Abstract
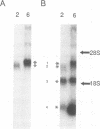
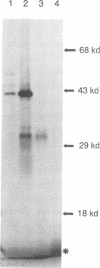
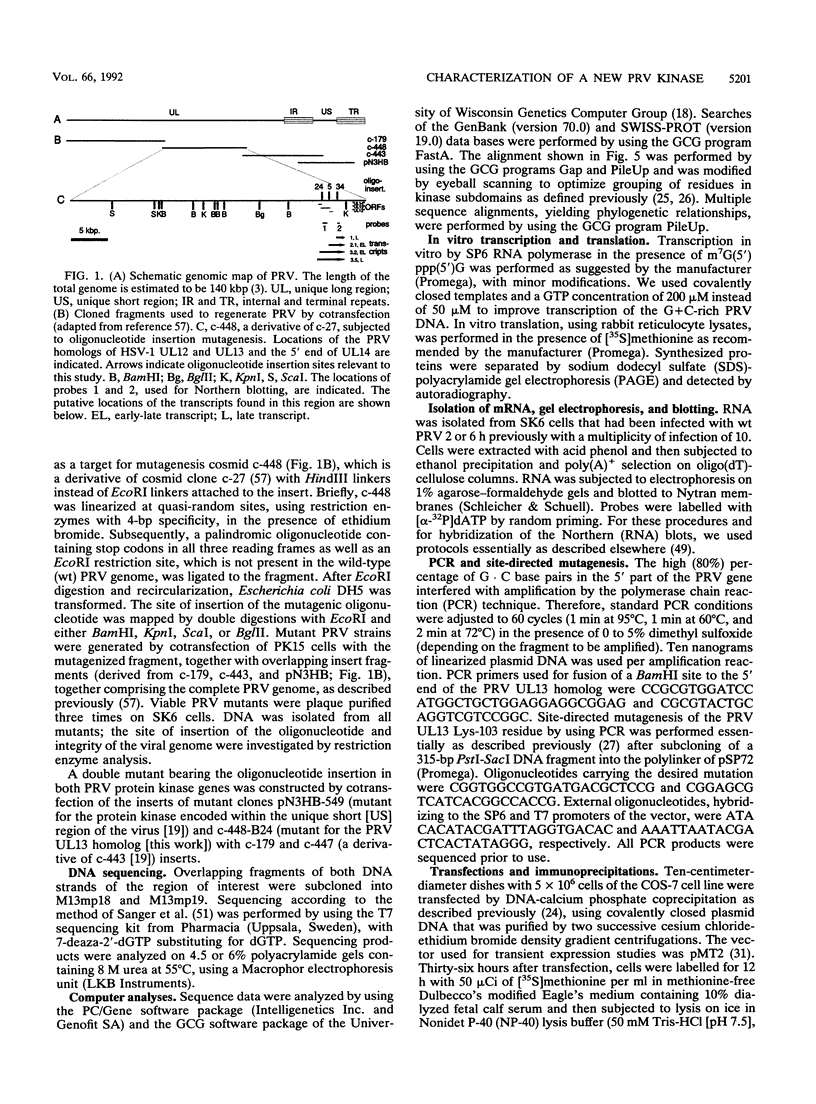
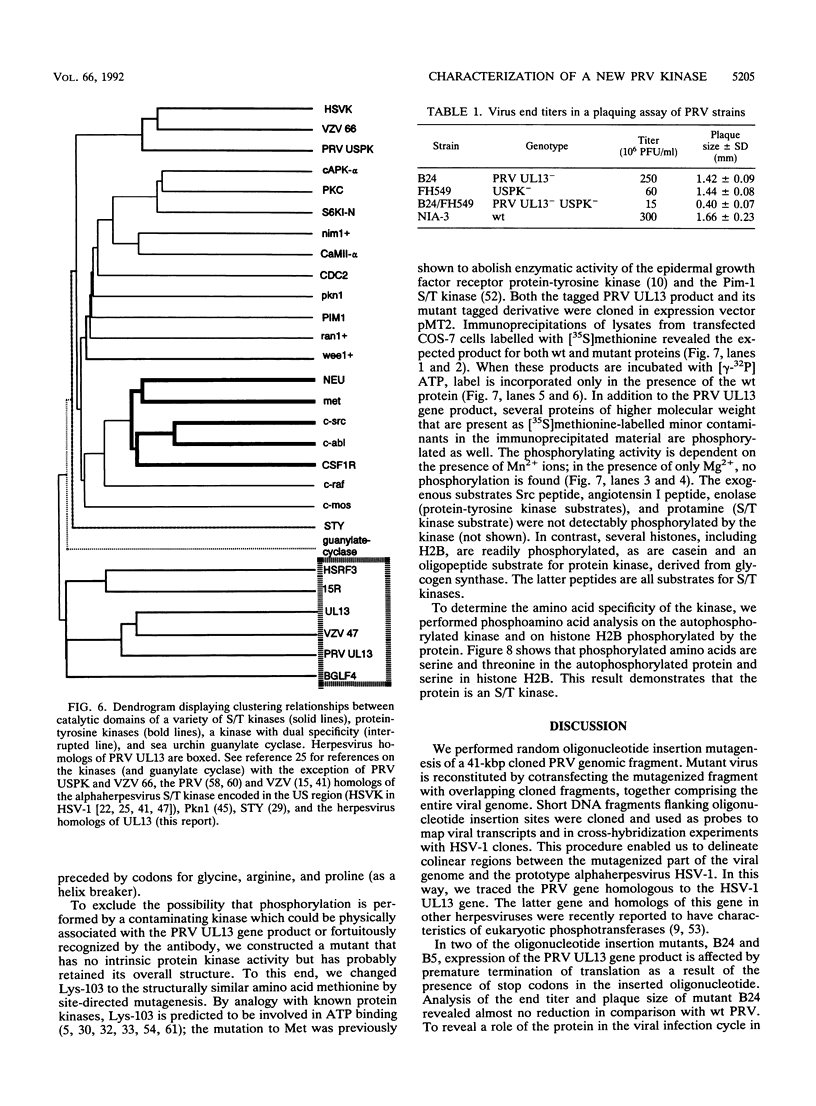
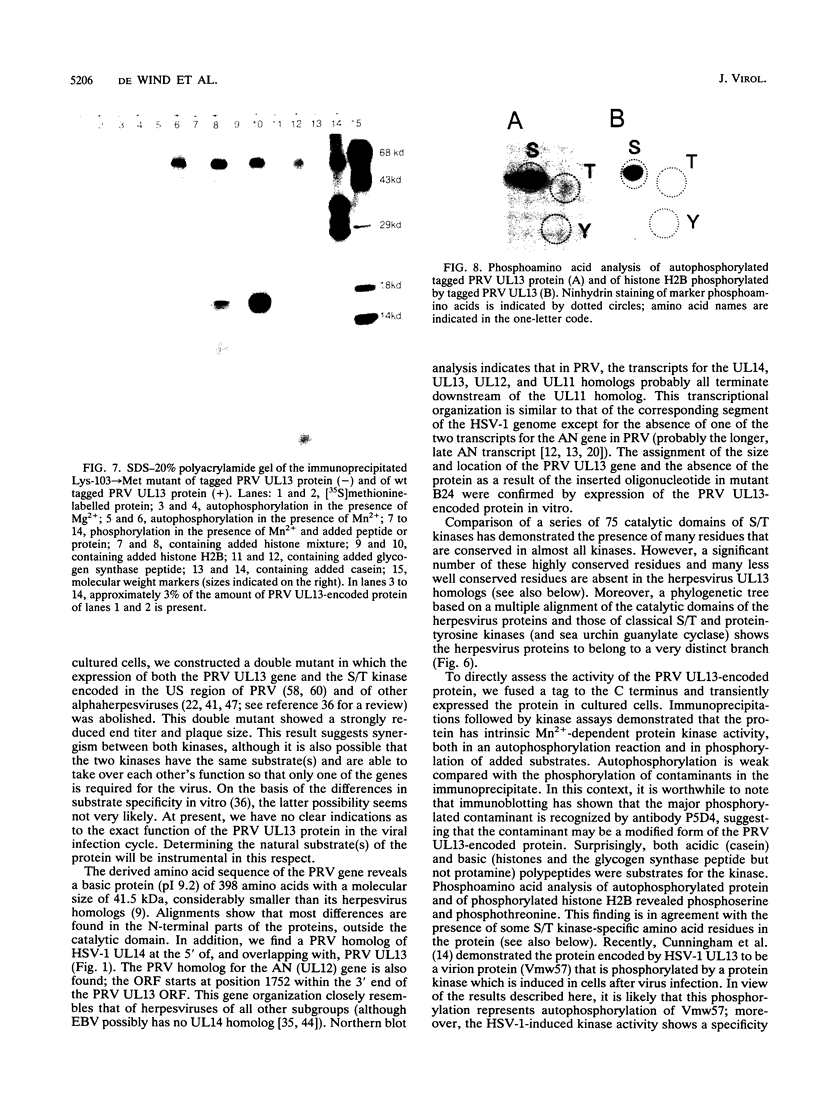
We have performed large-scale random oligonucleotide insertion mutagenesis on a 41-kbp genomic segment derived from the unique long (UL) region of the alphaherpesvirus pseudorabies virus (PRV). This procedure has resulted in the generation of a series of PRV strains, each carrying a single gene whose termination of translation is induced by the inserted oligonucleotide. To relate the genes that were involved in the mutagenization to genes previously identified in herpes simplex virus type 1, the prototype alphaherpesvirus, we have performed cross-hybridization studies. In this way, we have mapped the location of the homolog of a gene which was described to have sequence characteristics of a eukaryotic phosphotransferase. We characterized the phenotype of a mutant PRV strain lacking this putative phosphotransferase also the phenotype of a PRV strain lacking, in addition to the UL-encoded putative phosphotransferase, the protein kinase encoded within the unique short region of the virus. To assess the enzymatic activity of the UL region-encoded phosphotransferase, we expressed the gene transiently in a eukaryotic expression system. Immunoprecipitation of the protein followed by kinase assays and phosphoamino acid analyses revealed protein-serine/threonine kinase activity. Implications of sequence divergence of this protein from classical protein-serine/threonine kinases for kinase structure and function are discussed in view of the recent resolution of the structure of the catalytic domain of cyclic AMP-dependent protein kinase.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Ben-Porat T., Veach R. A., Ihara S. Localization of the regions of homology between the genomes of herpes simplex virus, type 1, and pseudorabies virus. Virology. 1983 May;127(1):194–204. doi: 10.1016/0042-6822(83)90383-5. [DOI] [PubMed] [Google Scholar]
- Bhatnagar D., Hartl F. T., Roskoski R., Jr, Lessor R. A., Leonard N. J. Adenosine cyclic 3',5'-monophosphate dependent protein kinase: nucleotide binding to the chemically modified catalytic subunit. Biochemistry. 1984 Sep 11;23(19):4350–4357. doi: 10.1021/bi00314a016. [DOI] [PubMed] [Google Scholar]
