Abstract
Though opening of the start site (+1) region of promoter DNA is required for transcription by RNA polymerase (RNAP), surprisingly little is known about how and when this occurs in the mechanism. Early events at the λPR promoter load this region of duplex DNA into the active site cleft of Escherichia coli RNAP, forming the closed, permanganate-unreactive intermediate I1. Conversion to the subsequent intermediate I2 overcomes a large enthalpic barrier. Is I2 open? Here we create a burst of I2 by rapidly destabilizing open complexes (RPo) with 1.1 M NaCl. Fast footprinting reveals that thymines at positions from -11 to +2 in I2 are permanganate-reactive, demonstrating that RNAP opens the entire initiation bubble in the cleft in a single step. Rates of decay of all observed thymine reactivities are the same as the I2 to I1 conversion rate determined by filter binding. In I2, permanganate reactivity of the +1 thymine on the template (t) strand is the same as the RPo control, whereas nontemplate (nt) thymines are significantly less reactive than in RPo. We propose that: (i) the +1(t) thymine is in the active site in I2; (ii) conversion of I2 to RPo repositions the nt strand in the cleft; and (iii) movements of the nt strand are coupled to the assembly and DNA binding of the downstream clamp and jaw that occurs after DNA opening and stabilizes RPo. We hypothesize that unstable open intermediates at the λPR promoter resemble the unstable, transcriptionally competent open complexes formed at ribosomal promoters.
Keywords: bottleneck step, transcription regulation, burst experiment, protein nucleic acid interactions
Interactions between RNA polymerase and specific promoter DNA sequences trigger a precise progression of conformational changes in both biomolecules. Taken together, these steps constitute the mechanism of DNA opening and the start of the transcription cycle. For Escherichia coli RNA polymerase holoenzyme (RNAP, subunit composition: α2ββ′ωσ70), binding free energy drives opening of the initiation bubble (-11 to +2, numbering relative to the start site base +1) in promoter DNA, placement of +1 template base in the active site of the enzyme, and subsequent conformational changes to form the stable open complex RPo. Each of these steps provides a checkpoint for regulatory input.
Over the past decade, structural (X-ray, FRET), single-molecule, and rapid mixing kinetic studies have greatly advanced the understanding of this machinery and these steps. Key advances include: (i) elucidation of the RNAP architecture at atomic resolution (1–4); (ii) dissection of composite forward and backward rate constants for RPo formation into individual rate and/or equilibrium constants for the steps leading to RPo (5, 6); (iii) single-molecule measurements of DNA topological changes (7); (iv) real-time determination of hydroxyl radial (HO•) protection patterns of DNA during stable open complex (RPo) formation (8–10); and (v) finding that unstable open complexes are stabilized by binding the initiating nucleoside triphosphate and greatly destabilized by the stress response factors ppGpp and DksA (11–13). These advances make it possible to address the key unresolved questions of initiation. How is the opening of 12–14 base pairs distributed between the steps of RPo formation? Does RNAP disrupt the DNA duplex in the active site cleft or does DNA melt outside the channel and enter as individual strands?
Evidence for at least two kinetically significant intermediates (generically designated I1 and I2) preceding RPo exists for a variety of promoters recognized by E. coli RNAP (cf. refs. 6, 8, and 14–17). Conversion of I1 to I2 is the rate-determining (bottleneck) step in forming the open complex at the λPR promoter and exhibits a 34-kcal activation enthalpy barrier (17). The reverse direction of this step is the bottleneck step in dissociation of RPo (6). Because I1 is a closed complex (9), determining when DNA opens requires trapping I2.
The minimal mechanism of RPo formation is formally analogous to minimal mechanisms of solute transport through membranes and enzyme catalysis. All involve three steps in which the initial step in each direction is rapidly reversible and a middle step that is the bottleneck in both directions. In RPo formation, as in mechanisms of catalysis and transport, ligands and solutes primarily act on the rapidly reversible steps and not on the central bottleneck step (6, 18). Given these analogies, is the central bottleneck step of open complex formation indeed DNA opening, just as transport is the central step in transporter mechanisms and catalysis is in enzyme mechanisms?
We address this fundamental mechanistic question by using a powerful method from physical enzymology, the burst experiment, which forms a transiently high concentration of an otherwise unobservable intermediate (preceding the bottleneck step). In the forward direction, a burst of I1 is generated by rapid mixing with a sufficiently high [RNAP] (cf. Fig. 1A). HO• and permanganate (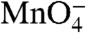 ) footprints of the population of the λPR promoter DNA in such a forward burst experiment demonstrate that I1 is a
) footprints of the population of the λPR promoter DNA in such a forward burst experiment demonstrate that I1 is a 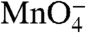 -unreactive complex in which downstream duplex DNA is protected to +20 from HO• attack (9). Structural modeling on the basis of these data and the X-ray structures of the bacterial RNAP (1, 2) indicates that duplex DNA in I1 is loaded in the active site cleft of RNAP but not yet open (9).
-unreactive complex in which downstream duplex DNA is protected to +20 from HO• attack (9). Structural modeling on the basis of these data and the X-ray structures of the bacterial RNAP (1, 2) indicates that duplex DNA in I1 is loaded in the active site cleft of RNAP but not yet open (9).
Fig. 1.
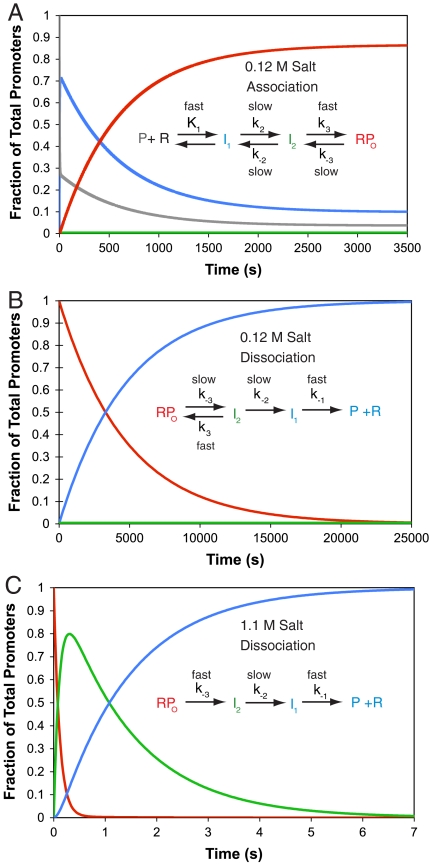
A burst dissociation experiment is required to characterize the unstable intermediate I2. Simulations of changes in the populations of RPo (Red), I2 (Green), I1 (Blue), and free λPR promoter DNA (P, Gray) at 10 °C, 0.01 M Mg2+ as a function of time are shown for: (A) reversible association at high [RNAP] (100 nM) and 0.12 M salt (KCl or NaCl); (B) irreversible (in excess competitor) dissociation at 0.12 M salt; and (C) irreversible dissociation after a rapid upshift to 1.1 M salt. Rate and equilibrium constants for the association simulation (A) are from ref. 17; at no point on the association time course is there a significant population of I2. Rate and equilibrium constants for the dissociation simulations are from ref. 6. The salt upshift (C) produces a large transient burst of I2, not observed in the low salt dissociation experiment (B). The species designated RPo also includes a minor population of the late intermediate I3 (6), which is not resolved from RPo in the present studies. Values of the parameters used to generate these simulations are given in Table S1.
