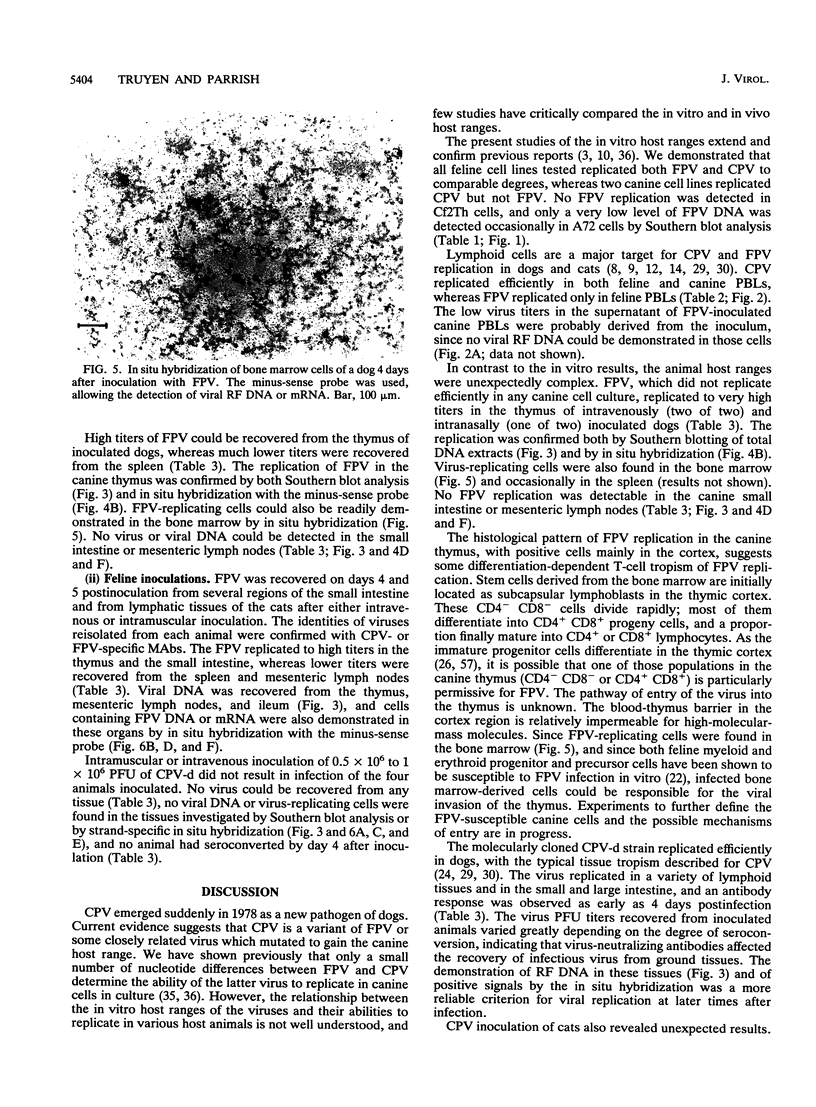
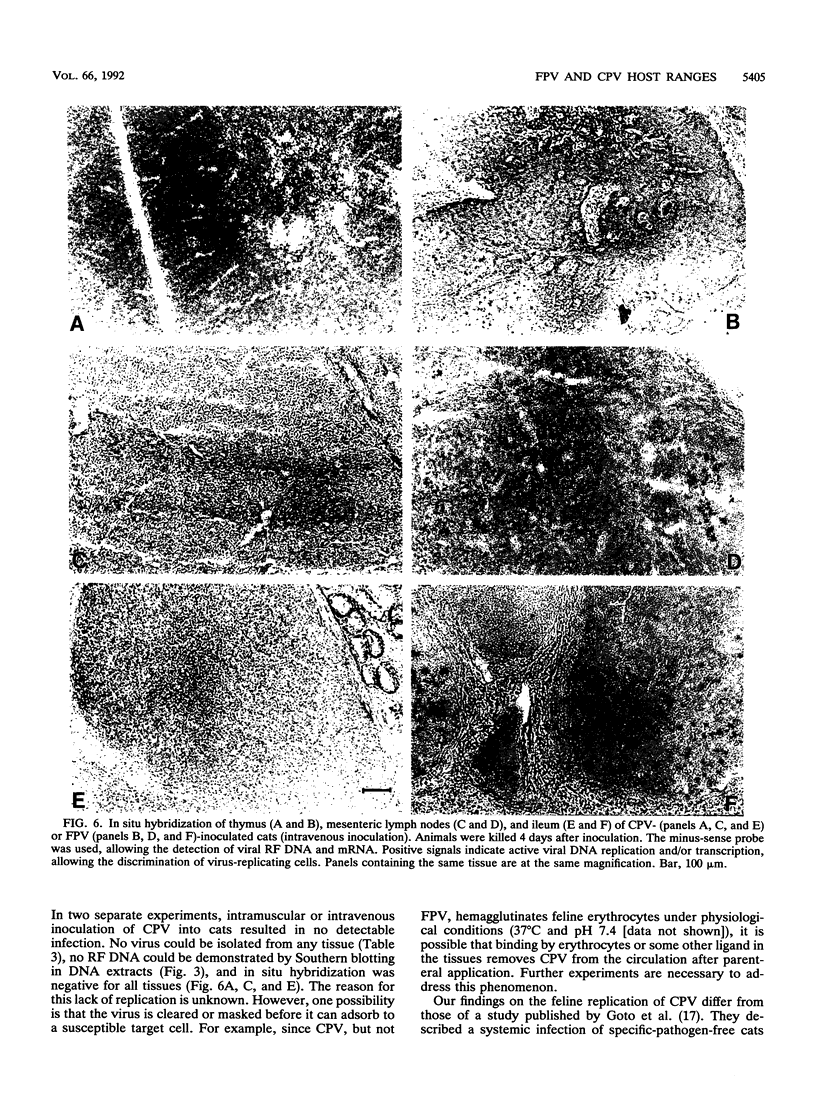
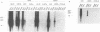Abstract
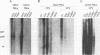
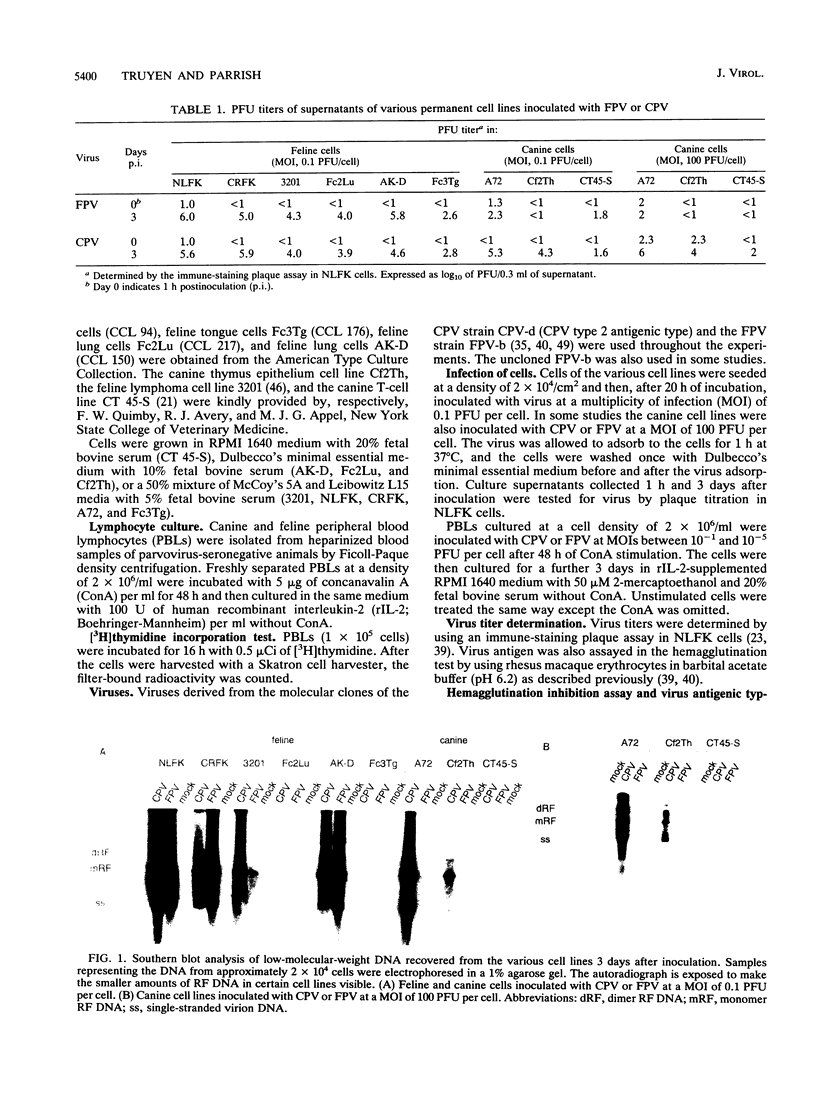
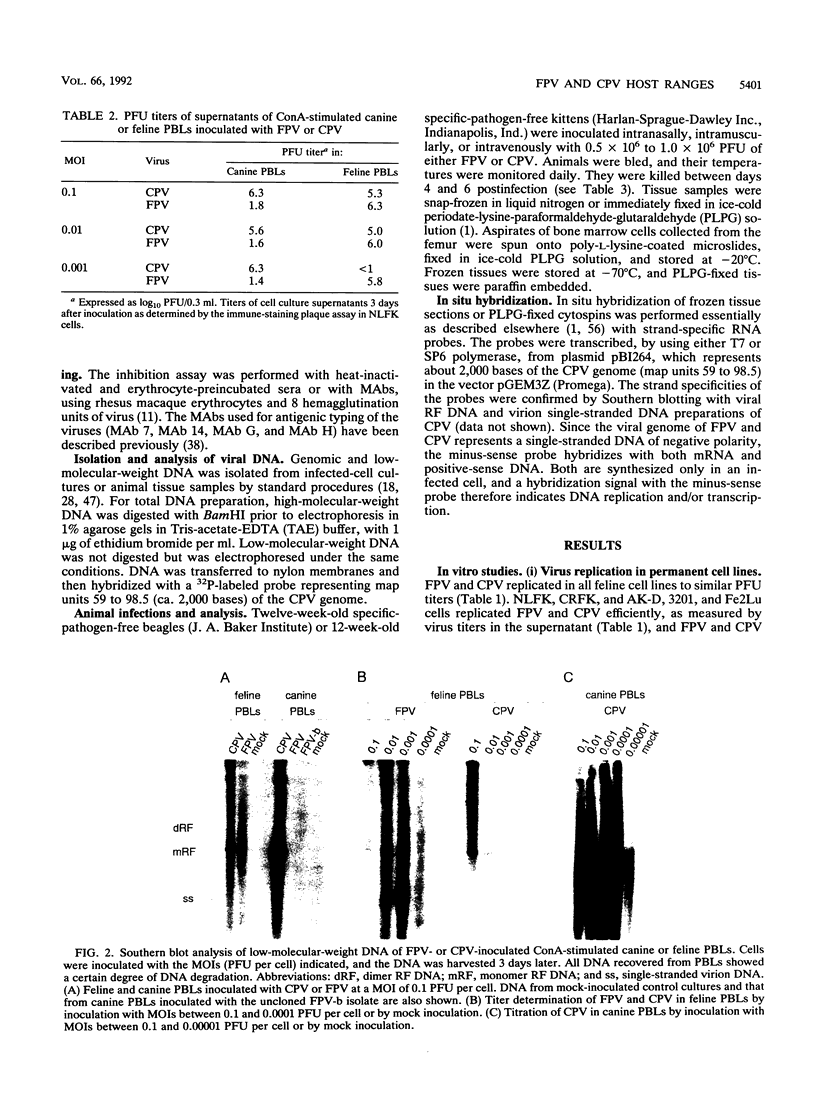
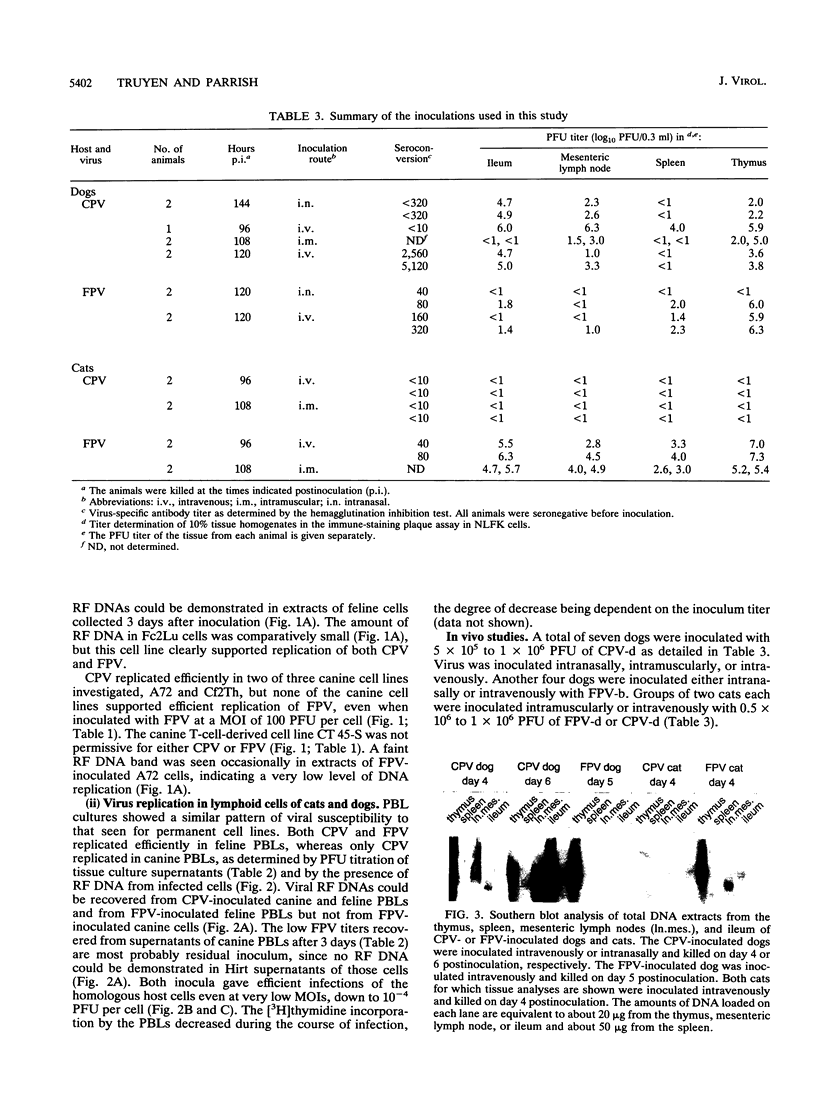
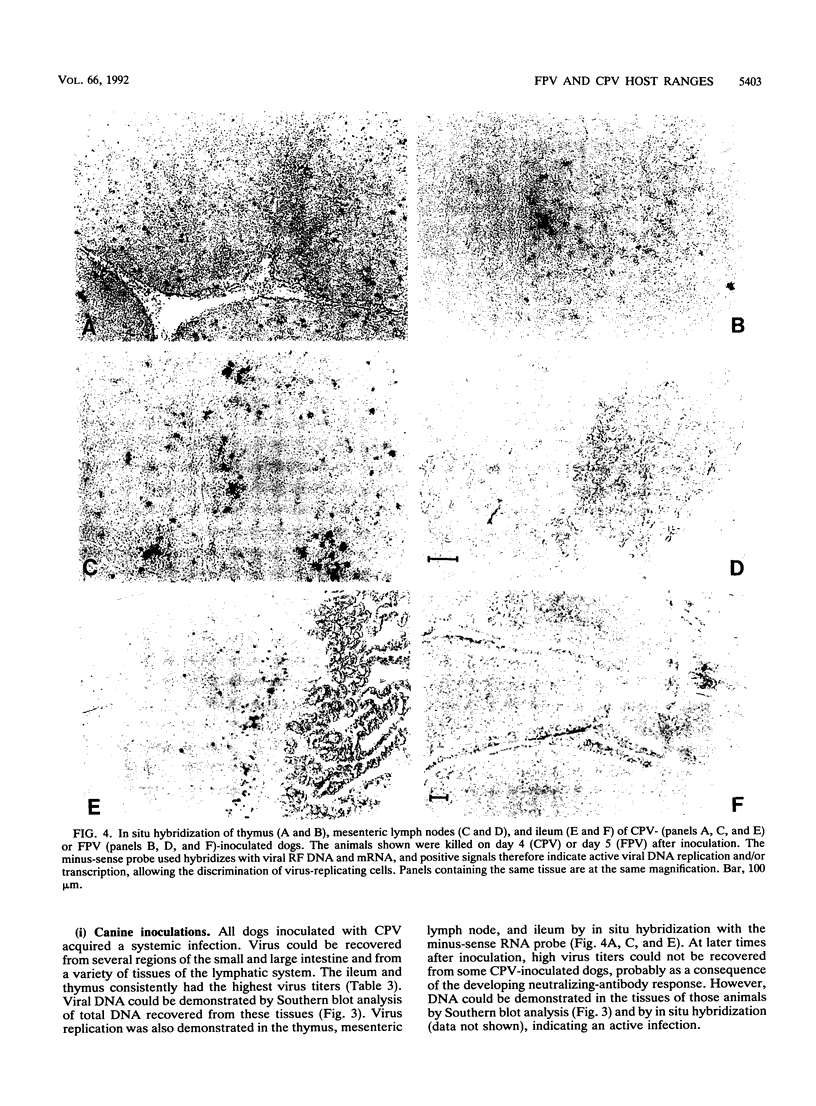
Canine parvovirus (CPV) emerged as an apparently new virus during the mid-1970s. The origin of CPV is unknown, but a variation from feline panleukopenia virus (FPV) or another closely related parvovirus is suspected. Here we examine the in vitro and in vivo canine and feline host ranges of CPV and FPV. Examination of three canine and six feline cell lines and mitogen-stimulated canine and feline peripheral blood lymphocytes revealed that CPV replicates in both canine and feline cells, whereas FPV replicates efficiently only in feline cells. The in vivo host ranges were unexpectedly complex and distinct from the in vitro host ranges. Inoculation of dogs with FPV revealed efficient replication in the thymus and, to some degree, in the bone marrow, as shown by virus isolation, viral DNA recovery, and Southern blotting and by strand-specific in situ hybridization. FPV replication could not be demonstrated in mesenteric lymph nodes or in the small intestine, which are important target tissues in CPV infection. Although CPV replicated well in all the feline cells tested in vitro, it did not replicate in any tissue of cats after intramuscular or intravenous inoculation. These results indicate that these viruses have complex and overlapping host ranges and that distinct tissue tropisms exist in the homologous and heterologous hosts.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandersen S., Bloom M. E., Wolfinbarger J., Race R. E. In situ molecular hybridization for detection of Aleutian mink disease parvovirus DNA by using strand-specific probes: identification of target cells for viral replication in cell cultures and in mink kits with virus-induced interstitial pneumonia. J Virol. 