Abstract
Assembly of the C(1-27) macrocyclic skeleton of rimocidinolide, the aglycone of (+)-rimocidin (1), has been achieved in convergent fashion. Key features of the synthetic strategy entail application of multicomponent Type 1 Anion Relay Chemistry (ARC), in conjunction with the SN2/SN2' reaction manifolds of vinyl epoxides, both employing 2-susbtituted-1,3-dithianes to construct the C(1-19) carbon backbone. Yamaguchi union of a C(20-27) vinyl borate ester, possessing the all trans triene, with an advanced C(1-19) vinyl iodide followed by macrocyclization via Suzuki-Miyaura cross-coupling completed construction of the C(1-27) rimocidinolide skeleton.
Introduction
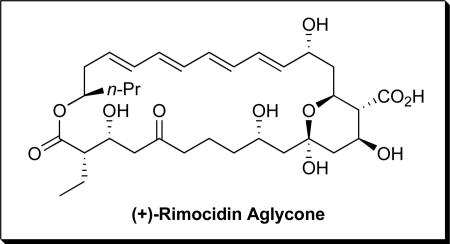
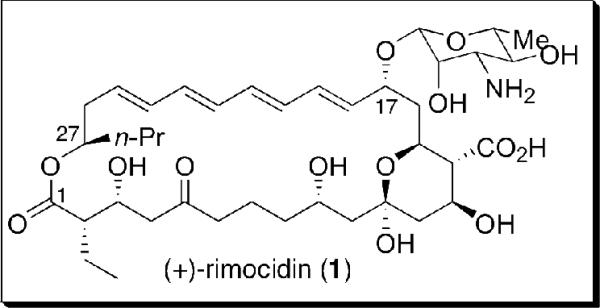
Rimocidin (Figure 1) member of a family of clinically important polyene macrolide antifungals first isolated in 1951 from the fermentation broths of Streptomyces rimosus,i embodies an architecturally interesting 28-membered macrocyclic skeleton comprised of an all trans tetraene, an internal hemi-ketal and nine stereogenic centers. Related members of this antifungal family include (+)-amphotericin B,ii nystatiniii and (+)-candidin.iv Structural studies of (+)-rimocidin (1), initially carried out in the laboratory of Copev and coworkers, established the absolute configuration of the C(27) stereocenter, as well as the presence of a conjugated tetraene. Subsequent studies, first by the Rinehartvi group and then Borowski and coworkers,vii led to assignment of the complete structure including relative and absolute stereochemistry.
Figure 1.
(+)-Rimocidin
Early interest in this class of architecturally complex natural products by the synthetic community resulted in pioneering contributions from the Nicolaouviii and Masamuneix groups, with the total synthesis of (+)-amphotericin B and the aglycone amphoterinolide B respectively. More recently, Rychnovsky and coworkers disclosed the utility of their elegant cyanohydrin acetonide union tactic with the total synthesis of rimocidinolide methyl ester.x We also became intrigued with the rimocidin aglycone, specifically as a vehicle to demonstrate the utility of what we now term Anion Relay Chemistry (ARC),xi a multicomponent reaction sequence, and the SN2/SN2' reaction manifolds of vinyl epoxides with 2-substituted-1,3-dithianes, xii two effective tactics recently introduced by our laboratory.
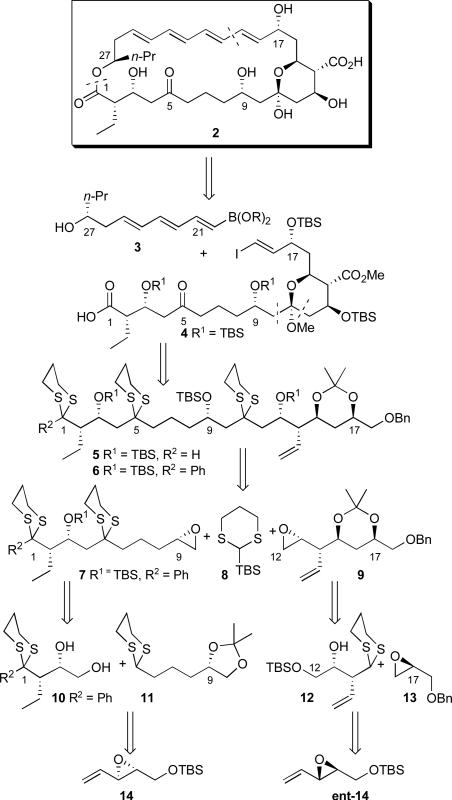
Synthetic Plan From the retro-synthetic perspective, disconnection of the (+)-rimocidin aglycone (2) at the C(19-20) and C(1-27) bonds reveals a C(1-19) carboxylic acid (4) and a C(20-27) vinyl borate ester (3), the latter possessing an all trans triene (Scheme 1). Access to the macrocycle was envisioned to entail union of 3 with 4 either via esterification followed by an intramolecular Suzuki-Miyaura cross-coupling reactionxiii or the reverse. Oxidation and protecting group reorganizations of 4 in the retrosynthetic sense, including removal of the (C18-19) vinyl iodide, reveal advanced tris-dithiane 6, which we envision would derive via what we now term the multicomponent Type 1 ARC tactic11m involving epoxides 7 and 9, employing 2-tert-butyldimethylsilyl-1,3-dithiane (8) as the bifunctional linchpin. In our original synthetic plan reported in 200311f the C(1) comprised a terminal dithiane moiety (cf. 5); however as presented in that report, the aldehydic proton was replaced with a phenyl group (cf. 6), with the expectation of eventual access to the requisite C(1) carboxylic acid via Baeyer-Villigerxiv oxidation followed by hydrolysis. As will be presented here, a further modification of the C(1) terminus would be required (vide infra). Access to advanced epoxides 7 and 9 in turn was envisioned to rely on the reactivity of the SN2 and SN2' manifolds of vinyl epoxide enantiomers 14 and ent-14 with 2-lithio-1,3-dithiane derivatives11f, thus lending a sense of symmetry to the overall synthetic plan.
Scheme 1.
Retrosynthetic Analysis of (+)-Rimocidin Aglycone 2
Results and Discussion
Execution of the First Synthetic Strategy: Construction of Vinyl Epoxide (+)-14 and the Antipode (−)-14
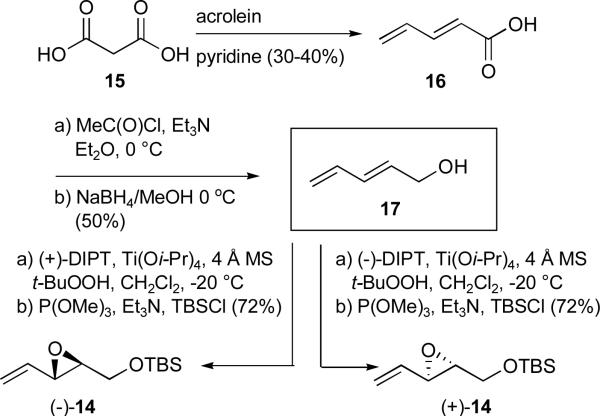
Although both epoxides could be readily prepared via asymmetric Sharpless epoxidationxv of commercially available 1,4-pentadien-3-ol, followed by Payne rearrangementxvi and TBS protection of the resulting primary alcohol, the cost of such an approach was prohibitive. An alternative method that proved both scalable and considerably less expensive entailed the known condensation of malonic acid (15) with acrolein to furnish (E)-2,4-pentadieneoic acid (16; Scheme 2),xvii followed by reduction to the corresponding alcohol and a “one-flask” Sharpless epoxidation/TBS protection protocol.xviii Absolute stereochemical assignments for (+)- and (−)-14 were based on the Sharpless model, in conjunction with subsequent X-ray analysis of an advanced intermediate.xix Employing this route over 50 g of (+)-14 and over 190 g of (−)-14 were prepared.
Scheme 2.
Preparation of vinyl epoxides (+)- and (−)-14 via Sharpless asymmetric epoxidation
Elaboration of Vinyl Epoxide (+)-14 and the Antipode Exploiting the SN2/SN2' Reaction Manifolds of Vinyl Epoxides: Construction of Epoxide 7
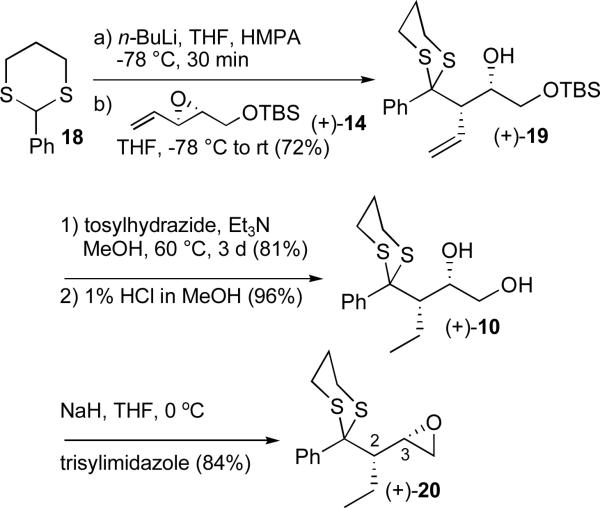
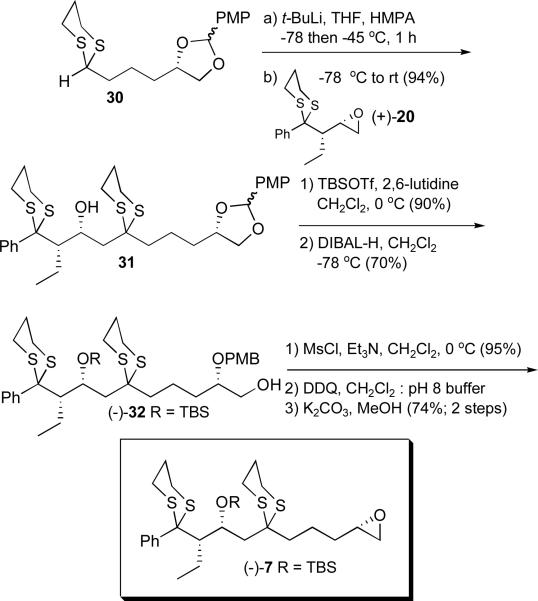
With both vinyl epoxide enantiomers in hand, we turned first to access epoxide 7. In what would prove to be the first of six planned dithiane coupling reactions, lithiation of 2-phenyl-1,3-dithiane (18), followed by treatment with (+)-14, as anticipated,12 furnished the SN2 product (+)-19 in good yield (Scheme 3). As previously reported, the phenyl substituent behaves as a small group providing the desired homoallylic alcohol.12 In a modification of the previous published synthetic sequence,11f the olefin was next reduced using tosylhydrazidexx rather than trisylhydrazide,xxi since on a larger scale the latter requires additional expenses in conjunction with both careful handling and storage. Treatment with methanolic HCl (1%) followed by the Fraser-Reid epoxidationxxii led to (+)-20. The overall yield for the six-step sequence was 17%. Conformation of the anticipated syn relationship between the ethyl and the C(3) hydroxy substituents was achieved via single crystal X-ray analysis of (+)-20.19
Scheme 3.
Construction of epoxide (+)-20 employing the SN2 reaction manifold of vinyl epoxides with 1,3-dithianes
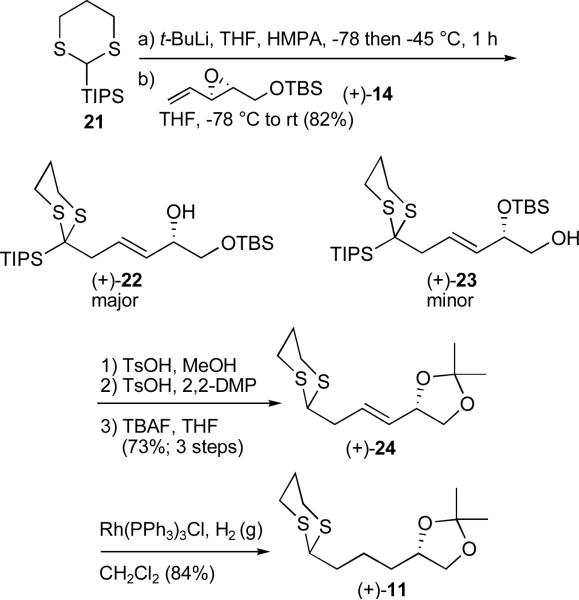
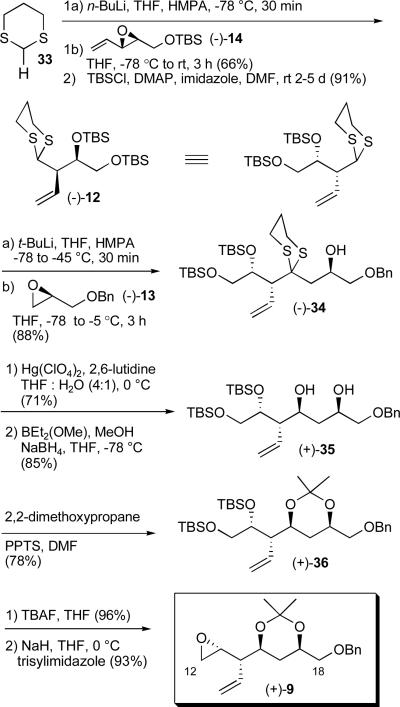
For construction of dithiane 11, reaction of vinyl epoxide (+)-14 with 1,3-dithiane 21, now carrying the sterically demanding TIPS group led to the SN2' product.12 A mixture of primary and secondary silyl ethers, (+)-22 and (+)-23, resulted due to silyl group migration (Scheme 4). Pleasingly, the reaction was both efficient and amenable to large scale. Acid mediated removal of the TBS group in the presence of the TIPS group employing p-toluenesulfonic acid next provided the corresponding diol which in turn was converted to the acetonide using 2,2-dimethoxypropane (2,2-DMP) and then treated with TBAF to furnish alkene (+)-24. Hydrogenation of the olefin promoted by the Wilkinson catalystxxiii completed the construction of dithiane (+)-11, with an overall yield for the six steps of 18%.
Scheme 4.
