Abstract
Recruitment of the Na,K-ATPase to the plasma membrane of alveolar epithelial cells results in increased active Na+ transport and fluid clearance in a process that requires an intact microtubule network. However, the microtubule motors involved in this process have not been identified. In the present report, we studied the role of kinesin-1, a plus-end microtubule molecular motor that has been implicated in the movement of organelles in the Na,K-ATPase traffic. We determined by confocal microscopy and biochemical assays that kinesin-1 and the Na,K-ATPase are present in the same membranous cellular compartment. Knockdown of kinesin-1 heavy chain (KHC) or the light chain-2 (KLC2), but not of the light chain-1 (KLC1), decreased the movement of Na,K-ATPase-containing vesicles when compared to sham siRNA-transfected cells (control group). Thus, a specific isoform of kinesin-1 is required for microtubule-dependent recruitment of Na,K-ATPase to the plasma membrane, which is of physiological significance—Trejo, H. E., Lecuona, E., Grillo, D., Szleifer, I., Nekrasova, O. E., Gelfand, V. I., Sznajder, J. I. Role of kinesin light chain-2 of kinesin-1 in the traffic of Na,K-ATPase-containing vesicles in alveolar epithelial cells.
Keywords: intracellular traffic, molecular motors, sodium pump
In the lungs an edema-free alveolar space is important for the maintenance of an appropriate gas-exchange function (1). Active Na+ transport and thus the clearance of liquid out of the alveolar space is regulated mostly by the basolateraly located Na,K-ATPase and the apical epithelial sodium channel (ENaC) (2,3,4). The Na,K-ATPase is a transmembrane protein located on the basolateral surface of mammalian epithelial cells, which is important for the preservation of cell volume, membrane potentials, and secondary active transport of other solutes (5,6,7).
Intracellular trafficking regulates the Na,K-ATPase abundance at the plasma membrane and its activity (8). We have previously reported that regulating Na,K-ATPase abundance in the basolateral membrane affects active Na+ transport and thus alveolar fluid reabsorption (2); however, the mechanisms regulating intracellular traffic of Na,K-ATPase are not fully understood.
Within the cell, organelles and vesicles are transported to their target destination using microtubule or actin tracks (9). We have previously reported that Na,K-ATPase-containing vesicle movement and incorporation into the plasma membrane are microtubule dependent (8, 10, 11); however, there are no studies on the specific molecular motor involved in this process.
The microtubule molecular motors utilize the energy of ATP hydrolysis to generate movement of particles along the microtubule network (12) and include the kinesin and dynein families. Among the kinesin family, kinesin-1, also known as conventional kinesin, is a tetrameric protein composed of 2 heavy chains (KHC) and 2 light chains (KLC) (13, 14). Three isoforms of KHC have been identified in humans: HsuKHC, HsxKHC, and HsnKHC, the first being ubiquitously expressed (15). KLCs are important for the cargo-binding and cargo-specificity function of the motor and may modulate binding affinity and cargo specificity (16, 17). There are 3 KLC genes: KLC1 and KLC2, ubiquitously expressed, and KLC3, which is expressed only in the testis (18).
In the present study, we explored whether kinesin motors regulate the intracellular trafficking of Na,K-ATPase toward the plasma membrane and which isoforms are involved. We utilized a stable alveolar epithelial cell (AEC) line expressing a GFP-tagged rat α1-Na,K-ATPase, which allowed us to track movement of Na,K-ATPase-containing vesicles; by using a siRNA approach, we provide evidence that Na,K-ATPase-containing vesicles move on microtubule tracks by means of KHC/KLC2, which is required for Na,K-ATPase recruitment into the plasma membrane.
MATERIALS AND METHODS
Reagents
All chemical reagents were purchased from Sigma (St. Louis, MO, USA) unless stated otherwise. Ouabain was purchased from ICN Biomedicals (Aurora, OH, USA). All cell culture reagents were from Mediatech (Herndon, VA, USA). MitoTracker was obtained from Invitrogen (Eugene, OR, USA). Normal goat serum was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA, USA). The following antibodies were used in this study: HD (19), SUK4 (20) (Developmental Studies Hybridoma Bank at the University of Iowa, Iowa City, IA, USA), pan-KLC (17), anti-KLC1 (L2) and anti-KLC2 (B2A5) (gifts from Dr. Gerardo Morfini, University of Illinois at Chicago, Chicago, IL, USA), Na,K-ATPase α1 (Upstate, Chicago, IL, USA), Actin (Sigma), GFP monoclonal (Santa Cruz Biotechnology, Santa Cruz, CA, USA), GFP polyclonal (Clontech, Palo Alto, CA, USA), AlexaFluor 568 (Invitrogen), and secondary goat anti-mouse-HRP and goat anti-rabbit-HRP (Bio-Rad, Hercules, CA, USA).
Cell culture
Human A549 cells (CCL 185; American Type Culture Collection, Manassas, VA, USA) expressing the endogenous human-Na,K-ATPase-α1-subunit (A549) or GFP tagged rat-Na,K- ATPase-α1-subunit (GFPα1-A549; a gift from Dr. Alejandro Bertorello, Karolinska Institute, Solna, Sweden) were used. A description of the Na,K-ATPase-α1 subunit tagged with GFP vector and the generation of a stable cell line expressing this construct can be found in ref. 21. GFPα1-A549 cells show the same enzymatic activity and respond similarly to stimuli as wild-type A549 cells (22). Cells were grown in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin, and for GFPα1-A549, 3 μM ouabain was added to suppress the endogenous Na,K-ATPase-α1 subunit. Cells were incubated in a humidified atmosphere of 5% CO2/95% air at 37°C.
Live-cell confocal imaging
GFPα1-A549 cells were transiently transfected with a vector encoding for tubulin-mCherry fusion protein (23). One day before the experiment, cells were transferred to a glass-bottom (no. 1.5 coverglass, 0.16–0.19 mm) microwell 35-mm dish. Images were obtained using a Nikon Eclipse Ti-E motorized inverted microscope using an ×100 VC Plan APO 1.4 NA oil objective (Nikon Instruments, Melville, NY, USA). The microscope was linked to a CSU-X1 confocal spinning disk unit (Yokogawa Corporation of America, Newnan, GA, USA), an iXon 897 EMCCD camera (Andor Technology, South Windsor, CT, USA), and a krypton/argon air-cooled excitation laser. Cells were visualized in vivo, and fluorescent images were collected every second using iQ multidimensional imaging software (Andor Technology) with exposure time of 300 ms and appropriate filters.