- Boyle W. J., van der Geer P., Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- Chee M. S., Bankier A. T., Beck S., Bohni R., Brown C. M., Cerny R., Horsnell T., Hutchison C. A., 3rd, Kouzarides T., Martignetti J. A. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr Top Microbiol Immunol. 1990;154:125–169. doi: 10.1007/978-3-642-74980-3_6. [DOI] [PubMed] [Google Scholar]
- Chee M. S., Lawrence G. L., Barrell B. G. Alpha-, beta- and gammaherpesviruses encode a putative phosphotransferase. J Gen Virol. 1989 May;70(Pt 5):1151–1160. doi: 10.1099/0022-1317-70-5-1151. [DOI] [PubMed] [Google Scholar]
- Chee M., Barrell B. Herpesviruses: a study of parts. Trends Genet. 1990 Mar;6(3):86–91. doi: 10.1016/0168-9525(90)90099-r. [DOI] [PubMed] [Google Scholar]
- Chen W. S., Lazar C. S., Poenie M., Tsien R. Y., Gill G. N., Rosenfeld M. G. Requirement for intrinsic protein tyrosine kinase in the immediate and late actions of the EGF receptor. 1987 Aug 27-Sep 2Nature. 328(6133):820–823. doi: 10.1038/328820a0. [DOI] [PubMed] [Google Scholar]
- Chung T. D., Wymer J. P., Smith C. C., Kulka M., Aurelian L. Protein kinase activity associated with the large subunit of herpes simplex virus type 2 ribonucleotide reductase (ICP10). J Virol. 1989 Aug;63(8):3389–3398. doi: 10.1128/jvi.63.8.3389-3398.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa R. H., Draper K. G., Banks L., Powell K. L., Cohen G., Eisenberg R., Wagner E. K. High-resolution characterization of herpes simplex virus type 1 transcripts encoding alkaline exonuclease and a 50,000-dalton protein tentatively identified as a capsid protein. J Virol. 1983 Dec;48(3):591–603. doi: 10.1128/jvi.48.3.591-603.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa R. H., Draper K. G., Kelly T. J., Wagner E. K. An unusual spliced herpes simplex virus type 1 transcript with sequence homology to Epstein-Barr virus DNA. J Virol. 1985 May;54(2):317–328. doi: 10.1128/jvi.54.2.317-328.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C., Davison A. J., Dolan A., Frame M. C., McGeoch D. J., Meredith D. M., Moss H. W., Orr A. C. The UL13 virion protein of herpes simplex virus type 1 is phosphorylated by a novel virus-induced protein kinase. J Gen Virol. 1992 Feb;73(Pt 2):303–311. doi: 10.1099/0022-1317-73-2-303. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Scott J. E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986 Sep;67(Pt 9):1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Taylor P. Genetic relations between varicella-zoster virus and Epstein-Barr virus. J Gen Virol. 1987 Apr;68(Pt 4):1067–1079. doi: 10.1099/0022-1317-68-4-1067. