Abstract
It is now well established that most cervical cancers are causally associated with HPV infection. This realization has led to efforts to control HPV-associated malignancy through prevention or treatment of HPV infection. Currently, commercially available HPV vaccines are not designed to control established HPV infection and associated premalignant and malignant lesions. To treat and eradicate pre-existing HPV infections and associated lesions which remain prevalent in the U.S. and worldwide, effective therapeutic HPV vaccines are needed. DNA vaccination has emerged as a particularly promising form of therapeutic HPV vaccines due to its safety, stability and ability to induce antigen-specific immunity. This review focuses on improving the potency of therapeutic HPV vaccines through modification of dendritic cells (DCs) by [1] increasing the number of antigen-expressing/antigen-loaded DCs, [2] improving HPV antigen expression, processing and presentation in DCs, and [3] enhancing DC and T cell interaction. Continued improvement in therapeutic HPV DNA vaccines may ultimately lead to an effective DNA vaccine for the treatment of HPV-associated malignancies.
Keywords: HPV, Therapeutic vaccine, HPV E6, HPV E7, Cervical cancer, DNA vaccines
Introduction
Association of HPV with cervical cancer
Cervical cancer is the second most common cancer in women worldwide, with an estimated 493,000 new diagnoses and approximately 270,000 deaths annually [1]. It is now clear that human papillomavirus (HPV) is the etiological agent implicated in cervical cancer and its precursor lesions (cervical intraepithelial neoplasia (CIN)), with HPV DNA present in 99.7% of cervical cancers [2]. There are more than 100 HPV genotypes identified, with forty of these commonly infecting anogenital epithelium, and fifteen thought to be carcinogenic [3]. Thus, these viruses are classified into low- and high-risk types depending on their propensity to cause cancer [4]. Among the high-risk types, HPV-16 is the most common type to be associated with cervical cancer, having been identified in approximately 50% of all tumors. HPV-18 accounts for an additional 10–15% and types 31, 33, 45, 52 and 58 each account for an estimated 2–5%. Thus, HPV-16 and 18 are the most frequent HPV types associated with cervical cancer and have subsequently been the focus of many recent HPV vaccines under development.
Molecular biology and molecular pathogenesis of HPV
HPV is a non-enveloped double-stranded DNA virus. Its genome contains approximately 8,000 base pairs, which encodes two classes of proteins: early and late proteins (for review, see [5]). Whereas late proteins (L1 and L2) are structural components of the viral capsid, early proteins (E1, E2, E4, E5, E6 and E7) are crucial regulators of the viral life cycle. E1 and E2 regulate replication of viral DNA, while E2 controls viral RNA transcription. E4 controls cytoskeletal reorganization and E5, E6 and E7 mediate cellular transformation. Among these, E2 is also called the ‘master regulator’ as it controls the transcription of all HPV viral proteins, particularly E6 and E7. In cervical cancer, viral integration into host genome often leads to the deletion of several early (E2, E4, E5) and late (L1, L2) genes, leading to constitutive upregulation of E6 and E7 oncogenes. E6 and E7 interact with and inactivate tumor suppressors p53 and Rb, respectively, thereby preventing cellular apoptosis, promoting cell growth and contributing to progression of HPV-associated malignancies [6].
Current preventive HPV vaccines
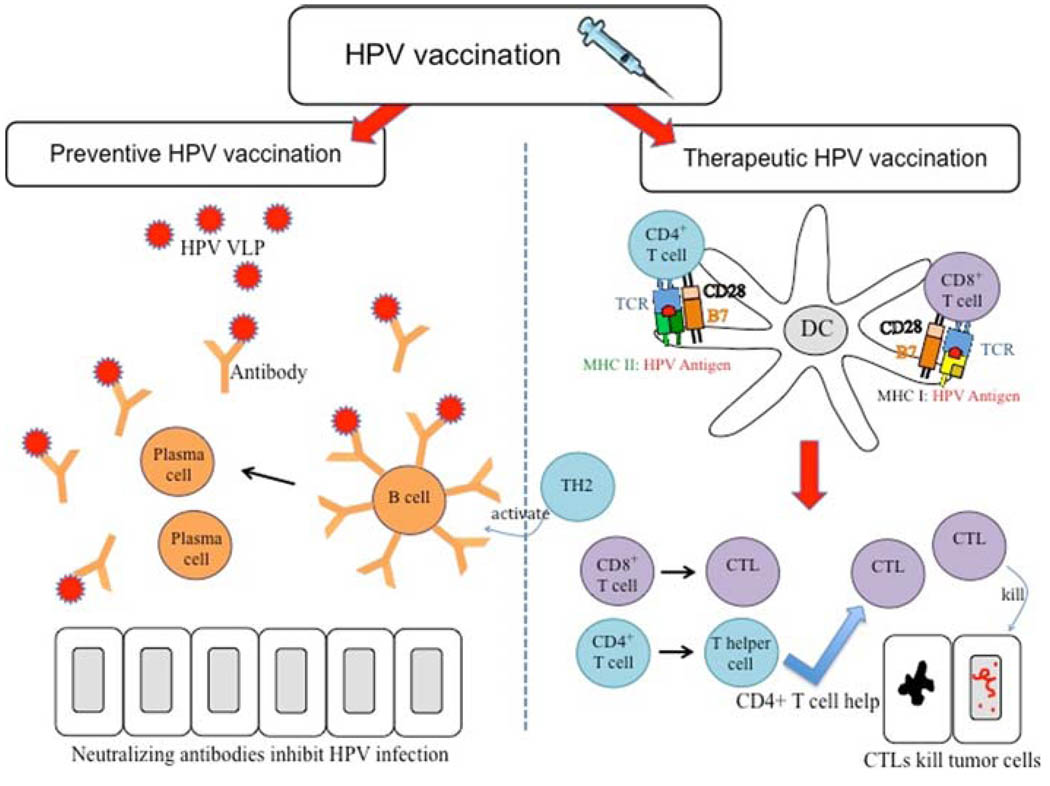
The realization of a close link between HPV infection and cervical cancer has led to the development of numerous HPV vaccines. Preventive HPV vaccines aim to prevent HPV infection by inducing a neutralizing antibody response (Fig. 1). Improved understanding of protective humoral immune response against primary HPV infection has led to the development of preventive HPV vaccines targeting L1 and/or L2 viral capsid proteins. Transfection of animal cell lines with the gene coding for L1 capsid protein leads to the assembly of virus-like particles (VLPs) in transfected cells. In preclinical models, VLPs were able to induce production of neutralizing antibodies, leading to the establishment of protective immunity [7, 8].
Fig. 1.
HPV vaccination. Vaccination can be categorized into two categories: preventive and therapeutic vaccination. Preventive HPV vaccines focus on the humoral immunity. Preventive HPV vaccines deliver HPV virus-like particles (VLPs) encoding L1 and/or L2 viral capsid proteins. B cells bind to the HPV VLPs and are activated by TH2 (differentiated CD4+ T helper cells) to become plasma cells, which secrete antibodies. These neutralizing antibodies block primary HPV infection, inducing protection against HPV. Therapeutic HPV vaccination focuses more on cell-mediated immunity. Cell-mediated immunity involves the interaction between professional antigen-presenting cells, particularly dendritic cells and T cells. Dendritic cells present the MHC:peptide complex to T cells and prime naїve T cells to become effector CD4+ T cells, if presented via MHC class II, or effector CD8+ T cells, if presented via MHC class I. These effector T cells mediate therapeutic effects, with effector CD8+ T cells, also known as cytotoxic T lymphocytes (CTL) mediating antigen-specific killing of tumor cells, and effector CD4+ T cells differentiating into T helper cells to either augment CTL immune response or activate B cells to make antibodies
There are two commercially available preventive HPV vaccines. Gardasil is a quadrivalent HPV L1 VLP vaccine containing L1 VLP derived from HPV-6, 11, 16, and 18. It has been shown to be extremely successful in protecting humans against infection with four of the most clinically relevant HPV types, namely HPV-6 and 11 for benign genital warts and HPV-16 and 18 for cervical cancers. Gardasil has been shown to reduce the incidence of high-grade CIN associated with HPV-16 and 18 [9] as well as HPV-related anogenital cancer [10]. Cervarix is another preventive HPV vaccine containing L1 VLP derived from HPV-16 and 18. The L1 VLP vaccine is administered in AS04 adjuvant (3-O-desacyl-4′-monophosphoryl lipid A and aluminium hydroxide). Cervarix also offers partial cross-protection against HPV types 31 and 45, which are not included in the vaccine [11]. Both Gardasil and Cervarix are highly immunogenic and are capable of generating high titers of neutralizing antibodies against L1 of the HPV types most frequently associated with cervical cancer, HPV-16 and 18 [10, 12, 13]. These vaccines have demonstrated efficacy over a 5-year period [11, 13].
Need for therapeutic HPV vaccines
While the preventive HPV vaccines represent a significant breakthrough in the control of cervical cancer, there is a high prevalence of existing HPV infection worldwide. Over 80% of cervical cancers occur in developing countries. Because of the high prevalence of existing HPV infection and years required for the development of cervical cancer, preventive HPV vaccines would need to be in widespread use for many years to reduce the number of HPV-associated cervical cancer. However, the high cost of the current HPV vaccines represents a major hurdle for implementation of HPV vaccines in developing countries, where screening programs are minimal and need is significant. An effective therapeutic vaccine could have an immediate impact on the mortality and morbidity of HPV-associated malignancies and its precursor lesions. Thus, there remains an urgent need for the development of therapeutic HPV vaccines.
Choice of HPV antigens for therapeutic vaccination
In order to develop an effective therapeutic DNA vaccine, there needs to be suitable target antigens against which an immune response may be mounted. Preventive HPV vaccines target HPV capsid proteins L1 and L2 to induce neutralizing antibody production. However, after primary HPV infection, the expression of L1 and L2 is undetectable in basal cell areas and in HPV-associated malignancies [14]. Therefore, therapeutic HPV vaccines should target HPV antigens constitutively expressed in HPV-associated malignancies and their precursor lesions. HPV E6 and E7 represent ideal targets for therapeutic HPV vaccination due to several properties. HPV E6 and E7 are tumor-specific antigens; they are present only on tumor cells and not on normal cells. In addition, they represent completely foreign proteins and therefore will not raise issues of immune tolerance. There is no risk of autoimmunity since they are not self-proteins. Additionally, as mentioned previously, HPV E6 and E7 are essential in cellular transformation and are constitutively expressed in malignant cells. Their crucial role in tumor pathogenesis makes it difficult for them to be lost. Thus, E6 and E7 have been the most commonly chosen target antigens in the development of therapeutic HPV vaccines (for review, see [15]).
Different forms of therapeutic HPV vaccines
Many different vaccine platforms have been used in the development of therapeutic HPV vaccines. These methods include peptide- or protein-based vaccines, live vector-based vaccines, DNA- or RNA replicon-based vaccines, whole cell vaccines and combined approaches. Each form has its own advantages and disadvantages. Peptide-based vaccines are known to be well tolerated, stable and easy to manufacture on a large scale. However, antigenic peptides delivered by these vaccines are restricted to a particular major histocompatibility complex (MHC) class I phenotype expressed in an individual; peptide-based vaccines may not necessarily be effective in all those who are immunized. Another issue for peptide-based vaccines is their low immunogenicity. It is therefore necessary for peptide-based vaccines to be administered with other immunomodulatory agents, such as Toll-like receptor (TLR) ligands, cytokines and co-stimulatory molecules. In contrast to peptide-based vaccines, protein-based vaccines circumvent the problem of being restricted to specific MHC class I molecules as they contain a wide range of epitopes [16–18]. However, they are also poorly immunogenic and have been shown to bias the antigen-specific immune response toward humoral rather than T cell immunity [19, 20].
Live vector-based vaccines, on the other hand, are able to generate strong cell-mediated and humoral immune responses [21–23]. Vectors such as adenoviruses and L. monocytogenes have been modified to enable preparation of such vaccines. However, pre-existing host immunity to such agents may potentially reduce effectiveness of live vector vaccines and limit the number of possible repeated vaccinations in the same subject. Additionally, the intrinsic pathogenic potential of viral and bacterial vectors may pose a risk should these vaccines be administered to immunocompromised individuals.
Whole cell vaccines include dendritic cell-based and tumor cell-based vaccines. Dendritic cell-based approach requires preparation of individual dendritic cells (DCs) pulsed with E6/E7 peptides, DNA or RNA encoding these peptides, or transfection with live vectors that carry E6/E7. However, this approach is labor-intensive and may prove to be expensive if applied to large-scale immunization programs. Tumor cell-based vaccines involve systemic administration of whole tumor cells in order to aid in the recognition of HPV-associated tumor antigens by the immune system. However, introducing new malignant cells into patients raise safety concerns. Several HPV vaccines based on these strategies have been tested in early phase clinical trials (for review, see [15]).
