Abstract
Developmental dyslexia is characterized by severe reading and spelling difficulties that are persistent and resistant to the usual didactic measures and remedial efforts. It is well established that a major cause of these problems lies in poorly specified representations of speech sounds. One hypothesis states that this phonological deficit results from a more fundamental deficit in auditory processing. Despite substantial research effort, the specific nature of these auditory problems remains debated. A first controversy concerns the speech specificity of the auditory processing problems: Can they be reduced to more basic auditory processing, or are they specific to the perception of speech sounds? A second topic of debate concerns the extent to which the auditory problems are specific to the processing of rapidly changing temporal information or whether they encompass a broader range of complex spectro-temporal processing. By applying a balanced design with stimuli that were adequately controlled for acoustic complexity, we show that adults with dyslexia are specifically impaired at categorizing speech and nonspeech sounds that differ in terms of rapidly changing acoustic cues (i.e., temporal cues), but that they perform adequately when categorizing steady-state speech and nonspeech sounds. Thus, we show that individuals with dyslexia have an auditory temporal processing deficit that is not speech-specific.
Keywords: auditory processing, categorical perception, speech perception
Speech contains a number of acoustic cues that are used to discriminate speech sounds belonging to different phonetic categories. For example, the acoustic cue that is critical for differentiating /bA/ versus /dA/, a stop consonant followed by a vowel, lies within the first 100 ms of the sounds, during which time the frequency of the second formant changes rapidly (i.e., a temporal cue). In contrast, the acoustic difference between two vowels such as /u/ versus /y/ lies in the frequency of the second formant, which stays relatively stable over time. Hence, an accurate perception of steady-state (i.e., nontemporal) spectral cues is essential for identification of these vowels. There is ample evidence that individuals with dyslexia exhibit problems in the representation of speech sounds (1), and that these may be rooted in a more fundamental auditory processing deficit (2). Originally, it was claimed that individuals with dyslexia have a deficit in processing auditory cues that are “temporal” in nature (i.e., rapidly changing), thereby causing problems in the accurate processing of rapid acoustic changes in speech (such as in stop consonants) (3). This speech perception problem was thought to consequently cause a cascade of effects, starting with the disruption of the normal development of the phonological system, eventually resulting in problems learning to read and spell. However, despite substantial research efforts, the literature is not concordant with respect to the specific nature of these auditory problems. In particular, it is unclear (i) whether the problem is specific to the perception of speech sounds (4) or whether it includes basic acoustic processing more generally (3), and (ii) whether the auditory problem is specific to rapid temporal processing (3, 5) or whether it encompasses a broader range of spectro-temporal processing abilities (6).
Although previous speech perception studies predominantly indicate that individuals with dyslexia are less categorical than normal readers in the way that they perceive phonetic contrasts, especially stop consonants (7–28; but see 6, 29–32 for contra-evidence), these studies are inconclusive as to whether these problems are exclusive to speech. Indeed, one cannot ignore the large body of literature that has demonstrated deficits in basic auditory perception of nonspeech sounds in individuals with dyslexia (reviewed in refs. 2, 33). Yet, some authors have argued that these deficits may depend on general factors such as stimulus complexity, and the use of specific task paradigms (34). To unequivocally answer the speech-specificity question, it is necessary to use stimuli with similar acoustic complexity and to administer speech and nonspeech tasks using identical paradigms. The few studies that used identical test paradigms (4, 6, 22, 24, 32) did not control for the acoustic complexity of the speech and nonspeech signals, and used either sine-wave-speech, isolated formants, or tones as the nonspeech counterpart. Consequently, although the results of these studies have mainly suggested that the auditory deficit is speech-specific, it may also be “that the auditory deficit in dyslexia is general but confined to stimuli that are more complex than the nonspeech analogues used here” (22).
With regard to the temporal-specificity issue, several studies have demonstrated that individuals with dyslexia tend to have difficulties in processing sequences of brief, rapidly presented sounds (reviewed in ref. 33), with more recent studies focusing on an impaired perception of dynamic aspects in the auditory signal itself (5). However, other scholars have failed to replicate findings of auditory temporal problems in individuals with dyslexia (35) or have demonstrated auditory problems in tasks that cannot be categorized as specifically temporal [e.g., frequency discrimination (36), backward notched-noise conditions (37)]. This suggests that the auditory deficit encompasses a rather broad range of complex spectro-temporal processing abilities that are not yet fully understood. Again, the best way to investigate this temporal-specificity issue is to evaluate the perception of temporal and nontemporal cues by means of identical test paradigms. One line of research addressed this question by manipulating the interstimulus interval (ISI) and/or duration of sounds (either speech or nonspeech). Here, findings are inconsistent. Some studies have demonstrated that the deficits are independent of ISI and stimulus duration (9, 12, 27, 38–42). Others, however, showed deficits exclusively for brief ISIs and stimulus durations, thus supporting the temporal-specificity hypothesis (3, 23, 43–46). A second line of studies examined the temporal-specific question by evaluating categorical perception of speech sounds, and compared performance on steady-state phonemes (i.e., vowels) versus temporal phonemes (i.e., stop consonants). Once again, findings were contradictory: One study suggested that there is no specific deficit (31), whereas other studies found evidence for a specific temporal deficit (22, 26).