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Wilkie N. M. Location and orientation of homologous sequences in the genomes of five herpesviruses. J Gen Virol. 1983 Sep;64(Pt 9):1927–1942. doi: 10.1099/0022-1317-64-9-1927. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper K. G., Devi-Rao G., Costa R. H., Blair E. D., Thompson R. L., Wagner E. K. Characterization of the genes encoding herpes simplex virus type 1 and type 2 alkaline exonucleases and overlapping proteins. J Virol. 1986 Mar;57(3):1023–1036. doi: 10.1128/jvi.57.3.1023-1036.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einberger H., Mertz R., Hofschneider P. H., Neubert W. J. Purification, renaturation, and reconstituted protein kinase activity of the Sendai virus large (L) protein: L protein phosphorylates the NP and P proteins in vitro. J Virol. 1990 Sep;64(9):4274–4280. doi: 10.1128/jvi.64.9.4274-4280.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame M. C., Purves F. C., McGeoch D. J., Marsden H. S., Leader D. P. Identification of the herpes simplex virus protein kinase as the product of viral gene US3. J Gen Virol. 1987 Oct;68(Pt 10):2699–2704. doi: 10.1099/0022-1317-68-10-2699. [DOI] [PubMed] [Google Scholar]
- Goldstein D. J., Weller S. K. Herpes simplex virus type 1-induced ribonucleotide reductase activity is dispensable for virus growth and DNA synthesis: isolation and characterization of an ICP6 lacZ insertion mutant. J Virol. 1988 Jan;62(1):196–205. doi: 10.1128/jvi.62.1.196-205.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M. Protein kinase catalytic domain sequence database: identification of conserved features of primary structure and classification of family members. Methods Enzymol. 1991;200:38–62. doi: 10.1016/0076-6879(91)00126-h. [DOI] [PubMed] [Google Scholar]
- Higuchi R., Krummel B., Saiki R. K. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988 Aug 11;16(15):7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House C., Wettenhall R. E., Kemp B. E. The influence of basic residues on the substrate specificity of protein kinase C. J Biol Chem. 1987 Jan 15;262(2):772–777. [PubMed] [Google Scholar]
- Howell B. W., Afar D. E., Lew J., Douville E. M., Icely P. L., Gray D. A., Bell J. C. STY, a tyrosine-phosphorylating enzyme with sequence homology to serine/threonine kinases. Mol Cell Biol. 1991 Jan;11(1):568–572. doi: 10.1128/mcb.11.1.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps M. P., Taylor S. S., Sefton B. M. Direct evidence that oncogenic tyrosine kinases and cyclic AMP-dependent protein kinase have homologous ATP-binding sites. Nature. 1984 Aug 16;310(5978):589–592. doi: 10.1038/310589a0. [DOI] [PubMed] [Google Scholar]
