Abstract
Shear stress imposed by blood flow is crucial for maintaining vascular homeostasis. We examined the role of shear stress in regulating SIRT1, an NAD+-dependent deacetylase, and its functional relevance in vitro and in vivo. The application of laminar flow increased SIRT1 level and activity, mitochondrial biogenesis, and expression of SIRT1-regulated genes in cultured endothelial cells (ECs). When the effects of different flow patterns were compared in vitro, SIRT1 level was significantly higher in ECs exposed to physiologically relevant pulsatile flow than pathophysiologically relevant oscillatory flow. These results are in concert with the finding that SIRT1 level was higher in the mouse thoracic aorta exposed to atheroprotective flow than in the aortic arch under atheroprone flow. Because laminar shear stress activates AMP-activated protein kinase (AMPK), with subsequent phosphorylation of endothelial nitric oxide synthase (eNOS) at Ser-633 and Ser-1177, we studied the interplay of AMPK and SIRT1 on eNOS. Laminar flow increased SIRT1-eNOS association and eNOS deacetylation. By using the AMPK inhibitor and eNOS Ser-633 and -1177 mutants, we demonstrated that AMPK phosphorylation of eNOS is needed to prime SIRT1-induced deacetylation of eNOS to enhance NO production. To verify this finding in vivo, we compared the acetylation status of eNOS in thoracic aortas from AMPKα2−/− mice and their AMPKα2+/+ littermates. Our finding that AMPKα2−/− mice had a higher eNOS acetylation indicates that AMPK phosphorylation of eNOS is required for the SIRT1 deacetylation of eNOS. These results suggest that atheroprotective flow, via AMPK and SIRT1, increases NO bioavailability in endothelium.
Keywords: NAD+-dependent deacetylase, AMP-activated protein kinase, endothelial nitric oxide synthase, NO bioavailability, endothelial homeostasis
SIRT1, also known as Sirtuin 1 (silent mating type information regulation 2 homolog), contributes to the caloric restriction (CR)-induced increase in lifespan in species ranging from yeast to mammals (1–3). Functioning as an NAD+-dependent class III histone deacetylase (4), SIRT1 deacetylates multiple targets in mammalian cells, including tumor suppressor p53, Forkhead box O1 and 3 (FOXO1 and FOXO3), peroxisome proliferator-activated receptor γ (PPARγ) coactivator 1α (PGC-1α), liver X receptor, and hypoxia-inducible factor 2α (5–14). By regulating these molecules involved in cell survival and in carbohydrate and lipid metabolism, SIRT1 functions as a master regulator of stress response and energy homeostasis.
SIRT1 is also an important modulator in cardiovascular functions in health and disease. The beneficial effects of SIRT1 on endothelial cell (EC) biology were demonstrated by several previous studies. Ota et al. (15) showed that overexpression of SIRT1 prevented oxidative stress-induced endothelial senescence, whereas inhibition of SIRT1 led to premature senescence. Treatment of human coronary arterial ECs with resveratrol (RSV), an SIRT1 activator, increased the mitochondrial mass and key factors mediating mitochondrial biogenesis, such as PGC-1α and nuclear respiratory factor 1 (NRF1) (16). Mattagajasingh et al. (17) demonstrated that inhibition of SIRT1 in rat arteries attenuated endothelium-dependent vasodilation, which might be due to the enhanced acetylation of endothelial nitric oxide synthase (eNOS). In mice, RSV administration increased aortic eNOS activity (18). Furthermore, EC-specific overexpression of SIRT1 decreased atherosclerosis in ApoE-knockout mice (19).
The endothelium forms a bioactive interface between the circulating blood and the vessel wall. The constant exposure of ECs to shear stress maintains vascular tone, which is mediated in part through its regulation of eNOS. Depending on the flow pattern, the associated shear stress can be atheroprotective or atheroprone. Steady pulsatile flow present in the straight parts of the arterial tree is atheroprotective, which increases the eNOS-derived NO bioavailability and exerts antiinflammatory and antioxidative effects. In bends and bifurcations, disturbed flow patterns induce the expression of molecules involved in atherogenesis and elevate the level of reactive oxygen species (ROS) in ECs (20). Using the flow channel as a model system, we have shown that laminar flow causes activation of AMP-activated protein kinase (AMPK), which in turn phosphorylates eNOS at Ser-633 and -1177, thus leading to enhanced NO bioavailability (21, 22).
In hepatocytes, SIRT1 regulates lipid metabolism through AMPK (23). In skeletal muscles, however, AMPK regulates SIRT1 by modulating the activity of nicotinamide phosphoribosyl transferase (24). A recent report by Canto et al. (25) showed that the phosphorylation of PGC-1α by AMPK primes the PGC-1α deacetylation by SIRT1 in myocytes. These studies suggest that AMPK may crosstalk with SIRT1 to modulate downstream targets. Because shear stress regulates the endothelium in health and disease, and both AMPK and SIRT1 play critical roles in endothelial biology, we investigated the effect of shear stress on SIRT1 in ECs and its functional consequences. Our results show that laminar flow elevates SIRT1 in ECs and that the laminar flow-activated SIRT1 acts synergistically with AMPK to increase NO bioavailability in vitro and in vivo.
Results
Shear Stress Increases SIRT1 Level/Activity in ECs.