Construction of dithiane (+)-11 employing the SN2' reaction manifold of vinyl epoxides with 1,3-dithianes
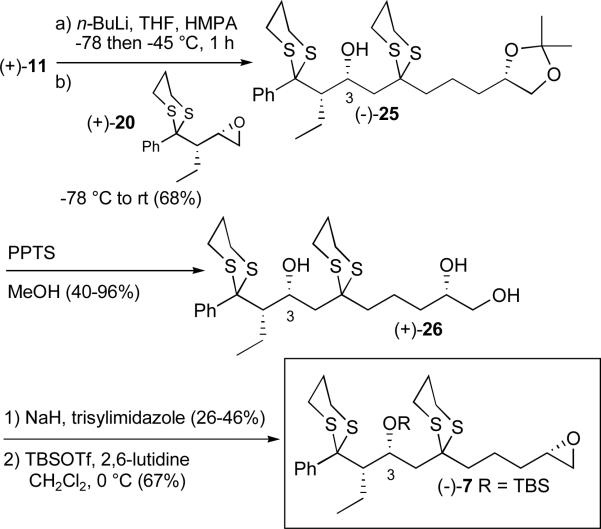
With both the epoxide and dithiane fragments in hand their union was achieved via lithiation of (+)-11 employing n-butyllithium followed by addition of (+)-20 (Scheme 5). Bis-dithiane (−)-25 comprising the C(1-10) (+)-rimocidin aglycone backbone was obtained in good yield. At this stage, we envisioned that protection of the C(3) oxygen prior to removal of the acetonide would lead to the corresponding diol. This transformation however proved problematic due to the lability of the C(3) TBS ether. Performing these transformations in revised order, that is removal of the acetonide to furnish triol (+)-26 prior to epoxide formation and then protection of the secondary alcohol led to the desired epoxide (−)-7, albeit the yield was modest. In addition, on preparative scale, removal of the acetonide proved highly variable, with epoxide formation remaining inefficient. We therefore chose to replace the acetonide with a more readily removable protecting group.
Scheme 5.
Initial synthesis of advanced epoxide (−)-7
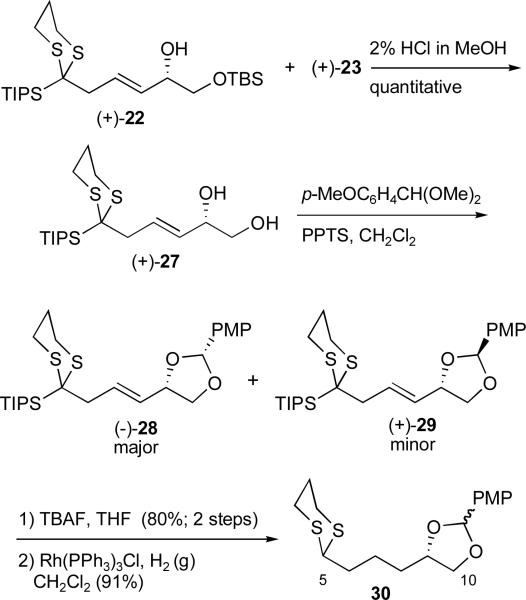
We selected a p-methoxybenzylidene (PMP) group based on two advantages. First, PMP acetals are more acid labile than acetonides, benefiting from a resonance stabilized oxocarbenium intermediate during the hydrolysis.xxiv Second, removal via a two-step protocol involving reduction to the PMB ether and oxidative removalxxv serve as backup, in the event that the hydrolysis proved problematic. To arrive at the PMP acetal, the SN2' adduct mixture of (+)-22 and (+)-23 was treated with HCl in methanol (2%) to provide diol (+)-27 in near quantitative yield (Scheme 6). Formation of the acetal then proceeded smoothly to furnish a diastereomeric mixture of (−)-28 and (+)-29 (2:1) at the acetal carbon. The acetals were readily separable via column chromatography and the relative stereochemistries determined by 1-D NMR nOe studies. In practice however, the diastereomeric mixture was carried forward without separation. Treatment with TBAF followed by hydrogenation in the presence of the Wilkinson catalyst23 then furnished the modified dithiane (30) as a mixture of diastereomers.
Scheme 6.
Preparation of the revised C(5-10) dithiane 30
Pleasingly, the anion derived from dithiane 30 proved to be an excellent nucleophile. Lithiation with tert-butyllithium, followed by addition of epoxide (+)-20 furnished 31 in 94% yield (Scheme 7). In previously unpublished studies we observed that (−)-7 was not stable to silica gel chromatography without Et3N buffering. A two-step acetal removal protocol was therefore developed. First, the secondary hydroxy group in 31 was protected as the TBS ether and the acetal reduced with DIBAL to provide the PMB ether (−)-32. Mesylation of the primary hydroxy group then proceeded in near quantitative yield. Oxidative removal of the PMB ether25 and exposure to K2CO3 in methanol completed construction of advanced coupling epoxide (−)-7 in 41% overall yield from 30. From the perspective of material advancement, the K2CO3/MeOH reaction proved very clean; moreover the resulting epoxide could be purified rapidly using silica gel buffered with Et3N. While this reaction sequence is slightly longer (cf. Scheme 5), the increase in overall yield represented a significant improvement.
Scheme 7.
An improved route for the preparation of epoxide (−)-7
Construction of Epoxide 9: A Third Application of the SN2/SN2' Reaction Manifold
With a scalable route to (−)-7 available, we initiated construction of epoxide 9, the second epoxide required for the multicomponent ARC tactic. Here an SN2 reaction of the lithiated anion derived from 1,3-dithiane 33 with vinyl epoxide (−)-14 was employed (Scheme 8). This transformation could be performed on a 50 gram scale to provide the desired adduct in 66% yield. However, due to silyl migration, the product was again a mixture of primary and secondary silyl ethers (2.4:1). Protection of the free hydroxy group was initially achieved with TBSOTf.11f However, the lower cost and ease of operation employing TBSCl was viewed more desirable. Thus, treatment of the mixture of alcohols with DMAP, imidazole and TBSCl in freshly distilled DMF furnished (−)-12. Lithiation followed by addition of benzyl-(R)-(−)- glycidyl ether (13) then furnished (−)-34 in 88% yield. This reaction proved highly reliable and was successfully performed on large scale (ca. 35-40g). Removal of the dithiane moiety, followed by β–hydroxy directed reduction of the resulting ketone next led to syn diol (+)-35,xxvi which upon formation of the acetonide, removal of the TBS groups with TBAF, and Fraser-Reid epoxide generation22 completed construction of advanced epoxide (+)-9 in ten steps from 2,4-pentadienoic acid 16, with an overall yield of 8.0%.
Scheme 8.
Construction of advanced epoxide (+)-9
Application of Type I Multicomponent Anion Relay Chemistry (ARC): Construction of Tris-Dithiane (+)-6
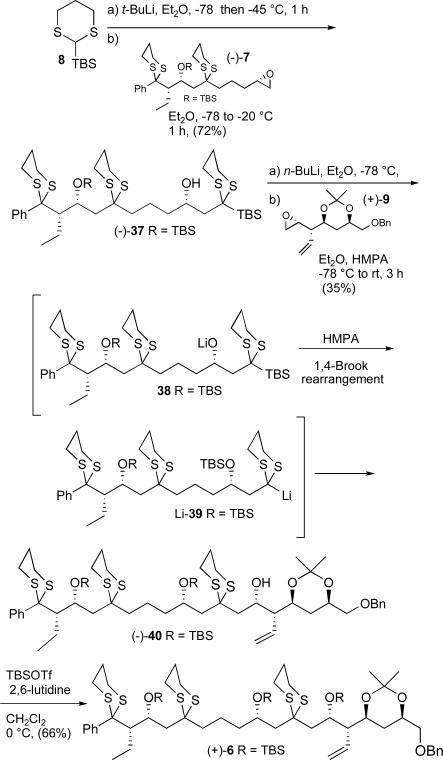
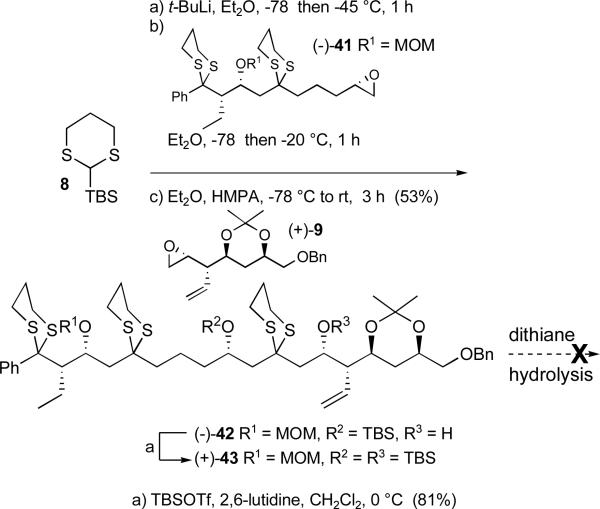
With reliable routes to epoxides (−)-7 and (+)-9 achieved, we turned to the Type I multicomponent ARC tactic.11d-g As found appropriate in several earlier cases, we first examined the individual steps of the ARC process. Addition of epoxide (−)-7 to the lithiated anion derived from 2-TBS-1,3-dithiane (8) proceeded smoothly to furnish bis-dithiane (−)-37 in 72% yield (Scheme 9). The second reaction in the ARC protocol calls for 1,4-Brook rearrangementxxvii of oxyanion 38, derived in this case via deprotonation of (−)-37, followed by reaction with a second electrophile (+)-9, in a solution of Et2O and HMPA, a solvent system which triggers the 1,4-Brook rearrangement. Unfortunately, the intermediate dithiane anion 39 did not react readily with (+)-9, furnishing the tris-dithiane (−)-40 in only modest yield (ca. 35%). The major product (−)-39 resulted from protonation of the intermediate rearranged lithiated anion 39. Not withstanding the modest yield of (−)-40, treatment with TBSOTf and 2,6-lutidine led to the fully protected adduct (+)-6 possessing the C(1-18) carbon backbone of the rimocidin aglycone. The linchpin union of 8 to (−)-7 and (+)-9 thus served as the last of six dithiane coupling reactions in the synthetic sequence to the aglycone skeleton.
Scheme 9.
Step-wise construction of tris-dithiane (+)-6
At this point, completion of the C(1-18) fragment required removal of the dithiane moieties to reveal the corresponding triketone, followed by hydrolysis of the acetonide with concomitant mixed ketal formation, and chemo- and regioselective Baeyer-Villiger oxidation14 at the C(1) carbonyl. Unfortunately, removal of the dithianes proved difficult in the extreme. A number of well known protocols [cf. tristrifluoracetoxyiodobenzene,xxviii mercury(II) salts, CH3I, I2 CANxxix and NCS/AgNO3xxx] in most cases led only to a complex mixture of products.
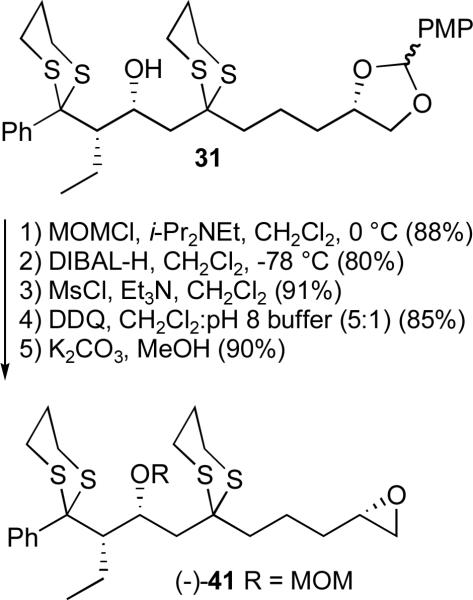
Postulating that steric congestion between the C(1) dithiane, the C(3) silyl ether, and the C(5) dithiane might contribute to the observed difficulties, the C(1-10) epoxide was re-designed by replacement of the large silyl group with a smaller MOM protecting group. The overall yield for the five-step sequence to (−)-41 (Scheme 10) was 49%.
Scheme 10.
Preparation of revised advanced epoxide (−)-41
The new epoxide (−)-41 proved to be a competent electrophile for the ARC Type I protocol, furnishing the desired tris-dithiane (−)-42 in 53% yield (Scheme 11). Protection of the C(9) hydroxy with TBSOTf then provided hydrolysis substrate (+)-43. Removal of the dithianes again proved difficult: use of harsh reagents such as Hg(ClO4)2 led to decomposition, whereas milder reagents such as Dess-Martin periodinanexxxi and 2-iodobenzoic acid (IBX),xxxii known to hydrolyze selectively aryl and allyl dithianes in the presence of alkyl dithianes, resulted in no reaction.
Scheme 11.
One-flask Type 1 multicomponent ARC union to provide tris-dithiane (+)-43
A Second Generation Strategy
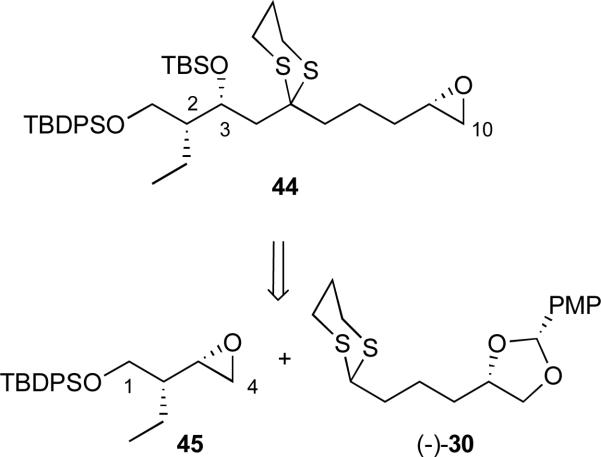
Based on the above results, a modified approach to the (+)-rimocidin aglycone was required. We reasoned that replacing either the C(1) or the C(5) dithiane with a moiety that could later be converted to the required functional group would be appropriate. A different ketone surrogate to replace the C(5) dithiane was not viewed feasible, without significantly altering the overall synthetic scheme. However, replacement of the C(1) dithiane with a primary hydroxy group that at a late stage could be converted to the required carboxylic acid would have minimal impact on the synthetic sequence, since only the C(1-4) epoxide would require revision (Scheme 12). The requisite C(1-10) fragment 44 was envisioned to arise via addition of epoxide 45, to the anion derived from dithiane (−)-30 (vide infra).
Scheme 12.
Retrosynthetic analysis revisited: C(1-10) epoxide
Execution of the Second Generation Strategy
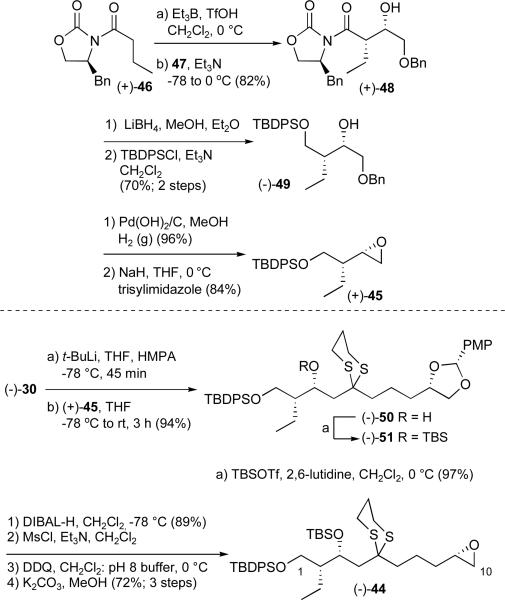
To construct epoxide 45 we began with an Evans aldol reactionxxxiii between known oxazolidinone (+)-46xxxiv and benzyloxyacetaldehyde (47); the reaction proceeded in good yield with excellent selectivity (Scheme 13). Reductive removal of the auxiliary and protection of the primary alcohol furnished alcohol (−)-49, which upon removal of the benzyl ether via hydrogenolysis, followed by epoxide formation led to epoxide (+)-45 in 47% yield for the five steps. Pleasingly, lithiation of (−)-30 followed by addition of (+)-45 afforded the coupled product (−)-50 in 94% yield; the latter was converted to the required advanced epoxide (−)-44 in five-steps (62% overall yield), employing the same reaction sequence as employed for (−)-7 in Scheme 7.