1987 Aug;61(8):2407–2419. doi: 10.1128/jvi.61.8.2407-2419.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel M. J., Parrish C. R. Raccoons are not susceptible to canine parvovirus. J Am Vet Med Assoc. 1982 Sep 1;181(5):489–489. [PubMed] [Google Scholar]
- Appel M. J., Scott F. W., Carmichael L. E. Isolation and immunisation studies of a canine parco-like virus from dogs with haemorrhagic enteritis. Vet Rec. 1979 Aug 25;105(8):156–159. doi: 10.1136/vr.105.8.156. [DOI] [PubMed] [Google Scholar]
- Azetaka M., Hirasawa T., Konishi S., Ogata M. Studies on canine parvovirus isolation, experimental infection and serologic survey. Nihon Juigaku Zasshi. 1981 Apr;43(2):243–255. doi: 10.1292/jvms1939.43.243. [DOI] [PubMed] [Google Scholar]
- Barker I. K., Povey R. C., Voigt D. R. Response of mink, skunk, red fox and raccoon to inoculation with mink virus enteritis, feline panleukopenia and canine parvovirus and prevalence of antibody to parvovirus in wild carnivores in Ontario. Can J Comp Med. 1983 Apr;47(2):188–197. [PMC free article] [PubMed] [Google Scholar]
- Binn L. N., Marchwicki R. H., Stephenson E. H. Establishment of a canine cell line: derivation, characterization, and viral spectrum. Am J Vet Res. 1980 Jun;41(6):855–860. [PubMed] [Google Scholar]
- Brownstein D. G., Smith A. L., Johnson E. A., Pintel D. J., Naeger L. K., Tattersall P. The pathogenesis of infection with minute virus of mice depends on expression of the small nonstructural protein NS2 and on the genotype of the allotropic determinants VP1 and VP2. J Virol. 1992 May;66(5):3118–3124. doi: 10.1128/jvi.66.5.3118-3124.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson J. H., Scott F. W., Duncan J. R. Feline panleukopenia. III. Development of lesions in the lymphoid tissues. Vet Pathol. 1978 May;15(3):383–392. doi: 10.1177/030098587801500314. [DOI] [PubMed] [Google Scholar]
