph nodes from anti-CD25 mAb–treated mice (A bottom panels) exhibited few Foxp3+ Tregs. Results are representative of 2 independent experiments demonstrating a decrease in Foxp3 levels in CD4+CD25+ Tregs in anti-CD25–treated mice. (B) BALB/c (H2d) mice were treated with control rat IgG or anti-CD25 mAb as described in panel A (3-4 mice/group) and were administered lethal TBI on day 0 and escalating doses of syngeneic TCD-BMCs (3-4 mice/group). Splenic CFU-IL3 were assessed on day 7. Triplicate cultures were established and the results presented as the average total CFU-IL3/spleen ± SD (ANOVA), after multiplying CFU frequency by the total number of nucleated splenocytes. Enhanced BM engraftment occurred after the depletion of host CD4+CD25+ FoxP3+ Tregs. One of 2 experiments is presented. (C) Depletion of CD25+ cells in recipients undergoing HSCT with donor B6-wt or B6-MHC class II−/− marrow differentially affects CFU-IL3 progenitor cell levels early after transplantation. B6 mice were prepared and treated with antibodies as in panel A, and then administered lethal TBI on day 0 (9.5 Gy) and escalating doses of B6-wt or B6-MHC class II−/− TCD-BMCs (3-4 mice/group). Splenic CFU-IL3 were assessed on day 7. Enhanced CFU-IL3 occurred after transplantation of B6-wt but not MHC class II–deficient TCD-BMC inoculum. P values illustrate groups that exhibited statistically significant (P < .05) differences.
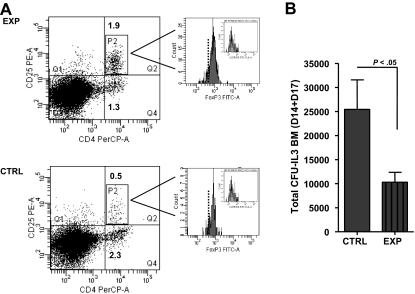
Coadministration of CD4+FoxP3+ T cells with bone marrow inhibits CFU-IL3 activity and diminishes circulating polymorphonuclear leukocyte levels in syngeneic transplant recipients
The findings thus far indicated that diminishment of the endogenous CD4+CD25+ T-cell compartment affected hematopoietic progenitor cell activity after HSCT. To definitively examine the capacity of CD4+FoxP3+ Tregs to modulate this progenitor cell activity after transplantation, B6 mice were TBI conditioned (9.5 Gy) and administered B6 TCD-BM together with highly purified (> 99.8%) B6 CD4+FoxP3+ T cells obtained after sorting (Figure 5A) from FoxP3gfp mice.27 One week after transplantation, the FoxP3+ infused cells were readily apparent in recipient spleens (Figure 5B). Notably, overall spleen cell numbers were diminished (49%, n = 4) in recipients of the Tregs versus controls. CFU-IL3 progenitor cell numbers in animals that received a cotransplant of Tregs were markedly diminished (P < .02) compared with control animals receiving marrow alone (Figure 5C). An experiment was performed to compare the regulatory activity of Tregs with a non-Treg (ie, “filler”) population. Cotransplantation of B6 CD4+CD25+ Tregs but not B6 CD19+ B cells into lethally irradiated recipients resulted in diminished spleen cell numbers and overall numbers of CFU-IL3 (supplemental Figure 4A-B).
Figure 5.
Recipients of syngeneic bone marrow and FoxP3+ T cells exhibit diminished CFU-IL3 progenitor cell activity after transplantation. CD4+GFP+ (Treg) cells were obtained from B6 Foxp3gfp donors after positive selection via cell sorting for allophycocyanin (CD4) and gfp (FoxP3) expression; purity = 99.8% (A). These highly purified Tregs were coinfused with syngeneic B6 TCD-BMCs at day 0 into lethally irradiated (9.5 Gy) B6 mice (n = 4). Control recipients received TCD-BM alone (n = 4). One week after HSCT, CD4+ GFP+ Tregs were readily identified in recipient spleens (B). Representative dot plots of control and experimental recipients are shown. Values in dot plots represent percentage of gfp+ cells. Control, 0%; experimental, 2.4%-2.9%. CFU-IL3 activity by spleen cells was assessed at day 7 after HSCT from triplicate cultures as described (C). Data are presented as total numbers of CFU/spleen from B6 animals that underwent transplantation. Statistical analysis comparing the mean values of the 2 transplant groups: P < .02.