Immunofluorescence confocal microscopy
GFPα1-A549 cells were allowed to grow on coverslips. Cell extraction was performed as described in Tsai et al. (24). Briefly, cells were extracted with 0.05% Triton-X-100 for 4 min at 37°C and then fixed in 2% paraformaldehyde. After fixation, cells were further extracted with 0.2% Triton-X-100 for 10 min, then blocked in PBS + 0.1% normal goat serum + 2% BSA for 1 h. Primary antibody (SUK4 for KHC) was diluted to a final concentration of 0.01 mg/ml in blocking solution and incubated at 37°C for 1 h. Secondary antibody was goat-anti-mouse AlexaFluor 568 (Invitrogen), diluted 1:100 in blocking solution and incubated for 1 h. Images were analyzed using a Zeiss LSM 510 laser-scanning confocal microscope using a Plan Apochromat, ×63/1.4 oil objective (Zeiss, Heidelberg, Germany). Contrast and brightness settings were adjusted so that all pixels were in the linear range.
Subcellular fractionation and sucrose gradient
Subcellular fractionation by sucrose gradient was performed as described previously (25). Briefly, GFPα1-A549 cells were placed on ice and washed 2 times with ice-cold PBS. Cells were scraped in homogenization buffer (0.3 M sucrose; 5 mM EDTA; 10 mM Tris-HCl, pH 7.4; 1 μg/ml leupeptin; 100 μg/ml TPCK; and 1 mM PMSF) and homogenized by using a motor pestle device and passing the homogenate 20 times through a 25-gauge fine needle. The homogenate was centrifuged at 500 g to remove nuclei and debris, and the supernatant was centrifuged at 100,000 g, 1 h at 4°C (TL-100 ultracentrifuge with TLA 100.2 rotor; Beckman Coulter Inc., Fullerton, CA, USA). The pellet containing the crude membrane fraction was resuspended in homogenization buffer and loaded on top of a sucrose gradient. Gradient consisted of seven 0.5-ml steps, with sucrose concentration increasing from 0.5 to 1.7 M, plus 1 ml 2 M sucrose cushion at the bottom. Gradient was centrifuged at 200,000 g for 16 h at 4°C (L7–80 ultracentrifuge with SW55 rotor; Beckman Coulter), and 8 different fractions were collected from the interfaces between the sucrose steps. Fractions were mixed with proper amounts of Laemmli’s sample buffer solution, loaded in an SDS-PAGE, and analyzed by Western blot.
Small interfering RNA against KHC, KLC1, and KLC2
shRNAs against KHC, KLC2, and a nontargeting negative control were generated using the mCherry version of the pG-SUPER vector (26, 27) (a gift from Dr. Shin Kojima, Northwestern University, Evanston, IL, USA). This vector coexpresses mCherry fluorescent protein under the SRα promoter and shRNA under the human H1 promoter, simultaneously so that the knockdown of our target proteins could be analyzed at the level of individual cells as well as by Western blot. The targeting sequences were as follows: for KHC (NM_004521), 5′-CCAGAAGGCATGGGAATTA-3′; and for KLC2 (NM_022822), 5′-GGGAGGAAAGCAAGGATAA-3′. For KLC1 (NM_005552), a pan-KLC1 siRNA pool of 3 different siRNA duplexes was obtained from Santa Cruz Biotechnology.
Live-cell imaging
GFPα1-A549 cells were allowed to grow on 40-mm coverslips to a density of 1 × 104 cells/coverslip. Time-lapse epifluorescent microscopy was performed using a Nikon TE2000 (Nikon Instruments) equipped with an environmental control system chamber (FCS2 system; Bioptechs, Butler, PA, USA), a 100 W halogen lamp, and a Planapo ×60 1.4-NA objective (Nikon Instruments). For visualization of GFP-tagged rat-α1, a 490-nm excitation, 515-nm emission filter was used. During imaging, the chamber was perfused with DMEM saturated with a gas mixture containing 5% CO2 and 21% O2. Images were collected with a Cascade EMCCD camera with on-chip multiplication gain (Photometrics, Tucson, AZ, USA) driven by MetaMorph software (Molecular Devices Corp., Downingtown, PA, USA). Images were acquired every second, with an exposure time of 0.5 s. To decrease phototoxic effects, a 1.5 neutral-density filter was used. To identify the GFPα1-A549 subpopulation of cells that were transfected with control mCherry-shRNA or targeting shRNA, a 560-nm excitation, 645-nm emission filter was used. For those experiments in which MitoTracker was used, A549 cells were loaded following the manufacturer’s instructions. For some experiments, A549 cells were transfected with a GFP-tagged Rab7 vector (Addgene plasmid 12605, generated by Dr. Richard Pagano, Mayo Clinic, Rochester, MN, USA; see ref. 28), and after 2 d, fluorescence images were taken as described above.
The movement of the GFP-α1-containing vesicles and MitoTracker-stained mitochondria was tracked and quantified using DiaTrack 3.0 software (Semasopht, Chavannes, Switzerland). The length of trajectory for each uninterrupted movement of each labeled particle was calculated; trajectories that could not be followed for ≥10 frames were discarded. For each GFPα1-A549 cell, we determined the mean and relative number of long trajectories, the previous determined as a ratio of the number of trajectories >6 μm to the total number of trajectories. The relative number of long trajectories was calculated for control shRNA and targeting shRNA-transfected cells, and the ratio of the 2 was calculated. For MitoTracker-loaded cells, the average length of trajectory and relative numbers were calculated for control shRNA and targeting shRNA-transfected cells. These measurements were averaged for groups of 7–10 cells for each experiment.
For single-vesicle displacement calculations, trajectories were obtained by single-particle tracking using MetaMorph 6 software. Twenty vesicles, out of 5 independent experiments/condition, were randomly selected among those showing linear plus-end directed movement, and those that could be followed for up to 60 s were analyzed. The displacement in micrometers per unit of time was obtained as follows: for each trajectory, the individual step displacements were added to obtain the total contour length as a function of time. Therefore, displacement as a function of time should be understood as the length that the motor covered on the microtubule. This distance then was plotted as a function of time, and we found a linear relationship in all cases (R2>0.94). The slopes of the trajectories for each case were averaged, and the presented displacements represent the calculated trajectory from the average slope. The dispersion on the observed values of the slopes is given by the calculated sd.
