Abstract
The Devonian marks a critical stage in the early evolution of vertebrates: It opens with an unprecedented diversity of fishes and closes with the earliest evidence of limbed tetrapods. However, the latter part of the Devonian has also been characterized as a period of global biotic crisis marked by two large extinction pulses: a “Big Five” mass extinction event at the Frasnian-Famennian stage boundary (374 Ma) and the less well-documented Hangenberg event some 15 million years later at the Devonian-Carboniferous boundary (359 Ma). Here, we report the results of a wide-ranging analysis of the impact of these events on early vertebrate evolution, which was obtained from a database of vertebrate occurrences sampling over 1,250 taxa from 66 localities spanning Givetian to Serpukhovian stages (391 to 318 Ma). We show that major vertebrate clades suffered acute and systematic effects centered on the Hangenberg extinction involving long-term losses of over 50% of diversity and the restructuring of vertebrate ecosystems worldwide. Marine and nonmarine faunas were equally affected, precluding the existence of environmental refugia. The subsequent recovery of previously diverse groups (including placoderms, sarcopterygian fish, and acanthodians) was minimal. Tetrapods, actinopterygians, and chondrichthyans, all scarce within the Devonian, undergo large diversification events in the aftermath of the extinction, dominating all subsequent faunas. The Hangenberg event represents a previously unrecognized bottleneck in the evolutionary history of vertebrates as a whole and a historical contingency that shaped the roots of modern biodiversity.
Keywords: gnathostome, Hangenberg, macroevolution, paleontology, Romer’s gap
The rise of jawed vertebrates (gnathostomes) throughout the Devonian (416 to 359 Ma) (1) and into the post-Devonian is one of the key episodes in vertebrate evolution (2, 3). This interval encompasses well-known early diversification events, including those of Osteichthyes (bony fishes: ray-finned Actinopterygii and lobe-finned Sarcopterygii, including tetrapods), Chondrichthyes (cartilaginous fishes: Elasmobranchii and Holocephalii), and Placodermi and Acanthodii (extinct groups of debated affinity to extant gnathostomes) (2–5). What is less well known, although apparent from cursory surveys, is that the gnathostome biota underwent major changes over the Devonian-Mississippian divide (3, 5). Placoderms, sarcopterygians, and acanthodians are replaced by chondrichthyans, actinopterygians, and tetrapods, occupying a wider range of ecological roles and dominating all succeeding biotas (3, 5, 6). This faunal transformation has been subjected to few analyses, and explanations have tended to focus on gradual replacement (2, 7) and competitive displacement (8).
However, the Devonian-Mississippian vertebrate succession also fits a characteristic pattern of large-scale mass extinction and subsequent refilling of niche space (9). The impact of global events on biodiversity has been established by studies of dinosaur-mammal turnover at the end-Cretaceous (3, 5, 9) and the decimation and restructuring of tetrapod ecosystems across the Permo-Triassic transition (10). Notably, one of the “Big Five” mass extinctions (11) occurred within the Late Devonian: the Kellwasser event of the Frasnian-Famennian stage boundary (374 Ma) (1, 12). This is associated with spectacular losses in marine diversity involving ∼13–40% of families and ∼50–60% of genera (11, 12). Furthermore, the Kellwasser event is followed by the apparently minor (11–14) Hangenberg event of the Devonian-Mississippian boundary (359 Ma) (1). This, too, has been linked to extinction on a global scale (13, 14) and, more recently, to climatic changes comparable to the Pleistocene ice ages (15).
Results
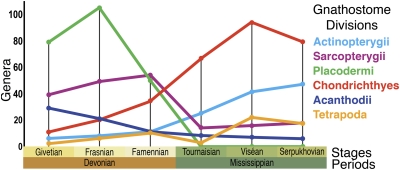
Key questions about vertebrate evolution in the Late Devonian concern the timing of the faunal turnover, the magnitude of extinction pulses (if any), and consequences on the ensuing development of the vertebrate biota. To these ends, we have constructed a dataset of gnathostome occurrences covering critical stages from the Givetian of the Devonian to the Serpukhovian of the Mississippian (391 to 318 Ma) (1) (Fig. 1 and SI Appendix). These data capture genera and species from all gnathostome divisions and environments: marine and nonmarine. Nonmarine is defined here as all environments with freshwater influence, including brackish and marginal marine. Diversity curves generated from the 1,019 genus-level entries (Fig. 1 and SI Appendix) do show large-scale turnover among vertebrate divisions between the Frasnian (385 to 374 Ma) (1) and Viséan (345 to 326 Ma) (1): the interval containing both the Kellwasser extinction (marked by a relatively small 19% loss in genus-level diversity) and the Hangenberg event (marked by an unexpectedly larger 32% loss in genus-level diversity).
