Abstract
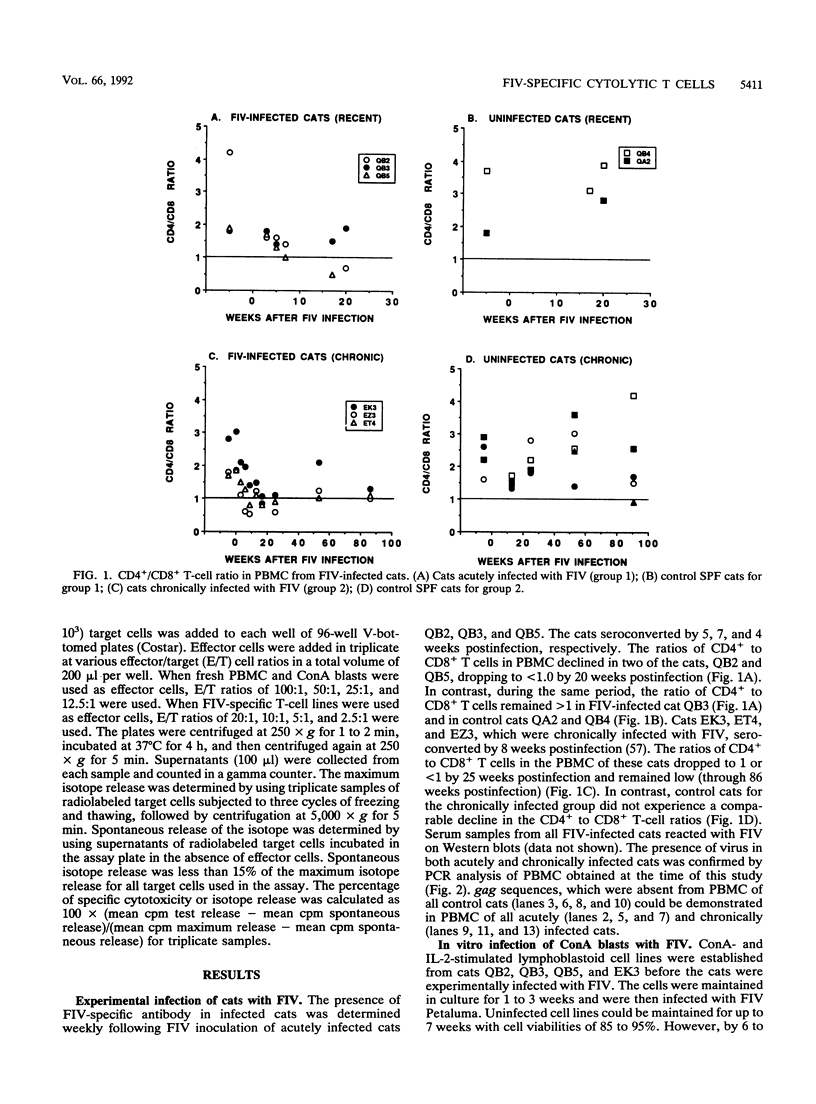
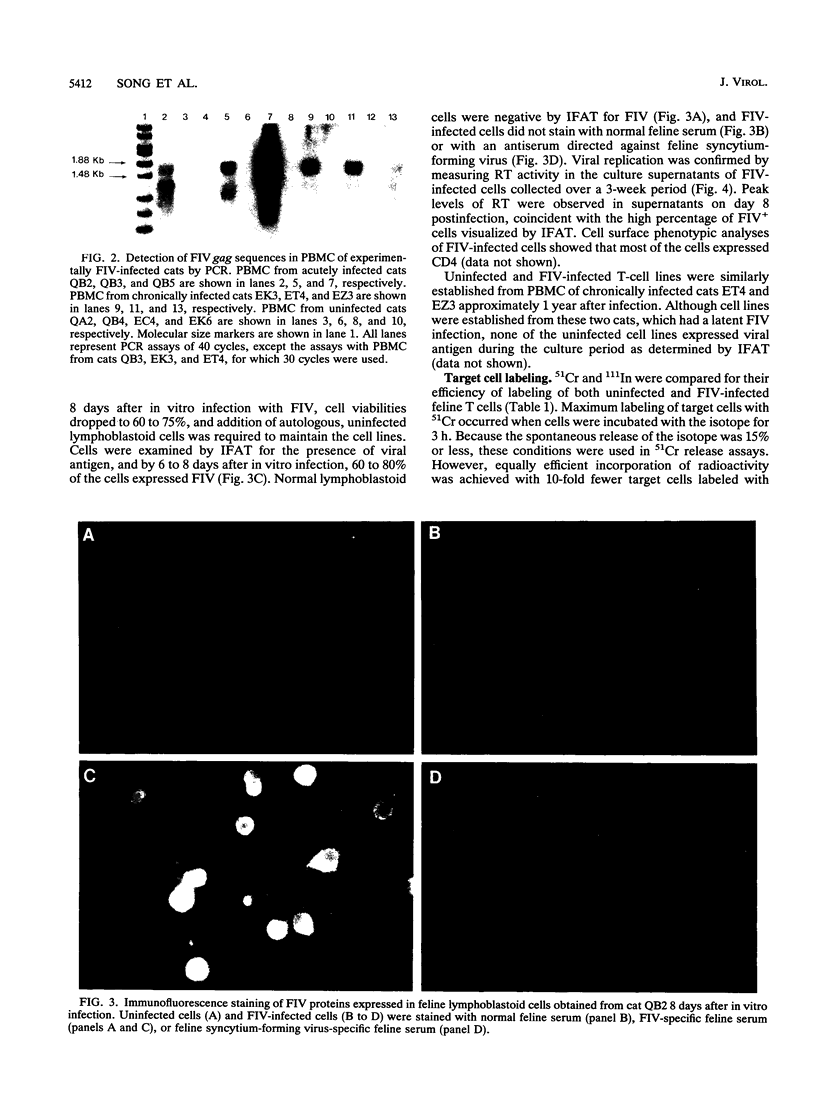
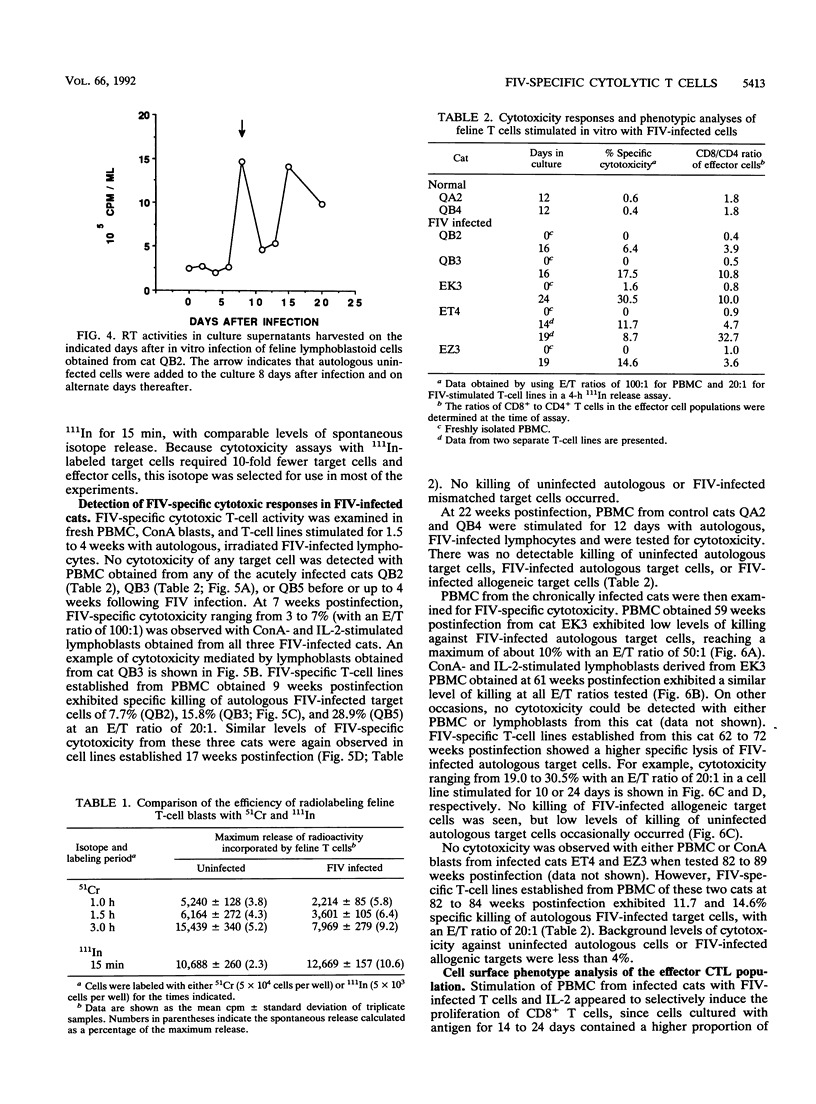
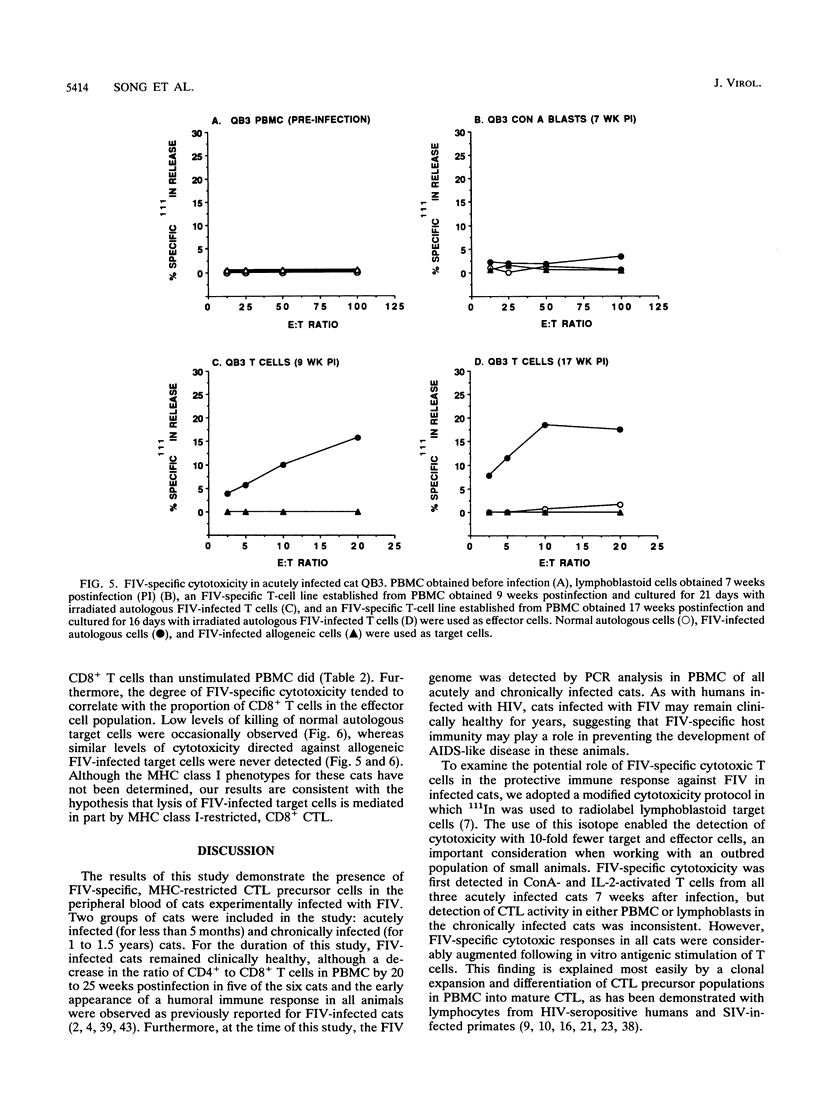
We have examined the in vitro induction and activity of feline immunodeficiency virus (FIV)-specific cytolytic T cells obtained from cats experimentally infected for 7 to 17 weeks or 20 to 22 months with the Petaluma isolate of FIV. Normal or FIV-infected autologous and allogeneic T lymphoblastoid cells were used as target cells in chromium-51 or indium-111 release assays. When effector cells consisted of either fresh peripheral blood mononuclear cells or concanavalin A- and interleukin-2-stimulated cells, only low levels of cytotoxicity were observed. However, the levels of FIV-specific cytotoxicity were consistently higher in both groups of cats following in vitro stimulation of the effector cells with irradiated, FIV-infected autologous T lymphoblastoid cells and interleukin-2. The effector cells lysed autologous but not allogeneic FIV-infected target cells and were composed predominantly of CD8+ T cells, indicating that the FIV-specific cytotoxicity measured in this system is mediated by CD8+, major histocompatibility complex class I-restricted T cells. These studies show that FIV-specific cytolytic T cells can be detected as early as 7 to 9 weeks postinfection, and they define a system to identify virus-encoded epitopes important in the induction of protective immunity against lentiviruses.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackley C. D., Hoover E. A., Cooper M. D. Identification of a CD4 homologue in the cat. Tissue Antigens. 1990 Feb;35(2):92–98. doi: 10.1111/j.1399-0039.1990.tb01762.x. [DOI] [PubMed] [Google Scholar]
- Ackley C. D., Yamamoto J. K., Levy N., Pedersen N. C., Cooper M. D. Immunologic abnormalities in pathogen-free cats experimentally infected with feline immunodeficiency virus. J Virol. 1990 Nov;64(11):5652–5655. doi: 10.1128/jvi.64.11.5652-5655.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldovini A., Young R. A. Humoral and cell-mediated immune responses to live recombinant BCG-HIV vaccines. Nature. 1991 Jun 6;351(6326):479–482. doi: 10.1038/351479a0. [DOI] [PubMed] [Google Scholar]