Scheme 13.
Construction of the revised C(1 10) advanced epoxide (−)-44
Construction of the Second-Generation C(1-18) Linear Fragment
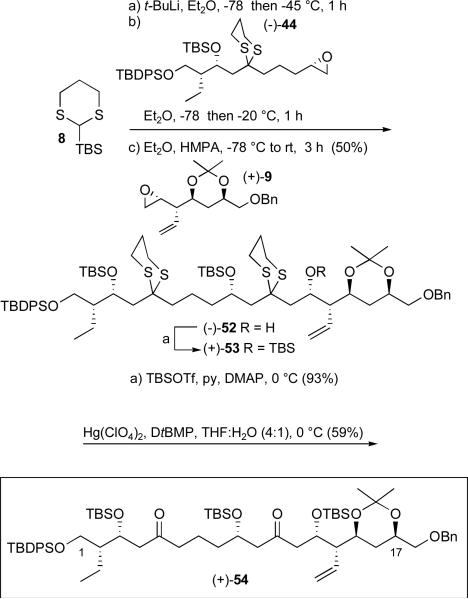
With both epoxides available we turned to the three component ARC Type I union. Lithiation of 2-TBS-1,3-dithiane (8) was followed by addition of (−)-44 dissolved in Et2O −78 °C. After stirring at −20 °C for one hour, the reaction was re-cooled to −78 °C and (+)-9 added as a solution in THF with HMPA, a solvent system known to trigger the 1,4-Brook rearrangement (Scheme 14). The desired three-component coupling product (−)-52 was isolated in 50% yield. While the ARC union was viewed as a strategic level transformation, the required dithiane hydrolysis would also prove crucial for further synthetic advancement. Towards this end, (−)-52 was treated with TBSOTf to furnish the fully protected bis-dithiane (+)-53, which when subjected to Hg(ClO4)2 and 2,6-di-tert-butyl-4-methyl pyridine (DtBMP)xxxv furnished diketone (+)-54 in 59% yield.
Scheme 14.
The Type 1 ARC tactic and dithiane hydrolysis to reveal diketone (+)-54
Elaboration of the C(11-16) Tetrahydropyran Mixed Methyl Ketal
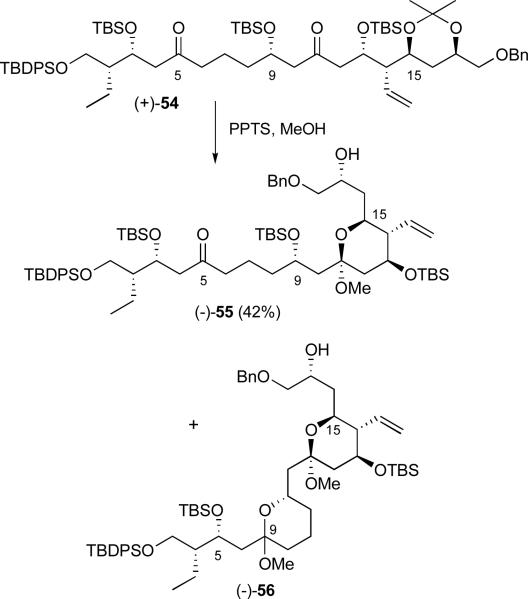
Construction of the C(11-16) tetrahydropyran hemiketal next called for removal of the acetonide from (+)-54. To this end, treatment of (+)-54 with PPTS in methanol furnished mixed methyl ketal (−)-55, albeit in modest yield along with 15% recovered diketone (+)-54 (Scheme 15). Extended reaction times only led to significant formation of the bis-ketal (−)-56, resulting from loss of the C(9) TBS group followed by cyclization at C(5). A number of related hydrolysis conditions (cf. HCl in methanol, Dowex, CSA, p-toluene sulfonic acid) were examined without significant improvement.
Scheme 15.
Acetonide hydrolysis
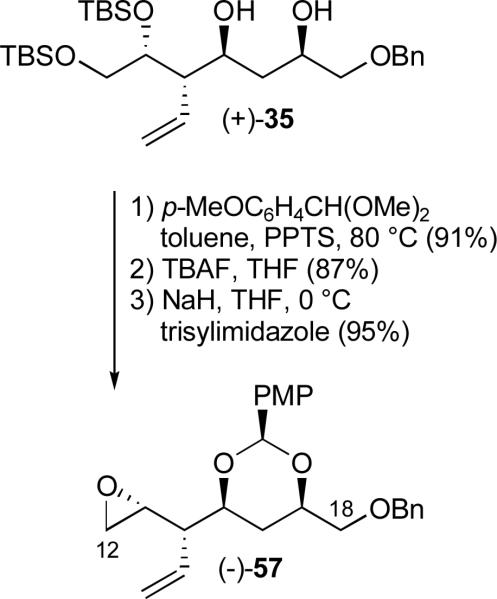
Given the modest overall yield of (−)-55, in conjunction with the need for material advancement, an alternative diol protecting group was introduced at the level of diol (+)-35 (Scheme 8). The PMP benzylidene acetal was again selected to facilitate the eventual hydrolysis process (vide infra). Preparation of epoxide (−)-57 was straightforward, requiring three-steps from (+)-35 (Scheme 16).
Scheme 16.
Preparation of advanced epoxide (−)-57
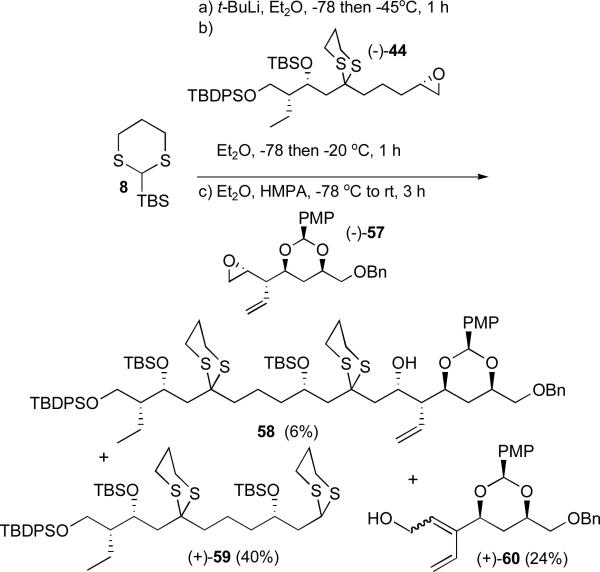
Revised epoxide (−)-57 was then subjected to the Type 1 ARC reaction conditions employed to unite successfully epoxides (+)-9 with (−)-41 (Scheme 11) and (−)-44 (Scheme 14). The results in this case were however disappointing; adduct 58 was obtained in only 6% yield (Scheme 17). The major product (40%) derived from initial dithiane addition followed by proton capture. In addition, diene (+)-60 was produced in 24%. The presence of diene (+)-60 suggested that the dithiane anion [Li-59] rather than acting as a nucleophile, deprotonated the allylic position of epoxide (−)-57 thereby leading, via ring opening, to diene (+)-60.
Scheme 17.
Type 1 ARC union exploiting revised epoxide (−)-57
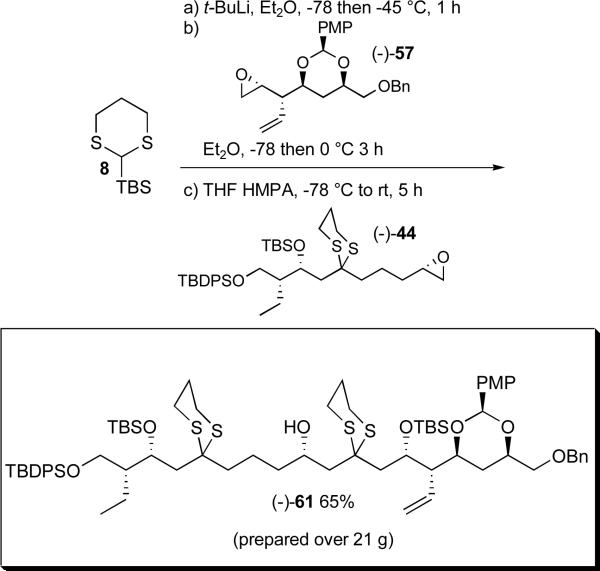
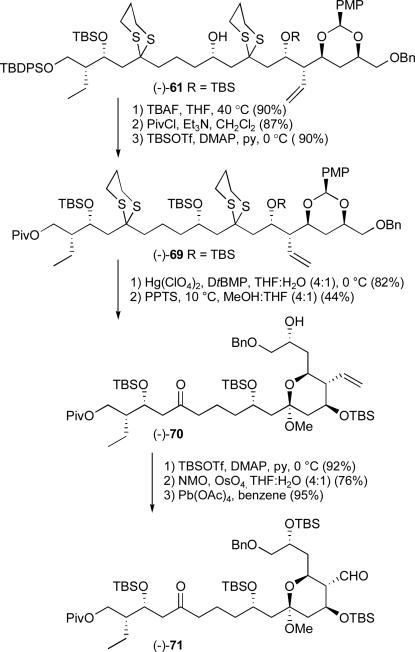
While unexpected, in the end this result served to highlight the versatility of the Type I ARC chemistry. Simply reversing the order of epoxide additions, to permit the less basic 2-Li-2-TBS-1,3-dithiane anionxxxvi to react first with epoxide (−)-57, followed in turn by triggering the 1,4-Brook rearrangement by solvent polarity change (i.e. HMPA) and addition of epoxide (−)-44, led to tricomponent adduct (−)-61 in 65% yield (Scheme 18). Importantly, this revised ARC protocol permitted the production of over 21 grams of bis-dithiane (−)-61.
Scheme 18.
The Type 1 ARC tactic; inverse order of epoxide addition
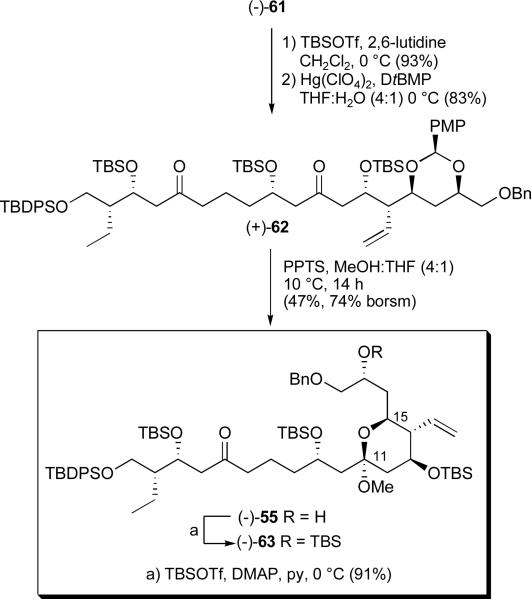
Protection of the secondary hydroxy group in (−)-61 as a TBS ether and exposure to Hg(ClO4)2 then led to diketone (+)-62 in 77% yield for the two steps (Scheme 19). Turning next to the required acetal hydrolysis, a number of acid-mediated conditions were explored. Ultimately, PPTS in anhydrous solvents (THF:MeOH, 4:1) at 10 °C over a period of 14 hours furnished (−)-55 in 47% yield (cf. 74% yield based on recovered starting material). By recycling the recovered starting material, the yield of ketal (−)-55 could be increased to 63% yield. Protection of the liberated hydroxy group was then achieved in pyridine, employing DMAP and TBSOTf to furnish the fully protected cyclic methyl ketal (−)-63.
Scheme 19.
Conversion of bis-dithiane (−)-61 into ketal (−)-63
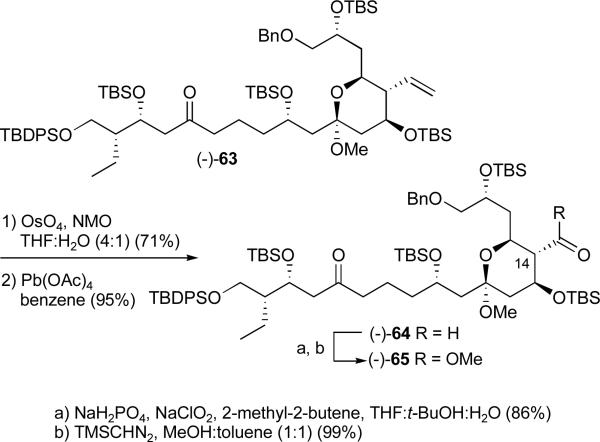
Conversion of the C(14) pendent olefin to the requisite methyl ester began with catalytic OsO4 dihydroxylation,xxxvii followed by oxidative cleavage to furnish aldehyde (−)-64 (Scheme 20).xxxviii Pinnick oxidationxxxix and esterification with trimethylsilyldiazomethane (TMSCHN2) completed construction of methyl ester (−)-65 in 58% yield for the four steps.
Scheme 20.
Elaboration of the pendent C(14) olefin to the requisite methyl ester
Elaboration of the C(18, 19) Vinyl Iodide and C(1) Carboxylic Acid: Non Trivial Operations
Generation of the C(18,19) vinyl iodide, proved to be surprisingly problematic. Hydrogenolysis of the benzyl ether using the Pearlman catalyst in ethanol afforded the debenzylated product albeit as a mixture of methyl and ethyl ketals at C(11) (1:1). Using methanol as a reaction solvent led to decomposition products, while ethyl acetate resulted in elimination of the methoxy group. Eventually we discovered that addition of a bulky amine, 2,6-di-tert-butyl-4-methyl pyridine (DtBMP), employing a mixture of methanol and ethyl acetate (1:1) provided primary alcohol (−)-66 in modest yield (Scheme 21). The latter was then subjected to Parikh-Doering oxidationxl to furnish the corresponding aldehyde. Unfortunately, Takai olefinationxli initially resulted in a mixture of products along with decomposition. After significant experimentation, we discovered that slow addition of a solution of the aldehyde, CHI3 (1.7 equiv), and DtBMP (2 equiv) in 1,4-dioxane to chromium dichloride (5 equiv) suspended in THFxlii suppressed decomposition (Scheme 22). Use of DtBMP as a base proved necessary to prevent the formation of the olefin-containing by-products analogous to those seen in the hydrogenolysis. With the DtBMP modification in place, the Takai olefination produced vinyl iodide (−)-67 in 54% yield for the two steps with excellent E to Z selectivity (>20:1).
Scheme 21.
Hydrogenolysis and Takai olefination: vinyl iodide (−)-67
Scheme 22.