Fig. 1.
Gnathostome genus level diversity curves for the Givetian to Serpukhovian (n = 1,018) (SI Appendix). Tetrapoda is defined here as all taxa closer to crown Tetrapoda than Rhizodontida based on the tree in the article by Coates et al. (31). This includes elpistostegalians. All other sarcopterygians are referred to Sarcopterygii.
Previously, curve-based techniques such as rarefaction and extinction rate calculations have been employed to investigate small-scale datasets of Devonian vertebrates, and these studies have linked losses among higher level marine placoderm groups to the Kellwasser event (12, 16). However, such techniques are highly sensitive to factors that include rock volume in the geological record, sample size bias, taxonomic assignment, and bin size (17, 18). These problems are illustrated by the peak in placoderm taxa during the Frasnian (Fig. 1), something mostly attributable to the existence of well-known Lagerstätten within that stage (e.g., Bad Wildungen and Gogo) (Fig. 2). Both of these localities are remote from any extinction pulses. In addition, the vast majority of Devonian and Mississippian vertebrate species are known from single localities within single intervals, a phenomenon sure to produce artificially high extinction and origination rates over any arbitrary interval (19). This precludes further investigation of the impact of the Devonian extinction events on gnathostomes using standard diversity curve methods.
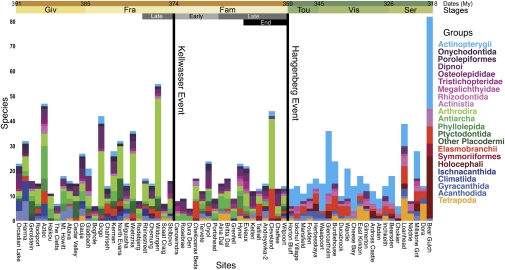
Fig. 2.
Histogram of species-level faunal composition for 66 well-sampled macrofossil localities (n = 1,267) (Dataset S1). Localities are arranged temporally, although some are concurrent. Restricted intervals used in some analyses are noted above the time scale. End-Devonian sites are from formations conformable to Hangenberg sediments and are treated as contemporaneous. The Hangenberg and Kellwasser events are represented by black lines at the relevant stage boundaries.
Here, we use a suite of ecological methods that focus on higher level faunal composition as an alternative and, arguably, more effective means of characterizing change through geological time and its causes (18) (Dataset S1, Dataset S2, and SI Appendix). A sudden break in worldwide biotic composition centered on a particular horizon, resulting in statistically significant and visually apparent differentiation of pre- and postextinction faunas, would be critical evidence for a Big Five magnitude mass extinction event. Significantly, just such a characteristic break coincides with the Hangenberg event at the Devonian-Mississippian boundary in a simple histogram of faunas (Fig. 2), whereas few differences in faunal composition are observed between Frasnian and Famennian sites bracketing the Kellwasser horizon. Every ecological ordination analysis applied to the total matrix [cluster analysis, canonical correspondence analysis (CCA), nonparametric multidimensional scaling (NMDS), and factor analysis (FA)] shows the same basic pattern (SI Appendix). Devonian faunas are distinct from Mississippian faunas, including those at sites conformable with Hangenberg event shales in the end-Famennian (e.g., Cleveland Shale) and earliest Tournaisian (e.g., Horton Bluff) (Figs. 3 and 4 and SI Appendix), whereas pre- and post-Kellwasser ecosystems overlap.
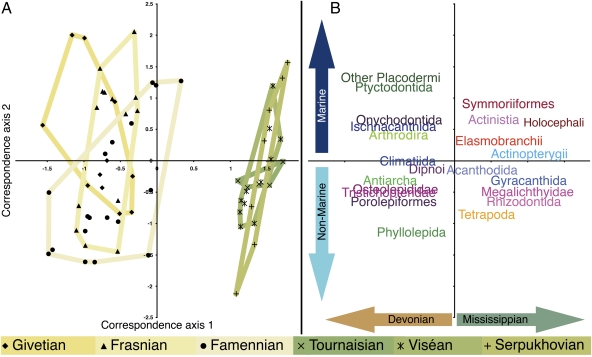
Fig. 3.