DNA vaccines
DNA vaccines have emerged as an attractive form of therapeutic HPV vaccines that display promising potential in treating HPV-associated lesions. DNA vaccines have several advantages over other forms of therapeutic HPV vaccines (Table 1). For example, compared to live vector-based and tumor cell-based vaccines, they are relatively safe and can be administered repeatedly to the same individual without losing efficacy. Since DNA vaccines do not elicit anti-vector immune responses in the vaccinated patient, they are well suited for indications likely to require multiple administrations in order to achieve and maintain target immune responses. DNA vaccines are also stable, easy to prepare at high purity, and inexpensive in terms of their storage and transportation [24, 25]. The presence of full-length complementary DNA provides multiple epitopes, thereby overcoming the limitation of MHC restriction associated with peptide-based vaccines. Plasmid DNA itself contains unmethylated CpG motifs that may act as potent immunological adjuvants. DNA vaccines are also able to provide sustained release of antigenic proteins, thereby enhancing immunological memory. Furthermore, they can be engineered to express HPV antigenic peptides or proteins and have a variety of delivery methods, enabling DNA vaccines to deliver HPV antigens to the antigen-presenting cells (APCs) and stimulate development of both CD4+ and CD8+ antigen–specific T cell responses in vivo.
Table 1.
Advantages of DNA vaccination
| Advantage | Attributes |
|---|---|
| Design | Optimization of plasmids through codon and RNA structure changes |
| Can generate effective cytotoxic T lymphocyte and antibody responses | |
| Can be engineered to express tumor antigenic peptides or proteins | |
| Enables prolonged expression of antigens and enhancement of immunologic memory | |
| Safety | Unable to revert into virulent forms, unlike live vector–based vaccines |
| Capacity for repeated administration safely and effectively | |
| No significant adverse events in any clinical trial | |
| Stability | Temperature-stable |
| Long shelf life | |
| Manufacture | Suitable for large scale production at high purity |
| Rapid production and formulation | |
| Easy to store and transport |
However, naked DNA suffers from insufficient intrinsic specificity for APCs and has a limited ability to spread between cells in vivo. These factors limit the potency of HPV DNA vaccines. Strategies to enhance therapeutic HPV DNA vaccines have focused on targeting DNA encoding antigens to professional APCs to boost vaccine-induced immune responses.
Strategies to enhance therapeutic HPV DNA vaccine potency
Professional antigen-presenting cells, particularly DCs, are central players in the initiation of the adaptive immune response. Thus, numerous efforts have been made to enhance the immunogenicity of these vaccines by focusing on (1) increasing the number of dendritic cells (DCs) transfected with HPV DNA plasmids (2) improving HPV antigen expression, processing and presentation by DCs through MHC class I and II pathways, and (3) enhancing the ability of HPV DNA transfected DCs to prime E6/E7-specific T cells in order to generate therapeutic effects against established HPV infections and HPV-associated lesions (Table 2).
Table 2.
Potential strategies to enhance therapeutic HPV DNA vaccine potency
| Strategies | Approach | Reference |
|---|---|---|
| Strategies to increase the number of HPV antigen-expressing/HPV antigen-loaded DCs | ||
| [1] Routes of administration | (a) Intradermal administration via gene gun | [26, 27, 55] |
| (b) Intramuscular injection followed by electroporation |
[29–33] | |
| (c) Intradermal injection followed by laser treatment |
[36, 37] | |
| (d) Intramuscular injection of DNA vaccine encapsulated by microparticles |
[39, 40] | |
| [2] Increasing intercellular spreading of HPV antigens to DCs |
(a) VP22 | [48–55] |
| (b) MVP22 | [56] | |
| (c) BVP22 | [57, 58] | |
| [3] Targeting Ag directly to DCs by linking Ag to DC-binding molecules |
(a) Flt3 Ligand | [60, 61] |
| (b) HSP70 | [63] | |
| [4] Enhancing the release of Ag into the DC milieu |
(a) IM-coadministration of DNA encoding xenogenic MHC I with DNA vaccine increases cross-presentation in DCs |
[67] |
| (b) Enhanced release of HPV antigen from tumor cells pre-treated with chemo-radiotherapy |
[69–74] | |
| Improving HPV antigen expression, processing and presentation in dendritic cells | ||
| [1] Enhancing the level of antigen expression in transfected DCs |
(a) Codon optimization | [32, 76–78] |
| (b) Employing demethylating agents | [80, 81] | |
| [2] Enhancing transcription of MHCI and MHC II |
Co-administration of DNA encoding CIITA with HPV DNA |
[82] |
| [3] Enhancing antigen processing through MHC I pathway |
(a) Targeting Ag for proteasomal degradation | [84, 85] |
| (b) Targeting antigen to endoplasmic reticulum | [87–89] | |
| (c) Targeting antigen for cross-presentation | [90] | |
| [4] Enhancing antigen processing through MHC II pathway |
Linking HPV antigens to LAMP-1 to target antigen to the endosomal/lysosomal compartment |
[93, 94] |
| [5] Bypassing antigen processing by MHC I pathway |
Linking HPV antigens to MHC I single chain trimer to generate stable antigen presentation in DC |
[95] |
| Strategies to enhance DC function and interaction with T cells | ||
| [1] Prolonging DC and T cell survival to enhance T cell priming and activation |
(a) Strategy to prolong DC survival | [96–98] |
| (b) Strategy to prolong T cell survival | [99] | |
| [2] Priming CD4+ T helper cells to enhance DC and T cell interaction | [100, 102] | |
| [3] Employing cytokines and co-stimulatory signals to enhance T cell and DC activation | [25, 105] | |
| [4] Promoting DC activation and expansion | [107–109, 111] | |
| [5] Eliminating immunosuppressive Treg to improve T cell priming | [115–117] | |
Strategies to increase the number of HPV antigen-expressing/HPV antigen-loaded DCs
Strategies improving techniques of delivering HPV DNA vaccines for increased antigen expression/antigen loading
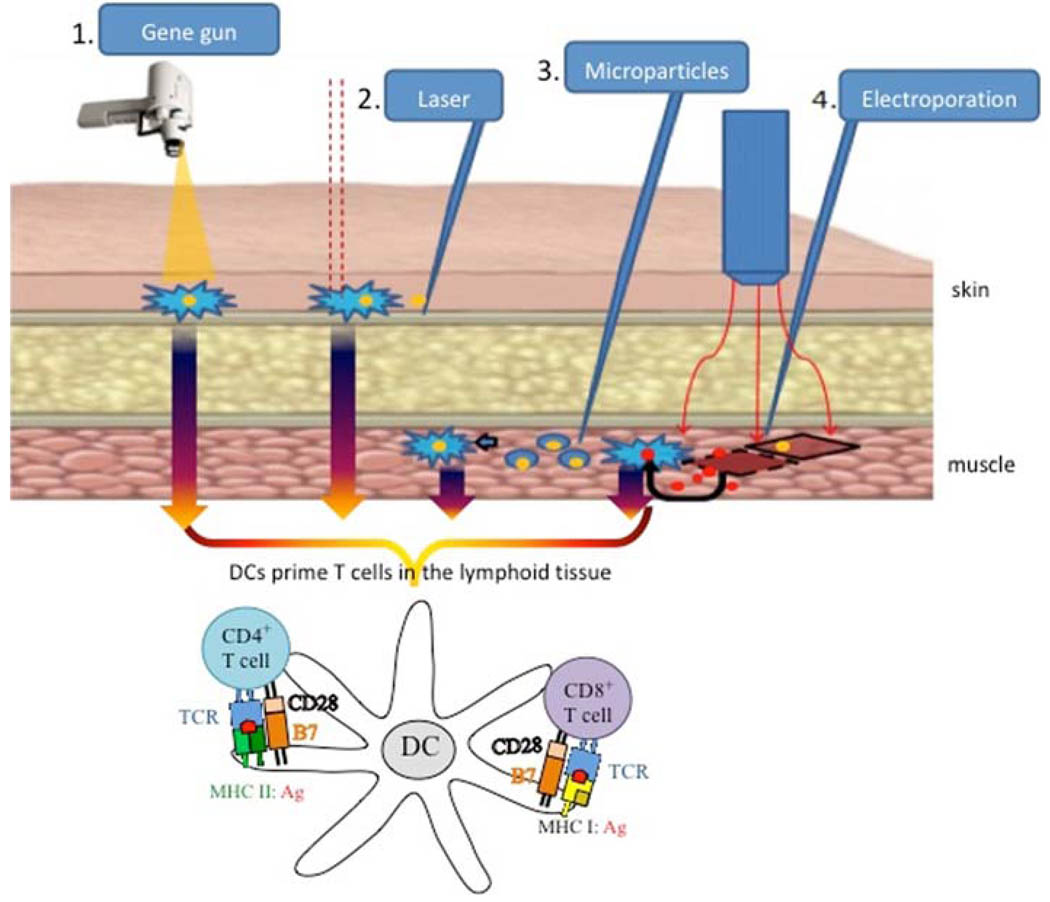
Several strategies of HPV DNA vaccine delivery have been developed to increase the number of antigen-expressing or antigen-loaded DCs. These strategies include intradermal administration through gene gun, intradermal injection followed by laser treatment, intramuscular injection, followed by electroporation and intramuscular injection of microencapsulated vaccine (Fig. 2). Table 2 summarizes their characteristics.
Fig. 2.
Routes of administration for therapeutic HPV DNA vaccines. There are several routes of administration currently used for HPV DNA vaccines. They focus on enhancing antigen uptake by dendritic cells (DCs), leading to priming of CD4+ and CD8+ T cells for an enhanced immune response. 1 Gene gun is a ballistic device that delivers gold particles coated with HPV DNA directly to Langerhans cells, which are immature DCs located under the skin. 2 Intradermal administration of HPV DNA followed by laser enhances DNA uptake into DCs. 3 Intramuscular injection with encapsulated microparticles containing HPV DNA potentiates uptake of the vaccine by DCs. 4 Intramuscular injection of naked DNA vaccine followed by electroporation enhances antigen expression by the muscle cells and generates a local inflammatory response, favoring antigen uptake by DCs
Intradermal administration of HPV DNA vaccines using gene gun
The gene gun is a biolistic (contraction of biological and ballistic) device that enables delivered DNA to directly transfect keratinocytes and epidermal Langerhans cells (immature dendritic cells). These events stimulate DC maturation and migration to the local lymphoid tissue, where DCs prime T cells for HPV antigen–specific immune responses. Gene gun is currently widely used in many immunotherapeutic applications in preclinical studies. Traditionally, gene gun technology is particle-mediated and involves using compressed helium to propel a stream of gold particles coated with DNA into the skin, where Langerhans cells are located. The delivery of HPV DNA vaccines by gene gun was shown to be the most dose-efficient method of vaccine administration in comparison with routine intramuscular and biojector injection [26]. Therefore, intradermal administration of HPV DNA vaccines by gene gun has the potential for increasing the effectiveness of these vaccines in humans.
More recently, gene gun has also been shown to be able to deliver non-carrier naked DNA under a low-pressure system. It has been shown that non-carrier naked therapeutic HPV DNA vaccine resulted in significantly less local skin damage than gold particle-coated DNA vaccination and was also able to enhance HPV antigen–specific T cell immunity and antibody response, as well as generate comparable antitumor effects as the gold particle-coated therapeutic HPV DNA vaccine did [27].
Intramuscular administration of HPV DNA vaccines followed by electroporation to increase number of antigen-loaded DCs
Electroporation represents another way of increasing the number of HPV DNA–transfected cells and enhancing the magnitude of gene expression, while reducing inter subject variability and requiring less time to reach a maximal immune response compared to conventional intramuscular injection of the vaccine (for review, see [28]). The technique increases cellular uptake of intramuscularly injected DNA through a combination of a transient increase in permeability of plasma membranes generated by a small electric current at the injection site and electrophoretic movement of negatively charged DNA into the cells, thus allowing expression of DNA plasmid [29, 30]. Additionally, electroporation generates a local inflammatory state by recruiting cytokines and mononuclear cells to the site of antigen production creating a favorable milieu for antigen presentation, establishment and maintenance of the immune response to the vaccine. In a head-to-head comparison study of the HPV DNA vaccine administered by different methods, electroporation has been shown to elicit the highest number of E7-specific cytotoxic CD8+ T cells and greatest antitumor immune response compared to intramuscular injection and intradermal gene gun delivery [31]. Furthermore, electroporation has been successfully used to administer several HPV DNA vaccines to mice [32] as well as rhesus macaques [33], which has prompted its use in an ongoing Phase I clinical trial of VGX-3100, a vaccine that includes plasmids targeting E6 and E7 proteins of both HPV subtypes 16 and 18, for treatment of patients with CIN 2 or 3 [34].