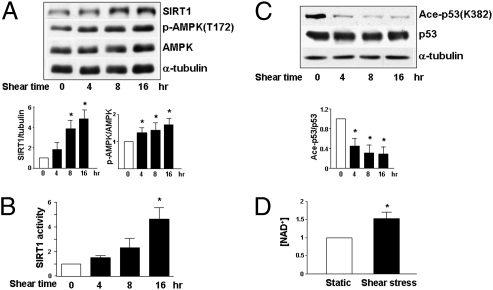
To explore whether shear stress regulates SIRT1 and AMPK in ECs, we applied a laminar flow at 12 dyn/cm2 to cultured human umbilical vein ECs (HUVECs) for various durations. Western blot analysis revealed that laminar flow increased the SIRT1 level and AMPK phosphorylation at Thr-172 and that these elevations lasted for at least 16 h (Fig. 1A). Concomitant with the increase in SIRT1 level, shear stress also augmented its deacetylase activity, as demonstrated by the decreased acetylation of Arg-His-Lys-Lys(ac) (Fig. 1B). Because p53 Lys-382 is an SIRT1 target site (26), the acetylation status of this Lys in ECs was examined. Shear stress decreased the acetylation of p53 Lys-382 in cells under shear stress, as compared to static controls (Fig. 1C). Because the enzymatic activity of SIRT1 depends on the cellular level of NAD+ and shear stress has been shown to increase the activity of NAD(P)H oxidase, which converts NADH to NAD+ (27), we compared the cellular level of NAD+ in ECs under shear stress and in controls. Compared with static control conditions, laminar flow increased NAD+ level by ∼50% (Fig. 1D). Together, Fig. 1 suggests that laminar flow elevates SIRT1 level with an attendant increase in SIRT1 deacetylation activity.
Fig. 1.
Laminar shear stress increases SIRT1 level/activity in ECs. Confluent monolayers of HUVECs were subjected to laminar flow at 12 dyn/cm2 for various times or kept as static controls represented as time 0. (A and C) Cell lysates were analyzed by Western blotting with anti-SIRT1, anti-phospho-AMPK (Thr-172; T172), anti-AMPK, anti-α-tubulin, anti-acetyl-p53 (Lys-382; K382), and anti-p53 antibodies. The bar graphs below are results of densitometry analyses of the ratios of SIRT1 to α-tubulin, phospho-AMPK to AMPK, and acetyl-p53 to p53. (B) SIRT1 deacetylase activity in HUVECs under shear stress or static conditions was assessed by using fluorogenic Arg-His-Lys-Lys (ac) as the substrate. The fluorescent intensities obtained from different groups were compared, with the value obtained at time 0 set as 1. (D) NAD+ level in HUVECs under laminar flow or static condition for 16 h was measured by spectrophotometry. The reading of the static group was set as 1. The bar graphs in all panels are mean ± SD averaged from three independent experiments. *, P < 0.05 vs. static control.
Shear Stress Increases Mitochondrial Biogenesis in ECs.
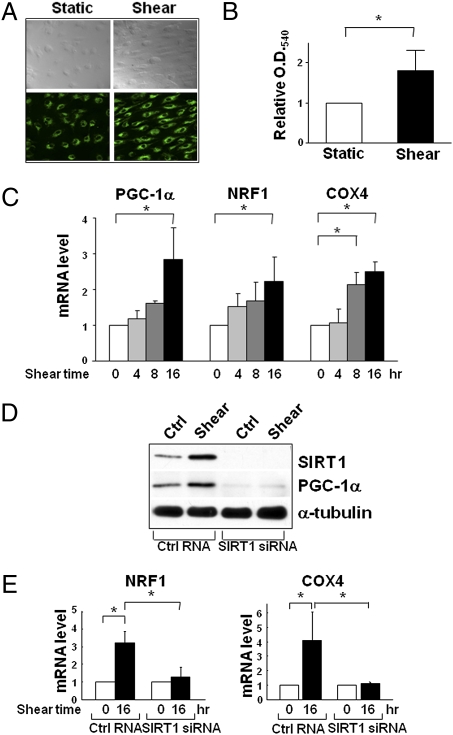
In several cell types, the elevation of SIRT1 is tightly correlated with increased mitochondrial biogenesis (12, 16, 25). In the present study, laminar flow indeed increased the mitochondrial mass (Fig. 2A), with attendant increase in the activity of mitochondrial reductase (Fig. 2B). Because PGC-1α and NRF1 are key regulators of mitochondrial biogenesis and COX4 is involved in the mitochondrial respiratory chain (28), we examined whether laminar flow increases the expression of these molecules in ECs. As shown in Fig. 2C, laminar flow increased the mRNA level of PGC-1α, NRF1, and COX4 in a time-dependent manner. Importantly, SIRT1 knockdown by small interfering RNA (siRNA) abolished the flow-induced PGC-1α, NRF1, and COX4 (Fig. 2 D and E). These results suggest that laminar flow increases mitochondrial biogenesis in ECs and that this effect is mediated through SIRT1.
Fig. 2.
Shear stress-increased mitochondrial biogenesis is mediated by SIRT1. Confluent monolayer of HUVECs were subjected to laminar flow for 16 h or kept under static condition. (A) Mitotracker Green FM staining. (B) Quantification of mitochondrial reductase activity by MTT assay from absorbance at 540 nm. (C) Assay of levels of mRNA encoding PGC-1α, NRF1, and COX4 in HUVECs subjected to laminar flow for various durations by quantitative RT-PCR (qRT-PCR). (D and E) HUVECs were transfected with SIRT1 siRNA or control RNA (20 nM) and then exposed to laminar flow for 16 h or were kept as static controls. SIRT1, PGC-1α, and α-tubulin levels were assessed by Western blot analysis in (D), and NRF1 and COX4 mRNA levels were analyzed by qRT-PCR in (E). Bar graphs are mean ± SD from three independent experiments. *, P < 0.05 between indicated groups.
