Abstract
GABAA receptors (GABAARs) regulate the majority of fast inhibition in the mammalian brain and are the target for multiple drug types, including sleep aids, anti-anxiety medication, anesthetics, alcohol, and neurosteroids. A variety of subunits, including the highly distributed γ2, allow for pharmacologic and kinetic differences in particular brain regions. The two common splice variants γ2S (short) and γ2L (long) show different patterns of regional distribution both in adult brain and during the course of development, but show few notable differences when incorporated into pentameric receptors. However, results presented here show that the γ2S variant can strongly affect both GABAAR pharmacology and kinetics by acting as an external modulator of fully formed receptors. Mutation of one serine residue can confer γ2S-like properties to γ2L subunits, and addition of a modified γ2 N-terminal polypeptide to the cell surface recapitulates the pharmacological effect. Thus, rather than incorporation of a separate accessory protein as with voltage-gated channels, this is an example of an ion channel using a common subunit for dual purposes. The modified receptor properties conferred by accessory γ2S have implications for understanding GABAAR pharmacology, receptor kinetics, stoichiometry, GABAergic signaling in the brain during development, and altered function in disease states such as epilepsy.
Introduction
Exquisite and precise signaling control in a healthy brain is achieved via complex network circuitry composed of excitatory and inhibitory neuronal connections. The majority of inhibitory signals are mediated by a Cl− conductance through ionotropic GABAA receptors (GABAARs). Despite myriad possible types of subunits and combinations, the most common GABAAR heteropentamers in the mammalian brain are composed of two α, two β, and one γ subunit (Akabas, 2004). GABAARs are also of particular interest as the targets of several classes of drugs and modulators, including benzodiazepines (BDZs), anesthetics, neurosteroids, and Zn2+ ions (Hevers and Luddens, 1998). However, different GABAAR subtypes respond to modulators with their own specificity (Mohler et al., 1995), and, to control the placement of different subtypes to their appropriate loci in the brain, elaborate (but not fully elaborated) mechanisms are in place to regulate assembly and trafficking.
One element of this fine control is the use of splice variants. The γ2 subunit has two forms, designated “short” (γ2S) and “long” (γ2L) that differ only in eight amino acids inserted into the large intracellular loop of the subunit (Whiting et al., 1990). The γ2S variant is more prevalent in early development (Wang and Burt, 1991) and is found at higher density in the cerebral cortex, olfactory bulb, and hippocampal formation (Gutiérrez et al., 1994; Miralles et al., 1994). In old age and in schizophrenia, γ2S levels fall (Gutiérrez et al., 1996; Huntsman et al., 1998). However, transgenic mice with only γ2S or γ2L appear thus far to have few if any clear phenotypic differences (Wick et al., 2000).
In previous experiments, BDZ potentiation was found to saturate as the ratio of GABAAR γ2 subunit was increased with fixed amounts of α1 and β2 subunits (Boileau et al., 2002) or by using subunit concatamers (Baumann et al., 2002; Boileau et al., 2005; Baur et al., 2006; Ericksen and Boileau, 2007). However, in transfections with lower γ2S ratios that displayed minimal BDZ potentiation and thus a large ratio of zinc-sensitive α1β2 receptors, the blockade by Zn2+ ions was unexpectedly low. In the present study, a striking difference between the two splice variants γ2S and γ2L was found in zinc sensitivity. In mixtures of αβ and αβγ2L GABAARs, the diazepam (DZ) potentiation of submaximal GABA-induced currents correlated well with zinc blockade of the mixture, whereas in mixtures of αβ and αβγ2S receptors, the zinc block was much less than predicted. Colocalization showed that γ2S subunits express on the cell surface alone, even in the presence of α and β subunits. Both the anomalous zinc sensitivity and surface expression of γ2S could be mimicked in γ2L by a single point mutation. Finally, external addition of a γ2 N-terminal polypeptide protected αβ GABAARs from zinc block. The “accessory” γ2S subunit also altered the kinetics of the expressed receptors. Together, the data demonstrate that the γ2S splice variant serves dual roles: as an integral subunit of the GABAAR and as an accessory protein acting as an external modulator of GABAAR function.