To address whether cotransplantation of Tregs could affect marrow progenitor activity, CFU-IL3 were assessed from B6 bone marrow 14 to 17 days after transplantation (Figure 6). To obtain sufficient numbers of Tregs for these experiments, we expanded CD4+CD25+ T cells from normal B6 mice using rmIL2 plus anti-IL2 mAb complexes.11,24 These Tregs express identical levels of FoxP3 as nonexpanded populations, and we have shown they possess virtually identical suppressive activity in Treg assays11,24 (data not shown). After transplantation, CD4+CD25+Foxp3+ B6 Tregs were identified in the marrow of both control and the experimental recipients of CD4+CD25+ B6 Tregs (Figure 6A). Notably, the marrow CD4 T-cell compartment of the Treg recipients contained approximately 60% CD25+Foxp3+ cells, whereas the control recipient's compartment contained approximately 18%. The numbers of Tregs were clearly higher in the former as well (2.3 × 106 vs 5.4 × 104). Overall CFU-IL3 numbers were diminished in the marrow of recipients of Tregs (Figure 6B). An experiment was performed to examine whether CD4+FoxP3+ knockin Tregs influenced the appearance of multipotential spleen CFUs. Although the decrease in numbers was modest, the overall size of spleen CFU colonies in recipients of Tregs was reduced (supplemental Figure 3B). To examine a comparable in vitro multipotent CFU population, a cocktail of growth factors to drive CFU–granulocyte, erythrocyte, monocyte, macrophages was used. Coculture with activated Tregs suppressed CFU numbers (supplemental Figure 1D). In total, these findings demonstrated that transplanted CD4+FoxP3+ Tregs possess the capacity to inhibit hematopoietic progenitor populations and function in situ after bone marrow transplantation in lethally conditioned recipients.
Figure 6.
Recipients of syngeneic bone marrow and Tregs have decreased CFU-IL3 activity in the marrow compartment 2 weeks after HSCT. Enriched CD4+CD25+ B6 Treg preparations were isolated from IL-2/anti-IL2 mAb complex–treated animals and coinfused with syngeneic TCD-BMCs on day 0 into lethally irradiated (9.5 Gy) B6 mice. (A) Approximately 2 weeks (days 14 and 17) after transplantation, markedly enhanced levels of CD4+CD25+ T cells (∼4×) were identified in the marrow compartment from recipients receiving Tregs and BMCs (experimental) versus BMCs (control) alone. The majority of these CD4+CD25+ T cells were FoxP3+ (inset histograms: isotype-FITC Ig staining; dotted line represents gate based on unstained control sample). (B) CFU-IL3 numbers were assessed from triplicate cultures as described. Results are presented as the mean CFU-IL3 numbers/marrow compartment in control (n = 6) and experimental (n = 6) groups. Error bars represent average CFU-IL3 ± SD from 18 replicate cultures. P < .05.
Lastly, to determine whether the diminished CFU-IL3 progenitor cell levels observed could result in altering the levels of a mature, reconstituting myeloid cell population, complete blood count (CBC) was performed for individual recipients (n = 7/group) in control mice and mice that received a Treg cotransplant (Figure 7). In vivo expanded Tregs were obtained from B6 mice and coinfused in experimental recipients. Circulating numbers of polymorphonuclear leukocytes (PMNs) were found to be decreased by 2 weeks after HSCT. Further analyses indicated that up to 5 weeks after transplantation, lower numbers of circulating PMNs (as well as decreased percentage of PMNs in the peripheral blood vs animals that did not undergo Treg transplantation, P < .03; data not shown) were observed in the experimental (ie, received a cotransplant with Tregs) recipient groups (Figure 7 inset). We also examined peripheral blood levels of monocytes and platelets (supplemental Figure 4C-D). Both of these parameters exhibited slower recovery and somewhat decreased numbers. the percentage of monocytes in blood was significantly decreased (P < .05), although the overall numbers did not reach statistical differences.
