Abstract
The Tec family tyrosine kinases regulate lymphocyte development, activation, and differentiation. In T cells, the predominant Tec kinase is Itk, which functions downstream of the T-cell receptor to regulate phospholipase C-γ. This review highlights recent advances in our understanding of Itk kinase structure and enzymatic regulation, focusing on Itk protein domain interactions and mechanisms of substrate recognition. We also discuss the role of Itk in the development of conventional versus innate T-cell lineages, including both αβ and γδ T-cell subsets. Finally, we describe the complex role of Itk signaling in effector T-cell differentiation and the regulation of cytokine gene expression. Together, these data implicate Itk as an important modulator of T-cell signaling and function.
Itk activates phospholipase C downstream of T-cell receptors. Recent work reveals the structural details of Itk activation and its distinct roles in different T-cell subsets.
The Tec family nonreceptor tyrosine kinases, Tec, Btk, Itk/Emt/Tsk, Rlk/Txk, and Bmx/Etk, are expressed primarily in hematopoietic cells and serve as important mediators of antigen receptor signaling in lymphocytes (Berg et al. 2005; Felices et al. 2007; Readinger et al. 2009). The demonstration that the human B-cell immunodeficiency, X-linked agammaglobulinemia (XLA), is caused by mutations in Btk first underscored the importance of this tyrosine kinase family in lymphocyte development and antigen receptor signaling (Rawlings et al. 1993; Thomas et al. 1993; Tsukada et al. 1993; Vetrie et al. 1993). T lymphocytes express three Tec kinases: Itk, Rlk and Tec. To date, only Itk has been found to have a clearly defined function in T cells, leading to the conclusion that Itk is the predominant Tec kinase in T cells. In this review, we will cover recent findings that highlight the critical role of Itk in T-cell signaling and function.
STRUCTURE AND REGULATION OF ITK ACTIVITY
Tec Kinase Domain Structure
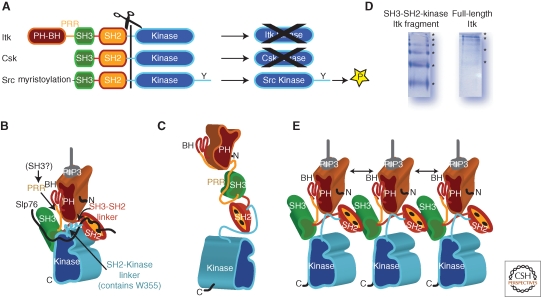
Itk (inducible T-cell kinase; also known as Emt, expressed in mast cells and T lymphocytes, and Tsk, T-cell-specific kinase) was cloned in the early 1990s (Siliciano et al. 1992; Gibson et al. 1993; Heyeck and Berg 1993; Tanaka et al. 1993; Yamada et al. 1993). The domain organization of Itk and related Tec kinase family members shares similarities with other tyrosine kinase families but also reveals unique features (Fig. 1A). Shared features include the common SH3-SH2-kinase cassette also found in the Src, Csk, and Abl kinases (Williams et al. 1998; Tsygankov 2003). Four of the five Tec tyrosine kinases (Itk, Btk, Tec, and Bmx) contain an amino-terminal region that includes a pleckstrin homology (PH) domain, followed by a Zn++ binding region termed the Btk homology (BH) motif, and (except for Bmx) a proline rich region (PRR) that conforms to the consensus sequence of an SH3 ligand. Instead of the PH-BH region, Txk (also known as Rlk) contains a cysteine string motif at its amino terminus.
Figure 1.
Itk domain organization and it's role in T cell signaling. (A) The domain structure of Itk. (B) Schematic of T-cell signaling emphasizing the proximal signaling complex downstream of the TCR as described in the text. The trio of tyrosine kinases, Zap-70, Lck, and Itk that initiate signaling following TCR engagement are shown as gray, green, and brown circles, respectively. Arrows indicate early phosphorylation events on TCR/pMHC recognition. The amino-terminal region of Lck binds to the cytoplasmic tail of CD4 via coordination of Zn++ (Huse et al. 1998). Active Lck phosphorylates the ITAMs, Zap-70, and Itk, which in turn phosphorylate their targets Slp-76, LAT (by Zap-70) and PLCγ1 (by Itk). Activated PLCγ1 then hydrolyzes PIP2 (dashed arrow) to produce DAG and IP3 leading to increased calcium flux. Multiple copies of these signaling molecules likely cooperate in the phosphorylation cascade and so stoichiometry as shown is overly simplified. Moreover, negative regulation of the signaling cascade in the form of phosphatases, kinases and other types of molecules also plays a significant role in T-cell function but are not represented here.
Itk Activation Downstream of the TCR
The Tec family kinases are involved in multiple receptor-mediated signaling pathways in many cell types; several recent reviews have covered these in depth (Berg et al. 2005; Felices et al. 2007; Gomez-Rodriguez et al. 2007; Mihara and Suzuki 2007; Gilfillan and Rivera 2009; Koprulu and Ellmeier 2009; Prince et al. 2009; Readinger et al. 2009). For Itk, significant attention has been given to activation via T-cell receptor (TCR) stimulation (see Fig. 1B). Interaction of the TCR with peptide-MHC complexes on antigen presenting cells (APC) activates the Src kinase Lck, leading to phosphorylation of the CD3 immunoreceptor tyrosine-based activation motifs (ITAMs). Zap-70 then binds to the phosphorylated ITAMs and is phosphorylated by Lck, resulting in Zap-70 activation and subsequent phosphorylation of the adaptors LAT and SLP-76 (Pitcher and van Oers 2003; Houtman et al. 2005; Au-Yeung et al. 2009; Smith-Garvin et al. 2009). Following activation of PI3K and accumulation of phosphatidylinositol (3,4,5) trisphosphate (PIP3) in the plasma membrane, Itk is recruited to the membrane via its PH domain (August et al. 1997; Ching et al. 1999; Yang et al. 2001). There, Itk interacts with the phosphorylated SLP-76/LAT adapter complex via Itk’s Src homology (SH3 and SH2) domains (Bubeck Wardenburg et al. 1996; Zhang et al. 1998; Shan and Wange 1999; Su et al. 1999; Bunnell et al. 2000; Ching et al. 2000; Sommers et al. 2004; Koretzky et al. 2006), permitting phosphorylation of Itk on its activation loop (Y511) by Lck (Heyeck et al. 1997).
Once activated, Itk undergoes cis autophosphorylation on Y180 in its SH3 domain (Wilcox and Berg 2003; Joseph et al. 2007a). Itk also interacts with and directly phosphorylates its downstream target, PLCγ1 (Perez-Villar and Kanner 1999; Joseph et al. 2007c), resulting in phospholipase activation (Liu et al. 1998; Schaeffer et al. 1999; Wilcox and Berg 2003; Bogin et al. 2007). The kinetics characterizing many of these early phosphorylation events have been delineated, providing an understanding of the sequential nature of signaling following TCR engagement (Houtman et al. 2005). It is not known, however, whether Itk autophosphorylation occurs prior to, or following, phosphorylation of PLCγ1. Because autophosphorylation on Y180 does not affect Itk catalytic activity but instead modulates binding of the Itk SH3 domain to different targets (Wilcox and Berg 2003; Joseph et al. 2007a), Itk autophosphorylation may serve as a trigger to alter protein interaction partners or localization either before or after PLCγ1 activation. PLCγ1, once activated, hydrolyzes PIP2 to produce the second messengers IP3 and DAG (Rhee 2001). Downstream consequences include Ca2+ flux (Lewis and Cahalan 1995), Erk activation (Schaeffer et al. 1999; Schaeffer et al. 2000; Miller and Berg 2002), transcription (Crabtree and Olson 2002), cytokine release (Liao and Littman 1995; Liu et al. 1998), and actin reorganization (Labno et al. 2003), all of which are impaired in the absence of Itk. Importantly, these downstream consequences of TCR activation are not ablated by a deficiency in Itk, but instead, are substantially reduced, leading to altered development and differentiation of distinct T-cell lineages, as discussed later.
The Itk Signaling Complex
The Itk/SLP-76 interaction likely involves the SH3 and SH2 domains of Itk targeting a proline-rich stretch within SLP-76 (residues 184-195) and an adjacent phosphorylated tyrosine (Y145) in SLP-76, respectively (Su et al. 1999; Bunnell et al. 2000). In an elegant knockin mouse study, tyrosine 145 of SLP-76 was replaced with the nonphosphorylatable phenylalanine to probe the contribution of the ItkSH2/pY145 interaction to signaling (Jordan et al. 2008). Mutation of Y145 resulted in markedly diminished PLCγ1 phosphorylation, decreased Ca2+ flux, lowered phospho-ERK levels and gave rise to T cells that resembled those lacking Itk, as discussed later. This observation (Jordan et al. 2008) is consistent with findings that the Itk/SLP-76 interaction is required to activate Itk kinase activity in Jurkat cells (Bogin et al. 2007). Intriguingly, the majority of the catalytically active Itk in the cell consists of only the small fraction of Itk that is bound to SLP-76 (Bogin et al. 2007). Surprisingly, the SLP-76 Y145F mutation did not lead to loss of the Itk/SLP-76 association (Jordan et al. 2008) despite altered signaling. This finding suggests that cooperative multivalent interactions preserve Itk localization in this signaling complex even when a single contact site is disrupted.
The importance of the Itk PH domain/PIP3 interaction (Fukuda et al. 1996; August et al. 1997) was also recently probed by inhibition of phosphatidylinositol-3 kinase (PI3K) activity in human peripheral blood T cells (Cruz-Orcutt and Houtman 2009). In a manner similar to SLP-76 Y145F, loss of the Itk PH domain/PIP3 interaction resulted in impaired downstream signaling but did not affect the ability of Itk and other PH domain containing signaling proteins to assemble into the signaling complex (Cruz-Orcutt and Houtman 2009). In a separate study, soluble IP4 was found to promote binding of the Itk PH domain to PIP3 (Huang et al. 2007). Reminiscent of the Itk/SLP-76 interaction (Bogin et al. 2007), these studies also showed that only a limited pool of Itk in the cell is capable of binding PIP3 even in the presence of excess PIP3 (Huang et al. 2007). These separate findings suggest that multiple interaction sites exist to properly localize Itk following TCR stimulation (Graef et al. 1997; Garcon and Nunes 2006; Beach et al. 2007). Furthermore, part of the function of Itk and other proteins in the LAT/SLP-76 complex may be to function as adaptors and stabilize proteins in the complex. Kinase-independent functions for Itk have been observed in the regulation of actin polymerization (Dombroski et al. 2005) and activation of the transcription factor, SRF (Hao et al. 2006); these functions may be secondary to effects on recruitment of the guanine-nucleotide exchange factor Vav to this complex (Labno et al. 2003).
