Abstract
Emerging evidence suggests that the neurotransmitter acetylcholine (ACh) negatively regulates the development of the neuromuscular junction, but it is not clear if ACh exerts its effects exclusively through muscle ACh receptors (AChRs). Here, we used genetic methods to remove AChRs selectively from muscle. Similar to the effects of blocking ACh biosynthesis, eliminating postsynaptic AChRs increased motor axon branching and expanded innervation territory, suggesting that ACh negatively regulates synaptic growth through postsynaptic AChRs. However, in contrast to the effects of blocking ACh biosynthesis, eliminating postsynaptic AChRs in agrin-deficient mice failed to restore deficits in pre- and postsynaptic differentiation, suggesting that ACh negatively regulates synaptic differentiation through nonpostsynaptic receptors. Consistent with this idea, the ACh agonist carbachol inhibited presynaptic specialization of motorneurons in vitro. Together, these data suggest that ACh negatively regulates axon growth and presynaptic specialization at the neuromuscular junction through distinct cellular mechanisms.
Keywords: negative regulation, postsynaptic, presynaptic, retrograde signal, neurotransmitter
Recent genetic evidence in flies, fish, nematodes, and mammals suggests that neurotransmitters not only mediate adult physiological function but also play a developmental role in the patterning and formation of the synapses that they subserve (1–3). For example, the neurotransmitter acetylcholine (ACh) is released from embryonic motor neurons (MNs) at the neuromuscular junction (NMJ), and it negatively regulates survival, axon branching, and synapse formation (4–7). ACh is also released from developing neurons even before they arrive at their target, and it acts in an autocrine and/or paracrine fashion to regulate growth (8), pathfinding (9), spontaneous activity (10), and target selection (11). Whereas the NMJ has served as an excellent model to elucidate the process of synapse formation, the cellular and molecular mechanisms by which ACh regulates this process are still unclear; this is in part because the expression of ACh receptor (AChR) subtypes varies over space and time during the development of this synapse. For example, each of the constituent parts of the NMJ (including the MN-derived presynaptic nerve terminal, the muscle-derived postsynaptic apparatus, and the perisynaptic Schwann cell) expres-ses AChR (12, 13). Therefore, in this study, we used genetic techniques to study the cellular mechanism by which ACh regulates formation of the vertebrate NMJ.
Development of the NMJ can be divided into the following five stages: (i) nerve-independent establishment of AChR accumulations within broad central regions of muscle, (ii) nerve-dependent refinement of these clusters and restriction of incoming motor innervation to narrow central endplate bands of muscle, (iii) motor axon branching onto specific regions of individual muscle fibers, (iv) presynaptic specialization of motor nerve terminals, and (v) postsynaptic stabilization of innervated AChR clusters. ACh negatively regulates the second and third (synaptic growth) steps, because mice lacking the ACh synthetic enzyme choline acetyltransferase (ChAT) exhibit increased motor endplate bandwidth and motor axon branching (14, 15). ACh also negatively regulates the fourth and fifth (synaptic differentiation) steps, because mice lacking ChAT and agrin reverse the deficit in these steps that is exhibited by mice lacking agrin alone (6, 7).
Because ACh is removed from all cell types and tissues in ChAT mutants, it remains to be shown if ACh exerts its effects on the NMJ through AChRs expressed by nerve, muscle, or Schwann cells. Furthermore, because it is widely assumed that the regulation of presynaptic specialization is largely dependent on stabilization of the postsynaptic apparatus (16, 17), ACh may exert its effects on NMJ formation indirectly from one cell type to the next. For example, nerve-derived agrin is believed to antagonize the declustering effects of nerve-derived ACh at the postsynaptic apparatus, and in so doing, it causes the stabilized apparatus to release a retrograde signal that induces specialization of proximal presynaptic terminals (6, 7). Therefore, this bottom-up model implies that ACh negatively regulates presynaptic differentiation indirectly and downstream of its effects on postsynaptic differentiation. Alternatively, ACh could regulate presynaptic specialization directly by activating AChRs expressed by presynaptic terminals or indirectly through Schwann cell-derived AChRs. To address this issue, we studied the effect of removing exclusively muscle-derived postsynaptic AChRs on synaptic growth and differentiation. Available data show that embryonic muscle expresses two types of nicotinic AChR complexes that use AChRα1 or AChRα7 as the ligand-binding subunits (18, 19), but no other nicotinic AChR complexes or muscarinic AChR subtypes are used (13, 20–22). Therefore, we analyzed mice deficient in AChRα1 and/or AChRα7 subunits.
Results
AChRα1 Mutants Lack a Functional Muscle AChR Complex and AChR Clustering.