Faunal comparison using CCA of the later Devonian and Mississippian sites and taxa. Data were not grouped a priori by either stage or environment. (A) Ordination of sites along the first two correspondence axes based on raw diversity of taxa (n = 66). Polygon colors adhere to the RGB color code of the Commission for the Geological Map of the World. (B) Ordination of taxonomic groups (n = 22) and representation of gradients determining placement of sites along the axes. The first correspondence axis represents a temporal gradient between the Devonian and Mississippian, whereas the second axis corresponds to relative salinity.
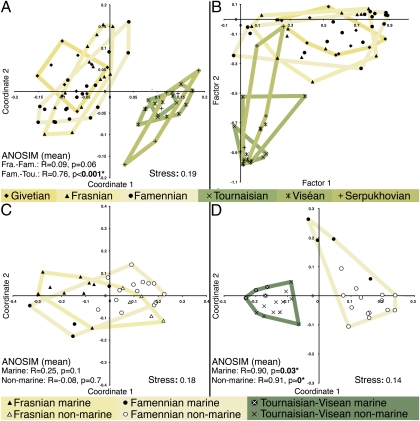
Fig. 4.
Faunal comparisons. (A) NMDS for all sites based on raw diversity and Bray–Curtis distances (n = 66). (B) FA plot for all sites based on raw diversity (n = 66). Factor 1 (39.68% of variance) is positively correlated with the Devonian nonmarine fauna presented in the CCA results (Fig. 2B and SI Appendix). Factor 2 (22.10% of variance) is negatively correlated with members of Mississippian fauna (SI Appendix). (C) NMDS of Frasnian and Famennian sites based on relative diversity and Bray–Curtis distance (n = 32). (D) NMDS of Famennian and Tournaisian-Viséan sites based on relative diversity and Bray–Curtis distance (n = 34).
CCA plots (Fig. 3 and SI Appendix) visualize transformation in pre- and postextinction ecosystem composition without a priori grouping by either environment or time interval (18). Therefore, faunal patterns shown in the results of CCA are unaffected by binning, stage length, or problematical environmental attribution. As mentioned above, in Fig. 3A, a major gap is shown along axis 1 between all Devonian and all Mississippian localities, including those immediately surrounding the period boundary (SI Appendix). This again pinpoints a major break in faunal composition to the short time span separating the latest Famennian site and the earliest Tournaisian fauna, an interval marked by the Hangenberg extinction. In contrast, clusters of localities from all stages of the Devonian overlap, as do all those from Mississippian. This precludes the existence of equivalent or even lesser turnover associated with any other stage boundary or extinction event. Consistent with these results, nonmarine faunas (as assigned by CCA along correspondence axis 2) of the Mississippian and Devonian are just as mutually disparate as the marine faunas, suggesting that large-scale turnover occurred in all environments (Fig. 3A).
No vertebrate division included in the correspondence analysis is distributed without significant change across the Devonian and Mississippian (Fig. 3B and SI Appendix). All sarcopterygian and acanthodian groups are restricted to one period or the other, indicating that even surviving divisions formerly abundant in the Devonian underwent comprehensive turnover. Meanwhile, taxa that dominate modern vertebrate faunas (tetrapods, actinopterygians, and chondrichthyans) (6) are precisely those that are associated with post-Devonian sites. Late Devonian and Mississippian vertebrate assemblages are recognizably and mutually distinct. Devonian and Mississippian sites also show great differentiation in taxonomic composition along a marine-nonmarine gradient (Fig. 3A, axis 2), suggesting that vertebrate assemblages could be used as crude proxies for environment and interval in future studies. The Tournaisian represents a notable exception: All sites are tightly clustered about the origin on axis 2, despite considerable geographical and environmental differentiation (Fig. 3A). This might indicate the presence of a homogeneous worldwide fauna during the recovery interval following the Hangenberg extinction.