Intradermal administration of HPV DNA vaccines followed by laser treatment
In vitro studies have shown that laser beam can deliver a focused amount of energy onto a target cell, modifying permeability of the cell membrane at the site of the impact by a local thermal effect. This transient perturbation is sufficient to allow a gene present in the surrounding medium to be transferred into the cell (for review, see [35]). The femtosecond infrared titanium sapphire laser beam was developed specifically for enhancing in vivo gene delivery without risks of tissue damage.
Intradermal administration of DNA vaccine followed by pulses of laser has been performed successfully inducing antigen-specific CD4+ and CD8+ T cell immune response as well as humoral immunity [36]. More recently, Tsen et al. found this novel technology to be an effective method of enhancing the transfection efficiency of injected plasmids administered intradermally [37]. Based upon a limited number of studies, this novel technology remains to hold a high potential for therapeutic HPV DNA vaccine development.
Intramuscular administration of HPV DNA vaccines encapsulated by microparticles
Another route of DNA vaccine delivery to APCs is facilitated by microencapsulation of plasmid DNA, which encodes HPV E6/E7 antigenic proteins [38]. The capsule is formed from polymeric microparticles of 1–2 µm in diameter consisting of Poly (D, L) Glycolic-Co-Lactic Acid (PGLA), a biocompatible polymer used in a number of pharmaceutical products, including sutures. These resulting microparticles have a greater propensity toward APC uptake compared to naked DNA, and several types of APC are known to phagocytose particulate matter in this micron to submicron-range [39]. This technique allows HPV DNA plasmid to be condensed inside the microparticle. The physical and chemical properties of the PGLA scaffold render DNA inaccessible to nuclease preventing degradation, allowing for a sustained release of DNA and enhancing transfection efficiency in vitro [40]. In mice, microspheres containing HPV plasmid encoding HPV E6/E7 antigens have been shown to elicit a strong antigen-specific cytotoxic T cell response [39]. Using this technology, microencapsulated DNA vaccine termed ZYC-101 encoding multiple HLA-A2 restricted HPV E7 epitopes has undergone Phase I trials in patients with CIN2/3 lesions [41] and high-grade anal intraepithelial neoplasia [39]. In both trials, intramuscularly administered vaccine was well tolerated, and in some patients had resulted in histological regression of the lesions as well as generation of E7-specific IFN-γ expressing T cells. A newer version of the DNA vaccine, ZYC-101a, which encodes HPV-16 and HPV-18 E6- and E7-derived epitopes has been used in phase II clinical trial in patients with CIN 2/3 lesions. This DNA vaccine has been shown to promote the resolution of CIN 2/3 in most (70%) of the patients younger than 25 years compared to the placebo group of the same age [42].
Other routes of administration with potential applications for HPV DNA vaccines include intradermal vaccination by tattooing [43, 44], skin patches to deliver DNA [45], microneedles [46], prime-boost vaccination utilizing priming of DNA complexed with cationic block copolymers and boosting with protein vaccine [47] (Table 3 and Table 4).
TABLE 3.
Routes of administration for therapeutic HPV DNA vaccine development
| Route of administration | Principle | Material | Advantages | Disadvantages |
|---|---|---|---|---|
| Intramuscular injection via hypodermic needle |
Manual injection into muscle | Hypodermic needle |
|
|
| Intramuscular administration via microencapsulation of plasmid DNA |
Biodegradable polymer to encapsulate DNA |
Polyglycolic-lactic acid microparticles, needle |
|
|
| Intramuscular administration via electroporation |
Electric field-induced cell membrane permeabilization and electrophoretic mobilityof DNA into cells |
Electrodes and pulse generator, needle |
|
|
| Intradermal administration via gene gun |
High-velocity particle bombardment |
Gene gun, gold beads coated with DNA |
|
|
| Low-pressured delivery of noncarrier naked DNA vaccine |
Gene gun, noncarrier naked DNA |
|
|
|
| Intradermal administration via laser treatment |
Laser beam–induced cell membrane permeabilization |
Low energy laser source, needle |
|
|
Table 4.
Selected clinical trials for therapeutic HPV DNA vaccines
| Vaccine construct | Antigen | HPV type(s) |
Route of administration |
Sponsor | Patient population | Reference |
|---|---|---|---|---|---|---|
| DNA (ZYC101) | E7 epitope (aa 83—95) |
HPV-16 | IM injection with microencapsulation of |
MGI Pharma (formerly Zycos) |
Phase I trial in 12 patients with anal HSIL |
[39] |
| HPV DNA vaccine | Phase I trial in 15 patients with CIN 2/3 |
[41] | ||||
| DNA (ZYClOla) | E6 and E7 | HPV-16, HPV-18 |
IM injection with microencapsulation of HPV DNA vaccine |
MGI Pharma (formerly Zycos) |
Phase II trial in 127 patients with CIN 2/3 |
[42] |
| DNA (pNGVL4a-Sig/E7 (detox)/HSP70) |
E7• | HPV-16 | IM injection | NCI | Phase I trial in 15 patients with CIN 2/3 |
[65] |
| Phase I clinical trial in patients with advanced HNSCC |
* | |||||
| Prime with DNA (pNGVL4a- Sig/E7(detox)/HSP70), boost with recombinant vaccinia virus (TA-HPV) ± imiquimod |
E7• in plasmid DNA + E6 and E7 (TA-HPV) |
HPV-16, HPV-18 |
IM injection (DNA vaccine and TA- HPV), Topical (imiquimod) |
NCI | Phase I trials in patients with CIN3 | [66] |
| DNA (pNGVL4a-CRT/E7(detox)) | E7• | HPV-16 | Intradermal injection via gene gun |
NCI | Plans for phase I trial in patients with CIN 2/3 |
** |
| DNA (VGX-3100) | E6 and E7 | HPV-16, HPV-18 |
IM injection with electroporation |
VGX Pharmaceuticals |
Ongoing Phase I trial in adult females, post- urgical or ablative treatment of CIN 2/3 |
[34] |
M Gillison, personal communication
W Huh and C Trimble, personal communication
Detox E7 mutated to abolish Rb binding site
aa Amino acid, CIN Cervical intraepithelial neoplasia, CRT Calreticulin, HNSCC head and neck squamous cell carcinoma, HPV Human papillomavirus, HSIL High-grade squamous intraepithelial lesions, HSP Heat shock protein, IM Intramuscular; NCI National Cancer Institute
Strategies to increase intercellular spreading of HPV antigens to DCs
Once naked DNA is introduced into the cell, it has a poor ability to spread the encoded antigen to other APCs in vivo. In order to enhance the spreading of antigen encoded by DNA vaccine, it is possible to link the antigen of interest to a protein capable of intercellular transport in the context of DNA vaccines. One such protein is herpes simplex virus type 1 (HSV-1) tegument protein, VP22. It is a transporter protein required for the pathogenesis of HSV-1 infection, importing HSV genome from one cell to its neighboring cells [48].
The significance of VP22 in intercellular spreading has been demonstrated through in vitro studies linking VP22 to p53 [49], thymidine kinase [50], cytosine deaminase [51] and Green Fluorescent Protein (GFP) [48]. The proteins were observed to be distributed to nuclei of surrounding cells. Concerns have been raised regarding the validity of results from these in vitro studies and attributing them to fixation artifacts [52]. Although this remains to be a controversial issue, Kim et al. have shown that in vivo transfection of mice with a fusion gene coding for HPV-16 E7 DNA and VP22 resulted in an increased number of E7-expressing DCs in the lymph nodes, thus priming more CD8+ T cells via MHC class I pathway [53]. Vaccination with DNA encoding E7 linked to VP22 was also shown to elicit enhanced E7-specific memory CD8+ T lymphocytes and enhanced antitumor effects against E7-expressing tumor cells [54]. Furthermore, VP22 has been used for HPV DNA vaccines targeting the E6 protein [55]. Bovine Herpesvirus VP22 (BVP22) and Marek’s disease virus VP22 (MVP-1) are both closely related by their structural homology to HSV-1 VP22 and may also have a significant role in intercellular spreading. Hung et al. have demonstrated that mice vaccinated with DNA encoding MVP22/E7 displayed significantly increased numbers of IFN-γ-secreting, E7-specific CD8+ T cell precursors compared to mice vaccinated with wild-type E7 DNA alone, which directly lead to a stronger tumor prevention response [56]. Similarly, immunization of mice [57] and cattle [58] with DNA vaccine coding for BVP22 linked to BVP-truncated glycoprotein D (tgD) was shown to generate a stronger tgD-specific immune response compared to animals vaccinated with tgD alone. Taken together, DNA vaccine encoding VP22 linked to antigens represents a promising approach to enhance DNA vaccine potency.
Strategies targeting Ag directly to DCs by means of linking Ag to molecules that bind directly to DCs
Another strategy to improve the potency of DNA vaccines is to engineer DNA encoding antigens linked to molecules that preferentially bind to dendritic cells. Molecules commonly employed in this strategy are DC receptor ligands. For example, Flt3 is a murine tyrosine kinase receptor expressed by DCs [59]. Flt3 Ligand (FL) was shown to have a potent growth stimulatory effect on DC precursors in vivo [60]. An HPV DNA vaccine encoding a recombinant chimera consisting of extracellular domain of FL linked to HPV-16 E7 has been shown to generate significantly higher levels of E7-specific cytotoxic immunity against E7-expressing tumors and reduce the size of established pulmonary metastases compared to wild-type E7 DNA [61]. This cytotoxic response was shown to significantly supercede the one generated by HPV E7 DNA vaccine alone.
Another molecule that is able to target antigen to DCs is HSP70. HSP70 is a chaperone protein capable of binding to scavenger receptor CD91 on the surface of DCs (for review, see [62]). Thus, linking HPV antigens to HSP70 may enable them to be targeted to DCs. The HPV antigen linked to HSP70 was shown to enter the cross-presentation pathway to be presented in the context of MHC I and prime CD8+ T cells [63]. Furthermore, when HSP70/E7 DNA vaccine construct was administered to mice, the CD8+ T cell immune response was shown to be independent of CD4+ T cell help. Additionally, HSP70 has been shown to activate the innate immune system, such as TLR 2 and TLR 4, which in turn could provide further signals for DC maturation (for review, see [64]) and result in more effective antigen cross-presentation. These features make HSP70 a potential tool in enhancing HPV DNA vaccine potency. DNA vaccine encoding a signal sequence linked to an attenuated form of HPV-16 E7 (with a mutation that bolishes the Rb binding site; E7(detox)) and fused to HSP70 (Sig/E7(detox)/HSP70) has been tested in Phase I clinical trials [65]. The vaccine was well tolerated in patients with CIN 2 and 3 lesions. Another Phase I trial using the same DNA vaccine (Sig/E7(detox)/HSP70) has been tested in HPV-16+ patients with advanced head and neck squamous cell carcinoma (M Gillison, personal communication). Additionally, a clinical trial employing a DNA vaccine encoding Sig/E7/ HSP70 boosted with recombinant vaccinia virus encoding HPV-16/18 E6/E7 fusion protein (TA-HPV) with or without imiquimod is in progress in patients with CIN III lesions [66].
Employing strategies to enhance the release of Ag into the DC milieu
IM co-administration of DNA encoding xenogenic MHC I with DNA vaccine increases cross-presentation in DCs
Intramuscular (IM) injection of DNA vaccines can generate antigen-specific immune responses predominantly through cross-priming mechanisms because muscle is not a professional APC. Cross-presentation involves the uptake, processing and presentation of extracellular antigens by MHC class I molecules to CD8+ T cells for antigen-specific T cell immune responses. It has been shown that DCs can be recruited to the local tissues for cross-presentation of CD8+ T cells [67]. Based on this understanding, Kang et al. have recently demonstrated an innovative strategy to enhance therapeutic HPV DNA vaccine potency by co-administration of an HPV DNA vaccine with DNA encoding xenogenic MHC class I molecules through intramuscular injection. This combination of DNA vaccine with DNA encoding xenogenic MHC I was shown to enhance E7-specific CD8+ T cell immune responses and antitumor effects against E7-expressing tumors in tumor-bearing mice [68]. Kang et al. also found that this strategy led to an increase in the number of infiltrating CD8+ T lymphocytes and activated APCs at the injection site. It is possible that the degree of local inflammation led to recruitment of APCs to the injection site, increasing the rate of apoptosis, leading to increased release of antigen into the DC milieu and cross-priming in local muscle tissue, subsequently leading to a greater number of primed antigen-specific CD8+ T cells in vaccinated mice. These data suggests that the IM co-administration of a xenogeneic MHC class I molecule with HPV DNA vaccine can enhance antigen-specific immune responses through the enhancement of cross presentation in DCs.