The present study is unique in addressing both the speech-specific and temporal-specific issues within one test paradigm, with stimuli adequately controlled for acoustic complexity. We tested 31 adults with dyslexia and 31 matched adults with normal reading (Table 1) on four types of stimuli: (i) a speech contrast exploiting temporal cues (/bA/-/dA/), (ii) a speech contrast defined by nontemporal, steady-state cues (/u/-/y/), (iii) a nonspeech temporal contrast (spectrally rotated /bA/-/dA/), and (iv) a nonspeech nontemporal contrast (spectrally rotated /u/-/y/) (Fig. 1). Given that the presence of auditory deficits may be related to stimulus complexity and/or to the use of specific task paradigms (34), we used stimuli containing the same degree of acoustic complexity and presented them using the same ABX identification paradigm. Matching the acoustic complexity of the speech and nonspeech sounds was achieved by spectrally rotating the former to create the latter (47, 48), resulting in unintelligible signals that are not perceived as speech but that show the same spectro-temporal complexity. The balanced design of the present study enabled us to disentangle some aspects of the existing controversy concerning auditory processing problems in dyslexia. According to the speech-specific hypothesis, it is predicted that individuals with dyslexia will show deficits on both the temporal and nontemporal speech contrasts while performing adequately on both nonspeech contrasts. Alternatively, according to the auditory temporal processing hypothesis, individuals with dyslexia will fail on both temporal contrasts but not on the nontemporal ones. In case the deficit is general or no deficit is present, we expect no differential effect on the four types of contrasts: either there will be a group difference on all of the four stimulus types, or there will be none.
Table 1.
Participant characteristics: Mean (SD) and test statistics (mixed-model pairwise comparison)
| Dyslexic readers (n = 31) | Normal readers (n = 31) | Test statistics | |
| Gender (men/women) | 9/22 | 9/22 | — |
| Age (y) | 21.4 (2.8) | 21.5 (3.0) | F(1, 30) = 0.12, P = 0.73 |
| Nonverbal IQ* (WAIS-III: Matrices) | 108 (13) | 106 (10) | F(1, 30) = 0.49, P = 0.49 |
| Word reading† | 67 (2) | 96 (13) | F(1, 30) = 116, P < 0.0001 |
| Pseudoword reading† | 67 (3) | 104 (11) | F(1, 30) = 240, P < 0.0001 |
| Spelling† | 72 (9) | 103 (10) | F(1, 30) = 87, P < 0.0001 |
*Standardized scores with population average (mean = 100, SD = 15).
†Standardized scores relative to a reference group of university students (mean = 100, SD =15).
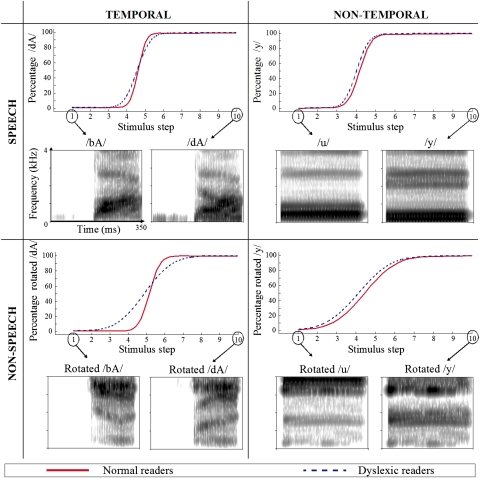
Fig. 1.
For each of the four conditions, the spectrograms of the two endpoint stimuli and the estimated identification curves are shown. The estimated identification curves are based on the averaged slope and category boundary parameters per group. Broken blue lines depict dyslexic reading group; unbroken red lines depict normal reading group. Percentage of /dA/, /y/, rotated /dA/ and rotated /y/ responses (y axis) is shown along the 10 stimulus steps (x axis). On spectrograms, x axis represents time (350 ms), y axis represents frequency (4 kHz), and intensity of gray scale represents amplitude.
Results
Categorical perception (CP) was used as the test paradigm. CP is based on the principle that different exemplars of speech sounds (phonemes) are consistently attributed to the same phonetic category despite small acoustic differences. CP may therefore be regarded as fundamental to the development of well-specified phonological representations. Categorical perception can also be obtained for nonspeech stimuli that emulate certain speech cues (49). The parameter of interest in this study was the slope of the identification curve at the category boundary. A high slope value indicates a small uncertainty range and suggests a highly consistent ability to categorize sounds, whereas a low slope value indicates a large range of uncertainty and suggests difficulties in identifying the sounds (19).
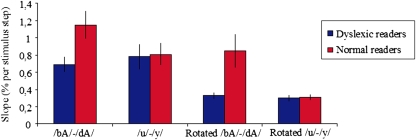
Data were analyzed by means of a 2 (dyslexic reading versus normal reading group) × 2 (temporal versus nontemporal) × 2 (speech versus nonspeech) factorial design. The analysis showed a main effect of group [F(1, 30) = 9.56, P = 0.004], with shallower slopes in the dyslexic reading group compared with the normal reading group. However, this main effect should be interpreted in light of the significant group × temporal/nontemporal interaction [F(1, 180) = 7.06, P = 0.009], which is shown in Fig. 2. First, posthoc analyses showed that individuals with dyslexia had a significantly shallower slope than individuals with normal reading on the temporal continua [t(78.9) = 4.08, P = 0.0001] but not on the nontemporal continua [t(78.9) = 0.69, P = 0.49]. Thus, the imprecise categorization in individuals with dyslexia is present only for sounds that are distinguished on the basis of temporal cues, and this both in the speech (/bA/-/dA/) and the nonspeech continua (rotated /bA/-/dA/), as no significant group × speech/nonspeech interaction was observed [F(1, 180) = 0.78, P = 0.38]. An important implication of the latter is that the categorization deficit in individuals with dyslexia is not speech-specific. Second, when inspecting the group × temporal/nontemporal interaction effect further, we found that normal readers showed a significantly steeper slope in the temporal conditions compared to the nontemporal ones [t(180) = −4.63, P < 0.0001], whereas the dyslexic reading group did not show such a differential effect [t(180) = −0.87, P = 0.38]. This effect was the same for the speech and nonspeech continua because there was no significant speech/nonspeech × temporal/nontemporal interaction [F(1, 180) = 0.72, P = 0.40]. Finally, no significant third-order interaction was found [F(1, 180) = 0.23, P = 0.63].