Construction of C(1) pivalate (−)-71
Removal of the primary TBDPS ether in the presence of the secondary TBS ethers was next required. Such selectivity usually entails use of either hydroxide ion,xliii buffered TBAF,xliv tris(diethylaminosulfonium difluorotrimethylsilicate (TAS-F),xlv or sodium hydride in HMPA.xlvi These conditions proved unsuccessful, most often leading to removal of one or more of the secondary silyl ethers. Several alternative tactics were explored, including global desilylation followed by selective oxidation of the C(1) primary alcohol or selective protection of the C(1) alcohol. Hemiketal formation between the C(5) ketone and the C(1) and C(9) hydroxy groups again complicated the outcome.
Return to an Earlier Intermediate: Access the C(1) Carboxylic Acid C(18,19) Vinyl Iodide
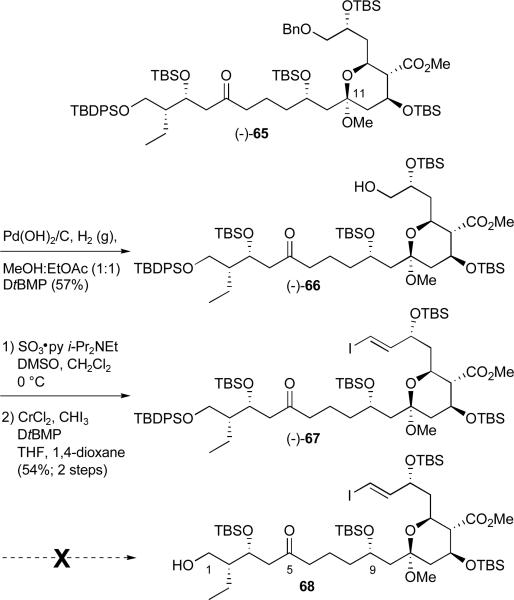
At this juncture we were forced to return to an earlier intermediate. Global desilylation of bis-dithiane (−)-61 (Scheme 18), followed in turn by selective protection of the primary alcohol as the pivalate ester and silylation of the remaining secondary hydroxy groups employing TBSOTf in pyridine furnished bis-dithiane (−)-69 (Scheme 22). Notably, while the reaction of primary alcohols with acid chlorides are generally facile, formation of this pivalate ester proved surprisingly slow, requiring over 24 hours at room temperature. The next five operations were then achieved in similar fashion as employed previously for the production of (−)-64 (cf. Scheme 19 and 20). Removal of the dithianes employing Hg(ClO4)2 provided the diketone in good yield, which upon exposure to PPTS furnished mixed methyl ketal (−)-70 in 44% yield. As with the previous route (cf. Scheme 19), the ketone could be recovered in 37% yield and resubjected to the reaction conditions thus permitting material advancement. Protection of the C(17) hydroxy group, followed by dihydroxylation of the pendent olefin employing catalytic OsO437 and Criegee oxidative cleavage38 completed construction of aldehyde (−)-71.
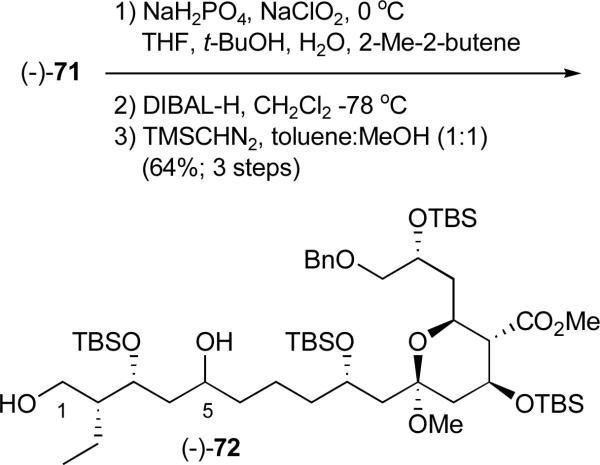
At this juncture Pinnick oxidation39 of aldehyde (−)-71 was followed by DIBAL-H mediated removal of the pivalate ester which proceeded with concomitant reduction of the C(5) ketone. Esterification with TMSCHN2 led to a diastereomeric mixture of diols [(−)-72; 3:1] that could be readily separated via silica gel chromatography (Scheme 23). Recognizing that the C(5) hydroxy group would ultimately be oxidized, the configuration of the C(5) diastereomers was not established. For material advancement however both isomers were carried forward independently. We describe here the results only for the major diastereomer.
Scheme 23.
Oxidation of the C(14) aldehyde and removal of the C(1) pivalate ester to afford diol (−)72
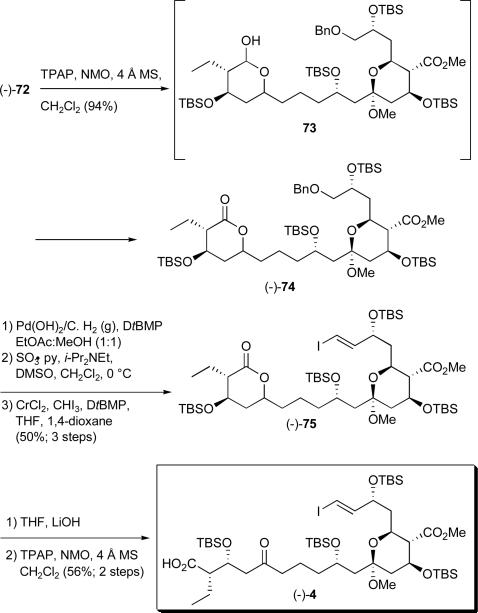
Oxidation of (−)-72 to the desired ketoacid employing a number of oxidants not-surprisingly was interrupted by the formation of lactol 73, which was then further oxidized to lactone (−)-74 (Scheme 24). Reasoning that the lactone could serve as a de facto protecting group for the carboxylic acid moiety, we screened several oxidants, however, tetrapropylammonium perruthenate (TPAP)xlvii employing NMO as the stoichiometric oxidant proved optimal, providing lactone (−)-74 in 94% yield. Removal of the benzyl group via hydrogenolysis then proceeded smoothly to furnish the corresponding alcohol, which upon Parikh-Doering oxidation40 and Takai olefination41, 42 exploiting our previously optimized reaction sequence (cf. Scheme 21) furnished vinyl iodide (−)-75 with excellent E/Z selectivity in 50% overall yield. Not surprisingly, opening the δ-lactone to reveal the carboxylic acid proved challenging, as the derived β-hydroxyacid quickly re-lactonized. Use of aqueous lithium hydroxide in THF, followed in turn by careful dilution with CH2Cl2, removal of the aqueous layer and immediate oxidation with TPAP and NMO furnished the advanced C(1)-keto-acid (−)-4 in 54% yield for the two steps.
Scheme 24.
Construction of keto acid (−)-4
Vinyl Borate Ester (−)-3: The C(20-27) Northwest Fragment
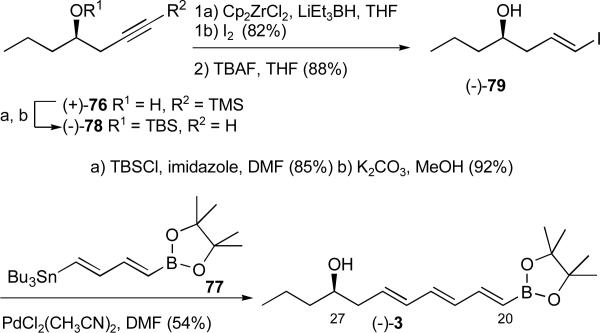
The requisite vinyl borate ester 3 required to complete the carbon skeleton of the rimocidin aglycone was prepared in five steps from known alkyne (+)-76xlviii exploiting the bidirectional linchpin of Coleman (cf. 77).xlix Towards this end, alcohol (+)-76 was protected as the TBS ether, followed by removal of the TMS group (Scheme 25). Hydrozirconation followed by iodination and removal of the silyl group furnished vinyl iodide (−)-79. The latter was then subjected to Stille cross-coupling with diene 77 to provide the C(20-27) borate ester (−)-3. The overall yield for the five-step sequence was 30%.
Scheme 25.
Construction of trienol (−)-3
Final Elaboration of (−)-80: The Macrocyclic Core of the (+)-Rimocidin Aglycone
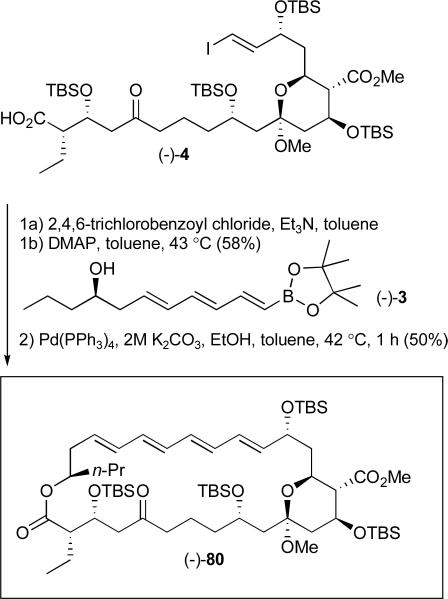
Union of (−)-3 with acid (−)-4 was successfully achieved employing the Yamaguchi esterification protocol (Scheme 26).l Intramolecular Suzuki-Miyaura cross-coupling13, 48 then provided macrocycle (−)-80 in 29% yield for the two steps, possessing the protected macrocyclic skeleton of the rimocidin aglycone. The spectral data for the macrocycle including 500 MHz 1H NMR, 125 MHz 13C NMR, 2D NMR (COSY, NOESY, HSQC) spectrum and high resolution mass spectrum are fully consistent with structure (−)-80.19
Scheme 26.
Completion of the fully functionalized macrocyclic core of (+)-rimocidin aglycone
Summary
A convergent synthesis of the C(1-19) carboxylic acid/vinyl iodide fragment of (+)-rimocidin aglycone (−)-4 has been achieved in 31 steps (longest linear sequence). This fragment was then successfully united with vinyl borate ester (−)-3 to form the macrocyclic core of the aglycone of (+)-rimocidin. The synthetic strategy highlights the utility of six dithiane coupling reactions, specifically the multicomponent Type 1 ARC tactic and the SN2/SN2' reaction manifolds of vinyl epoxides with 2-substituted-1,3-dithianes. Studies to extend the utility of Anion Relay Chemistry and the reactivity of the SN2/SN2' manifold of vinyl epoxides continues in our laboratory.
Experimental Section
2,4-Pentadien-1-ol (17)
Acid 16 (20.13 g, 205.2 mmol) was placed in a 3-necked round bottom flask equipped with a mechanical stirrer. Diethyl ether (2 L) was added to the flask and the suspension was cooled to 0 °C in an ice bath. Triethylamine (31.4 mL, 225.7 mmol) was added followed by methyl chloroformate (17.5 mL, 225.7 mmol) and the solution stirred for 30 min. Water was added to the flask to dissolve the salts and the remaining solids were filtered off as the solution was added to a separatory funnel. The layers were separated and the aqueous layer was extracted with Et2O until TLC showed the absence of product. The organic layer was returned to the flask and re-cooled to 0 °C. Sodium borohydride (15.5 g, 410.4 mmol) was added to chilled MeOH (155 mL). This solution was added rapidly with vigorous stirring to the flask. After stirring for 15 min. the reaction was quenched with saturated NH4Cl. The layers were separated and the aqueous layer was extracted until TLC showed that no product remained. The combined organic layers were washed with brine and the solvent evaporated using a stream of air. The allylic alcohol was purified using silica gel chromatography (100% hexanes to 1:1 hexanes:ether). The resulting liquid was distilled using a Kugelrohr distillation apparatus (40 mm, 85 °C) affording 9.40 g of 17 (54% yield, 111. 8 mmol) as a colorless liquid: The analytical data was consistent with data previously reported for this compound.li IR (film) 3331, 3087, 3040, 3007, 2864, 1604, 1414, 1086, 1004, 904 cm−1; 1H NMR (500 MHz, CDCl3) δ 6.36 (ddd, J = 10.3, 10.3, 16.8 Hz, 1H), 6.26 (dd, J = 10.6, 15.2 Hz, 1H), 5.86 (ddd, J = 5.8, 5.8, 11.6 Hz, 1H), 5.22 (d, J = 16.5 Hz, 1H), 5.11 (d, J = 9.6 Hz, 1H), 4.19 (s, 2H), 1.66 (s, 1H); 13C NMR (125 MHz, CDCl3) δ 141.8, 138.1, 137.5, 123.2, 68.8.
(−)-tert-Butyldimethyl(((2S,3S)-3vinyloxiran-2-yl-)methoxy)silane (14)
A flask was charged with 55 g of flame-dried 4 Å mesh molecular sieves and dry CH2Cl2 (1.7 L). The flask was cooled to −25 °C in a dry ice/isopropanol/water bath. (+)-Diisopropyl tartrate (11.98 g, 50.92 mmol), freshly distilled titanium(IV) isopropoxide (12.00 g, 42.43 mmol) and tert-butylhydroperoxide (stored over freshly activated molecular sieves for 24 hours 154.3 mL, 848.6 mmol) were added sequentially to the flask. The solution stirred for 30 min at −25 °C. Allylic alcohol 17 (35.69 g, 424.3 mmol) was placed in a flask and stored over activated sieves for 30 min. The alcohol was then added neat to the reaction mixture. After 1 h the epoxidation was judged complete via TLC. Trimethyl phosphite (79.00 g, 636.5 mmol) was added dropwise to quench the remaining peroxide. The reaction stirred 1 hour at −25 °C. A second flask was charged with TBSCl (95.90 g, 636.5 mmol), DMAP (2.59 g, 21.22 mmol), anhydrous CH2Cl2 (250 mL) and Et3N (89.3 mL, 636.5 mmol). The TBSCl solution was added to the reaction in one portion. The reaction warm slowly to 0 °C then was placed in a 0 °C refrigerator overnight. The following morning Et2O (500 mL) and silica gel (500 mL) were added to the flask and the slurry was filtered through Celite and rinsed with Et2O. The filtrate was re-filtered to remove the remaining solids. The filtrate was concentrated and the desired vinyl epoxide was purified using flash chromatography (0.5% Et2O in hexanes). Epoxide (−)-14 was isolated as a colorless oil in 72% yield (68.54 g, 319.8 mmol): (c = 1.25, CHCl3); IR (film) 2955, 2931, 2890, 2858, 1468, 1255, 1139, 1109, 838, 779 cm−1; 1H NMR (500 MHz, CDCl3) δ 5.60 (ddd, J = 7.5, 10.3, 17.3 Hz, 1H), 5.48 (dd, J = 2.2, 17.8 Hz, 1H), 5.29 (dd, J = 1.5, 10.6 Hz, 1H), 3.86 (dd, J = 3.2, 12.0 Hz, 1H), 3.72 (dd, J = 4.5, 12.0 Hz, 1H), 3.28 (dd, J = 2.1 7.5 Hz, 1H), 3.00 (ddd, J = 2.2, 3.3, 4.5 Hz, 1H), 0.90 (s, 9H), 0.083 (s, 6H); 13C NMR (125 MHz, CDCl3) δ 135.2, 119.4, 63.0, 60.3, 56.1, 25.8 (3C), 18.4, −5.3 (2C); high resolution mass spectrum (CI+) m/z 215.1464 [(MH+); calculated for C11H22OSi+H: 215.1467].