Regulation of Itk Activity
The regulatory mechanisms that control the activity of Lck and Zap-70 during TCR signaling have been the subject of intense investigation, leading to detailed insights into function (Palacios and Weiss 2004; Au-Yeung et al. 2009). In contrast, a mechanistic understanding of Itk regulation remains elusive, as Itk and other Tec kinases have, to date, resisted crystallization in their full-length form. Comparing the Tec and Src kinase families, it is evident that regulatory differences must exist. In spite of the shared SH3-SH2-Kinase cassette, the Tec kinases lack the carboxy-terminal regulatory tail that plays an important role in Src regulation (Brown and Cooper 1996; Xu et al. 1997; Xu et al. 1999; Cowan-Jacob et al. 2005). Moreover, removal of the noncatalytic domains from the Src kinase domain yields an active enzyme, whereas the isolated Itk and Btk kinase domains show poor catalytic activity (Fig. 2A) (LaFevre-Bernt et al. 1998; Hawkins and Marcy 2001; Guo et al. 2006; Joseph et al. 2007b). Mutation of a well-conserved tryptophan residue in the Itk and Btk SH2-kinase linkers (W355 in Itk, W395 in Btk) results in a significant decrease in kinase activity (Lin et al. 2005; Guo et al. 2006; Joseph et al. 2007b) whereas mutation of the corresponding tryptophan in the Src kinase Hck leads to a dramatic increase in the activity of that kinase (LaFevre-Bernt et al. 1998). In this regard, Itk is similar to Csk (carboxy-terminal Src kinase), another tyrosine kinase comprised of the common SH3-SH2-Kinase cassette and regulated in a manner opposite that of Src (Huang et al. 2009). The isolated Csk kinase domain shows negligible catalytic activity (Fig. 2A) and mutation of the conserved tryptophan (W188) decreases Csk activity (Lin et al. 2005). Based on similar biochemical profiles, a model for active Itk can be developed using the available structure of active Csk (Fig. 2B).
Figure 2.
Activation/inactivation of Itk. (A) Comparison of the domain structures of Itk, Csk and Src kinases. The domains are colored as follows: PH-BH is brown, PRR is yellow, SH3 is green, SH2 is orange, SH2-Kinase linker and Kinase domain are blue. All three kinases share the SH3-SH2-kinase cassette but differ with respect to resulting catalytic activity of the isolated kinase domain. Itk and Csk kinase domains alone show very poor catalytic activity whereas the Src kinase domain readily phosphorylates substrates. (B) Model of active Itk based on biochemical data and the high-resolution structure of Csk (PDB code: 1K9A). The SH2-Kinase linker and SH3-SH2 linker are both arranged along the back of the small kinase lobe. Activating interactions between the kinase domain and the SH2-kinase linker in particular are also supported by a recent structure of Btk (Marcotte et al. 2010). SLP-76 is depicted by the black line and is shown binding to both Itk SH3 and SH2. The PH-BH domain is placed so that its carboxy-terminus is close in space to the amino-terminus of the SH3 domain. The intervening sequence is the PRR, which would be available in this model for binding an SH3 domain from another protein. PH domain is shown bound to its PIP3 ligand. (C) Model of Itk adopting the extended conformation seen for Btk in SAXS studies (Marquez et al. 2003). Of note is the fact that the intramolecular PRR/SH3 interaction is sterically allowed and that the SH2-Kinase linker region is likely in an extended conformation and not the active conformation shown in (B). (D) Native gel electrophoresis for the SH3-SH2-Kinase fragment of Itk and for full length Itk showing ladders indicative of mulitmerization. Sample concentration for both purified proteins is approximately 5 micromolar. (E) Model of Itk multimerization based on the structure of the binary Itk SH3/SH2 complex (PDB code: 2K79). Doubled headed arrows indicate PH domain self-association. Whether Itk multimerization occurs exclusively at the membrane remains to be determined. It is of note that the SH3/SH2 interface defined in PDB 2K79 is accessible in the models shown in both (B) and (C) suggesting that either configuration could multimerize. In (B), (C) and (E) the amino and carboxy-termimi of full length Itk are indicated by N and C, respectively.
In agreement with the established interaction between active Itk and SLP-76 (Jordan et al. 2008; Bogin et al. 2007), the distance between the SH3 and SH2 binding pockets in the high-resolution structure of Csk closely matches the distance between the phosphotyrosine motif and proline-rich region in SLP-76 (residues 143–195). Moreover, the Itk PRR region adjacent to the Itk SH3 domain in the model of active Itk (Fig. 2B) could be available for binding to an SH3 domain from an exogenous signaling partner. The precise binding partner of the Itk PRR is not yet clear but candidates include the SH3 domains of Vav, Fyn, Src, PLCγ1 or Grb2 (Yang et al. 1995; Perez-Villar and Kanner 1999; Bunnell et al. 2000; Chamorro et al. 2001). Csk lacks the PH-BH region of the Tec kinases and so comparative model building for this region of Itk becomes speculative. It is interesting to note however, that the PH-BH domain of Itk extends from the amino-terminus of the PRR region and, within the context of the Csk-based model, the PH-BH domain could pack between the SH3 and SH2 domains making contact with the small lobe of the kinase domain and/or the SH3-SH2/SH2-kinase linker regions potentially affecting kinase activity (Joseph et al. 2007b) (Fig. 2B).
Mechanism of Itk Substrate Recognition
Once activated and assembled within the SLP-76/LAT signaling complex, Itk meets its downstream substrate, PLCγ1 (Liu et al. 1998; Schaeffer et al. 1999; Wilcox and Berg 2003; Houtman et al. 2005; Bogin et al. 2007). Itk mediated phosphorylation of Y783 in PLCγ1 requires a remote specificity-determining element within the second SH2 domain of PLCγ1 (Joseph et al. 2007c). Mutation in the PLCγ1 SH2 domain that disrupts the Itk/PLCγ1 interaction results in decreased PLCγ1 activation as measured by calcium flux in Jurkat T cells (Braiman et al. 2006; Min et al. 2009). Earlier work also identified a TCR-regulated association between Itk and PLCγ1 involving the SH3 domain of PLCγ1 (Perez-Villar and Kanner 1999). Although PLCγ1 is the most well established substrate of Itk, additional candidate substrates have been described and include TFII-I (August 2009; Sacristan et al. 2009), Tim-3 (van de Weyer et al. 2006) and T-bet (Hwang et al. 2005); the molecular details of Itk recognition of these substrates has not been elucidated.
Downregulation of Itk Kinase Activity
Inactivation of kinase activity is an important regulatory feature that must appropriately balance activating signals. Although few negative regulators of Itk have been described, expression of PTEN, a lipid phosphatase, can decrease Itk activity by reducing PIP3 levels (Shan et al. 2000). Negative regulation of Itk by the peptidyl prolyl isomerase, cyclophilin A, has been described (Brazin et al. 2002; Colgan et al. 2004). Interestingly, another peptidyl prolyl isomerase, Pin1 (protein interacting with NIMA1), regulates phosphorylation and steady state levels of Btk (Yu et al. 2006). However, the mechanism of action remains poorly understood for both Pin1 and CypA (Joseph and Andreotti 2009; Mohamed et al. 2009).
As already described, the regulatory domains of the Tec kinases exert a positive influence on the kinase domain in a manner similar to Csk (Fig. 2B) and so conformational rearrangements that uncouple the regulatory domains (particularly the SH2-kinase linker region) from the catalytic domain would down-regulate Itk activity. In this context, the only structural data available for any full length Tec kinase comes from small-angle X-ray scattering (SAXS) analysis of Btk (Marquez et al. 2003). These data indicate that an inactive preparation of Btk (Marquez et al. 2003; Lin et al. 2009) adopts an extended conformation in solution with negligible contacts between domains (Fig. 2C). Whether the fully extended configuration derived from SAXS data represent a physiologically relevant state remains to be determined but the available data suggest that a conformational shift of the regulatory domains away from the kinase domain could provide one layer of negative regulation.
In addition to negatively regulating the catalytic domain, the configuration of the inactive kinase might also serve to sequester its ligand binding sites (Pawson and Kofler 2009). The SH3/PXXP interaction, in particular, is constitutive and not controlled by transient phosphorylation/dephosphorylation events. In down-regulated Itk, the SH3 domain-binding site might be masked in an intramolecular fashion by the adjacent PRR (Andreotti et al. 1997; Laederach et al. 2003) (Fig. 2C). However, it is experimentally difficult to probe the precise role of the PRR in kinase regulation because it is also likely involved in activating interactions with SH3 domain-containing signaling partners (Yang et al. 1995; Bunnell et al. 2000). Efforts that use deletion of the PRR region to probe its regulatory role (Hao and August 2002) are therefore complicated by the dual nature of the PRR.
Regulation of Itk Activity by Multimerization
Structural studies using different Tec kinase fragments indicate that the noncatalytic regulatory domains interact in an intermolecular fashion driving multimerization in solution (Andreotti et al. 1997; Brazin et al. 2000; Hansson et al. 2001; Laederach et al. 2002; Okoh and Vihinen 2002; Pursglove et al. 2002; Laederach et al. 2003; Severin et al. 2009). For Itk, multimerization is mediated by self-association of the PH domain (Huang et al. 2007) and intermolecular interactions between the SH3 and SH2 domains of different Itk molecules (Brazin et al. 2000; Severin et al. 2009). Native gel electrophoresis indicates that the full-length Itk molecule, the SH3-SH2-kinase fragment, and the Itk SH3-SH2 fragment all form multimers (Severin et al. 2009) (Fig. 2D). The high resolution structure of the Itk SH3/SH2 complex has been solved and provides insight into how full length Itk might self-associate (Severin et al. 2009) (Fig. 2E). Functionally, disruption of the SH3/SH2 interface by mutation reduced oligomerization and increased Itk kinase activity both in vitro and in T cells (Severin et al. 2009; Min et al. 2010). Moreover, both binding experiments and structural data indicate that intermolecular association of Itk is mutually exclusive with binding of SLP-76 to the SH3 and SH2 domains (Brazin et al. 2000; Pletneva et al. 2006; Severin et al. 2009). Thus, down-regulation of Itk activity might involve intermolecular self-association whereas multimerization is disfavored for active, SLP-76 bound Itk. The interplay between specific clustering of signaling molecules (Houtman et al. 2006), the formation of TCR microclusters (Bunnell et al. 2006; Seminario and Bunnell 2008) and the specific role of Itk multimerization during T-cell signaling certainly deserve continued attention.
A split YFP/fluorescence complementation approach has been used to probe the different conformational and oligomeric states of Itk in cells (Qi et al. 2006; Qi and August 2009). Itk that is doubly tagged at the amino- and carboxy-termini results in fluorescence complementation in the cytosol and at the membrane. The authors suggest that the observed fluorescence complementation within a single Itk molecule is indicative of a folded compact structure. Taking the linker lengths between Itk and each of the split YFPs (two linkers each ∼40 Å) and the diameter of YFP itself (∼30 Å) into account, fluorescence complementation in this system would occur for distances of approximately 110 Å between the Itk termini. It should be noted that the distance between the amino- and carboxy-termini of Btk in the fully extended model derived from SAXS data is 125 Å and given the flexibility inherent in the extended model (Marquez et al. 2003), it seems likely that fluorescence complementation would be observed for almost any domain arrangement of Itk; compact or extended. Thus, the fluorescence “ruler” used in these experiments is unlikely to discriminate between different domain arrangements.