Fig. 2.
Average identification slopes of four stimulus continua for dyslexic reading and normal reading group. Error bars indicate ±1 SEM per group.
In addition to the factorial analysis, Pearson correlations were calculated between the categorical perception data of the four conditions and scores on the three literacy measures (word and pseudoword reading and spelling, discussed in Methods). For the whole sample, we found significant correlations between the reading and spelling tests and the slopes of the /bA/-/dA/ and rotated /bA/-/dA/ conditions, whereas we did not observe significant correlations with either of the nontemporal conditions (Table 2). This correlational analysis thus extends the findings of the group comparisons.
Table 2.
Whole-sample Pearson correlations among slopes of four conditions of categorical perception task and scores on word reading, pseudoword reading, and spelling
| Speech |
Nonspeech |
|||
| Temporal |
Nontemporal |
Temporal |
Nontemporal |
|
| /bA/-/dA/ | /u/-/y/ | Rotated /bA/-/dA/ | Rotated /u/-/y/ | |
| Word reading | 0.28* | −0.01 | 0.22 | −0.01 |
| Pseudoword reading | 0.30* | −0.04 | 0.27* | 0.09 |
| Spelling | 0.28* | 0.02 | 0.26* | −0.05 |
*P < 0.05.
Discussion
In the present study, a carefully selected group of participants was tested on four different stimulus types while task and test procedures were kept constant. The design involved creating four stimulus continua based on two dimensions: speech versus nonspeech and temporal versus nontemporal. Based on the performance on an identification task, we demonstrated that individuals with dyslexia have a temporal-specific deficit that is present both in the speech and nonspeech categorization tasks. These findings were extended by demonstrating significant correlations between the performance on both temporal conditions and reading and spelling ability.
A central implication of our findings is that they support the hypothesis that the core auditory deficit in dyslexia pertains to the processing of sounds containing rapidly changing temporal cues. Given that the task and test procedures were identical in all conditions, such factors cannot explain this temporal-specific group difference. The deficit therefore seems to depend on physical properties of the stimuli, namely whether rapidly changing cues are present or not present. Other studies that have focused on the categorization of rapidly changing versus steady-state cues are generally consistent with our results, and reveal a specific deficit in individuals with dyslexia for the categorization of stop consonants but not for vowels (22, 26). One study (31) could not find group differences for vowels or for stop consonants. In this study, the stop consonants differed along several cues, including nontemporal cues. The lack a group difference in this study in the stop consonant condition may thus have been due to methodological differences. In our study, we extend the finding of a temporal-specific speech by demonstrating that an analogous temporal processing deficit can be observed for nonspeech stimuli, matched for complexity with the speech stimuli. A second central implication of our study is thus that the deficit in individuals with dyslexia is not speech-specific. Previous studies that addressed this speech-specificity issue did not control for the acoustic complexity of the speech and nonspeech signals, and used either sine-wave-speech, isolated formants, or tones as the nonspeech counterpart (4, 6, 22, 24, 32). Consequently, although these studies mainly corroborated a speech-specific auditory deficit, they could not preclude the possibility that the auditory deficit in dyslexia might be more general but confined to relatively complex stimuli (22). This hypothesis is indeed supported by our study, in which we used spectrally rotated nonspeech analogs that contained exactly the same degree of complexity as the speech signal. It is plausible that problems with processing temporal properties in isolation or in simplified acoustic environments are less likely to occur in individuals with dyslexia, but that they show up when the relevant cues are concealed in a complex sound. A reasonable hypothesis is thus that individuals with dyslexia have problems extracting the relevant temporal cues, particularly when they are embedded within a complex acoustic signal. Otherwise stated, individuals with dyslexia might have problems extracting and distinguishing the relevant temporal cues (i.e., the signal) from the redundant acoustic information (i.e., the noise). Support for a noise-exclusion deficit in individuals with dyslexia, especially for temporal cues, has been provided by previous studies in both the visual (50) and auditory (51) modality.
We hypothesize that this temporal-specific impairment in individuals with dyslexia, which extends beyond the domain of language, might be explained by a lack of neural specialization of the auditory system for processing temporal cues. In line with prior research (52), our results confirm that individuals with normal reading label sounds containing temporal cues more categorically than steady-state sounds, and that this pattern extends beyond linguistic boundaries. Given that this pattern of categorization seems to be driven by the acoustic properties of the speech and nonspeech sounds, we hypothesize that this differential way of categorizing arises as a result of the different ways in which the auditory system processes temporal and steady-state cues (52). Several functional neuroimaging studies have shown greater left hemisphere involvement for processing temporal cues, and greater right hemisphere involvement for processing steady-state cues (53–56). This functional specialization appears to be based on low-level auditory cues rather than speech cues. Functional imaging studies in which both the speech and the temporal aspects were manipulated (57–60) replicated the pattern of left lateralization in response to rapidly changing speech and nonspeech sounds; no such left-hemisphere lateralization was observed for more steady-state or slowly changing sounds, irrespective of their speech value (reviewed in ref. 61). In addition, in a brain morphology study (62), a significant correlation was found between the amount of white matter in the left parietal cortex and the rate of learning to identify rapidly changing speech and nonspeech stimuli, whereas no correlation was observed with steady-state sounds.