Phenyl Dithiane (+)-19
2-Phenyl-1,3-dithiane (18) (7.23 g, 36.82 mmol) was azeotroped three times with benzene and placed under high vacuum overnight. Epoxide (+)-14 (6.34 g, 29.58 mmol) was azeotroped twice with benzene, dissolved in THF (50 mL) and allowed to stand with CaH2 for 30 min. Dithiane 18 was dissolved in dry THF (130 mL) and HMPA (15.4 mL, 88.7 mmol) was added. The solution was cooled to −78 °C before n-BuLi (2.15 M, 17.2 mL, 38.0 mmol) was added dropwise turning the solution orange. After stirring at −78 °C for 30 min the epoxide solution was added via cannula. The reaction turned red and was allowed to warm slowly in the dry ice bath over the course of 2 h. The bath was removed and the reaction stirred at room temperature for and additional 1 h before being quenched by addition of saturated NH4Cl (9 mL) and Et2O (230 mL). The layers were separated and the aqueous layer was extracted once with Et2O. Water (200 mL) was run through the combined organic layers to remove HMPA. The organic layer was washed with brine, dried over MgSO4 and concentrated. Flash chromatography (98:2 hexanes:EtOAc) afforded (+)-19 (10.88 g, 26.49 mmol) in 72% yield: (c = 1.19, CH2Cl2); IR (film) 3500, 3075, 2928, 2856, 1472, 1442, 1255, 1116, 838, 776 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.99 (m, 2H), 7.40 (m, 2H), 7.27 (m, 1H), 6.02 (ddd, J = 11.0, 11.0, 17.1 Hz, 1H), 5.24 (dd, J = 2.0, 10.1 Hz, 1H), 5.00 (dd, J = 2.0, 17.1 Hz, 1H), 4.06 (dddd, J = 2.4, 3.6, 6.8, 6.8 Hz, 1H), 3.36 (dd, J = 6.9, 9.8 Hz, 1H), 3.27 (dd, J = 6.8, 9.8 Hz, 1H), 2.76-2.62 (m, 5H), 1.98 (d, J = 3.4 Hz, 1H), 1.91 (m, 2H), 0.83 (s, 9H), −0.036 (s, 3H), −0.050 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 140.5, 132.1, 129.8 (2C), 128.6 (2C), 127.1, 120.5, 70.0, 64.7, 62.7, 58.4, 27.6, 27.5, 25.9 (3H), 24.9, 18.1, −5.4, −5.5; high resolution mass spectrum (ES+) m/z 433.1650 [(M+Na+); calculated for: C21H34OS2Si+Na 433.1667].
Alcohol 31
A mixture of dithiane 30 diastereomers (987 mg, 2.90 mmol) and epoxide (+)-20 (662 mg, 2.36 mmol) were azeotroped three times with toluene and placed under high vacuum overnight. The dithiane mixture was dissolved in dry THF (29 mL) and HMPA (1.23 mL, 7.08 mmol) was added. The solution was cooled to −78 °C in a dry ice/acetone bath and t-BuLi (1.35 M, 2.09 mL, 2.83 mmol) was added slowly, turning the solution dark orange, and stirred for 30 min. The epoxide in dry THF (15 mL) was added to the reaction mixture via cannula and the mixture was allowed to warm in the bath for 2.5 h. The bath was removed and the reaction stirred at room temperature for 30 min before addition of saturated NH4Cl (9 mL). The reaction was partitioned between Et2O (125 mL) and water (5 mL). The organic layer was washed twice with water and once with brine. The organic layer was dried over MgSO4 and concentrated. Flash chromatography (3:1 hexanes:EtOAc) afforded coupled product 31 as a 2:1 mixture of diastereomers at the acetal carbon (1.38g, 2.22 mmol) in 94% yield. The compounds were characterized as a mixture: IR (film) 3464, 2928, 1614, 1517, 1440, 1249, 1170, 1081, 1030, 831 cm−1; 1H NMR (500 MHz, C6D6) δ 7.51 (m, 2H), 7.47 (m, 1H), 7.41 (m, 3H), 7.15 (m, 3H), 7.05 (m, 1.5H), 6.80 (m, 3H), 5.94 (s, 0.5H), 5.75 (s, 1H), 3.95 (m, 0.5H), 3.91 (m, 1H), 3.85, (m, 1H), 3.73 (dd, J = 7.3, 7.3 Hz, 1H), 3.45 (dd, J = 7.0, 7.0 Hz, 1H), 3.32 (m, 0.5H), 3.31 (s, 1.5H), 3.30 (s, 3H), 3.15 (m, 1.5H), 3.05 (m, 4H), 2.53-2.38 (m, 7H), 2.17 (m, 3H), 2.04 (m, 4.5H), 1.88 (m, 3H), 1.70 (m, 4.5H), 1.54 (m, 4.5H), 1.32 (m, 1H), 1.21 (m, 3H), 1.17 (dd, J = 6.3, 6.3 Hz, 4.5H), 1.16 (m, 1H); 13C NMR (125 MHz, C6D6) δ 160.5, 160.3, 141.8, 131.5, 130.8, 130.5, 128.3, 128.0, 126.7, 113.6, 104.1, 103.2, 76.8, 75.9, 70.4, 69.8, 62.9, 54.6, 43.12, 43.08, 39.7, 34.8, 33.5, 33.2, 32.0, 31.0, 30.8, 28.7, 27.2, 25.0, 22.7, 21.1, 14.1; high resolution mass spectrum (ES+) m/z 643.2016 [(M+Na+); calculated for: C32H44O4S4+Na 643.2020].
Dithiane (12)
Epoxide (−)-14 (51.35 g, 239.6 mmol) and 1,3-dithiane 33 (43.21 g, 359.6 mmol) were both azeotroped three times with toluene. The dithiane was placed under high vacuum for 2 h before being dissolved in dry THF (1400 mL) and transferred into a dry three-necked flask equipped with an addition funnel. Hexamethylphosphoramide (111 mL, 638 mmol) was added and the flask was cooled to −78 °C in a dry ice/acetone bath. The addition funnel was charged with a solution of n-BuLi in hexanes (1.37 M, 245 mL, 335 mmol) and was added to the reaction mixture dropwise over 1 h, after which the reaction stirred an additional 30 min. The same funnel was then charged with (−)-14 and anhydrous THF (120 mL). This solution was added to the reaction mixture over 1 h and the bath was then allowed to warm to room temperature over 3 h. The reaction was quenched with saturated NH4Cl (100 mL) and Et2O (500 mL) was added. The layers were separated and the aqueous layer was extracted with Et2O (3 × 100 mL). The combined organic layers were washed with brine, dried over MgSO4 and concentrated to afford an orange oil. Using flash chromatography (97:3 to 9:1 hexanes:EtOAc) the alcohol was isolated as a 2.4:1 mixture of primary, and secondary silyl ethers in 66% total yield. Data for the major isomer is presented: (c = 0.47, CHCl3); IR (film) 3414, 2927, 1466, 1250, 1101, 836, 777, 668 cm−1; 1H NMR (CDCl3, 500 MHz) δ 5.87 (ddd, J = 10.0, 10.0, 17.1 Hz, 1H), 5.26 (dd, J = 1.8, 10.1 Hz, 1H), 5.16 (dd, J = 1.8, 17.1 Hz, 1H), 4.33 (d, J = 4.3 Hz, 1H), 4.10 (dddd, J = 3.4, 3.4, 6.2, 6.2 Hz, 1H), 3.56 (m, 2H), 2.95-2.83 (m, 4H), 2.45 (ddd, J = 3.4, 7.9, 11.0 Hz, 1H), 2.34 (d, J = 3.4 Hz, 1H), 2.10 (m, 1H), 1.90-1.85 (m, 1H), 0.90 (s, 9H), 0.07 (s, 6H); 13C NMR (CDCl3, 125 MHz), δ 133.9, 119.5, 70.6, 65.3, 51.1, 49.3, 30.7, 30.6, 26.0 (3C), 25.8, 19.2, −5.37, −5.42; high resolution mass spectrum (ES+) m/z 357.1362 [(M+Na+) calculated for: C15H30O2S2Si+Na 357.1354].
The mixture of alcohols (13.19 g, 39.42 mmol) was placed in a flask with TBSCl (8.91 g, 59.13 mmol), DMAP (1.20 g, 9.85 mmol) and imidazole (5.37 g, 78.84 mmol). The flask was then charged with 60 mL of DMF, freshly distilled from 4 Å molecular sieves, and allowed to stir at room temperature. After 3 days the reaction was diluted with EtOAc and saturated NH4Cl. The layers were separated and the aqueous layer was extracted three times with EtOAc. Distilled water (~300 mL) was run through the combined organic layers to remove the DMF. The organic layer was then washed with brine, dried over MgSO4 and concentrated to afford a yellow oil. Flash chromatography (99:1 hexanes:EtOAc) afforded the desired product (−)-12 (16.10 g, 35.87 mmol, 91%): (c = 0.30, CHCl3); IR (film) 2953, 2929, 2895, 2857, 1472, 1255, 1096, 836, 776 cm−1; 1H NMR (CDCl3, 500 MHz) δ 5.70 (ddd, J = 10.0, 10.0, 17.2 Hz, 1H), 5.22 (dd, J = 2.1, 10.0 Hz, 1H), 5.14 (dd, J = 2.0, 17.2 Hz, 1H), 4.17 (m, 1H), 4.14 (d, J = 10.8 Hz, 1H), 3.46 (dd, J = 5.4, 9.8 Hz, 1H), 3.40 (dd, J = 7.9, 9.7 Hz, 1H), 2.88-2.75 (m, 4H), 2.59 (ddd, J = 1.9, 10.4, 10.4 Hz, 1H), 2.12-2.07 (m, 1H), 1.92, 1.84 (m, 1H), 0.89 (s, 18H), 0.14 (s, 3H), 0.08 (s, 3H), 0.04 (s, 3H), 0.02 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 134.1, 119.0, 71.1, 64.8, 50.9, 47.9, 30.3, 30.0, 26.1, 25.94 (3C), 25.88 (3C), 18.2, 18.1, −4.2, −4,8, −5.35, −5.41; high resolution mass spectrum (ES+) m/z 471.2202 [(M+Na+) calculated for: C21H44O2S2Si2+Na 471.2219].
Alcohol (−)-34
Dithiane (−)-12 (13.30 g, 29.63 mmol) was azeotroped three times with toluene and placed under high vacuum overnight. Benzyl (R)-(−)-glycidyl ether 13 (2.43 g, 14.81 mmol) was azeotroped three times with toluene and placed under high vacuum for 1 h. The dithiane was dissolved in dry THF (420 mL) and HMPA (7.7 mL, 44.4 mmol) was added. The solution was cooled to −78 °C in a dry ice/acetone and t-BuLi (1.50 M, 20.7 mL, 31.10 mmol) was added dropwise. The solution turned orange and was then placed in a −45 °C in a dry ice/acetonitrile bath. After 30 min the solution was re-cooled to −78 °C and epoxide (−)-13 in dry THF (210 mL) was added via cannula. The solution was allowed to warm to −5 °C over the course of 3 h after which the reaction was quenched with saturated NH4Cl (6 mL). The THF was removed under reduced pressure and the resulting dark red oil was dissolved in Et2O (300 mL) and half saturated brine solution (100 mL) was added. The layers were separated and the aqueous layer was extracted twice with Et2O. The combined organics were washed with brine, dried over MgSO4 and concentrated to afford a dark orange oil. The desired alcohol, (−)-34, was isolated as an amorphous solid via flash chromatography (95:5 hexanes: EtOAc) in 88% yield (8.05 g, 13.13 mmol): (c = 1.4, CHCl3); IR (film) 3471, 2927, 2855, 1257, 1104, 836, 775 cm−1; 1H NMR (CDCl3, 500 MHz) δ 7.37-7.27 (m, 5H), 5.93 (ddd, J = 10.0, 10.0, 17.2 Hz, 1H), 5.30 (dd, J = 2.3, 10.0 Hz, 1H), 5.15 (dd, J = 2.2, 17.2 Hz, 1H), 4.57 (d, J = 2.4 Hz, 2H), 4.46 (dd, J = 4.6, 9.8 Hz, 1H), 4,43 (m, 1H), 3.47 (dd, J = 4.6, 9.0 Hz, 1H), 3.43 (m, 2H), 3.32 (dd, J = 9.5, 9.5 Hz, 1H), 3.22 (d, 9.8 Hz, 1H), 2.99 (ddd, J = 3.0, 10.8, 17.4 Hz, 1H), 2.90 (ddd, J = 3.1, 10.6, 17.5 Hz, 1H), 2.72-2.65 (m, 2H), 2.27 (dd, J = 2.1, 15.8 Hz, 1H), 2.03 (dd, J = 8.5, 15.8 Hz, 1H), 1.97 (m, 1H), 1.89 (m, 1H), 0.92 (s, 9H), 0.83 (s, 9H), 0.061 (s, 3H), 0.057 (s, 3H), 0.048 (s, 3H), 0.034 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 138.3, 133.0, 128.3 (2C), 127.8 (2C), 127.5, 119.6, 74.5, 73.3, 72.1, 67.6, 64.5, 55.9, 44.5, 40.3, 26.01, 25.94 (3C), 25.9 (3C), 25.5, 24.4, 18.2, 18.0, −3.4, −3.8, −5.38, −5.44; high resolution mass spectrum (ES+) m/z 635.3078 [(M+Na+) calculated for: C31H56O4S2Si2+Na 635.3056].