Figure 7.
Coinfusion of Tregs with bone marrow diminishes peripheral blood neutrophil numbers after transplantation. (Left panel) B6-wt mice (10-12 weeks old) were lethally irradiated (9.5 Gy TBI) and transplanted with 0.3 × 106 syngeneic TCD-BM. Animals were untreated (n = 7, 2 experiments) or coinfused with 1 × 106 in vivo IL2/anti-IL2 mAb complex–expanded Tregs (> 95% CD4+CD25+) at the time of transplantation (n = 7). Peripheral blood was obtained from animals at days 16 and 17 after HSCT and CBC were performed. Results of 2 independent transplantation experiments are presented. Data of individual mice and the average neutrophil counts/group are shown. (Right panel) PMN levels were obtained from individual transplant recipients as described (n = 4/group). Peripheral blood was obtained at the indicated days after HSCT and 40 μL was analyzed by the Department of Pathology (University of Miami Miller School of Medicine) using a Hemavet 950. There was also an overall decrease in the percentage of PMNs (P < .03) in the peripheral blood (data not shown).
Discussion
T cells have been proposed to contribute to the regulation of hematopoietic activity under both physiologic as well as transplant conditions.28–31 Hematopoietic growth factors including GM-CSF and IL-3 produced by T lymphocytes have been demonstrated to expand murine and human PCs ex vivo, and CD4 and NK/T lymphocytes have been reported to promote PC activity in vivo.28,29,32,33 The present results indicate that in contrast to findings regarding enhancement of hematopoietic progenitors by global CD4-activated and Th1 populations,30,31 CD4+CD25+ T cells possess the capacity to down-regulate functional IL-3–responsive progenitor cells to growth factor stimulation ex vivo. Several pathways may contribute to this regulation. The ex vivo findings here suggest that “direct” regulation of hematopoietic PC function may be mediated by Tregs. Although inclusion of anti-TCR (CD3) mAb and cytokines in our assays prevented meaningful analyses of bidirectional Treg-PC signaling, a recent study proposed that direct interaction of Tregs with mobilized Lin−Sca-1+c-kit+ mobilized PCs could signal expansion of the former via a notch-dependent pathway.34 In addition, results demonstrated that cotransplanted Tregs could also suppress progenitor cell populations in situ in syngeneic recipients conditioned with lethal TBI (but not sublethal TBI; data not shown). Thus, these findings support the notion that regulatory activity of CD4+FoxP3+ T cells extends to hematopoiesis under conditions of stress such as hematopoietic progenitor cell transplantation.
In typical lymphocyte suppression assays, Tregs are activated while in coculture with responding lymphocytes, typically by anti-CD3 mAb and other signals. Suppression of IL-3–driven progenitors in vitro occurred only using preactivated Tregs. Such “disparity” may reflect the different levels of cytokine(s) required to inhibit hematopoietic progenitor cells compared with lymphocytes and/or the time of susceptibility of these differing populations to regulation under the culture conditions used. The capacity of both fresh as well as “preactivated” Tregs to suppress CFUs in transplant recipients is not surprising, as the former presumably undergo activation involving antigen release and cytokines present in lethally conditioned recipients. Consistent with this interpretation, heightened levels of CD25 expression were apparent on CD4+FoxP3+ Tregs isolated from recipients after HSCT (supplemental Figure 3A). Nonetheless, it is not unlikely that Treg populations that are coinfused into conditioned recipients undergo activation because of a variety of potential signals dependent on the transplant conditions. We routinely detect 40% to 60% suppression of CFU-IL3 levels in experimental cultures even after addition of high numbers of Tregs/BMCs (ie, 5:1, 10:1). It is worthwhile to note that in the multiday coculture period, SCF and growth factors are included to maintain and support progenitor cell presence. Although the majority of progenitor cells express c-kit, there is considerable heterogeneity in the type and differentiated state of marrow populations present in the cultures that are then subject to signaling by the supplemented growth factors. Such heterogeneity is likely to contribute to the overall levels of suppression observed and inability to effect complete suppression (100% inhibition) in these assays. Similar circumstances are likely present during hematopoietic progenitor cell transplantations, resulting from diverse conditioning protocols as well as the source of progenitor cell populations (eg, marrow, mobilized peripheral blood, cord blood) used for clinical transplantation. Initial observations