Shear Stress Induction of SIRT1 Is Independent of AMPK.
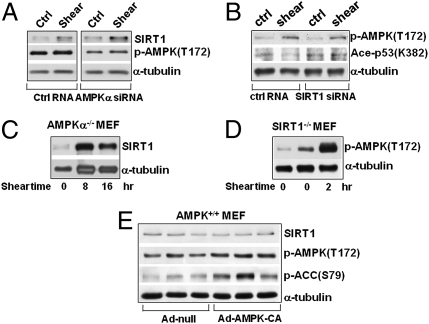
Depending on the stimuli, AMPK and SIRT1 can regulate each other reciprocally (23, 24). Because laminar flow concurrently activated AMPK and SIRT1, we investigated whether AMPK regulates SIRT1 or vice versa in ECs under shear stress. As shown in Fig. 3A, AMPKα knockdown by siRNA did not affect the laminar flow induction of SIRT1, indicating that AMPK is not upstream of SIRT1. That the shear-induced increase in SIRT1 level is independent of AMPK was further verified by the finding that ablation of both AMPKα1 and α2 in murine embryonic fibroblasts (MEFs) had little effect on the shear induction of SIRT1 (Fig. 3C). Furthermore, the level of SIRT1 was comparable in wild-type MEFs infected with Ad-null or Ad-AMPK-CA expressing the constitutively activated form of AMPK (Fig. 3E). These experiments demonstrate that AMPK is neither necessary nor sufficient for the laminar flow-induced SIRT1. We also knocked down SIRT1 in ECs and used MEFs isolated from SIRT1-null mouse embryos to determine whether SIRT1 regulates AMPK. As shown in Fig. 3 B and D, laminar flow was still able to activate AMPK in ECs with SIRT1 knocked down or in SIRT1−/− MEFs, indicating that SIRT1 is also not upstream to AMPK in our system. The results presented in Fig. 3 suggest that AMPK and SIRT1 are independently activated by laminar flow; neither is upstream to the other.
Fig. 3.
Shear stress-increased SIRT1 is AMPK independent. (A and B) HUVECs were transfected with AMPK α1 and α2 siRNAs (10 nM each), SIRT1 siRNA (20 nM), or with control RNA (20 nM) before being subjected to laminar flow for 16 h. (C and D) MEFs ablated with AMPKα or SIRT1 (i.e., AMPKα−/− and SIRT1−/−) were subjected to laminar flow, and cell extracts were collected at the indicated times. (E) Wild-type MEFs were infected with Ad-AMPK-CA [100 multiplicities of infection (MOIs)] or Ad-null for 48 h. Total cell proteins were then resolved by SDS/PAGE and subjected to Western blot analysis with various antibodies as indicated.
AMPK and SIRT1 Synergistically Activate eNOS.
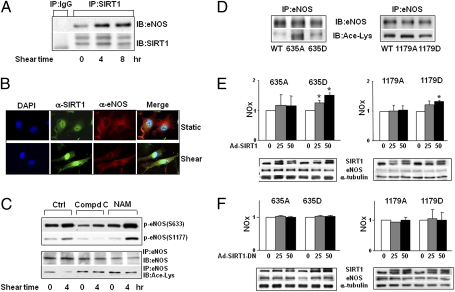
Although AMPK and SIRT1 were independently activated by laminar flow, there is the possibility that they may act synergistically on eNOS to increase NO bioavailability. Hence, we examined first whether laminar flow can increase the association of SIRT1 with eNOS. Following immunoprecipitation of SIRT1 from ECs, subsequent anti-eNOS immunoblotting showed an increase in the level of coimmunoprecipitated eNOS in lysates from ECs that had been subjected to laminar flow (Fig. 4A). The increased association between SIRT1 and eNOS in ECs exposed to laminar flow was further confirmed by double immunostaining (Fig. 4B).
Fig. 4.
Synergistic effects of AMPK and SIRT1 on eNOS. (A) HUVECs were exposed to laminar flow for the durations indicated or kept as static controls. SIRT1 was immunoprecipitated (IP) with anti-SIRT1, and immunoblotting (IB) was performed with anti-eNOS. In parallel, IgG was used as an IP control. The level of immunoprecipitated SIRT1 was also assessed by Western blot. (B) HUVECs under laminar flow or static condition for 4 h were subjected to immunostaining with anti-SIRT1 and anti-eNOS, followed by conjugated secondary antibodies. The cell nuclei were counterstained with DAPI. The colocalization of SIRT1 and eNOS is demonstrated by the merging of pseudocolors. (C) HUVECs were pretreated with DMSO, Compound C (10 μM), or nicotinamide (NAM) (5 mM) for 30 min before being exposed to laminar flow for 4 h. The phosphorylation of eNOS Ser-633 and Ser-1177 was probed in the input lysates. In addition, eNOS was immunoprecipitated from whole-cell lysates, and the acetylation of eNOS and total eNOS were assessed by immunoblotting with anti-Ace-Lys and anti-eNOS. (D–F) HEK293 cells were transfected with plasmids expressing bovine eNOS wild-type (WT), S635A, S635D, S1179A, or S1179D. (D) eNOS was immunoprecipitated and then examined by Western blot analysis with anti-eNOS and anti-Ace-Lys. (E and F) Transfected HEK293 cells were infected with Ad-(Flag)-SIRT1 or Ad-(Flag)-SIRT1-DN with various MOIs, as indicated. Twenty-four hours after infection, the NO bioavailability in the cells was determined by Griess assay and expressed as NOx. The expressions of SIRT1 and eNOS were examined by Western blot.