- Carman P. S., Povey R. C. Pathogenesis of canine parvovirus-2 in dogs: histopathology and antigen identification in tissues. Res Vet Sci. 1985 Mar;38(2):141–150. [PubMed] [Google Scholar]
- Carmichael L. E., Binn L. N. New enteric viruses in the dog. Adv Vet Sci Comp Med. 1981;25:1–37. [PubMed] [Google Scholar]
- Carmichael L. E., Joubert J. C., Pollock R. V. Hemagglutination by canine parvovirus: serologic studies and diagnostic applications. Am J Vet Res. 1980 May;41(5):784–791. [PubMed] [Google Scholar]
- Cooper B. J., Carmichael L. E., Appel M. J., Greisen H. Canine viral enteritis. II. Morphologic lesions in naturally occurring parvovirus infection. Cornell Vet. 1979 Apr;69(3):134–144. [PubMed] [Google Scholar]
- Crandell R. A., Fabricant C. G., Nelson-Rees W. A. Development, characterization, and viral susceptibility of a feline (Felis catus) renal cell line (CRFK). In Vitro. 1973 Nov-Dec;9(3):176–185. doi: 10.1007/BF02618435. [DOI] [PubMed] [Google Scholar]
- Csiza C. K., Scott F. W., De Lahunta A., Gillespie J. H. Pathogenesis of feline panleukopenia virus in susceptible newborn kittens I. Clinical signs, hematology, serology, and virology. Infect Immun. 1971 Jun;3(6):833–837. doi: 10.1128/iai.3.6.833-837.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engers H. D., Louis J. A., Zubler R. H., Hirt B. Inhibition of T cell-mediated functions by MVM(i), a parvovirus closely related to minute virus of mice. J Immunol. 1981 Dec;127(6):2280–2285. [PubMed] [Google Scholar]
- Evermann J. F., Foreyt W., Maag-Miller L., Leathers C. W., McKeirnan A. J., LeaMaster B. Acute hemorrhagic enteritis associated with canine coronavirus and parvovirus infections in a captive coyote population. J Am Vet Med Assoc. 1980 Nov 1;177(9):784–786. [PubMed] [Google Scholar]