Intriguingly, the present study demonstrates that individuals with dyslexia do not show this differential pattern of categorization of steady-state versus rapidly changing sounds. This may suggest that individuals with dyslexia do not use a distinct neural mechanism for processing temporal versus steady-state sounds. Support for this view is provided by electrophysiological studies showing that individuals with dyslexia do not present the typical left hemispheric dominance in response to speech or rapidly changing signals (63). In addition, functional brain imaging studies demonstrated leftward brain lateralization for rapid versus slow transitions in individuals with normal reading but not in individuals with dyslexia (64–66).
It is demonstrated that auditory training can result in plastic changes in the auditory cortex (66, 67) and, importantly, in improvements in phonological (68) and reading (67) skills. On the basis of our study, it is recommended that these auditory training studies should particularly focus on the processing of temporal cues in complex speech and nonspeech sounds.
Methods
Participants.
A total of 62 native Dutch-speaking university students with normal hearing participated in the study (Table 1), 31 of whom were in the dyslexic reading group. In line with current practice in Belgium and the Netherlands (69), the criterion used for the diagnosis of dyslexia took into account both the severity and the persistence of the literacy problem. Every individual with dyslexia had received a formal diagnosis of developmental dyslexia during childhood by a qualified psychologist. Second, when these individuals started their studies at the university, the diagnosis was verified and confirmed by the diagnostic center of our university (Katholieke Universiteit Leuven). Third, each of these individuals with dyslexia scored below the fifth percentile on a standardized pseudoword reading test (70), which we administered (university norm group, ref. 71). This resulted in a group of 31 individuals with dyslexia, for whom the diagnosis was independently confirmed three times. The group of students with dyslexia scored ~2 SD below the university reference group on standardized word reading (70) and spelling (71) tests. The other half of our participants had no history of reading difficulties and had average reading and spelling skills compared with the university norm group. Every student with dyslexia was individually matched to a student with normal reading based on education (i.e., same discipline and year), nonverbal intelligence (72), age, and gender. Participants had no history of other neurological, psychiatric, or language pathology. Based on the Edinburgh Handedness Inventory (73), all but three participants (of whom two were normal readers) were right-handed.
Stimuli.
The intensity level (RMS), duration and cutoff frequency of the spectra (<4 kHz) were identical for the four stimulus types. The endpoints, or “prototypes,” of each of the four continua are displayed in Fig. 1.
Speech continua.
Two 10-step phonetic continua were created using Praat (74): One started from a naturalistic spoken /bA/ and was interpolated to /dA/, and one started from a naturalistic spoken /u/ and was interpolated to /y/. The signals were down-sampled to 11025 Hz for linear predictive coding analyses of the formant frequencies. The linear predictive coding analysis was done with 10 linear prediction parameters, a window width of 25 ms, a time step of 5 ms, and preemphasis of +6 dB/octave starting at 50 Hz.
The acoustic difference between /bA/ and /dA/ lies within the transition of the second formant (F2), which is rising for /bA/ and falling for /dA/. To create a speech continuum that would gradually move from /bA/ to /dA/ in 10 acoustically identical steps, the transition of F2 was linearly interpolated from /b/ to /d/. The manipulated part of the signal was a 100-ms interval at the beginning of the sound. The F2 onset ranged from 830 to 1,906 Hz, whereas the steady-state part of the vowel was kept at 1,100 Hz. F1 and F3 were constant for all 10 stimuli at 680 Hz and 2,620 Hz, respectively. Each item of the resulting 10-step continuum had a total length of 350 ms.
The acoustic difference between /u/ and /y/ lies within the frequency of F2. During the total length of the phonemes, F2 stays relatively stable but is at a lower frequency in /u/ than in /y/. To create a gradual vowel continuum, the frequency of F2 was linearly interpolated from /u/ to /y/ in 10 acoustically identical steps ranging from 800 Hz to 2,100 Hz across the 10 stimuli, and remaining constant throughout the vowel within each stimulus. F1 and F3 and were kept stable at 680 Hz and 2,620 Hz, respectively. Stimuli were once again 350 ms in duration.
Nonspeech continua.
Nonphonetic contrasts with the same spectro-temporal complexity as the speech continua were obtained by flipping both the /bA/-/dA/ and the /u/-/y/ continua along a frequency axis of 2 kHz (47, 48) (Fig. 1). In addition, the spectra of the rotated stimuli were filtered to the long-term average spectra of the nonrotated original speech stimuli to equalize the overall spectral cues of the continua. To minimize the effects of nonspecific performance factors such as attention and effort, we ensured that performance levels on the endpoint stimuli were identical across the four stimulus types. For this reason, and based on behavioral pilot data, the discriminability of the rotated /bA/-/dA/ continuum was enhanced by additionally manipulating, in 10 equal steps, the onset of F2 of the rotated continuum (i.e., corresponding to F3 in the nonrotated continuum). This resulted in a falling F2 for the rotated /bA/ and a rising F2 for the rotated /dA/. The distances from the onset of this formant transition to its steady-state part were equal to those in the nonrotated /bA/-/dA/-continuum (similar approach in ref. 75). For the rotated /u/-/y/ continuum, no additional manipulation was needed to obtain similar performance levels as in the speech condition.