- Barlough J. E., Ackley C. D., George J. W., Levy N., Acevedo R., Moore P. F., Rideout B. A., Cooper M. D., Pedersen N. C. Acquired immune dysfunction in cats with experimentally induced feline immunodeficiency virus infection: comparison of short-term and long-term infections. J Acquir Immune Defic Syndr. 1991;4(3):219–227. [PubMed] [Google Scholar]
- Brown W. C., Bissey L., Logan K. S., Pedersen N. C., Elder J. H., Collisson E. W. Feline immunodeficiency virus infects both CD4+ and CD8+ T lymphocytes. J Virol. 1991 Jun;65(6):3359–3364. doi: 10.1128/jvi.65.6.3359-3364.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. C., Grab D. J. Biological and biochemical characterization of bovine interleukin 2. Studies with cloned bovine T cells. J Immunol. 1985 Nov;135(5):3184–3190. [PubMed] [Google Scholar]
- Brown W. C., Sugimoto C., Conrad P. A., Grab D. J. Differential response of bovine T-cell lines to membrane and soluble antigens of Theileria parva schizont-infected cells. Parasite Immunol. 1989 Nov;11(6):567–583. doi: 10.1111/j.1365-3024.1989.tb00921.x. [DOI] [PubMed] [Google Scholar]
- Brunner D., Pedersen N. C. Infection of peritoneal macrophages in vitro and in vivo with feline immunodeficiency virus. J Virol. 1989 Dec;63(12):5483–5488. doi: 10.1128/jvi.63.12.5483-5488.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenciner N., Michel F., Dadaglio G., Langlade-Demoyen P., Hoffenbach A., Leroux A., Garcia-Pons F., Rautmann G., Guy B., Guillon J. M. Multiple subsets of HIV-specific cytotoxic T lymphocytes in humans and in mice. Eur J Immunol. 1989 Sep;19(9):1537–1544. doi: 10.1002/eji.1830190904. [DOI] [PubMed] [Google Scholar]
- Clerici M., Lucey D. R., Zajac R. A., Boswell R. N., Gebel H. M., Takahashi H., Berzofsky J. A., Shearer G. M. Detection of cytotoxic T lymphocytes specific for synthetic peptides of gp160 in HIV-seropositive individuals. J Immunol. 1991 Apr 1;146(7):2214–2219. [PubMed] [Google Scholar]
- Gardner M. B., Luciw P. A. Animal models of AIDS. FASEB J. 1989 Dec;3(14):2593–2606. doi: 10.1096/fasebj.3.14.2556312. [DOI] [PubMed] [Google Scholar]