Materials and Methods
Cell culture and transfection.
HEK293 cells (CRL 1573; American Type Culture Collection) were grown on 100 or 35 mm tissue culture dishes in Minimum Essential Medium with Earle's salts (Invitrogen) containing 10% fetal bovine serum (Hyclone Laboratories) in a 37°C incubator under a 5% CO2 atmosphere. Transfections with cDNAs for rat GABAAR α1, β2, γ2S, and γ2L subunits, tandem subunits, mutant subunits, and enhanced green fluorescent protein (EGFP) in pCEP4 (Invitrogen) were performed as described previously (Boileau et al., 2005). For example, transfection with free subunits used 200 ng of pCEP4–α1 plus 200 ng of pCEP4–β2 plus a varied weight ratio of pCEP4–γ2S, pCEP4–γ2L, or pCEP4–γ2L mutant; thus, a 1:1:10 ratio had 2000 ng of pCEP4–γ2, and γ2S or γ2L were used in the same amounts and yielded a similar range of current amplitudes (1–10 nA of peak current at −40 mV). Cells were cotransfected at 70–90% confluence using Lipofectamine 2000 (Invitrogen), washed, and replated to coverslips after 4–8 h.
Drug application and recording.
Solutions were applied to excised outside-out patches using a four-barrel square glass application pipette or theta pipette connected to a piezoelectric stacked translator (Physik Instrumente) as described previously (Boileau et al., 2005). The voltage input to the high-voltage amplifier (Physik Instrumente) driving the stacked translator was filtered at 90 Hz using an eight-pole Bessel filter (Frequency Devices) to reduce oscillations arising from rapid acceleration of the pipette. The open-tip solution exchange time (typically 100–300 μs) was estimated using a solution of lower ionic strength in the drug barrel after experiments were completed.
The recording chamber was perfused continuously with HEPES-buffered saline containing the following (in mm): 135 NaCl, 5.4 KCl, 1 MgCl2, 1.8 CaCl2, and 5 HEPES, pH 7.2. Recording pipettes were filled with the following (in mm): 129 KCl, 9 NaCl, 5 EGTA, 10 HEPES, and 4 MgCl2, pH 7.2. The Cl− equilibrium potential across the patch was ∼0 mV. GABA solutions were prepared daily from powder and diluted to desired concentrations in the same control/bath solution. Recordings were performed at room temperature (22–25°C).
Recording electrodes were fabricated from KG-33 glass (Garner Glass) using a multistage puller (Flaming-Brown model P-97; Sutter Instruments) and coated with Sylgard to reduce electrode capacitance. The tips were fire polished. Open-tip electrode resistance was typically 2–4 MΩ when filling with standard recording solution. Outside-out patches were held at −40 mV using a low noise patch amplifier (Axopatch 200A; Molecular Devices). Data were low-pass filtered at 2–5 kHz using amplifier circuitry, sampled at 5–10 kHz, and stored on the computer hard drive.
Axograph, pClamp (Molecular Devices), Excel (Microsoft), and Prism (GraphPad Software) were used for data acquisition and analysis. For comparisons of multiple datasets, one-way ANOVA was used, with Dunnett's or Bonferroni's multiple comparison post hoc tests for significance of difference (GraphPad Software), and confirmed with a false discovery rate test at http://www.unt.edu/benchmarks/archives/2002/april02/rss.htm (Benjamini and Hochberg, 1995).