have not detected differences in apoptosis of marrow cells in cocultures lacking or containing Tregs (data not shown). These observations are consistent with the present findings that Tregs simultaneously lacking functional perforin and FasL retain this hematopoietic inhibitory activity and with prior reports that did not detect apoptosis by TGF-β1 in multipotent CFU–high proliferative potential (HPP) populations.35 CFU activity could be inhibited as a result of a diminution in growth factor receptors. Studies have reported that TGF-β1 can modulate (ie, down-regulate) IL-3 and CSF-1 receptors.36 TFGβ1 has also been reported to down modulate c-kit expression and thus could dampen SCF signals to progenitor populations.37 Alternatively, but not mutually exclusively, cell cycle arrest of CD34+ human cord blood–derived hematopoietic PCs has recently been reported after TGF-β signaling. In contrast to nonhematopoietic populations in which p15 and p21 are important for TGF-β–mediated cytostasis, a recent study reported TGF-β receptor type I (TβR-I) inhibition of the human CD34+ populations was linked to up-regulation of the cyclin-dependent kinase inhibitor p57KIP2.38 P27−/− mice express an increased level of PC proliferation and overall pool size numbers.39 The use of purified PCs together with CD4+CD25+ T regulatory cells in transwell experiments may enable analyses of TβR signaling and involvement of p57/p27 pathways in the inhibition observed.
It may be noteworthy that in mice deficient in TGF-β1, concurrent with a relative increase in CD4+ T cells, there is elevated myelopoiesis present in the bone marrow and spleen in the absence of extreme inflammation.40 A recent study noted that after depletion of FoxP3+ T cells in adult mice, expansion was observed in the myeloid dendritic cell compartment.2 Because the CD11c+CD11b+ dendritic cell populations are mostly nonproliferative, the generation of the cells was examined and labeling studies suggested that their enhanced numbers resulted via heightened generation from precursors after elimination of Tregs in these mice.2 Finally, bipotential megakaryocytic erythroid progenitors can give rise to both megakaryocyte CFUs and erythroid CFUs.41 Because thrombopoietin is known to up-regulate TGF-β receptors on megakaryocytic precursors as part of negative feedback inhibition of platelet production, it will be interesting to determine where during the erythroid/megakaryocytic lineage Treg modulation may be occurring.42,43 We have observed that Tregs were also found to be capable of enhancing erythroid CFUs and this pathway is IL-9 dependent (Urbieta et al, manuscript in preparation).
The inhibition of CFU-IL3 required MHC class II expression on the targeted populations. MHC class II expression on some hematopoietic progenitor cells has been reported and its cytokine induction by interferon-γ can also occur.44–46 If direct Treg-PC and not non-PC interaction is required for the inhibition observed, the distribution and level of MHC class II expression may influence the extent of regulation observed. Although no evidence of a qualitative or quantitative difference in hematopoiesis has been reported in MHC class II–deficient mice, these animals clearly lack physiologic levels of CD4+CD25+Foxp3+ T cells. It may be noteworthy that we have frequently observed somewhat higher overall numbers of CFU-IL3 from highly purified MHC class II−/− versus wild-type BMCs (Figure 3), but studies necessary to carefully assess a causal physiologic relationship of greater numbers of CFU-IL3 with decreased Tregs in mice that have not undergone transplantation (eg, IL-2, IL-2R, CD4, or MHC class II deficiency) have not been performed. Our findings suggest that individuals lacking this population may have altered/imbalanced levels of selected CFU progenitor populations consistent with observations addressing the role of CD4 T-cell activity during normal hematopoiesis.31
Tregs have been identified in healthy mouse and human bone marrow and with respect to transplantation, ex vivo–activated Tregs up-regulate CXCR4.47 Based on these observations, it is likely that both endogenous as well as anti-CD3–stimulated and infused Tregs possess the potential to migrate to hematopoietic tissues via stromal-derived factor-1 (SDF-1 or CXCL12). We have found endogenous, that is, host CD4+CD25+FoxP3+ cells in the marrow and spleen compartments of mice after TBI at levels greater than those used in the present studies (eg, ≥ 9.5 Gy) as well as after busulfan with or without cyclophosphamide conditioning.48 These CD4+CD25+Foxp3+ cells are of recipient origin and represent a small number of surviving Tregs, which then rapidly undergo expansion assessed by bromodeoxyuridine incorporation.48 