Enhanced release of HPV antigen from tumor cells pre-treated with chemo/radiotherapy
Chemotherapeutic agents and radiation can potentially induce apoptosis of tumor cells, leading to the release of HPV-E6/E7 antigen into circulation for uptake into DCs to cross-prime CD8+ T cells and increasing the frequency of HPV E6/E7-specific CD8+ T cells, which can significantly enhance the antigen-specific immune responses generated by the HPV DNA vaccine. Previously, it has been shown that E7-expressing tumor-bearing mice pretreated with various chemotherapeutic agents such as cisplatin [69] and bortezomib [70] enhanced E7-specific CD8+ T cell immune response induced by HPV E7 DNA vaccination. Kang et al. have demonstrated a similar effect when using epigallocatechin-3-gallate (EGCG), a compound derived from green tea, which in turn induces tumor cell apoptosis in a dose-dependent manner [71]. The combination of oral treatment of EGCG with HPV E7 DNA vaccine administered via gene gun led to the production of greater E7-specific CD8+ immune responses and anti-tumor effects than either chemotherapy or immunotherapy alone. Recently, Chuang et al. have used apigenin, another flavenoid chemotherapeutic compound shown to have lower intrinsic toxicity than EGCG to enhance therapeutic HPV DNA vaccine potency [72]. They showed that treatment with apigenin rendered E7-expressing tumor cells more susceptible to the killing mediated by E7-specific CD8+ cytotoxic T lymphocytes. Furthermore, when used in mice, this approach generated the highest frequency of primary and memory E7-specific CD8+ T cells compared to the controls, which in turn led to enhanced anti-tumor effects against E7-expressing tumors. Other techniques which increase the rate of tumor cell apoptosis, such as death receptor 5 monoclonal antibody [73] and low-dose radiation [74] have been used in combination with therapeutic HPV DNA vaccines and have also enhanced HPV antigen-specific cytotoxic T cell immunity against E7-expressing tumors. Taken together, these results demonstrate that treatment of tumor-bearing mice combined with chemotherapeutic agents or radiotherapy that are capable of increasing the rate of tumor cell apoptosis may enhance the potency of therapeutic HPV DNA vaccines.
Strategies to improve HPV antigen expression, processing and presentation in dendritic cells
Enhancing the level of antigen expression in transfected DCs through codon optimization and use of demethylating agents
Codon optimization
In order to enhance translation of genes coding for HPV antigens in cells transfected by HPV DNA vaccines, codon optimization has been used. Cell lines transfected with wild-type HPV-16 E6 DNA have been shown to have a low level of E6 protein expression, which may signify that natural E6 gene sequence is poorly recognized by cellular translational machinery [75, 76]. This phenomenon results from the selection pressure imposed on HPV that has probably caused the virus to retain a unique pattern of codon usage distinct from that of their host cells [5]. This in turn compromises excessive viral early protein expression, enabling the virus to evade the immune system [77]. Transfection with codon optimized versions of the E6 [76] and/or E7 DNA [32, 77, 78] strongly enhanced E6 and/or E7 protein expression, improving translation of HPV DNA vaccines in DCs and provoking enhanced antigen-specific CD8+ T cell immune responses in vaccinated mice.
Employing demethylating agents
Employment of demethylating agents may enhance therapeutic HPV DNA vaccine potency by enhancing the level of expression of antigen encoded in the DNA vaccine. Methylated CpG motifs located in the CMV promoter regions of DNA have been previously shown to silence gene expression [79]. Since CMV promoter is used in the majority of expression vectors of DNA vaccines, this silencing is likely to have a negative impact on the potency of these vaccines by reducing the level of target antigen produced by the host. Thus, reducing the level of methylation in the HPV DNA construct may represent a way of overcoming this problem. Demethylating agents such as nucleoside analogue 5-aza-2′-deoxycystidine (DAC) have been shown to inhibit DNA methyltransferase, which resulted in reactivation of methylation-silenced genes [80]. Employing DAC in the context of a DNA vaccine encoding calreticulin linked to HPV 16-E7 (CRT/E7) led to the upregulation of CRT/E7 expression and an enhanced E-7 specific CD8+ T cell immune response generated by HPV DNA vaccine in vaccinated mice [81]. Therefore, pre-treatment of HPV DNA vaccines with DAC represents another promising approach to enhancing the DNA vaccine potency.
Strategy to enhance transcription of MHC I and MHC II
MHC CIITA is a known master regulator for MHC II expression. It has also been demonstrated that MHC CIITA upregulates the expression of MHC I molecules on the surface of DC. Therefore, co-administration of CIITA with HPV DNA vaccines represents a potential strategy to enhance antigen presentation through both MHC class I and MHC class II pathways. Kim et al. have shown that this strategy was able to potentiate a stronger anti-tumor CD4+ and CD8+ T cell immune responses, prolonging the survival of mice better than the vaccine given without DNA encoding CIITA [82]. Thus, this innovative strategy to increase MHC class I and II expression can potentially be used in the design of a more potent HPV DNA vaccine.
Strategy to enhance antigen processing through MHC class I pathway
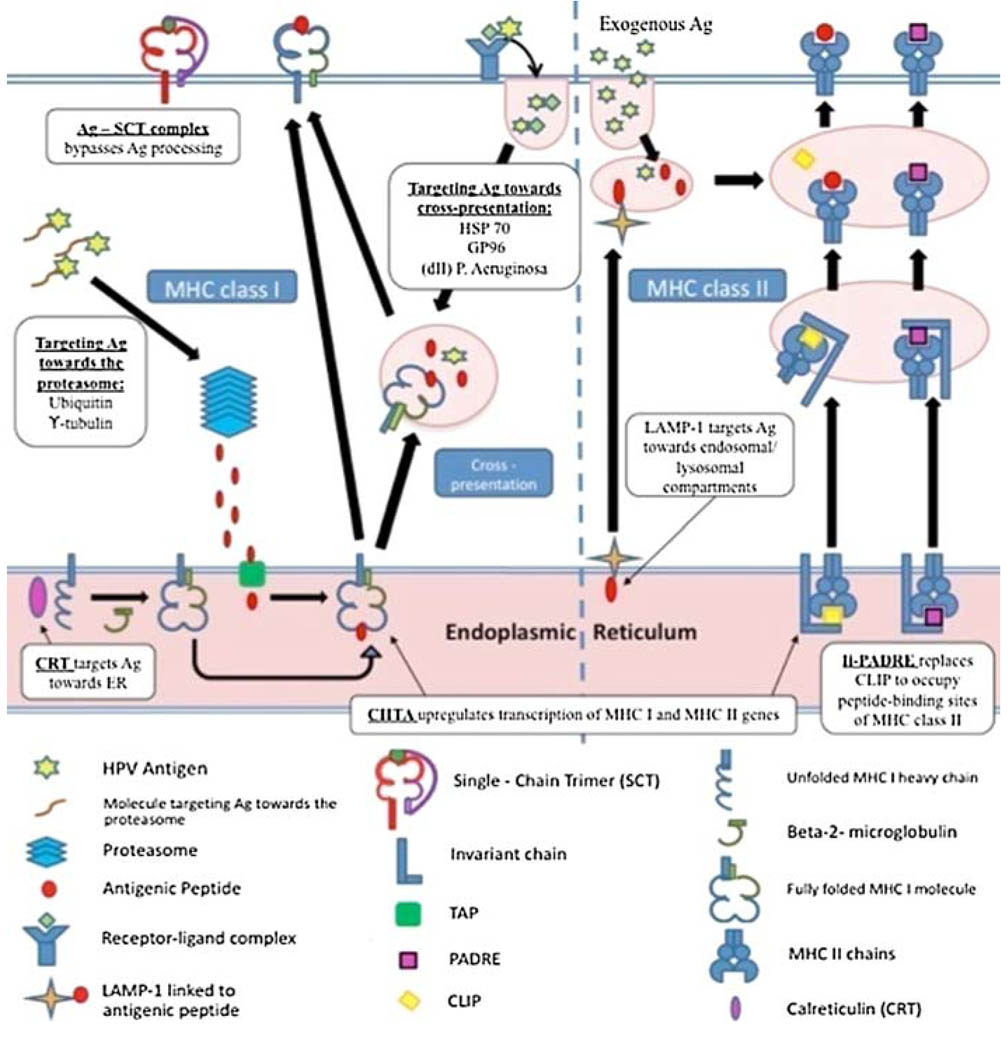
Understanding of HPV antigen processing and presentation pathway has created opportunities to design new strategies for enhancing HPV DNA potency (Fig. 3). Antigen processing and presentation by the MHC class I pathway is crucial in the priming of antigen-specific CD8+ T cells for antigen-specific killing and antitumor effects. Various strategies have been designed to enhance MHC class I presentation of HPV antigens. Particularly, HPV antigens have been linked to proteins that target antigen for proteasomal degradation, entry into endoplasmic reticulum or for cross-presentation.
Fig. 3.
Strategies to enhance antigen processing and presentation through MHC class I and II pathways. There are a variety of strategies to enhance DNA vaccine potency through enhancing antigen processing and presentation. These include enhancing the transcription of MHC class I and MHC class II molecules, enhancing or bypassing antigen processing through MHC I pathway and enhancing antigen processing and presentation through MHC II pathway
Targeting Ag for proteasomal degradation
The rate of antigen degradation by the ubiquitin–proteasome pathway has been shown to influence MHC class I presentation [83]. Therefore, HPV antigens have been linked to various molecules that directly increase this rate and amplify the amount of antigen processed and presented to CD8+ cells through MHC class I. These molecules include potato virus × coat protein sequence, y-tubulin and ubiquitin (Fig 3). Massa et al. have demonstrated that fusion of HPV-16 E7 DNA with a gene encoding potato virus × coat protein sequence resulted in increased instability of the expressed product and faster degradation via the proteasome [84]. This fusion vaccine also inhibited growth of E7-expressing tumors in vivo better than DNA vaccine alone and demonstrated enhanced levels of humoral and cell-mediated immune responses. Another molecule that could potentially target antigens to the proteasome is the γ-tubulin. The centrosome is a peri-nuclear organelle that has been shown to contain a high density of proteasomes. Several molecules of interest are located in the centrosome, including γ-tubulin. Therefore, Hung et al. have created a DNA vaccine encoding HPV-16 E7 linked to γ-tubulin. Cells transfected with this DNA construct target E7 to centrosome for proteasomal degradation in the centrosomes. They have shown that this approach resulted in enhanced MHC I presentation of E7 antigen and a more potent anti-tumor response independent of CD4+ T cell help [85].
Targeting antigen to the endoplasmic reticulum
Endoplasmic reticulum is an important focus for MHC class I presentation. DNA vaccine encoding HPV E6/E7 antigen linked to molecules targeting endoplasmic reticulum has been shown to improve MHC class I presentation of E6/E7 antigens [86]. One of those kinds of molecules is calreticulin (CRT), a ‘polypeptide chaperone’ molecule located within the ER which interacts with peptide folding in the context of MHC class I heavy chain. Vaccinating mice with CRT/E7 DNA has been shown to significantly increase E7-specific CD8+ T cell precursors and exhibit an impressive antitumor effect in E7-expressing tumors compared to mice vaccinated with wild-type E7 DNA or CRT DNA [87]. Furthermore, in a head-to-head comparison of HPV-16 E7 DNA vaccines employing intracellular targeting strategies, Kim et al. found that a DNA vaccine encoding CRT/E7 generated the greatest E7-specific CD8+ T cell immune responses and antitumor effects against E7-expressing tumors in vaccinated mice [88]. DNA vaccines encoding CRT linked to other HPV antigens have been reported including E6 and E6/E7/L2 fusion proteins [55, 89]. The linkage of antigen to proteins that target the antigen processing and presentation pathway represents an innovative strategy to enhance therapeutic HPV DNA vaccine potency, with significant clinical applications.
Targeting antigen for cross-presentation
HPV DNA constructs involve the linkage of HPV E7 to various proteins including HSP 70, GP96, and domain II of P. Aeruginosa [90] have been shown to result in enhanced E7-specific CD8+ T cell immune response. It is believed that these proteins stimulate antigen processing through the cross-presentation pathway by inducing its translocation from the endosomal/lysosomal compartments of DCs to the cytoplasm, resulting in enhanced MHC class I presentation of HPV antigens.