- Goto H., Uchida E., Ichijo S., Shimizu K., Morohoshi Y., Nakano K. Experimental infection of canine parvovirus in specific pathogen-free cats. Nihon Juigaku Zasshi. 1984 Oct;46(5):729–731. doi: 10.1292/jvms1939.46.729. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kimsey P. B., Engers H. D., Hirt B., Jongeneel C. V. Pathogenicity of fibroblast- and lymphocyte-specific variants of minute virus of mice. J Virol. 1986 Jul;59(1):8–13. doi: 10.1128/jvi.59.1.8-13.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koptopoulos G., Papadopoulos O., Papanastasopoulou M., Cornwell H. J. Presence of antibody cross-reacting with canine parvovirus in the sera of dogs from Greece. Vet Rec. 1986 Mar 22;118(12):332–333. doi: 10.1136/vr.118.12.332. [DOI] [PubMed] [Google Scholar]
- Krakowka S., Olsen R., Cockerell G. The effect of cell synchronization upon the detection of T and B lymphoid cell receptors on two continuous lymphoid cell lines. In Vitro. 1977 Feb;13(2):119–124. doi: 10.1007/BF02615076. [DOI] [PubMed] [Google Scholar]
- Kurtzman G. J., Platanias L., Lustig L., Frickhofen N., Young N. S. Feline parvovirus propagates in cat bone marrow cultures and inhibits hematopoietic colony formation in vitro. Blood. 1989 Jul;74(1):71–81. [PubMed] [Google Scholar]
- Lemon S. M., Binn L. N., Marchwicki R. H. Radioimmunofocus assay for quantitation of hepatitis A virus in cell cultures. J Clin Microbiol. 1983 May;17(5):834–839. doi: 10.1128/jcm.17.5.834-839.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macartney L., McCandlish I. A., Thompson H., Cornwell H. J. Canine parvovirus enteritis 1: Clinical, haematological and pathological features of experimental infection. Vet Rec. 1984 Sep 1;115(9):201–210. doi: 10.1136/vr.115.9.201. [DOI] [PubMed] [Google Scholar]