Linchpin Product (−)-42
Epoxide (−)-41 (174 mg, 0.329 mmol) and epoxide (+)-9 (164 mg, 0.515 mmol) were both azeotroped three times with benzene and placed under high vacuum overnight. 2-TBS-1,3- dithiane 8 (91 mg, .39 mmol) was azeotroped three times with benzene and left under high vacuum for 1h before being dissolved in dry Et2O (1.6 mL) and cooled to −78 °C in a dry ice/acetone bath. A solution of t-BuLi (1.40 M, 0.26 mL, 0.36 mmol) was added and the reaction remained colorless. After 3 min the flask was placed in a −45 °C dry ice / acetonitrile bath and stirred for 1 h. The flask was re-cooled to −78 °C and epoxide (−)-41 in anhydrous Et2O (2.6 mL) was added via cannula. The reaction stirred at −20 °C (dry ice / acetone) for 1 hour and was then cooled back down to −78 °C. The solution was pale yellow. Epoxide (+)-9 in dry Et2O (4 mL) and HMPA (0.18 mL, 0.98 mmol) was added via cannula and the reaction turned dark orange. The solution was warmed to −20 °C and stirred 1 h before being warmed to 0 °C for 30 min and then room temperature for an additional 30 min. The reaction was quenched with saturated NH4Cl and diluted with Et2O. The aqueous layer was extracted twice with Et2O. The combined organic layers were washed with brine, dried over MgSO4 and concentrated. Flash chromatography afforded the desired coupled product (−)-42 in 53% yield (188 mg, 0.174 mmol): (c = 0.16, CH2Cl2); IR (film) 3502, 2928, 2856, 1456, 1436, 1379, 1257, 1200, 1173, 1107, 1049, 837, 775, 700 cm−1- ; 1H NMR (500 MHz, C6H6) δ 7.47 (m, 2H), 7.30 (m, 1H), 7.17 (m, 5H), 7.09 (m, 2H), 6.01 (ddd, J = 10.1, 10.1, 17.3 Hz, 1H), 5.16 (dd, J = 2.2, 10.3 Hz, 1H), 5.05 (dd, J = 2.1, 17.3 Hz, 1H), 4.86 (d, J = 8.3 Hz, 1H), 4.42 (d, J = 6.5 Hz, 1H), 4.41 (m, 1H), 4.39 (d, J = 3.8 Hz, 2H), 4.37 (d, J = 6.4 Hz, 1H), 4.19 (ddd, J = 2.2, 8.4, 13.8 Hz, 1H), 4.02 (dddd, J = 2.5, 5.1, 5.1, 11.6 Hz, 1H), 3.51 (dd, J = 5.5, 9.8 Hz, 1H), 3.41 (d, J = 2.8 Hz, 1H), 3.26 (dd, J = 4.9, 9.8 Hz, 1H), 3.23 (dd, J = 6.0, 10.6 Hz, 1H), 3.12 (dd, J = 8.2, 10.6 Hz, 1H), 3.11 (s, 3H), 3.05 (m, 2H), 2.61-2.40 (m, 8H), 2.36 (dd, J = 5.0, 15.0 Hz, 1H), 2.32 (m, 2H), 2.19-2.01 (m, 8H), 1.94 (m, 1H), 1.83-1.67 (m, 5H), 1.58-1.31 (m, 7H), 1.54 (s, 3H), 1.40 (s, 3H), 1.18 (ddd, J = 6.8, 6.8, 6.8 Hz, 1H), 1.13 (dd, J = 7.4, 7.4 Hz, 3H), 1.09 (s, 9H), 0.33 (s, 3H), 0.28 (s, 3H); 13C NMR (125 MHz, C6D6) δ 141.7, 138.9, 134.8, 130.8 (2C), 128.2 (2C), 128.1 (2C), 128.0 (2C), 127.3, 126.8, 118.7, 98.7, 96.3, 74.0, 73.2, 69.7, 69.0, 68.4, 68.3, 66.2, 63.1, 57.2, 54.7, 52.1, 47.5, 45.3, 43.7, 39.7, 39.3, 33.0, 32.3, 32.1, 31.3, 30.9, 30.2, 28.8, 27.2 (2C), 26.5, 26.2 (3C), 26.0, 25.0, 24.6, 22.8, 20.1, 19.5, 18.2, 14.1, −3.6, −4.0; high resolution mass spectrum (ES+) m/z 1103.4515 [(M+Na+); calculated for C55H88O7S6Si+Na 1103.4521].
Dithiane Adduct (−)-50
Dithianes 30 (6.00 g, 17.62 mmol) and epoxide (+)-45 (5.20 g, 14.68 mmol) were both azeotroped three times with toluene then placed under high vacuum for 14 hours. The dithiane was dissolved in dry THF (100 mL) and HMPA (7.6 mL, 44.0 mmol) was added. The solution was cooled to −78 °C in a dry ice/acetone bath. The dithiane was treated with t-BuLi (1.40 M, 12.7 mL, 17.7 mmol) and was allowed to stir at −78 °C for 30 min. Epoxide (+)-45 was dissolved in anhydrous THF (75 mL) and added to the dithiane solution via cannula. The reaction was allowed to warm to room temperature over 3 h during which time the color changed from yellow to dark orange. The reaction was then quenched by addition of saturated NH4Cl and diluted with Et2O. The layers were separated and the aqueous layer was extracted three times with ether. The combined organic layers were washed with water to remove the HMPA and with brine prior to drying with MgSO4 and concentration under reduced pressure. The product mixture was isolated as a colorless oil in 94% yield (9.59 g, 13.80 mmol) via flash chromatography (9:1 hexanes : EtOAc): (major) (−)-50 (c = 0.145, C6H6); IR (film) 3493, 2932, 1613, 1428, 1304, 1248, 1080, 826, 741 cm−1; 1H NMR (500 MHz, C6D6) δ 7.81 (m, 4H), 7.45 (m, 2H), 7.24 (m, 6H), 6.80 (m, 2H), 5.80 (s, 1H), 4.50 (m, 1H), 3.94 (m, 1H), 3.90 (dd, J = 4.2, 10.1 Hz, 1H), 3.87 (dd, J = 7.0, 10.3 Hz, 1H), 3.78 (dd, J = 7.3, 7.3 Hz, 1H), 3.51 (dd, J = 7.2, 7.2 Hz, 1H), 3.26 (s, 3H), 2.50 (m, 3H), 2.23 (m, 2H), 2.18 (m, 2H), 2.09 (m, 1H), 1.87 (m, 2H), 1.67 (m, 1H), 1.59 (m, 1H), 1.52-1.35 (m, 6H), 1.19 (s, 9H), 0.80 (dd, J = 7.5, 7.5 Hz, 3H); 13C NMR (125 MHz, C6D6) δ 160.8, 136.1 (2C), 136.0 (2C), 134.0, 133.8, 131.3, 130.1, 130.0, 128.5 (2C), 127.9 (4C), 113.9 (2C), 104.3, 77.2, 70.2, 69.3, 64.5, 54.7, 53.4, 49.4, 43.1, 39.8, 34.1, 27,2 (3C), 26.3, 26,1, 25.4, 21.4, 19.5, 19.2, 12.6; (minor) (c = 0.16, C6H6); IR (film) 3510, 2933, 1612, 1248, 1172, 1087, 825, 703 cm−1; 1H NMR (500 MHz, C6D6) δ 7.82 (m, 4H), 7.51 (m, 2H), 7.24 (m, 6H), 6.79 (m, 2H), 6.00 (s, 1H), 4.52 (ddd, J = 3.1, 3.1, 7.3 Hz, 1H), 4.04 (m, 1H), 3.96 (dd, J = 6.2, 7.9 Hz, 1H), 3.88 (dd, J = 4.4, 6.2 Hz, 1H), 3.88 (m, 1H), 3.38 (dd, J = 7.7, 7.7 Hz, 1H), 3.28 (dd, J = 3.1, 17.1 Hz, 1H), 3.27 (s, 3H), 2.51 (m, 3H), 2.39-2.28 (m, 2H), 2.25 −2.09 (m, 3H), 1.86 (m, 2H), 1.62 (m, 2H), 1.50 (m, 3H), 1.38 (ddd, J = 7.4, 7.4, 9.4 Hz, 1H), 1.34 (m, 2H), 1.19 (s, 9H), 0.80 (dd, J = 7.5, 7.5 Hz, 3H); 13C NMR (125 MHz, C6D6) δ 160.6, 136.1 (2C), 136.0 (2C), 133.9, 133.8, 131.9, 130.1, 130.0, 128.3 (2C), 127.5 (4C), 113.9 (2C), 103.5, 76.3, 70.8, 69.3, 64.5, 54.7, 53.4, 49.4, 43.0, 39.8, 33.9, 27.2 (3C), 26.3, 26.1, 25.4, 21.4, 19.4, 19.2, 12.6; high resolution mass spectrum (ES+) m/z 717.3096 [(M+Na+) calculated for C39H54O5S2Si+Na 717.3080].
Bis-dithiane (−)-61
2-tert-Butyldimethylsilyl-1,3-dithiane 8 (1.72 g, 7.34 mmol), epoxide (−)-57 (2.9 1g, 7.34 mmol) and epoxide (−)-44 (3.53 g, 5.24 mmol) were each azeotroped three times with toluene and dried under high vacuum for 12 hours. The dithiane was dissolved in dry Et2O (43 mL) and was cooled to −78 °C in a dry ice / acetone bath. t-Butyllithium (1.49 M, 4.9 mL, 7.4 mmol) was added dropwise and the colorless solution turned pale yellow. The −78 °C bath was removed and the reaction stirred at −45 °C (dry ice/acetonitrile) for 55 min. The reaction was re-cooled to −78 °C and epoxide (−)-57 in anhydrous Et2O (43 mL) was added via cannula. The now yellow reaction mixture was placed in an ice water bath for 3 h after which it was re-cooled to −78 °C. Epoxide (−)-44 was then added via cannula in THF (43 mL) with HMPA (3.8 mL, 22.0 mmol); the reaction turned orange upon addition. The flask warmed slowly to −45 °C over 1 h. The dry ice/acetone bath was removed and the reaction stirred for 1 hour at −45 °C in a dry ice/acetonitrile bath. After which the bath was allowed to warm to room temperature over the course of 3 h. The reaction was quenched with saturated NH4Cl (6 mL) and distilled H2O (10 mL). The mixture was poured into a separatory funnel and Et2O (30 mL) was added. The layers were separated and the aqueous layer was extracted once with Et2O. The combined organic layers were washed with brine, dried over MgSO4 and concentrated to afford a yellow oil. The desired bis-dithiane (−)-61 was isolated as a white foam in 65% yield (4.48 g, 3.43 mmol) following flash column chromatography (9:1 hexanes : EtOAc): (c = 0.35, C6H6); IR (film) 3453, 3069, 2952, 2859, 1615, 1517, 1467, 1427, 1250, 1108, 1007, 933, 831, 776, 740, 703 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.70 (m, 4H), 7.54 (m, 2H), 7.37 (m, 10H), 7.28 (m, 1H), 6.88 (m, 2H), 5.38 (ddd, J = 10.2, 10.2, 17.3 Hz, 1H), 5.51 (s, 1H), 5.29 (dd, J = 2.1, 10.4 Hz, 1H), 5.25 (dd, J = 2.1, 17.4 Hz, 1H), 4.79 (d, J = 9.0 Hz, 1H), 4.61 (d, J = 12.2 Hz, 1H), 4.56 (d, J = 12.2 Hz, 1H), 4.38 (ddd, J = 2.6, 5.5, 5.5 Hz, 1H), 4.04 (m, 1H), 3.94 (m, 1H), 3.88 (m, 1H), 3.80 (s, 3H), 3.68 (m, 2H), 3.63 (dd, J = 6.2, 10.4 Hz, 1H), 3.56 (bs, 1H), 3.49 (dd, J = 4.6, 10.3 Hz, 1H), 3.01 (dddd, J = 2.8, 11.1, 14.1, 25.5 Hz, 2H), 2.74 (m, 7H), 2.51 (dd, J = 9.6, 15.4 Hz, 1H), 2.24 (dd, J = 9.2, 15.3 Hz, 1H), 2.02 (m, 1H), 1.87 (m, 7H), 1.77 (m, 1H), 1.70 (d, J = 14.9 Hz, 1H), 1.64 (m, 2H), 1.46 (m, 1H), 1.37 (m, 2H), 1.30 (m, 3H), 1.07 (s, 9H), 0.89 (s, 9H), 0.86 (dd, J = 7.5, 7.5 Hz, 3H), 0.83 (s, 9H), 0.12 (s, 3H), 0.097 (s, 3H), 0.057 (s, 3H), 0.033 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 159.7, 138.2, 135.69 (2C), 135.65 (2C), 134.0, 133.8, 133.4, 131.4, 129.4 (2C), 128.3 (2C), 127.7 (2C), 127.6, 127.5 (6C), 121.0, 113.3 (2C), 100.0, 76.2, 74.5, 73.5, 73.1, 69.4, 68.4, 66.2, 62.7, 56.6, 55.2, 53.1, 51.2, 49.2, 47.1, 45.7, 42.5, 39.2, 37.7, 32.9, 26.9 (3C), 26.8, 26.5, 26.3, 26.1 (4C), 26.0 (3C), 25.1, 24.3, 21.0, 19.4, 19.2, 18.2, 18.1, 12.6, −3.49, −3.51, −4.2, −4.9; high resolution mass spectrum (ES+) m/z 1325.6230 [(M+Na+) calculated for C71H110O8S4Si3+Na 1325.6289].
Diketone (+)-62
A round bottom flask was charged with bis-dithiane (773 mg, 0.545 mmol), DtBMP (840 mg, 4.08 mmol) and a THF:H2O solution (4:1, 15 mL). The solution was cooled to 0 °C prior to portion-wise addition of Hg(ClO4)2 (762 mg, 1.91 mmol). The reaction stirred for 1 h at 0 °C at which point it was diluted with EtOAc and filtered through Celite. Saturated NH4Cl was added to the slightly cloudy filtrate. The layers were separated and the aqueous layer was extracted twice with EtOAc. The combined organic extracts were washed with brine and dried over MgSO4. The solution was filtered through Celite and concentrated to provide a yellow oil. Column chromatography (95:5 to 9:1 hexanes:EtOAc) furnished diketone (+)-62 as a colorless oil in 83% yield (562 mg, 0.454 mmol): (c = 0.17, CDCl3); IR (film) 2955, 2930, 2857, 1716, 1616, 1516, 1467, 1428, 1385, 1251, 1108, 833, 777, 740, 703 cm−1; 1H NMR (500 MHz, C6D6) δ 7.84 (m, 4H), 7.72 (m, 2H), 7.28 (m, 6H), 7.17 (m, 4H), 7.10 (m, 1H), 6.83 (m, 2H), 5.79 (ddd, J = 10.2, 10.2, 17.3 Hz, 1H), 5.63 (s, 1H), 5.15 (dd, J = 2.2, 10.2 Hz, 1H), 5.13 (m, 1H), 5.05 (dd, J = 2.1, 17.4 Hz, 1H), 4.70 (ddd, J = 3.7, 3.7, 7.7 Hz, 1H), 4.44 (d, J = 12.2 Hz, 1H), 4.41 (d, J = 12.2 Hz, 1H), 4.30 (m, 1H), 3.97 (m, 2H), 3.89 (dd, J = 5.0, 10.1 Hz, 1H), 3.85 (dd, J= 5.3, 10.8 Hz, 1H), 2.87 (dd, J = 5.7, 10.1 Hz, 1H), 3.39 (dd, J = 4.7, 10.1 Hz, 1H), 3.27 (s, 3H), 2.62 (m, 3H), 2.52 (dd, J = 6.1, 16.3 Hz, 1H), 2.37 (m, 3H), 2.16 (dd, J = 7.2, 7.2 Hz, 2H), 1.70-1.58 (m, 4H), 1.57-1.40 (m, 4H), 1.33 (m, 1H), 1.22 (s, 9H), 1.01 (s, 9H), 0.98 (s, 9H), 0.95 (s, 9H), 0.87 (dd, J = 7.5, 7.5 Hz, 3H), 0.19 (s, 3H), 0.16 (s, 3H). 0.15 (s, 3H), 0.13 (s, 3H), 0.12 (s, 3H), 0.12 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 209.5, 207.1, 159.7, 138.2, 135.60 (2C), 135.58 (2C), 133.73, 133.65, 133.5, 131.2, 129.5 (2C), 128.3 (2C), 127.73 (2C), 127.72 (2C), 127.54 (4C), 127.49, 119.9, 113.4 (2C), 100.4, 76.2, 74.0, 73.4, 73.1, 69.0, 68.2, 64.9, 62.0, 55.6, 55.2, 51.0, 50.5, 48.4, 47.0, 44.5, 36.8, 32.8, 26.9 (3C), 26.1, 25.9 (3C), 25.8 (6C), 19.9, 19.2, 18.9, 18.1, 17.9, 12.3, −4.3, −4.67, −4.71, −4.76, −4.83, −5.0; high resolution mass spectrum (ES+) m/z 1259.7169 [(M+Na+) calculated for C71H112O10Si4+Na 1259.7230].