Next, we examined the functional consequence of the SIRT1-eNOS association. eNOS immunoprecipitation followed by immunoblotting with an antibody recognizing acetylated Lys revealed that acetylation of eNOS was decreased in ECs exposed to laminar flow (Fig. 4C). We have shown that the application of laminar flow causes AMPK activation before SIRT1 elevation (22) (Fig. 1), suggesting that eNOS phosphorylation by AMPK may prime eNOS for deacetylation by SIRT1. This possibility was verified by the finding that the increase in eNOS phosphorylation and deacetylation induced by laminar flow were blocked by the AMPK-inhibitor Compound C (Fig. 4C). In contrast, SIRT1 inhibition by nicotinamide (NAM in Fig. 4C) abolished eNOS deacetylation without affecting eNOS phosphorylation.
To further delineate the requirement of eNOS phosphorylation for SIRT1 deacetylation, we transfected human embryonic kidney 293 (HEK293) cells with plasmids overexpressing gain- or loss-of-function mutants of bovine eNOS (bovine eNOS Ser-635 and -1179 corresponds to human eNOS Ser-633 and -1177). S635D and S1179D (Asp replacing Ser) are the phosphomimetics that simulate the phosphorylation by AMPK, whereas S635A and S1179A (Ala replacing Ser) are the nonphosphorylatable mutants. As shown in Fig. 4D, eNOS S635A and S1179A were more acetylated than the wild-type, S635D, or S1179D. The transfected cells were also infected with adenovirus overexpressing the wild-type SIRT1 (Ad-SIRT1) to mimic the induction by flow (Fig. 4E). In parallel control experiments, cells were infected with a dominant negative mutant of SIRT1 (Ad-SIRT1-DN) (Fig. 4F). Overexpression of Ad-SIRT1, but not Ad-SIRT1-DN, increased NO production by cells overexpressing S635D or S1179D. In cells transfected with S635A or S1179A, the NO production was not changed by overexpressing SIRT1, nor by using SIRT1-DN (Fig. 4 E and F).
Pulsatile and Oscillatory Flows Have Opposite Effects on Regulating SIRT1.
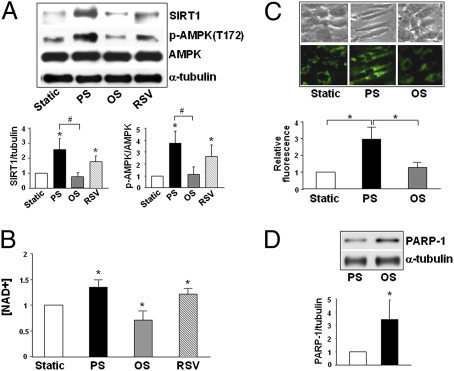
To correlate the results obtained from flow channel experiments with physiological and pathophysiological flow conditions in arteries, we compared the SIRT1 level in ECs subjected to pulsatile flow (12 ± 4 dyn/cm2) and oscillatory flow (1 ± 4 dyn/cm2) representing steady flow that has a distinct direction and disturbed flow with little net direction, respectively. As shown in Fig. 5A, pulsatile flow, but not oscillatory flow, increased the SIRT1 level in ECs. As a positive control, RSV treatment increased the SIRT1 level in ECs similar to that with pulsatile flow. Consistent with the increase in SIRT1 level, the NAD+ level in ECs was higher under pulsatile than oscillatory flow (Fig. 5B). Mitochondrial mass, revealed by Mitotracker Green staining, was also increased by pulsatile, but not oscillatory, flow (Fig. 5C).
Fig. 5.
SIRT1 is differentially regulated by pulsatile flow vs. oscillatory flow. HUVECs were exposed to pulsatile flow (PS) (12 ± 4 dyn/cm2, 1 Hz) or oscillatory flow (OS) (1 ± 4 dyn/cm2, 1 Hz), treated with resveratrol (RSV) (100 μM), or kept under static condition for 16 h. (A) The levels of SIRT1, phospho-AMPK (Thr-172), AMPK, and α-tubulin were assessed by Western blot analysis. *, P < 0.05 compared to static control; #, P < 0.05 between PS and OS. (B) NAD+ level was measured spectrophotometrically. *, P < 0.05 compared to static control. (C) The mitochondria in static cells and those exposed to pulsatile or oscillatory flow were stained with Mitotracker Green FM. *, P < 0.05 between indicated groups. (D) PARP-1 expression in HUVECs exposed to pulsatile or oscillatory flow was revealed by Western blot analysis. *, P < 0.05 compared to PS. Bar graphs represent densitometry analysis of three independent experiments, with the static group set as 1 in (A), (B), and (C). In (D), the level of PARP-1/α-tubulin in ECs exposed to pulsatile flow was set as 1.
Poly(ADP ribose) polymerase-1 (PARP-1), which is highly sensitive to ROS, consumes NAD+ for catalysis of poly(ADP ribosyl)ation on target proteins (29, 30). Because oscillatory flow causes a sustained activation of NAD(P)H oxidase and a high level of intracellular ROS (27, 31), we considered the possibility that PARP-1 may be involved in the differential regulation of SIRT1 in ECs subjected to different flow patterns. Fig. 5D shows the level of PARP-1 was indeed higher under oscillatory than pulsatile flow.