We first generated mice deficient in AChRα1 subunit as shown in SI Results and Fig. S1. Whereas neither AChRα1 mRNA nor protein is detected in these mice, mRNA for other subunits, such as the AChRδ subunit, is expressed; however, the protein is not aggregated on the muscle cell membrane (Fig. S2 A and B). Electrophysiological analysis showed that spontaneous miniature and nerve-evoked endplate potentials are not detected in AChRα1 mutant muscle (Fig. 1 A and B). In contrast, mice deficient in the AChRα7 subunit exhibit normal AChR clusters in muscle (23). Consistent with the absence of synaptic transmission, when stained with Texas Red-conjugated α-bungarotoxin (TR-αBTX), whole-mount preparations of E17.5 mouse diaphragm from AChRα1 mutants also fail to exhibit AChR clustering (Fig. 2). These results contrast with those obtained from mice deficient in the AChRγ subunit (23, 24), the AChRε subunit (25, 26), and phosphorylation of the AChRβ subunit (3, 27), in which a varying degree of AChR clustering is detected; thus, this supports the idea that the α1 subunit is necessary for other subunits to be located to the membrane (28). Together, these data provide anatomical and physiological evidence that AChRs are absent in muscle of AChRα1 null mutant mice. Remarkably, zebrafish nic-1 mutants that harbor a deletion within the α1 AChR subunit display a similar phenotype (29, 30).
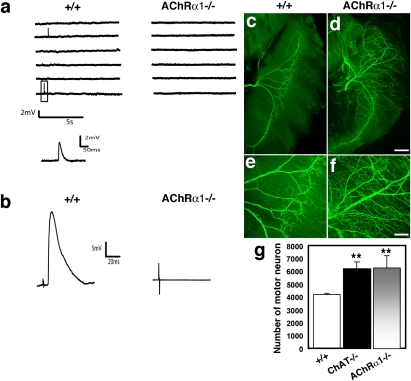
Fig. 1.
Postsynaptic transmission deficiency, muscle hyperinnervation, and increased motor neuron number in AChRα1 mutant mice. (A) Spontaneous miniature endplate potentials (MEPPs) were observed in control (+/+) diaphragm but not in AChRα1 mutant (AChRα1−/−) diaphragm. One MEPP is expanded below. (B) Nerve-evoked endplate potentials (EPPs) were observed in control but not in mutant muscle. (C–F) E17.5 whole-mount diaphragm muscles from controls and AChRα1 mutants were immunostained with antineurofilament (anti-NF) antibodies (green). Both (C and D) low- and (E and F) high-power magnifications showed that the phrenic nerve is highly branched in mutant muscle (D and F). (Scale bars: 200 μm for C and D; 100 μm for E and F.) (G) A similar increase in the number of lumbar motor neurons was observed in ChAT (ChAT−/−) and AChRα1 mutants compared with controls. **, P < 0.01.
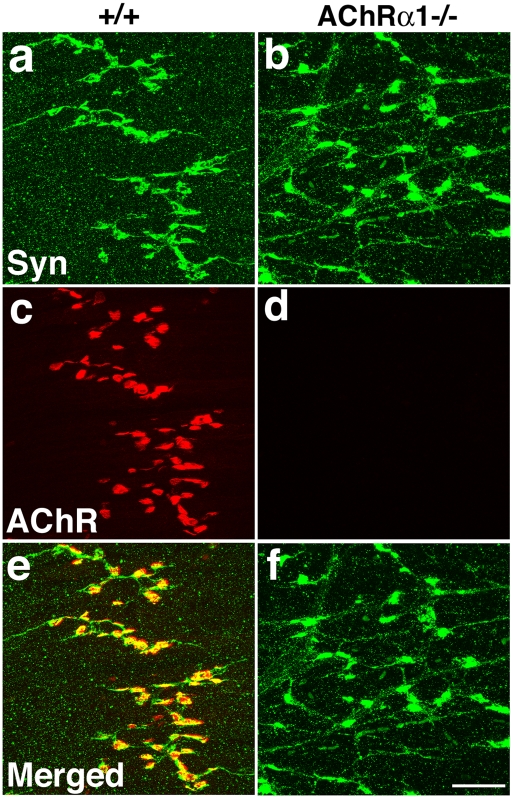
Fig. 2.
Presynaptic nerve terminals differentiate in AChRα1 mutants. Control (+/+; A, C, and E) and AChRα1 mutant (AChRα1−/−; B, D, and F) E17.5 whole-mount diaphragm muscles were immunostained with antisynapto-physin antibodies (A, B, and green in E and F) and costained with Texas Red-conjugated α-bungarotoxin (C, D, and red in E and F). Synaptophysin immunoreactivity is accumulated at the nerve terminals (arrows in A) and colocalized with receptor clusters in control diaphragms. Mutant diaphragms lack receptor clusters but maintain synaptophysin accumulations at the nerve terminals. (Scale bar: 50 μm.)
Loss of Muscle Receptors Results in Increased Nerve Branching and Motor Neuron Number.
Mice lacking ChAT display increased endplate bandwidth, motor axon branching, and MN number (14, 15). To determine if these effects are mediated by postsynaptic AChRs, we immunostained diaphragm muscles with neurofilament (NF) antibodies (to assess branching) and quantified the number of vesicular acetylcholine (VAChT) mRNA-positive cells in the lumbar lateral motor column (to assess MN number) of AChRα1 mutant mice. In contrast to the centrally restricted pattern of innervation observed in the control diaphragm at E17.5, AChRα1 mutant mice exhibit a greater number of nerve branches that supply a wider region of muscle (Fig. 1 C–F). Additionally, MN number is increased by about 60% in both ChAT and AChRα1 mutants compared with wild type (WT) (Fig. 1G). These results are similar in magnitude to those reported in ChAT mutants and support the idea that postsynaptic AChRs limit the growth, branching, and survival of developing MNs; additionally, they are consistent with those results observed in chicken embryos treated with a toxin that selectively blocks fetal muscle AChRs (31), but they contrast with the results obtained on studies of primary MNs in zebrafish nic-1 mutants (29).