- Mann P. C., Bush M., Appel M. J., Beehler B. A., Montali R. J. Canine parvovirus infection in South American canids. J Am Vet Med Assoc. 1980 Nov 1;177(9):779–783. [PubMed] [Google Scholar]
- Martinez C., Gutierrez-Ramos J. C., de la Pompa J. L., Leonardo E., Sanchez M. J., Alonso J. M., Toribio M. L. The thousand and one ways of being a T cell. Thymus. 1990 Nov-Dec;16(3-4):173–185. [PubMed] [Google Scholar]
- Martyn J. C., Davidson B. E., Studdert M. J. Nucleotide sequence of feline panleukopenia virus: comparison with canine parvovirus identifies host-specific differences. J Gen Virol. 1990 Nov;71(Pt 11):2747–2753. doi: 10.1099/0022-1317-71-11-2747. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Beard P., Engers H. D., Hirt B. Characterization of an immunosuppressive parvovirus related to the minute virus of mice. J Virol. 1981 Apr;38(1):317–326. doi: 10.1128/jvi.38.1.317-326.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier P. C., Cooper B. J., Appel M. J., Lanieu M. E., Slauson D. O. Pathogenesis of canine parvovirus enteritis: sequential virus distribution and passive immunization studies. Vet Pathol. 1985 Nov;22(6):617–624. doi: 10.1177/030098588502200617. [DOI] [PubMed] [Google Scholar]
- Meunier P. C., Cooper B. J., Appel M. J., Slauson D. O. Pathogenesis of canine parvovirus enteritis: the importance of viremia. Vet Pathol. 1985 Jan;22(1):60–71. doi: 10.1177/030098588502200110. [DOI] [PubMed] [Google Scholar]
- Naeger L. K., Cater J., Pintel D. J. The small nonstructural protein (NS2) of the parvovirus minute virus of mice is required for efficient DNA replication and infectious virus production in a cell-type-specific manner. J Virol. 1990 Dec;64(12):6166–6175. doi: 10.1128/jvi.64.12.6166-6175.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterhaus A. D., Drost G. A., Wirahadiredja R. M., van den Ingh T. S. Canine viral enteritis: prevalence of parvo-, corona- and rotavirus infections in dogs in the Netherlands. Tijdschr Diergeneeskd. 1980 Oct 15;105(20):181–190. [PubMed] [Google Scholar]