Transient transfections
Plasmids containing shRNA constructs, tubulin-mCherry, and GFP-Rab7 were transfected into A549 and GFPα1-A549 using Lipofectamin LTX (Invitrogen) per the manufacturer’s recommended protocols for A549 cells. For the time-course knockdown by shRNA experiments, cells were harvested after 24, 48, and 72 h. For the rest of the experiments in which shRNA was used, cells were harvested after 72 h.
Cell lysates
Cells were washed twice with ice-cold PBS and lysed in modified RIPA (mRIPA) buffer (50 mM Tris-HCl, pH 8; 150 mM NaCl; 1% Nonidet P-40; 1% sodium deoxycholate; 1 mM phenylmethylsulfonyl fluoride; 100 μg/ml N-tosyl-l-phenylalanine chloromethyl ketone; and 10 μg/ml leupeptin, pH 7.4), and centrifuged 10 min at 20,000 g. Supernatant was considered the cell lysate.
Western blot analysis
Protein was quantified by Bradford assay (Bio-Rad) (29) and resolved in 10–15% polyacrylamide gels (SDS-PAGE). Thereafter, proteins were transferred onto nitrocellulose membranes (Optitran; Schleider & Schuell, Keene, NH, USA) using a semidry transfer apparatus (Bio-Rad). Incubation with specific antibodies was performed overnight at 4°C. When more than one primary antibody was used on the same membrane, blots were stripped by incubating 1 h at 55°C in stripping solution (62.5 mM Tris-HCl, 2% SDS, and 100 mM 2-mercaptoethanol, pH 6.8). Blots were developed with a chemiluminescence detection kit (PerkinElmer Life Sciences, Boston, MA, USA) used as recommended by the manufacturer. The band signals in the linear range were quantified by densitometric scan (ImageJ 1.37; National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data are presented as means ± se. Comparisons between 2 groups of values were evaluated by Student’s t test. Results were considered significant when P < 0.05. For statistical significance between the slopes, a 2-sample t significance test was performed.
RESULTS
Na,K-ATPase-containing vesicles move along microtubules and colocalize with kinesin-1
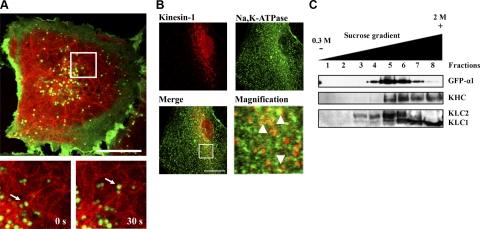
It has been previously reported that disruption of the microtubule network affects the traffic of Na,K,ATPase-containing vesicles toward the plasma membrane in AECs (8, 30). To further determine the role of microtubules in the traffic of Na,K-ATPase, we performed live-cell confocal imaging experiments in GFPα1-A549 cells transiently transfected with tubulin-mCherry. As depicted in Fig. 1A and Supplemental Video 1, GFPα1-containing vesicles (green) localize with tubulin-mCherry (red); moreover, GFPα1-containing vesicles were observed to move along the microtubules, demonstrating that Na,K-ATPase-containing vesicles use the microtubule network to displace within the cell. To determine whether kinesin-1 and Na,K-ATPase-containing vesicles colocalize, first, we performed immunofluorescence experiments in which GFPα1-A549 cells were immunostained for kinesin-1 after detergent extraction, and as shown in Fig. 1B, fluorescence confocal microscopy images depict a punctuate distribution of kinesin-1 (red), as has been previously described (24, 31), with some Na,K-ATPase-containing vesicles (green) overlapping with kinesin-1 (yellow) more predominantly around the perinuclear area. To further demonstrate that Na,K-ATPase and kinesin-1 localize within compartments with similar density, the total membrane fraction from GFPα1-A549 cells was layered on a sucrose gradient. Na,K-ATPase and kinesin-1 distributions were evaluated, and as shown in Fig. 1C, Na,K-ATPase and KHC were found to overlap in the gradient. The fact that both have similar buoyancy suggests that kinesin-1 is present in the same subcellular fraction that Na,K-ATPase-containing vesicles. Interestingly, KLC1 and KLC2 showed different distribution profile within the gradient with KLC2 overlapping in more fractions with Na,K-ATPase than KLC1, which was present toward the highest sucrose concentration in the gradient.
Figure 1.
Na,K-ATPase-containing vesicles move using the microtubule network via the molecular motor kinesin-1. A) Live-cell confocal imaging of GFPα1-A549 cells (green) transiently transfected with tubulin-mCherry (red). Movement of GFP-containing vesicles was recorded as multidimensional acquisition stacks. Top panel: localization of Na,K-ATPase-containing vesicles and microtubules in a single cell. Bottom panels: magnification (white square) of 2 stacks showing a single vesicle (white arrow) displacing over the microtubule over time (0 and 30 s). B) Immunofluorescence confocal microscopy images from detergent-permeabilized GFPα1-A549 cells labeled for kinesin-1 using multichannel acquisitions. Red, kinesin-1; green, Na,K-ATPase. Right bottom panel shows an ×4 digital view of the area delineated with a white square. Arrowheads indicate colocalization of kinesin-1 and Na,K-ATPase. C) Total membrane fraction of GFPα1-A549 cells was loaded on top of a sucrose gradient, and 8 fractions were recovered. Distribution of Na,K-ATPase, KHC, KLC1, and KLC2 was analyzed by Western blotting with specific antibodies. Representative Western blot is shown. Scale bars = 10 μm.
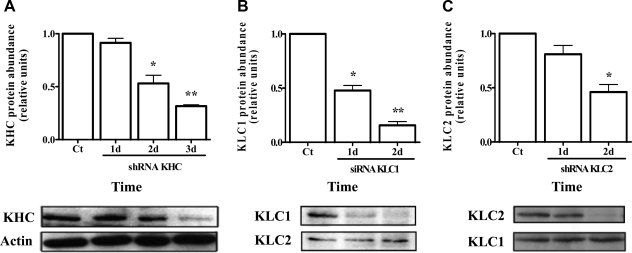
KHC, KLC1, and KLC2 protein knockdown in A549 cells
To determine whether kinesin-1 has functional importance in the trafficking of Na,K-ATPase-containing vesicles, we designed shRNAs against KHC and KLC2 using the mCherry-pG-SUPER vector. KLC1 isoforms underwent knockdown with a mixture of 3 different siRNA duplexes for targeting all isoforms of KLC1. As shown in Fig. 2, ∼75% knockdown was achieved in KHC and KLC2 shRNA plasmid-transfected cells and ∼90% of knockdown for KLC1 in siRNA-treated cells at 2–3 d post-transfection, with a transfection efficiency close to 90%.