- Gibbs C. J., Jr, Peters R., Gravell M., Johnson B. K., Jensen F. C., Carlo D. J., Salk J. Observations after human immunodeficiency virus immunization and challenge of human immunodeficiency virus seropositive and seronegative chimpanzees. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3348–3352. doi: 10.1073/pnas.88.8.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotch F. M., Hovell R., Delchambre M., Silvera P., McMichael A. J. Cytotoxic T-cell response to simian immunodeficiency virus by cynomolgus macaque monkeys immunized with recombinant vaccinia virus. AIDS. 1991 Mar;5(3):317–320. doi: 10.1097/00002030-199103000-00012. [DOI] [PubMed] [Google Scholar]
- Gotch F. M., Nixon D. F., Alp N., McMichael A. J., Borysiewicz L. K. High frequency of memory and effector gag specific cytotoxic T lymphocytes in HIV seropositive individuals. Int Immunol. 1990;2(8):707–712. doi: 10.1093/intimm/2.8.707. [DOI] [PubMed] [Google Scholar]
- Gruters R. A., Terpstra F. G., De Jong R., Van Noesel C. J., Van Lier R. A., Miedema F. Selective loss of T cell functions in different stages of HIV infection. Early loss of anti-CD3-induced T cell proliferation followed by decreased anti-CD3-induced cytotoxic T lymphocyte generation in AIDS-related complex and AIDS. Eur J Immunol. 1990 May;20(5):1039–1044. doi: 10.1002/eji.1830200514. [DOI] [PubMed] [Google Scholar]
- Hoffenbach A., Langlade-Demoyen P., Dadaglio G., Vilmer E., Michel F., Mayaud C., Autran B., Plata F. Unusually high frequencies of HIV-specific cytotoxic T lymphocytes in humans. J Immunol. 1989 Jan 15;142(2):452–462. [PubMed] [Google Scholar]
- Jarrett O., Yamamoto J. K., Neil J. C. Feline immunodeficiency virus as a model for AIDS vaccination. AIDS. 1990;4 (Suppl 1):S163–S165. [PubMed] [Google Scholar]
- Kannagi M., Chalifoux L. V., Lord C. I., Letvin N. L. Suppression of simian immunodeficiency virus replication in vitro by CD8+ lymphocytes. J Immunol. 1988 Apr 1;140(7):2237–2242. [PubMed] [Google Scholar]
- Klotz F. W., Cooper M. D. A feline thymocyte antigen defined by a monoclonal antibody (FT2) identifies a subpopulation of non-helper cells capable of specific cytotoxicity. J Immunol. 1986 Apr 1;136(7):2510–2514. [PubMed] [Google Scholar]