Intermolecular fluorescence complementation between differentially tagged Itk molecules is consistently observed only at the cell membrane and not in the cytosol (Qi et al. 2006; Qi and August 2009). Although this data alone does not rule out multimerization in the cytosol, the spatial segregation of the stronger fluorescence signal to the membrane is certainly interesting and suggests that Itk clusters are at least stabilized upon or after membrane localization. Notably, Itk autophosphorylation enhances the affinity between the SH3 and SH2 domains (Joseph et al. 2007a), possibly triggering sequestration of Itk into clusters and down-regulation of kinase activity. Release of Itk from the membrane, promoted by dephosphorylation of PIP3 and the SLP-76/LAT complex (Shan et al. 2000; Baker et al. 2001; Yang et al. 2001; Tomlinson et al. 2004), would then result in decreased local Itk concentration, which could cause Itk oligomers to disassociate into inactive, monomeric species in the cytosol. Regardless of aggregation state, the inactive species would adopt a conformation that decouples the regulatory region (in particular the SH2-kinase linker) from the Itk kinase domain.
ITK REGULATION OF T-CELL FUNCTION
Naïve T-Cell Activation is Impaired in the Absence of Itk
The central role of Itk in the LAT-SLP-76 complex suggests that Itk is an important component of TCR signaling. Early studies using primary murine Itk-/- T cells showed that Itk is required for robust T-cell activation in response to TCR plus costimulatory receptor signaling. However, these studies also revealed that Itk is not absolutely required for all responses downstream of the TCR (Liao and Littman 1995; Liu et al. 1998; Schaeffer et al. 1999). One aspect of this modulatory role for Itk is the partial redundancy between Itk and a second Tec kinase family member, Rlk. Thus, a combined deficiency in both Itk and Rlk results in TCR signaling defects that are significantly more profound than is seen in T cells lacking Itk alone (Schaeffer et al. 1999). Overall, the message from these experiments was that Itk functions to amplify TCR signaling, rather than as an all-or-nothing component of the TCR signaling pathway.
T-Cell Differentiation and Effector Functions are Regulated by Itk
In spite of the relatively modest impact of the Itk deficiency on naïve T-cell activation, numerous studies have shown that Itk signaling plays a critical role in regulating T-cell differentiation and T-cell effector functions (Fig. 3). The first of these studies showed that Itk-/- Balb/c mice were unable to generate the TH2 response to Leishmania major infection typically seen in wild-type Balb/c mice and instead, mounted a protective TH1 response that cleared the infection (Fowell et al. 1999). These results highlighted the importance of Itk signaling in the generation of TH2 responses, a function of Itk that has been studied in more detail both in vitro and in vivo.
Figure 3.
Itk function in T helper cell differentiation. At the left, the three major lineages of effector T cells are shown, along with the cytokine signals and transcription factors that regulate their differentiation from naïve CD4+ T cells. Unlike TH1 cells, which coexpress Itk and Rlk, TH2 cells express only Itk. The status of Rlk expression in TH17 cells is currently unknown. In the absence of Itk (right panel), NFATc1 activity is greatly reduced, leading to impaired production of IL-4 by TH2 cells and IL-17A by TH17 cells. Itk-/- TH1 cells, which continue to express Rlk, have a more modest defect in effector cytokine production.
Itk Promotes TH2 Differentiation and Cytokine Production
The initial findings that Itk-deficient mice were unable to mount a protective TH2 response to infection were verified in a number of distinct infectious disease systems including Leishmania major in Itk-/- Balb/c mice, as well as Nippostrongylus brasiliensis and Schistosoma mansoni, two parasites that are eliminated by TH2-dependent recruitment and activation of granulocytes (Fowell et al. 1999; Schaeffer et al. 2001). Analyses of cytokine responses of the parasite-specific T cells from these infected mice indicated that Itk-/- T cells produced decreased IL-4, IL-5, and IL-10 (Schaeffer et al. 2001). Similarly, in vitro experiments showed that Itk-/- Balb/c CD4+ T cells stimulated under TH2-polarizing conditions produced much less IL-4 than their wild-type counterparts (Fowell et al. 1999). These cytokine responses correlated with defective TCR-induced activation of the calcium-dependent transcription factor, NFAT, by Itk-/- T cells, providing a potential explanation for the reduced Il4 transcription (Fig. 3).
However, it is difficult to infer from these experiments the precise nature of the T-cell defect resulting in impaired protection to parasitic infections. Reductions in T-cell activation, T-cell differentiation, T-cell migration, or elicitation of recall responses at the site of infection could each contribute to the poor protection observed in Itk-/- mice. An additional complication is the presence of a large population of previously activated/memory CD4+ T cells in Itk-/- mice; global gene expression analysis has confirmed that Itk-/- CD4+ T cells are not comparable to wild-type CD4+ T cells prior to their activation (Blomberg et al. 2009), and thus, might respond differently than naïve T cells to parasite infections.
Nonetheless, useful information has been gleaned from direct infection of Itk-/- mice with pathogenic microorganisms. One elegant approach to evaluating T cell defects in the absence of Itk was taken by Au-Yeung and colleagues, who crossed Balb/c Itk-/- mice to reporter mice that express GFP under the control of the IL-4 promoter (Au-Yeung et al. 2006). Following infection of these mice with Leishmania major, equal numbers of CD4+ GFP+ T cells were found at the sites of infection in wild-type versus Itk-/- mice, indicating that Itk was not required for the initiation of the TH2 response or for the migration of IL-4-competent T cells to these sites. Instead, the effector T-cell recall response at the sites of infection were impaired in the absence of Itk, leading to reduced IL-4 production by Itk-/- T cells following restimulation. However, these studies are not in complete agreement with an independent set of experiments examining T-cell-mediated airway hyperresponsiveness in Itk-/- mice (Mueller and August 2003). In this system, in addition to impaired TH2 cytokine production, T-cell recruitment to the lungs was also impaired following inhaled antigen challenge in Itk-/- mice compared to controls. Interestingly, a partial inhibition of Itk kinase activity was able to block T-cell migration to the lung in this model system, but had no effect on the priming of TH2 effector responses in secondary lymphoid organs (Sahu et al. 2008a). These findings indicate roles for Itk in both T-cell priming and T-cell migration, findings that are supported by studies showing a role for Itk in signaling downstream of chemokine receptors (Fischer et al. 2004; Takesono et al. 2004).
These in vivo experiments have been complemented by a series of in vitro experiments performed with naïve Itk-/- CD4+ T cells. All of these studies agreed that Itk-/- CD4+ T cells produced substantially less IL-4, IL-5, and IL-13 than wild-type T cells, even after differentiation in a powerful TH2 polarizing environment (Fowell et al. 1999; Schaeffer et al. 2001; Miller et al. 2004; Au-Yeung et al. 2006). In these studies, the defects in effector cytokine production were not because of impaired TH2 differentiation, as molecular analyses indicated that Itk-/- T cells polarized into the TH2 lineage showed all of the hallmarks of bona fide TH2 effector cells (Schaeffer et al. 2001; Miller et al. 2004; Au-Yeung et al. 2006). Interestingly, the requirement for Itk in effector cytokine production is shared by αβTCR+ NKT cells specific for α-galactosyl ceramide bound to CD1d; in the absence of Itk, NKT cells stimulated in vivo or in vitro produce substantially less IL-4 or IFNγ than wild-type NKT cells (Au-Yeung and Fowell 2007; Felices and Berg 2008).
TH1 Differentiation and Effector Functions are Modestly Impaired in the Absence of Itk
Unlike the essential role for Itk in protective immunity to helminthic parasites, Itk signaling in T cells is largely dispensible for protective CD4+ TH1 responses to intracellular pathogens. Thus, Itk-/- mice clear infections of Leishmania major, and are only modestly more susceptible to sublethal doses of Toxoplasma gondii than wild-type mice (Fowell et al. 1999; Schaeffer et al. 1999). These observations may reflect the fact that IFNγ production by Itk-/- CD4+ T cells is not reduced to the same extent as production of TH2 cytokines (Fowell et al. 1999; Schaeffer et al. 2001; Miller et al. 2004; Au-Yeung et al. 2006). Indeed, under certain conditions that normally lead to TH2 cytokine production, such as stimulation with altered peptide ligands, Itk-/- CD4+ T cells produce IFN-γ (Miller et al. 2004). A second aspect of this differential requirement for Itk in TH1 versus TH2 effector responses is the interesting expression pattern of the two predominant Tec kinases in T cells, Itk and Rlk (Fig. 3). Unlike naïve T cells which express both Itk and Rlk, TH2 effector cells lose Rlk and express only Itk; in fact, the levels of Itk are several-fold increased in TH2 cells compared to naïve T cells (Miller et al. 2004). In contrast, TH1 effector cells continue to express both of these Tec kinases. As a consequence, whereas Itk-/- TH1 cells continue to express Rlk, Itk-/- TH2 cells lack both Itk and Rlk, thus accounting for the more severe functional defect observed in Itk-/- TH2 responses. Corroborating this notion, ectopic expression of Rlk in effector TH2 cells restores wild-type T cell responses in Itk-/- mice injected with Schistosoma mansoni eggs or immunized to induce allergic airway hypersensitivity (Sahu et al. 2008b).
Itk Is Required for IL-17A Production in TH17 Effector Cells
In addition to the global defects in effector T-cell responses described above for TH2, and to a lesser extent, TH1 responses, T cells lacking Itk display more selective alterations in cytokine production that highlight the complex nature of the signaling pathways that regulate T-cell effector responses. One recent example is the role of Itk in the regulation of cytokine production by TH17 cells, a recently recognized CD4 effector T-cell lineage that differentiates in response to IL-6 and TGF-β and expresses the pro-inflammatory cytokines IL-17A, IL-17F, IL-21 and IL-22 (Korn et al. 2009). TH17 cells are important for antimicrobial activity, particularly in the gastrointestinal system, but are also hallmarks of inflammation involved in multiple autoimmune disorders.
Although the requirements for cytokine signals for TH17 differentiation have been extensively examined, the contribution of TCR signaling to the regulation of TH17 cytokine production was unknown. To evaluate this question, naïve CD4+ T cells from Itk-/- and Rlk-/-Itk-/- mice were differentiated under TH1 and TH17 polarizing conditions, and cytokine production evaluated by intracellular staining (Gomez-Rodriguez et al. 2009). Although both cell types could generate large percentages of IFNγ producing TH1 cells, Itk-/- and even more dramatically Rlk-/-Itk-/- T cells showed reduced IL-17A production. These defects did not appear to be because of altered thymic development, because they could be reversed by re-introduction of Itk by retroviral transduction before TH17 differentiation. Further analyses showed decreased Il17a message in Itk-/- T cells (Fig. 3). However, surprisingly, expression of other TH17 cytokines including the closely linked Il17f gene, was relatively intact, as was the expression of genes encoding the master regulators RORγt and RORα. Although phosphorylation of STAT3 in response to IL-6 was slightly attenuated in Itk-deficient T cells, re-expression of a constitutively activated STAT3 construct did not restore normal IL-17A production.