We depleted endogenous CD25+ Tregs before HSCT and observed modulation of PC activity consistent with our in vitro observations, that is, removal of suppression resulted in increased CFU-IL3 numbers. Notably, host “residual” Tregs are known to persist even after ablative conditioning and mediate protection from autoimmune disease as well as regulation of marrow allograft rejection.10,49–51 These observations support the notion that surviving host Tregs undergo activation in transplant recipients perhaps due to cytokine and or self-antigen signaling. To address the question of whether Tregs coinfused at the time of transplantation could alter hematopoietic activity, highly purified Treg populations including Foxp3gfp knockin cells were cotransplanted with syngeneic progenitor cells and found to reduce splenic and marrow levels of CFU-IL3 1 and 2 weeks, respectively, after transplantation. These results demonstrate the potential of FoxP3+ regulatory cells to modulate hematopoietic activity under transplant conditions and together with the depletion studies indicate that both donor as well as recipient Tregs may be important. Currently, clinical trials have begun administering donor CD4+CD25+FoxP3+ T cells at the time of MHC-mismatched HSCT to regulate GVHD. When potent alloreactive T-cell activity is ongoing, Treg cell affects on T-cell responses may obfuscate or make assessment of hematopoietic activity difficult to assess. However, we did observe diminution of CFU activity when FoxP3+ Tregs were added to a MiHA-mismatched transplant under conditions where weak alloreactivity is engendered early after transplantation (supplemental Figure 4). Present studies observed decreased levels of circulating PMNs for several weeks posttransplantation in recipients of CD4+CD25+ Tregs; Treg effects may be more pronounced in situations where more limited PCs are present. It is thus interesting to consider cord blood transplant settings where GVHD is often reduced and neutrophil and platelet reconstitution can be delayed.52–54 Previous studies have clearly demonstrated that Tregs can influence HSCT via regulation of donor (GVHD) and host (host vs graft) alloimmune T/NK-cell responses.6–8 Delayed infusion of Tregs, which has been shown to inhibit GVHD, may provide one approach to circumvent early regulation of hematopoiesis after transplantation while maintaining desired affects on adaptive and innate immune responses.20
Our results suggest that CD4+CD25+FoxP3+ T cells are not restricted in their regulatory actions within the adaptive and innate immune systems. The ability of these cells to inhibit progenitor cell activity illustrates the complex interactions between the immune and hematopoietic compartments. The findings support the notion that Treg involvement in hematopoiesis may occur under conditions of stress to the hematopoietic compartment, such as accompanies hematopoietic cell transplantations, infections, and others. Interestingly, in a nontransplantation model, a recent study noted that depletion of CD25+ Tregs resulted in elevated myeloid progenitors during an Staphylococcus enterotoxin B–induced immune response.55 In total, the present study suggests that negative regulation of myeloid progenitors can occur in the presence of CD4+FoxP3+ regulatory cells after ablative conditioning and HSCT. Thus, the intentional addition (ie, “donor”) and/or deletion (ie, “recipient”) of this population may be important to consider within the context of the hematopoietic and immunologic reconstitution of the patient after HSCT.
Supplementary Material
Acknowledgments
The authors thank The Sylvester Cancer Center for support of the Flow Cytometry Facility, the Diabetes Research Institute (DRI) for help with flow sorting, and Dr Allison Bayer (Department of Microbiology and Immunology, University of Miami and the DRI) for technical advice and FoxP3gfp mice.
This work was supported by National Institutes of Health grants AI46689 and CA 120776 (R.B.L.), HL089905 (W.J.M.), and HL63452 and CA72669 (B.R.B.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.U. and I.B. designed and performed experiments, collected and analyzed results, and wrote and revised the paper; M.J. performed experiments and revised the paper; R.J. helped performed experiments and revised the paper; A.P.-M. collected data; B.R.B. collected data and revised the paper; W.J.M. designed experiments, analyzed results, and revised the paper; and R.B.L. designed experiments, analyzed data, and wrote and revised the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert B. Levy, University of Miami Miller School of Medicine, Department of Microbiology & Immunology, 1600 NW 10th Ave, PO Box 016960, Miami, FL 33101; e-mail: rlevy@med.miami.edu.