Muscle Receptors Are Not Required for Presynaptic Specialization.
In mice lacking muscle-specific kinase (MuSK) or agrin, AChR is not clustered at synaptic sites, and motor axons fail to arborize (Results) or elaborate specialized synaptic terminals, suggesting that AChR clustering may be required for presynaptic differentiation (16, 17). To test this idea, we costained E17.5 diaphragm with TR-αBTX and antibodies against the synaptic vesicle protein synaptophysin (Syn) (Fig. 2). In control diaphragms, Syn is highly concentrated in nerve terminals closely opposed to AChR clusters. Surprisingly, despite the absence of receptor clusters in AChRα1 mutant muscle, Syn immunoreactivity is accumulated in presynaptic nerve terminals. Although Syn can also be detected in embryonic axon bundles, the higher level of punctate staining observed in the nerve terminal is clearly distinct from the lower level of diffuse reactivity characteristic of axonal staining (32, 33). Furthermore, when antibodies against the synaptic vesicle protein SV2 were used, similar results were obtained (Fig. S3B). Finally, accumulations of synaptic vesicles within presynaptic nerve terminals are observed by electron microscopy (EM) in AChRα1 mutant mice (Fig. S4B; SI Results and Table S1 show additional EM analysis). Similar to the pattern of NF-immunostaining, Syn-rich nerve terminals occupy a broader region of the diaphragm. These results indicate that although agrin–MuSK signaling is necessary for both expression of AChR in postsynaptic clusters and specialization of presynaptic nerve terminals, expression of AChR in clusters per se is not an essential intermediary in this process. Interestingly, synaptic vesicles are also clustered in zebrafish AChR cluster mutants nic-1 (29) and sop, which are deficient in the AChRδ subunit (34, 35). Although AChRs are required for the postsynaptic clustering of several of the proteins observed in the NMJ such as acetylcholinesterase (AChE), syntrophin isoforms, and rapsyn (36–38), we found that AChE and MuSK are, whereas rapsyn is not, clustered at the postsynaptic membrane (Fig. S3), consistent with previous findings (35, 39–41).
ACh Inhibits Pre- and Postsynaptic Differentiation Through Nonpost-synaptic AChR.
Previous studies have shown that whereas agrin mutant mice lack both pre- and postsynaptic differentiation (42), removing ACh from agrin mutants by deleting ChAT restores both of these deficits (6, 7). The presence of presynaptic specialization in ChAT/agrin double mutants may be indirect and result from the restoration of postsynaptic differentiation, or ACh may be direct and inhibit presynaptic specialization through nonpostsynaptic receptors. In striking contrast to ChAT/agrin double mutants (Fig. 3C and Table S2), AChRα1/agrin double mutants fail to exhibit punctate Syn immunostaining and thus, presynaptic specialization (Fig. 3A). To exclude the possibility that the AChRα7 subunit, which is transiently expressed in embryonic muscle at low levels and nonpostsynaptic sites, plays a compensatory role to mediate inhibition by ACh in AChRα1/agrin double mutants, we analyzed AChRα1/AChRα7/agrin triple mutant mice. Presynaptic specialization was not observed in either AChRα7/agrin double or AChRα1/AChRα7/agrin triple mutants (Fig. S5). Finally, to determine if AChRα1/agrin double mutants maintain postsynaptic differentiation even in the absence of presynaptic differentiation, we examined the expression of AChE and MuSK in E17.5 muscle. In contrast to results obtained from ChAT/agrin double mutant mice (7), both AChRα1/agrin (Fig. 3B and Fig. S6) and AChRα1/AChRα7/agrin mutants fail to exhibit clustering of either AChE or MuSK. Therefore, because (α7)5 and (α1)2βδγ pentamers are the only AChR complexes present in embryonic muscle (18, 19), our results indicate that ACh inhibits both pre- and postsynaptic differentiation by a mechanism that does not involve postsynaptic receptor clusters; instead, we suggest that the inhibitory activity is likely mediated by AChRs on nerve terminals or Schwann cells.
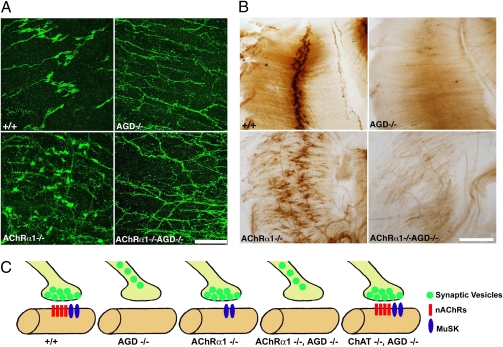
Fig. 3.
Absence of presynaptic differentiation in AChRα1/agrin double mutants. (A) Diaphragm muscles from control, agrin-deficient (AGD), AChRα1, and AChRα1/AGD mutants were immunostained with antibodies against synaptophysin. Presynaptic specialization is not observed in either AGD single or AChRα1/AGD double mutants. (Scale bar: 100 μm.) (B) Absence of AChE clusters in AGD and AChRα1/AGD mutants. (Scale bar: 200 μm.) (C) Summary analysis of accumulation of synaptic vesicles in control, AGD, AChRα1−/−, AChRα1−/−, AGD, and ChAT−/−, AGD mutants.
