Abstract
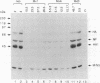
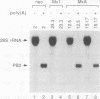
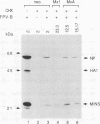
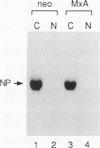
Human MxA and mouse Mx1 are interferon-induced proteins capable of inhibiting the multiplication of influenza virus. MxA protein is localized in the cytoplasm, whereas Mx1 protein accumulates in the nucleus. Taking advantage of stably transfected cell lines that constitutively express either MxA or Mx1 protein, we examined the steps at which these proteins block influenza A viruses. In infected cells expressing MxA protein, all viral mRNAs synthesized as a result of primary transcription in the nucleus by the virion-associated RNA polymerase accumulated to normal levels. These primary viral transcripts were polyadenylated, were active in directing viral protein synthesis in vitro, and appeared to be efficiently transported to the cell cytoplasm. Yet viral protein synthesis and genome amplification were strongly inhibited, suggesting that MxA protein interfered with either intracytoplasmic transport of viral mRNAs, viral protein synthesis, or translocation of newly synthesized viral proteins to the cell nucleus. However, in infected cells expressing Mx1 protein, the concentrations of the longest primary transcripts encoding the three influenza virus polymerase proteins PB1, PB2, and PA were at least 50-fold reduced. Accumulation of the shorter primary transcripts encoding the other viral proteins was also inhibited but to a lesser extent. These results demonstrate that the mouse Mx1 protein interferes with primary transcription of influenza virus in the nucleus, whereas the human MxA protein inhibits a subsequent step that presumably takes place in the cytoplasm of infected cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi M., Fäh J., Hurt N., Samuel C. E., Thomis D., Bazzigher L., Pavlovic J., Haller O., Staeheli P. cDNA structures and regulation of two interferon-induced human Mx proteins. Mol Cell Biol. 1989 Nov;9(11):5062–5072. doi: 10.1128/mcb.9.11.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnheiter H., Meier E. Mx proteins: antiviral proteins by chance or by necessity? New Biol. 1990 Oct;2(10):851–857. [PubMed] [Google Scholar]
- Bean W. J., Jr, Simpson R. W. Primary transcription of the influenza virus genome in permissive cells. Virology. 1973 Dec;56(2):646–651. doi: 10.1016/0042-6822(73)90067-6. [DOI] [PubMed] [Google Scholar]
- Broni B., Julkunen I., Condra J. H., Davies M. E., Berry M. J., Krug R. M. Parental influenza virion nucleocapsids are efficiently transported into the nuclei of murine cells expressing the nuclear interferon-induced Mx protein. J Virol. 1990 Dec;64(12):6335–6340. doi: 10.1128/jvi.64.12.6335-6340.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. S., Obar R. A., Schroeder C. C., Austin T. W., Poodry C. A., Wadsworth S. C., Vallee R. B. Multiple forms of dynamin are encoded by shibire, a Drosophila gene involved in endocytosis. Nature. 1991 Jun 13;351(6327):583–586. doi: 10.1038/351583a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dreiding P., Staeheli P., Haller O. Interferon-induced protein Mx accumulates in nuclei of mouse cells expressing resistance to influenza viruses. Virology. 1985 Jan 15;140(1):192–196. doi: 10.1016/0042-6822(85)90460-x. [DOI] [PubMed] [Google Scholar]
- Haller O., Arnheiter H., Lindenmann J., Gresser I. Host gene influences sensitivity to interferon action selectively for influenza virus. Nature. 1980 Feb 14;283(5748):660–662. doi: 10.1038/283660a0. [DOI] [PubMed] [Google Scholar]
- Haller O. Inborn resistance of ice to orthomyxoviruses. Curr Top Microbiol Immunol. 1981;92:25–52. doi: 10.1007/978-3-642-68069-4_3. [DOI] [PubMed] [Google Scholar]
- Horisberger M. A., Haller O., Arnheiter H. Interferon-dependent genetic resistance to influenza virus in mice: virus replication in macrophages is inhibited at an early step. J Gen Virol. 1980 Sep;50(1):205–210. doi: 10.1099/0022-1317-50-1-205. [DOI] [PubMed] [Google Scholar]
- Israël A. Preliminary characterization of the particles from productive and abortive infections of L cells by fowl plague virus. Ann Microbiol (Paris) 1979 Jul;130B(1):85–100. [PubMed] [Google Scholar]