References
- 1.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155(3):1151–1164. [PubMed] [Google Scholar]
- 2.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8(2):191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 3.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445(7129):766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 4.Williams LM, Rudensky AY. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat Immunol. 2007;8(3):277–284. doi: 10.1038/ni1437. [DOI] [PubMed] [Google Scholar]
- 5.Kingsley CI, Karim M, Bushell AR, Wood KJ. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J Immunol. 2002;168(3):1080–1086. doi: 10.4049/jimmunol.168.3.1080. [DOI] [PubMed] [Google Scholar]
- 6.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99(10):3493–3499. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196(3):389–399. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edinger M, Hoffmann P, Ermann J, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9(9):1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 9.Hanash AM, Levy RB. Donor CD4+CD25+ T cells promote engraftment and tolerance following MHC-mismatched hematopoietic cell transplantation. Blood. 2005;105(4):1828–1836. doi: 10.1182/blood-2004-08-3213. [DOI] [PubMed] [Google Scholar]
- 10.Barao I, Hanash AM, Hallett W, et al. Suppression of natural killer cell-mediated bone marrow cell rejection by CD4+CD25+ regulatory T cells. Proc Natl Acad Sci U S A. 2006;103(14):5460–5465. doi: 10.1073/pnas.0509249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shatry A, Levy RB. In situ activation and expansion of host Tregs: a new approach to enhance donor chimerism and stable engraftment in MHC-matched allogeneic HCT. Biol Blood Marrow Transplant. 2009;15(10):1239–1243. doi: 10.1016/j.bbmt.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woo EY, Chu CS, Goletz TJ, et al. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61(12):4766–4772. [PubMed] [Google Scholar]
- 13.Yu P, Lee Y, Liu W, et al. Intratumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. J Exp Med. 2005;201(5):779–791. doi: 10.1084/jem.20041684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen ML, Pittet MJ, Gorelik L, et al. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc Natl Acad Sci U S A. 2005;102(2):419–424. doi: 10.1073/pnas.0408197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piccirillo CA, Shevach EM. Cutting edge: control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J Immunol. 2001;167(3):1137–1140. doi: 10.4049/jimmunol.167.3.1137. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194(5):629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piccirillo CA, Letterio JJ, Thornton AM, et al. CD4+CD25+ regulatory T cells can mediate suppressor function in the absence of transforming growth factor {beta}1 production and responsiveness. J Exp Med. 2002;196(2):237–246. doi: 10.1084/jem.20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uhlig HH, Coombes J, Mottet C, et al. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J Immunol. 2006;177(9):5852–5860. doi: 10.4049/jimmunol.177.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu LF, Lind EF, Gondek DC, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442(7106):997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 20.Jones SC, Murphy GF, Korngold R. Post-hematopoietic cell transplantation control of graft-versus-host disease by donor CD425 T cells to allow an effective graft-versus-leukemia response. Biol Blood Marrow Transplant. 2003;9(4):243–256. doi: 10.1053/bbmt.2003.50027. [DOI] [PubMed] [Google Scholar]
- 21.Johnson BD, Konkol MC, Truitt RL. CD25(+) immunoregulatory T-cells of donor origin suppress alloreactivity after BMT. Biol Blood Marrow Transplant. 2002;8(10):525–535. doi: 10.1053/bbmt.2002.v8.pm12434947. [DOI] [PubMed] [Google Scholar]
- 22.Spielman J, Lee RK, Podack ER. Perforin/Fas-ligand double deficiency is associated with macrophage expansion and severe pancreatitis. J Immunol. 1998;161(12):7063–7070. [PubMed] [Google Scholar]