- Parrish C. R., Aquadro C. F., Carmichael L. E. Canine host range and a specific epitope map along with variant sequences in the capsid protein gene of canine parvovirus and related feline, mink, and raccoon parvoviruses. Virology. 1988 Oct;166(2):293–307. doi: 10.1016/0042-6822(88)90500-4. [DOI] [PubMed] [Google Scholar]
- Parrish C. R., Aquadro C. F., Strassheim M. L., Evermann J. F., Sgro J. Y., Mohammed H. O. Rapid antigenic-type replacement and DNA sequence evolution of canine parvovirus. J Virol. 1991 Dec;65(12):6544–6552. doi: 10.1128/jvi.65.12.6544-6552.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish C. R., Carmichael L. E., Antczak D. F. Antigenic relationships between canine parvovirus type 2, feline panleukopenia virus and mink enteritis virus using conventional antisera and monoclonal antibodies. Arch Virol. 1982;72(4):267–278. doi: 10.1007/BF01315223. [DOI] [PubMed] [Google Scholar]
- Parrish C. R., Carmichael L. E. Antigenic structure and variation of canine parvovirus type-2, feline panleukopenia virus, and mink enteritis virus. Virology. 1983 Sep;129(2):401–414. doi: 10.1016/0042-6822(83)90179-4. [DOI] [PubMed] [Google Scholar]
- Parrish C. R., Carmichael L. E. Characterization and recombination mapping of an antigenic and host range mutation of canine parvovirus. Virology. 1986 Jan 15;148(1):121–132. doi: 10.1016/0042-6822(86)90408-3. [DOI] [PubMed] [Google Scholar]
- Parrish C. R. Emergence, natural history, and variation of canine, mink, and feline parvoviruses. Adv Virus Res. 1990;38:403–450. doi: 10.1016/S0065-3527(08)60867-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish C. R., Have P., Foreyt W. J., Evermann J. F., Senda M., Carmichael L. E. The global spread and replacement of canine parvovirus strains. J Gen Virol. 1988 May;69(Pt 5):1111–1116. doi: 10.1099/0022-1317-69-5-1111. [DOI] [PubMed] [Google Scholar]
