Abstract
We have previously reported the inhibitory effect of NCX-4016, a nitro derivative of aspirin, on the proliferation of cisplatin-resistant human ovarian cancer cells, in vitro (Bratasz et al., Proc Natl Acad Sci USA 2006; 103:3914–9). In this report we present the results of our study on the mechanistic aspects of drug action including the molecular and signaling pathways involved in an in vitro cell line, as well as in a murine tumor xenograft. We report, for the first time, that NCX-4016 significantly inhibited the growth of cisplatin-resistant human ovarian cancer xenografts in mice. We observed that the inhibitory effect of NCX-4016 on cell proliferation was associated with G1 phase cell cycle arrest with increased activity of p53, p21 and p27 proteins. NCX-4016 modulated the Bcl-2 family of proteins, and induced apoptosis by activating Bax and cytochrome c release in a time-dependent manner. In addition, NCX-4016 selectively down-regulated the phosphorylated forms of EGFR (Tyr845, Tyr992), pAkt (Ser473, Thr305), and STAT3 (Tyr705, Ser727), in vitro and in vivo. Taken together, the results clearly suggested that NCX-4016 causes significant induction of cell cycle arrest and apoptosis in cisplatin-resistant human ovarian cancer cells via down-regulation of EGFR/PI3K/STAT3 signaling and modulation of Bcl-2 family proteins. Thus, NCX-4016 appears to be a potential therapeutic agent for treating recurrent human ovarian carcinoma.
Keywords: NCX-4016, ovarian cancer, cisplatin resistance, apoptosis, EGFR, STAT3, Bcl-2
Introduction
Ovarian cancer is the fourth most lethal cancer among women and the leading cause of gynecologic cancer deaths in the United States.1 Surgical cytoreduction followed by treatment with chemotherapeutic agents, such as cisplatin and paclitaxel (Taxol), is commonly used to treat this malignancy.2 Although this therapy is effective for the first time, most ovarian cancer survivors eventually suffer from recurrent disease with acquired resistance to subsequent chemotherapy. Cisplatin is an effective and widely used chemotherapeutic agent against various human cancers, including ovarian cancer.3 Despite its great efficacy at treating ovarian cancer, a major limitation of cisplatin treatment is the development of drug-resistance. Even a slight increase in the cisplatin resistance of the tumor may pose a clinically challenging problem requiring administration of large doses of the drug that may lead to multiorgan toxicities.2,4,5 Therefore, a new adjuvant treatment is necessary to specifically overcome drug-resistance in the treatment of recurrent ovarian cancer.
Multiple mechanisms have been proposed for the development of cisplatin-resistance in ovarian cancer. The mechanisms include reduced intracellular accumulation of the drug, increased levels of glutathione, upregulation of anti-apoptotic proteins, and downregulation of pro-apoptotic proteins.6-9 Recent studies have shown that constitutive activation of STAT3 (signal transducers and activators of transcription 3) and EGFR (epidermal growth factor receptor) confers resistance to chemotherapy-induced apoptosis in epithelial malignancies.10,11 STAT3 can be activated by various protein tyrosine kinases including Janus tyrosine kinases (JAK) and the proto-oncogene tyrosine kinase (Src), as well as membrane-bound growth factor receptor tyrosine kinases such as EGFR.12,13 EGFR is over-expressed in a wide variety of epithelial malignancies including non-small cell lung, head and neck, colon, and breast cancers.14,15 Over-expression and/or increased activity of EGFR are key characteristics of human tumors and are frequently linked to more aggressive tumor behaviors, including increased proliferation, metastasis, and therapeutic resistance.10 As such, EGFR is considered to be a key therapeutic target for human cancers.16 Recently, some anticancer drugs and synthetic compounds that inhibit EGFR tyrosine kinase activity have been shown to be effective in preclinical studies and in late stages of clinical trials for non-small cell lung cancer, breast, and ovarian cancer. Although this efficacy is associated with a certain population, specific types of histology, and activation of somatic mutations in the tyrosine kinase domain of EGFR.17,18
Both EGF and activated STAT3 translocate to the perinuclear region via endosomes,19 implying that EGFR and STAT3 may interact in cellular locations other than the plasma membrane. STAT3 activates the transcription of genes involved in cell cycle progression and anti-apoptosis.20 Recent work suggests that the deregulated iNOS/NO pathway may partly contribute to the malignant biology of tumor cells with high levels of nuclear EGFR and STAT3.21 A direct and consistent link between the EGFR and iNOS/NO pathways has not been established. For example, EGF is known to induce expression of iNOS in normal astrocytes22 and head and neck squamous cell carcinomas.23 However, such regulation was not found in other cellular systems.24 A previous report showed that NO donors inhibited the EGF-induced EGFR transphosphorylation without affecting the binding of the ligand to the receptor.21 In ovarian carcinoma cells, both exogenously applied NO and endogenously synthesized NO have been shown to inhibit tumor growth.25,26 NO has been shown to inhibit the proliferation of vascular smooth muscle cells by blocking ornithine decarboxylase, an enzyme involved in the synthesis of polyamines (spermine, spermidine, and putrescine) that are required for cell proliferation.27 It is conceivable that different NO-generating profiles result in different susceptibility of these cells to NO-mediated inhibition of cell-proliferation and killing by activating pro-apoptotic and down-regulating antiapoptotic genes.25,28
We have recently reported that NCX-4016 (see Fig. 1A inset for chemical structure), a nitro derivative of aspirin capable of NO-release in cells, caused a significant reduction in the colony-forming ability of cisplatin-resistant (CR) human ovarian cancer cells in vitro.6 The CR cells subjected to NCX-4016 treatment showed a significant reduction in the surviving fraction and cellular GSH levels. The antiproliferative effect of NCX-4016 was attributed to its enhanced NO-releasing ability under the ‘thiol-rich’ cellular environment in the CR cell.6 However, the precise mechanism by which the NO-releasing could exert its cytotoxic effect in the cisplatin resistant ovarian cancer carcinoma is unclear.29 In this study, we have investigated the molecular mechanism and pathways responsible for the antiproliferative activity of NCX-4016 against cisplatin-resistant human ovarian cancer cells in vitro and in vivo. We show that NCX-4016 induces apoptosis in the cancer cells by selectively inhibiting the activity of EGFR/PI3K and STAT3 and by altering the downstream signaling pathway through Bcl-2 family of proteins.
Figure 1.