- 23.Jones M, Komatsu M, Levy RB. Cytotoxically impaired transplant recipients can efficiently resist major histocompatibility complex-matched bone marrow allografts. Biol Blood Marrow Transplant. 2000;6:456–464. doi: 10.1016/s1083-8791(00)70038-3. [DOI] [PubMed] [Google Scholar]
- 24.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311(5769):1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 25.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 26.Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat Immunol. 2006;7(4):401–410. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- 27.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22(3):329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 28.Geissler D, Lu L, Bruno E, Yang HH, Broxmeyer HE, Hoffman R. The influence of T lymphocyte subsets and humoral factors on colony formation by human bone marrow and blood megakaryocyte progenitor cells in vitro. J Immunol. 1986;137(8):2508–2513. [PubMed] [Google Scholar]
- 29.Kanz L, Lohr GW, Fauser AA. Lymphokine (s) from isolated T lymphocyte subpopulations support multilineage hematopoietic colony and megakaryocytic colony formation. Blood. 1986;68(5):991–995. [PubMed] [Google Scholar]
- 30.Broxmeyer HE, Bruns HA, Zhang S, et al. Th1 cells regulate hematopoietic progenitor cell homeostasis by production of oncostatin M. Immunity. 2002;16(6):815–825. doi: 10.1016/s1074-7613(02)00319-9. [DOI] [PubMed] [Google Scholar]
- 31.Monteiro JP, Benjamin A, Costa ES, Barcinski MA, Bonomo A. Normal hematopoiesis is maintained by activated bone marrow CD4+ T cells. Blood. 2005;105(4):1484–1491. doi: 10.1182/blood-2004-07-2856. [DOI] [PubMed] [Google Scholar]
- 32.Murphy WJ, Raziuddin A, Mason L, Kumar V, Bennett M, Longo DL. NK cell subsets in the regulation of murine hematopoiesis. I. 5E6+ NK cells promote hematopoietic growth in H-2d strain mice. J Immunol. 1995;155(6):2911–2917. [PubMed] [Google Scholar]
- 33.Leite-de-Moraes MC, Lisbonne M, Arnould A, et al. Ligand-activated natural killer T lymphocytes promptly produce IL-3 and GM-CSF in vivo: relevance to peripheral myeloid recruitment. Eur J Immunol. 2002;32(7):1897–1904. doi: 10.1002/1521-4141(200207)32:7<1897::AID-IMMU1897>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 34.Kared H, Adle-Biassette H, Fois E, et al. Jagged2-expressing hematopoietic progenitors promote regulatory T cell expansion in the periphery through notch signaling. Immunity. 2006;25(5):823–834. doi: 10.1016/j.immuni.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 35.Batard P, Monier MN, Fortunel N, et al. TGF-(beta)1 maintains hematopoietic immaturity by a reversible negative control of cell cycle and induces CD34 antigen up-modulation. J Cell Sci. 2000;113(pt 3):383–390. doi: 10.1242/jcs.113.3.383. [DOI] [PubMed] [Google Scholar]
- 36.Jacobsen SE, Ruscetti FW, Roberts AB, Keller JR. TGF-beta is a bidirectional modulator of cytokine receptor expression on murine bone marrow cells: differential effects of TGF-beta 1 and TGF-beta 3. J Immunol. 1993;151(9):4534–4544. [PubMed] [Google Scholar]
- 37.Dubois CM, Ruscetti FW, Stankova J, Keller JR. Transforming growth factor-beta regulates c-kit message stability and cell-surface protein expression in hematopoietic progenitors. Blood. 1994;83(11):3138–3145. [PubMed] [Google Scholar]
- 38.Scandura JM, Boccuni P, Massague J, Nimer SD. Transforming growth factor beta-induced cell cycle arrest of human hematopoietic cells requires p57KIP2 up-regulation. Proc Natl Acad Sci U S A. 2004;101(42):15231–15236. doi: 10.1073/pnas.0406771101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng T, Rodrigues N, Dombkowski D, Stier S, Scadden DT. Stem cell repopulation efficiency but not pool size is governed by p27(kip1). Nat Med. 2000;6(11):1235–1240. doi: 10.1038/81335. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi S, Yoshida K, Ward JM, et al. Beta 2-microglobulin-deficient background ameliorates lethal phenotype of the TGF-beta 1 null mouse. J Immunol. 1999;163(7):4013–4019. [PubMed] [Google Scholar]