Procedure.
Participants performed a two-alternative forced-choice ABX identification task in which they had to indicate whether the third presented stimulus (X) was most similar to the first (A) or second (B) stimulus, by pressing “1” or “2,” respectively. Reference stimuli (A and B) were always endpoints of the tested continuum. The three presented sounds in each trial were accompanied by simultaneously highlighting “1,” “2,” and “?” on a computer screen. Each of the 10 stimuli of the continuum was presented eight times in a random sequence. The amount of time to respond was unlimited, and no feedback was given. Stimuli were presented monaurally at 70 dB SPL over calibrated TDH-39 headphones using the integrated audio card from a PC routed to an audiometer. The presentation order of the continua was counterbalanced, with the restriction that participants always started with one of the two speech continua.
Analyses.
To calculate the slope, individual identification data were submitted to a logistic fitting using Psignifit toolbox (76). Before analysis, slope parameters were log10-transformed to approach normally distributed data. In addition, one normal reader in the /bA/-/dA/ condition and two normal readers in the rotated /bA/-/dA/ condition were outperforming outliers, and their scores were adjusted to a value corresponding to +3 SD. (Identical results were obtained when these outlying subjects were excluded from the analyses.) Results were analyzed in a pairwise manner, comparing matched dyslexic reading and normal reading individuals. As such, we analyzed the data using linear mixed model analysis (77) with pair number as the random variable (pair 1 to 31) and participant group (dyslexic reading versus normal reading group) as the fixed between-subjects variable. Kenward-Roger correction was applied to approximate the error degrees of freedom. Mixed model analysis was chosen not merely to allow a pairwise comparison but also because of its robustness in analyzing seminormally distributed data. All statistical tests were two-tailed (α = 0.05). Results of the posthoc tests were interpreted as significant if they survived a Bonferroni correction for multiple comparisons. Finally, Pearson correlations were calculated to determine the relationship between the (log)slope of the four conditions and the three literacy tests (word and pseudoword reading and spelling).
Acknowledgments
We are grateful to all participants. We especially thank A. van Wieringen and T. Vandenbogaert for assistance in constructing the stimuli and T. Francart for technical help with Psignifit. M.V. is a research assistant and B.B. is a postdoctoral research Fellow of the Research Foundation Flanders, Belgium. The research was financed by the fund for Scientific Research Flanders (Grant G.0331.08) and by the Research Council of Katholieke Universiteit Leuven (Grant OT/07/034).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Snowling M. Dyslexia. 2nd Ed. Malden, MA: Blackwell Publishers; 2000. [Google Scholar]
- 2.Hämäläinen J, Salminen H, Leppänen P. Basic auditory processing deficits in dyslexia: Review of the behavioral and event-related potential/field evidence. J Learn Disabil. doi: 10.1177/0022219411436213. in press. [DOI] [PubMed] [Google Scholar]
- 3.Tallal P. Auditory temporal perception, phonics, and reading disabilities in children. Brain Lang. 1980;9:182–198. doi: 10.1016/0093-934x(80)90139-x. [DOI] [PubMed] [Google Scholar]
- 4.Mody M, Studdert-Kennedy M, Brady S. Speech perception deficits in poor readers: Auditory processing or phonological coding? J Exp Child Psychol. 1997;64:199–231. doi: 10.1006/jecp.1996.2343. [DOI] [PubMed] [Google Scholar]
- 5.Talcott JB, et al. Dynamic sensory sensitivity and children's word decoding skills. Proc Natl Acad Sci USA. 2000;97:2952–2957. doi: 10.1073/pnas.040546597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramus F, et al. Theories of developmental dyslexia: Insights from a multiple case study of dyslexic adults. Brain. 2003;126:841–865. doi: 10.1093/brain/awg076. [DOI] [PubMed] [Google Scholar]
- 7.Bogliotti C, Serniclaes W, Messaoud-Galusi S, Sprenger-Charolles L. Discrimination of speech sounds by children with dyslexia: Comparisons with chronological age and reading level controls. J Exp Child Psychol. 2008;101:137–155. doi: 10.1016/j.jecp.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Breier JI, et al. Perception of voice and tone onset time continua in children with dyslexia with and without attention deficit/hyperactivity disorder. J Exp Child Psychol. 2001;80:245–270. doi: 10.1006/jecp.2001.2630. [DOI] [PubMed] [Google Scholar]
- 9.Breier JI, Gray LC, Fletcher JM, Foorman B, Klaas P. Perception of speech and nonspeech stimuli by children with and without reading disability and attention deficit hyperactivity disorder. J Exp Child Psychol. 2002;82:226–250. doi: 10.1016/s0022-0965(02)00005-x. [DOI] [PubMed] [Google Scholar]