Our results indicate a massive turnover in the vertebrate biota limited to the Hangenberg sediments between the latest Devonian and earliest Mississippian sites. Analysis of similarity (ANOSIM) performed using raw diversity data and seven distinct similarity metrics (18) generated high R statistics (measuring faunal similarity on a scale up to 1, where 0 = highly similar and 1 = highly dissimilar) and significant P values (α = 0.05) for comparisons between all Famennian and all Tournaisian sites (mean R = 0.76, mean P < 0.001*) (SI Appendix). These same analyses failed to differentiate between Frasnian and Famennian vertebrate faunas (mean R = 0.09, mean P = 0.06) (SI Appendix). The same pattern was observed in comparisons of nonadjacent stages around the Hangenberg (Famennian-Viséan: mean R = 0.77, mean P < 0* in 1 million; Frasnian-Tournaisian: mean R = 0.75, mean P < 0.001*) and Kellwasser (Givetian-Famennian: mean R = 0.11, mean P = 0.06). In addition, little faunal change was detected at other stage boundaries, including those associated with other more obscure events such as the Taghanic (Givetian-Frasnian: mean R = −0.02, mean P = 0.6) and early Serpukhovian (mean R = 0.05, P = 0.3), which have at other times also been promoted as possible first-order extinction events (14). Although similarity percentage (SIMPER) results show only relative increases and decreases of various environmentally localized taxa between the Frasnian and Famennian, the Devonian-Mississippian transition involved the total loss of 44% of higher level mostly monophyletic groups (SI Appendix). These results show that the vertebrate turnover associated with the Hangenberg extinction is highly anomalous compared with both background rates and other events during the later Devonian and Mississippian.
To examine the possibility of gradual change between faunas, and to characterize more fully the environmental extent of these extinctions, we analyzed subsets of the site matrix. These consisted of marine and nonmarine localities from restricted intervals adjacent to the Kellwasser and Hangenberg events (SI Appendix). As in the CCA results (Fig. 3A), NMDS plots of Frasnian and Famennian sites overlap in all spaces, with marine faunas, the supposed locus of the extinction (2, 12, 16), displaying no significant difference across the stage boundary (ANOSIM: mean R = 0.25, mean P = 0.1) (Fig. 4C and SI Appendix). In contrast to previous hypotheses (12, 16), SIMPER analyses revealed that arthrodire placoderm diversity remains stable, with marine faunal changes spanning the Kellwasser largely driven by an apparent increase in chondrichthyans and the absence of primarily nonmarine taxa (Fig. 3B and SI Appendix).
Complements of nonmarine vertebrates in both stages are notably similar, despite geographic differences (ANOSIM: mean R = −0.08, mean P = 0.7) (SI Appendix). Comparisons of values generated from abundance (marine: mean R = 0.22, mean P = 0.08; nonmarine: mean R = −0.01, P = 0.5) and presence-absence distances (ANOSIM marine: mean R = 0.27, mean P = 0.1; nonmarine: R = −0.13, mean P = 0.8) show that dissimilarity between Frasnian and Famennian ecosystems depends mostly on changes to relative diversity rather than extinction (SI Appendix). When latest Frasnian and earliest Famennian sites are considered alone, faunal similarity is even greater than that for the longer two-stage interval (ANOSIM: mean R = 0.19, mean P = 0.1) (SI Appendix) but less than the average for the individual environments. SIMPER results show that although the per site diversity of certain marine taxa such as placoderms decreases by two-thirds over the boundary, primarily nonmarine taxa such as antiarchs increase by the same amount (SI Appendix). This pattern is similar to that seen in the SIMPER comparison of entire stages around the extinction (SI Appendix). This suggests that gradual factors and environmental sampling are responsible for observed diversity changes around the Kellwasser event.
In contrast to the Frasnian-Famennian interval, large interfaunal gaps are revealed by temporally restricted comparisons of faunas from the Tournaisian and both the late Famennian (ANOSIM: mean R = 0.71, mean P < 0.001*) (Fig. 4D and SI Appendix) and end-Famennian conformable with the Hangenberg horizon (ANOSIM: mean R = 0.64, mean P = 0.002*). Accordingly, the time period for complete faunal turnover is confined to an interval between the start of the extinction event and the earliest Tournaisian locality. This corresponds closely to sea level changes and glaciation associated with the Hangenberg (15). As suggested by the CCA results, acute turnover is characteristic of both marine realms (ANOSIM: mean R = 0.90, mean P = 0.03*) and nonmarine realms (ANOSIM: mean R = 0.91, mean P ≈ 0* in 1 million permutations) (Fig. 4D and SI Appendix). SIMPER results show that every taxonomic group included in these analyses was present at a minimum of one locality from the end-Devonian and that arthrodires remained the most diverse component of the global vertebrate fauna right up to the event horizon (SI Appendix). Therefore, the sudden and complete loss of 44% of higher taxa in both marine and nonmarine environments between the latest Devonian and earliest Tournaisian faunas must be coincident with the Hangenberg event (SI Appendix).