Differential Regulation of SIRT1-eNOS in the Vessel Wall.
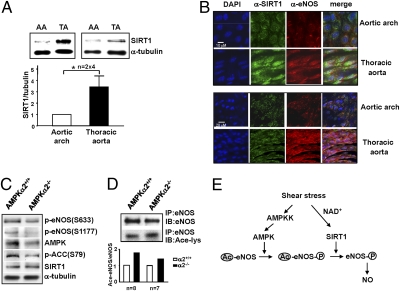
To determine whether SIRT1 is differentially regulated in atheroprone vs. atheroprotective areas in the arterial tree, we isolated the aortic arch and thoracic aorta from C57BL6 mice. These areas correspond to regions under disturbed and pulsatile flows, respectively (32). Western blot analysis showed that the SIRT1 level was higher in the thoracic aorta (under pulsatile flow) than in the aortic arch (under disturbed flow) (Fig. 6A). Because the isolated tissues were a mixture of ECs, vascular smooth muscle cells, and connective tissues, we performed en face staining on the mouse arterial tree. Confocal microscopy revealed that the level of SIRT1 in the endothelium of the thoracic aorta was significantly higher than that in the inner curvature of the aortic arch (Fig. 6B). Furthermore, colocalization of SIRT1 and eNOS was noticeably higher in the thoracic aorta than aortic arch endothelium. To confirm the synergistic effect of AMPK and SIRT1 on eNOS in vivo, we compared the phosphorylation and acetylation of eNOS in thoracic aortas from AMPKα2−/− mice and their AMPKα2+/+ littermates. As seen in Fig. 6 C and D, acetyl-CoA carboxylase (ACC), the AMPK canonical target, and eNOS phosphorylations were lower, whereas eNOS acetylation level was higher in the thoracic aortas of AMPKα2−/− mice, as compared with their AMPKα2+/+ littermates. However, the SIRT1 level was comparable between the two lines of mice. These results suggest that eNOS deacetylation by SIRT1 in the vessel wall requires AMPK.
Fig. 6.
Differential regulation of SIRT1/eNOS in the vessel wall in vivo. (A) Tissue extracts of the aortic arch (AA) and thoracic aorta (TA) from two C57BL6 male mice were pooled and subjected to Western blot analysis with anti-SIRT1 and α-tubulin antibodies. Data shown represent results from a total of eight mice. (B) Confocal microscope images of en face immunostaining of SIRT1 and eNOS in the endothelium of the aortic arch and thoracic aorta of C57BL6 mice. The primary antibodies were anti-SIRT1 and anti-eNOS, and secondary antibodies were Alexa Fluor 488-conjugated chicken anti-rabbit or Alexa Fluor 555-conjugated chicken anti-mouse, respectively. Nuclei were counterstained with DAPI. Shown are representative images from six animals. (C and D) Tissue extracts were isolated from the thoracic aorta of AMPKα2−/− and their AMPKα2+/+ littermates. In (C), the level of phospho-eNOS Ser-633, Ser-1177, AMPKα, phosphorylated ACC, SIRT1, and α-tubulin was determined by Western blot. In (D), eNOS was immunoprecipitated and the acetylation of eNOS in the immunoprecipitates was determined by immunoblotting with anti-Ace-Lys. After stripping, the blot was reprobed with anti-eNOS. The presented results on AMPKα2−/− and AMPKα2+/+ mice were pooled from eight aortas in each group. The experiments were repeated with another group of seven animals per mouse line. Bar graphs below are densitometry analysis of the ratios of acetyl-eNOS to eNOS from the two experiments. (E) Schematic model of the synergistic effects of AMPK and SIRT1 on eNOS activity. Laminar/pulsatile shear stress increases the NAD+ level in ECs, thus leading to elevation of SIRT1. Concomitantly, AMPK is activated by upstream AMPK kinase (AMPKK), which in turn phosphorylates eNOS. The phosphorylated eNOS enhances its affinity toward SIRT1 so that eNOS is deacetylated. Such a synergism between AMPK and SIRT1 augments the eNOS-derived NO bioavailability.
Discussion
Dysfunctional ECs, signified by enhanced inflammation and oxidative stress, prelude atherosclerosis and other vascular impairments. The effects of shear stress on ECs can be atheroprotective or atheroprone depending on the associated flow patterns. Because SIRT1 exerts antiinflammatory and antioxidative effects on various cell types, including ECs, we studied the shear stress regulation of SIRT1 in ECs. Our results indicate that laminar flow causes a marked increase in SIRT1 level, and that AMPK and SIRT1 synergistically increase the eNOS-derived NO bioavailability in ECs responding to laminar flow.
Consistent with its effects of elevating the levels of SIRT1 and NAD+, laminar flow increased the mitochondria mass and levels of PGC-1α, NRF1, and COX4. siRNA knockdown experiments presented in Fig. 2 showed that these key regulators of mitochondrial biogenesis are up-regulated by the flow-elevated SIRT1 (Fig. 2 C–E). Increased mitochondrial biogenesis is implicated in the balance of the endothelial redox state (33). By scavenging free radicals (e.g., ROS and reactive nitrogen species) through PGC-1α-induced ROS detoxifying enzymes (34), the laminar flow-enhanced mitochondrial biogenesis may protect ECs against oxidative stress. We have previously shown that laminar flow activates endogenous PPARγ and its target genes in ECs and that these effects are ligand-dependent (35). Together with the ligand-activated PPARγ, the elevated PGC-1α, functioning as a transcriptional coactivator of PPARγ, may also contribute to the positive effects of PPARγ on EC biology, including reverse cholesterol transport, antiinflammation, and antiatherosclerosis (36). In addition, laminar flow is known to arrest the EC cycle at the G0/G1 phase (37), which may explain the quiescence of ECs in the straight part of the arterial tree. Inhibition of SIRT1 by sirtinol or siRNA causes premature senescence-like growth arrest in ECs (15). The implication of this notion in the current study is that the elevated SIRT1 by laminar flow may ameliorate aging-related senescence of ECs, even if they are quiescent.