- 10.Breier Categorical perception of speech stimuli in children at risk for reading difficulty. J Exp Child Psychol. 2004;88:152–170. doi: 10.1016/j.jecp.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Cheung H, et al. Perception of tone and aspiration contrasts in Chinese children with dyslexia. J Child Psychol Psychiatry. 2009;50:726–733. doi: 10.1111/j.1469-7610.2008.02001.x. [DOI] [PubMed] [Google Scholar]
- 12.Chiappe R, Stringer R, Siegel L, Stanovich K. Why the timing deficit hypothesis does not explain reading disability in adults. Read Writ. 2002;15:73–107. [Google Scholar]
- 13.de Gelder B, Vroomen J. Impaired speech perception in poor readers: Evidence from hearing and speech reading. Brain Lang. 1998;64:269–281. doi: 10.1006/brln.1998.1973. [DOI] [PubMed] [Google Scholar]
- 14.Dufor O, Serniclaes W, Sprenger-Charolles L, Démonet JF. Top-down processes during auditory phoneme categorization in dyslexia: A PET study. Neuroimage. 2007;34:1692–1707. doi: 10.1016/j.neuroimage.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 15.Gerrits E, de Bree E. Speech perception and production in dyslexia and SLI: Evidence from 3-4 year olds. J Commun Disord. 2009;42:180–194. doi: 10.1016/j.jcomdis.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Godfrey JJ, Syrdal-Lasky AK, Millay KK, Knox CM. Performance of dyslexic children on speech perception tests. J Exp Child Psychol. 1981;32:401–424. doi: 10.1016/0022-0965(81)90105-3. [DOI] [PubMed] [Google Scholar]
- 17.Kraus N, et al. Auditory neurophysiologic responses and discrimination deficits in children with learning problems. Science. 1996;273:971–973. doi: 10.1126/science.273.5277.971. [DOI] [PubMed] [Google Scholar]
- 18.Liu W, Shu H, Yang Y. Speech perception deficits by Chinese children with phonological dyslexia. J Exp Child Psychol. 2009;103:338–354. doi: 10.1016/j.jecp.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Maassen B, Groenen P, Crul T, Assman-Hulsmans C, Gabreëls F. Identification and discrimination of voicing and place-of-articulation in developmental dyslexia. Clin Linguist Phon. 2001;15:319–339. [Google Scholar]
- 20.Manis FR, et al. Are speech perception deficits associated with developmental dyslexia? J Exp Child Psychol. 1997;66:211–235. doi: 10.1006/jecp.1997.2383. [DOI] [PubMed] [Google Scholar]
- 21.Paul I, Bott C, Heim S, Wienbruch C, Elbert TR. Phonological but not auditory discrimination is impaired in dyslexia. Eur J Neurosci. 2006;24:2945–2953. doi: 10.1111/j.1460-9568.2006.05153.x. [DOI] [PubMed] [Google Scholar]
- 22.Rosen S, Manganari E. Is there a relationship between speech and nonspeech auditory processing in children with dyslexia? J Speech Lang Hear Res. 2001;44:720–736. doi: 10.1044/1092-4388(2001/057). [DOI] [PubMed] [Google Scholar]
- 23.Reed MA. Speech perception and the discrimination of brief auditory cues in reading disabled children. J Exp Child Psychol. 1989;48:270–292. doi: 10.1016/0022-0965(89)90006-4. [DOI] [PubMed] [Google Scholar]
- 24.Serniclaes W, Sprenger-Charolles L, Carré R, Demonet JF. Perceptual discrimination of speech sounds in developmental dyslexia. J Speech Lang Hear Res. 2001;44:384–399. doi: 10.1044/1092-4388(2001/032). [DOI] [PubMed] [Google Scholar]
- 25.Serniclaes W, Van Heghe S, Mousty Ph, Carré R, Sprenger-Charolles L. Allophonic mode of speech perception in dyslexia. J Exp Child Psychol. 2004;87:336–361. doi: 10.1016/j.jecp.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Steffens ML, Eilers RE, Gross-Glenn K, Jallad B. Speech perception in adult subjects with familial dyslexia. J Speech Hear Res. 1992;35:192–200. doi: 10.1044/jshr.3501.192. [DOI] [PubMed] [Google Scholar]
- 27.van Beinum FJ, Schwippert CE, Been PH, van Leeuwen TH, Kuijpers CTL. Development and application of a /bAk-/dAk/ continuum for testing auditory perception within the Dutch longitudinal study. Speech Commun. 2005;47:124–142. [Google Scholar]
- 28.Werker JF, Tees RC. Speech perception in severely disabled and average reading children. Can J Exp Psychol. 1987;41:48–61. doi: 10.1037/h0084150. [DOI] [PubMed] [Google Scholar]
- 29.Blomert L, Mitterer H, Paffen C. In search of the auditory, phonetic, and/or phonological problems in dyslexia: Context effects in speech perception. J Speech Lang Hear Res. 2004;47:1030–1047. doi: 10.1044/1092-4388(2004/077). [DOI] [PubMed] [Google Scholar]
- 30.Joanisse MF, Manis FR, Keating P, Seidenberg MS. Language deficits in dyslexic children: Speech perception, phonology, and morphology. J Exp Child Psychol. 2000;77:30–60. doi: 10.1006/jecp.1999.2553. [DOI] [PubMed] [Google Scholar]