The selective role for Itk in IL-17A production resulted from a requirement for robust calcium signaling, leading to NFAT activation, in the transcription of the Il17a gene (Fig. 3). Thus, differentiation of Itk-/- T cells in the presence of anti-CD3 plus ionomycin, which can rescue Ca2+ mobilization in Itk-deficient T cells (Liu et al. 1998), did restore IL-17A production, directly implicating defective TCR-driven Ca2+ mobilization in this phenotype (Gomez-Rodriguez et al. 2009). Supporting this idea, stimulation of wild-type T cells with decreasing amounts of anti-CD3 during differentiation preferentially reduced expression of IL-17A, whereas leaving IL-17F relatively spared. Similarly, differentiation of wild-type T cells in the presence of low-dose CyclosporinA or FK506, two inhibitors of Calcineurin that prevent NFAT activation (Winslow et al. 2003), also preferentially decreased IL-17A expression. Conversely, expression of a constitutively active mutant of NFATc1 (Neal and Clipstone 2003) restored IL-17A production by Itk-/- TH17 cells, suggesting that Itk-mediated activation of NFAT contributes to the regulation of IL-17A. Sequence analyses showed a conserved NFAT binding site located 3.5 kb upstream of the start site of the Il17a gene and ChIP analyses showed that this site was occupied in wild-type but not Itk-deficient cells differentiated under TH17 conditions. Although potential NFAT binding sites were found upstream of the Il17f gene, none were conserved cross-species. Nonetheless, conserved noncoding sequences in the region around both the linked Il17a and Il17f genes showed epigenetic modifications suggestive of open chromatin conformations (Wilson et al. 2009).
These results suggest that a TCR-driven pathway involving Itk-mediated regulation of Ca2+ and NFAT is required for full expression of IL-17A and that this pathway distinguishes expression of different TH17 cytokines. These data support the growing view that there are distinct subclasses of TH17 cells that produce overlapping sets of cytokines based upon distinct inputs. Thus, TCR signaling may be particularly important for expression of IL-17A, which is more strongly proinflammatory than IL-17F (Yang et al. 2008; Ishigame et al. 2009). This regulation operates during the differentiation of TH17 cells, because the defect in IL-17A production is seen even upon restimulation of cells with PMA and ionomycin (Gomez-Rodriguez et al. 2009). NFATc1 autoregulates its own expression and previous data showed defective NFATc1 induction in Itk-deficient cells (Nurieva et al. 2007), perhaps accounting for the requirement for Itk during TH17 differentiation. Alternatively, other chromatin remodeling factors that are recruited by transcription factors, including the BRG proteins, may not assemble properly on the IL-17A locus in the absence of efficient NFAT activation (Berg 2009). Although the exact mechanism of this defect awaits further study, these detailed investigations of Itk-deficient T lymphocytes have revealed another feature of how TCR signaling can affect the differentiation of distinct cytokine producing cells.
TCR Signaling in CD8+ T Cells Requires Itk
Although fewer studies have addressed the role of Itk in CD8+ T-cell responses, the conclusions thus far indicate that primary responses to viral infection are largely intact in the absence of Itk, or Itk and Rlk (Bachmann et al. 1997; Atherly et al. 2006). Biochemical studies show that Itk-/- and Rlk-/-Itk-/- CD8+ T cells share a similar deficiency in TCR-induced PLC-γ1 activation, calcium mobilization, ERK activation, and cytokine production as CD4+ T cells lacking the Tec kinases (Atherly et al. 2006). Nonetheless, Itk-/- mice do mount protective immune responses to LCMV, vaccinia virus, and VSV, and Rlk-/-Itk-/- mice to LCMV, although kinetics of viral clearance were often slightly delayed. Tracking of virus-specific CD8+ T cells indicated that Itk-/- and Rlk-/-Itk-/- T cells were unable to expand to the extent seen with wild-type T cells, resulting in an overall deficit in the magnitude of the CD8+ T-cell response. This reduction in T-cell expansion could not be accounted for by impaired CD4+ T cell help in Itk-/- mice, as adoptive transfer of wild-type LCMV-immune T cells (CD4+ and CD8+) into Itk-/- mice prior to LCMV challenge was unable to rescue the CD8+ T-cell defects. In addition, the impaired response of Itk-/- CD8+ T-cells was not because of the predominant population of “innate-like” CD8+ T-cells present in these mice (see the following), as OT-1 TCR transgenic Itk-/- CD8+ T-cells, which develop as conventional naïve T-cells, also shared the defect seen with polyclonal Itk-/- CD8+ T-cells in non-TCR transgenic mice. It still remains to be determined whether Itk-/- CD8+ T-cells can generate functional memory cells. Although virus-specific T-cells persist in Itk-/- mice well after virus has been cleared from the animals, the ability of these cells to provide protection, and to mount an effective recall response, has not yet been tested. This issue is complex, as defects in Itk-/- CD4+ T-cells could impact the generation of robust memory CD8+ T-cells; thus, appropriate studies will be required to distinguish CD4+ T-cell-intrinsic from CD8+ T-cell-intrinsic contributions to antiviral memory responses.
TEC KINASES IN T-CELL DEVELOPMENT
Role of Itk and Rlk in Conventional T-cell Development
TCR signaling plays a critical role in the development of T cells within the thymus, where the strength or duration of TCR signaling helps determine the survival, maturation, and differentiation of thymocytes into mature T cells. Double negative CD4−CD8− (DN) cells that have rearranged their TCRβ chains must receive competent signals through the pre-TCR to progress through the DN3 stage and expand in the DN4 to double positive (DP) transition (Ciofani and Zuniga-Pflucker 2006). At the double positive stage, TCR signaling regulates cell survival and differentiation of conventional T cells (Jameson et al. 1995). Thymocytes that receive very poor or no TCR signals undergo “death by neglect.” Thymocytes that receive strong TCR signals, as from agonist peptides, particularly in the context of costimulation, undergo negative selection and also die within the thymus. Only thymocytes that receive weak but adequate signals will progress onto CD4/CD8 selection, where the duration of TCR signaling, in large part dictated by the expression patterns of the co-receptors CD4 and CD8 upon TCR engagement, will determine their ultimate lineage as CD4 or CD8 single positive (SP) cells (Bosselut 2004).
It is therefore not surprising that Itk-/- and Rlk-/-Itk-/- T cells show altered thymocyte development (Fig. 4). Itk-/- mice have smaller thymi and show phenotypes consistent with impaired pre-TCR signaling. Although these defects are relatively subtle, competitive bone marrow transfers using mixtures of WT and Itk-/- bone marrow indicated that both Itk-/- and Rlk-/-Itk-/- cells had a selective disadvantage at the DN-DP transition (Lucas et al. 2007). However, Rlk-/-Itk-/- T cells also showed a competitive advantage compared to WT cells at earlier stages (prior to DN3), perhaps reflecting a negative signaling role for Rlk in cell survival/cytokine signaling pathways.
Figure 4.
Regulation of T-cell lineages by Itk. In the thymus, progenitor T cells (CD4−CD8−CD3−; DN) develop into γδ T cells and αβ T cells. Cells developing in the αβ T-cell lineage progress through an intermediate CD4+CD8+ (DP) stage prior to their maturation into mature conventional αβ T cells, CD1d-restricted αβ NKT cells and other subsets of innate-like αβ T cells. Conventional αβ T cells, which are selected on classical MHC molecules expressed on thymic stromal cells, and αβ NKT cells, which are selected on the MHC class IB molecule, CD1d, expressed on hematopoietic cells (HC) and dependent on SLAM receptor signaling, are greatly reduced in the absence of Itk. In contrast, innate-like αβ T cells, which are also selected by recognition of MHC molecules on HC and require SLAM receptor signaling, and “innate-like” γδ T cells (γδ NKT) are substantially increased in number in Itk-/- mice. A third subset of cells, illustrated by conventional γδ T cells, appear unaffected by the presence versus the absence of Itk. Currently, the cell–cell interactions required for γδ T-cell development are unknown.
Defects in positive selection of thymocytes lacking Itk have been shown using a variety of MHC Class I and Class II-restricted TCR transgenes (Liao and Littman 1995; Schaeffer et al. 2000; Lucas et al. 2002; Lucas et al. 2003). The extent of these defects depended on the selecting TCR, with the weakest selecting TCRs showing the largest effects (Lucas et al. 2002). Defects in positive selection were exacerbated in Rlk-/-Itk-/- mice, providing evidence that these genes are partially redundant, and supporting the idea that decreased TCR signaling reduces positive selection of conventional T cells (Schaeffer et al. 2000). Supporting partial redundancy of these genes, overexpression of Txk/Rlk partially rescued positive selection defects in Itk-/- mice (Sommers et al. 1999).
Itk-deficient animals also show reduced or delayed negative selection in multiple TCR transgenic systems (Liao and Littman 1995; Schaeffer et al. 2000; Lucas et al. 2002). In Rlk-/-Itk-/- mice, negative selection is more clearly impaired, leading to positive selection of normally deleted HY TCR transgenic cells in male Rlk-/-Itk-/- mice (Schaeffer et al. 2000). These data support a model where negative selection is converted to positive selection in the context of impaired TCR signaling and may account for the increased SP cells in Rlk-/-Itk-/- mice.
Development of Innate T-Lymphocyte Populations
Recent data have revealed surprising new findings on thymic selection in mice deficient in Itk and Rlk (Atherly et al. 2006; Broussard et al. 2006; Dubois et al. 2006; Berg 2007; Hu et al. 2007). Although Itk-/- and Rlk-/-Itk-/- mice show decreased positive selection of conventional T cells using both MHC Class I and Class II-restricted TCR transgenes, surprisingly, Itk-/- and Rlk-/-Itk-/- mice have increased numbers of CD8 SP cells (Liao and Littman 1995; Schaeffer et al. 2000; Lucas et al. 2002). Further examination of these CD8 SP populations suggested that these cells do not resemble conventional naive CD8 SP thymocytes. Instead, virtually all of the CD8 SP thymocytes in these mice showed markers seen on memory or activated T cells, including CD44 and CD122 (the IL-2Rβ chain), and rapidly expressed cytokines upon activation (Atherly et al. 2006; Broussard et al. 2006; Dubois et al. 2006; Hu et al. 2007). These phenotypes are characteristic of memory cells in the periphery—however, developmental studies and fetal thymic organ cultures provided evidence that Itk-deficient CD8 cells developed these characteristics within the thymus (Atherly et al. 2006; Broussard et al. 2006; Dubois et al. 2006). Although genetic crosses showed a requirement for MHC Class I for their development, transfers of bone marrow from Itk-/- or Rlk-/-Itk-/- mice into irradiated β2m-deficient mice, which lack MHC-Class I on their radioresistant thymic stroma, showed that Itk-/- and Rlk-/-Itk-/- CD8+ thymocytes could be selected on hematopoietically derived cells (Broussard et al. 2006). Although these observations are atypical for conventional thymocytes, these features are all reminiscent of NKT cells and other related innate T-cell lineages; thus, many innate T-cell lineages have been shown to be selected on double-positive thymocytes, to express activation or memory cell markers, and to rapidly produce cytokines even in the thymus (Berg 2007). Like NKT cells, Itk-/- and Rlk-/-Itk-/- CD8+ cells also expressed NK cell markers and high levels of the transcription factor Eosmesodermin, and were dependent on IL-15 (Atherly et al. 2006; Dubois et al. 2006). Moreover, like NKT cells, development of these CD8 cells was dependent on SAP, a small adaptor molecule that is required for signaling from the SLAM family of receptors that are expressed on hematopoietic cells, but not on the thymic epithelium (Horai et al. 2007). Together these characteristics suggested that Itk-deficiency led to the preferential selection of an innate CD8 cell population on hematopoietic cells within the thymus (Fig. 4).