- 41.Nakorn TN, Miyamoto T, Weissman IL. Characterization of mouse clonogenic megakaryocyte progenitors. Proc Natl Acad Sci U S A. 2003;100(1):205–210. doi: 10.1073/pnas.262655099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fortunel NO, Hatzfeld JA, Monier MN, Hatzfeld A. Control of hematopoietic stem/progenitor cell fate by transforming growth factor-beta. Oncol Res. 2003;13(6-10):445–453. doi: 10.3727/096504003108748483. [DOI] [PubMed] [Google Scholar]
- 43.Sakamaki S, Hirayama Y, Matsunaga T, et al. Transforming growth factor-beta1 (TGF-beta1) induces thrombopoietin from bone marrow stromal cells, which stimulates the expression of TGF-beta receptor on megakaryocytes and, in turn, renders them susceptible to suppression by TGF-beta itself with high specificity. Blood. 1999;94(6):1961–1970. [PubMed] [Google Scholar]
- 44.Bhattacharya D, Rossi DJ, Bryder D, Weissman IL. Purified hematopoietic stem cell engraftment of rare niches corrects severe lymphoid deficiencies without host conditioning. J Exp Med. 2006;203(1):73–85. doi: 10.1084/jem.20051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mammolenti M, Gajavelli S, Tsouflas P, Levy RB. The absence os MHC class I on neural stem cells does not permit NK killing and prevents recognititon by alloreactive cytotoxic T cells in vitro. Stem Cells. 2004;22:1101–1110. doi: 10.1634/stemcells.22-6-1101. [DOI] [PubMed] [Google Scholar]
- 46.Broxmeyer HE. Relationship of cell-cycle expression of Ia-like antigenic determinants on normal and leukemia human granulocyte-macrophage progenitor cells to regulation in vitro by acidic isoferritins. J Clin Invest. 1982;69(3):632–642. doi: 10.1172/JCI110490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zou L, Barnett B, Safah H, et al. Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer Res. 2004;64(22):8451–8455. doi: 10.1158/0008-5472.CAN-04-1987. [DOI] [PubMed] [Google Scholar]
- 48.Bayer AL, Jones M, Chirinos J, et al. Host CD4+CD25+ T cells can expand and comprise a major component of the Treg compartment following experimental HCT. Blood. 2009;113(3):733–743. doi: 10.1182/blood-2008-08-173179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Komatsu N, Hori S. Full restoration of peripheral Foxp3+ regulatory T cell pool by radioresistant host cells in scurfy bone marrow chimeras. Proc Natl Acad Sci U S A. 2007;104(21):8959–8964. doi: 10.1073/pnas.0702004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bayer AL, Jones M, Chirinos J, et al. Host CD4+CD25+ T cells can expand and comprise a major component of the Treg compartment after experimental HCT. Blood. 2009;113(3):733–743. doi: 10.1182/blood-2008-08-173179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anderson BE, McNiff JM, Matte C, Athanasiadis I, Shlomchik WD, Shlomchik MJ. Recipient CD4+ T cells that survive irradiation regulate chronic graft-versus-host disease. Blood. 2004;104(5):1565–1573. doi: 10.1182/blood-2004-01-0328. [DOI] [PubMed] [Google Scholar]
- 52.Frassoni F, Podesta M, Maccario R, et al. Cord blood transplantation provides better reconstitution of hematopoietic reservoir compared with bone marrow transplantation. Blood. 2003;102(3):1138–1141. doi: 10.1182/blood-2003-03-0720. [DOI] [PubMed] [Google Scholar]
- 53.Rocha V, Cornish J, Sievers EL, et al. Comparison of outcomes of unrelated bone marrow and umbilical cord blood transplants in children with acute leukemia. Blood. 2001;97(10):2962–2971. doi: 10.1182/blood.v97.10.2962. [DOI] [PubMed] [Google Scholar]
- 54.Barker M. Who should get cord blood transplants. Biol Blood Marrow Transplant. 2007;13:78–82. [Google Scholar]
- 55.Lee JH, Wang C, Kim CH. FoxP3+ regulatory T cells restrain splenic extramedullary myelopoiesis via suppression of hemopoietic cytokine-producing T cells. J Immunol. 2009;183(10):6377–6386. doi: 10.4049/jimmunol.0901268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.