- 31.McArthur GM, Ellis D, Atkinson CM, Coltheart M. Auditory processing deficits in children with reading and language impairments: Can they (and should they) be treated? Cognition. 2008;107:946–977. doi: 10.1016/j.cognition.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 32.White S, et al. The role of sensorimotor impairments in dyslexia: A multiple case study of dyslexic children. Dev Sci. 2006;9:237–255. doi: 10.1111/j.1467-7687.2006.00483.x. discussion 265–269. [DOI] [PubMed] [Google Scholar]
- 33.Habib M. The neurobiological basis of developmental dyslexia: An overview and working hypothesis. Brain. 2000;123:2373–2399. doi: 10.1093/brain/123.12.2373. [DOI] [PubMed] [Google Scholar]
- 34.Banai K, Ahissar M. Auditory processing deficits in dyslexia: Task or stimulus related? Cereb Cortex. 2006;16:1718–1728. doi: 10.1093/cercor/bhj107. [DOI] [PubMed] [Google Scholar]
- 35.McArthur GM, Hogben JH. Auditory backward recognition masking in children with a specific language impairment and children with a specific reading disability. J Acoust Soc Am. 2001;109:1092–1100. doi: 10.1121/1.1338559. [DOI] [PubMed] [Google Scholar]
- 36.De Weirdt W. Speech perception and frequency discrimination in good and poor readers. Appl Psycholinguist. 1988;9:163–183. [Google Scholar]
- 37.Montgomery CR, Morris RD, Sevcik RA, Clarkson MG. Auditory backward masking deficits in children with reading disabilities. Brain Lang. 2005;95:450–456. doi: 10.1016/j.bandl.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 38.Amitay S, Ben-Yehudah G, Banai K, Ahissar M. Disabled readers suffer from visual and auditory impairments but not from a specific magnocellular deficit. Brain. 2002;125:2272–2285. doi: 10.1093/brain/awf231. [DOI] [PubMed] [Google Scholar]
- 39.Bretherton L, Holmes VM. The relationship between auditory temporal processing, phonemic awareness, and reading disability. J Exp Child Psychol. 2003;84:218–243. doi: 10.1016/s0022-0965(03)00023-7. [DOI] [PubMed] [Google Scholar]
- 40.Marshall CM, Snowling MJ, Bailey PJ. Rapid auditory processing and phonological ability in normal readers and readers with dyslexia. J Speech Lang Hear Res. 2001;44:925–940. doi: 10.1044/1092-4388(2001/073). [DOI] [PubMed] [Google Scholar]
- 41.Nittrouer S. Do temporal processing deficits cause phonological processing problems? J Speech Lang Hear Res. 1999;42:925–942. doi: 10.1044/jslhr.4204.925. [DOI] [PubMed] [Google Scholar]
- 42.Waber DP, et al. Processing of rapid auditory stimuli in school-age children referred for evaluation of learning disorders. Child Dev. 2001;72:37–49. doi: 10.1111/1467-8624.00264. [DOI] [PubMed] [Google Scholar]
- 43.Cohen-Mimran R, Sapir S. Auditory temporal processing deficits in children with reading disabilities. Dyslexia. 2007;13:175–192. doi: 10.1002/dys.323. [DOI] [PubMed] [Google Scholar]
- 44.De Martino SD, Espesser R, Rey V, Habib M. The “temporal processing deficit” hypothesis in dyslexia: New experimental evidence. Brain Cogn. 2001;46:104–108. doi: 10.1016/s0278-2626(01)80044-0. [DOI] [PubMed] [Google Scholar]
- 45.Heiervang E, Stevenson J, Hugdahl K. Auditory processing in children with dyslexia. J Child Psychol Psychiatry. 2002;43:931–938. doi: 10.1111/1469-7610.00097. [DOI] [PubMed] [Google Scholar]
- 46.Rey V, De Martino S, Espesser R, Habib M. Temporal processing and phonological impairment in dyslexia: Effect of phoneme lengthening on order judgment of two consonants. Brain Lang. 2002;80:576–591. doi: 10.1006/brln.2001.2618. [DOI] [PubMed] [Google Scholar]
- 47.Blesser B. Speech perception under conditions of spectral transformation. I. Phonetic characteristics. J Speech Hear Res. 1972;15:5–41. doi: 10.1044/jshr.1501.05. [DOI] [PubMed] [Google Scholar]
- 48.Scott SK, Blank CC, Rosen S, Wise RJS. Identification of a pathway for intelligible speech in the left temporal lobe. Brain. 2000;123:2400–2406. doi: 10.1093/brain/123.12.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pisoni DB. Identification and discrimination of the relative onset time of two component tones: Implications for voicing perception in stops. J Acoust Soc Am. 1977;61:1352–1361. doi: 10.1121/1.381409. [DOI] [PubMed] [Google Scholar]
- 50.Sperling AJ, Lu Z, Manis FR, Seidenberg MS. Motion perception deficits and reading impairment: It's the noise, not the motion. Psychol Sci. 2006;1:1047–1053. doi: 10.1111/j.1467-9280.2006.01825.x. [DOI] [PubMed] [Google Scholar]
- 51.Ziegler JC, Pech-Georgel C, George F, Lorenzi C. Speech-perception-in-noise deficits in dyslexia. Dev Sci. 2009;12:732–745. doi: 10.1111/j.1467-7687.2009.00817.x. [DOI] [PubMed] [Google Scholar]
- 52.Mirman D, Holt LL, McClelland JL. Categorization and discrimination of nonspeech sounds: Differences between steady-state and rapidly-changing acoustic cues. J Acoust Soc Am. 2004;116:1198–1207. doi: 10.1121/1.1766020. [DOI] [PubMed] [Google Scholar]
- 53.Allard F, Scott BL. Burst cues, transition cues, and hemispheric specialization with real speech sounds. Q J Exp Psychol A. 1975;27:487–497. [Google Scholar]
- 54.Belin P, et al. Lateralization of speech and auditory temporal processing. J Cogn Neurosci. 1998;10:536–540. doi: 10.1162/089892998562834. [DOI] [PubMed] [Google Scholar]