Zn2+ blockade tests were performed as follows: patches were tested with 200 ms pulses of 1 mm GABA, and peak current was measured for three or more trials to assess rundown. Patches exhibiting >10% rundown were discarded. Patches were then preincubated for ≥30 s in control solution containing 30 μm ZnCl2 and then exposed to 1 mm GABA plus 30 μm ZnCl2, and peak current was measured. Cells were then washed back into control solutions to recover from Zn2+ block and retested. If the post hoc test peaks were decreased by >10% from the original control peaks, the test was repeated for that patch or discarded. Percentage block was calculated as [1 − (peak current in Zn2+)/(peak current in control)] × 100%.
Potentiation of IGABA by DZ was performed as follows: patches were exposed repeatedly to a low concentration of GABA (3 μm) for 500 ms until a stable current level was achieved. Patches were next preincubated ≥30 s with control solution plus 1 μm DZ and then pulsed 500 ms with 3 μm GABA plus 1 μm DZ until a stable current level was achieved. Potentiation was calculated from peak currents before and during DZ exposure using the equation (IGABA + DZ/IGABA) − 1.
Immunostaining.
Transfected cells were replated on coverslips, washed with PBS, fixed for 15 min in 4% paraformaldehyde in PBS, blocked with 1% BSA in PBS, and incubated with M2 monoclonal 1° antibody (Sigma-Aldrich) against FLAG-tagged γ2 subunits, chimeras, or truncation mutants. After multiple washes, coverslips were incubated with 2° goat anti-mouse antibodies labeled with 655 nm peak emission quantum dots (Invitrogen). Images were acquired using a Bio-Rad MRC 1024 confocal laser-scanning microscope with 488 nm laser excitation at 15–30% power and emission filters at 522 nm (bandpass, 35 nm) for EGFP and 680 nm (bandpass, 32 nm) for red quantum dots.
Colocalization measurements.
Transfected cells were replated on coverslips, washed with PBS, fixed for 15 min in 4% paraformaldehyde in PBS, blocked with 1% BSA in PBS, and incubated simultaneously with polyclonal 1° antibody against α1 subunits and monoclonal 1° antibody against FLAG-tagged γ2 subunits. After multiple washes, coverslips were incubated with 2° antibodies labeled with quantum dots at 565 nm (green, goat anti-rabbit) for α1 subunits and 655 nm (red, goat anti-mouse) for γ2 subunits. Images were acquired using a Bio-Rad MRC 1024 confocal laser scanning microscope with 488 nm laser excitation at 15–30% power and emission filters at 522 nm (bandpass, 35 nm) and 680 nm (bandpass, 32 nm), respectively. Colocalization between green and red signals was measured using ColocalizerPro software on selected regions of interest (whole-cell edges or nonoverlapping membrane segments) after background correction. Values reported correspond to M1 (see Fig. 2B) and M2 (see Fig. 2C), where M1 is the ΣRcoloc/ΣRtotal and M2 is ΣGcoloc/ΣGtotal (R represents red pixels, and G represents green pixels).
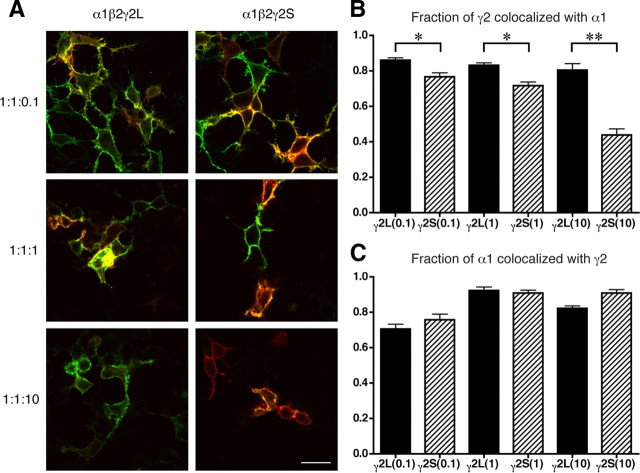
Figure 2.