Because the rate limiting forward step is the conversion of I1 to I2, no subsequent burst of I2 occurs in the forward direction experiment (Fig. 1A). Hence the dissociation direction must be investigated to obtain a sufficient population of I2 to characterize. The time course of a standard dissociation experiment in which a competitor such as heparin is added to make dissociation irreversible is shown in Fig. 1B. Because I2 is an unstable intermediate, rapidly converting back to the stable open complex on the time scale of its conversion to I1, it never accumulates to a significant level in this experiment. Kontur et al. (6) discovered that rapid destabilization of the stable open complex with moderately high concentrations of urea or salt generates a dramatic transient buildup (burst) of I2 ∼0.5 s after mixing (Fig. 1C). (A RPo-destabilizing temperature downshift cannot be performed rapidly enough to detect such bursts.) Because the rate of conversion of I2 to I1 is found to be independent of urea or salt concentration, the burst of I2 persists for a period of approximately 1 s, ample time for characterization of the extent of opening of bases in I2 and of the decay of I2 to I1 by fast 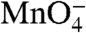 footprinting.
footprinting.
In Fig. 1 (all panels) at 10 °C, the stable open complex (labeled RPo) may be an equilibrium mixture containing some of the intermediate complex I3 identified previously (6). An increase in the population of I3 is expected early in the solute upshifts in Fig. 1C, decaying to I2 in less than 100 ms. Simulations on the basis of kinetic data (6) show that the time resolution of the three-syringe burst/fast footprinting experiments reported in this research is insufficient to investigate I3, and its population is therefore combined with that of RPo in Fig. 1.
Results
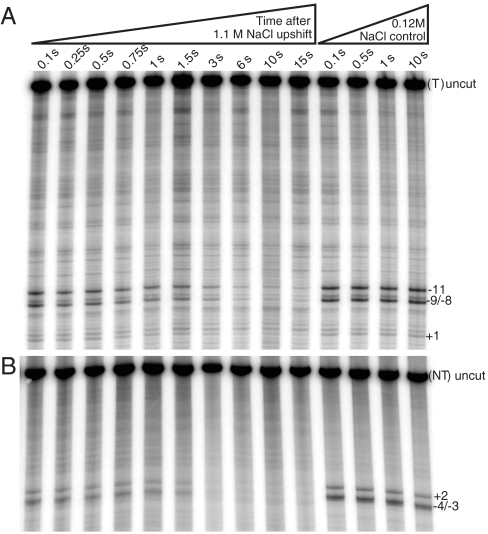
To determine whether the DNA in I2 is closed or partially or fully open in the region of the initiation bubble, we footprinted thymines with a constant dose of 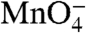 (19). Complexes were probed as a function of time after rapidly destabilizing open complexes with 1.1 M NaCl or after mixing them with 0.12 M NaCl (control reaction). The sequencing gels in Fig. 2 compare the time-dependent behavior of all
(19). Complexes were probed as a function of time after rapidly destabilizing open complexes with 1.1 M NaCl or after mixing them with 0.12 M NaCl (control reaction). The sequencing gels in Fig. 2 compare the time-dependent behavior of all 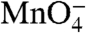 -reactive positions on each strand after the upshift with that of the control reaction.
-reactive positions on each strand after the upshift with that of the control reaction.
Fig. 2.
Visualizing open thymines on template and nontemplate strands during the burst and subsequent decay of intermediate promoter complex I2 after an upshift of RPo to 1.1 M NaCl. Sequencing gels (representative of three independent experiments) show the decay of permanganate reactivity of individual open thymine bases (left lanes) on the template (A) and nontemplate (B) strands as a function of time after upshift, probed with a constant dose of NaMnO4 (see Material and Methods). Right lanes show corresponding low salt RPo reactivity controls for both strands as a function of time after mixing with 0.12 M NaCl.
For the template (t) strand (Fig. 2A) the control lanes indicate that thymines at positions +1, -8/-9 (doublet band), and -11 are 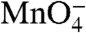 -reactive in RPo. After the upshift to 1.1 M NaCl, a monotonic decay of
-reactive in RPo. After the upshift to 1.1 M NaCl, a monotonic decay of 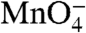 reactivity of these bands is observed in the time range 0.1–10 s. For the nontemplate (nt) strand (Fig. 2B), the control lanes indicate that thymines at positions +2 and -3/-4 (doublet band) are
reactivity of these bands is observed in the time range 0.1–10 s. For the nontemplate (nt) strand (Fig. 2B), the control lanes indicate that thymines at positions +2 and -3/-4 (doublet band) are 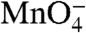 -reactive in RPo; the kinetics of the decay in
-reactive in RPo; the kinetics of the decay in 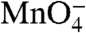 reactivity with time after the NaCl upshift is very similar to that of the t strand. Thymines at -7 and -10 on the nt strand are not detected, though the DNA is open in this region (as judged by reactivity at positions -11 and -8/-9 on the t strand). We infer that interactions of these upstream nt strand thymines with σ70 region 2 (cf. ref. 20 and references therein) protect them from reacting with
reactivity with time after the NaCl upshift is very similar to that of the t strand. Thymines at -7 and -10 on the nt strand are not detected, though the DNA is open in this region (as judged by reactivity at positions -11 and -8/-9 on the t strand). We infer that interactions of these upstream nt strand thymines with σ70 region 2 (cf. ref. 20 and references therein) protect them from reacting with 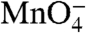 in I2 as well as in RPo.
in I2 as well as in RPo.