Figure 2.
siRNAs against KHC, KLC1, and KLC2 effectively reduce protein abundance. GFPα1-A549 cells were transfected with specific shRNA against KHC (A), a pool of 3 different siRNAs against KLC1 (B), or specific shRNA against KLC2 (C) using lipofectamine LTX. Cell lysates from the transfected cells were obtained at the indicated times. Protein concentration was measured, equal amounts of proteins were resolved by SDS-PAGE, and Western blots were analyzed with specific antibodies for KHC, KLC1, and KLC2. For KHC, actin was used as loading control; KLC2 and KLC1 served as loading control for KLC1 and KLC2, respectively. *P < 0.05; **P < 0.001.
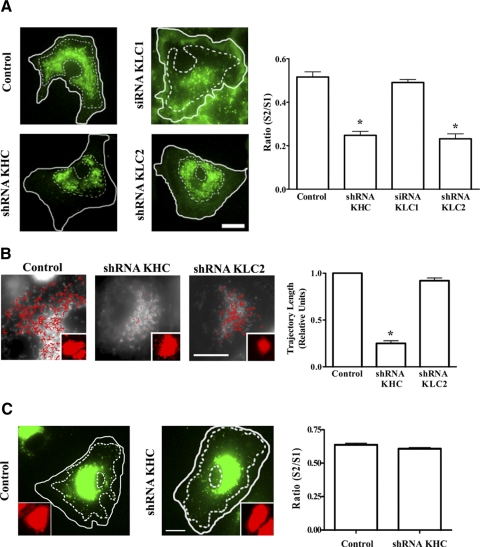
KHC and KLC2 are necessary for the traffic of the Na,K-ATPase
We investigated whether kinesin-1 was required for the movement of Na,K-ATPase-containing vesicles by transfecting GFP-α1-A549 cells with either a control shRNA or an shRNA against KHC and then performed live-cell imaging experiments. We first determined the ratio between the area of the cell occupied by Na,K-ATPase-containing vesicles (S2) and the total area of the cytoplasm (S1, nucleus excluded), as a change of this ratio is an indicator that vesicle displacement and organization have been affected. As shown in Fig. 3A, a significant decrease in the S2/S1 ratio was observed in kinesin-1-depleted cells when compared with control cells, which correlated with a redistribution of vesicles toward the cell center and their perinuclear accumulation.
Figure 3.
Knockdown of KHC or KLC2 (but not KLC1) causes redistribution of GFPα1-containing vesicles toward the nucleus. A) GFPα1-A549 cells were transfected with shRNA against KHC and KLC2, or siRNA against KLC1, and at 3 d post-transfection, fluorescence images were taken of 20 cells for each condition. Outer solid line delineates total area of the cell (S1); inner dotted line delineates area of the cell occupied by Na,K-ATPase-containing vesicles (S2). Graph represents ratio of S2 to S1 for each cell. B) MitoTracker-loaded A549 cells were transfected with shRNA against KHC or KLC2, and at 3 d post-transfection, time-lapse fluorescence images were taken of 20 cells for each condition using an inverted fluorescence microscope and MetaMorph software. Trajectories were analyzed using Diatrack 3.0 software. Still fluorescence images were taken of a representative cell for each condition. Red lines show length of each individual trajectory. Graph depicts the average of all trajectories for each condition (relative units). C) A549 cells were transiently transfected with a vector encoding for GFP-Rab7 fusion protein, and 3 d post-transfection, fluorescence images were taken of 20 cells for each condition. Outer line delineates total area of the cell (S1); inner dotted line delineates area of the cell occupied by GFP-Rab7 late endosomes (S2). Graph represents the ratio of S2 to S1 for each cell. Insets: transfections with mCherry shRNA. Scale bars = 10 μm. *P < 0.05.
Kinesin-1 is known to be responsible for mitochondria movement (32). Therefore, to determine whether a KHC shRNA effect was specific for kinesin-1, we performed live-cell imaging experiments in MitoTracker-loaded A549 cells transfected with KHC shRNA. As shown in Fig. 3B, we found decreased mitochondrial motility, confirming the specificity of the shRNA. Next, we analyzed whether KHC depletion affected the movement of an organelle with kinesin-1-independent traffic, such as late endosomes (33). We transiently transfected A549 cells with a vector encoding for Rab7-GFP fusion protein, which allowed us to visualize late-endosome motility, and as depicted in Fig. 3C, late-endosome distribution within the cell was not altered by KHC depletion.
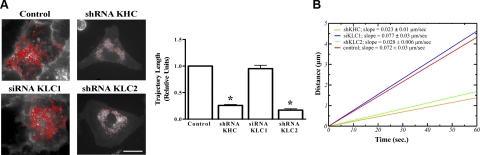
We determined the length of the trajectories of GFPα1-containing vesicles, and as shown in Fig. 4A (Supplemental Videos 2–5), the motility, assessed as the relative occurrence of long trajectories (total trajectory>6 μm in 60 s), was significantly reduced in cells depleted of KHC when compared with control cells. Moreover, the velocity of the GFPα1-containing vesicles showing long-range linear movement (Fig. 4B) in KHC-depleted cells was lower when compared with control cells (0.02±0.01 vs. 0.07±0.02 μm/s), suggesting that displacement of the Na,K-ATPase-containing vesicles is facilitated by kinesin-1.
Figure 4.
Knockdown of KHC or KLC2 causes a reduction in the length of GFPα1-containing vesicle trajectories. A) GFPα1-A549 cells were transfected with shRNA against KHC or KLC2, or siRNA against KLC1; at 3 d post-transfection, time-lapse fluorescence images were taken of 20 cells for each condition, using an inverted fluorescence microscope and MetaMorph software. Trajectories were analyzed using Diatrack 3.0 software. Left panel: still fluorescence images of a representative cell for each condition. Red lines show individual trajectories. Right panel: average of long trajectories (>6 μm) for each condition (relative units). B) Average contour length traveled by vesicles as a function of time, calculated as described in Materials and Methods. Scale bar = 10 μm. *P < 0.05.