Effect of NCX-4016 on cell cycle distribution and cell cycle-related protein expression in cisplatin-resistant human ovarian cancer cells in vitro. (A) Cell cycle distribution. Cells were treated with either DMSO (vehicle, Control) or NCX-4016 (100 μM) for 24 h and then subjected to flow cytometric analysis. *significantly different (p < 0.05) compared to Control G1 population. The molecular structure of NCX-4016 is shown in the inset. (B) Immunoblot images of cell cycle regulatory molecules p53, p21, p27, CycA, CycD1, cdk2, and cdk4 in the CR cells treated with NCX-4016 (100 μM) for 6, 12 or 24 h. The results show significant G1 arrest and alterations in the G1 cell cycle-related protein expressions in the CR cells treated with NCX-4016.
Results
NCX-4016 induces G1 arrest and alters cell cycle-related protein expression in cisplatin-resistant human ovarian cancer cells, in vitro
Perturbations in cell cycle progression, mediated by alterations in cell cycle-related protein expression, play a vital role in the proliferation of cancer cells. We determined the effect of NCX-4016 on cell cycle progression in CR ovarian cancer cells. The distribution of cell cycle population in cells treated with NCX-4016 (100 μM, 24 h) was measured by flow cytometry. The results showed that NCX-4016 induced G1 arrest (64.5% as compared control at 48.4%) in CR cells (Fig. 1A). Western blot analysis of total lysates of cells treated with NCX-4016 showed a time-dependent increase in the expression of p53, p21, and p27, while there was a decrease in the expression of cyclinD1, cdk2, cdk4, and cyclinA (Fig. 1B). The results suggested that NCX-4016 induced G1 arrest, possibly by altering the G1 cell cycle-related protein expression in the CR human ovarian cancer cells.
NCX-4016 induces apoptosis by activating the intrinsic apoptotic pathway in ovarian cancer cells
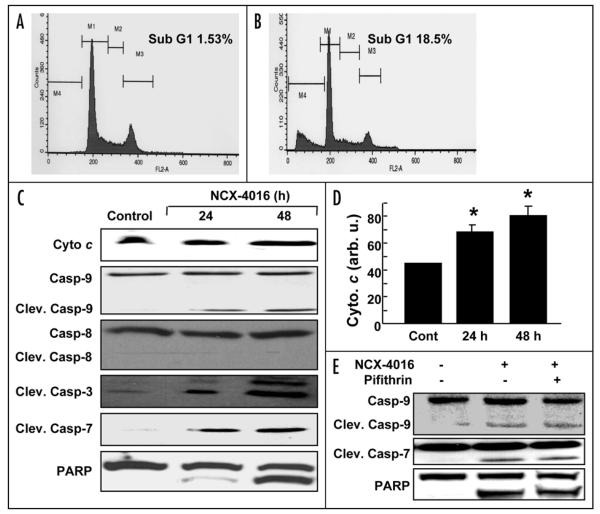
The number of cells with sub-G1 DNA content, which is a marker of apoptosis, was significantly higher in cells treated with NCX-4016 for 48 h when compared to control (Fig. 2, Panels A and B). Caspases are cysteine proteases that are activated through several apoptotic pathways. The activation of different initiator and executor caspases are commonly used to characterize the involvement of distinct apoptotic signaling pathways. Western blotting showed an increase in the active form of caspase-9, but not caspase-8 (Fig. 2C). Exposure of the CR cells to NCX-4016 for 12 or 24 h did not show any change in protein levels of the death receptors Fas, FasL, or DR5 (data not shown) suggesting that extrinsic apoptosis pathways did not contribute to the NCX-4016-induced apoptosis in CR cells. We further observed that NCX-4016 also induced activation of caspase-3 and caspase-7, and cleavage of their substrate PARP. The increased levels of cleaved caspase-9 suggested that it became activated, while, in parallel, cytochrome c was released from the mitochondria (Fig. 2C and 2D). Many chemotherapeutic agents induce apoptosis by activation of the p53 dependent pathway.31,32 In order to determine whether or not the NCX-4016-induced apoptosis was dependent on p53, CR cancer cells were pretreated with the pifithrin (pifithrin-α; Sigma) for 1 h, and then treated with NCX-4016 for 48 h. The cells were analyzed for cleaved caspase-7, caspase-9 and PARP by immunoblot assay. Pretreatment with pifithrin did not block NCX-4016-induced apoptosis (Fig. 2E). These results indicate that the NCX-4016-induced apoptosis in the CR cells is not mediated by p53.
Figure 2.
NCX-4016-induced apoptosis in CR ovarian cancer cells. Cells were treated with NCX-4016 (100 μM) for 48 h and the sub-G1 population for 20,000 events within a fixed gate was analyzed. Representative flow-cytometry profile for (A) control (vehicle-treated) and (B) NCX-4016-treated groups. (C) Immunoblot analysis of apoptotic proteins. Cleavages in caspase-9, caspase-8, caspase-3, caspase-7, cytochorome c, and PARP are shown in cells treated with NCX-4016 (100 μM) for 24 or 48 h. (D) Intensity of cytochrome c band as quantified by densitometry. (E) Inhibition of p53 by pifithrin did not reverse the NCX-4016 induced apoptosis. The CR cancer cells were pretreated with the pifithrin 20 μM for 1 h and then treated with NCX-4016 for 48 h. The cells were analyzed for cleaved caspase-9, caspases-7 and PARP by immunoblot assay.