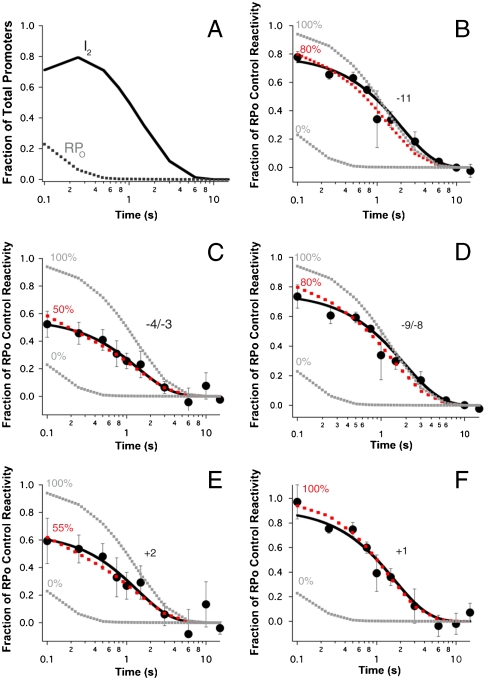
In addition to providing visual demonstrations of the positions of reactive thymines in I2 and of the time course of their decay as I2 converts to products (I1 and then free promoter DNA), Fig. 2 allows a visual comparison of the reactivities of these thymines in I2 [judged by the early time points (0.1–0.25 s) after the upshift], both relative to other thymines in I2 and to the RPo control. At 0.25 s, the population distribution of promoter DNA (Fig. 3A) is 80% I2, 10% RPo and I3, and 10% closed I1 and free promoter DNA. Strikingly, in the early time lanes where I2 is the major species, thymines at both +2 and -3/-4 on the nt strand appear much less reactive than in the RPo control, whereas reactive thymines at +1 and other positions on the t strand appear nearly as reactive as in the RPo control.
Fig. 3.
Kinetics of the open to closed transition of individual thymines as the burst population of I2 decays to I1 and promoter DNA. (A) Predicted populations of RPo and I2 (see SI Text) are plotted versus time (log scale) after NaCl upshift (0.1–10 s). (B–F) Relative 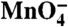 reactivities of each thymine singlet or doublet band (calculated from three independent experiments on each strand; see Fig. 2) as a function of time (log scale). Solid curves are single exponential fits of these data, yielding the rate constants for the decay of I2 in Table 1. Simulations of the decay using the population distribution in Fig. 1C and varying the
reactivities of each thymine singlet or doublet band (calculated from three independent experiments on each strand; see Fig. 2) as a function of time (log scale). Solid curves are single exponential fits of these data, yielding the rate constants for the decay of I2 in Table 1. Simulations of the decay using the population distribution in Fig. 1C and varying the 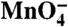 reactivity of each thymine singlet or doublet in I2 relative to the RPo control are shown as dashed lines. The best fit is shown in red; the limits of the fit (I2 closed (0% reactive) and I2 as open as RPo (100%)) are shown in gray.
reactivity of each thymine singlet or doublet in I2 relative to the RPo control are shown as dashed lines. The best fit is shown in red; the limits of the fit (I2 closed (0% reactive) and I2 as open as RPo (100%)) are shown in gray.
To explore the behavior of each 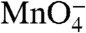 -reactive thymine during dissociation, we quantified their individual decay kinetics and reactivities relative to the RPo control (Fig. 3 and Table 1). In Fig. 3A, the populations of I2 and of RPo (including I3) are plotted as a function of time after the upshift on a logarithmic time scale (two decades, from 0.1 to 10 s) to compare with the analysis of the individual thymines shown in the subsequent panels. For all thymines, the observed change in
-reactive thymine during dissociation, we quantified their individual decay kinetics and reactivities relative to the RPo control (Fig. 3 and Table 1). In Fig. 3A, the populations of I2 and of RPo (including I3) are plotted as a function of time after the upshift on a logarithmic time scale (two decades, from 0.1 to 10 s) to compare with the analysis of the individual thymines shown in the subsequent panels. For all thymines, the observed change in 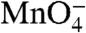 reactivity is well fit by a single exponential decay (Solid Curve, Fig. 3 B–F). Although I2 is initially the dominant species after the upshift, the presence of some RPo, I3, and closed promoter DNA requires that the data be deconvoluted to quantify the
reactivity is well fit by a single exponential decay (Solid Curve, Fig. 3 B–F). Although I2 is initially the dominant species after the upshift, the presence of some RPo, I3, and closed promoter DNA requires that the data be deconvoluted to quantify the 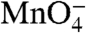 reactivity of each thymine in I2. A series of simulations of the observed decay kinetics were performed in which these reactivities were systematically varied from 0% (unreactive in I2) to 100% (as reactive in I2 as in RPo). Simulated time courses calculated by using the best-fit reactivities of each thymine in the bubble in I2 are compared with the 0% and 100% limiting cases and with the experimental data in Fig. 3 B–F. Best-fit reactivities are listed in Table 1 together with the rate constants for decay of each reactive thymine in the conversion of I2 to I1.
reactivity of each thymine in I2. A series of simulations of the observed decay kinetics were performed in which these reactivities were systematically varied from 0% (unreactive in I2) to 100% (as reactive in I2 as in RPo). Simulated time courses calculated by using the best-fit reactivities of each thymine in the bubble in I2 are compared with the 0% and 100% limiting cases and with the experimental data in Fig. 3 B–F. Best-fit reactivities are listed in Table 1 together with the rate constants for decay of each reactive thymine in the conversion of I2 to I1.
Table 1.
Permanganate reactivities of thymine bases in the open region of I2 and rate constants for closing these positions in I2 to I1
| Strand | Template | Nontemplate | |||
| Position | −11 | −9/−8 | +1 | −4/−3 | +2 |
| Rate constant (s-1)* | 0.50 (±0.04) | 0.52 (±0.05) | 0.6 (±0.1) | 0.7 (±0.2) | 0.7 (±0.3) |
| Predicted reactivity of I2 (relative to RPo control)† | 80% | 80% | 100% | 50% | 55% |
*Fit parameters from the decay kinetics (solid lines) in Fig. 3. The rate constant for the conversion of I2 to I1 (k-2) determined by nitrocellulose filter-binding experiments at 10 °C is 0.72 ( ± 0.07) s-1 (6).
†Uncertainty in these reactivities, estimated from the range of triplicate determinations at a given time point in the range from 0.1 to 0.75 s, is ± 10%. See Material and Methods.
Comparison of the results reveals that:
Decay rate constants are the same for all thymines (0.6 ± 0.1 s-1) and agree within the uncertainty with the rate constant for the conversion of I2 to I1 (k-2 = 0.72 ± 0.07 s-1) determined by filter binding of quenched samples as a function of time after exposure to a 1.1 M salt upshift (6).
The
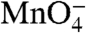 reactivity of the start site (+1) thymine on the t strand in I2 is the same as in the RPo control.
reactivity of the start site (+1) thymine on the t strand in I2 is the same as in the RPo control. 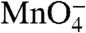 reactivities of upstream thymines on the t strand in I2 are about 80% as large as in the RPo control.
reactivities of upstream thymines on the t strand in I2 are about 80% as large as in the RPo control. 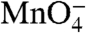 reactivities of downstream thymines on the nt strand in I2 are 50–55% as large as in the RPo control. (These conclusions are insensitive to assumptions regarding the amount and
reactivities of downstream thymines on the nt strand in I2 are 50–55% as large as in the RPo control. (These conclusions are insensitive to assumptions regarding the amount and 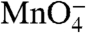 reactivity of the I3 present in the residual population labeled RPo in Fig. 3A) Thymines at -7 and -10 on the nt strand are fully protected (zero
reactivity of the I3 present in the residual population labeled RPo in Fig. 3A) Thymines at -7 and -10 on the nt strand are fully protected (zero 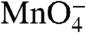 reactivity) in both I2 and RPo.
reactivity) in both I2 and RPo.