- Kaufman R. J., Davies M. V., Pathak V. K., Hershey J. W. The phosphorylation state of eucaryotic initiation factor 2 alters translational efficiency of specific mRNAs. Mol Cell Biol. 1989 Mar;9(3):946–958. doi: 10.1128/mcb.9.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knighton D. R., Zheng J. H., Ten Eyck L. F., Ashford V. A., Xuong N. H., Taylor S. S., Sowadski J. M. Crystal structure of the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science. 1991 Jul 26;253(5018):407–414. doi: 10.1126/science.1862342. [DOI] [PubMed] [Google Scholar]
- Knighton D. R., Zheng J. H., Ten Eyck L. F., Xuong N. H., Taylor S. S., Sowadski J. M. Structure of a peptide inhibitor bound to the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science. 1991 Jul 26;253(5018):414–420. doi: 10.1126/science.1862343. [DOI] [PubMed] [Google Scholar]
- Kreis T. E. Microinjected antibodies against the cytoplasmic domain of vesicular stomatitis virus glycoprotein block its transport to the cell surface. EMBO J. 1986 May;5(5):931–941. doi: 10.1002/j.1460-2075.1986.tb04306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence G. L., Chee M., Craxton M. A., Gompels U. A., Honess R. W., Barrell B. G. Human herpesvirus 6 is closely related to human cytomegalovirus. J Virol. 1990 Jan;64(1):287–299. doi: 10.1128/jvi.64.1.287-299.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leader D. P., Purves F. C. The herpesvirus protein kinase: a new departure in protein phosphorylation? Trends Biochem Sci. 1988 Jul;13(7):244–246. doi: 10.1016/0968-0004(88)90157-0. [DOI] [PubMed] [Google Scholar]
- Maru Y., Witte O. N. The BCR gene encodes a novel serine/threonine kinase activity within a single exon. Cell. 1991 Nov 1;67(3):459–468. doi: 10.1016/0092-8674(91)90521-y. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Dalrymple M. A., Davison A. J., Dolan A., Frame M. C., McNab D., Perry L. J., Scott J. E., Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Jul;69(Pt 7):1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Davison A. J. Alphaherpesviruses possess a gene homologous to the protein kinase gene family of eukaryotes and retroviruses. Nucleic Acids Res. 1986 Feb 25;14(4):1765–1777. doi: 10.1093/nar/14.4.