NCX-4016 modulates the expression of Bcl-2 proteins in ovarian cancer cells
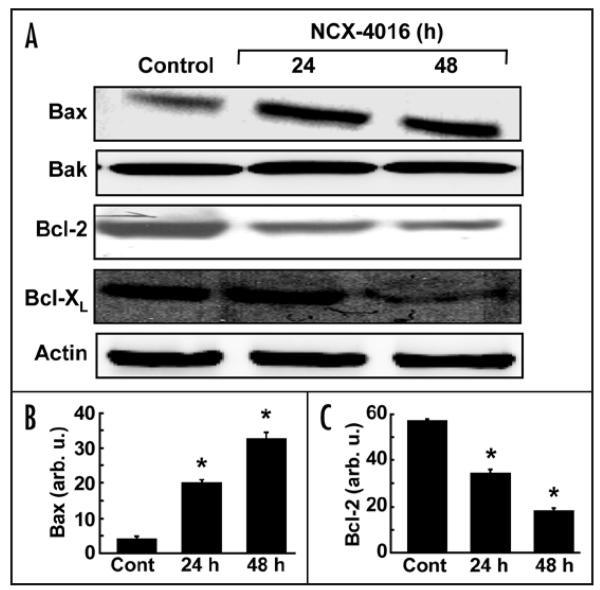
To determine whether or not the NCX-4016-induced apoptosis in the CR cells was associated with the modulation of the Bcl-2 family of proteins, we examined the expression of Bcl-2, Bak, Bax, and Bcl-XL proteins. As shown in Fig. 3A, treatment with NCX-4016 resulted in a time-dependent decrease in the expression of Bcl-2 and Bcl-XL, and an increase in the expression of Bax. However, the expression of Bak was not affected by NCX-4016 treatment. Quantitation of band density showed a significant increase in Bax (Fig. 3B) and a significant decrease in Bcl-2 (Fig. 3C) expression at 24 or 48 h when compared to controls.
Figure 3.
Effect of NCX-4016 on Bcl-2 family protein expressions in CR ovarian cancer cells. Cells were treated with NCX-4016 (100 μM) for 24 or 48 h and analyzed for various Bcl-2 family proteins. (A) Representative Western blot images showing time-dependent expressions of Bax, Bak, Bcl-2, Bcl-XL, and actin. Intensity of Bax (B) and Bcl-2 (C) as quantified by densitometry are also shown. Data represent mean ± SE of three independent experiments. *p < 0.05 as compared to the Control group. The results reveal a significant contribution of the Bcl-2 proteins, particularly Bax/Bcl-2, to the induction of apoptosis in the CR cancer cells treated with NCX-4016.
NCX-4016 selectively inhibits pEGFR, pAkt and pSTAT3 in ovarian cancer cells
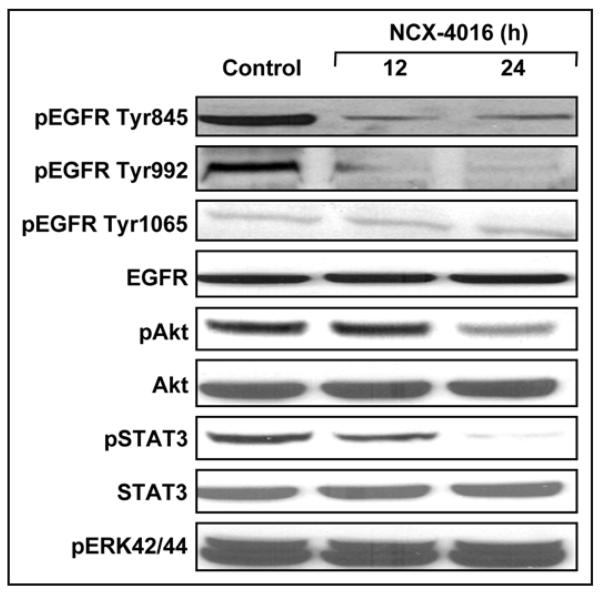
Drug-resistance is a major problem in chemotherapy.33 Potential mechanisms of drug-resistance include the activation of EGFR/PI3K and STAT3 signal transduction cascades.10,34 Over-expression of EGFR and STAT3, which occurs frequently in CR ovarian cancer cells, is an adverse prognostic factor. Hence, we studied the involvement of EGFR/PI3K- and STAT3- signaling pathways. Western blotting showed that NCX-4016 selectively downregulated pEGFR (Tyr845 & Tyr992), pAkt, and pSTAT3 (Tyr705 & Ser727), although the total EGFR, Akt, and STAT3 levels remained unchanged (Fig. 4).
Figure 4.
Effect of NCX-4016 on EGFR/PI3K and STAT3 signaling in CR ovarian cancer cells. Representative immunoblot images showing the expression levels of pEGFR, pAkt and pSTAT3 and their phosphorylated counterparts are shown. The cells were treated with NCX-4016 (100 μM) for 12 or 24 h. The results show inhibition of EGFR and STAT3 activation, but no effect on MAPK pathway, in the CR cells treated with NCX-4016.
NCX-4016 inhibits tumor growth and modulates cell cycle- and Bcl-2-related protein expressions in ovarian cancer xenografts in mice
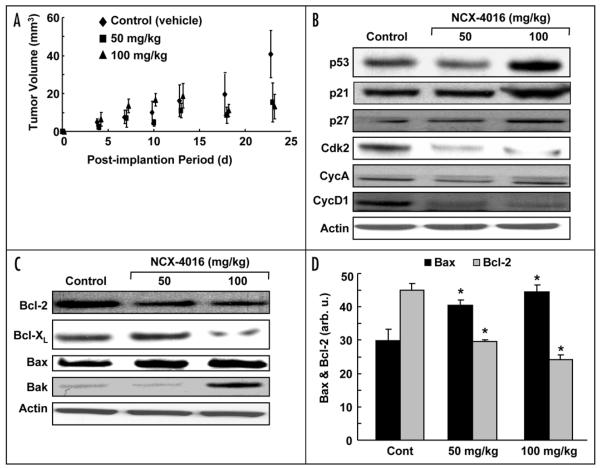
We evaluated the efficacy of NCX-4016 in the inhibition of tumor growth and modulation of cell cycle and apoptotic protein expression in an in vivo xenograft mouse model of the human ovarian cancer. After 3 weeks of NCX-4016 treatment, a significant reduction in the tumor volume was observed (Fig. 5A). The excised tumors, after 4 weeks of NCX-4016 treatment, showed increased levels of p53, p21, and p27 and decreased levels of cyclinD1, cdk2, and cyclinA (Fig. 5B). We further observed a downregulation in the expression of antiapoptotic proteins Bcl-2 and Bcl-XL, and an upregulation of the expression of proapoptotic proteins Bax and Bak (Fig. 5C). Quantitation of the band density showed a significant increase in Bax and a significant decrease in Bcl-2 (Fig. 5D) expression in treated animals when compared to controls.
Figure 5.