- Koenig S., Earl P., Powell D., Pantaleo G., Merli S., Moss B., Fauci A. S. Group-specific, major histocompatibility complex class I-restricted cytotoxic responses to human immunodeficiency virus 1 (HIV-1) envelope proteins by cloned peripheral blood T cells from an HIV-1-infected individual. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8638–8642. doi: 10.1073/pnas.85.22.8638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koup R. A., Pikora C. A., Luzuriaga K., Brettler D. B., Day E. S., Mazzara G. P., Sullivan J. L. Limiting dilution analysis of cytotoxic T lymphocytes to human immunodeficiency virus gag antigens in infected persons: in vitro quantitation of effector cell populations with p17 and p24 specificities. J Exp Med. 1991 Dec 1;174(6):1593–1600. doi: 10.1084/jem.174.6.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koup R. A., Sullivan J. L., Levine P. H., Brettler D., Mahr A., Mazzara G., McKenzie S., Panicali D. Detection of major histocompatibility complex class I-restricted, HIV-specific cytotoxic T lymphocytes in the blood of infected hemophiliacs. Blood. 1989 May 15;73(7):1909–1914. [PubMed] [Google Scholar]
- Langlade-Demoyen P., Michel F., Hoffenbach A., Vilmer E., Dadaglio G., Garicia-Pons F., Mayaud C., Autran B., Wain-Hobson S., Plata F. Immune recognition of AIDS virus antigens by human and murine cytotoxic T lymphocytes. J Immunol. 1988 Sep 15;141(6):1949–1957. [PubMed] [Google Scholar]
- Letvin N. L. Animal models for AIDS. Immunol Today. 1990 Sep;11(9):322–326. doi: 10.1016/0167-5699(90)90127-u. [DOI] [PubMed] [Google Scholar]
- Luzuriaga K., Koup R. A., Pikora C. A., Brettler D. B., Sullivan J. L. Deficient human immunodeficiency virus type 1-specific cytotoxic T cell responses in vertically infected children. J Pediatr. 1991 Aug;119(2):230–236. doi: 10.1016/s0022-3476(05)80732-2. [DOI] [PubMed] [Google Scholar]
- McGraw T. P., Vowels B. R., Gershwin M. E., Gardner M. B. Cellular immune response of SIV-infected rhesus macaques. J Med Primatol. 1990;19(3-4):177–187. [PubMed] [Google Scholar]
- Miller M. D., Lord C. I., Stallard V., Mazzara G. P., Letvin N. L. The gag-specific cytotoxic T lymphocytes in rhesus monkeys infected with the simian immunodeficiency virus of macaques. J Immunol. 1990 Jan 1;144(1):122–128. [PubMed] [Google Scholar]