Agrin Induces Expression of Fibroblast Growth Factors.
The presence of an alternative pathway by which ACh negatively regulates synaptic differentiation prompted us to examine if and how agrin might antagonize this pathway. Recent studies show that muscle-derived organizing molecules, including selective members of the fibroblast growth factor (FGF) family, play essential roles in regulating presynaptic specialization (43, 44). To investigate if the retrograde signal induced by agrin was a member of this family, RNA was isolated from C2C12 myotubes treated with agrin and subjected to real-time quantitative PCR analysis. As shown in Fig. 4B, mRNA levels for FGF7 and FGF9 are significantly elevated after agrin treatment. Conversely, levels of FGF7 and FGF9 mRNA are reduced in agrin mutant diaphragm compared with control (Fig. 4C). These results show that agrin regulates expression of FGFs in muscle and suggest that these molecules may oppose the negative effects of nonpostsynaptic ACh signaling on presynaptic differentiation.
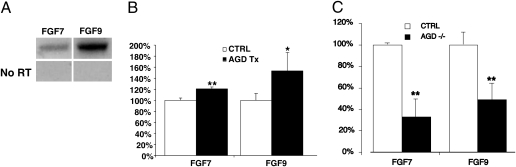
Fig. 4.
Induced FGF mRNA expression by agrin in C2C12 myotubes and decreased FGF mRNA expression in agrin mutant muscle. (A) RT-PCR showed that FGF7 and FGF9 mRNAs are expressed in E18.5 diaphragm muscles. The results illustrate that the primer sets used for the RT-PCR experiments are specific because no signal is detected when reverse transcriptase (RT) is omitted (No RT) in the reaction. For the simplicity of presentation, we cropped the original scan to show only FGF7 and FGF9 expression in control samples. (B) Real-time quantitative RT-PCR showed that agrin induces expression of FGF7 and FGF9 mRNA in C2C12 myotube cultures. Expression level of control without agrin treatment is set as 100%. The results were expressed as percentage of control (n = 4). *, P < 0.05; **, P < 0.01. (C) RNA was isolated from E18.5 controls or agrin mutant muscles for real-time quantitative RT-PCR analysis. Expression level of control embryos is set as 100%. The results were expressed as percentage of control embryos. The results showed reduced levels of FGF7 and FGF9 mRNA in muscle from agrin mutants, relative to controls (n = 4). *, P < 0.05; **, P < 0.01.
ACh Agonist Disperses FGF-Induced Synaptic Vesicle-Rich Varicosities.
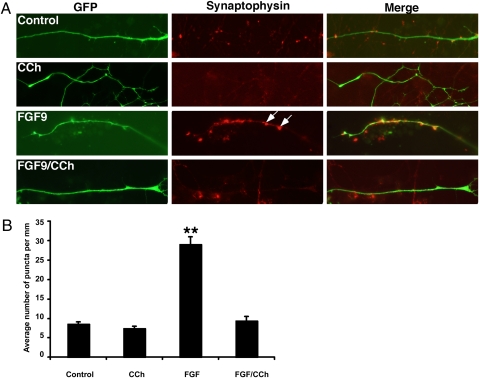
To show that ACh inhibits presynaptic specialization through non-postsynaptic AChRs, we used embryonic stem (ES) cell-derived MNs to determine if ACh inhibits FGF-induced presynaptic specialization (45). The HBG3 (HB9-green fluorescence protein) ES cell line was derived from transgenic mice expressing an enhanced GFP under the control of the MN-specific homeobox protein 9 promoter (45). MNs were treated overnight with FGF9 or FGF22 to induce aggregation of synaptic vesicles, washed with medium to remove FGFs, and then treated with the ACh agonist carbachol (CCh). Consistent with previous studies (43, 44), FGFs induce formation of Syn- and SV2-immunoreactive varicosities (Fig. 5A, arrows), but treatment with CCh markedly reduces the number of these varicosities (Fig. 5 A and B). Interestingly, FGFs are incapable of preventing inhibition of CCh-induced dispersion of varicosities (Fig. 5B). These results support the idea that ACh inhibits presynaptic specialization directly by activating presynaptic AChRs such as those present on motor axons.
Fig. 5.
Dispersion of FGF-induced aggre-gates of synaptic vesicles by the ACh agonist carbachol in ES-derived motor neurons. (A) Compared with control cultures of ES cell-derived motor neurons (Control), treatment with the ACh agonist CCh did not induce aggregation of synaptic vesicles in motor neurons (CCh). ES-derived motor neurons treated with FGF9 exhibited synaptic vesicle-rich varicosities (FGF9; arrows). CCh destabilized FGF-induced aggregation of synaptic vesicles (FGF9/CCh). (B) Quantitative analysis of the effect of CCh in the maintenance of synaptic varicosity (n = 4). **, P < 0.01.