To identify which isoform of KLC participates in the traffic of Na,K-ATPase-containing vesicles, we used siRNA and shRNA to knock down KLC1 and KLC2, respectively. GFPα1-A549 cells treated with KLC2 shRNA but not with KLC1 siRNA had a redistribution of vesicles toward the nucleus with the corresponding reduction of the S2/S1 ratio (Fig. 3A) and decreased vesicle motility (Fig. 4A). Furthermore, single-vesicle tracking and velocity measurements (Fig. 4B) revealed that control cells and KLC1-depleted cells had the highest velocity (0.072±0.03 and 0.077±0.03 μm/s, respectively), while the velocity in KLC2-depleted cells was reduced to the same extent as in KHC-depleted cells (0.028±0.01 μm/s and 0.023±0.01 μm/s, respectively).
Mitochondrial movement within the cell is known to be independent of KLC and to be performed by KHC alone (34, 35). Therefore, to rule out a nonspecific effect of KLC2 shRNA, we performed experiments in A549 cells loaded with MitoTracker and assessed mitochondria movement. As shown in Fig. 3B, mitochondria displacement was decreased in KHC-depleted cells but not in KLC2-depleted cells, confirming that the KLC2 effect on Na,K-ATPase traffic was specific.
DISCUSSION
The Na,K-ATPase generates the osmotic gradient that drives the reabsorption of edema across the alveolar epithelium (1,2,3, 30, 36, 37). To exert its function, Na,K-ATPase must be transported from intracellular compartments to the plasma membrane (38). Understanding the processes involved in the intracellular traffic of the Na,K-ATPase in AECs is of physiological and clinical importance. We have previously reported that the traffic of the Na,K-ATPase-containing vesicles depends on the integrity of the microtubule cytoskeleton, with the long range plus-end directed movement, suggesting a microtubule-based process (8). Here we provide evidence for the role of the plus-end directed microtubule molecular motor kinesin-1 in the traffic of Na,K-ATPase in AECs.
Intracellular microtubule-dependent transport has been shown to play a role in recruiting ion transport-related proteins to the plasma membrane (39, 40). It has been shown that microtubules are required for the insertion of aquaporin-1 and the proton pump into the plasma membrane in kidney epithelial cells (41) and for the traffic of the GLUT4 cotransporter in adipocytes (42). We found that Na,K-ATPase-containing vesicles move using microtubules as tracks in AECs, which is consistent with our previous findings that disruption of the microtubule network impairs alveolar fluid reabsorption in rat lungs (30) and the traffic of Na,K-ATPase-containing vesicles in AECs (8).
Among the plus-end-directed microtubule molecular motors, kinesin-1 is ubiquitously expressed and has been shown to have a role in intracellular traffic (40, 43). Since its description in 1985 (44), proteins such as aquaporin-1 (31), GLUT4 (45), and the Na+-taurocholate cotransporting polypeptide (46) have been shown to have kinesin-1-dependent traffic. We observed that kinesin-1 colocalizes with Na,K-ATPase-containing vesicles, and both are present in the same subcellular fractions (see Fig. 1). The possibility that the Na,K-ATPase-kinesin-1 association resulted from redistribution during homogenization (24) was ruled out by detecting their colocalization by confocal immunofluorescence. Lack of complete merge between kinesin-1 and Na,K-ATPase was not surprising, because many other cargoes are moved by kinesin-1, and ∼30% of the total membrane-bound Na,K-ATPase is present in the plasma membrane (47), where it does not colocalize with kinesin-1.
Kinesin-1 inhibition can be achieved by blocking antibodies (48), dominant negative constructs (49, 50), and more recently siRNA (51). We used an siRNA approach, because it lacks the secondary effects on the microtubules that are seen with dominant negative constructs (52), and performed live-cell imaging experiments using a mCherry tagged shRNA plasmid, which allowed us to visualize both the GFP-α1-Na,K-ATPase and the shRNA plasmid-transfected cell. We used this vector for KHC and KLC2 knockdown; however, for KLC1, we used a pool of 3 siRNA, because KLC1 has several splice forms (17), and a single shRNA construct did not achieve optimal knockdown. As shown in Fig. 2, we achieved ∼75% silencing of KHC and KLC2 and > 90% silencing for KLC1. In steady-state conditions in GFP-α1-A549 cells, Na,K-ATPase is located at the plasma membrane, with a pool of vesicles scattered throughout the intracellular compartment. The majority of Na,K-ATPase-containing vesicles move constantly, following a “biased random walk” pattern within the intracellular pool, with some of the vesicles displaying unidirectional linear movement with a mean velocity of 0.07 μm/s (8). This speed is within the lower range of motility for other organelles with microtubule-dependent movement, which can displace as fast as 3 μm/s in astrocytes (53) or as slow as 0.03 μm/s for kinethocore poleward movement during anaphase (54). The slow-range movement of Na,K-ATPase-containing vesicles can be due to the role of actin-based Myosin-Va in restraining Na,K-ATPase-containing vesicle movement in AECs (55).
KHC silencing promoted the redistribution of the Na,K-ATPase-containing vesicles toward the nucleus, and the length of trajectory and speed of vesicles were dramatically reduced. These findings concur with the morphological changes reported in mitochondria distribution (32) and intermediate filaments (56) after KHC depletion.
The fact that motility of late endosomes was not affected by kinesin-1 depletion demonstrates that traffic of other organelles within the cell remained unaffected (see Figs. 3 and 4). Other kinesin family members have been reported in vesicle traffic; for example, kinesin-2 has been involved in melanosome trafficking (57) as well as in late endosome and lysosome movement (33).
KLC regulates the binding of membrane-bound organelles to kinesin (58). Because there is considerable variability in the C terminus of KLC due to alternate splicing (59), it has been suggested that the C-terminal region of the different KLC isoforms is the determinant for targeting kinesin-1 to the specific cargo (16, 60). Moreover, the tetrameric kinesin-1 always contains 2 identical KLC isoforms (61), which favors the hypothesis that any given cargo is moved by only one type of kinesin. To determine which KLC isoform plays a role in the traffic of Na,K-ATPase-containing vesicles, we performed live-cell imaging experiments in GFP-α1-A549 cells, where either KLC1 or KLC2 genes were silenced. As shown in Figs. 3 and 4, only in KLC2-silenced cells did we observe redistribution of vesicles toward the nucleus as well as reduction in the length of trajectory and speed of vesicles, as occurred in KHC-depleted cells, suggesting that KLC2 but not KLC1 participates in the traffic of Na,K-ATPase-containing vesicles in AECs. Moreover, the fact that KLC2 but not KLC1 showed the same cellular distribution as the Na,K-ATPase in the sucrose gradient further supports the role of KLC2 in Na,K-ATPase traffic.