- Miller M. D., Yamamoto H., Hughes A. L., Watkins D. I., Letvin N. L. Definition of an epitope and MHC class I molecule recognized by gag-specific cytotoxic T lymphocytes in SIVmac-infected rhesus monkeys. J Immunol. 1991 Jul 1;147(1):320–329. [PubMed] [Google Scholar]
- Mills K. H., Barnard A. L., Williams M., Page M., Ling C., Stott E. J., Silvera P., Taffs F., Kingsman A. S., Adams S. E. Vaccine-induced CD4+ T cells against the simian immunodeficiency virus gag protein. Epitope specificity and relevance to protective immunity. J Immunol. 1991 Nov 15;147(10):3560–3567. [PubMed] [Google Scholar]
- Nixon D. F., Townsend A. R., Elvin J. G., Rizza C. R., Gallwey J., McMichael A. J. HIV-1 gag-specific cytotoxic T lymphocytes defined with recombinant vaccinia virus and synthetic peptides. Nature. 1988 Dec 1;336(6198):484–487. doi: 10.1038/336484a0. [DOI] [PubMed] [Google Scholar]
- Orentas R. J., Hildreth J. E., Obah E., Polydefkis M., Smith G. E., Clements M. L., Siliciano R. F. Induction of CD4+ human cytolytic T cells specific for HIV-infected cells by a gp160 subunit vaccine. Science. 1990 Jun 8;248(4960):1234–1237. doi: 10.1126/science.2190315. [DOI] [PubMed] [Google Scholar]
- Pantaleo G., De Maria A., Koenig S., Butini L., Moss B., Baseler M., Lane H. C., Fauci A. S. CD8+ T lymphocytes of patients with AIDS maintain normal broad cytolytic function despite the loss of human immunodeficiency virus-specific cytotoxicity. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4818–4822. doi: 10.1073/pnas.87.12.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N. C., Ho E. W., Brown M. L., Yamamoto J. K. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science. 1987 Feb 13;235(4790):790–793. doi: 10.1126/science.3643650. [DOI] [PubMed] [Google Scholar]
- Phillips R. E., Rowland-Jones S., Nixon D. F., Gotch F. M., Edwards J. P., Ogunlesi A. O., Elvin J. G., Rothbard J. A., Bangham C. R., Rizza C. R. Human immunodeficiency virus genetic variation that can escape cytotoxic T cell recognition. Nature. 1991 Dec 12;354(6353):453–459. doi: 10.1038/354453a0. [DOI] [PubMed] [Google Scholar]