Ketal (−)-55
Diketone (+)-62 (1.34 g, 1.08 mmol) was dissolved in dry THF (12 mL) and dry MeOH (48 mL) was added. The solution was cooled to 10 °C before the addition of PPTS (0.50 g, 2.16 mmol). The reaction stirred at 10 °C for 14 hours at which point TLC indicated that the reaction was about 50% complete and that formation of byproduct (−)-56 had begun. The reaction was quenched with saturated NaHCO3 and the solvent was removed under reduced pressure. The resulting slurry was partitioned between distilled water and EtOAc. The aqueous layer was extracted twice with EtOAc and the combined organic layers were washed with brine, dried over MgSO4 and concentrated to afford a colorless oil. Flash chromatography (95:5 to 9:1 hexanes:EtOAc) provided ketal (−)-55 in 44% yield (533 mg, 0.470 mmol) along with 38% recovered diketone (+)-62 (507 mg, 0.409 mmol). Resubjection of the starting material to the same reaction conditions gave an additional 221 mg of (−)-55 along with 209 mg of recovered (+)-62 bringing the total yield to 63%. (c = 0.43, toluene); IR (film) 3515, 2955, 2928, 2856, 1717, 1467, 1375, 1254, 1093, 836, 775, 739, 703 cm−1; 1H NMR (C6D6, 500 MHz) δ 7.85 (m, 4H), 7.30 (m, 7H), 7.21 (m, 3H), 7.10 (m, 1H), 5.28 (ddd, J = 9.9, 9.9, 17.1 Hz, 1H), 5.10 (dd, J = 2.1, 17.1 Hz, 1H), 5.05 (dd, J = 2.1, 10.1 Hz, 1H), 4.72 (ddd, J = 3.6, 3.6, 7.8 Hz, 1H), 4.39 (s, 2H), 4.19 (m, 1H), 4.00 (m, 2H), 3.91 (dd, J = 5.0, 10.1 Hz, 1H), 3.87 (dd, J = 5.2, 10.1 Hz, 1H), 3.67 (dd, J = 2.4, 10.1 Hz, 1H), 3.56 (dd, J = 5.8, 9.3 Hz, 1H), 3.43 (dd, J = 6.3, 9.3 Hz, 1H), 3.31 (s, 1H), 3.07 (s, 3H), 2.67 (dd, J = 8.3, 16.1 Hz, 1H), 2.38 (dd, J = 3.7, 16.1 Hz, 1H), 2.34 (dd, J = 4.8, 13.1 Hz, 1H), 2.23 (apt t, J = 7.0 Hz, 2H), 2.13 (ddd, J = 2.6, 3.7, 14.6 Hz, 1H), 2.02 (m, 2H), 1.90 (dd, J = 5.5, 14.9 Hz, 1H), 1.79 (dd, J = 10.9, 13.1 Hz, 1H), 1.76-1.61 (m, 5H), 1.57-1.48 (m, 2H), 1.34 (m, 1H), 1.23 (s, 9H), 1.04 (s, 9H) 1.01 (s, 9H), 0.97 (s, 9H), 0.89 (dd, J = 7.5, 7.5 Hz, 3H), 0.19 (s, 3H), 0.175 (s, 3H), 0.166 (s, 6H), 0.15 (s, 3H), 0.068 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 209.5, 138.2, 136.6, 135.6 (4C), 133.75, 133.67, 129.4 (2C), 128.3 (2C), 127.2 (2C), 127.54 (4C), 127.48, 119.3, 100.6, 74.1, 72.1, 70.4, 69.0, 68.4, 67.9, 62.1, 55.7, 48.4, 47.6, 47.0, 44.6, 43.1, 42.7, 37.1, 37.0, 26.93, 26.86 (3C), 26.1, 25.9 (3C), 25.8 (6C), 19.9, 19.2, 19.1, 18.0, 17.8, 12.3, −4.2 (4C), −4.7, −4.8; high resolution mass spectrum (ES+) m/z 1155.6967 [(M+Na+) calculated for: C64H108O9Si4+Na 1155.6968].
Carboxylic Acid (−)-4
Lactone (−)-75 (60 mg, 0.056 mmol) was dissolved in a THF:H2O solution (4:1, 1.6 mL)) and LiOH (27 mg, 1.12 mmol) was added. After stirring at room temperature for 5 h the reaction was partitioned between pH 8 buffer and CH2Cl2. The aqueous layer was extracted with CH2Cl2 (6 × 2 mL). The organic layers were washed with brine and dried over MgSO4. The organic layer was filtered through cotton into a flask containing CH2Cl2 (5 mL), NMO (19 mg, 0.17 mmol) and activated 4 Å powdered molecular sieves (60 mg). A catalytic amount of TPAP was added to the flask and the reaction mixture was concentrated under reduced pressure to a volume of approximately 1 mL. After stirring at room temperature for 2 h the solvent was removed and the desired acid was isolated using flash chromatography (high quality silica gel, 9:1 to 4:1 hexanes:EtOAc). Acid (−)-4 and was obtained in 56% yield over two steps (36 mg, 0.033 mmol): (c = 0.05, C6H6); IR (film) 2953, 2929, 2856, 1736, 1715, 1651, 1540, 1460, 1363, 1255, 1095, 837, 776 cm−1; 1H NMR (500 MHz, C6D6) δ 6.72 (dd, J = 5.5, 14.3 Hz, 1H), 6.24 (dd, J = 1.3, 14.3 Hz, 1H), 4.67 (ddd, J = 4.9, 10.7, 10.7 Hz, 1H), 4.64 (m, 1H), 4.23 (m, 1H), 4.13 (m, 1H), 4.03 (m, 1H), 3.41 (s, 3H), 3.16 (s, 3H), 2.72 (dd, J = 7.5, 16.7 Hz, 1H), 2.55 (dd, J = 10.3, 10.3 Hz, 1H), 2.55 (m, 1H), 2.46 (dd, J = 3.8, 16.7 Hz, 1H), 2.42 (dd, J = 4.8, 13.0 Hz, 1H), 2.16 (m, 2H), 1.93 (dd, J = 5.7, 15.0 Hz, 1H), 1.81-1.67 (m, 7H), 1.52 (m, 1H), 1.39 (m, 2H), 1.06 (s, 9H), 1.00 (s, 9H), 0.98 (s, 9H), 0.93 (s, 9H), 0.91 (dd, J = 7.6, 7.6, Hz, 3H), 0.21 (s, 3H), 0.20 (s, 3H), 0.183 (s, 3H), 0.180 (s, 3H), 0.14 (s, 3H), 0.12 (s, 3H), 0.046 (s, 3H), 0.0084 (s, 3H); 13C NMR (125 MHz, C6D6) δ 207.3, 176.8, 172.9, 149.5, 101.0, 75.4, 72.3, 69.7, 69.3, 68.3, 66.8, 57.6, 54.1, 51.2, 48.2, 47.1, 44.2, 43.6, 43.2, 42.0, 37.4, 26.3 (3C), 26.1 (3C), 26.0 (3C), 25.9 (3C), 21.8, 19.7, 18.33, 18.32, 18.2, 18.0, 12.4, −3.8, −3.9, −4.0, −4.4, −4.7 (2C), −4.8, −4.9; high resolution mass spectrum (ES-) m/z 1109.4865 [(M+Na+) calculated for C48H95IO11Si4+Na 1109.4894].
Lactone (−)-80
Acid (−)-4 (15 mg, 0.014 mmol), alcohol (−)-3 (16 mg, 0.055 mmol) and DMAP (7 mg, 0.06 mmol) were each azeotroped three times with toluene and placed under high vacuum for 2 h. The acid was then dissolved in dry toluene (200 μL) and Et3N (8 μL) was added followed by the addition of a solution of 2,4,6-tricholorbenzoyl chloride in toluene (1.37 M, 20 μL, 2 equiv). After 2 h, DMAP in toluene (100 μL) and alcohol (−)-3 in toluene (200 μL) were added sequentially. The reaction was heated to 42 °C overnight. The reaction was then quenched with a saturated solution of NaHCO3 and diluted with stabilizer free ether (solvent purification system). The aqueous layer was extracted three times with ether and the combined organic extracts were washed with brine, dried over MgSO4 and concentrated. The desired ester was isolated in 58% yield (11 mg, 0.0081 mmol) following column chromatography (high quality silica gel, 98:2 hexanes:EtOAc). (c = 0.12, C6H6); IR (film) 3449, 2955, 2929, 2856, 1735, 1615, 1584, 1471, 1362, 1324, 1156, 1144, 1104, 1035, 1008, 970, 938, 837, 776 cm−1; 1H NMR (500 MHz, C6D6) δ 7.44 (dd, J = 9.7, 17.5 Hz, 1 H), 6.72 (dd, J = 5.4, 14.3 Hz, 1H), 6.24 (dd, J = 1.4, 14.3 Hz, 1H), 6.20 (dd, J = 8.7, 9.6 Hz, 1H), 5.99 (m, 1H), 5.92 (d, J = 17.6 Hz, 1H), 5.54 (ddd, J = 7.4, 7.4, 14.9 Hz, 1H), 5.04 (m, 1H), 4.68 (ddd, J = 4.7, 10.6, 10.6 Hz, 1H), 4.61 (m, 1H), 4.23 (m, 1H), 4.19 (m, 1H), 4.01 (m, 1H), 3.42 (s, 3H), 3.13 (s, 3H), 2.89 (dd, J = 8.2, 16.6 Hz, 1H), 2.61 (m, 2H), 2.54 (dd, J = 10.3, 10.3 Hz, 1H), 2.41 (dd, J = 4.8, 13.1 Hz, 1H), 2.23 (m, 4 H), 2.16 (dd, J = 4.8, 15.1 Hz, 1H), 1.92 (dd, J = 6.1, 15.1 Hz, 1H), 1.80 (m, 2H), 1.74-1.53 (m, 7H), 1.37 (m, 5H), 1.10 (s, 12H), 1.06 (s, 9H), 1.02 (s, 9H), 1.00 (s, 9H), 0.99 (s, 9H), 0.89 (m, 6H), 0.22 (s, 3H), 0.21 (s, 3H), 0.19 (s, 6H), 0.17 (s, 3H), 0.087 (2, 3H), 0.049 (s, 3H), 0.011 (s, 3H); 13C (125 MHz, C6D6) δ 207.5, 173.4, 172.8, 150.2, 149.5, 136.3, 133.7, 133.3, 132.1, 127.4, 100.9, 83.0, 75.3, 73.1, 72.3, 70.0, 69.1, 68.2, 66.7, 57.6, 54.4, 51.1, 48.1, 46.9, 44.5, 43.5, 43.2, 38.1, 37.7, 36.4, 26.3 (3C), 26.1 (3C), 25.9 (3C), 25.8 (3C), 24.8 (4C), 22.9, 19.5, 18.9, 18.29, 18.25 (2C), 17.9, 14.0, 12.4, −3.9 (2C), −4.0, −4.46, −4.52, −4.8, −4.9, −5.0; high resolution mass spectrum (ES-) m/z 1383.7010 [(M+Na+) calculated for C65H122BIO13Si4+Na 1383.6998].
The ester (6.6 mg, 0.0048 mmol) was placed in a flask and the flask was evacuated and back-filled with argon three times. Tetrakis(triphenylphosphine)palladium(0) (catalytic) was added and the flask was again evacuated and back-filled three times. Oxygen-free toluene (400 μL) was added followed by a 2 M oxygen-free aqueous solution of K2CO3 (50 μL) and oxygen free EtOH (50 μL). The reaction was heated to 40 °C for 2 h at which point the reaction was cooled, diluted with Et2O (from the solvent purification system) and washed with saturated NaHCO3. The organic layer was washed with brine and dried over MgSO4. The solvent was removed under reduced pressure and macrocycle (−)-80 was isolated in 50% yield (2.7 mg, 0.0024 mmol) following column chromatography (high quality silica gel, 98:2 to 95:5 hexanes:EtOAc). lii (c = 0.08, C6H6); IR (film) 2929, 1733, 1646, 1463, 1383, 1255, 1095, 837, 776 cm−1; 1H NMR (500 MHz, C6D6) δ 6.39-6.14 (m, 6H), 5.77 (dd, J = 3.3, 14.0 Hz, 1H), 5.67 (ddd, J = 5.3, 9.2, 14.9 Hz, 1H), 5.10-5.06 (m, 1H), 4.73 (ddd, J = 4.7, 10.5, 10.5 Hz, 1H), 4.50 (ddd, J = 1.8, 6.2, 9.6 Hz, 1H), 4.38 (br s, 1H), 4.30 (ddd, J = 1.6, 10.3, 10.3 Hz, 1H), 4.09-4.06 (m, 1H), 3.50 (s, 3H), 3.24 (s, 3H), 2.97 (dd, J = 9.8, 16.0, Hz, 1H), 2.73 (dt, J = 4.9, 11.5 Hz, 1H), 2.64 (ddd, J = 3.1, 9.1, 15.7 Hz, 1H), 2.56-2.50 (m, 2H), 2.41 (dt, J = 6.2, 18.9 Hz, 1H), 2.32 (dd, J = 1.7, 16.0 Hz, 1H), 2.28-2.22 (m, 1H), 2.18-2.12 (m, 1H), 2.01 (dd, J = 1.2, 15.4 Hz, 1H), 1.86-1.14 (series of m, 14H), 1.10 (s, 9H), 1.07 (s, 9H), 1.02 (s, 9H), 1.01 (s, 9H), 0.87 (t, J = 7.4 Hz, 3H), 0.82 (t, J = 7.0 Hz, 3H), 0.30 (s, 3H), 0.28 (s, 3H), 0.27 (s, 3H), 0.21 (s, 3H), 0.18 (s, 3H), 0.082 (s, 3H), 0.078 (s, 3H), 0.073 (s, 3H); 13C NMR (125 MHz, C6D6) δ 207.8, 173.2, 171.8, 140.3, 134.5, 134.0, 132.8, 132.3, 130.7, 128.9, 125.6, 101.0, 73.2, 70.1, 69.5, 68.7, 68.5, 66.0, 57.9, 56.6, 51.2, 48.5, 46.6, 45.7, 44.0, 43.7, 41.0, 39.2, 36.8, 35.2, 30.2, 26.5 (3C), 26.3 (3C), 26.1 (3C), 26.0 (3C), 22.9, 19.4, 18.6, 18.5, 18.4, 18.1, 14.0, 12.4, −3.3, −3.8, −4.0, −4.1, −4.5, −4.8, −4.9, −5.0; high resolution mass spectrum (ES-) m/z 1129.7023 [(M+Na+) calculated for C59H110O11Si4+Na 1129.7003.