Discussion
Opening of the Initiation Bubble Occurs in the Active Site Cleft to Convert the Closed Complex I1 to I2.
The architecture of multisubunit RNA polymerases appears to have evolved a series of steric blocks to prevent access of nonpromoter DNA to the active site (2–4, 9, 21, 22). For example, bacterial RNAP recognizes promoter DNA by interactions between the σ subunit and hexameric sequences (-10 and -35 regions) that are upstream of +1 (cf. ref. 23 and references therein). Placement of σ70 with respect to the channel requires the DNA to bend sharply at -11/-12 to enter the active site cleft formed by the β and β′ “pincers” (or jaws) (2, 17). Does double-stranded DNA bind in the cleft at this point in the mechanism prior to being opened by RNAP? Or does the DNA open above the cleft, allowing the template strand to then descend down to the active site “floor” (2, 23)?
The relatively “closed” state of the pincers (less than 25 Å apart) observed in crystal structures of the bacterial RNAP (2, 22) and the transcription factor TFIIB bound to the 12-subunit eukaryotic RNAP (24, 25) has motivated proposals that DNA must open outside of the cleft. For the bacterial RNAP, which lacks a helicase cofactor, opening outside the active site cleft is proposed to be nucleated by a thermal breathing mechanism (23). In this proposal, transient opening and closing of the A/T-rich -10 hexamer leads to capture of the nt strand by σ70 region 2 at the upstream entrance (23), followed by entry of only the t strand into the cleft (2, 23). Indeed an RNAP subassembly consisting of σ70 region 2 and an N-terminal fragment of β′ was observed to form an open (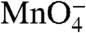 -reactive) complex with a highly A/T-rich promoter set in negatively supercoiled DNA; this polymerase subassembly did not open this promoter on linear DNA (26). Whereas these structure- or equilibrium-based mechanistic hypotheses are appealing and could apply to promoters under highly negative supercoiling stress and/or high temperature, E. coli RNAP readily forms open complexes on linear promoter fragments in vitro.
-reactive) complex with a highly A/T-rich promoter set in negatively supercoiled DNA; this polymerase subassembly did not open this promoter on linear DNA (26). Whereas these structure- or equilibrium-based mechanistic hypotheses are appealing and could apply to promoters under highly negative supercoiling stress and/or high temperature, E. coli RNAP readily forms open complexes on linear promoter fragments in vitro.
Kinetic-mechanistic studies of RPo formation and dissociation at the λPR promoter combined with footprinting data argue that interactions of regions of E. coli RNAP with promoter DNA bound in the cleft actively distort and open the initiation bubble. HO• and DNase I cleavage of the DNA backbone of the early intermediate I1 demonstrate that both the t and nt DNA strands are protected without interruption in this region (-15 to +25), whereas 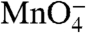 does not detect any unstacked thymine bases (9, 27, 28). For the T7A1 promoter, time-resolved HO• footprints of populations of intermediates during formation of stable open complexes have been interpreted in terms of a mechanism involving three classes of intermediates before the rate-determining step (10). Comparison of the kinetics of development of downstream
does not detect any unstacked thymine bases (9, 27, 28). For the T7A1 promoter, time-resolved HO• footprints of populations of intermediates during formation of stable open complexes have been interpreted in terms of a mechanism involving three classes of intermediates before the rate-determining step (10). Comparison of the kinetics of development of downstream 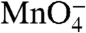 reactivity with the kinetics of downstream HO• protection led the authors to propose that DNA opening occurs outside the cleft and before the rate-determining step (10). Further research is needed to determine the nature of the rate-determining step at T7A1 and to understand the origins of the apparent mechanistic differences between these two promoters.
reactivity with the kinetics of downstream HO• protection led the authors to propose that DNA opening occurs outside the cleft and before the rate-determining step (10). Further research is needed to determine the nature of the rate-determining step at T7A1 and to understand the origins of the apparent mechanistic differences between these two promoters.
Experiments presented here demonstrate that 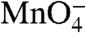 -reactive thymines appear in the conversion of I1 to I2 at λPR. We conclude from these results that the pincers of RNAP are sufficiently flexible in solution to allow DNA to enter as a double helix, where it is then opened via binding interactions with elements on RNAP. Additional evidence that DNA opening occurs in the active site cleft and not in solution is provided by the observation that the [salt] dependence of the DNA opening step (conversion of I1 to I2) is much smaller in magnitude than that of melting 12–14 base pairs of DNA in solution (18). We proposed that the N-terminal polyanionic domain of σ70 (σ region 1.1) must move in the cleft, allowing the duplex DNA to descend (18). These movements would position -2/-1(t) near the highly conserved region on the downstream lobe of β known as fork loop 2. By analogy with base-flipping enzymes, we speculate that fork loop 2 inserts in the minor groove, creating a 90° bend and unwinding the DNA helix to form the I1-I2 transition state. Interactions between the DNA phosphate backbone and positive regions in the cleft provide an additional driving force for opening. A bind-bend-open mechanism has also been proposed for RPo formation by the single subunit T7 phage RNAP and tested by stopped flow kinetic and FRET experiments (29, 30).
-reactive thymines appear in the conversion of I1 to I2 at λPR. We conclude from these results that the pincers of RNAP are sufficiently flexible in solution to allow DNA to enter as a double helix, where it is then opened via binding interactions with elements on RNAP. Additional evidence that DNA opening occurs in the active site cleft and not in solution is provided by the observation that the [salt] dependence of the DNA opening step (conversion of I1 to I2) is much smaller in magnitude than that of melting 12–14 base pairs of DNA in solution (18). We proposed that the N-terminal polyanionic domain of σ70 (σ region 1.1) must move in the cleft, allowing the duplex DNA to descend (18). These movements would position -2/-1(t) near the highly conserved region on the downstream lobe of β known as fork loop 2. By analogy with base-flipping enzymes, we speculate that fork loop 2 inserts in the minor groove, creating a 90° bend and unwinding the DNA helix to form the I1-I2 transition state. Interactions between the DNA phosphate backbone and positive regions in the cleft provide an additional driving force for opening. A bind-bend-open mechanism has also been proposed for RPo formation by the single subunit T7 phage RNAP and tested by stopped flow kinetic and FRET experiments (29, 30).