The effects of our shRNA constructs were specific, because KLC2 knockdown in A549 cells did not affect mitochondria movement, whereas KHC did, which is in agreement with data reported for mitochondria traffic that is independent of the KLC (62). We understand the limitations of using two different delivery systems for protein knockdown (plasmid delivered shRNA vs. siRNA) and thus have optimized our siRNA duplex transfection efficiency in A549 cells, which resulted in >90% knockdown of KLC1. We studied >100 cells in 10 different experiments without observing either vesicle redistribution toward the nucleus or a reduction in the trajectory length in the KLC1-depleted cells. In many models, cargo specificity is determined by the KLC isoform; for example, it has been reported that KLC1B is necessary for endoplasmic reticulum movement (16), whereas KLC1D is specific for Golgi traffic (60), and KLC2 has been shown to be important for kinesin-1 binding to vesicles in neurons (63). Here we provide functional evidence that a specific KLC isoform (KLC2) targets kinesin-1 to Na,K-ATPase-containing vesicles.
We are aware that using a nonpolarized cell line is a limitation; however, we have previously demonstrated that these cells depict directed traffic of the Na,K-ATPase-containing vesicles toward the plasma membrane after stimulation with G-protein-coupled receptor agonists (8), suggesting that these cells are capable of directed vectorial transport.
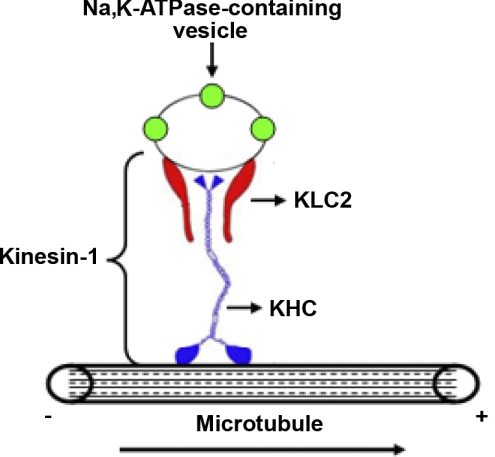
As summarized in Fig. 5, we provide the first evidence that Na,K-ATPase traffic toward the plasma membrane in AECs is driven by kinesin-1, where KLC2 is the isoform regulating this process, which is of physiological importance.
Figure 5.
Proposed model for the role of kinesin-1 in the traffic of Na,K-ATPase-containing vesicles. Kinesin-1 (represented as a tetramer of 2 KHC and 2 KLC2) moves Na,K-ATPase-containing vesicles from intracellular pools (minus end) into the plasma membrane (plus end), using microtubules as tracks.
Acknowledgments
The authors acknowledge the valuable insights and comments to this manuscript of Lynn Welch. This work was supported in part by U.S. National Institutes of Health grants HL-48129, HL-71643, and GM-52111. H.T. is a recipient of an American Heart Association Postdoctoral Fellowship grant. D.G. is a recipient of a National Science Foundation Graduate Research Fellowship.
References
- Matthay M A, Folkesson H G, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev. 2002;82:569–600. doi: 10.1152/physrev.00003.2002. [DOI] [PubMed] [Google Scholar]
- Mutlu G M, Sznajder J I. Mechanisms of pulmonary edema clearance. Am J Physiol. 2005;289:L685–L695. doi: 10.1152/ajplung.00247.2005. [DOI] [PubMed] [Google Scholar]
- Lecuona E, Trejo H E, Sznajder J I. Regulation of Na,K-ATPase during acute lung injury. J Bioenerg Biomembr. 2007;39:391–395. doi: 10.1007/s10863-007-9102-1. [DOI] [PubMed] [Google Scholar]
- Xie Z. Molecular mechanisms of Na/K-ATPase-mediated signal transduction. Ann N Y Acad Sci. 2003;986:497–503. doi: 10.1111/j.1749-6632.2003.tb07234.x. [DOI] [PubMed] [Google Scholar]
- Skou J C. Nobel lecture: the identification of the sodium pump. Biosci Rep. 1998;18:155–169. doi: 10.1023/a:1020196612909. [DOI] [PubMed] [Google Scholar]
- Shoshani L, Contreras R G, Roldan M L, Moreno J, Lazaro A, Balda M S, Matter K, Cereijido M. The polarized expression of Na+,K+-ATPase in epithelia depends on the association between beta-subunits located in neighboring cells. Mol Biol Cell. 2005;16:1071–1081. doi: 10.1091/mbc.E04-03-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feschenko M S, Wetzel R K, Sweadner K J. Phosphorylation of Na,K-ATPase by protein kinases. Sites, susceptibility, and consequences. Ann N Y Acad Sci. 1997;834:479–488. doi: 10.1111/j.1749-6632.1997.tb52306.x. [DOI] [PubMed] [Google Scholar]
- Bertorello A M, Komarova Y, Smith K, Leibiger I B, Efendiev R, Pedemonte C H, Borisy G, Sznajder J I. Analysis of Na+,K+-ATPase motion and incorporation into the plasma membrane in response to G protein-coupled receptor signals in living cells. Mol Biol Cell. 2003;14:1149–1157. doi: 10.1091/mbc.E02-06-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apodaca G. Endocytic traffic in polarized epithelial cells: role of the actin and microtubule cytoskeleton. Traffic (Cph) 2001;2:149–159. doi: 10.1034/j.1600-0854.2001.020301.x. [DOI] [PubMed] [Google Scholar]
- Saldias F J, Comellas A, Ridge K M, Lecuona E, Sznajder J I. Isoproterenol improves ability of lung to clear edema in rats exposed to hyperoxia. J Appl Physiol. 1999;87:30–35. doi: 10.1152/jappl.1999.87.1.30. [DOI] [PubMed] [Google Scholar]
- Cai T, Wang H, Chen Y, Liu L, Gunning W T, Quintas L E, Xie Z J. Regulation of caveolin-1 membrane trafficking by the Na/K-ATPase. J Cell Biol. 2008;182:1153–1169. doi: 10.1083/jcb.200712022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliwa M, Woehlke G. Molecular motors. Nature. 2003;422:759–765. doi: 10.1038/nature01601. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Pfister K K, Yorifuji H, Wagner M C, Brady S T, Bloom G S. Submolecular domains of bovine brain kinesin identified by electron microscopy and monoclonal antibody decoration. Cell. 1989;56:867–878. doi: 10.1016/0092-8674(89)90691-0. [DOI] [PubMed] [Google Scholar]
- Scholey J M, Heuser J, Yang J T, Goldstein L S. Identification of globular mechanochemical heads of kinesin. Nature. 1989;338:355–357. doi: 10.1038/338355a0. [DOI] [PubMed] [Google Scholar]
- Niclas J, Navone F, Hom-Booher N, Vale R D. Cloning and localization of a conventional kinesin motor expressed exclusively in neurons. Neuron. 1994;12:1059–1072. doi: 10.1016/0896-6273(94)90314-x. [DOI] [PubMed] [Google Scholar]