Effect of NCX-4016 on ovarian cancer xenograft tumor in mice. (A) Effect of tumor volume growth in mice treated with NCX-4016. Three groups (N = 5 each) of mice were implanted with the CR ovarian cancer cells (s.c.) in the back. Five days after implantation, two groups were administered (i.p.) with 50 or 100 mg/kg NCX-4016 daily, while the control group received vehicle (DMSO) only. The results show a dose-dependent inhibition of tumor growth in the drug-treated groups, which was significantly different from the Control group (*p < 0.05) on day 18 and beyond. (B and C) show immunoblots and analysis of Bcl-2 and cell cycle-related proteins in the tissue lysates of tumor xenografts. (D) The Bcl-2 and Bax band signals were quantified by densitometry. Data represent mean ± SE of three independent experiments. *p < 0.05 as compared to the respective Control groups.
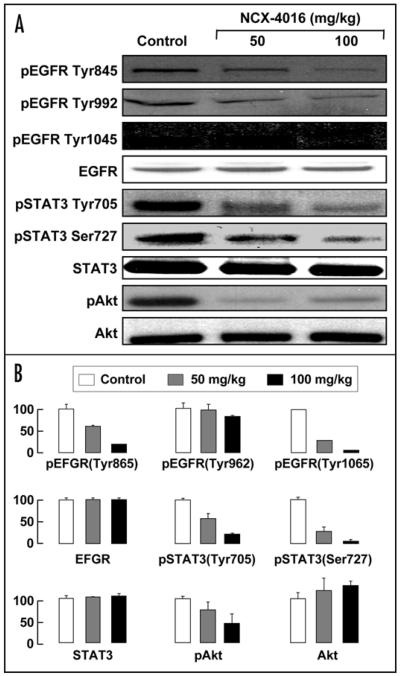
NCX-4016 downregulates EGFR, STAT3 and Akt in ovarian cancer xenografts in mice
EGFR and STAT3 signaling pathways impact many aspects of tumor biology, including proliferation, invasion, metastasis, and apoptosis.35,36 The activation of EGFR has been shown to inhibit apoptosis, and enhance tumor growth, invasion, and metastasis. Specifically, a majority of pancreatic and ovarian cancers over-express the EGFR receptor. Therefore, we analyzed the EGFR and STAT3 proteins by Western blotting in the ovarian cancer xenografts from mice treated with NCX-4016. The results from tissue taken from tumors grown in vivo showed that NCX-4016 downregulated the expression of pEGFR (Tyr845, Tyr992, Tyr1045), pSTAT3 (Tyr705, Ser727), and pAkt (Ser473) in the tumor xenografts (Fig. 6). The in vivo results suggest that administration of NCX-4016 inhibits tumor-growth through the downregulation of EGFR, STAT3 and PI3K signaling in the cisplatin-resistant ovarian cancer xenografts in mice.
Figure 6.
Effect of NCX-4016 on EGFR/PI3K and STAT3 signaling in ovarian cancer xenograft tumors in mice. (A) Immunoblot images of tumor tissue lysates, obtained as in Fig. 5 and subjected to immunoblot analysis of EGFR/ PI3K and STAT3 signaling pathways. (B) Quantification. NCX-4016, at both 50 and 100 mg/kg doses, downregulated the phosphorylated EGFR, Akt and STAT3 without significant change in their total levels.
Discussion
The present study clearly demonstrated that treatment of cisplatin-resistant human ovarian carcinoma with NCX-4016 resulted in the inhibition of cell proliferation and tumor growth in a murine xenograft model. The results established the involvement of cell cycle arrest and apoptosis mediated by modulation of the Bcl-2 family proteins and downregulation of EGFR/PI3K and STAT3 phosphorylation, both in vitro and in vivo.
We have previously shown that NCX-4016 inhibited cell proliferation in both the cisplatin-sensitive and resistant cell lines.6 The present study indicated that the NCX-4016-induced cell-growth inhibition was due to the induction of G1 arrest mediated by modulation of G1 cell cycle-related proteins including p53, p21, cdk2 and cyclin D1. We further observed that NCX-4016 led to the down-regulation of EGFR/PI3K levels in CR cells. Indeed, the blockage of EGFR-signaling cascades seemingly increased the levels of p27 and p21 proteins, which in turn inhibited the CDK family proteins and repressed cellular proliferation by impairment of G1 phase progression and entry into S phase.37,38 It has been shown that blockage of EGFR cascades resulted in the inhibition of proliferation of prostate cancer cell lines via G1 arrest.39
Both PI3K and Akt, a serine/threonine protein kinase and downstream effecter of PI3K, are part of a signaling pathway that can be initiated by EGFR activation. Akt is frequently activated and/or overexpressed in ovarian cancer.40 A recent study showed that Akt-mediated chemoresistance in CR ovarian cancer cells might be critically dependent on the suppression of p53 function.8 We observed that the expression of p53 was significantly enhanced by NCX-4016 treatment, which is consistent with the fact that Akt negatively regulates the transcription of p53. There are also other reports that have demonstrated the activation of p53 and induction of cell cycle arrest by inhibition of Akt.41,42 Taken together, NCX-4016 induced G1 growth arrest via downregulating the phosphorylation of EGFR and Akt in CR cells.
Several mechanisms, including ROS production, increased p53 levels, loss of mitochondrial membrane potential and mitochondrial permeability transition, have been proposed to explain the induction of apoptosis by aspirin and NCX related compounds.43-46 In the present study we observed that the activation of Bax protein was the first proapoptotic event following drug exposure. The activation of Bax induced cytochrome c release, which was followed by caspase-9, caspase-7, caspase-3, and PARP cleavages. Since p53 can activate apoptotic cell death pathways by mechanisms that are both dependent47 and independent48 of its transactivation potential, we examined whether the p53 activation in response to NCX-4016 was required for the cytotoxic effects of NCX-4016. The results revealed that the NCX-4016-mediated induction of apoptosis was independent of the p53 status.
The anti-apoptotic protein Bcl-2 is associated with inhibition of apoptosis, whereas the increased expression of the pro-apoptotic protein, Bax, is associated with the induction of apoptosis. Rather than the expressions of individual proteins, the ratio of Bax:Bcl-2 is considered as the determining factor in the transmission of the apoptotic signal. We, therefore, calculated the ratio of these proteins from the data shown in Fig. 3B and C. We observed that the ratio Bax/Bcl-2 exhibited a significant dose-dependent increase both at 24-h (2-fold; p < 0.05) and 48-h (2.5-fold; p < 0.05) treatments. The results suggest that the ratio of the pro-apoptotic proteins to anti-apoptotic proteins is the critical determinant of the apoptotic fate of cells treated with NCX-4016.