Single-molecule DNA magnetic tweezer experiments revealed that unwinding of ∼1 turn of the helix in an E. coli RNAP–promoter complex occurred in a single process at all promoters studied on both negatively and positively supercoiled DNA (7). Because the time resolution of the assay was ∼1 s, these experiments could not resolve whether untwisting occurred in a single kinetic step, or whether the mechanism involved a sequence of unwinding steps. Our results show that opening (unpairing, partial unstacking, and therefore unwinding) occurs in the bottleneck step of RPo formation (I1 to I2) at the λPR promoter.
Proposed DNA Conformations in the Steps in Open Complex Formation.
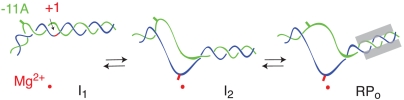
Fig. 4 shows a schematic of the proposed series of conformational changes in the downstream DNA in the RNAP active site channel as the reaction proceeds from the closed intermediate I1 to the unstable open complex I2 and finally to the stable open complex RPo. DNA in I1 is shown as double-stranded with the exception of a distortion at -11(t), which flips out -11A(nt) (20). However, because -11(t) is not 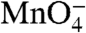 -reactive, we infer that it remains stacked with other DNA bases and/or is interacting with RNAP (9). In our model of I1, the +1 base pair lies ∼40 Å above the active site Mg2+ (9, 17). Further descent of duplex DNA in I1 is blocked by σ region 1.1 and the β′ “bridge” helix that spans the channel.
-reactive, we infer that it remains stacked with other DNA bases and/or is interacting with RNAP (9). In our model of I1, the +1 base pair lies ∼40 Å above the active site Mg2+ (9, 17). Further descent of duplex DNA in I1 is blocked by σ region 1.1 and the β′ “bridge” helix that spans the channel.
Fig. 4.
Proposal for DNA conformational changes during the steps of opening and stabilizing the initiation bubble at the λPR promoter. Proposed location (relative to the active site Mg2+, shown as a red dot) and extent of bending and/or opening of DNA strands (template, blue; nontemplate, green; -20 to +20) in the bent and wrapped closed complex I1, in the relatively unstable initial open complex I2, and in the stable open complex RPo. The transcription start site thymine is shown as a red rectangle. -11A (Green Rectangle) on the nontemplate strand is proposed to be flipped out in the 90° bend that directs the duplex into the active site cleft in I1. Formation of I1 loads downstream duplex in the active site cleft. RNAP opens the DNA in the cleft and positions the t strand start site base (+1) in the active site, forming I2. Final loading of the nt strand and assembly of a clamp/jaw (Gray Rectangles) on the downstream duplex DNA greatly stabilize RPo relative to I2.
Because the 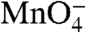 reactivity of +1 appears to be the same in both I2 and RPo, we propose that opening of the bubble in I1 → I2 places the +1(t) base in the active site (near the catalytic Mg2+). However, the reduced reactivities of other thymines, especially those on the nt strand (Table 1), motivate the proposal that the surrounding protein environment and/or degree of stacking of these thymines in I2 differs from that in RPo. Large conformational changes in RNAP accompany the conversion of I2 to RPo (5, 15). Very large solute effects on the dissociation rate constant kd and the large activation heat capacity of kd provide evidence that the downstream clamp/jaw is assembled and tightened on the downstream DNA duplex (+5 to +20) in these late steps of RPo formation, after the DNA has been opened (6, 18, 31) (see Fig. 4). The twofold increase in permanganate reactivity of nt strand thymines at -4/-3 and +2 in the conversion of I2 to RPo indicates that the nt strand is repositioned and/or unstacked in these steps. These local changes in the nt strand in the cleft may be coupled to changes in positioning of σ70 region 1.1 and the switch regions (3, 32) and to the large scale downstream conformational changes involved in assembly and DNA binding of the clamp/jaw. Together these events greatly stabilize RPo relative to I2.
reactivity of +1 appears to be the same in both I2 and RPo, we propose that opening of the bubble in I1 → I2 places the +1(t) base in the active site (near the catalytic Mg2+). However, the reduced reactivities of other thymines, especially those on the nt strand (Table 1), motivate the proposal that the surrounding protein environment and/or degree of stacking of these thymines in I2 differs from that in RPo. Large conformational changes in RNAP accompany the conversion of I2 to RPo (5, 15). Very large solute effects on the dissociation rate constant kd and the large activation heat capacity of kd provide evidence that the downstream clamp/jaw is assembled and tightened on the downstream DNA duplex (+5 to +20) in these late steps of RPo formation, after the DNA has been opened (6, 18, 31) (see Fig. 4). The twofold increase in permanganate reactivity of nt strand thymines at -4/-3 and +2 in the conversion of I2 to RPo indicates that the nt strand is repositioned and/or unstacked in these steps. These local changes in the nt strand in the cleft may be coupled to changes in positioning of σ70 region 1.1 and the switch regions (3, 32) and to the large scale downstream conformational changes involved in assembly and DNA binding of the clamp/jaw. Together these events greatly stabilize RPo relative to I2.
Opening of the entire bubble (-11 to +2) is rate-determining at the λPR promoter and has the properties of a single (elementary) kinetic step. At other promoters, opening may be separated into several kinetically distinguishable steps and may or may not be rate-determining (10, 20). For the λPR promoter, the activation enthalpy barrier is very high for the forward direction of the opening step [34 kcal; (17)]; and the overall enthalpy change for this step is also large [∼24 kcal; (6)]. For comparison, the enthalpy of melting 13 bp of DNA in solution is approximately 75 kcal at 25 °C (33). One interpretation of the 34-kcal activation barrier is that approximately half the bubble is open and unstacked in the transition state and that few enthalpically favorable interactions with RNAP have formed at this stage. Alternatively, the majority of bases in the bubble may be unpaired but not unstacked or, if unstacked, may be engaged in enthalpically favorable interactions with RNAP. Opening in the cleft is likely initiated at the -11 bend by interactions between the -10 region of the nt strand and aromatic residues of σ70 region 2 (20). Because these interactions presumably are enthalpically favorable, we infer that the majority of the bubble is open in the transition state. Conversion of this very unstable transition state to I2 likely establishes additional interactions with RNAP, possibly including those between the t strand and switch 2 of β′ (32).
Proposed Physiological Relevance of the Unstable Open Complex I2.
Under physiological conditions, I2 is highly unstable relative to RPo at the λPR promoter and converts to RPo so rapidly that it never accumulates (Fig. 1 A and B). Yet the conversion of I1 to I2 opens the entire transcription bubble and may correctly load the start site base in the active site. Do multiple open complexes (RPo, I3, and I2) with large differences in stability play distinct functional roles in the regulation of transcription initiation? We propose that the answer to this question is strongly affirmative.