Enhancing antigen processing through MHC class II pathway
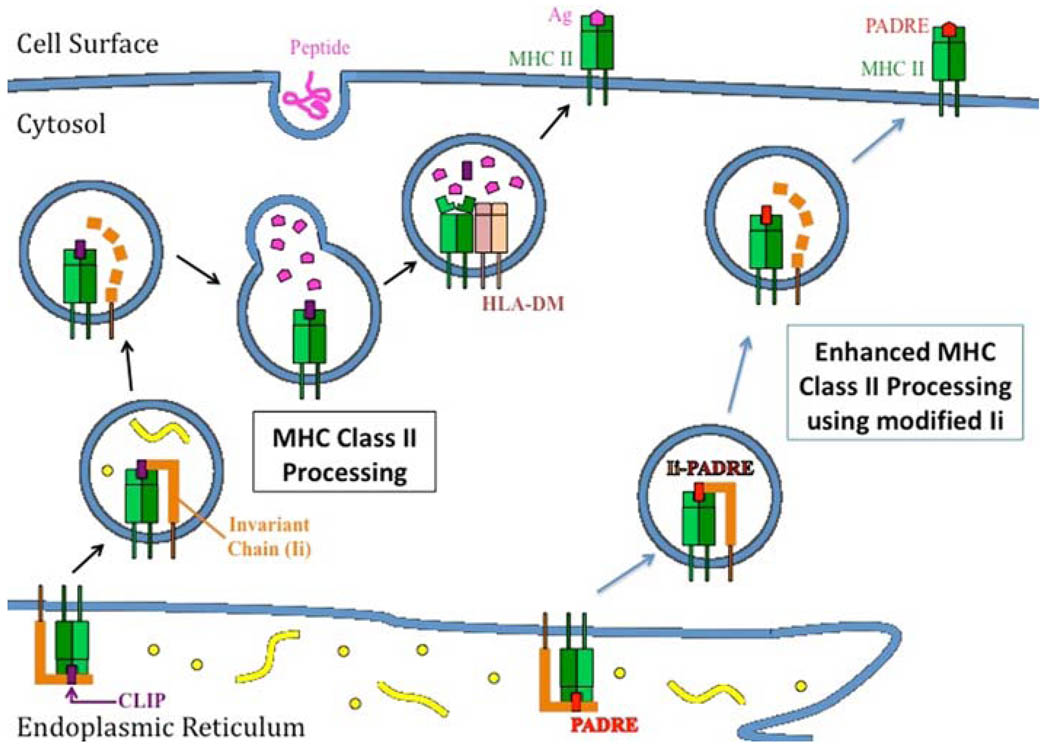
CD4+ T helper cells play an important role in providing auxiliary signals for the activation of antigen-specific CD8+ immune response against cervical cancer [91] and generation of long-term immunity [92]. In order to enhance presentation of antigens encoded by DNA vaccines to the CD4+ T helper cells, strategies have been developed to enhance antigen processing by the MHC class II pathway. For example, Wu et al. have developed a novel DNA vaccine encoding HPV-16 E7 protein linked to the sorting signal of lysosomal-associated membrane protein type 1 (LAMP-1) [93]. Cells transfected with this DNA construct have been shown to reroute E7 antigen from cytoplasmic/nuclear localization to cellular endosomal/lysosomal compartments, enabling a more efficient presentation of E7 in the context of MHC class II pathway (Fig 4). Mice vaccinated with E7/LAMP-1 generated greater E7-specific CD4+ and CD8+ effector cells compared to those vaccinated with DNA vaccine encoding wild-type E7 alone [94].
Fig. 4.
Employment of modified MHC class II–associated invariant chain for improving class II presentation of antigen in dendritic cells. In the endoplasmic reticulum, the major histocompatibility complex (MHC) class II–associated invariant chain (Ii) binds with MHC class II molecules and the class II-associated peptide (CLIP) region of Ii occupies the peptide-binding groove of the MHC molecule, preventing premature binding of antigenic peptides into the groove. In the endosomal/lysosomal compartments, HLA-DM facilitates the release of CLIP and CLIP is replaced by a peptide antigen. The MHC class II molecule/antigenic peptide complex is then presented on the cell surface. By replacing the CLIP region of Ii with a CD4+ T-helper epitope such as the pan human leukocyte antigen (HLA)-DR-binding epitope (PADRE), PADRE can be presented efficiently through the MHC class II pathway in dendritic cells for the stimulation of PADRE-specific CD4+ T cells. This vaccine, termed Ii-PADRE, has been shown to generate significantly greater T-cell immune responses compared to vaccination with DNA encoding unmodified Ii
Bypassing antigen processing by MHC class I pathway by linking HPV antigens to MHC class I single chain trimer to generate stable antigen presentation in DC
Novel single chain trimer (SCT) technology allows HPV antigen to bypass antigen processing and presentation through the MHC class I pathway. This technology involves the linkage of the genes encoding E6 antigenic peptide linked to β2 microglobulin and MHC I heavy chain in order to produce a stable single-chain construct encoding antigenic peptide fused to an MHC class I molecule. This approach may allow for a more stable MHC class I presentation of E6 on the surface of DCs. DNA vaccine coding for the HPV-16 E6 CTL epitope linked to the SCT genes (β2 microglobulin and H-2 Kb MHC class I heavy chain) has been shown to produce a greater E6-specific CD8+ T cell immune response in vaccinated mice than wild-type HPV-16 E6 DNA [95]. Furthermore, mice vaccinated with E6 SCT DNA vaccine exhibited complete protection against a lethal challenge of E6-expressing tumor cells, while all mice administered wild-type E6 DNA developed tumors.
Strategies to enhance DC function and interaction with T cells
Strategies to prolong DC and T cell survival in order to enhance T cell priming and activation
Strategy to prolong DC survival
DC cells become potential targets of T cell–mediated apoptosis after T cell priming. In order to prolong survival of DCs transfected with DNA vaccine, strategies have focused on co-administration of DNA vaccine with anti-apoptotic proteins such as Bcl-xL, Bcl-2, X-linked inhibitor of apoptosis protein and dominant negative mutants (dn) of caspase-9, dn caspase-8 [96] and connective tissue growth factor [97]. However, co-administration of HPV DNA vaccines with DNA encoding anti-apoptotic molecules may not be a suitable option for clinical trials as this raises safety concerns for possible cellular transformations. Thus, Kim et al. have employed small interfering RNA (siRNA), to co-administer with HPV DNA vaccine, as an effective mode of transiently silencing gene expression of pro-apoptotic proteins Bak and Bax in transfected DCs in order to alleviate these concerns of oncogenicity [98]. As a result, administration of the DNA vaccine encoding HPV-16 E7 with siRNA targeting Bak and Bax has prolonged the life of DCs in the draining lymph nodes, stimulating stronger E7-specific CD8+ T cell responses and eliciting more potent antitumor effects relative to the mice given HPV-16 E7 DNA alone. Thus, these data indicate that using siRNA to target key pro-apoptotic molecules in combination with HPV DNA vaccines may effectively prolong DC survival and improve therapeutic HPV DNA vaccine potency.
Strategy to prolong T cell survival
Another approach to increase the number of activated T cells is to prohibit apoptotic signals to T cells. It is known that DCs produce Fas Ligand (FasL), which induces apoptosis of naive T cells at the immunological synapse during antigen presentation. This interaction is enabled by FasL binding to Fas, a cognate death receptor expressed by the T cells. Huang et al. showed that by blocking FasL on the HPV-16 E7 peptide-loaded DCs has reduced the apoptosis of E7-specific CD8+ T cells [99]. Thus, in order to augment antigen-specific CD8+ T cell response to HPV-16 E7 they have co-administered a therapeutic HPV DNA vaccine construct with DNA coding for short hairpin RNA (shRNA) targeting FasL. This approach has generated a much stronger E7-specific CD8+ T cell response in mice in comparison with HPV DNA vaccine alone and resulted in a more potent cytotoxic response against E7-expressing tumors. Therefore, HPV DNA vaccine potency may be enhanced through anti-apoptotic signals to APCs as well as inhibition of apoptosis in T cells.
Priming CD4+ T helper cells to enhance DC and T cell interaction
CD4+ T cells are known to provide help to activate primed CD8+ T cells. Recently, Hung et al. have developed a technique to enhance CD4+ T cell activation by engineering MHC class II-associated invariant chain (Ii) [100]. A region of the invariant chain known as CLIP (Class II-associated peptide) prevents premature binding of the antigenic peptide to the MHC class II molecules by occupying their peptide-binding groove (for review, see [101]). CLIP is replaced by the peptide sequence only when the MHC class II molecules are located in the endosomal/lysosomal compartments. Hung et al. have substituted CLIP with Pan-DR helper T lymphocyte epitope (PADRE) in the invariant chain (Ii-PADRE), which enabled MHC class II molecules to present this epitope more efficiently on the surface of DC cells for activation of a strong PADRE-specific CD4+ T cell immune response (Fig. 4). Mice vaccinated with HPV-16 E7 DNA in conjunction with DNA coding for Ii-PADRE have shown to generate significantly greater E7-specific CD8+ T cell response compared to those immunized with E7 DNA in conjunction with DNA coding for unmodified Ii [100]. Furthermore, Kim et al. have shown that CD4+ T cells activated by PADRE are able to secrete IL-2, a cytokine known to potentiate activation of the neighboring CD8+ T cells [102]. Therefore, this suggests a prominent role for CD4+ T helper cells when developing methods to enhance the potency of HPV DNA vaccines. Indeed, Kim et al. have promoted the use of Ii-PADRE DNA in combination with strategies to enhance DC life and various intracellular targeting strategies to further enhance antigen-specific CD8+ T cell immune responses generated by HPV DNA vaccine [103]. It has also been demonstrated that coadministration of Ii-PADRE DNA with CRT/E6 DNA vaccination in mice pretreated with doxorubicin reversed doxorubicin-mediated immunosuppression of antigen-specific immune responses, leading to enhanced antitumor effects and prolonged survival in tumor-bearing mice [104].
Strategies employing cytokines and co-stimulatory signals to enhance T cell and DC activation
Dendritic cells are able to provide the following three signals that are crucial in T cell priming: (1) Interaction of an appropriate peptide-MHC complex with TCR (2) co-stimulation of T cell receptors by molecules such as B7 and (3) provision of a favorable cytokine milieu for T cell proliferation and survival. For example, co-administration of HPV DNA vaccines with DNA encoding codon-optimized cytokines, including IL-2 and IL-12 have been shown to enhance antigen-specific cytotoxic T cell–mediated immune responses [105].
Strategies to promote DC activation and expansion
Dendritic cell activation is a vital pre-requisite to T cell priming. Provision of appropriate immunomodulatory signals to DCs in order to enhance this process thus represents a potential target which can be exploited in the development of strategies to increase potency of HPV DNA vaccines.
Membrane-associated and intracellular Toll-like receptors play an important role in controlling the innate and adaptive arms of immune response (for review, see [106]). Activation of various TLRs on DCs has been shown to induce maturation signals [107]. This provides a rationale for the future use of TLR ligands as adjuvants of HPV DNA vaccines. CpG motifs are bacterial-derived immunostimulatory gene sequences that serve as ligands for the activation of DCs through TLR9 and are able to further upregulate expression of the TLR9 transcript [108]. Transfection of DCs with HPV DNA encoding plasmids that contain high numbers of CpG motifs may thus promote more effective DC maturation signals and enhance DNA vaccine potency [109]. Other TLR ligands may also be useful for enhancing HPV DNA vaccine potency through their actions on DC TLRs.
An alternative way to promote activation of DCs is to block molecules that naturally downregulate the activation of DCs. It is known that SOCS-1 inhibits signaling through Jak-STAT pathway inducing suppression of DC function (for review, see [110]). Therefore, by using siRNA technique to target and inactivate SOCS-1 in DCs could potentially enhance CD8+ immune response elicited by the HPV DNA vaccines in the future [111].
Strategies to eliminate immunosuppressive regulatory T cells in order to improve T cell priming
Regulatory T cells (Treg) are known to limit effector immune responses against malignancies by inhibiting DC and CD8+ T cell responses (for review, see [112]). Increased number of Treg cells found within HPV-cervical lesions and lymph nodes of women with CIN and cervical cancer may prevent immune clearance of oncogenic HPV infection and promote viral persistence [43, 113, 114]. Therefore, eliminating Treg cells may potentially enhance magnitude of HPV-specific immune responses induced by the DNA vaccines and reverse tolerogenic state of the immune system caused by HPV. Several agents including administration of cyclophosphamide, Cimetidine, COX-2 inhibitors and Foxp3-transfected DC have been used to deplete Treg cells [115, 116]. These agents used in vivo in conjunction with DNA vaccines have been shown to result in enhancement in antigen-specific CD8+ T cell responses and anti-tumor effects. Recently, Chuang et al. have successfully used anti-CD25 monoclonal antibody to deplete Treg cells in cervical cancer mouse model, enabling a more potent CTL-mediated response to the HSP70/E7 DNA fusion vaccine [117]. Thus, this novel approach represents a valuable strategy to be used in the development of a more efficacious therapeutic HPV DNA vaccine.