Discussion
In this study, we provide genetic evidence that ACh negatively regulates synaptic growth and differentiation by distinct cellular mechanisms. Specifically, ACh inhibits motor endplate bandwidth and motor axon branching (synaptic growth) by activating postsynaptic AChRs, and it inhibits presynaptic nerve terminal specialization and postsynaptic AChR clustering (synaptic differentiation) by activating nonpostsynaptic AChRs. A schematic model summarizing these findings is presented in Fig. S7. These results are unexpected and have several important implications. First, they strengthen the hypothesis that aneural AChR clusters detected at E14.5 along a narrow central band of muscle are a component of the muscle intrinsic mechanism for prepatterning of neuromuscular synapses (46). We suggest that their func-tion is to restrict nerve branching and nerve terminal growth within a limited, central region of muscle fiber, thereby contributing to the control of the boundary for the formation and distribution of mature synapses (15). Second, the effects of AChRα1 inactivation on branching and survival strengthen the idea that MN activity regulates these aspects of development through postsynaptic AChR and thus, a peripheral mechanism, at least in chick and mouse (47). These findings are, therefore, consistent with the neurotrophic tenet that retrograde distribution by muscle of branching- and survival-promoting molecules is regulated by MN activity. Interestingly, results obtained from studies of primary MNs in zebrafish AChRα1 mutants failed to show an effect on MN branching and terminal arborization, which may reflect species differences or unique characteristics of zebrafish primary MNs (29). Indeed, recent evidence suggests that the cues regulating primary and secondary motor axon branching in zebrafish are different (48, 49).
In contrast to its effects on endplate bandwidth and branching, ACh inhibits pre- and postsynaptic differentiation through nonpostsynaptic AChRs in the absence of postsynaptic AChR and agrin. This is unexpected, because ACh directly inhibits postsynaptic differentiation by activating and dispersing postsynaptic AChR clusters (50). Together with the findings that agrin is required not only for postsynaptic but also presynaptic differentiation (42) and that ChAT/agrin double mutants restore these pre- and postsynaptic deficits, these data led to the proposition that agrin at the NMJ inhibits the declustering activity of ACh (6, 7). According to this model, presynaptic specialization occurs only in nerve terminals closely opposed to these agrin-stabilized postsynaptic AChR clusters and thus, is downstream of postsynaptic differentiation (16). This bottom-up model is also consistent with the idea that agrin-stabilized AChR clusters release a retrograde factor that specializes presynaptic terminals (43, 44). However, the current results suggest that this model may be too simple and that a parallel top-down pathway also exists to regulate NMJ development. Therefore, whereas ACh may disperse agrin-unstabilized AChR clusters directly through interactions with postsynaptic AChRs, ACh also eliminates these clusters indirectly in the absence of agrin through interactions with nonpostsynaptic AChRs, presumably through an indirect, orthograde signal released from unspecialized nerve terminals. The extent to which this indirect, top-down pathway occurs exclusively, in tandem with the direct bottom-up pathway, or latently (i.e., only in the absence of the bottom-up pathway) in control animals is still unclear, and it will require the selective removal of presynaptic and/or perisynaptic AChRs in agrin mutants.
These studies also suggest that, in addition to opposing the direct postsynaptic ACh pathway by stabilizing nerve terminal-opposed AChR clusters, agrin is capable of inhibiting the indirect, presynaptic ACh pathway; mice lacking only AChRα1 exhibit normal expression of MuSK, AChE, and Syn at the NMJ, whereas those lacking both AChRα1 and agrin do not. Because nerve-derived agrin acts on muscle receptors and because the indirect ACh pathway originates within Schwann cells or motor axon terminals, the ability of agrin to inhibit this latter pathway is likely mediated by the induction of a retrograde signal from muscle to presynaptic nerve terminal. However, compared with the retrograde factor hypothesized to induce presynaptic specialization (bottom-up pathway), this signal more likely achieves presynaptic specialization through the disinhibition of ACh effects through nonpostsynaptic AChRs. Moreover, whereas the retrograde factor in the bottom-up pathway is proposed to be dependent on agrin-mediated stabilization of postsynaptic AChR clusters, the retrograde signal in the top-down pathway is released through an agrin-induced mechanism independent of cluster stabilization; this is because clusters are not stabilized in the AChRα1/agrin double mutants in which this pathway is revealed (Fig. S7). In support of the idea that agrin induces the release of a retrograde signal capable of disinhibiting presynaptic specialization, we found that agrin induces FGF expression in muscle and conversely, that muscle from agrin-deficient mice expresses less FGF than muscle from control mice. However, other retrograde factors such as β-catenin are required for antagonizing ACh-induced inhibition of presynaptic differentiation such as β-catenin–dependent signals (51).
Although the data in this study strongly support the idea that ACh regulates NMJ formation in nAChRα1-deficient mice by nonpostsynaptic nicotinic or muscarinic AChRs, it remains a formal possibility that ACh regulates postsynaptic muscarinic AChRs, although evidence supporting the embryonic expression of muscle-derived mAChRs has not been reported. ACh may also mediate these effects by activating nonpostsynaptic muscarinic AChRs in embryonic MNs or Schwann cells, because distinct muscarinic AChR subtypes are expressed by adult MNs, Schwann cells, and muscle (13, 21, 22, 52) and play a role in the stability of adult NMJs (52). A more detailed analysis of both nicotinic and muscarinic AChRs in embryonic NMJ cell types will help clarify the molecular mechanism by which ACh regulates presynaptic differentiation in nAChRα1-deficient mice. Similarly, whereas the similarity of electrophysiological results between nAChRα1 and ChAT null mutant mice strongly suggests that the effects of deleting nAChRα1 are caused by the lack of ACh-mediated neurotransmission at the NMJ, it is possible that other ACh-independent events, such as altered muscle development, may contribute to these effects.