- Wozniak M J, Allan V J. Cargo selection by specific kinesin light chain 1 isoforms. EMBO J. 2006;25:5457–5468. doi: 10.1038/sj.emboj.7601427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodjakov A, Lizunova E M, Minin A A, Koonce M P, Gyoeva F K. A specific light chain of kinesin associates with mitochondria in cultured cells. Mol Biol Cell. 1998;9:333–343. doi: 10.1091/mbc.9.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junco A, Bhullar B, Tarnasky H A, van der Hoorn F A. Kinesin light-chain KLC3 expression in testis is restricted to spermatids. Biol Reprod. 2001;64:1320–1330. doi: 10.1095/biolreprod64.5.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodionov V I, Gyoeva F K, Gelfand V I. Kinesin is responsible for centrifugal movement of pigment granules in melanophores. Proc Natl Acad Sci U S A. 1991;88:4956–4960. doi: 10.1073/pnas.88.11.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingold A L, Cohn S A, Scholey J M. Inhibition of kinesin-driven microtubule motility by monoclonal antibodies to kinesin heavy chains. J Cell Biol. 1988;107:2657–2667. doi: 10.1083/jcb.107.6.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dada L A, Chandel N S, Ridge K M, Pedemonte C, Bertorello A M, Sznajder J I. Hypoxia-induced endocytosis of Na,K-ATPase in alveolar epithelial cells is mediated by mitochondrial reactive oxygen species and PKC-zeta. J Clin Invest. 2003;111:1057–1064. doi: 10.1172/JCI16826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Krmar R T, Dada L, Efendiev R, Leibiger I B, Pedemonte C H, Katz A I, Sznajder J I, Bertorello A M. Phosphorylation of adaptor protein-2 mu2 is essential for Na+,K+-ATPase endocytosis in response to either G protein-coupled receptor or reactive oxygen species. Am J Respir Cell Mol Biol. 2006;35:127–132. doi: 10.1165/rcmb.2006-0044OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaner N C, Campbell R E, Steinbach P A, Giepmans B N, Palmer A E, Tsien R Y. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- Tsai M Y, Morfini G, Szebenyi G, Brady S T. Release of kinesin from vesicles by hsc70 and regulation of fast axonal transport. Mol Biol Cell. 2000;11:2161–2173. doi: 10.1091/mbc.11.6.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose S D, Lejen T, Casaletti L, Larson R E, Pene T D, Trifaro J M. Myosins II and V in chromaffin cells: myosin V is a chromaffin vesicle molecular motor involved in secretion. J Neurochem. 2003;85:287–298. doi: 10.1046/j.1471-4159.2003.01649.x. [DOI] [PubMed] [Google Scholar]
- Brummelkamp T R, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- Kojima S, Vignjevic D, Borisy G G. Improved silencing vector co-expressing GFP and small hairpin RNA. BioTechniques. 2004;36:74–79. doi: 10.2144/04361ST02. [DOI] [PubMed] [Google Scholar]
- Choudhury A, Dominguez M, Puri V, Sharma D K, Narita K, Wheatley C L, Marks D L, Pagano R E. Rab proteins mediate Golgi transport of caveola-internalized glycosphingolipids and correct lipid trafficking in Niemann-Pick C cells. J Clin Invest. 2002;109:1541–1550. doi: 10.1172/JCI15420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Saldias F, Lecuona E, Friedman E, Barnard M L, Ridge K M, Sznajder J I. Modulation of lung liquid clearance by isoproterenol in rat lungs. Am J Physiol. 1998;274:L694–L701. doi: 10.1152/ajplung.1998.274.5.L694. [DOI] [PubMed] [Google Scholar]
- Tietz P S, McNiven M A, Splinter P L, Huang B Q, Larusso N F. Cytoskeletal and motor proteins facilitate trafficking of AQP1-containing vesicles in cholangiocytes. Biol Cell. 2006;98:43–52. doi: 10.1042/BC20040089. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Kanai Y, Okada Y, Nonaka S, Takeda S, Harada A, Hirokawa N. Targeted disruption of mouse conventional kinesin heavy chain, kif5B, results in abnormal perinuclear clustering of mitochondria. Cell. 1998;93:1147–1158. doi: 10.1016/s0092-8674(00)81459-2. [DOI] [PubMed] [Google Scholar]
- Brown C L, Maier K C, Stauber T, Ginkel L M, Wordeman L, Vernos I, Schroer T A. Kinesin-2 is a motor for late endosomes and lysosomes. Traffic (Cph) 2005;6:1114–1124. doi: 10.1111/j.1600-0854.2005.00347.x. [DOI] [PubMed] [Google Scholar]
- Glater E E, Megeath L J, Stowers R S, Schwarz T L. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J Cell Biol. 2006;173:545–557. doi: 10.1083/jcb.200601067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick R L, Shaw J M. Moving mitochondria: establishing distribution of an essential organelle. Traffic (Cph) 2007;8:1668–1675. doi: 10.1111/j.1600-0854.2007.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthiaume Y, Broaddus V C, Gropper M A, Tanita T, Matthay M A. Alveolar liquid and protein clearance from normal dog lungs. J Appl Physiol. 1988;65:585–593. doi: 10.1152/jappl.1988.65.2.585. [DOI] [PubMed] [Google Scholar]
- Lecuona E, Saldias F, Comellas A, Ridge K, Guerrero C, Sznajder J I. Ventilator-associated lung injury decreases lung ability to clear edema and downregulates alveolar epithelial cell Na,K-adenosine triphosphatase function. Chest. 1999;116:29S–30S. doi: 10.1378/chest.116.suppl_1.29s. [DOI] [PubMed] [Google Scholar]
- Teixeira V L, Katz A I, Pedemonte C H, Bertorello A M. Isoform-specific regulation of Na+,K+-ATPase endocytosis and recruitment to the plasma membrane. Ann N Y Acad Sci. 2003;986:587–594. doi: 10.1111/j.1749-6632.2003.tb07257.x. [DOI] [PubMed] [Google Scholar]
- Luna E J, Hitt A L. Cytoskeleton–plasma membrane interactions. Science. 1992;258:955–964. doi: 10.1126/science.1439807. [DOI] [PubMed] [Google Scholar]