- Krug R. M., Shaw M., Broni B., Shapiro G., Haller O. Inhibition of influenza viral mRNA synthesis in cells expressing the interferon-induced Mx gene product. J Virol. 1985 Oct;56(1):201–206. doi: 10.1128/jvi.56.1.201-206.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marsh M., Helenius A. Virus entry into animal cells. Adv Virus Res. 1989;36:107–151. doi: 10.1016/S0065-3527(08)60583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin K., Helenius A. Transport of incoming influenza virus nucleocapsids into the nucleus. J Virol. 1991 Jan;65(1):232–244. doi: 10.1128/jvi.65.1.232-244.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier E., Fäh J., Grob M. S., End R., Staeheli P., Haller O. A family of interferon-induced Mx-related mRNAs encodes cytoplasmic and nuclear proteins in rat cells. J Virol. 1988 Jul;62(7):2386–2393. doi: 10.1128/jvi.62.7.2386-2393.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T., Horisberger M. A. Combined action of mouse alpha and beta interferons in influenza virus-infected macrophages carrying the resistance gene Mx. J Virol. 1984 Mar;49(3):709–716. doi: 10.1128/jvi.49.3.709-716.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obar R. A., Collins C. A., Hammarback J. A., Shpetner H. S., Vallee R. B. Molecular cloning of the microtubule-associated mechanochemical enzyme dynamin reveals homology with a new family of GTP-binding proteins. Nature. 1990 Sep 20;347(6290):256–261. doi: 10.1038/347256a0. [DOI] [PubMed] [Google Scholar]
- Pavlovic J., Banz E., Parish R. W. The effects of transcription on the nucleosome structure of four Dictyostelium genes. Nucleic Acids Res. 1989 Mar 25;17(6):2315–2332. doi: 10.1093/nar/17.6.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovic J., Staeheli P. The antiviral potentials of Mx proteins. J Interferon Res. 1991 Aug;11(4):215–219. doi: 10.1089/jir.1991.11.215. [DOI] [PubMed] [Google Scholar]
- Pavlovic J., Zürcher T., Haller O., Staeheli P. Resistance to influenza virus and vesicular stomatitis virus conferred by expression of human MxA protein. J Virol. 1990 Jul;64(7):3370–3375. doi: 10.1128/jvi.64.7.3370-3375.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotch S. J., Bouloy M., Ulmanen I., Krug R. M. A unique cap(m7GpppXm)-dependent influenza virion endonuclease cleaves capped RNAs to generate the primers that initiate viral RNA transcription. Cell. 1981 Mar;23(3):847–858. doi: 10.1016/0092-8674(81)90449-9. [DOI] [PubMed] [Google Scholar]
- Repik P., Flamand A., Bishop D. H. Effect of interferon upon the primary and secondary transcription of vesicular stomatitis and influenza viruses. J Virol. 1974 Nov;14(5):1169–1178. doi: 10.1128/jvi.14.5.1169-1178.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. H., Raymond C. K., Gilbert T., O'Hara P. J., Stevens T. H. A putative GTP binding protein homologous to interferon-inducible Mx proteins performs an essential function in yeast protein sorting. Cell. 1990 Jun 15;61(6):1063–1074. doi: 10.1016/0092-8674(90)90070-u. [DOI] [PubMed] [Google Scholar]
- Shapiro G. I., Gurney T., Jr, Krug R. M. Influenza virus gene expression: control mechanisms at early and late times of infection and nuclear-cytoplasmic transport of virus-specific RNAs. J Virol. 1987 Mar;61(3):764–773. doi: 10.1128/jvi.61.3.764-773.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staeheli P., Dreiding P., Haller O., Lindenmann J. Polyclonal and monoclonal antibodies to the interferon-inducible protein Mx of influenza virus-resistant mice. J Biol Chem. 1985 Feb 10;260(3):1821–1825. [PubMed] [Google Scholar]
- Staeheli P., Haller O., Boll W., Lindenmann J., Weissmann C. Mx protein: constitutive expression in 3T3 cells transformed with cloned Mx cDNA confers selective resistance to influenza virus. Cell. 1986 Jan 17;44(1):147–158. doi: 10.1016/0092-8674(86)90493-9. [DOI] [PubMed] [Google Scholar]
- Staeheli P., Haller O. Interferon-induced human protein with homology to protein Mx of influenza virus-resistant mice. Mol Cell Biol. 1985 Aug;5(8):2150–2153. doi: 10.1128/mcb.5.8.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staeheli P. Interferon-induced proteins and the antiviral state. Adv Virus Res. 1990;38:147–200. doi: 10.1016/s0065-3527(08)60862-3. [DOI] [PubMed] [Google Scholar]
- Staeheli P., Pavlovic J. Inhibition of vesicular stomatitis virus mRNA synthesis by human MxA protein. J Virol. 1991 Aug;65(8):4498–4501. doi: 10.1128/jvi.65.8.4498-4501.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh E., Driscoll R., Coltrera M., Olins A., Bloom K. A dynamin-like protein encoded by the yeast sporulation gene SPO15. Nature. 1991 Feb 21;349(6311):713–715. doi: 10.1038/349713a0. [DOI] [PubMed] [Google Scholar]
- van der Bliek A. M., Meyerowitz E. M. Dynamin-like protein encoded by the Drosophila shibire gene associated with vesicular traffic. Nature. 1991 May 30;351(6325):411–414. doi: 10.1038/351411a0. [DOI] [PubMed] [Google Scholar]