Also it has been shown that Bax is activated by p53 via a transcription-dependent and/or transcription-independent mechanism.49 In addition, activated Akt can protect cells from apoptosis by suppressing (i) Bax translocation to the mitochondria50 and (ii) caspase-9 cleavage. Caspase-9 was reported to be directly phosphorylated by activated Akt and the pro-apoptotic function was suppressed.51 Our data, as shown in Fig. 2, indicated that NCX-4016 activated Bax expression and caspase-9 cleavage through the inhibition of activated Akt, thereby leading to cytochrome c release and apoptosis in CR cancer cells.
Our data also showed that NCX-4016 inhibits phospo-STAT3 activation in CR cancer cells. STAT3 has a wide variety of biological functions, including acceleration of cell proliferation and activation of antiapoptotic proteins such as Bcl-XL, Bcl-2, Mcl-1, c-Myc, and Survivin.52-54 STAT3 is constitutively activated in a variety of tumor cell types including CR ovarian cancer cells.55 The constitutively active STAT3 has been implicated in the induction of resistance to apoptosis, possibly through the expression of Bcl-2 and Bcl-XL.53 Bcl-XL, which is regulated by both STAT3 Tyr705 and Ser727, is known to be overexpressed in CR cells.34 Bcl-XL can inhibit cell death induced by a variety of chemotherapeutic agents, and expression of Bcl-XL has been correlated with chemoresistance in patients with ovarian cancer.11 Recently, the oncogenic transcription factor STAT3 has attracted much attention as a pharmacologic target.53 Several reports have demonstrated that inhibition of STAT3 by flavonoids and synthetic compounds results in tumor cell apoptosis in vitro.34,56,57 NCX-4016 inhibited EGFR, STAT3 and STAT3-regulated antiapoptotic genes such as Bcl-2 and Bcl-XL which contributed to the growth inhibition of xenograft tumors. In contrast, the increase in the Bax and decrease in Bcl-2 and Bcl-XL expressions by the NCX-4016-induced downregulation of EGFR/PI3K and STAT3 appear to contribute less to the apoptosis of xenograft ovarian tumors in vivo.
In conclusion, we report, for the first time, that NCX-4016 significantly inhibited tumor growth by inhibiting the activation of EGFR/PI3K and STAT3 which are normally overexpressed in cisplatin-resistant ovarian cancer. These effects on phosphorylated EGFR, Akt, and STAT3s may trigger apoptosis in cancer cells, although minimally in vivo, and/or inhibit tumor growth by upregulation of Bax and downregulation of cyclinD1, Bcl-2 and Bcl-XL.
Materials and Methods
Materials
NCX-4016 (2-(acetyloxy)benzoic acid 3-(nitrooxymethyl)phenyl ester) was obtained from NicOx (Sophia Antipolis, France). DMSO (dimethyl sulfoxide) and MTT powder (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) were obtained from Sigma (St. Louis, MO). Cell-culture medium (RPMI 1640), fetal bovine serum, antibiotics, sodium pyruvate, trypsin, and phosphate-buffered saline (PBS) were purchased from Gibco, BRL (Grand Island, NY). Polyvinylidene fluoride (PVDF) membrane and molecular weight markers were obtained from Bio-Rad (Hercules, CA). Antibodies for pEGFR (Tyr845, Tyr992, Tyr1065), EGFR, pSTAT3 (Tyr705 and Ser727), pAkt Ser473 and Thr308, poly-adenosine diphosphate ribose polymerase (PARP), and cleaved caspases-3, 7, 8 and 9, Bcl-2, Bcl-XL, Bax, Bak, and cytochorome c were purchased from Cell Signaling Technology (Beverly, MA). Antibodies for CycA, p53, p21, p27, cdk2, cdk4, cycD1, cycD2, and STAT3 were purchased from Santa Cruz (Santa Cruz, CA). Recombinant Protein G agarose and lipofectamine kits were purchased from Invitrogen (Carlsbad, CA). Enhanced chemiluminescence (ECL) reagents were obtained from Amersham Pharmacia Biotech (Buckinghamshire, UK).
Cell lines and cultures
The cisplatin-resistant human ovarian carcinoma cell line, A2780/cDDP, was used. The cell line was originally developed from an in vivo A2780 tumor model by treating with cisplatin.30 The cisplatin resistance of the cell line was confirmed by a cell-proliferation assay (MTT). Cells were grown in RPMI 1640 medium supplemented with 10% FBS, 2% sodium pyruvate, 1% penicillin and 1% streptomycin. Cells were grown in a 100-mm dish to 70% confluence at 37°C in an atmosphere of 5% CO2 and 95% air. Cells were routinely trypsinized (0.05% trypsin/EDTA) and counted using an automated counter (NucleoCounter, New Brunswick Scientific, Edison, NJ).
Analysis of cell cycle distribution and quantification of cell-death by flow cytometry
Cells were treated with NCX-4016 (100 μM) for 24 or 48 h and then trypsinized, washed in PBS and fixed in ice-cold 75% ethanol/PBS. The DNA was labeled with propidium iodide. Cells were sorted by flow-cytometry analysis, and cell cycle profiles were determined using a PC software (ModFit LT, Becton Dickinson, San Diego, CA).
Immunoblot analysis
Cells were grown in RPMI 1640 medium and treated with DMSO (control) or NCX-4016 (100 μM) for the desired times. Equal volumes of DMSO (0.1% v/v) were present in each treatment. Following NCX-4016 exposure, the cell lysates were prepared in nondenaturing lysis buffer (10 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 1 mM EGTA, 0.3 mM phenylmethylsulfonyl fluoride (PMSF), 0.2 mM sodium orthovanadate, 0.5% NP40, aprotinin (1 μg/ml) and leupetin (1 μg/ml). Cell lysates were centrifuged at 10,000 × g for 20 min at 4°C, and the supernatant was separated. The protein concentration in the lysates was determined using a Pierce detergent-compatible protein assay kit (Rockford, IL). For Western blotting, 25 to 50 μg of protein lysate per sample was denatured in 2x SDS-PAGE sample buffer and subjected to SDS-PAGE on a 10% or 12% tris-glycine gel. The separated proteins were transferred onto a PVDF membrane and then the membrane was blocked with 5% nonfat milk powder (w/v) in TBST (10 mM Tris, 100 mM NaCl, 0.1% Tween 20) for 1 h at room temperature or overnight at 4°C. The membranes were incubated with the primary antibodies described above. The bound antibodies were detected with horseradish peroxidase (HRP)-labeled sheep anti-mouse IgG or HRP-labeled donkey anti-rabbit IgG (Amersham Pharmacia Biotech) using an ECL detection system. The ECL image was digitized using a scanner (ScanJet 7400c, Hewlett-Packard, Palo Alto, CA) and quantified using a densitometry software (Scion Image v0.4.0.2, Scion, Frederick, MD).