Although this phenotype is primarily seen in CD8 cells, there is also a small population of CD4 cells that show these properties in Itk-/- mice. These CD44hiCD4+ cells are also dependent on SAP and express PLZF, a transcription factor responsible for the innate characteristics of NKT cells (PLS and LJB, unpublished data and Broussard et al. 2006; Hu and August 2008; Raberger et al. 2008). Although the selecting cell population for the innate type T cells in Itk-/- mice is yet undetermined, the large number of CD8 cells with this characteristic (and relatively low numbers of CD4 cells) suggests that it is likely to be DP thymocytes, which express MHC Class I but not Class II, and are the selecting cells for NKT cells (Bendelac 1995).
Why loss of Itk leads to the preferential development of CD8 cells with this phenotype is not clear. Reciprocal transfers of Itk-/-β2m-/- bone marrow provided evidence that the development of these innate phenotypes required selection on hematopoietic cells (Horai et al. 2007). One possibility is that poor selection of conventional thymocytes could permit selection or expansion of cells selected on hematopoietic cells. However, transfer of WT bone marrow into irradiated β2m-deficient hosts prevents selection of conventional CD8+ T cells and yet does not give rise to a large number of innate CD8 cells, suggesting that development of these cells is normally suppressed. Given the rapid production of cytokines showed by these cells, it is likely that the generation of these cells may be under tight control.
Several theories could account for the development of this population in Itk-/- mice. Itk could play a negative role in signaling pathways required for the development of these lineages, such those downstream of SLAM family receptors. Alternatively, these cells might normally be deleted in wild-type animals—negative selection can occur on hematopoietic cells, which express costimulatory molecules, such as CD28, thought to be important in this process (Punt et al. 1994). Alternatively, defective TCR signaling may make these cells more susceptible to signals from costimulatory molecules, such as SLAM family members, or cytokines that may be required either for selection or development of these phenotypes (Atherly et al. 2006; Dubois et al. 2006; Horai et al. 2007). Further analyses will provide clues to the regulation of these interesting lineages that appear to be at the boundary of innate and adaptive immune responses (Berg 2007).
Itk Signaling Regulates αβ and γδ NKT-Cell Maturation
In addition to defects in the development and selection of conventional naïve CD4+ and CD8+ T-cells, αβTCR+ NKT-cell maturation is also impaired in the absence of Itk and Rlk (Gadue and Stein 2002; Au-Yeung and Fowell 2007; Felices and Berg 2008; and Fig. 5). In these Tec kinase-deficient mice, αβ NKT cell numbers are substantially reduced; furthermore, of the cells that remain, few represent the most mature αβ NKT subset characterized by high expression of NK1.1 and CD122, and down-regulation of CD4. Itk-/- and Itk-/-Rlk-/- NKT cells also show poor responses to TCR stimulation, producing little to no IL-4 and IFNγ following in vivo activation. Thus, similar to conventional αβ T cells, αβ NKT cells require Tec kinases for their development and function.
In stark contrast to the requirements for αβTCR+ T cells, the development of γδ T cells does not require Itk signaling (Fig. 4). The major γδ T-cell populations that arise in the fetus and adult are largely unchanged in Itk-/- mice (Felices et al. 2009; C. Yin and LJB, unpub. observ.). Indeed, overall γδ̇T cell numbers in Itk-/- mice are significantly increased compared to wild-type mice, because of dramatic increases in one particular γδ T-cell subset, the γδ NKT cells (Felices et al. 2009; Qi et al. 2009). This subset expresses the Vγ1.1/Vδ6.3 TCR; however, in all other regards, these γδ T cells share characteristics of CD1d-specific αβ NKT cells rather than traits associated with most adult-derived γδ T cells (Vicari et al. 1996; Azuara et al. 1997; Lees et al. 2001). For instance, the majority of γδ NKT cells express CD4, NK1.1, and the signature NKT cell transcription factor, PLZF (Felices et al. 2009; Kreslavsky et al. 2009). As a result, γδ NKT cells, like their αβ NKT cell counterparts, produce both IFNγ and TH2 cytokines. In addition, these cells preferentially home to the liver, rather than to lymphoid organs such as the spleen (Lees et al. 2001; Felices et al. 2009).
Surprisingly, this expanded population of γδ NKT cells is responsible for a spontaneous hyper-IgE syndrome observed in Itk-/- mice (Felices et al. 2009; Qi et al. 2009). When Itk-/- mice are crossed to mice lacking γδ T cells, the elevated serum IgE and germinal center hyperplasia seen in Itk-/- mice disappear. Itk-/- splenic γδ NKT cells constitutively express ICOS, and readily upregulate CD40-ligand and OX40 following γδ TCR stimulation in vitro (Felices et al. 2009). These findings suggest that Itk-/- γδ NKT cells are being activated in vivo, allowing them to provide T cell help to B cells and secrete cytokines that promote switching to IgE. As with the majority of γδ T cells, the ligand recognized by the Vγ1.1/Vδ6.3+ TCR is unknown, nor is it known whether activation of these cells requires signaling through the γδ TCR.
These findings raise several interesting issues regarding the development and signaling properties of γδ T cells. In contrast to αβ T cells, γδ T cells are not reduced in the absence of Itk; further, the γδ NKT cell population is, in fact, greatly expanded. These data indicate that the signals regulating γδ T-cell development do not require Itk, a situation comparable to that seen for innate CD8+ αβ T cells. The expansion of both the γδ NKT and the innate CD8+ population in Itk-/- mice may reflect the fact that the majority of these cells normally undergo negative selection, but that their deletion is impaired in the absence of Itk.
A second interesting issue is the apparently robust TCR signaling in Itk-/- γδ T cells. αβ T cells lacking Itk have substantially reduced responses to TCR signaling, particularly in their ability to synthesize effector cytokines. Itk-/- γδ T cells, however, produce copious amounts of cytokines in response to γδ TCR stimulation. These data suggest that the biochemical pathways downstream of the γδ TCR are likely distinct from those in αβ T cells. Consistent with this idea, mice carrying a mutant version of the adapter protein LAT also have few αβ T cells but greatly increased numbers of γδ T cells that overproduce TH2 cytokines; further, these mice share the hyper-IgE syndrome seen in Itk-/- mice (Nunez-Cruz et al. 2003). This LAT mutant is lacking a critical tyrosine in the cytoplasmic tail that is essential for efficient TCR signaling in αβ T cells, reinforcing the notion that αβ̇ and γδ TCR signaling have distinct biochemical requirements.
ITK AND HUMAN DISEASE
Although mutations affecting Btk were identified as the cause of X-linked Agammaglobulinemia 17 years ago (Rawlings et al. 1993; Thomas et al. 1993; Tsukada et al. 1993; Vetrie et al. 1993), alterations in Itk have only recently been associated with human disorders. These include Itk promoter polymorphisms associated with atopy (Graves et al. 2005) and the expression of an Itk-Syk fusion protein generated by a chromosomal translocation in T-cell lymphomas (Streubel et al. 2006); in this latter case, the Itk PH domain becomes fused to the kinase domain of Syk, leading to constitutive activation of the kinase. Most recently, a mutation affecting Itk was found in 2 siblings who died from fatal Epstein-Barr Virus infection (Huck et al. 2009). This mutation changes a single amino acid in the Itk SH2 domain and destabilizes the Itk protein. Although the basis for this particular immunodeficiency is unknown, it may be related to Itk function in cytotoxic CD8+ T cells (Bachmann et al. 1997; Atherly et al. 2006). Importantly, the implication of Itk in a human primary immunodeficiency has validated its role as a critical regulator of T lymphocyte function. Given the depth of information on lymphocyte biology provided through the study of Tec kinases, it is clear that their study will continue to provide important insights into the signaling pathways involved in immune cell development, homeostasis and function.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (AI37584 and AI46564 to LJB and AI043957 and AI075150 to AHA) and by intramural funds from the NHGRI/NIH (PLS). We thank Julia Fekecs for assistance with graphic illustrations.
Footnotes
Editors: Lawrence E. Samelson and Andrey Shaw
Additional Perspectives on Immunoreceptor Signaling available at www.cshperspectives.org
REFERENCES
- Andreotti AH, Bunnell SC, Feng S, Berg LJ, Schreiber SL 1997. Regulatory intramolecular association in a tyrosine kinase of the Tec family. Nature 385:93–97 [DOI] [PubMed] [Google Scholar]
- Atherly LO, Brehm MA, Welsh RM, Berg LJ 2006. Tec kinases Itk and Rlk are required for CD8+ T cell responses to virus infection independent of their role in CD4+ T cell help. J Immunol 176:1571–1581 [DOI] [PubMed] [Google Scholar]
- Au-Yeung BB, Deindl S, Hsu LY, Palacios EH, Levin SE, Kuriyan J, Weiss A 2009. The structure, regulation, and function of ZAP-70. Immunol Rev 228:41–57 [DOI] [PubMed] [Google Scholar]
- Au-Yeung BB, Fowell DJ 2007. A key role for Itk in both IFN gamma and IL-4 production by NKT cells. J Immunol 179:111–119 [DOI] [PubMed] [Google Scholar]
- Au-Yeung BB, Katzman SD, Fowell DJ 2006. Cutting edge: Itk-dependent signals required for CD4+ T cells to exert, but not gain, Th2 effector function. J Immunol 176:3895–3899 [DOI] [PubMed] [Google Scholar]
- August A 2009. IL-2-inducible T-cell kinase (ITK) finds another (dance) partner … TFII-I. Eur J Immunol 39:2354–2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- August A, Sadra A, Dupont B, Hanafusa H 1997. Src-induced activation of inducible T cell kinase (ITK) requires phosphatidylinositol 3-kinase activity and the Pleckstrin homology domain of inducible T cell kinase. Proc Natl Acad Sci U S A 94:11227–11232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuara V, Levraud JP, Lembezat MP, Pereira P 1997. A novel subset of adult gamma delta thymocytes that secretes a distinct pattern of cytokines and expresses a very restricted T cell receptor repertoire. Eur J Immunol 27:544–553 [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Littman DR, Liao XC 1997. Antiviral immune responses in Itk-deficient mice. J Virol 71:7253–7257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JE, Majeti R, Tangye SG, Weiss A 2001. Protein tyrosine phosphatase CD148-mediated inhibition of T-cell receptor signal transduction is associated with reduced LAT and phospholipase Cgamma1 phosphorylation. Mol Cell Biol 21:2393–2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach D, Gonen R, Bogin Y, Reischl IG, Yablonski D 2007. Dual role of SLP-76 in mediating T cell receptor-induced activation of phospholipase C-gamma1. J Biol Chem 282:2937–2946 [DOI] [PubMed] [Google Scholar]