- 55.Cutting JE. Two left-hemisphere mechanisms in speech perception. Percept Psychophys. 1974;16:601–612. [Google Scholar]
- 56.Jamison HL, Watkins KE, Bishop DVM, Matthews PM. Hemispheric specialization for processing auditory nonspeech stimuli. Cereb Cortex. 2006;16:1266–1275. doi: 10.1093/cercor/bhj068. [DOI] [PubMed] [Google Scholar]
- 57.Boemio A, Fromm S, Braun A, Poeppel D. Hierarchical and asymmetric temporal sensitivity in human auditory cortices. Nat Neurosci. 2005;8:389–395. doi: 10.1038/nn1409. [DOI] [PubMed] [Google Scholar]
- 58.Husain FT, et al. Neural bases of categorization of simple speech and nonspeech sounds. Hum Brain Mapp. 2006;27:636–651. doi: 10.1002/hbm.20207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Joanisse MF, Gati JS. Overlapping neural regions for processing rapid temporal cues in speech and nonspeech signals. Neuroimage. 2003;19:64–79. doi: 10.1016/s1053-8119(03)00046-6. [DOI] [PubMed] [Google Scholar]
- 60.Zaehle T, Wüstenberg T, Meyer M, Jäncke L. Evidence for rapid auditory perception as the foundation of speech processing: A sparse temporal sampling fMRI study. Eur J Neurosci. 2004;20:2447–2456. doi: 10.1111/j.1460-9568.2004.03687.x. [DOI] [PubMed] [Google Scholar]
- 61.Zatorre RJ, Gandour JT. Neural specializations for speech and pitch: Moving beyond the dichotomies. Philos Trans R Soc Lond B Biol Sci. 2008;363:1087–1104. doi: 10.1098/rstb.2007.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Golestani N, Zatorre RJ. Learning new sounds of speech: Reallocation of neural substrates. Neuroimage. 2004;21:494–506. doi: 10.1016/j.neuroimage.2003.09.071. [DOI] [PubMed] [Google Scholar]
- 63.Lyytinen H, et al. Psychophysiology of developmental dyslexia: A review of findings including studies of children at risk for dyslexia. J Neurolinguist. 2005;18:167–195. [Google Scholar]
- 64.Gaab N, Gabrieli JDE, Deutsch GK, Tallal P, Temple E. Neural correlates of rapid auditory processing are disrupted in children with developmental dyslexia and ameliorated with training: An fMRI study. Restor Neurol Neurosci. 2007;25:295–310. [PubMed] [Google Scholar]
- 65.Ruff SCA, Cardebat D, Marie N, Démonet JF. Enhanced response of the left frontal cortex to slowed down speech in dyslexia: An fMRI study. Neuroreport. 2002;13:1285–1289. doi: 10.1097/00001756-200207190-00014. [DOI] [PubMed] [Google Scholar]
- 66.Temple E, et al. Disruption of the neural response to rapid acoustic stimuli in dyslexia: Evidence from functional MRI. Proc Natl Acad Sci USA. 2000;97:13907–13912. doi: 10.1073/pnas.240461697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kujala T, et al. Plastic neural changes and reading improvement caused by audiovisual training in reading-impaired children. Proc Natl Acad Sci USA. 2001;98:10509–10514. doi: 10.1073/pnas.181589198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moore DR, Rosenberg JF, Coleman JS. Discrimination training of phonemic contrasts enhances phonological processing in mainstream school children. Brain Lang. 2005;94:72–85. doi: 10.1016/j.bandl.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 69.Gersons-Wolfensberger DCM, Ruijssenaars WAJJM. Definition and treatment of dyslexia: A report by the Committee on Dyslexia of the Health Council of The Netherlands. J Learn Disabil. 1997;30:209–213. doi: 10.1177/002221949703000208. [DOI] [PubMed] [Google Scholar]
- 70.van den Bos KP, Spelberg HCL, Scheepstra AJM, De Vries JR. De Klepel. Vorm A en B. Een Test Voor de Leesvaardigheid Van Pseudowoorden. Verantwoording, Handleiding, Diagnostiek en Behandeling [Word and Nonword Reading Test A and B manual] Nijmegen: Berkhout; 1994. [Google Scholar]
- 71.Depessemier P, Andries C. Gletschr, test voor gevorderd lezen et schrijven [A test for advanced reading and writing skills] Antwerpen: Garant; 2009. [Google Scholar]
- 72.Wechsler D. Wechsler Adult Intelligence Scale, 3rd Ed. NL. London: Psychological Corp; 1999. [Google Scholar]
- 73.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 74.Boersma P, Weenink D. Praat: Doing phonetics by computer. PRAAT. Institute of Phonetic Sciences, University of Amsterdam. 2000 Available at http://www.fon.let.uva.nl/praat/. Accessed August, 2008. [Google Scholar]
- 75.Liebenthal E, Binder JR, Spitzer SM, Possing ET, Medler DA. Neural substrates of phonemic perception. Cereb Cortex. 2005;15:1621–1631. doi: 10.1093/cercor/bhi040. [DOI] [PubMed] [Google Scholar]
- 76.Wichmann FA, Hill NJ. The psychometric function: Fitting, sampling and goodness of fit. Percept Psychofys. 2001;63:1293–1313. doi: 10.3758/bf03194544. Available at http://bootstrap-software.org/psignifit/. Accessed March, 2009. [DOI] [PubMed] [Google Scholar]
- 77.Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for Mixed Models. 2nd Ed. Cary, NC: SAS Institute; 2006. [Google Scholar]