Discussion
In light of the results, we conclude that the end-Devonian Hangenberg event was a first-order magnitude extinction for jawed vertebrates comparable to Big Five events, including the end-Cretaceous and end-Permian (11). Furthermore, the Hangenberg extinction was a global phenomenon, affecting all ecosystems no matter how the data are analyzed. Taxa primarily associated with nonmarine environments in the CCA results (Fig. 3B), including tetrapods as well as the more widespread and abundant antiarch placoderms and porolepiform sarcopterygians (Fig. 3B), were as likely to succumb as their marine contemporaries. In contrast, we find the original Big Five Frasnian-Famennian Kellwasser event to be of minor significance with no major losses (SI Appendix). For vertebrates at least, the Kellwasser event resembles an instance of “backsmearing” (20) caused by insufficient sampling of Famennian marine localities relative to those of the Frasnian (four vs. nine; SI Appendix). This sampling bias might be linked to observed losses in reef habitats (12). However, any coincident change in invertebrate marine biotas seems, thus far, to have had unexpectedly little effect on the vertebrates.
If the hypothesized acute Hangenberg extinction is correct, then “Romer's gap” (21), a widely discussed hiatus in the earliest post-Devonian tetrapod fossil record, emerges as a postextinction trough—a lull in abundance and diversity that can last for millions of years after such an event (10, 22). As noted above, Tournaisian sites exhibit low disparity in all ordination plots despite their large geographical spread (Figs. 3A and 4 and SI Appendix). This implies the existence of a characteristic recovery fauna (10, 12, 22): a worldwide depauperate assemblage of, in this case, vertebrate survivors. Corresponding lack of Tournaisian material from other terrestrial groups (e.g., insects) supports this conclusion (23), and Devonian levels of faunal disparity are only regained in the Serpukhovian (Figs. 3A and 4 and SI Appendix). Even late Mississippian assemblages of vertebrate species from both sides of the newly formed Pangaea are strikingly similar (e.g., Bearsden and Bear Gulch localities; SI Appendix), likewise for associated invertebrate assemblages (24).
Once again, these patterns are consistent with long-term consequences of an extraordinary faunal bottleneck. This is further corroborated by the contingent distribution of traits in surviving groups: Extinctions remove characters from the pool of varying morphologies (25). For example, digit number is known to be variable among late Famennian tetrapods (26) but stabilizes with a maximum limit of five among all later forms.
There is some indication that even the acute and pervasive signal of end-Devonian vertebrate extinction obtained here represents a conservative estimate of the Hangenberg's true impact. Most of the vertebrate survivors, aside from actinopterygians, chondrichthyans, and tetrapods, persisted only into the late Paleozoic. Moreover, these groups are never represented by more than a few relict genera such as Megalichthys and Acanthodes (Figs. 2 and 3). Notwithstanding the fact that rhizodont and megalichthyid stem-tetrapods, as well as acanthodid and gyracanth acanthodians, are widespread and well represented in the fossil record, these vestiges of Devonian diversity are classic instances of “dead clades walking” (27): seemingly niche-restricted and failing to radiate into unique forms (thus easily characterized and long established in fossil collections).
We note that the fossil records of Mississippian chondrichthyans and actinopterygians are undersampled: Many species are either undescribed at the species level or have not been revised in the past 50–100 years. Specimens are often grouped as form taxa alongside their Devonian relatives or are neglected in favor of the aforementioned Devonian holdovers and tetrapods present in the same deposits (compare East Kirkton with Ducabrook, Niddrie, and Dora; SI Appendix). Both of these phenomena result from nonrecognition of the Hangenberg turnover by vertebrate workers, leading to an underestimation of the diversity of actinopterygians and chondrichthyans during the prolonged recovery interval, and therefore a dampening of extinction signal.