In conclusion, our results show that although ACh negatively regulates synaptic growth exclusively through postsynaptic AChRs, ACh inhibits synaptic differentiation by activating nonpostsynaptic AChRs. These data reveal an unexpected complexity in the mechanism by which ACh regulates synaptic differentiation and suggest that ACh directly prevents the specialization of presynaptic terminals not opposing a postsynaptic apparatus in addition to directly eliminating postsynaptic AChR clusters that are not opposed to presynaptic terminals,. Conversely, agrin directly stabilizes postsynaptic AChR clusters that are opposed to synaptic terminals and indirectly stimulates the presynaptic specialization of nerve terminals opposing a postsynaptic apparatus. This reciprocal control of pre- and postsynaptic elements of the developing NMJ by positive and negative nerve-derived signals may represent a homeostatic mechanism preventing the development of inappropriate and thus deleterious neuromuscular circuits.
Materials and Methods
Mice.
AChRα1 mutant mice were generated by standard procedures. For details, see (SI Materials and Methods.
Electrophysiology.
Intracellular sharp-electrode recording was performed blind to genotype on phrenic nerve/diaphragm preparations from E17.5 embryos. For details, see SI Materials and Methods.
Motor Neuron Counts.
The total number of VAChT mRNA-positive motoneurons in the lumbar spinal cord was quantified according to details available in SI Materials and Methods.
Embryonic Stem Cell-Derived Motor Neurons, FGF, and CCh Treatments.
HBG3 mouse ES cells were used to generate motor neurons according to details described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Richard Rotundo for providing the protocol for labeling fasciculin with fluorochrome to detect AChE clusters, Steven Burden for the in situ probes and anti-MuSK antibodies, Zuo-Zhong Wang for anti-AChRδ subunit antibodies, Stanley Froehner and Margaret Maimone for antirapsyn antibodies, Mariella De Biasi for AChRα7 mutant mice, and Thomas Jessell for HBG3 cells. This work was supported by a fellowship from the Muscular Dystrophy Association (J.Y.) and grants from the Robert Packard Center for ALS Research (R.W.O.) and the Muscular Dystrophy Association (K.-F.L.). This work was also supported by National Institutes of Health Grants HD034534, NS047345, and NS044420 (to K.-F.L.) and NS055028 (to W.L.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1004956107/-/DCSupplemental.
References
- 1.Nguyen L, et al. Neurotransmitters as early signals for central nervous system development. Cell Tissue Res. 2001;305:187–202. doi: 10.1007/s004410000343. [DOI] [PubMed] [Google Scholar]
- 2.Broadie KS, Richmond JE. Establishing and sculpting the synapse in Drosophila and C. elegans. Curr Opin Neurobiol. 2002;12:491–498. doi: 10.1016/s0959-4388(02)00359-8. [DOI] [PubMed] [Google Scholar]
- 3.Behra M, et al. Acetylcholinesterase is required for neuronal and muscular development in the zebrafish embryo. Nat Neurosci. 2002;5:111–118. doi: 10.1038/nn788. [DOI] [PubMed] [Google Scholar]
- 4.Pittman RH, Oppenheim RW. Neuromuscular blockade increases motoneurone survival during normal cell death in the chick embryo. Nature. 1978;271:364–366. doi: 10.1038/271364a0. [DOI] [PubMed] [Google Scholar]
- 5.Dahm LM, Landmesser LT. The regulation of intramuscular nerve branching during normal development and following activity blockade. Dev Biol. 1988;130:621–644. doi: 10.1016/0012-1606(88)90357-0. [DOI] [PubMed] [Google Scholar]
- 6.Lin W, et al. Neurotransmitter acetylcholine negatively regulates neuromuscular synapse formation by a Cdk5-dependent mechanism. Neuron. 2005;46:569–579. doi: 10.1016/j.neuron.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Misgeld T, Kummer TT, Lichtman JW, Sanes JR. Agrin promotes synaptic differentiation by counteracting an inhibitory effect of neurotransmitter. Proc Natl Acad Sci USA. 2005;102:11088–11093. doi: 10.1073/pnas.0504806102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pugh PC, Berg DK. Neuronal acetylcholine receptors that bind α-bungarotoxin mediate neurite retraction in a calcium-dependent manner. J Neurosci. 1994;14:889–896. doi: 10.1523/JNEUROSCI.14-02-00889.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng JQ, Felder M, Connor JA, Poo M-M. Turning of nerve growth cones induced by neurotransmitters. Nature. 1994;368:140–144. doi: 10.1038/368140a0. [DOI] [PubMed] [Google Scholar]
- 10.Milner LD, Landmesser LT. Cholinergic and GABAergic inputs drive patterned spontaneous motoneuron activity before target contact. J Neurosci. 1999;19:3007–3022. doi: 10.1523/JNEUROSCI.19-08-03007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang H, Kunes S. Nonvesicular release of acetylcholine is required for axon targeting in the Drosophila visual system. Proc Natl Acad Sci USA. 2004;101:15213–15218. doi: 10.1073/pnas.0308141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowman WC, Prior C, Marshall IG. Presynaptic receptors in the neuromuscular junction. Ann N Y Acad Sci. 1990;604:69–81. doi: 10.1111/j.1749-6632.1990.tb31983.x. [DOI] [PubMed] [Google Scholar]