Ovarian cancer tumor xenografts in mice
Cultured CR cancer cells (5×106 cells in 60 μl of PBS) were subcutaneously (s.c.) injected into the back of 6-week-old BALB/c nude mice from the National Cancer Institute. On the 5th day when the tumor size reached approximately 2–4 mm, the mice were injected (i.p.) with 50 or 100 mg/kg NCX-4016, daily. The control group received vehicle only. The doses were chosen based on an initial dose-response study optimized to produce an observable effect on tumor growth. The size of the tumor was measured three times per week using a digital Vernier caliper. The tumor volume was determined from the orthogonal dimensions (d1, d2, d3) using the formula (d1x d2 x d3) π/6. Nineteen days after the beginning of NCX-4016 treatment, the mice were sacrificed, and the tumors were resected. The tumor tissues were then subjected to immunoblotting assay.
Statistical analysis
All data were expressed as mean ± SE. Comparisons among groups were performed using a Student's t-test. The significance level was set at p < 0.05.
Acknowledgements
We acknowledge the financial support from NIH grant CA102264.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Parmar MK, Ledermann JA, Colombo N, du Bois A, Delaloye JF, Kristensen GB, Wheeler S, Swart AM, Qian W, Torri V, Floriani I, Jayson G, Lamont A, Trope C. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099–106. doi: 10.1016/s0140-6736(03)13718-x. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal R, Linch M, Kaye SB. Novel therapeutic agents in ovarian cancer. Eur J Surg Oncol. 2006;32:875–86. doi: 10.1016/j.ejso.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 4.Brewer CA, Blessing JA, Nagourney RA, Morgan M, Hanjani P. Cisplatin plus gemcitabine in platinum-refractory ovarian or primary peritoneal cancer: a phase II study of the Gynecologic Oncology Group. Gynecol Oncol. 2006;103:446–50. doi: 10.1016/j.ygyno.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 5.Pani E, Stojic L, El-Shemerly M, Jiricny J, Ferrari S. Mismatch repair status and the response of human cells to cisplatin. Cell Cycle. 2007;6:1796–802. doi: 10.4161/cc.6.14.4472. [DOI] [PubMed] [Google Scholar]
- 6.Bratasz A, Weir NM, Parinandi NL, Zweier JL, Sridhar R, Ignarro LJ, Kuppusamy P. Reversal to cisplatin sensitivity in recurrent human ovarian cancer cells by NCX-4016, a nitro derivative of aspirin. Proc Natl Acad Sci USA. 2006;103:3914–9. doi: 10.1073/pnas.0511250103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez RP, Hamilton TC, Ozols RF, Young RC. Mechanisms and modulation of resistance to chemotherapy in ovarian cancer. Cancer. 1993;71:1571–80. doi: 10.1002/cncr.2820710424. [DOI] [PubMed] [Google Scholar]
- 8.Yang X, Fraser M, Moll UM, Basak A, Tsang BK. Akt-mediated cisplatin resistance in ovarian cancer: modulation of p53 action on caspase-dependent mitochondrial death pathway. Cancer Res. 2006;66:3126–36. doi: 10.1158/0008-5472.CAN-05-0425. [DOI] [PubMed] [Google Scholar]
- 9.Henkels KM, Turchi JJ. Induction of apoptosis in cisplatin-sensitive and -resistant human ovarian cancer cell lines. Cancer Res. 1997;57:4488–92. [PubMed] [Google Scholar]
- 10.Coley HM, Shotton CF, Ajose-Adeogun A, Modjtahedi H, Thomas H. Receptor tyrosine kinase (RTK) inhibition is effective in chemosensitising EGFR-expressing drug resistant human ovarian cancer cell lines when used in combination with cytotoxic agents. Biochem Pharmacol. 2006;72:941–8. doi: 10.1016/j.bcp.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 11.Duan Z, Foster R, Bell DA, Mahoney J, Wolak K, Vaidya A, Hampel C, Lee H, Seiden MV. Signal transducers and activators of transcription 3 pathway activation in drug-resistant ovarian cancer. Clin Cancer Res. 2006;12:5055–63. doi: 10.1158/1078-0432.CCR-06-0861. [DOI] [PubMed] [Google Scholar]
- 12.Lo HW, Hsu SC, Ali-Seyed M, Gunduz M, Xia W, Wei Y, Bartholomeusz G, Shih JY, Hung MC. Nuclear interaction of EGFR and STAT3 in the activation of the iNOS/NO pathway. Cancer Cell. 2005;7:575–89. doi: 10.1016/j.ccr.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Park OK, Schaefer TS, Nathans D. In vitro activation of Stat3 by epidermal growth factor receptor kinase. Proc Natl Acad Sci USA. 1996;93:13704–8. doi: 10.1073/pnas.93.24.13704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craven RJ, Lightfoot H, Cance WG. A decade of tyrosine kinases: from gene discovery to therapeutics. Surg Oncol. 2003;12:39–49. doi: 10.1016/s0960-7404(03)00004-5. [DOI] [PubMed] [Google Scholar]
- 15.Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- 16.Woodworth CD, Michael E, Marker D, Allen S, Smith L, Nees M. Inhibition of the epidermal growth factor receptor increases expression of genes that stimulate inflammation, apoptosis, and cell attachment. Mol Cancer Ther. 2005;4:650–8. doi: 10.1158/1535-7163.MCT-04-0238. [DOI] [PubMed] [Google Scholar]
- 17.Ariyama H, Qin B, Baba E, Tanaka R, Mitsugi K, Harada M, Nakano S. Gefitinib, a selective EGFR tyrosine kinase inhibitor, induces apoptosis through activation of Bax in human gallbladder adenocarcinoma cells. J Cell Biochem. 2006;97:724–34. doi: 10.1002/jcb.20678. [DOI] [PubMed] [Google Scholar]