eNOS-derived NO bioavailability, which is critically important for EC physiological functions, is regulated by multiple mechanisms at transcriptional and posttranslational levels (38). Among the various mechanisms, phosphorylations at Ser-633 and Ser-1177 enhance eNOS activity, and AMPK can phosphorylate both sites (21, 22, 39). Although the shear stress activation of AMPK and SIRT1 seems to be independent processes, the activated AMPK and SIRT1 converge at eNOS in that AMPK phosphorylation may prime eNOS for SIRT1 deacetylation (depicted in Fig. 6E). Such a model is supported by the following observations: eNOS was no longer deacetylated following inhibition of AMPK activity by Compound C (Fig. 4C), and eNOS S635A and S1179A were much more acetylated than eNOS S635D and S1179D (Fig. 4D). This mode of action is reminiscent of the synergistic effects of AMPK and SIRT1 on PGC-1α (25). By using AMPKα2−/− mice, we showed that this synergism occurs in vivo. Because shear stress appears to preferentially activate AMPKα2 in ECs (40), and AMPKα1 ablation caused anemia and severe splenomegaly in the knockout animals (41), AMPKα2−/− instead of AMPKα1−/− mice were used. AMPKα2 ablation resulted in a lower level of eNOS phosphorylation and a higher level of eNOS acetylation, even though SIRT1 was as abundant as in the wild type (Fig. 6 C and D). These results indicate that AMPK phosphorylation of eNOS is required for the deacetylation by SIRT1 in the mouse thoracic aorta. SIRT1 inhibition by nicotinamide resulted in superphosphorylation of eNOS (Fig. 4C). It seems that eNOS deacetylation is also correlated with its dephosphorylation. Such a mechanism may restore the set point for eNOS activation. Despite the fact that eNOS phosphorylation sites have been extensively studied, the acetylation sites remain unclear. Two potential residues are Lys-494 and Lys-504 (17). The identification of SIRT1-targeted sites in eNOS and their interplay with phosphorylation sites warrant future research.
Cellular NAD+ level can modulate SIRT1 activity and expression (42, 43). Thus, one possible mechanism by which laminar/pulsatile flow augments SIRT1 level and/or activity is through the elevated NAD+ level. Such an enhanced oxidized state in ECs may be caused by the increase in NAD+ (Figs. 1D and 5B) resulting from an increased activity of NAD(P)H oxidase. Indeed, laminar flow induces a transient increase in the activity of NAD(P)H oxidase (27). Previously, Nisoli et al. (44) found that the SIRT1 induction by CR involves eNOS-derived NO, as evidenced by the attenuated SIRT1 in white adipose tissue of the eNOS-null mice. However, laminar flow was able to increase SIRT1 level in eNOS-null MEFs and in HUVECs treated with the NOS inhibitor nitro-l-arginine methyl ester (L-NAME) (Fig. S1). These results suggest that the flow induction of SIRT1 does not depend on eNOS. One possible explanation for this independency is that the moderate and transient increase in ROS by pulsatile flow is able to induce SIRT1. This is in line with the recent finding that oxidative stress generated by short-term H2O2 treatment enhanced SIRT1 (45). In contrast to pulsatile flow, oscillatory flow causes a sustained activation of NAD(P)H oxidase with an attendant strong elevation of ROS (27, 31, 46). This pathophysiological ROS level should induce or activate PARP-1 (29), which in turn depletes cellular NAD+ to diminish the expression or activity of SIRT1 as seen in Fig. 5 A and B.
The differential effects of pulsatile vs. oscillatory flow on SIRT1 expression was verified in the mouse arterial tree (Fig. 6). SIRT1 level was higher, with increased eNOS colocalized in the atheroprotective areas. It is known that endothelial dysfunction (which is signified by increased oxidative and inflammatory responses) predisposes the arteries to atherosclerosis. Hence, SIRT1 activation by pulsatile flow may prevent EC dysfunction and counteract the risk factors associated with atherosclerosis. Compared with therapeutic interventions such as RSV and several small molecules developed for SIRT1 activation, shear stress would be more physiologically relevant. The molecular basis underlying the marked differences between pulsatile and oscillatory flows in the modulation of SIRT1 may provide new insights into the mechanism of regulation of EC biology by shear stress.
Materials and Methods
The resources of antibodies and reagents, as well as detailed methods for cell culture, adenoviral infection, siRNA knockdown, transient transfection, Western blot analysis, immunoprecipitation, qRT-PCR, NAD+ measurement, MTT assay, and NO bioavailability assay are described in SI Materials and Methods.
Fluid Shear Stress Experiments.
The shear stress experiments were performed as previously described (22, 47, 48). Various types of cells were exposed to laminar flow at 12 dyn/cm2 for different times. In some experiments, a reciprocating syringe pump was connected to the circulating system to introduce a sinusoidal component with a frequency of 1 Hz onto the shear stress. Pulsatile shear flow or oscillatory shear flow was applied to ECs with a shear stress of 12 ± 4 dyn/cm2 or 1 ± 4 dyn/cm2, respectively.