- 13.Rochon D, Rousse I, Robitaille R. Synapse-glia interactions at the mammalian neuromuscular junction. J Neurosci. 2001;21:3819–3829. doi: 10.1523/JNEUROSCI.21-11-03819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Misgeld T, et al. Roles of neurotransmitter in synapse formation: Development of neuromuscular junctions lacking choline acetyltransferase. Neuron. 2002;36:635–648. doi: 10.1016/s0896-6273(02)01020-6. [DOI] [PubMed] [Google Scholar]
- 15.Brandon EP, et al. Aberrant patterning of neuromuscular synapses in choline acetyltransferase-deficient mice. J Neurosci. 2003;23:539–549. doi: 10.1523/JNEUROSCI.23-02-00539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glass DJ, Yancopoulos GD. Sequential roles of agrin, MuSK and rapsyn during neuromuscular junction formation. Curr Opin Neurobiol. 1997;7:379–384. doi: 10.1016/s0959-4388(97)80066-9. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen QT, Son Y-J, Sanes JR, Lichtman JW. Nerve terminals form but fail to mature when postsynaptic differentiation is blocked: In vivo analysis using mammalian nerve-muscle chimeras. J Neurosci. 2000;20:6077–6086. doi: 10.1523/JNEUROSCI.20-16-06077.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romano SJ, Pugh PC, McIntosh JM, Berg DK. Neuronal-type acetylcholine receptors and regulation of alpha 7 gene expression in vertebrate skeletal muscle. J Neurobiol. 1997;32:69–80. [PubMed] [Google Scholar]
- 19.Fischer U, Reinhardt S, Albuquerque EX, Maelicke A. Expression of functional alpha7 nicotinic acetylcholine receptor during mammalian muscle development and denervation. Eur J Neurosci. 1999;11:2856–2864. doi: 10.1046/j.1460-9568.1999.00703.x. [DOI] [PubMed] [Google Scholar]
- 20.Wessler I. Acetylcholine at motor nerves: Storage, release, and presynaptic modulation by autoreceptors and adrenoceptors. Int Rev Neurobiol. 1992;34:283–384. doi: 10.1016/s0074-7742(08)60100-2. [DOI] [PubMed] [Google Scholar]
- 21.Minic J, Molgó J, Karlsson E, Krejci E. Regulation of acetylcholine release by muscarinic receptors at the mouse neuromuscular junction depends on the activity of acetylcholinesterase. Eur J Neurosci. 2002;15:439–448. doi: 10.1046/j.0953-816x.2001.01875.x. [DOI] [PubMed] [Google Scholar]
- 22.Garcia N, Santafé MM, Salon I, Lanuza MA, Tomàs J. Expression of muscarinic acetylcholine receptors (M1-, M2-, M3- and M4-type) in the neuromuscular junction of the newborn and adult rat. Histol Histopathol. 2005;20:733–743. doi: 10.14670/HH-20.733. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, et al. Essential roles of the acetylcholine receptor gamma-subunit in neuromuscular synaptic patterning. Development. 2008;135:1957–1967. doi: 10.1242/dev.018119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi M, et al. Spontaneous muscle action potentials fail to develop without fetal-type acetylcholine receptors. EMBO Rep. 2002;3:674–681. doi: 10.1093/embo-reports/kvf128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Witzemann V, et al. Acetylcholine receptor epsilon-subunit deletion causes muscle weakness and atrophy in juvenile and adult mice. Proc Natl Acad Sci USA. 1996;93:13286–13291. doi: 10.1073/pnas.93.23.13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Missias AC, et al. Deficient development and maintenance of postsynaptic specializations in mutant mice lacking an ‘adult’ acetylcholine receptor subunit. Development. 1997;124:5075–5086. doi: 10.1242/dev.124.24.5075. [DOI] [PubMed] [Google Scholar]
- 27.Friese MB, Blagden CS, Burden SJ. Synaptic differentiation is defective in mice lacking acetylcholine receptor beta-subunit tyrosine phosphorylation. Development. 2007;134:4167–4176. doi: 10.1242/dev.010702. [DOI] [PubMed] [Google Scholar]
- 28.Green WN, Millar NS. Ion-channel assembly. Trends Neurosci. 1995;18:280–287. [PubMed] [Google Scholar]
- 29.Westerfield M, Liu DW, Kimmel CB, Walker C. Pathfinding and synapse formation in a zebrafish mutant lacking functional acetylcholine receptors. Neuron. 1990;4:867–874. doi: 10.1016/0896-6273(90)90139-7. [DOI] [PubMed] [Google Scholar]
- 30.Sepich DS, Wegner J, O'Shea S, Westerfield M. An altered intron inhibits synthesis of the acetylcholine receptor alpha-subunit in the paralyzed zebrafish mutant nic1. Genetics. 1998;148:361–372. doi: 10.1093/genetics/148.1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oppenheim RW, et al. The rescue of developing avian motoneurons from programmed cell death by a selective inhibitor of the fetal muscle-specific nicotinic acetylcholine receptor. Dev Neurobiol. 2008;68:972–980. doi: 10.1002/dneu.20636. [DOI] [PubMed] [Google Scholar]