- 18.Helfrich BA, Raben D, Varella-Garcia M, Gustafson D, Chan DC, Bemis L, Coldren C, Baron A, Zeng C, Franklin WA, Hirsch FR, Gazdar A, Minna J, Bunn PA., Jr Antitumor activity of the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor gefitinib (ZD1839, Iressa) in non-small cell lung cancer cell lines correlates with gene copy number and EGFR mutations but not EGFR protein levels. Clin Cancer Res. 2006;12:7117–25. doi: 10.1158/1078-0432.CCR-06-0760. [DOI] [PubMed] [Google Scholar]
- 19.Bild AH, Turkson J, Jove R. Cytoplasmic transport of Stat3 by receptor-mediated endocytosis. Embo J. 2002;21:3255–63. doi: 10.1093/emboj/cdf351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choudhari SR, Khan MA, Harris G, Picker D, Jacob GS, Block T, Shailubhai K. Deactivation of Akt and STAT3 signaling promotes apoptosis, inhibits proliferation, and enhances the sensitivity of hepatocellular carcinoma cells to an anticancer agent, Atiprimod. Mol Cancer Ther. 2007;6:112–21. doi: 10.1158/1535-7163.MCT-06-0561. [DOI] [PubMed] [Google Scholar]
- 21.Torroglosa A, Murillo-Carretero M, Romero-Grimaldi C, Matarredona ER, Campos-Caro A, Estrada C. Nitric oxide decreases subventricular zone stem cell proliferation by inhibition of epidermal growth factor receptor and phosphoinositide-3-kinase/Akt pathway. Stem Cells. 2007;25:88–97. doi: 10.1634/stemcells.2006-0131. [DOI] [PubMed] [Google Scholar]
- 22.Liu B, Neufeld AH. Activation of epidermal growth factor receptor signals induction of nitric oxide synthase-2 in human optic nerve head astrocytes in glaucomatous optic neuropathy. Neurobiol Dis. 2003;13:109–23. doi: 10.1016/s0969-9961(03)00010-x. [DOI] [PubMed] [Google Scholar]
- 23.Gallo O, Fabbroni V, Sardi I, Magnelli L, Boddi V, Franchi A. Correlation between nitric oxide and cyclooxygenase-2 pathways in head and neck squamous cell carcinomas. Biochem Biophys Res Commun. 2002;299:517–24. doi: 10.1016/s0006-291x(02)02683-9. [DOI] [PubMed] [Google Scholar]
- 24.Wang T, FitzGerald TJ, Haregewoin A. Differential expression of nitric oxide synthases in EGF-responsive mouse neural precursor cells. Cell Tissue Res. 1999;296:489–97. doi: 10.1007/s004410051309. [DOI] [PubMed] [Google Scholar]
- 25.Rieder J, Jahnke R, Schloesser M, Seibel M, Czechowski M, Marth C, Hoffmann G. Nitric oxide-dependent apoptosis in ovarian carcinoma cell lines. Gynecol Oncol. 2001;82:172–6. doi: 10.1006/gyno.2001.6242. [DOI] [PubMed] [Google Scholar]
- 26.Wink DA, Cook JA, Christodoulou D, Krishna MC, Pacelli R, Kim S, DeGraff W, Gamson J, Vodovotz Y, Russo A, Mitchell JB. Nitric oxide and some nitric oxide donor compounds enhance the cytotoxicity of cisplatin. Nitric Oxide. 1997;1:88–94. doi: 10.1006/niox.1996.0108. [DOI] [PubMed] [Google Scholar]
- 27.Ignarro LJ, Buga GM, Wei LH, Bauer PM, Wu G, del Soldato P. Role of the arginine-nitric oxide pathway in the regulation of vascular smooth muscle cell proliferation. Proc Natl Acad Sci USA. 2001;98:4202–8. doi: 10.1073/pnas.071054698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Sun H, Li C. Nitric oxide induces promyelocytic cell growth arrest and apoptosis through deactivation of Akt pathway. Leuk Res. 2007;31:653–60. doi: 10.1016/j.leukres.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 29.Turchi JJ. Nitric oxide and cisplatin resistance: NO easy answers. Proc Natl Acad Sci USA. 2006;103:4337–8. doi: 10.1073/pnas.0601001103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andrews PA, Jones JA, Varki NM, Howell SB. Rapid emergence of acquired cis-diammin edichloroplatinum(II) resistance in an in vivo model of human ovarian carcinoma. Cancer Commun. 1990;2:93–100. doi: 10.3727/095535490820874641. [DOI] [PubMed] [Google Scholar]
- 31.Abeysinghe RD, Greene BT, Haynes R, Willingham MC, Turner J, Planalp RP, Brechbiel MW, Torti FM, Torti SV. p53-independent apoptosis mediated by tachpyridine, an anti-cancer iron chelator. Carcinogenesis. 2001;22:1607–14. doi: 10.1093/carcin/22.10.1607. [DOI] [PubMed] [Google Scholar]
- 32.Kojima K, Konopleva M, Samudio IJ, Ruvolo V, Andreeff M. Mitogen-activated protein kinase kinase inhibition enhances nuclear proapoptotic function of p53 in acute myelogenous leukemia cells. Cancer Res. 2007;67:3210–9. doi: 10.1158/0008-5472.CAN-06-2712. [DOI] [PubMed] [Google Scholar]
- 33.Alli E, Yang JM, Ford JM, Hait WN. Reversal of stathmin-mediated resistance to paclitaxel and vinblastine in human breast carcinoma cells. Mol Pharmacol. 2007;71:1233–40. doi: 10.1124/mol.106.029702. [DOI] [PubMed] [Google Scholar]
- 34.Alas S, Bonavida B. Inhibition of constitutive STAT3 activity sensitizes resistant non-Hodgkin's lymphoma and multiple myeloma to chemotherapeutic drug-mediated apoptosis. Clin Cancer Res. 2003;9:316–26. [PubMed] [Google Scholar]
- 35.Arteaga C. Targeting HER1/EGFR: a molecular approach to cancer therapy. Semin Oncol. 2003;30:3–14. [PubMed] [Google Scholar]
- 36.Danial NN, Pernis A, Rothman PB. Jak-STAT signaling induced by the v-abl oncogene. Science. 1995;269:1875–7. doi: 10.1126/science.7569929. [DOI] [PubMed] [Google Scholar]