The results of this study raise questions about the peri-Hangenberg fossil record, extinction selectivity among gnathostomes, the failure of previously diverse clades to reradiate (27), and the ultimate causes of this global event. Significantly, the post-Hangenberg configuration of vertebrate biodiversity persists to the present day: chondrichthyans, actinopterygians, and tetrapods thrived (6). In conclusion, narrative explanations of early vertebrate evolution, with passing reference to a long-term biotic crisis toward the end of the Devonian, are no longer sufficient.
Materials and Methods
A gnathostome occurrence dataset for the Givetian-Serpukhovian interval was compiled from the literature and contained taxonomic, species, and geographical data for 1,019 genus-level entries. Genus-level diversity curves binned by stage were generated for six gnathostome divisions: Placodermi, Acanthodii, Actinopterygii, Sarcopterygii, Tetrapoda, and Chondrichthyes (Fig. 1). For faunal composition analyses, data were subsampled to produce species lists for macrofossil sites containing at least five named species in three groups in two divisions, resulting in a set of 66 geographically widespread localities (mean species count = 19.37, SD = 13.10, median = 15.00, range: 6–82; SI Appendix). These data were arranged into sites as samples and species counts for major gnathostome clades and/or well-differentiated groups extant by the Frasnian as variables (Dataset S1, Dataset S2, and SI Appendix).
Data were characterized and analyzed using R (version 2.8.1) (28), the ecological package vegan (version 1.15) (29), and the paleobiological program PAST (version 2) (30). Ecological ordination analyses used in this study included cluster analysis, CCA, detrended correspondence analysis, FA, and NMDS. These are useful for detecting true faunal breaks because they do not use a priori assignments of either age or environment (18). Pairwise P values (α = 0.05) and R statistics for patterns observed in ordination analyses were generated by one-tailed ANOSIM from 1 million permutations of site faunas grouped a priori by interval (18). SIMPER was used to show the relative contribution of taxonomic groups to observed differences between intervals, and therefore the association of turnover with extinction. Bray–Curtis abundance distance and Kulczynski presence-absence similarity metrics were used in cluster analysis and NMDS (18). ANOSIM was performed using these and five similar metrics; P values and R statistics in the text are the mean from comparisons using all seven metrics based on raw diversity data. All ecological analyses were performed using both raw species counts and relative diversities based on percentage of the site total (Dataset S1 and Dataset S2). Subsets containing only localities from restricted intervals around specific events and from single environments were analyzed to pinpoint the turnover. Detailed methods, along with the results of all analyses, are presented in SI Appendix.
Supplementary Material
Acknowledgments
We thank O. Lebedev, G. Hunt, M. Friedman, C. K. Boyce, M. Webster, and W. L. Smith for helpful discussions of the data and methods and M. Foote, D. Jablonski, M. LaBarbera, and two anonymous reviewers for their input and critical reading of the manuscript. This research was supported by grants from the National Science Foundation (DDIG DEB-1011002), University of Chicago, Paleontological Society, Palaeontological Association, Evolving Earth Foundation, and American Society of Ichthyologists and Herpetologists (to L.C.S.) and grant DEB-0917922 from the National Science Foundation (to M.I.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.C. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0914000107/-/DCSupplemental.
References
- 1.Gradstein FM, Ogg JG, Smith AG. A Geologic Time Scale. Cambridge, U.K: Cambridge Univ Press; 2004. [Google Scholar]
- 2.Janvier P. Early Vertebrates. Oxford: Clarendon; 1996. [Google Scholar]
- 3.Benton MJ. The Fossil Record II. London: Chapman & Hall; 1993. [Google Scholar]
- 4.Brazeau MD. The braincase and jaws of a Devonian ‘acanthodian’ and modern gnathostome origins. Nature. 2009;457:305–308. doi: 10.1038/nature07436. [DOI] [PubMed] [Google Scholar]