- Plata F., Autran B., Martins L. P., Wain-Hobson S., Raphaël M., Mayaud C., Denis M., Guillon J. M., Debré P. AIDS virus-specific cytotoxic T lymphocytes in lung disorders. Nature. 1987 Jul 23;328(6128):348–351. doi: 10.1038/328348a0. [DOI] [PubMed] [Google Scholar]
- Riviere Y., Tanneau-Salvadori F., Regnault A., Lopez O., Sansonetti P., Guy B., Kieny M. P., Fournel J. J., Montagnier L. Human immunodeficiency virus-specific cytotoxic responses of seropositive individuals: distinct types of effector cells mediate killing of targets expressing gag and env proteins. J Virol. 1989 May;63(5):2270–2277. doi: 10.1128/jvi.63.5.2270-2277.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L., Chen Z. W., Miller M. D., Stallard V., Mazzara G. P., Panicali D. L., Letvin N. L. Recombinant virus vaccine-induced SIV-specific CD8+ cytotoxic T lymphocytes. Science. 1991 Apr 19;252(5004):440–443. doi: 10.1126/science.1708168. [DOI] [PubMed] [Google Scholar]
- Shepp D. H., Daguillard F., Mann D., Quinnan G. V. Human class I MHC-restricted cytotoxic T-lymphocytes specific for human immunodeficiency virus envelope antigens. AIDS. 1988 Apr;2(2):113–117. doi: 10.1097/00002030-198804000-00007. [DOI] [PubMed] [Google Scholar]
- Siebelink K. H., Chu I. H., Rimmelzwaan G. F., Weijer K., van Herwijnen R., Knell P., Egberink H. F., Bosch M. L., Osterhaus A. D. Feline immunodeficiency virus (FIV) infection in the cat as a model for HIV infection in man: FIV-induced impairment of immune function. AIDS Res Hum Retroviruses. 1990 Dec;6(12):1373–1378. doi: 10.1089/aid.1990.6.1373. [DOI] [PubMed] [Google Scholar]
- Siliciano R. F., Lawton T., Knall C., Karr R. W., Berman P., Gregory T., Reinherz E. L. Analysis of host-virus interactions in AIDS with anti-gp120 T cell clones: effect of HIV sequence variation and a mechanism for CD4+ cell depletion. Cell. 1988 Aug 12;54(4):561–575. doi: 10.1016/0092-8674(88)90078-5. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Nakagawa Y., Pendleton C. D., Houghten R. A., Yokomuro K., Germain R. N., Berzofsky J. A. Induction of broadly cross-reactive cytotoxic T cells recognizing an HIV-1 envelope determinant. Science. 1992 Jan 17;255(5042):333–336. doi: 10.1126/science.1372448. [DOI] [PubMed] [Google Scholar]