- 37.Zhu XF, Wang JS, Cai LL, Zeng YX, Yang D. SUCI02 inhibits the erbB-2 tyrosine kinase receptor signaling pathway and arrests the cell cycle in G1 phase in breast cancer cells. Cancer Sci. 2006;97:84–9. doi: 10.1111/j.1349-7006.2006.00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weir NM, Selvendiran K, Kutala VK, Tong L, Vishwanath S, Rajaram M, Tridandapani S, Anant S, Kuppusamy P. Curcumin Induces G(2)/M Arrest and Apoptosis in Cisplatin-Resistant Human Ovarian Cancer Cells by Modulating Akt and p38 MAPK. Cancer Biol Ther. 2007;6:178–84. doi: 10.4161/cbt.6.2.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peng D, Fan Z, Lu Y, DeBlasio T, Scher H, Mendelsohn J. Anti-epidermal growth factor receptor monoclonal antibody 225 up-regulates p27KIP1 and induces G1 arrest in prostatic cancer cell line DU145. Cancer Res. 1996;56:3666–9. [PubMed] [Google Scholar]
- 40.Meng Q, Xia C, Fang J, Rojanasakul Y, Jiang BH. Role of PI3K and AKT specific isoforms in ovarian cancer cell migration, invasion and proliferation through the p70S6K1 pathway. Cell Signal. 2006;18:2262–71. doi: 10.1016/j.cellsig.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 41.Singh RP, Dhanalakshmi S, Agarwal C, Agarwal R. Silibinin strongly inhibits growth and survival of human endothelial cells via cell cycle arrest and downregulation of survivin, Akt and NF-kappaB: implications for angioprevention and antiangiogenic therapy. Oncogene. 2005;24:1188–202. doi: 10.1038/sj.onc.1208276. [DOI] [PubMed] [Google Scholar]
- 42.Skladanowski A, Bozko P, Sabisz M, Larsen AK. Dual Inhibition of PI3K/Akt Signaling and the DNA Damage Checkpoint in p53-Deficient Cells with Strong Survival Signaling: Implications for Cancer Therapy. Cell Cycle. 2007:6. doi: 10.4161/cc.6.18.4705. [DOI] [PubMed] [Google Scholar]
- 43.Konturek PC, Kania J, Burnat G, Hahn EG. NO-releasing aspirin exerts stronger growth inhibitory effect on Barrett's adenocarcinoma cells than traditional aspirin. J Physiol Pharmacol. 2006;12:15–24. [PubMed] [Google Scholar]
- 44.Rao CV, Reddy BS, Steele VE, Wang CX, Liu X, Ouyang N, Patlolla JM, Simi B, Kopelovich L, Rigas B. Nitric oxide-releasing aspirin and indomethacin are potent inhibitors against colon cancer in azoxymethane-treated rats: effects on molecular targets. Mol Cancer Ther. 2006;5:1530–8. doi: 10.1158/1535-7163.MCT-06-0061. [DOI] [PubMed] [Google Scholar]
- 45.Tesei A, Ricotti L, Ulivi P, Medri L, Amadori D, Zoli W. NCX 4016, a nitric oxide-releasing aspirin derivative, exhibits a significant antiproliferative effect and alters cell cycle progression in human colon adenocarcinoma cell lines. Int J Oncol. 2003;22:1297–302. [PubMed] [Google Scholar]
- 46.Tanaka T, Kurose A, Halicka HD, Huang X, Traganos F, Darzynkiewicz Z. Nitrogen oxide-releasing aspirin induces histone H2AX phosphorylation, ATM activation and apoptosis preferentially in S-phase cells: involvement of reactive oxygen species. Cell Cycle. 2006;5:1669–74. doi: 10.4161/cc.5.15.3100. [DOI] [PubMed] [Google Scholar]
- 47.Fisher DE. Apoptosis in cancer therapy: crossing the threshold. Cell. 1994;78:539–42. doi: 10.1016/0092-8674(94)90518-5. [DOI] [PubMed] [Google Scholar]
- 48.Willingham MC. Cytochemical methods for the detection of apoptosis. J Histochem Cytochem. 1999;47:1101–10. doi: 10.1177/002215549904700901. [DOI] [PubMed] [Google Scholar]
- 49.Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–4. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 50.Tsuruta F, Masuyama N, Gotoh Y. The phosphatidylinositol 3-kinase (PI3K)-Akt pathway suppresses Bax translocation to mitochondria. J Biol Chem. 2002;277:14040–7. doi: 10.1074/jbc.M108975200. [DOI] [PubMed] [Google Scholar]
- 51.Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–21. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 52.Bhardwaj A, Sethi G, Vadhan-Raj S, Bueso-Ramos C, Takada Y, Gaur U, Nair AS, Shishodia S, Aggarwal BB. Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down-regulation of STAT3 and nuclear factor-kappaB-regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Blood. 2007;109:2293–302. doi: 10.1182/blood-2006-02-003988. [DOI] [PubMed] [Google Scholar]
- 53.Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 54.Kojima K, Konopleva M, Samudio IJ, Schober WD, Bornmann WG, Andreeff M. Concomitant inhibition of MDM2 and Bcl-2 protein function synergistically induce mitochondrial apoptosis in AML. Cell Cycle. 2006;5:2778–86. doi: 10.4161/cc.5.23.3520. [DOI] [PubMed] [Google Scholar]
- 55.Rosen DG, Mercado-Uribe I, Yang G, Bast RC, Jr., Amin HM, Lai R, Liu J. The role of constitutively active signal transducer and activator of transcription 3 in ovarian tumorigenesis and prognosis. Cancer. 2006;107:2730–40. doi: 10.1002/cncr.22293. [DOI] [PubMed] [Google Scholar]
- 56.Nielsen M, Kaestel CG, Eriksen KW, Woetmann A, Stokkedal T, Kaltoft K, Geisler C, Ropke C, Odum N. Inhibition of constitutively activated Stat3 correlates with altered Bcl-2/Bax expression and induction of apoptosis in mycosis fungoides tumor cells. Leukemia. 1999;13:735–8. doi: 10.1038/sj.leu.2401415. [DOI] [PubMed] [Google Scholar]
- 57.Selvendiran K, Koga H, Ueno T, Yoshida T, Maeyama M, Torimura T, Yano H, Kojiro M, Sata M. Luteolin promotes degradation in signal transducer and activator of transcription 3 in human hepatoma cells: an implication for the antitumor potential of flavonoids. Cancer Res. 2006;66:4826–34. doi: 10.1158/0008-5472.CAN-05-4062. [DOI] [PubMed] [Google Scholar]