- 5.Sepkoski JJ. A compendium of fossil marine animal genera. Bull Am Paleontol. 2002;363:1–560. [Google Scholar]
- 6.Nelson JS. Fishes of the World. 4th Ed. Hoboken, NJ: Wiley; 2006. [Google Scholar]
- 7.Zhao W-J, Zhu M. Diversification and faunal shift of Siluro-Devonian vertebrates in China. Geol J. 2007;42:351–369. [Google Scholar]
- 8.Janvier P, Clement G. A new groenlandaspid arthrodire (Vertebrata: Placodermi) from the Famennian of Belgium. Geol Belg. 2005;8:51–67. [Google Scholar]
- 9.Jablonski D. Mass extinctions and macroevolution. Paleobiology. 2005;31:192–210. [Google Scholar]
- 10.Sahney S, Benton MJ. Recovery from the most profound mass extinction of all time. Proc Biol Sci. 2008;275:759–765. doi: 10.1098/rspb.2007.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raup DM, Sepkoski JJ., Jr Mass extinctions in the marine fossil record. Science. 1982;215:1501–1503. doi: 10.1126/science.215.4539.1501. [DOI] [PubMed] [Google Scholar]
- 12.McGhee GR. The Late Devonian Mass Extinction: The Frasnian-Famennian Crisis. Columbia, New York: 1996. [Google Scholar]
- 13.Caplan ML, Bustin RM. Devonian-Carboniferous mass extinction event, widespread organic-rich mudrock and anoxia: Causes and consequences. Palaeogeogr Palaeoclimatol Palaeoecol. 1999;148:187–207. [Google Scholar]
- 14.Bambach RK. Phanerozoic biodiversity mass extinctions. Annu Rev Earth Planet Sci. 2006;34:127–155. [Google Scholar]
- 15.Brezinski DK, Cecil CB, Skema VW. Late Devonian glacigenic and associated facies from the central Appalachian Basin, eastern United States. GSA Bull. 2010;122:265–281. [Google Scholar]
- 16.Long JA. In: Palaeozoic Vertebrate Biostratigraphy and Biogeography. Long JA, editor. Baltimore: Johns Hopkins Univ Press; 1993. pp. 54–66. [Google Scholar]
- 17.Peters SE, Foote M. Determinants of extinction in the fossil record. Nature. 2002;416:420–424. doi: 10.1038/416420a. [DOI] [PubMed] [Google Scholar]
- 18.Hammer Ø, Harper DAT. Paleontological Data Analysis. Malden, MA: Blackwell; 2006. [Google Scholar]
- 19.Smith AB, Patterson C. The influence of taxonomic method on the perception of patterns of evolution. Evol Biol. 1988;23:127–216. [Google Scholar]
- 20.Foote M. Extinction and quiescence in marine animal genera. Paleobiology. 2007;33:261–272. [Google Scholar]
- 21.Coates MI, Clack JA. Romer's gap: Tetrapod origins and terrestriality. Bull Mus Nat d'Hist Nat Paris. 1995;17:373–388. [Google Scholar]
- 22.Erwin DH. The end and the beginning: recoveries from mass extinctions. Trends Ecol Evol. 1998;13:344–349. doi: 10.1016/s0169-5347(98)01436-0. [DOI] [PubMed] [Google Scholar]
- 23.Ward P, Labandeira C, Laurin M, Berner RA. Confirmation of Romer's Gap as a low oxygen interval constraining the timing of initial arthropod and vertebrate terrestrialization. Proc Natl Acad Sci USA. 2006;103:16818–16822. doi: 10.1073/pnas.0607824103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schram FR. In: Mazon Creek Fossils. Nitecki MH, editor. New York: Academic; 1979. pp. 159–190. [Google Scholar]
- 25.Wagner PJ, Ruta M, Coates MI. Evolutionary patterns in early tetrapods. II. Differing constraints on available character space among clades. Proc Biol Sci. 2006;273:2113–2118. doi: 10.1098/rspb.2006.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coates MI, Clack JA. Polydactyly in the earliest known tetrapod limbs. Nature. 1990;347:66–69. [Google Scholar]
- 27.Jablonski D. Survival without recovery after mass extinctions. Proc Natl Acad Sci USA. 2002;99:8139–8144. doi: 10.1073/pnas.102163299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.R Development Core Team R: A language and environment for statistical computing. 2008. Available at http://www.R-project.org. Accessed January 6, 2009.
- 29.Oksanen J, et al. Vegan: Community Ecology Package. R package version 1.15-1. 2008. Available at http://cran.r-project.org/, http://vegan.r-forge.r-project.org/. Accessed January 6, 2009.
- 30.Hammer Ø, Harper DAT, Ryan PD. PAST: Paleontological software package for education and data analysis. Palaeont Electr. 2001;4:9. [Google Scholar]
- 31.Coates MI, Ruta M, Friedman M. Ever since Owen: Changing perspectives on the early evolution of tetrapods. Annu Rev Ecol Evol Syst. 2008;39:571–592. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.