- Talbott R. L., Sparger E. E., Lovelace K. M., Fitch W. M., Pedersen N. C., Luciw P. A., Elder J. H. Nucleotide sequence and genomic organization of feline immunodeficiency virus. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5743–5747. doi: 10.1073/pnas.86.15.5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torten M., Franchini M., Barlough J. E., George J. W., Mozes E., Lutz H., Pedersen N. C. Progressive immune dysfunction in cats experimentally infected with feline immunodeficiency virus. J Virol. 1991 May;65(5):2225–2230. doi: 10.1128/jvi.65.5.2225-2230.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubota H., Lord C. I., Watkins D. I., Morimoto C., Letvin N. L. A cytotoxic T lymphocyte inhibits acquired immunodeficiency syndrome virus replication in peripheral blood lymphocytes. J Exp Med. 1989 Apr 1;169(4):1421–1434. doi: 10.1084/jem.169.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vowels B. R., Gershwin M. E., Gardner M. B., Ahmed-Ansari A., McGraw T. P. Characterization of simian immunodeficiency virus-specific T-cell-mediated cytotoxic response of infected rhesus macaques. AIDS. 1989 Dec;3(12):785–792. doi: 10.1097/00002030-198912000-00002. [DOI] [PubMed] [Google Scholar]
- Walker B. D., Chakrabarti S., Moss B., Paradis T. J., Flynn T., Durno A. G., Blumberg R. S., Kaplan J. C., Hirsch M. S., Schooley R. T. HIV-specific cytotoxic T lymphocytes in seropositive individuals. Nature. 1987 Jul 23;328(6128):345–348. doi: 10.1038/328345a0. [DOI] [PubMed] [Google Scholar]
- Walker B. D., Flexner C., Birch-Limberger K., Fisher L., Paradis T. J., Aldovini A., Young R., Moss B., Schooley R. T. Long-term culture and fine specificity of human cytotoxic T-lymphocyte clones reactive with human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9514–9518. doi: 10.1073/pnas.86.23.9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker B. D., Flexner C., Paradis T. J., Fuller T. C., Hirsch M. S., Schooley R. T., Moss B. HIV-1 reverse transcriptase is a target for cytotoxic T lymphocytes in infected individuals. Science. 1988 Apr 1;240(4848):64–66. doi: 10.1126/science.2451288. [DOI] [PubMed] [Google Scholar]