- Bendelac A 1995. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J Exp Med 182:2091–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg LJ 2007. Signalling through TEC kinases regulates conventional versus innate CD8(+) T-cell development. Nat Rev Immunol 7:479–485 [DOI] [PubMed] [Google Scholar]
- Berg LJ 2009. Strength of T cell receptor signaling strikes again. Immunity 31:529–531 [DOI] [PubMed] [Google Scholar]
- Berg LJ, Finkelstein LD, Lucas JA, Schwartzberg PL 2005. Tec family kinases in T lymphocyte development and function. Annu Rev Immunol 23:549–600 [DOI] [PubMed] [Google Scholar]
- Blomberg KE, Boucheron N, Lindvall JM, Yu L, Raberger J, Berglof A, Ellmeier W, Smith CE 2009. Transcriptional signatures of Itk-deficient CD3+, CD4+ and CD8+ T-cells. BMC Genomics 10:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogin Y, Ainey C, Beach D, Yablonski D 2007. SLP-76 mediates and maintains activation of the Tec family kinase ITK via the T cell antigen receptor-induced association between SLP-76 and ITK. Proc Natl Acad Sci U S A 104:6638–6643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosselut R 2004. CD4/CD8-lineage differentiation in the thymus: from nuclear effectors to membrane signals. Nat Rev Immunol 4:529–540 [DOI] [PubMed] [Google Scholar]
- Braiman A, Barda-Saad M, Sommers CL, Samelson LE 2006. Recruitment and activation of PLCgamma1 in T cells: a new insight into old domains. EMBO J 25:774–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazin KN, Fulton DB, Andreotti AH 2000. A specific intermolecular association between the regulatory domains of a Tec family kinase. J Mol Biol 302:607–623 [DOI] [PubMed] [Google Scholar]
- Brazin KN, Mallis RJ, Fulton DB, Andreotti AH 2002. Regulation of the tyrosine kinase Itk by the peptidyl-prolyl isomerase cyclophilin A. Proc Natl Acad Sci U S A 99:1899–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard C, Fleischacker C, Horai R, Chetana M, Venegas AM, Sharp LL, Hedrick SM, Fowlkes BJ, Schwartzberg PL 2006. Altered development of CD8+ T cell lineages in mice deficient for the Tec kinases Itk and Rlk. Immunity 25:93–104 [DOI] [PubMed] [Google Scholar]
- Brown MT, Cooper JA 1996. Regulation, substrates and functions of src. Biochim Biophys Acta 1287:121–149 [DOI] [PubMed] [Google Scholar]
- Bubeck Wardenburg J, Fu C, Jackman JK, Flotow H, Wilkinson SE, Williams DH, Johnson R, Kong G, Chan AC, Findell PR 1996. Phosphorylation of SLP-76 by the ZAP-70 protein-tyrosine kinase is required for T-cell receptor function. J Biol Chem 271:19641–19644 [DOI] [PubMed] [Google Scholar]
- Bunnell SC, Diehn M, Yaffe MB, Findell PR, Cantley LC, Berg LJ 2000. Biochemical interactions integrating Itk with the T cell receptor-initiated signaling cascade. J Biol Chem 275:2219–2230 [DOI] [PubMed] [Google Scholar]
- Bunnell SC, Singer AL, Hong DI, Jacque BH, Jordan MS, Seminario MC, Barr VA, Koretzky GA, Samelson LE 2006. Persistence of cooperatively stabilized signaling clusters drives T-cell activation. Mol Cell Biol 26:7155–7166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro M, Czar MJ, Debnath J, Cheng G, Lenardo MJ, Varmus HE, Schwartzberg PL 2001. Requirements for activation and RAFT localization of the T-lymphocyte kinase Rlk/Txk. BMC Immunol 2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching KA, Grasis JA, Tailor P, Kawakami Y, Kawakami T, Tsoukas CD 2000. TCR/CD3-Induced activation and binding of Emt/Itk to linker of activated T cell complexes: requirement for the Src homology 2 domain. J Immunol 165:256–262 [DOI] [PubMed] [Google Scholar]
- Ching KA, Kawakami Y, Kawakami T, Tsoukas CD 1999. Emt/Itk associates with activated TCR complexes: role of the pleckstrin homology domain. J Immunol 163:6006–6013 [PubMed] [Google Scholar]
- Ciofani M, Zuniga-Pflucker JC 2006. A survival guide to early T cell development. Immunol Res 34:117–132 [DOI] [PubMed] [Google Scholar]
- Colgan J, Asmal M, Neagu M, Yu B, Schneidkraut J, Lee Y, Sokolskaja E, Andreotti A, Luban J 2004. Cyclophilin A regulates TCR signal strength in CD4+ T cells via a proline-directed conformational switch in Itk. Immunity 21:189–201 [DOI] [PubMed] [Google Scholar]
- Cowan-Jacob SW, Fendrich G, Manley PW, Jahnke W, Fabbro D, Liebetanz J, Meyer T 2005. The crystal structure of a c-Src complex in an active conformation suggests possible steps in c-Src activation. Structure 13:861–871 [DOI] [PubMed] [Google Scholar]
- Crabtree GR, Olson EN 2002. NFAT signaling: choreographing the social lives of cells. Cell 109 Suppl:S67–79 [DOI] [PubMed] [Google Scholar]
- Cruz-Orcutt N, Houtman JC 2009. PI3 kinase function is vital for the function but not formation of LAT-mediated signaling complexes. Mol Immunol 46:2274–2283 [DOI] [PubMed] [Google Scholar]
- Dombroski D, Houghtling RA, Labno CM, Precht P, Takesono A, Caplen NJ, Billadeau DD, Wange RL, Burkhardt JK, Schwartzberg PL 2005. Kinase-independent functions for Itk in TCR-induced regulation of Vav and the actin cytoskeleton. J Immunol 174:1385–1392 [DOI] [PubMed] [Google Scholar]
- Dubois S, Waldmann TA, Muller JR 2006. ITK and IL-15 support two distinct subsets of CD8+ T cells. Proc Natl Acad Sci U S A 103:12075–12080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felices M, Berg LJ 2008. The Tec kinases Itk and Rlk regulate NKT cell maturation, cytokine production, and survival. J Immunol 180:3007–3018 [DOI] [PubMed] [Google Scholar]
- Felices M, Falk M, Kosaka Y, Berg LJ 2007. Tec kinases in T cell and mast cell signaling. Adv Immunol 93:145–184 [DOI] [PubMed] [Google Scholar]
- Felices M, Yin CC, Kosaka Y, Kang J, Berg LJ 2009. Tec kinase Itk in γδ T cells is pivotal for controlling IgE production in vivo. Proc Natl Acad Sci 106:8308–8313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AM, Mercer JC, Iyer A, Ragin MJ, August A 2004. Regulation of CXC chemokine receptor 4-mediated migration by the Tec family tyrosine kinase ITK. J Biol Chem 279:29816–29820 [DOI] [PubMed] [Google Scholar]
- Fowell DJ, Shinkai K, Liao XC, Beebe AM, Coffman RL, Littman DR, Locksley RM 1999. Impaired NFATc translocation and failure of Th2 development in Itk-deficient CD4+ T cells. Immunity 11:399–409 [DOI] [PubMed] [Google Scholar]
- Fukuda M, Kojima T, Kabayama H, Mikoshiba K 1996. Mutation of the pleckstrin homology domain of Bruton’s tyrosine kinase in immunodeficiency impaired inositol 1,3,4,5-tetrakisphosphate binding capacity. J Biol Chem 271:30303–30306 [DOI] [PubMed] [Google Scholar]
- Gadue P, Stein PL 2002. NK T cell precursors exhibit differential cytokine regulation and require Itk for efficient maturation. J Immunol 169:2397–2406 [DOI] [PubMed] [Google Scholar]
- Garcon F, Nunes JA 2006. Travel informations on the Tec kinases during lymphocyte activation. Adv Exp Med Biol 584:15–27 [DOI] [PubMed] [Google Scholar]
- Gibson S, Leung B, Squire JA, Hill M, Arima N, Goss P, Hogg D, Mills GB 1993. Identification, cloning, and characterization of a novel human T-cell-specific tyrosine kinase located at the hematopoietin complex on chromosome 5q. Blood 82:1561–1572 [PubMed] [Google Scholar]
- Gilfillan AM, Rivera J 2009. The tyrosine kinase network regulating mast cell activation. Immunol Rev 228:149–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Rodriguez J, Readinger JA, Viorritto IC, Mueller KL, Houghtling RA, Schwartzberg PL 2007. Tec kinases, actin, and cell adhesion. Immunol Rev 218:45–64 [DOI] [PubMed] [Google Scholar]
- Gomez-Rodriguez J, Sahu N, Handon R, Davidson TS, Anderson SM, Kirby MR, August A, Schwartzberg PL 2009. Differential expression of interleukin-17A and -17F is coupled to T cell receptor signaling via inducible T cell kinase. Immunity 31:587–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graef IA, Holsinger LJ, Diver S, Schreiber SL, Crabtree GR 1997. Proximity and orientation underlie signaling by the non-receptor tyrosine kinase ZAP70. EMBO J 16:5618–5628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves PE, Siroux V, Guerra S, Klimecki WT, Martinez FD 2005. Association of atopy and eczema with polymorphisms in T-cell immunoglobulin domain and mucin domain-IL-2-inducible T-cell kinase gene cluster in chromosome 5 q 33. J Allergy Clin Immunol 116:650–656 [DOI] [PubMed] [Google Scholar]
- Guo S, Wahl MI, Witte ON 2006. Mutational analysis of the SH2-kinase linker region of Bruton’s tyrosine kinase defines alternative modes of regulation for cytoplasmic tyrosine kinase families. Int Immunol 18:79–87 [DOI] [PubMed] [Google Scholar]
- Hansson H, Okoh MP, Smith CI, Vihinen M, Hard T 2001. Intermolecular interactions between the SH3 domain and the proline-rich TH region of Bruton’s tyrosine kinase. FEBS Lett 489:67–70 [DOI] [PubMed] [Google Scholar]
- Hao S, August A 2002. The proline rich region of the Tec homology domain of ITK regulates its activity. FEBS Lett 525:53–58 [DOI] [PubMed] [Google Scholar]
- Hao S, Qi Q, Hu J, August A 2006. A kinase independent function for Tec kinase ITK in regulating antigen receptor induced serum response factor activation. FEBS Lett 580:2691–2697 [DOI] [PubMed] [Google Scholar]
- Hawkins J, Marcy A 2001. Characterization of Itk tyrosine kinase: contribution of noncatalytic domains to enzymatic activity. Protein Expr Purif 22:211–219 [DOI] [PubMed] [Google Scholar]
- Heyeck SD, Berg LJ 1993. Developmental regulation of a murine T-cell-specific tyrosine kinase gene, Tsk. Proc Natl Acad Sci U S A 90:669–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyeck SD, Wilcox HM, Bunnell SC, Berg LJ 1997. Lck phosphorylates the activation loop tyrosine of the Itk kinase domain and activates Itk kinase activity. J Biol Chem 272:25401–25408 [DOI] [PubMed] [Google Scholar]