Conclusion
While considerable progress has been made in the development of preventive HPV vaccines with the commercialization of Gardasil and Cervarix, the existing population of those afflicted with HPV-associated malignances and precursor lesions underscores the need for advances in the field of therapeutic HPV vaccines. We have discussed several strategies involving the modification of dendritic cells to enhance therapeutic HPV DNA vaccine potency by increasing the number of antigen-expressing/antigen-loaded DCs, improving antigen expression, processing and presentation in DCs and enhancing DC and T cell interaction. Increased understanding of dendritic cell biology will lead to the further development of innovative strategies to optimize vaccine-elicited T-cell immune responses.
The availability of different forms of therapeutic HPV vaccines creates opportunities for prime-boost regimens to further enhance therapeutic HPV DNA vaccine potency. Studies have shown that priming with a HPV-16 E6/E7 DNA vaccine followed by boosting with recombinant vaccinia [118] or adenovirus [119] or with the HPV-16 E6/E7 expressing tumor cell-based vaccine [120] elicited greater HPV antigen-specific CD8+ T cell immune responses in vaccinated mice compared to vaccination with DNA vaccine, viral vector vaccine or tumor cell-based vaccine alone. More recently, a clinical trial using pNGVL4a/ Sig/E7(detox)/HSP70 DNA prime followed by TA-HPV boost is currently underway in patients with CIN 2/3 lesions, evaluating whether or not the topical application of imiquimod can further enhance prime-boost administration [66]. In general, the promising preclinical data for therapeutic HPV DNA vaccine development has led to several early phase clinical trials. Continuing progress into advanced phases of clinical trials is crucial for the success of the therapeutic HPV DNA vaccine.
It is important to consider the use of immunomodulatory agents in combination with therapeutic HPV DNA vaccines, since there are many factors within the tumor microenvironment that may hinder the success of effective immune therapies. For example, Treg cells can release immune suppressive cytokines such as IL-10 and TGF-β, which can inhibit T cell function. The depletion of Treg in the tumor microenvironment has been shown to significantly enhance therapeutic HPV vaccine potency [117]. Other factors contributing to tumor immune suppression in tumor microenvironment include B7 homolog-1 (B7-H1), signal transducer and activator of transcription 3 (STAT3), MHC class I polypeptide-related sequence (MIC)-A and B, indoleamine 2,3-dioxygenase (IDO) enzyme, and galectin-1. These factors may serve as potential targets for immune modulation to enhance therapeutic HPV vaccine potency (for review, see [121]). Further study into the tumor microenvironment and molecular mechanisms impeding immune attack against HPV will lead to novel targets for therapeutic intervention in the future.
Therapeutic HPV vaccines may potentially be combined with other therapeutic modalities, such as chemotherapy and radiation therapy to augment the therapeutic vaccine effects. Chemotherapeutic agents such as cisplatin [69], bortezomib [70], epigallocatechin-3-gallate [71] and apigenin [72] have been shown to render E7-expressing tumor cells more susceptible to lysis by E7-specific cytotoxic T lymphocytes. Low-dose radiotherapy has also been combined with therapeutic HPV DNA vaccine for the control of E7-expressing tumor in a preclinical model and significantly enhanced therapeutic antitumor effects generated by HPV DNA vaccine [74]. Hence, the use of combined therapies such as prime-boost regimens, immunomodulatory agents and chemotherapy/radiation therapy will allow HPV vaccines to be used in a synergistic manner in the future. Continuing progress in these endeavors may allow for the eventual control of HPV-associated malignancies.
Acknowledgments
This review is not intended to be an encyclopedic one, and the authors apologize to those not cited. The work is supported by the NCI SPORE in Cervical Cancer P50 CA098252 and NCI 1RO1 CA114425-01.
Contributor Information
Ken Lin, Department of Obstetrics, Gynecology and Reproductive Sciences, Yale University School of Medicine, New Haven, CT, USA.
Elena Roosinovich, Imperial College School of Medicine, London, UK.
Barbara Ma, Department of Pathology, The Johns Hopkins University School of Medicine, CRBII 309, 1550 Orleans Street, Baltimore, MD 21231, USA.
Chien-Fu Hung, Department of Pathology, The Johns Hopkins University School of Medicine, CRBII 309, 1550 Orleans Street, Baltimore, MD 21231, USA; Department of Oncology, The Johns Hopkins Medical Institutions, Baltimore, MD, USA.
T.-C. Wu, Email: wutc@jhmi.edu, Department of Pathology, The Johns Hopkins University School of Medicine, CRBII 309, 1550 Orleans Street, Baltimore, MD 21231, USA; Department of Obstetrics and Gynecology, The Johns Hopkins Medical Institutions, Baltimore, MD, USA; Department of Molecular Microbiology and Immunology, The Johns Hopkins Medical Institutions, Baltimore, MD, USA; Department of Oncology, The Johns Hopkins Medical Institutions, Baltimore, MD, USA.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 3.Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007;121:621–632. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 4.de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004;324:17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 5.Tindle RW. Immune evasion in human papillomavirus-associated cervical cancer. Nat Rev Cancer. 2002;2:59–65. doi: 10.1038/nrc700. [DOI] [PubMed] [Google Scholar]
- 6.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 7.Roden R, Wu TC. How will HPV vaccines affect cervical cancer? Nat Rev Cancer. 2006;6:753–763. doi: 10.1038/nrc1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roden RB, Monie A, Wu TC. Opportunities to improve the prevention and treatment of cervical cancer. Curr Mol Med. 2007;7:490–503. doi: 10.2174/156652407781387127. [DOI] [PubMed] [Google Scholar]
- 9.Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–1927. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 10.Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928–1943. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 11.Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006;367:1247–1255. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 12.Villa LL, Costa RL, Petta CA, Andrade RP, Ault KA, Giuliano AR, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6:271–278. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- 13.Villa LL, Ault KA, Giuliano AR, Costa RL, Petta CA, Andrade RP, et al. Immunologic responses following administration of a vaccine targeting human papillomavirus Types 6, 11, 16, and 18. Vaccine. 2006;24:5571–5583. doi: 10.1016/j.vaccine.2006.04.068. [DOI] [PubMed] [Google Scholar]
- 14.Schiller JT, Castellsague X, Villa LL, Hildesheim A. An update of prophylactic human papillomavirus L1 virus-like particle vaccine clinical trial results. Vaccine. 2008;26 Suppl 10:K53–K61. doi: 10.1016/j.vaccine.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hung CF, Ma B, Monie A, Tsen SW, Wu TC. Therapeutic human papillomavirus vaccines: current clinical trials and future directions. Expert Opin Biol Ther. 2008;8:421–439. doi: 10.1517/14712598.8.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenstone HL, Nieland JD, de Visser KE, De Bruijn ML, Kirnbauer R, Roden RB, et al. Chimeric papillomavirus virus-like particles elicit antitumor immunity against the E7 oncoprotein in an HPV16 tumor model. Proc Natl Acad Sci USA. 1998;95:1800–1805. doi: 10.1073/pnas.95.4.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng S, Frazer IH, Fernando GJ, Zhou J. Papillomavirus virus-like particles can deliver defined CTL epitopes to the MHC class I pathway. Virology. 1998;240:147–157. doi: 10.1006/viro.1997.8912. [DOI] [PubMed] [Google Scholar]
- 18.Schafer K, Muller M, Faath S, Henn A, Osen W, Zentgraf H, et al. Immune response to human papillomavirus 16 L1E7 chimeric virus-like particles: induction of cytotoxic T cells and specific tumor protection. Int J Cancer. 1999;81:881–888. doi: 10.1002/(sici)1097-0215(19990611)81:6<881::aid-ijc8>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 19.Fausch SC, Da Silva DM, Eiben GL, Le Poole IC, Kast WM. HPV protein/peptide vaccines: from animal models to clinical trials. Front Biosci. 2003;8:s81–s91. doi: 10.2741/1009. [DOI] [PubMed] [Google Scholar]
- 20.Gerard CM, Baudson N, Kraemer K, Bruck C, Garcon N, Paterson Y, et al. Therapeutic potential of protein and adjuvant vaccinations on tumour growth. Vaccine. 2001;19:2583–2589. doi: 10.1016/s0264-410x(00)00486-2. [DOI] [PubMed] [Google Scholar]
- 21.Lin CW, Lee JY, Tsao YP, Shen CP, Lai HC, Chen SL. Oral vaccination with recombinant Listeria monocytogenes expressing human papillomavirus type 16 E7 can cause tumor growth in mice to regress. Int J Cancer. 2002;102:629–637. doi: 10.1002/ijc.10759. [DOI] [PubMed] [Google Scholar]
- 22.Gunn GR, Zubair A, Peters C, Pan ZK, Wu TC, Paterson Y. Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumours immortalized by HPV-16. J Immunol. 2001;167:6471–6479. doi: 10.4049/jimmunol.167.11.6471. [DOI] [PubMed] [Google Scholar]
- 23.Jabbar IA, Fernando GJ, Saunders N, Aldovini A, Young R, Malcolm K, et al. Immune responses induced by BCG recombinant for human papillomavirus L1 and E7 proteins. Vaccine. 2000;18:2444–2453. doi: 10.1016/s0264-410x(99)00550-2. [DOI] [PubMed] [Google Scholar]
- 24.Donnelly JJ, Ulmer JB, Liu MA. DNA vaccines. Life Sci. 1997;60:163–172. doi: 10.1016/s0024-3205(96)00502-4. [DOI] [PubMed] [Google Scholar]
- 25.Gurunathan S, Klinman DM, Seder RA. DNA vaccines: immunology, application, and optimization*. Annu Rev Immunol. 2000;18:927–974. doi: 10.1146/annurev.immunol.18.1.927. [DOI] [PubMed] [Google Scholar]
- 26.Trimble C, Lin CT, Hung CF, Pai S, Juang J, He L, et al. Comparison of the CD8+ T cell responses and antitumor effects generated by DNA vaccine administered through gene gun, biojector, and syringe. Vaccine. 2003;21:4036–4042. doi: 10.1016/s0264-410x(03)00275-5. [DOI] [PubMed] [Google Scholar]
- 27.Chen CA, Chang MC, Sun WZ, Chen YL, Chiang YC, Hsieh CY, et al. Noncarrier naked antigen-specific DNA vaccine generates potent antigen-specific immunologic responses and antitumor effects. Gene Ther. 2009;16:776–787. doi: 10.1038/gt.2009.31. [DOI] [PubMed] [Google Scholar]
- 28.Luxembourg A, Evans CF, Hannaman D. Electroporation-based DNA immunisation: translation to the clinic. Expert Opin Biol Ther. 2007;7:1647–1664. doi: 10.1517/14712598.7.11.1647. [DOI] [PubMed] [Google Scholar]
- 29.Roos AK, Eriksson F, Timmons JA, Gerhardt J, Nyman U, Gudmundsdotter L, et al. Skin electroporation: effects on transgene expression, DNA persistence, local tissue environment. PLoS One. 2009;4:e7226. doi: 10.1371/journal.pone.0007226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roos AK, Eriksson F, Walters DC, Pisa P, King AD. Optimization of skin electroporation in mice to increase tolerability of DNA vaccine delivery to patients. Mol Ther. 2009;17:1637–1642. doi: 10.1038/mt.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Best SR, Peng S, Juang CM, Hung CF, Hannaman D, Saunders JR, et al. Administration of HPV DNA vaccine via electroporation elicits the strongest CD8+ T cell immune responses compared to intramuscular injection and intradermal gene gun delivery. Vaccine. 2009;27:5450–5459. doi: 10.1016/j.vaccine.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seo SH, Jin HT, Park SH, Youn JI, Sung YC. Optimal induction of HPV DNA vaccine-induced CD8(+) T cell responses and therapeutic antitumor effect by antigen engineering and electroporation. Vaccine. 2009;27:5906–5912. doi: 10.1016/j.vaccine.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 33.Yan J, Harris K, Khan AS, Draghia-Akli R, Sewell D, Weiner DB. Cellular immunity induced by a novel HPV18 DNA vaccine encoding an E6/E7 fusion consensus protein in mice and rhesus macaques. Vaccine. 2008;26:5210–5215. doi: 10.1016/j.vaccine.2008.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.VGX pharmaceuticals, Inc. Phase I of human papillomavirus (HPV) DNA plasmid (VGX-3100) + Electroporation for CIN 2 or 3 [Clinicaltrials.gov identifier NCT00685412] [Accessed 2009 October 13];US National Institutes of Health. ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov.