SIRT1 Activity Assay.
SIRT1 activity was assessed as previously described (24), with minor modification. Briefly, 10–20 μg of EC extracts were used in a deacetylation assay, with Fluor de Lys-SIRT1 as the substrate (Biomol). The deacetylation of the substrate was measured by use of a microplate-reading fluorimeter, with excitation set at 380 nm and emission at 460 nm.
Animal Experiments.
The animal experimental protocols were approved by the Institutional Animal Care and Use Committee of University of California, Riverside. The thoracic aorta and aortic arch from male C57BL6 mice older than 20 weeks were isolated for both Western blot analysis and en face immunostaining as previously described (47, 48). In addition, thoracic aortas were dissected from 20-week-old AMPKα2−/− mice and their AMPKα2+/+ littermates. Tissue extracts from aortas were pooled for eNOS immunoprecipitation and immunoblotting.
Mitotracker and Immunofluorescence Staining.
Mitotracker Green FM (Invitrogen) was used to stain mitochondria in HUVECs as previously described (49). The cells were washed twice with PBS and then incubated with 20 nM Mitotracker Green FM for 30 min. After 3 washes with PBS, the cells were subjected to fluorescence detection (excitation, 490 nm; emission, 516 nm) by using a Leica SP2 confocal microscope.
For immunostaining, HUVECs were fixed with 4% paraformaldehyde in PBS (pH 7.4) for 30 min at room temperature. For en face staining, mouse aortas were perfused with 4% paraformaldehyde in PBS for 15 min, and the dissected specimens were fixed for another 60 min after the removal of tunica adventitia. Primary antibodies, i.e., rabbit anti-SIRT1 and mouse anti-eNOS, were applied at 1:100 dilution to the specimens, which were incubated at 4 °C overnight and then washed off with PBS and 0.1% Tween-20 (PBST), followed by the application of Alexa Fluor 488-conjugated chicken anti-rabbit or Alexa Fluor 555-conjugated chicken anti-mouse (Invitrogen). After PBST wash, coverslips were mounted with Prolong Gold Antifade Reagent (Invitrogen) and viewed with a Leica SP2 confocal microscope. For all immunofluorescence experiments, parallel groups of cells or aorta specimens were stained with only primary antibody or secondary antibody as a control. Multiple images were captured from three to four regions in the aortic arch and thoracic aorta of every mouse.
Statistical Analysis.
The significance of variability was determined by Student's t test or one-way ANOVA. All results are presented as mean ± SD from at least three independent experiments. Unless otherwise indicated, P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Dr. Michael McBurney (Department of Medicine, University of Ottawa) for providing SIRT1-null MEF, and Mr. Phu Nguyen (Department of Bioengineering, University of California at San Diego) for technical services. This work was supported in part by National Institutes of Health Grants HL89940 (to J.Y.-J.S.), HL93731 (to J.Y.-J.S.), HL080518 (to S.C.), and HL085159 (to S.C.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1003833107/-/DCSupplemental.
References
- 1.Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- 2.Bordone L, et al. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;6:759–767. doi: 10.1111/j.1474-9726.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- 3.Boily G, et al. SirT1 regulates energy metabolism and response to caloric restriction in mice. PLoS One. 2008;3:e1759. doi: 10.1371/journal.pone.0001759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 5.Motta MC, et al. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- 6.Brunet A, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 7.Nemoto S, Fergusson MM, Finkel T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science. 2004;306:2105–2108. doi: 10.1126/science.1101731. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y, Hou H, Haller EM, Nicosia SV, Bai W. Suppression of FOXO1 activity by FHL2 through SIRT1-mediated deacetylation. EMBO J. 2005;24:1021–1032. doi: 10.1038/sj.emboj.7600570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo J, et al. Negative control of p53 by Sir2α promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- 10.Vaziri H, et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- 11.Rodgers JT, et al. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 12.Lagouge M, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Li X, et al. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol Cell. 2007;28:91–106. doi: 10.1016/j.molcel.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 14.Dioum EM, et al. Regulation of hypoxia-inducible factor 2α signaling by the stress-responsive deacetylase sirtuin 1. Science. 2009;324:1289–1293. doi: 10.1126/science.1169956. [DOI] [PubMed] [Google Scholar]
- 15.Ota H, et al. Sirt1 modulates premature senescence-like phenotype in human endothelial cells. J Mol Cell Cardiol. 2007;43:571–579. doi: 10.1016/j.yjmcc.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Csiszar A, et al. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H13–H20. doi: 10.1152/ajpheart.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mattagajasingh I, et al. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 2007;104:14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orimo M, et al. Protective role of SIRT1 in diabetic vascular dysfunction. Arterioscler Thromb Vasc Biol. 2009;29:889–894. doi: 10.1161/ATVBAHA.109.185694. [DOI] [PubMed] [Google Scholar]