Extensive studies of transcription initiation at the ribosomal rrnB P1 promoter by Gourse, Ross, and coworkers reveal that the open complex formed at this promoter in the absence of NTPs and negative supercoiling is highly unstable, with a short lifetime, existing in an equilibrium shifted toward closed complexes (cf. ref. 34 and references therein). If open complex formation at λPR were blocked at I2 or I3, unable to progress to the stable RPo, then a similar situation would be observed. An equilibrium would exist between these unstable open intermediates and the closed complex I1 shifted toward I1 at 25 °C and with a lifetime of I2 of only a few seconds. On the basis of this kinetic/thermodynamic analogy, and because I2 at λPR and the functional open complex at rrnB P1 both appear to be the first open complex formed from a closed complex at these promoters, we propose that the unstable intermediate(s) I2 (and/or I3) at λPR behave functionally and structurally like the unstable open complex characterized at rrnB P1.
A key functional property of the open complex at rrnB P1 is the ability to initiate synthesis of a full-length transcript rapidly and efficiently, without any short, abortive product synthesis, upon addition of all four NTPs (35). A key structural property of the rrnB P1 open complex is its shorter downstream boundary of the hydroxyl radical footprint (∼+12 to +15 of the (t) strand for the rrnB P1 (36), compared to +20 to +25 for RPo at λPR (9). This difference in downstream boundaries likely reflects a difference in the extent of assembly and DNA binding of downstream mobile domains in β′ including the clamp, jaw, and sequence insertion 3, which we propose stabilize RPo at λPR (6, 18, 31). We hypothesize that these hallmarks of the open complex at rrnB P1 will be observed for I2 (and/or I3) at λPR.
The instability (relative to the closed complex) and short lifetime of the open complex at ribosomal promoters makes it a target of regulation by proteins like DksA (12) and by ligands including the stress factor ppGpp (37, 38) and the initiating NTP (11). Clearly, ribosomal expression has been tuned to respond rapidly to changes in conditions, including changes in NTP concentrations. We hypothesize that complete assembly and tightening of the clamp/jaw on downstream DNA characteristic of the stable open complex RPo at λPR is disfavored at the rrnB P1 promoter [possibly because of differences in interactions with the nt strand in the cleft (39, 40)], explaining its instability relative to λPR. If so, tight downstream interactions would not need to be broken for RNAP to escape from the rrnB P1 promoter, allowing highly efficient production of full-length transcripts under exponential growth conditions. In contrast, a fully assembled and tightened clamp at λPR in RPo may impede escape and favor abortive initiation, consistent with the observation that downstream DNA from +1 to +20 plays a key role in determining the efficiency of the transition to elongation (41).
For a series of promoters that form stable binary open complexes, Hsu, Chamberlin and colleagues obtained evidence for two ternary initial transcription complexes (ITCs): One ITC makes only abortive products and the other primarily makes full length transcripts. For these promoters, the overall abortive:productive ratio is large and promoter-sequence-dependent [(42); see also ref. 43]. Abortive transcripts are made in vivo (44); their cellular function as small RNAs is not yet known.
What is the relationship between multiple binary open complexes (I2, I3, and RPo) and multiple ITCs? We propose that stable RPo, with its tightly gripping downstream clamp/jaw, is capable of only abortive synthesis, whereas less-stable I2 and I3, in which the clamp/jaw is less assembled and/or less tightly bound to downstream DNA, are capable of productive initiation. In this model, the relative populations of stable (RPo) and less stable (I2 and I3) open complexes determine the observed abortive:productive ratio. The fact that the clamp/jaw assembles only after DNA opening and untwisting is complete indicates that its grip likely impedes the movement of duplex DNA within it. Promoter sequence, sigma factor, concentrations of regulatory ligands, and solution conditions all must affect the relative stability and lifetime of each initiation intermediate and hence are determinants of initiation rate and the abortive:productive ratio, providing potent and relatively unexplored avenues for the regulation of transcription.
Material and Methods
Solutions and Materials.
Standard methods of purifying RNAP and of obtaining 32P-labeled DNA fragments were used. See SI Text.
High [NaCl] Fast-Kinetic MnO4 DNA Footprinting.
Labeled DNA promoter fragments in BB were mixed with RNAP in SB at a final concentration of 10 nM and incubated at room temperature (∼20 °C) for 90 min to preform open complexes. These complexes were loaded into the sample A tube of a KinTek Corporation RQF-3 Rapid Chemical Quench-Flow instrument cooled to 10 °C by a circulating water bath. Push syringe A was loaded with BB supplemented to 4% SB. The sample B tube and push syringe B were loaded with BB supplemented to 1.96 M NaCl, 400 μg/mL heparin, and 4% SB. Push syringe C was loaded with a solution containing 200 mM NaMnO4 and 900 mM NaCl. Collection tubes were filled with 300 μL of a quench solution containing 3.75 M ammonium acetate and 7.1 M β-mercaptoethanol. The quench-flow instrument was operated in a push-pause-push-pause-push mode. The first push rapidly mixed the preformed open complexes with the high salt solution resulting in a final [NaCl] of 1.1 M. This solution was held in reaction loop 7 for the desired perturbation time. The second push mixed the contents of reaction loop with the solution in push syringe C resulting in a final [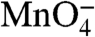 ] of 66.7 mM. This solution was held in the exit tube for 150 ms before the final push expelled the solution into the collection tube containing quench solution. Quenched reactions were immediately ethanol precipitated. Each load–reaction cycle took 250 min. Low salt control reactions were performed as above with the exception of loading solutions that keep [NaCl] at 120 mM. DNA fragments were washed with 70% ethanol, resuspended, reprecipitated, and washed. Modified fragments were cleaved by incubation at 90 °C in 1 M piperidine. Reactions were evaporated and resuspended in TE buffer (10 mM Tris HCl, 1 mM EDTA, pH 8.0) three times. The resulting DNA was resuspended in urea loading buffer and resolved on an 8% acrylamide gel. The gel was dried and exposed to a storage phosphor screen. The screen was scanned on a Typhoon scanner, and the resulting data analyzed with ImageQuant software. Phosphoimager intensities of each
] of 66.7 mM. This solution was held in the exit tube for 150 ms before the final push expelled the solution into the collection tube containing quench solution. Quenched reactions were immediately ethanol precipitated. Each load–reaction cycle took 250 min. Low salt control reactions were performed as above with the exception of loading solutions that keep [NaCl] at 120 mM. DNA fragments were washed with 70% ethanol, resuspended, reprecipitated, and washed. Modified fragments were cleaved by incubation at 90 °C in 1 M piperidine. Reactions were evaporated and resuspended in TE buffer (10 mM Tris HCl, 1 mM EDTA, pH 8.0) three times. The resulting DNA was resuspended in urea loading buffer and resolved on an 8% acrylamide gel. The gel was dried and exposed to a storage phosphor screen. The screen was scanned on a Typhoon scanner, and the resulting data analyzed with ImageQuant software. Phosphoimager intensities of each 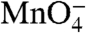 reactive thymine band (or doublet) as a function of time after the salt upshift were fit to a first order (single exponential) rate equation in which the long-time plateau value was floated. To obtain normalized θ values [fraction of that base remaining in an open (I2) condition], this long-time plateau intensity, arising from background/duplex reactivity of the thymine, was subtracted from the observed phosphoimager intensity at each time and divided by the background corrected intensity of that position in a low salt RPo control. Data fitting was performed by using IgorPro 5 software.
reactive thymine band (or doublet) as a function of time after the salt upshift were fit to a first order (single exponential) rate equation in which the long-time plateau value was floated. To obtain normalized θ values [fraction of that base remaining in an open (I2) condition], this long-time plateau intensity, arising from background/duplex reactivity of the thymine, was subtracted from the observed phosphoimager intensity at each time and divided by the background corrected intensity of that position in a low salt RPo control. Data fitting was performed by using IgorPro 5 software.