Supplementary Material
Acknowledgment
Financial support was provided by the National Institutes of Health (Institutes of General Medicinal Sciences) through Grant No. GM-29028. We also thank Drs. George Furst, R. Kohli and Patrick Carroll in obtaining the NMR spectra, the high resolution mass spectra, and the x-ray crystal analyses, respectively
Footnotes
Supporting Information Available. Experimental procedures and analytical and spectroscopic data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- i.Davisson JW, Tanner FW, Jr., Finlay AC, Solomons IA. Antibiotics and Chemotherapy. 1951;1:289. [PubMed] [Google Scholar]
- ii.a Gold W, Stout HA, Pagano JF, Donovick R. Antobiotics Annual. 1955-1956:579. [PubMed] [Google Scholar]; b Vandeputte J, Wachtel JL, Stiller ET. Antiobiotics Annual. 1955-1956:587. [PubMed] [Google Scholar]; c Dutcher JD, Young MB, Sherman JH, Hibbits W, Walters DR. Antiobiotics Annual. 1956-1957:866. [PubMed] [Google Scholar]; d Mechlinsker W, Schaffner CP, Ganis P, Avitabile G. Tetrahedron Lett. 1970;11:3873. [Google Scholar]
- iii.a Hazel EL, Brown R. Science. 1950;112:423. [PubMed] [Google Scholar]; b Lancelin J-M, Beau J-M. Tetrahedron Lett. 1989;30:4521. [Google Scholar]
- iv.a Taber WA, Vining LC, Waksman SA. Antiobiot. Chemother. 1954;4:455. [PubMed] [Google Scholar]; b Vining LC, Taber WA. Can. J. Chem. 1956;34:1163. [Google Scholar]; c Pawlak J, Sowinski P, Borowski E. J. Antibiot. 1993;46:1598. doi: 10.7164/antibiotics.46.1598. [DOI] [PubMed] [Google Scholar]
- v.a Cope AC, Burrows EP, Derieg ME, Moon S, Wirth W. J. Am. Chem. Soc. 1965;87:5452. doi: 10.1021/ja00951a036. [DOI] [PubMed] [Google Scholar]; b Cope AC, Axen U, Burrows EP. J. Am. Chem. Soc. 1966;88:4221. [Google Scholar]
- vi.Pandey R, Rinehart KL., Jr. J. Antiobiot. 1977;30:146. doi: 10.7164/antibiotics.30.146. [DOI] [PubMed] [Google Scholar]
- vii.Sowinski P, Pawlak J, Borowski E. J. Antiobiot. 1995;48:1288. doi: 10.7164/antibiotics.48.1288. [DOI] [PubMed] [Google Scholar]
- viii.a Nicolaou KC, Chakraborty TK, Daines RA, Simpkins NS. J. Chem. Soc., Chem. Commun. 1986;5:413. [Google Scholar]; b Nicolaou KC, Daines RA, Chakraborty TK, Ogawa Y. J. Am. Chem. Soc. 1987;109:2821. [Google Scholar]; c Chemtracts: Organic Chemistry. 1990;3:327. [Google Scholar]
- ix.a Masamune S, Ma P, Okumoto H, Ellingboe JW, Ito Y. J. Org. Chem. 1984;49:2834. [Google Scholar]; b Boschelli D, Takemasa T, Nishitani Y, Masamune S. Tetrahedron Lett. 1985;26:5239. [Google Scholar]; c Masamune S. Ann. N.Y. Acad. Sci. 1988;544:168. doi: 10.1111/j.1749-6632.1988.tb40399.x. [DOI] [PubMed] [Google Scholar]
- x.Packard GK, Hu Y, Vescvsi A, Rychnovsky SD. Angew. Chem. Int. Ed. 2004;43:2822. doi: 10.1002/anie.200453697. [DOI] [PubMed] [Google Scholar]
- xi.Anion Relay Chemistry (ARC) as recently defined11p entails the migration of a negative charge within a molecule (i.e., through bond or through space, the latter employing a transfer element such as a trisubstituted silyl group). Two types of through space ARC process are possible. In Type I ARC the anion after initial migration returns to the site of origin, whereas in Type II ARC the anion is relayed to a new remote site. For an account on the evolution of Type I and Type II Anion Relay Chemistry, see ref. 11p. For early examples of Type I Anion Relay Chemistry seeMatsuda, et al. Chem. Soc. Perkin Trans. 1. 1979;1:26.Tietze LF, Geissler H, Gewert JA, Jakobi U. Synlett. 1994:511. and Shinokubo H, Miura K, Oshima K, Utimoto K. Tetrahedron. 1996;52:503.Smith AB, III, Boldi AM. J. Am. Chem. Soc. 1997;119:6925.Smith AB, III, Pitram SM. Org. Lett. 1999;1:2001. doi: 10.1021/ol991166b.Smith AB, III, Pitram MS, Furetes MJ. Org. Lett. 2003:2751. doi: 10.1021/ol034989g.Smith AB, III, Pitram SM, Boldi AM, Gaunt MJ, Sfouggatakis C, Moser WH. J. Am. Chem. Soc. 2003;125:14435. doi: 10.1021/ja0376238.Smith AB, III, Duffey MO. Synlett. 2004:1363.Smith AB, III, Kim D-S. Org. Lett. 2004;6:1493. doi: 10.1021/ol049601b.Smith AB, III, Kim D-S. Org. Lett. 2005;7:3247. doi: 10.1021/ol0510264.Smith AB, III, Kim D-S. J. Org. Chem. 2006;71:2547. doi: 10.1021/jo052314g.Smith AB, III, Xian M. J. Am. Chem. Soc. 2006;128:66. doi: 10.1021/ja057059w.Smith AB, III, Xian M, Kim W-S, Kim D-S. J. Am. Chem. Soc. 2006;128:12368. doi: 10.1021/ja065033e.Smith AB, III, Kim D-S, Xian M. Org. Lett. 2007;9:3307. doi: 10.1021/ol071281j.Smith AB, III, Kim D-S. Org. Lett. 2007;17:3311. doi: 10.1021/ol071282b.Smith AB, III, Wuest WM. Chem. Commun. 2008:5883. doi: 10.1039/b810394a.Smith AB, III, Kim W-S, Wuest WM. Angew. Chem. Int. Ed. 2008;47:7082. doi: 10.1002/anie.200802301.Bevarie-Baez NO, Kim W-S, Smith AB, III, Xian M. Org. Lett. 2009;11:1861. doi: 10.1021/ol900434k. For examples of Type I and II ARC developed by Smith and co-workers, see references 11d-h
- xii.Smith AB, III, Pitram SM, Gaunt MJ, Kozmin SA. J. Am. Chem. Soc. 2002;124:14516. doi: 10.1021/ja0283100. [DOI] [PubMed] [Google Scholar]
- xiii.Miyaura N, Yamada K, Suzuki A. Tetrahedron Lett. 1979;36:3437. [Google Scholar]
- xiv.a Baeyer A, Villiger V. Ber. Dtsch. Chem. Ges. 1899;32:3625. [Google Scholar]; b Hawthorne MF, Emmons WD, McCallum KS. J. Am. Chem. Soc. 1958;80:6393. [Google Scholar]
- xv.a Katsuki T, Sharpless KB. J. Am. Chem. Soc. 1980;102:5974. [Google Scholar]; b Romero A, Wong C-H. J. Org. Chem. 2000;65:8264. doi: 10.1021/jo000933d. [DOI] [PubMed] [Google Scholar]
- xvi.Payne G. J. Org. Chem. 1962;27:3819. [Google Scholar]
- xvii.a Kohler EP, Butler FR. J. Am. Chem. Soc. 1926;48:1036. [Google Scholar]; b Woodward RB, Bader FE, Bickel H, Frey AJ, Kierstead RW. Tetrahedron. 1958;2:1. [Google Scholar]; c Cannizzaro CE, Ashley JA, Janda KD, Houk KN. J. Am. Chem. Soc. 2003;125:2489. doi: 10.1021/ja020879d. [DOI] [PubMed] [Google Scholar]
- xviii.Gao Y, Klunder JM, Hanson RM, Ko SY, Masamune H, Sharpless KB. J. Am. Chem. Soc. 1987;109:5765. [Google Scholar]
- xix.See Supporting Information
- xx.Dewey RS, van Tamelen EE. J. Am. Chem. Soc. 1961;83:3729. [Google Scholar]
- xxi.Cusack NJ, Reese CB, Risius AC, Roozpeikar B. Tetrahedron. 1976;32:2157. [Google Scholar]
- xxii.a Hicks DR, Fraser-Reid B. Synthesis. 1974:203. [Google Scholar]; b Corey EJ, Weigel LO, Chamberlin AR, Lipshutz B. J. Am. Chem. Soc. 1980;102:1439. [Google Scholar]
- xxiii.a Osborn JA, Jardine FH, Young JF, Wilkinson G. J. Chem. Soc. (A) 1966:1711. [Google Scholar]; b Birch AJ, Walker KAM. Tetrahedron Lett. 1967;20:1935. [Google Scholar]
- xxiv.Smith M, Rammler DH, Goldberg IH, Khorana HG. J. Am. Chem. Soc. 1961;84:430. [Google Scholar]
- xxv.Oikawa Y, Yoshioka T, Yonemitsu O. Tetrahedron Lett. 1982;23:885. [Google Scholar]
- xxvi.Chen K-M, Hardtmann GE, Prasad K, Repic O, Shapiro MJ. Tetrahedron Lett. 1987;28:155. [Google Scholar]
- xxvii.a Brook AG. J. Am. Chem. Soc. 1958;80:1886. [Google Scholar]; b Brook AG, Warner CM, McGriskin ME. J. Am. Chem. Soc. 1959;81:981. [Google Scholar]
- xxviii.Stork G, Zhao K. Tetrahedron Lett. 1989;30:287. [Google Scholar]
- xxix.Cristau H-J, Chabaud B, Labaudinière R, Christol H. Synth. Commun. 1981;11:423. [Google Scholar]
- xxx.Corey EJ, Erickson BW. J. Org. Chem. 1971;36:3553. [Google Scholar]
- xxxi.a Dess DB, Martin JC. J. Org. Chem. 1983;48:4155. [Google Scholar]; b Langille NF, Dakin LA, Panek JS. Org. Lett. 2003;5:575. doi: 10.1021/ol027518n. [DOI] [PubMed] [Google Scholar]
- xxxii.Wu Y, Shen X, Huang J-H, Tang C-J, Liu H-H, Hu Q. Tetrahedron Lett. 2002;43:6443. [Google Scholar]
- xxxiii.a Evans DA, Bartroli J, Shih TL. J. Am. Chem. Soc. 1981;103:2127. [Google Scholar]; b Evans DA, Nelson JV, Vogel E, Taber TR. J. Am. Chem. Soc. 1981;103:3099. [Google Scholar]
- xxxiv.Evans DA, Rieger DL, Jones TK, Kaldor SW. J. Org. Chem. 1990;55:6260. [Google Scholar]
- xxxv.2,6-Di-tert-butyl-4-methylpyridine is a non-nucleophilic pyridine derivative that is also relatively non-polar, allowing for facile purification of diketone (+)-54.
- xxxvi.Streitwiesser A, Xie L, Wang P, Bachrach SM. J. Org. Chem. 1993;58:1778. [Google Scholar]
- xxxvii.Pappo R, Allen DS, Jr., Lemieux RU, Johnson WS. J. Org. Chem. 1956;21:478. [Google Scholar]
- xxxviii.Criegee R. Ber. 1931;64:260. [Google Scholar]
- xxxix.Bal BS, Childers WE, Jr, Pinnick HW. Tetrahedron. 1981;37:2091. [Google Scholar]
- xl.Parikh JR, Doering W. v. E. J. Am. Chem. Soc. 1967;89:5505. [Google Scholar]
- xli.Takai K, Nitta K, Utimoto K. J. Am. Chem. Soc. 1986;108:7408. doi: 10.1021/ja00279a068. [DOI] [PubMed] [Google Scholar]
- xlii.Evans DA, Black WC. J. Am. Chem. Soc. 1993;115:4497. [Google Scholar]
- xliii.Smith AB, III, Minbiole KP, Verhoest PR, Schelhaas M. J. Am. Chem. Soc. 2001;123:10942. doi: 10.1021/ja011604l. [DOI] [PubMed] [Google Scholar]
- xliv.a Nakamura R, Tanino K, Miyashita M. Org. Lett. 2003;5:3583. doi: 10.1021/ol0352299. [DOI] [PubMed] [Google Scholar]; b Evans DA, Starr JT. J. Am. Chem. Soc. 2003;125:13531. doi: 10.1021/ja037643+. [DOI] [PubMed] [Google Scholar]
- xlv.Scheidt KA, Chen H, Follows BC, Chemler SR, Coffey DS, Roush WR. J. Org. Chem. 1998;63:6436. [Google Scholar]
- xlvi.a Shekhani MS, Khan KM, Mahmood K, Shah PM, Malik S. Tetrahedron Lett. 1990;31:1669. [Google Scholar]; b Marshall JA, Eidam PM. Org. Lett. 2008;10:93. doi: 10.1021/ol702024b. [DOI] [PubMed] [Google Scholar]
- xlvii.Griffith WP, Ley SV. Aldrichimica Acta. 1990;23:13. [Google Scholar]
- xlviii.Ivanov IV, Romanov SG, Shevchenko VP, Rozhkova EA, Maslov MA, Groza NV, Myasoedov NF, Kuhn H, Myagkova GI. Tetrahedron. 2003;59:8091. [Google Scholar]
- xlix.Coleman RS, Walczak MC. Org. Lett. 2005;7:2289. doi: 10.1021/ol050768u. [DOI] [PubMed] [Google Scholar]
- l.Inanaga J, Hirara K, Saeki H, Katsuki T, Yamaguchi M. Bull. Chem. Soc. Jpn. 1979;52:1989. [Google Scholar]
- li.For 1H and IR data see Ainsworth PJ, Craig D, Reader JC, Slawin AMZ, White AJP, Williams DJ. Tetrahedron. 1995;51:11601.Constantine MG, de Oliveira KT, Polo EC, da Silva GVJ, Brockson TJ. J. Org. Chem. 2006;71:9880. doi: 10.1021/jo061722x. For 1H and 13C NMR see
- lii.See Supporting Information for 1H and 13C NMR assignments.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.