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Dolan A., Donald S., Rixon F. J. Sequence determination and genetic content of the short unique region in the genome of herpes simplex virus type 1. J Mol Biol. 1985 Jan 5;181(1):1–13. doi: 10.1016/0022-2836(85)90320-1. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Dolan A., Frame M. C. DNA sequence of the region in the genome of herpes simplex virus type 1 containing the exonuclease gene and neighbouring genes. Nucleic Acids Res. 1986 Apr 25;14(8):3435–3448. doi: 10.1093/nar/14.8.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J. Evolutionary relationships of virion glycoprotein genes in the S regions of alphaherpesvirus genomes. J Gen Virol. 1990 Oct;71(Pt 10):2361–2367. doi: 10.1099/0022-1317-71-10-2361. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J. The genomes of the human herpesviruses: contents, relationships, and evolution. Annu Rev Microbiol. 1989;43:235–265. doi: 10.1146/annurev.mi.43.100189.001315. [DOI] [PubMed] [Google Scholar]
- Muñoz-Dorado J., Inouye S., Inouye M. A gene encoding a protein serine/threonine kinase is required for normal development of M. xanthus, a gram-negative bacterium. Cell. 1991 Nov 29;67(5):995–1006. doi: 10.1016/0092-8674(91)90372-6. [DOI] [PubMed] [Google Scholar]
- Perry L. J., McGeoch D. J. The DNA sequences of the long repeat region and adjoining parts of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Nov;69(Pt 11):2831–2846. doi: 10.1099/0022-1317-69-11-2831. [DOI] [PubMed] [Google Scholar]
- Purves F. C., Longnecker R. M., Leader D. P., Roizman B. Herpes simplex virus 1 protein kinase is encoded by open reading frame US3 which is not essential for virus growth in cell culture. J Virol. 1987 Sep;61(9):2896–2901. doi: 10.1128/jvi.61.9.2896-2901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B., Jenkins F. J. Genetic engineering of novel genomes of large DNA viruses. Science. 1985 Sep 20;229(4719):1208–1214. doi: 10.1126/science.2994215. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saris C. J., Domen J., Berns A. The pim-1 oncogene encodes two related protein-serine/threonine kinases by alternative initiation at AUG and CUG. EMBO J. 1991 Mar;10(3):655–664. doi: 10.1002/j.1460-2075.1991.tb07994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. F., Smith T. F. Identification of new protein kinase-related genes in three herpesviruses, herpes simplex virus, varicella-zoster virus, and Epstein-Barr virus. J Virol. 1989 Jan;63(1):450–455. doi: 10.1128/jvi.63.1.450-455.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg M. J., Taylor W. R. Modelling the ATP-binding site of oncogene products, the epidermal growth factor receptor and related proteins. FEBS Lett. 1984 Oct 1;175(2):387–392. doi: 10.1016/0014-5793(84)80774-7. [DOI] [PubMed] [Google Scholar]