- 35.Mehier-Humbert S, Guy RH. Physical methods for gene transfer: improving the kinetics of gene delivery into cells. Adv Drug Deliv Rev. 2005;57:733–753. doi: 10.1016/j.addr.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 36.Zeira E, Manevitch A, Manevitch Z, Kedar E, Gropp M, Daudi N, et al. Femtosecond laser: a new intradermal DNA delivery method for efficient, long-term gene expression and genetic immunization. Faseb J. 2007;21:3522–3533. doi: 10.1096/fj.06-7528com. [DOI] [PubMed] [Google Scholar]
- 37.Tsen SW, Wu CY, Meneshian A, Pai SI, Hung CF, Wu TC. Femtosecond laser treatment enhances DNA transfection efficiency in vivo. J Biomed Sci. 2009;16:36. doi: 10.1186/1423-0127-16-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hedley ML, Curley J, Urban R. Microspheres containing plasmid-encoded antigens elicit cytotoxic T-cell responses. Nat Med. 1998;4:365–368. doi: 10.1038/nm0398-365. [DOI] [PubMed] [Google Scholar]
- 39.Klencke B, Matijevic M, Urban RG, Lathey JL, Hedley ML, Berry M, et al. Encapsulated plasmid DNA treatment for human papillomavirus 16-associated anal dysplasia: a phase I study of ZYC101. Clin Cancer Res. 2002;8:1028–1037. [PubMed] [Google Scholar]
- 40.Storrie H, Mooney DJ. Sustained delivery of plasmid DNA from polymeric scaffolds for tissue engineering. Adv Drug Deliv Rev. 2006;58:500–514. doi: 10.1016/j.addr.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 41.Sheets EE, Urban RG, Crum CP, Hedley ML, Politch JA, Gold MA, et al. Immunotherapy of human cervical high-grade cervical intraepithelial neoplasia with microparticle-delivered human papillomavirus 16 E7 plasmid DNA. Am J Obstet Gynecol. 2003;188:916–926. doi: 10.1067/mob.2003.256. [DOI] [PubMed] [Google Scholar]
- 42.Garcia F, Petry KU, Muderspach L, Gold MA, Braly P, Crum CP, et al. ZYC101a for treatment of high-grade cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol. 2004;103:317–326. doi: 10.1097/01.AOG.0000110246.93627.17. [DOI] [PubMed] [Google Scholar]
- 43.van den Berg JH, Nujien B, Beijnen JH, Vincent A, van Tinteren H, Kluge J, et al. Optimization of intradermal vaccination by DNA tattooing in human skin. Hum Gene Ther. 2009;20:181–189. doi: 10.1089/hum.2008.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pokorna D, Rubio I, Muller M. DNA-vaccination via tattooing induces stronger humoral and cellular immune responses than intramuscular delivery supported by molecular adjuvants. Genet Vaccines Ther. 2008;6:4. doi: 10.1186/1479-0556-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Su X, Kim BS, Kim SR, Hammond PT, Irvine DJ. Layer-by-Layer-assembled multilayer films for transcutaneous drug and vaccine delivery. ACS Nano. 2009 Oct 13; doi: 10.1021/nn900928u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prausnitz MR, Mikszta JA, Cormier M, Andrianov AK. Microneedle-based vaccines. Curr Top Microbiol Immunol. 2009;333:369–393. doi: 10.1007/978-3-540-92165-3_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Voltan R, Castaldello A, Brocca-Cofano E, De Michele R, Triulzi C, Altavilla G, et al. Priming with a very low dose of DNA complexed with cationic block copolymers followed by protein boost elicits broad and long-lasting antigen-specific humoral and cellular responses in mice. Vaccine. 2009;27:4498–4507. doi: 10.1016/j.vaccine.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 48.Elliott G, O’Hare P. Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell. 1997;88:223–233. doi: 10.1016/s0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- 49.Phelan A, Elliott G, O’Hare P. Intercellular delivery of functional p53 by the herpesvirus protein VP22. Nat Biotechnol. 1998;16:440–443. doi: 10.1038/nbt0598-440. [DOI] [PubMed] [Google Scholar]
- 50.Dilber MS, Phelan A, Aints A, Mohamed AJ, Elliott G, Smith CI, et al. Intercellular delivery of thymidine kinase prodrug activating enzyme by the herpes simplex virus protein, VP22. Gene Ther. 1999;6:12–21. doi: 10.1038/sj.gt.3300838. [DOI] [PubMed] [Google Scholar]
- 51.Wybranietz WA, Gross CD, Phelan A, O’Hare P, Spiegel M, Graepler F, et al. Enhanced suicide gene effect by adenoviral transduction of a VP22-cytosine deaminase (CD) fusion gene. Gene Ther. 2001;8:1654–1664. doi: 10.1038/sj.gt.3301564. [DOI] [PubMed] [Google Scholar]
- 52.Lundberg M, Johansson M. Is VP22 nuclear homing an artifact? Nat Biotechnol. 2001;19:713–714. doi: 10.1038/90741. [DOI] [PubMed] [Google Scholar]
- 53.Kim TW, Hung CF, Kim JW, Juang J, Chen PJ, He L, et al. Vaccination with a DNA vaccine encoding herpes simplex virus type 1 VP22 linked to antigen generates long-term antigen-specific CD8-positive memory T cells and protective immunity. Hum Gene Ther. 2004;15:167–177. doi: 10.1089/104303404772679977. [DOI] [PubMed] [Google Scholar]
- 54.Hung CF, Cheng WF, Chai CY, Hsu KF, He L, Ling M, et al. Improving vaccine potency through intercellular spreading and enhanced MHC class I presentation of antigen. J Immunol. 2001;166:5733–5740. doi: 10.4049/jimmunol.166.9.5733. [DOI] [PubMed] [Google Scholar]
- 55.Peng S, Trimble C, Ji H, He L, Tsai YC, Macaes B, et al. Characterization of HPV-16 E6 DNA vaccines employing intracellular targeting and intercellular spreading strategies. J Biomed Sci. 2005;12:689–700. doi: 10.1007/s11373-005-9012-3. [DOI] [PubMed] [Google Scholar]
- 56.Hung CF, He L, Juang J, Lin TJ, Ling M, Wu TC. Improving DNA vaccine potency by linking Marek’s disease virus type 1 VP22 to an antigen. J Virol. 2002;76:2676–2682. doi: 10.1128/JVI.76.6.2676-2682.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng CF, Brownlie R, Huang DY, Babiuk LA, den Hurk S. Intercellular trafficking of the major tegument protein VP22 of bovine herpesvirus-1 and its application to improve a DNA vaccine. Arch Virol. 2006;151:985–993. doi: 10.1007/s00705-005-0694-7. [DOI] [PubMed] [Google Scholar]
- 58.Zheng C, Babiuk LA, den Hurk S. Bovine herpesvirus 1 VP22 enhances the efficacy of a DNA vaccine in cattle. J Virol. 2005;79:1948–1953. doi: 10.1128/JVI.79.3.1948-1953.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Onai N, Obata-Onai A, Schmid MA, Manz MG. Flt3 in regulation of type I interferon-producing cell and dendritic cell development. Ann N Y Acad Sci. 2007;1106:253–261. doi: 10.1196/annals.1392.015. [DOI] [PubMed] [Google Scholar]
- 60.Shurin MR, Pandharipande PP, Zorina TD, Haluszczak C, Subbotin VM, Hunter O, et al. FLT3 ligand induces the generation of functionally active dendritic cells in mice. Cell Immunol. 1997;179:174–184. doi: 10.1006/cimm.1997.1152. [DOI] [PubMed] [Google Scholar]
- 61.Hung CF, Hsu KF, Cheng WF, Chai CY, He L, Ling M, et al. Enhancement of DNA vaccine potency by linkage of antigen gene to a gene encoding the extracellular domain of Fms-like tyrosine kinase 3-ligand. Cancer Res. 2001;61:1080–1088. [PubMed] [Google Scholar]
- 62.Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- 63.Pandya MJ, Bendz H, Manzenrieder F, Noessner E, Kessler H, Buchner J, et al. Interaction of human heat shock protein 70 with tumor-associated peptides. Biol Chem. 2009;390:305–312. doi: 10.1515/BC.2009.038. [DOI] [PubMed] [Google Scholar]
- 64.Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the toll/interleukin-1 receptor signal pathway. J Biol Chem. 2002;277:15107–15112. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- 65.Trimble CL, Peng S, Kos F, Gravitt P, Viscidi R, Sugar E, et al. A phase I trial of a human papillomavirus DNA vaccine for HPV16+ cervical intraepithelial neoplasia 2/3. Clin Cancer Res. 2009;15:361–367. doi: 10.1158/1078-0432.CCR-08-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Johns Hopkins University. Vaccine therapy with or without imiquimod in treating patients with grade 3 cervical intraepithelial neoplasia [ClinicalTrials.gov identifier NCT00788164] [Accessed 2009 June 4];US Natioanl Institutes of Health. ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov.