- Horai R, Mueller KL, Handon RA, Cannons JL, Anderson SM, Kirby MR, Schwartzberg PL 2007. Requirements for selection of conventional and innate T lymphocyte lineages. Immunity 27:775–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtman JC, Houghtling RA, Barda-Saad M, Toda Y, Samelson LE 2005. Early phosphorylation kinetics of proteins involved in proximal TCR-mediated signaling pathways. J Immunol 175:2449–2458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtman JC, Yamaguchi H, Barda-Saad M, Braiman A, Bowden B, Appella E, Schuck P, Samelson LE 2006. Oligomerization of signaling complexes by the multipoint binding of GRB2 to both LAT and SOS1. Nat Struct Mol Biol 13:798–805 [DOI] [PubMed] [Google Scholar]
- Hu J, August A 2008. Naive and innate memory phenotype CD4+ T cells have different requirements for active Itk for their development. J Immunol 180:6544–6552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Sahu N, Walsh E, August A 2007. Memory phenotype CD8+ T cells with innate function selectively develop in the absence of active Itk. Eur J Immunol 37:2892–2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K, Wang YH, Brown A, Sun G 2009. Identification of N-terminal lobe motifs that determine the kinase activity of the catalytic domains and regulatory strategies of Src and Csk protein tyrosine kinases. J Mol Biol 386:1066–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YH, Grasis JA, Miller AT, Xu R, Soonthornvacharin S, Andreotti AH, Tsoukas CD, Cooke MP, Sauer K 2007. Positive regulation of Itk PH domain function by soluble IP4. Science 316:886–889 [DOI] [PubMed] [Google Scholar]
- Huck K, Feyen O, Niehues T, Ruschendorf F, Hubner N, Laws HJ, Telieps T, Knapp S, Wacker HH, Meindl A, et al. 2009. Girls homozygous for an IL-2-inducible T cell kinase mutation that leads to protein deficiency develop fatal EBV-associated lymphoproliferation. J Clin Invest 119:1350–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huse M, Eck MJ, Harrison SC 1998. A Zn2+ ion links the cytoplasmic tail of CD4 and the N-terminal region of Lck. J Biol Chem 273:18729–18733 [DOI] [PubMed] [Google Scholar]
- Hwang ES, Szabo SJ, Schwartzberg PL, Glimcher LH 2005. T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science 307:430–433 [DOI] [PubMed] [Google Scholar]
- Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, Fujikado N, Tanahashi Y, Akitsu A, Kotaki H, et al. 2009. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity 30:108–119 [DOI] [PubMed] [Google Scholar]
- Jameson SC, Hogquist KA, Bevan MJ 1995. Positive selection of thymocytes. Annu Rev Immunol 13:93–126 [DOI] [PubMed] [Google Scholar]
- Jordan MS, Smith JE, Burns JC, Austin JE, Nichols KE, Aschenbrenner AC, Koretzky GA 2008. Complementation in trans of altered thymocyte development in mice expressing mutant forms of the adaptor molecule SLP76. Immunity 28:359–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph RE, Andreotti AH 2009. Conformational snapshots of Tec kinases during signaling. Immunol Rev 228:74–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph RE, Fulton DB, Andreotti AH 2007a. Mechanism and functional significance of Itk autophosphorylation. J Mol Biol 373:1281–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph RE, Min L, Andreotti AH 2007b. The linker between SH2 and kinase domains positively regulates catalysis of the Tec family kinases. Biochemistry 46:5455–5462 [DOI] [PubMed] [Google Scholar]
- Joseph RE, Min L, Xu R, Musselman ED, Andreotti AH 2007c. A remote substrate docking mechanism for the tec family tyrosine kinases. Biochemistry 46:5595–5603 [DOI] [PubMed] [Google Scholar]
- Koprulu AD, Ellmeier W 2009. The role of Tec family kinases in mononuclear phagocytes. Crit Rev Immunol 29:317–333 [DOI] [PubMed] [Google Scholar]
- Koretzky GA, Abtahian F, Silverman MA 2006. SLP76 and SLP65: complex regulation of signalling in lymphocytes and beyond. Nat Rev Immunol 6:67–78 [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK 2009. IL-17 and Th17 Cells. Annu Rev Immunol 27:485–517 [DOI] [PubMed] [Google Scholar]
- Kreslavsky T, Savage AK, Hobbs R, Gounari F, Bronson R, Pereira P, Pandolfi PP, Bendelac A, von Boehmer H 2009. TCR-inducible PLZF transcription factor required for innate phenotype of a subset of gammadelta T cells with restricted TCR diversity. Proc Natl Acad Sci U S A 106:12453–12458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labno CM, Lewis CM, You D, Leung DW, Takesono A, Kamberos N, Seth A, Finkelstein LD, Rosen MK, Schwartzberg PL, et al. 2003. Itk functions to control actin polymerization at the immune synapse through localized activation of Cdc42 and WASP. Curr Biol 13:1619–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laederach A, Cradic KW, Brazin KN, Zamoon J, Fulton DB, Huang XY, Andreotti AH 2002. Competing modes of self-association in the regulatory domains of Bruton’s tyrosine kinase: intramolecular contact versus asymmetric homodimerization. Protein Sci 11:36–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laederach A, Cradic KW, Fulton DB, Andreotti AH 2003. Determinants of intra versus intermolecular self-association within the regulatory domains of Rlk and Itk. J Mol Biol 329:1011–1020 [DOI] [PubMed] [Google Scholar]
- LaFevre-Bernt M, Sicheri F, Pico A, Porter M, Kuriyan J, Miller WT 1998. Intramolecular regulatory interactions in the Src family kinase Hck probed by mutagenesis of a conserved tryptophan residue. J Biol Chem 273:32129–32134 [DOI] [PubMed] [Google Scholar]
- Lees RK, Ferrero I, MacDonald HR 2001. Tissue-specific segregation of TCRgamma delta+ NKT cells according to phenotype TCR repertoire and activation status: parallels with TCR alphabeta+NKT cells. Eur J Immunol 31:2901–2909 [DOI] [PubMed] [Google Scholar]
- Lewis RS, Cahalan MD 1995. Potassium and calcium channels in lymphocytes. Annu Rev Immunol 13:623–653 [DOI] [PubMed] [Google Scholar]
- Liao XC, Littman DR 1995. Altered T cell receptor signaling and disrupted T cell development in mice lacking Itk. Immunity 3:757–769 [DOI] [PubMed] [Google Scholar]
- Lin L, Czerwinski R, Kelleher K, Siegel MM, Wu P, Kriz R, Aulabaugh A, Stahl M 2009. Activation loop phosphorylation modulates Bruton’s tyrosine kinase (Btk) kinase domain activity. Biochemistry 48:2021–2032 [DOI] [PubMed] [Google Scholar]
- Lin X, Ayrapetov MK, Lee S, Parang K, Sun G 2005. Probing the communication between the regulatory and catalytic domains of a protein tyrosine kinase, Csk. Biochemistry 44:1561–1567 [DOI] [PubMed] [Google Scholar]
- Liu KQ, Bunnell SC, Gurniak CB, Berg LJ 1998. T cell receptor-initiated calcium release is uncoupled from capacitative calcium entry in Itk-deficient T cells. J Exp Med 187:1721–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas JA, Atherly LO, Berg LJ 2002. The absence of Itk inhibits positive selection without changing lineage commitment. J Immunol 168:6142–6151 [DOI] [PubMed] [Google Scholar]
- Lucas JA, Felices M, Evans JW, Berg LJ 2007. Subtle defects in pre-TCR signaling in the absence of the Tec kinase Itk. J Immunol 179:7561–7567 [DOI] [PubMed] [Google Scholar]
- Lucas JA, Miller AT, Atherly LO, Berg LJ 2003. The role of Tec family kinases in T cell development and function. Immunol Rev 191:119–138 [DOI] [PubMed] [Google Scholar]
- Marcotte DJ, Liu YT, Arduini RM, Hession CA, Miatkowski K, Wildes CP, Cullen PF, Hong V, Hopkins BT, Mertsching E, et al. 2010. Structures of human Bruton’s tyrosine kinase in active and inactive conformations suggest a mechanism of activation for TEC family kinases. Protein Sci 19:429–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez JA, Smith CI, Petoukhov MV, Lo Surdo P, Mattsson PT, Knekt M, Westlund A, Scheffzek K, Saraste M, Svergun DI 2003. Conformation of full-length Bruton tyrosine kinase (Btk) from synchrotron X-ray solution scattering. EMBO J 22:4616–4624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara S, Suzuki N 2007. Role of Txk, a member of the Tec family of tyrosine kinases, in immune-inflammatory diseases. Int Rev Immunol 26:333–348 [DOI] [PubMed] [Google Scholar]
- Miller AT, Berg LJ 2002. Defective Fas ligand expression and activation-induced cell death in the absence of IL-2-inducible T cell kinase. J Immunol 168:2163–2172 [DOI] [PubMed] [Google Scholar]
- Miller AT, Wilcox HM, Lai Z, Berg LJ 2004. Signaling through Itk promotes T helper 2 differentiation via negative regulation of T-bet. Immunity 21:67–80 [DOI] [PubMed] [Google Scholar]
- Min L, Joseph RE, Fulton DB, Andreotti AH 2009. Itk tyrosine kinase substrate docking is mediated by a nonclassical SH2 domain surface of PLCgamma1. Proc Natl Acad Sci U S A 106:21143–21148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min L, Wu W, Joseph RE, Fulton DB, Berg L, Andreotti AH 2010. Disrupting the intermolecular self-association of Itk enhances T cell signaling. J Immunology 184:4228–4235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed AJ, Yu L, Backesjo CM, Vargas L, Faryal R, Aints A, Christensson B, Berglof A, Vihinen M, Nore BF, et al. 2009. Bruton’s tyrosine kinase (Btk): function, regulation, and transformation with special emphasis on the PH domain. Immunol Rev 228:58–73 [DOI] [PubMed] [Google Scholar]
- Mueller C, August A 2003. Attenuation of Immunological Symptoms of Allergic Asthma in Mice Lacking the Tyrosine Kinase ITK. J Immunol 170:5056–5063 [DOI] [PubMed] [Google Scholar]
- Neal JW, Clipstone NA 2003. A constitutively active NFATc1 mutant induces a transformed phenotype in 3T3-L1 fibroblasts. J Biol Chem 278:17246–17254 [DOI] [PubMed] [Google Scholar]
- Nunez-Cruz S, Aguado E, Richelme S, Chetaille B, Mura AM, Richelme M, Pouyet L, Jouvin-Marche E, Xerri L, Malissen B, et al. 2003. LAT regulates gammadelta T cell homeostasis and differentiation. Nat Immunol 4:999–1008 [DOI] [PubMed] [Google Scholar]
- Nurieva RI, Chuvpilo S, Wieder ED, Elkon KB, Locksley R, Serfling E, Dong C 2007. A costimulation-initiated signaling pathway regulates NFATc1 transcription in T lymphocytes. J Immunol 179:1096–1103 [DOI] [PubMed] [Google Scholar]
- Okoh MP, Vihinen M 2002. Interaction between Btk TH and SH3 domain. Biopolymers 63:325–334 [DOI] [PubMed] [Google Scholar]
- Palacios EH, Weiss A 2004. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene 23:7990–8000 [DOI] [PubMed] [Google Scholar]
- Pawson T, Kofler M 2009. Kinome signaling through regulated protein-protein interactions in normal and cancer cells. Curr Opin Cell Biol 21:147–153 [DOI] [PubMed] [Google Scholar]
- Perez-Villar JJ, Kanner SB 1999. Regulated association between the tyrosine kinase Emt/Itk/Tsk and phospholipase-C gamma 1 in human T lymphocytes. J Immunol 163:6435–6441 [PubMed] [Google Scholar]