- Sugden B. Herpes viruses: human transducing viruses. Trends Biochem Sci. 1991 Feb;16(2):45–46. doi: 10.1016/0968-0004(91)90019-r. [DOI] [PubMed] [Google Scholar]
- Sánchez A., De B. P., Banerjee A. K. In vitro phosphorylation of NS protein by the L protein of vesicular stomatitis virus. J Gen Virol. 1985 May;66(Pt 5):1025–1036. doi: 10.1099/0022-1317-66-5-1025. [DOI] [PubMed] [Google Scholar]
- Taylor S. S., Buechler J. A., Yonemoto W. cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu Rev Biochem. 1990;59:971–1005. doi: 10.1146/annurev.bi.59.070190.004543. [DOI] [PubMed] [Google Scholar]
- Wu J. Y., Zhou Z. Y., Judd A., Cartwright C. A., Robinson W. S. The hepatitis B virus-encoded transcriptional trans-activator hbx appears to be a novel protein serine/threonine kinase. Cell. 1990 Nov 16;63(4):687–695. doi: 10.1016/0092-8674(90)90135-2. [DOI] [PubMed] [Google Scholar]
- Zhang G., Stevens R., Leader D. P. The protein kinase encoded in the short unique region of pseudorabies virus: description of the gene and identification of its product in virions and in infected cells. J Gen Virol. 1990 Aug;71(Pt 8):1757–1765. doi: 10.1099/0022-1317-71-8-1757. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Nelson N. C., Taylor S. S. Affinity labeling of cAMP-dependent protein kinase with p-fluorosulfonylbenzoyl adenosine. Covalent modification of lysine 71. J Biol Chem. 1981 Nov 10;256(21):10837–10842. [PubMed] [Google Scholar]
- de Wind N., Zijderveld A., Glazenburg K., Gielkens A., Berns A. Linker insertion mutagenesis of herpesviruses: inactivation of single genes within the Us region of pseudorabies virus. J Virol. 1990 Oct;64(10):4691–4696. doi: 10.1128/jvi.64.10.4691-4696.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zijl M., Quint W., Briaire J., de Rover T., Gielkens A., Berns A. Regeneration of herpesviruses from molecularly cloned subgenomic fragments. J Virol. 1988 Jun;62(6):2191–2195. doi: 10.1128/jvi.62.6.2191-2195.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zijl M., van der Gulden H., de Wind N., Gielkens A., Berns A. Identification of two genes in the unique short region of pseudorabies virus; comparison with herpes simplex virus and varicella-zoster virus. J Gen Virol. 1990 Aug;71(Pt 8):1747–1755. doi: 10.1099/0022-1317-71-8-1747. [DOI] [PubMed] [Google Scholar]