- Walker B. D., Plata F. Cytotoxic T lymphocytes against HIV. AIDS. 1990 Mar;4(3):177–184. doi: 10.1097/00002030-199003000-00001. [DOI] [PubMed] [Google Scholar]
- Walker C. M., Moody D. J., Stites D. P., Levy J. A. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science. 1986 Dec 19;234(4783):1563–1566. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]
- Weintrub P. S., Ulrich P. P., Edwards J. R., Boucher F., Levy J. A., Cowan M. J., Vyas G. N. Use of polymerase chain reaction for the early detection of HIV infection in the infants of HIV-seropositive women. AIDS. 1991 Jul;5(7):881–884. doi: 10.1097/00002030-199107000-00014. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Miller M. D., Tsubota H., Watkins D. I., Mazzara G. P., Stallard V., Panicali D. L., Aldovini A., Young R. A., Letvin N. L. Studies of cloned simian immunodeficiency virus-specific T lymphocytes. gag-specific cytotoxic T lymphocytes exhibit a restricted epitope specificity. J Immunol. 1990 May 1;144(9):3385–3391. [PubMed] [Google Scholar]
- Yamamoto J. K., Hansen H., Ho E. W., Morishita T. Y., Okuda T., Sawa T. R., Nakamura R. M., Pedersen N. C. Epidemiologic and clinical aspects of feline immunodeficiency virus infection in cats from the continental United States and Canada and possible mode of transmission. J Am Vet Med Assoc. 1989 Jan 15;194(2):213–220. [PubMed] [Google Scholar]
- Yamamoto J. K., Okuda T., Ackley C. D., Louie H., Pembroke E., Zochlinski H., Munn R. J., Gardner M. B. Experimental vaccine protection against feline immunodeficiency virus. AIDS Res Hum Retroviruses. 1991 Nov;7(11):911–922. doi: 10.1089/aid.1991.7.911. [DOI] [PubMed] [Google Scholar]
- Yamamoto J. K., Sparger E., Ho E. W., Andersen P. R., O'Connor T. P., Mandell C. P., Lowenstine L., Munn R., Pedersen N. C. Pathogenesis of experimentally induced feline immunodeficiency virus infection in cats. Am J Vet Res. 1988 Aug;49(8):1246–1258. [PubMed] [Google Scholar]
- Zarling J. M., Eichberg J. W., Moran P. A., McClure J., Sridhar P., Hu S. L. Proliferative and cytotoxic T cells to AIDS virus glycoproteins in chimpanzees immunized with a recombinant vaccinia virus expressing AIDS virus envelope glycoproteins. J Immunol. 1987 Aug 15;139(4):988–990. [PubMed] [Google Scholar]