Population Modeling.
See SI Text.
Supplementary Material
Acknowledgments.
We thank Dr. C. Davis for preliminary KMnO4 experiments. We are grateful to our colleagues for many fruitful discussions and to the reviewers and the editor for their comments on the manuscript. This work was supported by National Institutes of Health Grant GM23467 (to M.T.R.). T.J.G. gratefully acknowledges the support of the William R. and Dorothy E. Sullivan Distinguished Graduate Fellowship. W.S.K. acknowledges support from National Institutes of Health Biotechnology Training Grant (NIH 5 T32 GM08349).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1000967107/-/DCSupplemental.
References
- 1.Murakami KS, Masuda S, Darst SA. Structural basis of transcription initiation: RNA polymerase holoenzyme at 4 Å resolution. Science. 2002;296:1280–1284. doi: 10.1126/science.1069594. [DOI] [PubMed] [Google Scholar]
- 2.Vassylyev DG, et al. Crystal structure of a bacterial RNA polymerase holoenzyme at 2.6 Å resolution. Nature. 2002;417:712–719. doi: 10.1038/nature752. [DOI] [PubMed] [Google Scholar]
- 3.Cramer P, Bushnell DA, Kornberg RD. Structural basis of transcription: RNA polymerase II at 2.8 Å resolution. Science. 2001;292:1863–1876. doi: 10.1126/science.1059493. [DOI] [PubMed] [Google Scholar]
- 4.Zhang G, et al. Crystal structure of Thermus aquaticus core RNA polymerase at 3.3 Å resolution. Cell. 1999;98:811–824. doi: 10.1016/s0092-8674(00)81515-9. [DOI] [PubMed] [Google Scholar]
- 5.Saecker RM, Record MT., Jr Protein surface salt bridges and paths for DNA wrapping. Curr Opin Struct Biol. 2002;12:311–319. doi: 10.1016/s0959-440x(02)00326-3. [DOI] [PubMed] [Google Scholar]
- 6.Kontur WS, Saecker RM, Capp MW, Record MT., Jr Late steps in the formation of E. coli RNA polymerase—λPR promoter open complexes: characterization of conformational changes by rapid [perturbant] upshift experiments. J Mol Biol. 2008;376:1034–1047. doi: 10.1016/j.jmb.2007.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Revyakin A, Ebright RH, Strick TR. Promoter unwinding and promoter clearance by RNA polymerase: detection by single-molecule DNA nanomanipulation. Proc Natl Acad Sci USA. 2004;101:4776–4780. doi: 10.1073/pnas.0307241101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sclavi B, et al. Real-time characterization of intermediates in the pathway to open complex formation by Escherichia coli RNA polymerase at the T7A1 promoter. Proc Natl Acad Sci USA. 2005;102:4706–4711. doi: 10.1073/pnas.0408218102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis CA, Bingman CA, Landick R, Record MT, Jr, Saecker RM. Real-time footprinting of DNA in the first kinetically significant intermediate in open complex formation by Escherichia coli RNA polymerase. Proc Natl Acad Sci USA. 2007;104:7833–7838. doi: 10.1073/pnas.0609888104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rogozina A, Zaychikov E, Buckle M, Heumann H, Sclavi B. DNA melting by RNA polymerase at the T7A1 promoter precedes the rate-limiting step at 37 °C and results in the accumulation of an off-pathway intermediate. Nucleic Acids Res. 2009;37:5390–5404. doi: 10.1093/nar/gkp560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaal T, Bartlett MS, Ross W, Turnbough CL, Jr, Gourse RL. Transcription regulation by initiating NTP concentration: rRNA synthesis in bacteria. Science. 1997;278:2092–2097. doi: 10.1126/science.278.5346.2092. [DOI] [PubMed] [Google Scholar]
- 12.Paul BJ, et al. DksA: a critical component of the transcription initiation machinery that potentiates the regulation of rRNA promoters by ppGpp and the initiating NTP. Cell. 2004;118:311–322. doi: 10.1016/j.cell.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Paul BJ, Berkmen MB, Gourse RL. DksA potentiates direct activation of amino acid promoters by ppGpp. Proc Natl Acad Sci USA. 2005;102:7823–7828. doi: 10.1073/pnas.0501170102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buc H, McClure WR. Kinetics of open complex formation between Escherichia coli RNA polymerase and the lacUV5 promoter. Evidence for a sequential mechanism involving three steps. Biochemistry. 1985;24:2712–2723. doi: 10.1021/bi00332a018. [DOI] [PubMed] [Google Scholar]
- 15.Roe JH, Burgess RR, Record MT., Jr Temperature dependence of the rate constants of the Escherichia coli RNA polymerase—λPR promoter interaction. Assignment of the kinetic steps corresponding to protein conformational change and DNA opening. J Mol Biol. 1985;184:441–453. doi: 10.1016/0022-2836(85)90293-1. [DOI] [PubMed] [Google Scholar]
- 16.Li XY, McClure WR. Characterization of the closed complex intermediate formed during transcription initiation by Escherichia coli RNA polymerase. J Biol Chem. 1998;273:23549–23557. doi: 10.1074/jbc.273.36.23549. [DOI] [PubMed] [Google Scholar]
- 17.Saecker RM, et al. Kinetic studies and structural models of the association of E. coli σ70 RNA polymerase with the λPR promoter: Large scale conformational changes in forming the kinetically significant intermediates. J Mol Biol. 2002;319:649–671. doi: 10.1016/S0022-2836(02)00293-0. [DOI] [PubMed] [Google Scholar]
- 18.Kontur WS, Capp MW, Gries TJ, Saecker RM, Record MT., Jr Probing DNA binding, DNA opening and assembly of a downstream clamp/jaw in Escherichia coli RNA polymerase—λPR promoter complexes using salt and the physiological anion glutamate. Biochemistry. 2010 doi: 10.1021/bi100092a. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borowiec JA, Zhang L, Sasse-Dwight S, Gralla JD. DNA supercoiling promotes formation of a bent repression loop in lac DNA. J Mol Biol. 1987;196:101–111. doi: 10.1016/0022-2836(87)90513-4. [DOI] [PubMed] [Google Scholar]