- 19.Zhang QJ, et al. Endothelium-specific overexpression of class III deacetylase SIRT1 decreases atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc Res. 2008;80:191–199. doi: 10.1093/cvr/cvn224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chien S. Effects of disturbed flow on endothelial cells. Ann Biomed Eng. 2008;36:554–562. doi: 10.1007/s10439-007-9426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, et al. AMP-activated protein kinase is involved in endothelial NO synthase activation in response to shear stress. Arterioscler Thromb Vasc Biol. 2006;26:1281–1287. doi: 10.1161/01.ATV.0000221230.08596.98. [DOI] [PubMed] [Google Scholar]
- 22.Chen Z, et al. AMP-activated protein kinase functionally phosphorylates endothelial nitric oxide synthase Ser633. Circ Res. 2009;104:496–505. doi: 10.1161/CIRCRESAHA.108.187567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hou X, et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem. 2008;283:20015–20026. doi: 10.1074/jbc.M802187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fulco M, et al. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14:661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cantó C, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langley E, et al. Human SIR2 deacetylates p53 and antagonizes PML/p53-induced cellular senescence. EMBO J. 2002;21:2383–2396. doi: 10.1093/emboj/21.10.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Keulenaer GW, et al. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state: role of a superoxide-producing NADH oxidase. Circ Res. 1998;82:1094–1101. doi: 10.1161/01.res.82.10.1094. [DOI] [PubMed] [Google Scholar]
- 28.Hock MB, Kralli A. Transcriptional control of mitochondrial biogenesis and function. Annu Rev Physiol. 2009;71:177–203. doi: 10.1146/annurev.physiol.010908.163119. [DOI] [PubMed] [Google Scholar]
- 29.Chiarugi A. Poly(ADP-ribose) polymerase: killer or conspirator? The ‘suicide hypothesis’ revisited. Trends Pharmacol Sci. 2002;23:122–129. doi: 10.1016/S0165-6147(00)01902-7. [DOI] [PubMed] [Google Scholar]
- 30.Ha HC, Snyder SH. Poly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proc Natl Acad Sci USA. 1999;96:13978–13982. doi: 10.1073/pnas.96.24.13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hwang J, et al. Pulsatile versus oscillatory shear stress regulates NADPH oxidase subunit expression: implication for native LDL oxidation. Circ Res. 2003;93:1225–1232. doi: 10.1161/01.RES.0000104087.29395.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suo J, et al. Hemodynamic shear stresses in mouse aortas: implications for atherogenesis. Arterioscler Thromb Vasc Biol. 2007;27:346–351. doi: 10.1161/01.ATV.0000253492.45717.46. [DOI] [PubMed] [Google Scholar]
- 33.Davidson SM, Duchen MR. Endothelial mitochondria: contributing to vascular function and disease. Circ Res. 2007;100:1128–1141. doi: 10.1161/01.RES.0000261970.18328.1d. [DOI] [PubMed] [Google Scholar]
- 34.St-Pierre J, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, et al. Laminar flow activates peroxisome proliferator-activated receptor-gamma in vascular endothelial cells. Circulation. 2004;110:1128–1133. doi: 10.1161/01.CIR.0000139850.08365.EC. [DOI] [PubMed] [Google Scholar]
- 36.Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 37.Lin K, et al. Molecular mechanism of endothelial growth arrest by laminar shear stress. Proc Natl Acad Sci USA. 2000;97:9385–9389. doi: 10.1073/pnas.170282597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sessa WC. eNOS at a glance. J Cell Sci. 2004;117:2427–2429. doi: 10.1242/jcs.01165. [DOI] [PubMed] [Google Scholar]
- 39.Boo YC, et al. Coordinated regulation of endothelial nitric oxide synthase activity by phosphorylation and subcellular localization. Free Radic Biol Med. 2006;41:144–153. doi: 10.1016/j.freeradbiomed.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 40.Fisslthaler B, Fleming I. Activation and signaling by the AMP-activated protein kinase in endothelial cells. Circ Res. 2009;105:114–127. doi: 10.1161/CIRCRESAHA.109.201590. [DOI] [PubMed] [Google Scholar]
- 41.Föller M, et al. Regulation of erythrocyte survival by AMP-activated protein kinase. FASEB J. 2009;23:1072–1080. doi: 10.1096/fj.08-121772. [DOI] [PubMed] [Google Scholar]
- 42.Lin SJ, Ford E, Haigis M, Liszt G, Guarente L. Calorie restriction extends yeast life span by lowering the level of NADH. Genes Dev. 2004;18:12–16. doi: 10.1101/gad.1164804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Q, et al. Metabolic regulation of SIRT1 transcription via a HIC1:CtBP corepressor complex. Proc Natl Acad Sci USA. 2007;104:829–833. doi: 10.1073/pnas.0610590104. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Nisoli E, et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 45.Nasrin N, et al. JNK1 phosphorylates SIRT1 and promotes its enzymatic activity. PLoS One. 2009 doi: 10.1371/journal.pone.0008414. 10.1371/journal.pone.0008414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hwang J, et al. Oscillatory shear stress stimulates endothelial production of O2- from p47phox-dependent NAD(P)H oxidases, leading to monocyte adhesion. J Biol Chem. 2003;278:47291–47298. doi: 10.1074/jbc.M305150200. [DOI] [PubMed] [Google Scholar]
- 47.Guo D, Chien S, Shyy JY. Regulation of endothelial cell cycle by laminar versus oscillatory flow: distinct modes of interactions of AMP-activated protein kinase and Akt pathways. Circ Res. 2007;100:564–571. doi: 10.1161/01.RES.0000259561.23876.c5. [DOI] [PubMed] [Google Scholar]
- 48.Young A, et al. Flow activation of AMP-activated protein kinase in vascular endothelium leads to Krüppel-like factor 2 expression. Arterioscler Thromb Vasc Biol. 2009;29:1902–1908. doi: 10.1161/ATVBAHA.109.193540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schulz E, et al. Suppression of the JNK pathway by induction of a metabolic stress response prevents vascular injury and dysfunction. Circulation. 2008;118:1347–1357. doi: 10.1161/CIRCULATIONAHA.108.784298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.