- 67.Le Borgne M, Etchart N, Goubier A, Lira SA, Sirard JC, van Rooijen N, et al. Dendritic cells rapidly recruited into epithelial tissues via CCR6/CCL20 are responsible for CD8+ T cell crosspriming in vivo. Immunity. 2006;24:191–201. doi: 10.1016/j.immuni.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 68.Kang TH, Chung JY, Monie A, Pai SI, Hung CF, Wu TC. Enhancing DNA vaccine potency by co-administration of xenogenic MHC-class-I DNA. Gene Ther. 2009 doi: 10.1038/gt.2009.152. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tseng CW, Hung CF, Alvarez RD, Trimble C, Huh WK, Kim D, et al. Pretreatment with cisplatin enhances E7-specific CD8+ T-Cell-mediated antitumor immunity induced by DNA vaccination. Clin Cancer Res. 2008;14:3185–3192. doi: 10.1158/1078-0432.CCR-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tseng CW, Monie A, Wu CY, Huang B, Wang MC, Hung CF, et al. Treatment with proteasome inhibitor bortezomib enhances antigen-specific CD8+ T-cell-mediated antitumor immunity induced by DNA vaccination. J Mol Med. 2008;86:899–908. doi: 10.1007/s00109-008-0370-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kang TH, Lee JH, Song CK, Han HD, Shin BC, Pai SI, et al. Epigallocatechin-3-gallate enhances CD8+ T cell-mediated antitumor immunity induced by DNA vaccination. Cancer Res. 2007;67:802–811. doi: 10.1158/0008-5472.CAN-06-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chuang CM, Monie A, Wu A, Hung CF. Combination of apigenin treatment with therapeutic HPV DNA vaccination generates enhanced therapeutic antitumor effects. J Biomed Sci. 2009;16:49. doi: 10.1186/1423-0127-16-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tseng CW, Monie A, Trimble C, Alvarez RD, Huh WK, Buchsbaum DJ, et al. Combination of treatment with death receptor 5-specific antibody with therapeutic HPV DNA vaccination generates enhanced therapeutic anti-tumor effects. Vaccine. 2008;26:4314–4319. doi: 10.1016/j.vaccine.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tseng CW, Trimble C, Zeng Q, Monie A, Alvarez RD, Huh WK, et al. Low-dose radiation enhances therapeutic HPV DNA vaccination in tumor-bearing hosts. Cancer Immunol Immunother. 2009;58:737–748. doi: 10.1007/s00262-008-0596-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stacey SN, Jordan D, Williamson AJ, Brown M, Coote JH, Arrand JR. Leaky scanning is the predominant mechanism for translation of human papillomavirus type 16 E7 oncoprotein from E6/E7 bicistronic mRNA. J Virol. 2000;74:7284–7297. doi: 10.1128/jvi.74.16.7284-7297.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin CT, Tsai YC, He L, Calizo R, Chou HH, Chang TC, et al. A DNA vaccine encoding a codon-optimized human papillomavirus type 16 E6 gene enhances CTL response and anti-tumor activity. J Biomed Sci. 2006;13:481–488. doi: 10.1007/s11373-006-9086-6. [DOI] [PubMed] [Google Scholar]
- 77.Cid-Arregui A, Juarez V, zur Hausen H. A synthetic E7 gene of human papillomavirus type 16 that yields enhanced expression of the protein in mammalian cells and is useful for DNA immunization studies. J Virol. 2003;77:4928–4937. doi: 10.1128/JVI.77.8.4928-4937.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yan J, Reichenbach DK, Corbitt N, Hokey DA, Ramanathan MP, McKinney KA, et al. Induction of antitumor immunity in vivo following delivery of a novel HPV-16 DNA vaccine encoding an E6/E7 fusion antigen. Vaccine. 2009;27:431–440. doi: 10.1016/j.vaccine.2008.10.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hirasawa Y, Arai M, Imazeki F, Tada M, Mikata R, Fukai K, et al. Methylation status of genes upregulated by demethylating agent 5-aza-2′-deoxycytidine in hepatocellular carcinoma. Oncology. 2006;71:77–85. doi: 10.1159/000100475. [DOI] [PubMed] [Google Scholar]
- 80.Arai M, Imazeki F, Sakai Y, Mikata R, Tada M, Seki N, et al. Analysis of the methylation status of genes up-regulated by the demethylating agent, 5-aza-2′-deoxycytidine, in esophageal squamous cell carcinoma. Oncol Rep. 2008;20:405–412. [PubMed] [Google Scholar]
- 81.Lu D, Hoory T, Monie A, Wu A, Wang MC, Hung CF. Treatment with demethylating agent, 5-aza-2′-deoxycytidine enhances therapeutic HPV DNA vaccine potency. Vaccine. 2009;27:4363–4369. doi: 10.1016/j.vaccine.2009.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim D, Hoory T, Monie A, Ting JP, Hung CF, Wu TC. Enhancement of DNA vaccine potency through coadministration of CIITA DNA with DNA vaccines via gene gun. J Immunol. 2008;180:7019–7027. doi: 10.4049/jimmunol.180.10.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grant EP, Michalek MT, Goldberg AL, Rock KL. Rate of antigen degradation by the ubiquitin-proteasome pathway influences MHC class I presentation. J Immunol. 1995;155:3750–3758. [PubMed] [Google Scholar]
- 84.Massa S, Simeone P, Muller A, Benvenuto E, Venuti A, Franconi R. Antitumor activity of DNA vaccines based on the human papillomavirus-16 E7 protein genetically fused to a plant virus coat protein. Hum Gene Ther. 2008;19:354–364. doi: 10.1089/hum.2007.122. [DOI] [PubMed] [Google Scholar]
- 85.Hung CF, Cheng WF, He L, Ling M, Juang J, Lin CT, et al. Enhancing major histocompatibility complex class I antigen presentation by targeting antigen to centrosomes. Cancer Res. 2003;63:2393–2398. [PubMed] [Google Scholar]
- 86.Smahel M, Polakova I, Pokorna D, Ludvikova V, Duskova M, Vlasak J. Enhancement of T cell-mediated and humoral immunity of beta-glucuronidase-based DNA vaccines against HPV16 E7 oncoprotein. Int J Oncol. 2008;33:93–101. [PubMed] [Google Scholar]
- 87.Cheng WF, Hung CF, Chai CY, Hsu KF, He L, Ling M, et al. Tumor-specific immunity and antiangiogenesis generated by a DNA vaccine encoding calreticulin linked to a tumor antigen. J Clin Invest. 2001;108:669–678. doi: 10.1172/JCI12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim JW, Hung CF, Juang J, He L, Kim TW, Armstrong DK, et al. Comparison of HPV DNA vaccines employing intracellular targeting strategies. Gene Ther. 2004;11:1011–1018. doi: 10.1038/sj.gt.3302252. [DOI] [PubMed] [Google Scholar]
- 89.Kim D, Gambhira R, Karanam B, Monie A, Hung CF, Roden R, et al. Generation and characterization of a preventive and therapeutic HPV DNA vaccine. Vaccine. 2008;26:351–360. doi: 10.1016/j.vaccine.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hung CF, Cheng WF, Hsu KF, Chai CY, He L, Ling M, et al. Cancer immunotherapy using a DNA vaccine encoding the translocation domain of a bacterial toxin linked to a tumor antigen. Cancer Res. 2001;61:3698–3703. [PubMed] [Google Scholar]
- 91.Lin CT, Chang TC, Shaw SW, Cheng PJ, Huang CT, Chao A, et al. Maintenance of CD8 effector T cells by CD4 helper T cells eradicates growing tumors and promotes long-term tumor immunity. Vaccine. 2006;24:6199–6207. doi: 10.1016/j.vaccine.2006.05.108. [DOI] [PubMed] [Google Scholar]
- 92.Castellino F, Germain RN. Cooperation between CD4+ and CD8+ T cells: when, where, and how. Annu Rev Immunol. 2006;24:519–540. doi: 10.1146/annurev.immunol.23.021704.115825. [DOI] [PubMed] [Google Scholar]
- 93.Wu TC, Guarnieri FG, Staveley-O’Carroll KF, Viscidi RP, Levitsky HI, Hedrick L, et al. Engineering an intracellular pathway for major histocompatibility complex class II presentation of antigens. Proc Natl Acad Sci USA. 1995;92:11671–11675. doi: 10.1073/pnas.92.25.11671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ji H, Wang TL, Chen CH, Pai SI, Hung CF, Lin KY, et al. Targeting human papillomavirus type 16 E7 to the endosomal/lysosomal compartment enhances the antitumor immunity of DNA vaccines against murine human papillomavirus type 16 E7-expressing tumors. Hum Gene Ther. 1999;10:2727–2740. doi: 10.1089/10430349950016474. [DOI] [PubMed] [Google Scholar]
- 95.Huang CH, Peng S, He L, Tsai YC, Boyd DA, Hansen TH, et al. Cancer immunotherapy using a DNA vaccine encoding a single-chain trimer of MHC class I linked to an HPV-16 E6 immunodominant CTL epitope. Gene Ther. 2005;12:1180–1186. doi: 10.1038/sj.gt.3302519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim TW, Hung CF, Ling M, Juang J, He L, Hardwick JM, et al. Enhancing DNA vaccine potency by coadministration of DNA encoding antiapoptotic proteins. J Clin Invest. 2003;112:109–117. doi: 10.1172/JCI17293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cheng WF, Chang MC, Sun WZ, Lee CN, Lin HW, Su YN, et al. Connective tissue growth factor linked to the E7 tumor antigen generates potent antitumor immune responses mediated by an anti-apoptotic mechanism. Gene Ther. 2008;15:1007–1016. doi: 10.1038/gt.2008.25. [DOI] [PubMed] [Google Scholar]
- 98.Kim TW, Lee JH, He L, Boyd DA, Hardwick JM, Hung CF, et al. Modification of professional antigen-presenting cells with small interfering RNA in vivo to enhance cancer vaccine potency. Cancer Res. 2005;65:309–316. [PubMed] [Google Scholar]
- 99.Huang B, Mao CP, Peng S, Hung CF, Wu TC. RNA interference-mediated in vivo silencing of Fas ligand as a strategy for the enhancement of DNA vaccine potency. Hum Gene Ther. 2008;19:763–773. doi: 10.1089/hum.2007.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hung CF, Tsai YC, He L, Wu TC. DNA vaccines encoding Ii-PADRE generates potent PADRE-specific CD4+ T-cell immune responses and enhances vaccine potency. Mol Ther. 2007;15:1211–1219. doi: 10.1038/sj.mt.6300121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cresswell P. Assembly, transport, and function of MHC class II molecules. Annu Rev Immunol. 1994;12:259–293. doi: 10.1146/annurev.iy.12.040194.001355. [DOI] [PubMed] [Google Scholar]
- 102.Kim D, Monie A, He L, Tsai YC, Hung CF, Wu TC. Role of IL-2 secreted by PADRE-specific CD4+ T cells in enhancing E7-specific CD8+ T-cell immune responses. Gene Ther. 2008;15:677–687. doi: 10.1038/sj.gt.3303102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim D, Hoory T, Wu TC, Hung CF. Enhancing DNA vaccine potency by combining a strategy to prolong dendritic cell life and intracellular targeting strategies with a strategy to boost CD4+ T cell. Hum Gene Ther. 2007;18:1129–1139. doi: 10.1089/hum.2007.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim D, Monie A, Tsai YC, He L, Wang MC, Hung CF, et al. Enhancement of CD4+ T-cell help reverses the doxorubicin-induced suppression of antigen-specific immune responses in vaccinated mice. Gene Ther. 2008;15:1176–1183. doi: 10.1038/gt.2008.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ohlschlager P, Quetting M, Alvarez G, Durst M, Gissmann L, Kaufmann AM. Enhancement of immunogenicity of a therapeutic cervical cancer DNA-based vaccine by co-application of sequence-optimized genetic adjuvants. Int J Cancer. 2009;125:189–198. doi: 10.1002/ijc.24333. [DOI] [PubMed] [Google Scholar]
- 106.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4(7):499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 107.Larange A, Antonios D, Pallardy M, Kerdine-Romer S. TLR7 and TLR8 agonists trigger different signaling pathways for human dendritic cell maturation. J Leukoc Biol. 2009;85:673–683. doi: 10.1189/jlb.0808504. [DOI] [PubMed] [Google Scholar]
- 108.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 109.Kojima Y, Xin KQ, Ooki T, Hamajima K, Oikawa T, Shinoda K, et al. Adjuvant effect of multi-CpG motifs on an HIV-1 DNA vaccine. Vaccine. 2002;20:2857–2865. doi: 10.1016/s0264-410x(02)00238-4. [DOI] [PubMed] [Google Scholar]
- 110.Alexander WS. Suppressors of cytokine signalling (SOCS) in the immune system. Nat Rev Immunol. 2002;2:410–416. doi: 10.1038/nri818. [DOI] [PubMed] [Google Scholar]
- 111.Song XT, Evel-Kabler K, Rollins L, Aldrich M, Gao F, Huang XF, et al. An alternative, effective HIV vaccination approach based on inhibition of antigen presentation attenuators in dendritic cells. PLoS Med. 2006;3:e11. doi: 10.1371/journal.pmed.0030011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Petrausch U, Poehlein CH, Jensen SM, Twitty C, Thompson JA, Assmann I, et al. Cancer immunotherapy: the role regulatory T cells play and what can be done to overcome their inhibitory effects. Curr Mol Med. 2009;9:673–682. doi: 10.2174/156652409788970670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Piersma SJ, Jordanova ES, van Poelgeest MI, Kwappenberg KM, van der Hulst JM, Drijfhout JW, et al. High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Res. 2007;67:354–361. doi: 10.1158/0008-5472.CAN-06-3388. [DOI] [PubMed] [Google Scholar]
- 114.Molling JW, de Gruijl TD, Glim J, Moreno M, Rozendaal L, Meijer CJ, et al. CD4(+)CD25hi regulatory T-cell frequency correlates with persistence of human papillomavirus type 16 and T helper cell responses in patients with cervical intraepithelial neoplasia. Int J Cancer. 2007;121:1749–1755. doi: 10.1002/ijc.22894. [DOI] [PubMed] [Google Scholar]
- 115.Beyer M, Schultze JL. Regulatory T cells in cancer. Blood. 2006;108:804–811. doi: 10.1182/blood-2006-02-002774. [DOI] [PubMed] [Google Scholar]
- 116.Nair S, Boczkowski D, Fassnacht M, Pisetsky D, Gilboa E. Vaccination against the fork head family transcription factor Foxp3 enhances tumor immunity. Cancer Res. 2007;67:371–380. doi: 10.1158/0008-5472.CAN-06-2903. [DOI] [PubMed] [Google Scholar]
- 117.Chuang CM, Hoory T, Monie A, Wu A, Wang MC, Hung CF. Enhancing therapeutic HPV DNA vaccine potency through depletion of CD4+ CD25+ T regulatory cells. Vaccine. 2009;27:684–689. doi: 10.1016/j.vaccine.2008.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen CH, Wang TL, Hung CF, Yang Y, Young RA, Pardoll DM, et al. Enhancement of DNA vaccine potency by linkage of antigen gene to an HSP70 gene. Cancer Res. 2000;60:1035–1042. [PubMed] [Google Scholar]
- 119.Wlazlo AP, Deng H, Giles-Davis W, Ertl HC. DNA vaccines against the human papillomavirus type 16 E6 or E7 oncoproteins. Cancer Gene Ther. 2004;11:457–464. doi: 10.1038/sj.cgt.7700723. [DOI] [PubMed] [Google Scholar]
- 120.Rittich S, Duskova M, Mackova J, Pokorna D, Jinoch P, Smahel M. Combined immunization with DNA and transduced tumor cells expressing mouse GM-CSF or IL-2. Oncol Rep. 2005;13:311–317. [PubMed] [Google Scholar]
- 121.Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51–81. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]