- 20.Schroeder LA, et al. Evidence for a tyrosine-adenine stacking interaction and for a short-lived open intermediate subsequent to initial binding of Escherichia coli RNA polymerase to promoter DNA. J Mol Biol. 2009;385:339–349. doi: 10.1016/j.jmb.2008.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bushnell DA, Kornberg RD. Complete, 12-subunit RNA polymerase II at 4.1 Å resolution: Implications for the initiation of transcription. Proc Natl Acad Sci USA. 2003;100:6969–6973. doi: 10.1073/pnas.1130601100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murakami KS, Masuda S, Campbell EA, Muzzin O, Darst SA. Structural basis of transcription initiation: An RNA polymerase holoenzyme-DNA complex. Science. 2002;296:1285–1290. doi: 10.1126/science.1069595. [DOI] [PubMed] [Google Scholar]
- 23.Murakami KS, Darst SA. Bacterial RNA polymerases: The wholo story. Curr Opin Struct Biol. 2003;13:31–39. doi: 10.1016/s0959-440x(02)00005-2. [DOI] [PubMed] [Google Scholar]
- 24.Kostrewa D, et al. RNA polymerase II-TFIIB structure and mechanism of transcription initiation. Nature. 2009;462:323–330. doi: 10.1038/nature08548. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Bushnell DA, Wang D, Calero G, Kornberg RD. Structure of an RNA polymerase II-TFIIB complex and the transcription initiation mechanism. Science. 2010;327:206–209. doi: 10.1126/science.1182015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young BA, Gruber TM, Gross CA. Minimal machinery of RNA polymerase holoenzyme sufficient for promoter melting. Science. 2004;303:1382–1384. doi: 10.1126/science.1092462. [DOI] [PubMed] [Google Scholar]
- 27.Craig ML, et al. DNA footprints of the two kinetically significant intermediates in formation of an RNA polymerase-promoter open complex: evidence that interactions with start site and downstream DNA induce sequential conformational changes in polymerase and DNA. J Mol Biol. 1998;283:741–756. doi: 10.1006/jmbi.1998.2129. [DOI] [PubMed] [Google Scholar]
- 28.Davis CA, Capp MW, Record MT, Jr, Saecker RM. The effects of upstream DNA on open complex formation by Escherichia coli RNA polymerase. Proc Natl Acad Sci USA. 2005;102:285–290. doi: 10.1073/pnas.0405779102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang GQ, Patel SS. Rapid binding of T7 RNA polymerase is followed by simultaneous bending and opening of the promoter DNA. Biochemistry. 2006;45:4947–4956. doi: 10.1021/bi052292s. [DOI] [PubMed] [Google Scholar]
- 30.Tang GQ, Patel SS. T7 RNA polymerase-induced bending of promoter DNA is coupled to DNA opening. Biochemistry. 2006;45:4936–4946. doi: 10.1021/bi0522910. [DOI] [PubMed] [Google Scholar]
- 31.Kontur WS, Saecker RM, Davis CA, Capp MW, Record MT., Jr Solute probes of conformational changes in open complex (RPo) formation by Escherichia coli RNA polymerase at the λPR promoter: Evidence for unmasking of the active site in the isomerization step and for large-scale coupled folding in the subsequent conversion to RPo. Biochemistry. 2006;45:2161–2177. doi: 10.1021/bi051835v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belogurov GA, et al. Transcription inactivation through local refolding of the RNA polymerase structure. Nature. 2009;457:332–335. doi: 10.1038/nature07510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holbrook JA, Capp MW, Saecker RM, Record MT., Jr Enthalpy and heat capacity changes for formation of an oligomeric DNA duplex: interpretation in terms of coupled processes of formation and association of single-stranded helices. Biochemistry. 1999;38:8409–8422. doi: 10.1021/bi990043w. [DOI] [PubMed] [Google Scholar]
- 34.Haugen SP, Ross W, Gourse RL. Advances in bacterial promoter recognition and its control by factors that do not bind DNA. Nat Rev Microbiol. 2008;6:507–519. doi: 10.1038/nrmicro1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gourse RL. Visualization and quantitative analysis of complex formation between E. coli RNA polymerase and an rRNA promoter in vitro. Nucleic Acids Res. 1988;16:9789–9809. doi: 10.1093/nar/16.20.9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rutherford ST, Villers CL, Lee JH, Ross W, Gourse RL. Allosteric control of Escherichia coli rRNA promoter complexes by DksA. Genes Dev. 2009;23:236–248. doi: 10.1101/gad.1745409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barker MM, Gaal T, Gourse RL. Mechanism of regulation of transcription initiation by ppGpp. II. Models for positive control based on properties of RNAP mutants and competition for RNAP. J Mol Biol. 2001;305:689–702. doi: 10.1006/jmbi.2000.4328. [DOI] [PubMed] [Google Scholar]
- 38.Barker MM, Gaal T, Josaitis CA, Gourse RL. Mechanism of regulation of transcription initiation by ppGpp. I. Effects of ppGpp on transcription initiation in vivo and in vitro. J Mol Biol. 2001;305:673–688. doi: 10.1006/jmbi.2000.4327. [DOI] [PubMed] [Google Scholar]
- 39.Haugen SP, et al. rRNA promoter regulation by nonoptimal binding of sigma region 1.2: An additional recognition element for RNA polymerase. Cell. 2006;125:1069–1082. doi: 10.1016/j.cell.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 40.Feklistov A, et al. A basal promoter element recognized by free RNA polymerase sigma subunit determines promoter recognition by RNA polymerase holoenzyme. Mol Cell. 2006;23:97–107. doi: 10.1016/j.molcel.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 41.Hsu LM, Vo NV, Kane CM, Chamberlin MJ. In vitro studies of transcript initiation by Escherichia coli RNA polymerase. 1. RNA chain initiation, abortive initiation, and promoter escape at three bacteriophage promoters. Biochemistry. 2003;42:3777–3786. doi: 10.1021/bi026954e. [DOI] [PubMed] [Google Scholar]
- 42.Vo NV, Hsu LM, Kane CM, Chamberlin MJ. In vitro studies of transcript initiation by Escherichia coli RNA polymerase. 2. Formation and characterization of two distinct classes of initial transcribing complexes. Biochemistry. 2003;42:3787–3797. doi: 10.1021/bi0269613. [DOI] [PubMed] [Google Scholar]
- 43.Kubori T, Shimamoto N. A branched pathway in the early stage of transcription by Escherichia coli RNA polymerase. J Mol Biol. 1996;256:449–457. doi: 10.1006/jmbi.1996.0100. [DOI] [PubMed] [Google Scholar]
- 44.Goldman SR, Ebright RH, Nickels BE. Direct detection of abortive RNA transcripts in vivo. Science. 2009;324:927–928. doi: 10.1126/science.1169237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.