- Vale R D, Milligan R A. The way things move: looking under the hood of molecular motor proteins. Science. 2000;288:88–95. doi: 10.1126/science.288.5463.88. [DOI] [PubMed] [Google Scholar]
- Brown D, Sabolic I. Endosomal pathways for water channel and proton pump recycling in kidney epithelial cells. J Cell Sci Suppl. 1993;17:49–59. doi: 10.1242/jcs.1993.supplement_17.8. [DOI] [PubMed] [Google Scholar]
- Oatey P B, Van Weering D H, Dobson S P, Gould G W, Tavare J M. GLUT4 vesicle dynamics in living 3T3 L1 adipocytes visualized with green-fluorescent protein. Biochem J. 1997;327:637–642. doi: 10.1042/bj3270637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapp B J. Trafficking of signaling modules by kinesin motors. J Cell Sci. 2003;116:2125–2135. doi: 10.1242/jcs.00488. [DOI] [PubMed] [Google Scholar]
- Vale R D, Reese T S, Sheetz M P. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985;42:39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semiz S, Park J G, Nicoloro S M, Furcinitti P, Zhang C, Chawla A, Leszyk J, Czech M P. Conventional kinesin KIF5B mediates insulin-stimulated GLUT4 movements on microtubules. EMBO J. 2003;22:2387–2399. doi: 10.1093/emboj/cdg237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Bananis E, Nath S, Anwer M S, Wolkoff A W, Murray J W. PKCzeta is required for microtubule-based motility of vesicles containing the ntcp transporter. Traffic (Cph) 2006;7:1078–1091. doi: 10.1111/j.1600-0854.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- Lecuona E, Sun H, Vohwinkel C, Ciechanover A, Sznajder J I. Ubiquitination participates in the lysosomal degradation of the Na,K-ATPase in steady-state conditions [E-pub ahead of print] Am J Respir Cell Mol Biol. 2009 doi: 10.1165/rcmb.2008-0365OC. doi: 10.1165/rcmb.2008-0365OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady S T, Pfister K K, Bloom G S. A monoclonal antibody against kinesin inhibits both anterograde and retrograde fast axonal transport in squid axoplasm. Proc Natl Acad Sci U S A. 1990;87:1061–1065. doi: 10.1073/pnas.87.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wubbolts R, Fernandez-Borja M, Jordens I, Reits E, Dusseljee S, Echeverri C, Vallee R B, Neefjes J. Opposing motor activities of dynein and kinesin determine retention and transport of MHC class II-containing compartments. J Cell Sci. 1999;112:785–795. doi: 10.1242/jcs.112.6.785. [DOI] [PubMed] [Google Scholar]
- Nakata T, Hirokawa N. Point mutation of adenosine triphosphate-binding motif generated rigor kinesin that selectively blocks anterograde lysosome membrane transport. J Cell Biol. 1995;131:1039–1053. doi: 10.1083/jcb.131.4.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colin E, Zala D, Liot G, Rangone H, Borrell-Pages M, Li X J, Saudou F, Humbert S. Huntingtin phosphorylation acts as a molecular switch for anterograde/retrograde transport in neurons. EMBO J. 2008;27:2124–2134. doi: 10.1038/emboj.2008.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepis A, Stauber T, Krijnse Locker J. Kinesin-1 plays multiple roles during the vaccinia virus life cycle. Cell Microbiol. 2007;9:1960–1973. doi: 10.1111/j.1462-5822.2007.00927.x. [DOI] [PubMed] [Google Scholar]
- Potokar M, Kreft M, Pangrsic T, Zorec R. Vesicle mobility studied in cultured astrocytes. Biochem Biophys Res Commun. 2005;329:678–683. doi: 10.1016/j.bbrc.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Mitchison T J. The role of microtubule polarity in the movement of kinesin and kinetochores. J Cell Sci Suppl. 1986;5:121–128. doi: 10.1242/jcs.1986.supplement_5.7. [DOI] [PubMed] [Google Scholar]
- Lecuona E, Minin A, Trejo H E, Chen J, Comellas A P, Sun H, Grillo D, Nekrasova O E, Welch L, Szleifer I, Gelfand V I, Sznajder J I. Myosin Va restrains Na,K-ATPase-containing vesicles traffic in alveolar epithelial cells. J Cell Sci. 2009 doi: 10.1242/jcs.046953. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfand B T, Mendez M G, Pugh J, Delsert C, Goldman R D. A role for intermediate filaments in determining and maintaining the shape of nerve cells. Mol Biol Cell. 2003;14:5069–5081. doi: 10.1091/mbc.E03-06-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuma M C, Gelfand V I. Molecular mechanisms of pigment transport in melanophores. Pigment Cell Res. 1999;12:283–294. doi: 10.1111/j.1600-0749.1999.tb00762.x. [DOI] [PubMed] [Google Scholar]
- Verhey K J, Lizotte D L, Abramson T, Barenboim L, Schnapp B J, Rapoport T A. Light chain-dependent regulation of Kinesin’s interaction with microtubules. J Cell Biol. 1998;143:1053–1066. doi: 10.1083/jcb.143.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCart A E, Mahony D, Rothnagel J A. Alternatively spliced products of the human kinesin light chain 1 (KNS2) gene. Traffic (Cph) 2003;4:576–580. doi: 10.1034/j.1600-0854.2003.00113.x. [DOI] [PubMed] [Google Scholar]
- Gyoeva F K, Bybikova E M, Minin A A. An isoform of kinesin light chain specific for the Golgi complex. J Cell Sci. 2000;113:2047–2054. doi: 10.1242/jcs.113.11.2047. [DOI] [PubMed] [Google Scholar]
- Gyoeva F K, Sarkisov D V, Khodjakov A L, Minin A A. The tetrameric molecule of conventional kinesin contains identical light chains. Biochem. 2004;43:13525–13531. doi: 10.1021/bi049288l. [DOI] [PubMed] [Google Scholar]
- Boldogh I R, Pon L A. Mitochondria on the move. Trends Cell Biol. 2007;17:502–510. doi: 10.1016/j.tcb.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Morfini G, Szebenyi G, Elluru R, Ratner N, Brady S T. Glycogen synthase kinase 3 phosphorylates kinesin light chains and negatively regulates kinesin-based motility. EMBO J. 2002;21:281–293. doi: 10.1093/emboj/21.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]