- Pitcher LA, van Oers NS 2003. T-cell receptor signal transmission: who gives an ITAM? Trends Immunol 24:554–560 [DOI] [PubMed] [Google Scholar]
- Pletneva EV, Sundd M, Fulton DB, Andreotti AH 2006. Molecular details of Itk activation by prolyl isomerization and phospholigand binding: the NMR structure of the Itk SH2 domain bound to a phosphopeptide. J Mol Biol 357:550–561 [DOI] [PubMed] [Google Scholar]
- Prince AL, Yin CC, Enos ME, Felices M, Berg LJ 2009. The Tec kinases Itk and Rlk regulate conventional versus innate T-cell development. Immunol Rev 228:115–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punt JA, Osborne BA, Takahama Y, Sharrow SO, Singer A 1994. Negative selection of CD4+CD8+ thymocytes by T cell receptor-induced apoptosis requires a costimulatory signal that can be provided by CD28. J Exp Med 179:709–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pursglove SE, Mulhern TD, Mackay JP, Hinds MG, Booker GW 2002. The solution structure and intramolecular associations of the Tec kinase SRC homology 3 domain. J Biol Chem 277:755–762 [DOI] [PubMed] [Google Scholar]
- Qi Q, August A 2009. The tec family kinase itk exists as a folded monomer in vivo. J Biol Chem 284:29882–29892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Q, Sahu N, August A 2006. Tec kinase Itk forms membrane clusters specifically in the vicinity of recruiting receptors. J Biol Chem 281:38529–38534 [DOI] [PubMed] [Google Scholar]
- Qi Q, Xia M, Hu J, Hicks E, Iyer A, Xiong N, August A 2009. Enhanced development of CD4+ gammadelta T cells in the absence of Itk results in elevated IgE production. Blood 114:564–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raberger J, Schebesta A, Sakaguchi S, Boucheron N, Blomberg KE, Berglof A, Kolbe T, Smith CI, Rulicke T, Ellmeier W 2008. The transcriptional regulator PLZF induces the development of CD44 high memory phenotype T cells. Proc Natl Acad Sci U S A 105:17919–17924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings DJ, Saffran DC, Tsukada S, Largaespada DA, Grimaldi JC, Cohen L, Mohr RN, Bazan JF, Howard M, Copeland NG, et al. 1993. Mutation of unique region of Bruton’s tyrosine kinase in immunodeficient XID mice. Science 261:358–361 [DOI] [PubMed] [Google Scholar]
- Readinger JA, Mueller KL, Venegas AM, Horai R, Schwartzberg PL 2009. Tec kinases regulate T-lymphocyte development and function: new insights into the roles of Itk and Rlk/Txk. Immunol Rev 228:93–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SG 2001. Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem 70:281–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacristan C, Schattgen SA, Berg LJ, Bunnell SC, Roy AL, Rosenstein Y 2009. Characterization of a novel interaction between transcription factor TFII-I and the inducible tyrosine kinase in T cells. Eur J Immunol 39:2584–2595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu N, Mueller C, Fischer A, August A 2008a. Differential sensitivity to Itk kinase signals for T helper 2 cytokine production and chemokine-mediated migration. J Immunol 180:3833–3838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu N, Venegas AM, Jankovic D, Mitzner W, Gomez-Rodriguez J, Cannons JL, Sommers C, Love P, Sher A, Schwartzberg PL, et al. 2008b. Selective expression rather than specific function of Txk and Itk regulate Th1 and Th2 responses. J Immunol 181:6125–6131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer EM, Broussard C, Debnath J, Anderson S, McVicar DW, Schwartzberg PL 2000. Tec family kinases modulate thresholds for thymocyte development and selection. J Exp Med 192:987–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer EM, Debnath J, Yap G, McVicar D, Liao XC, Littman DR, Sher A, Varmus HE, Lenardo MJ, Schwartzberg PL 1999. Requirement for Tec kinases Rlk and Itk in T cell receptor signaling and immunity. Science 284:638–641 [DOI] [PubMed] [Google Scholar]
- Schaeffer EM, Yap GS, Lewis CM, Czar MJ, McVicar DW, Cheever AW, Sher A, Schwartzberg PL 2001. Mutation of Tec family kinases alters T helper cell differentiation. Nat Immunol 2:1183–1188 [DOI] [PubMed] [Google Scholar]
- Seminario MC, Bunnell SC 2008. Signal initiation in T-cell receptor microclusters. Immunol Rev 221:90–106 [DOI] [PubMed] [Google Scholar]
- Severin A, Joseph RE, Boyken S, Fulton DB, Andreotti AH 2009. Proline isomerization preorganizes the Itk SH2 domain for binding to the Itk SH3 domain. J Mol Biol 387:726–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan X, Czar MJ, Bunnell SC, Liu P, Liu Y, Schwartzberg PL, Wange RL 2000. Deficiency of PTEN in Jurkat T cells causes constitutive localization of Itk to the plasma membrane and hyperresponsiveness to CD3 stimulation. Mol Cell Biol 20:6945–6957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan X, Wange RL 1999. Itk/Emt/Tsk activation in response to CD3 cross-linking in Jurkat T cells requires ZAP-70 and Lat and is independent of membrane recruitment. J Biol Chem 274:29323–29330 [DOI] [PubMed] [Google Scholar]
- Siliciano JD, Morrow TA, Desiderio SV 1992. itk, a T-cell-specific tyrosine kinase gene inducible by interleukin 2. Proc Natl Acad Sci U S A 89:11194–11198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Garvin JE, Koretzky GA, Jordan MS 2009. T cell activation. Annu Rev Immunol 27:591–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommers CL, Rabin RL, Grinberg A, Tsay HC, Farber J, Love PE 1999. A role for the Tec family tyrosine kinase Txk in T cell activation and thymocyte selection. J Exp Med 190:1427–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommers CL, Samelson LE, Love PE 2004. LAT: a T lymphocyte adapter protein that couples the antigen receptor to downstream signaling pathways. Bioessays 26:61–67 [DOI] [PubMed] [Google Scholar]
- Streubel B, Vinatzer U, Willheim M, Raderer M, Chott A 2006. Novel t(5;9)(q33;q22) fuses ITK to SYK in unspecified peripheral T-cell lymphoma. Leukemia 20:313–318 [DOI] [PubMed] [Google Scholar]
- Su YW, Zhang Y, Schweikert J, Koretzky GA, Reth M, Wienands J 1999. Interaction of SLP adaptors with the SH2 domain of Tec family kinases. Eur J Immunol 29:3702–3711 [DOI] [PubMed] [Google Scholar]
- Takesono A, Horai R, Mandai M, Dombroski D, Schwartzberg PL 2004. Requirement for Tec kinases in chemokine-induced migration and activation of Cdc42 and Rac. Curr Biol 14:917–922 [DOI] [PubMed] [Google Scholar]
- Tanaka N, Asao H, Ohtani K, Nakamura M, Sugamura K 1993. A novel human tyrosine kinase gene inducible in T cells by interleukin 2. FEBS Lett 324:1–5 [DOI] [PubMed] [Google Scholar]
- Thomas JD, Sideras P, Smith CI, Vorechovsky I, Chapman V, Paul WE 1993. Colocalization of X-linked agammaglobulinemia and X-linked immunodeficiency genes. Science 261:355–358 [DOI] [PubMed] [Google Scholar]
- Tomlinson MG, Heath VL, Turck CW, Watson SP, Weiss A 2004. SHIP family inositol phosphatases interact with and negatively regulate the Tec tyrosine kinase. J Biol Chem 279:55089–55096 [DOI] [PubMed] [Google Scholar]
- Tsukada S, Saffran DC, Rawlings DJ, Parolini O, Allen RC, Klisak I, Sparkes RS, Kubagawa H, Mohandas T, Quan S, et al. 1993. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell 72:279–290 [DOI] [PubMed] [Google Scholar]
- Tsygankov AY 2003. Non-receptor protein tyrosine kinases. Front Biosci 8:595–635 [DOI] [PubMed] [Google Scholar]
- van de Weyer PS, Muehlfeit M, Klose C, Bonventre JV, Walz G, Kuehn EW 2006. A highly conserved tyrosine of Tim-3 is phosphorylated upon stimulation by its ligand galectin-9. Biochem Biophys Res Commun 351:571–576 [DOI] [PubMed] [Google Scholar]
- Vetrie D, Vorechovsky I, Sideras P, Holland J, Davies A, Flinter F, Hammarstrom L, Kinnon C, Levinsky R, Bobrow M, et al. 1993. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature 361:226–233 [DOI] [PubMed] [Google Scholar]
- Vicari AP, Mocci S, Openshaw P, O’Garra A, Zlotnik A 1996. Mouse gamma delta TCR+NK1.1+ thymocytes specifically produce interleukin-4, are major histocompatibility complex class I independent, and are developmentally related to alpha beta TCR+NK1.1+ thymocytes. Eur J Immunol 26:1424–1429 [DOI] [PubMed] [Google Scholar]
- Wilcox HM, Berg LJ 2003. Itk phosphorylation sites are required for functional activity in primary T cells. J Biol Chem 278:37112–37121 [DOI] [PubMed] [Google Scholar]
- Williams JC, Wierenga RK, Saraste M 1998. Insights into Src kinase functions: structural comparisons. Trends Biochem Sci 23:179–184 [DOI] [PubMed] [Google Scholar]
- Wilson CB, Rowell E, Sekimata M 2009. Epigenetic control of T-helper-cell differentiation. Nat Rev Immunol 9:91–105 [DOI] [PubMed] [Google Scholar]
- Winslow MM, Neilson JR, Crabtree GR 2003. Calcium signalling in lymphocytes. Curr Opin Immunol 15:299–307 [DOI] [PubMed] [Google Scholar]
- Xu W, Doshi A, Lei M, Eck MJ, Harrison SC 1999. Crystal structures of c-Src reveal features of its autoinhibitory mechanism. Mol Cell 3:629–638 [DOI] [PubMed] [Google Scholar]
- Xu W, Harrison SC, Eck MJ 1997. Three-dimensional structure of the tyrosine kinase c-Src. Nature 385:595–602 [DOI] [PubMed] [Google Scholar]
- Yamada N, Kawakami Y, Kimura H, Fukamachi H, Baier G, Altman A, Kato T, Inagaki Y, Kawakami T 1993. Structure and expression of novel protein-tyrosine kinases, Emb and Emt, in hematopoietic cells. Biochem Biophys Res Commun 192:231–240 [DOI] [PubMed] [Google Scholar]
- Yang W, Malek SN, Desiderio S 1995. An SH3-binding site conserved in Bruton’s tyrosine kinase and related tyrosine kinases mediates specific protein interactions in vitro and in vivo. J Biol Chem 270:20832–20840 [DOI] [PubMed] [Google Scholar]
- Yang WC, Ching KA, Tsoukas CD, Berg LJ 2001. Tec kinase signaling in T cells is regulated by phosphatidylinositol 3-kinase and the Tec pleckstrin homology domain. J Immunol 166:387–395 [DOI] [PubMed] [Google Scholar]
- Yang XO, Chang SH, Park H, Nurieva R, Shah B, Acero L, Wang YH, Schluns KS, Broaddus RR, Zhu Z, et al. 2008. Regulation of inflammatory responses by IL-17F. J Exp Med 205:1063–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Mohamed AJ, Vargas L, Berglof A, Finn G, Lu KP, Smith CI 2006. Regulation of Bruton tyrosine kinase by the peptidylprolyl isomerase Pin1. J Biol Chem 281:18201–18207 [DOI] [PubMed] [Google Scholar]
- Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE 1998. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell 92:83–92 [DOI] [PubMed] [Google Scholar]