- Parrish C. R. Mapping specific functions in the capsid structure of canine parvovirus and feline panleukopenia virus using infectious plasmid clones. Virology. 1991 Jul;183(1):195–205. doi: 10.1016/0042-6822(91)90132-u. [DOI] [PubMed] [Google Scholar]
- Parrish C. R., O'Connell P. H., Evermann J. F., Carmichael L. E. Natural variation of canine parvovirus. Science. 1985 Nov 29;230(4729):1046–1048. doi: 10.1126/science.4059921. [DOI] [PubMed] [Google Scholar]
- Pollock R. V., Carmichael L. E. Use of modified live feline panleukopenia virus vaccine to immunize dogs against canine parvovirus. Am J Vet Res. 1983 Feb;44(2):169–175. [PubMed] [Google Scholar]
- Reed A. P., Jones E. V., Miller T. J. Nucleotide sequence and genome organization of canine parvovirus. J Virol. 1988 Jan;62(1):266–276. doi: 10.1128/jvi.62.1.266-276.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Nucleotide sequence of the coat protein gene of canine parvovirus. J Virol. 1985 May;54(2):630–633. doi: 10.1128/jvi.54.2.630-633.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojko J. L., Kociba G. J., Abkowitz J. L., Hamilton K. L., Hardy W. D., Jr, Ihle J. N., O'Brien S. J. Feline lymphomas: immunological and cytochemical characterization. Cancer Res. 1989 Jan 15;49(2):345–351. [PubMed] [Google Scholar]
- Siegl G., Bates R. C., Berns K. I., Carter B. J., Kelly D. C., Kurstak E., Tattersall P. Characteristics and taxonomy of Parvoviridae. Intervirology. 1985;23(2):61–73. doi: 10.1159/000149587. [DOI] [PubMed] [Google Scholar]