- 32.Lupa MT, Gordon H, Hall ZW. A specific effect of muscle cells on the distribution of presynaptic proteins in neurites and its absence in a C2 muscle cell variant. Dev Biol. 1990;142:31–43. doi: 10.1016/0012-1606(90)90148-c. [DOI] [PubMed] [Google Scholar]
- 33.Polo-Parada L, Bose CM, Landmesser LT. Alterations in transmission, vesicle dynamics, and transmitter release machinery at NCAM-deficient neuromuscular junctions. Neuron. 2001;32:815–828. doi: 10.1016/s0896-6273(01)00521-9. [DOI] [PubMed] [Google Scholar]
- 34.Li W, Ono F, Brehm P. Optical measurements of presynaptic release in mutant zebrafish lacking postsynaptic receptors. J Neurosci. 2003;23:10467–10474. doi: 10.1523/JNEUROSCI.23-33-10467.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ono F, Mandel G, Brehm P. Acetylcholine receptors direct rapsyn clusters to the neuromuscular synapse in zebrafish. J Neurosci. 2004;24:5475–5481. doi: 10.1523/JNEUROSCI.0851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De La Porte S, et al. Accumulation of acetylcholine receptors is a necessary condition for normal accumulation of acetylcholinesterase during in vitro neuromuscular synaptogenesis. Eur J Neurosci. 1998;10:1631–1643. doi: 10.1046/j.1460-9568.1998.00165.x. [DOI] [PubMed] [Google Scholar]
- 37.Grow WA, Ferns M, Gordon H. Agrin-independent activation of the agrin signal transduction pathway. J Neurobiol. 1999;40:356–365. [PubMed] [Google Scholar]
- 38.Marangi PA, et al. Acetylcholine receptors are required for agrin-induced clustering of postsynaptic proteins. EMBO J. 2001;20:7060–7073. doi: 10.1093/emboj/20.24.7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burden SJ, DePalma RL, Gottesman GS. Crosslinking of proteins in acetylcholine receptor-rich membranes: Association between the beta-subunit and the 43 kd subsynaptic protein. Cell. 1983;35:687–692. doi: 10.1016/0092-8674(83)90101-0. [DOI] [PubMed] [Google Scholar]
- 40.Maimone MM, Merlie JP. Interaction of the 43 kd postsynaptic protein with all subunits of the muscle nicotinic acetylcholine receptor. Neuron. 1993;11:53–66. doi: 10.1016/0896-6273(93)90270-2. [DOI] [PubMed] [Google Scholar]
- 41.Ono F, Higashijima S, Shcherbatko A, Fetcho JR, Brehm P. Paralytic zebrafish lacking acetylcholine receptors fail to localize rapsyn clusters to the synapse. J Neurosci. 2001;21:5439–5448. doi: 10.1523/JNEUROSCI.21-15-05439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gautam M, et al. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell. 1996;85:525–535. doi: 10.1016/s0092-8674(00)81253-2. [DOI] [PubMed] [Google Scholar]
- 43.Umemori H, Linhoff MW, Ornitz DM, Sanes JR. FGF22 and its close relatives are presynaptic organizing molecules in the mammalian brain. Cell. 2004;118:257–270. doi: 10.1016/j.cell.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 44.Fox MA, et al. Distinct target-derived signals organize formation, maturation, and maintenance of motor nerve terminals. Cell. 2007;129:179–193. doi: 10.1016/j.cell.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 45.Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 46.Lin W, et al. Distinct roles of nerve and muscle in postsynaptic differentiation of the neuromuscular synapse. Nature. 2001;410:1057–1064. doi: 10.1038/35074025. [DOI] [PubMed] [Google Scholar]
- 47.Oppenheim RW, et al. Rescue of developing spinal motoneurons from programmed cell death by the GABA(A) agonist muscimol acts by blockade of neuro-muscular activity and increased intramuscular nerve branching. Mol Cell Neurosci. 2003;22:331–343. doi: 10.1016/s1044-7431(02)00020-9. [DOI] [PubMed] [Google Scholar]
- 48.Panzer JA, et al. Neuromuscular synaptogenesis in wild-type and mutant zebrafish. Dev Biol. 2005;285:340–357. doi: 10.1016/j.ydbio.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 49.Panzer JA, Song Y, Balice-Gordon RJ. In vivo imaging of preferential motor axon outgrowth to and synaptogenesis at prepatterned acetylcholine receptor clusters in embryonic zebrafish skeletal muscle. J Neurosci. 2006;26:934–947. doi: 10.1523/JNEUROSCI.3656-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bloch RJ. Loss of acetylcholine receptor clusters induced by treatment of cultured rat myotubes with carbachol. J Neurosci. 1986;6:691–700. doi: 10.1523/JNEUROSCI.06-03-00691.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li XM, et al. Retrograde regulation of motoneuron differentiation by muscle beta-catenin. Nat Neurosci. 2008;11:262–268. doi: 10.1038/nn2053. [DOI] [PubMed] [Google Scholar]
- 52.Wright MC, et al. Distinct muscarinic acetylcholine receptor subtypes contribute to stability and growth, but not compensatory plasticity, of neuromuscular synapses. J Neurosci. 2009;29:14942–14955. doi: 10.1523/JNEUROSCI.2276-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.