- Spalholz B. A., Tattersall P. Interaction of minute virus of mice with differentiated cells: strain-dependent target cell specificity is mediated by intracellular factors. J Virol. 1983 Jun;46(3):937–943. doi: 10.1128/jvi.46.3.937-943.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surleraux M., Bodeus M., Burtonboy G. Study of canine parvovirus polypeptides by immunoblot analysis. Arch Virol. 1987;95(3-4):271–281. doi: 10.1007/BF01310785. [DOI] [PubMed] [Google Scholar]
- Tattersall P., Bratton J. Reciprocal productive and restrictive virus-cell interactions of immunosuppressive and prototype strains of minute virus of mice. J Virol. 1983 Jun;46(3):944–955. doi: 10.1128/jvi.46.3.944-955.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao J., Chapman M. S., Agbandje M., Keller W., Smith K., Wu H., Luo M., Smith T. J., Rossmann M. G., Compans R. W. The three-dimensional structure of canine parvovirus and its functional implications. Science. 1991 Mar 22;251(5000):1456–1464. doi: 10.1126/science.2006420. [DOI] [PubMed] [Google Scholar]
- Uttenthal A., Larsen S., Lund E., Bloom M. E., Storgård T., Alexandersen S. Analysis of experimental mink enteritis virus infection in mink: in situ hybridization, serology, and histopathology. J Virol. 1990 Jun;64(6):2768–2779. doi: 10.1128/jvi.64.6.2768-2779.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker S. T., Feilen C. P., Sabine M., Love D. N., Jones R. F. A serological survey of canine parvovirus infection in New South Wales, Australia. Vet Rec. 1980 Apr 12;106(15):324–325. doi: 10.1136/vr.106.15.324. [DOI] [PubMed] [Google Scholar]
- Zarnke R. L., Ballard W. B. Serologic survey for selected microbial pathogens of wolves in Alaska, 1975-1982. J Wildl Dis. 1987 Jan;23(1):77–85. doi: 10.7589/0090-3558-23.1.77. [DOI] [PubMed] [Google Scholar]
- von Boehmer H. The developmental biology of T lymphocytes. Annu Rev Immunol. 1988;6:309–326. doi: 10.1146/annurev.iy.06.040188.001521. [DOI] [PubMed] [Google Scholar]