γ2S subunits can also express independently, even in the presence of α1 and β2 subunits. A, Selected images from colocalization experiments performed on HEK293 cells transfected with α1β2γ2 cDNAs at 1:1:0.1, 1:1:1, and 1:1:10 ratios. Cells were labeled with 565 nm quantum dot-labeled antibodies (green) for α1 subunits and 655 nm (far red, pseudocolored red) for γ2 subunits (see Materials and Methods). Colocalized signal ranges from yellow–green to orange–red. Scale bar, 20 μm. B, Measurements of colocalization of γ2L or γ2S with α1 signal. At each transfection ratio, γ2S subunits were significantly different from γ2L subunits in the amount of red signal that was not colocalized with green (α1) signal. Data are mean ± SEM for n ≥ 50 cells per condition. *p < 0.01, **p < 0.001. C, Control measurements of α1 colocalization with γ2L or γ2S. At no transfection ratio does γ2S differ significantly from γ2L for this measurement.
Truncation and point mutant construction, purification, and quantitation.
Point mutants in γ2L (S343D, S343V) were made in pCEP4 using recombinant PCR overlap extension with diagnostic restriction sites engineered into the mutating oligonucleotides. N-terminal truncated subunit constructs were made by PCR with an upstream oligonucleotide and a downstream oligonucleotide including coding for six lysines (or arginines) and a stop codon. For γ2N–K6 (supplemental Fig. S3K, available at www.jneurosci.org as supplemental material), the native sequence ends at Met 233 (in the mature rat protein sequence) with six lysines added before the new stop codon, whereas γ2N–R6 includes Arg 231 and Arg 232 plus four more arginines. α1N–K6 ends at I222 plus six lysines, and β2N–K6 ends at I218 plus six lysines using FLAG-tagged α1 (Horenstein et al., 2001) or β2 subunit cDNA as template. A FLAG-tagged β2 subunit was constructed for this study and used as template for β2N–K6 with FLAG inserted between the third and fourth amino acid residues of the mature rat β2 subunit. Coexpression of β2–FLAG with α1 gave currents indistinguishable from nontagged β2. Constructs for γ2N+M1 ends at I257 with K6 or R6 tails (supplemental Fig. S3J, available at www.jneurosci.org as supplemental material). All constructs were subcloned into pCEP4 and were confirmed with double-stranded DNA sequencing.
For protein production, HEK293 cells were transfected with N-terminal truncated subunit plasmid constructs at 500–1000 ng cDNA/35 mm plate. Forty-eight to 72 h after transfection, intact cells were washed with ice-cold PBS (in mm: 2.7 KCl, 1.5 KH2PO4, 0.5 MgCl2, 137 NaCl, and 14 Na2HPO4, pH 7.1). Sulfhydryl groups were blocked by incubating cells with 10 mm N-ethylmaleimide (NEM) in PBS (15 min, room temperature). Cells were solubilized (overnight, 4°C) in lysis buffer (2% Triton X-100, 50 mm Tris-HCl, 150 mm NaCl, and 5 mm EDTA, pH7.5) supplemented with protease inhibitors (Complete, mini; Roche Diagnostics) and 10 mm NEM. Lysates were cleared by centrifugation (16,000 × g, 10 min, 4°C). The FLAG-tagged N-terminal truncations were immunopurified from the cell lysates by incubating (4–6 h, 4°C, rotating) with 40 μl of EZview Red ANTI-FLAG M2 affinity gel (Sigma-Aldrich), washed six times with 1 ml of wash buffer (150 mm NaCl and 50 mm Tris-Cl, pH 7.5). The FLAG-tagged subunits were eluted from the beads with 100 μl of 400 μg/ml FLAG peptide in recording buffer (1 h, 4°C rotating). After the incubation, the samples were centrifuged (8200 × g, 10 min, 4°C), and the eluate was collected and stored at −20°C.
For protein concentration determinations, samples were denatured with 2× Laemli's sample buffer (3% SDS, 0.6 m sucrose, 0.325 m Tris-HCl, pH 6.8, and 10 mm DTT) and then separated by SDS-PAGE on 4–15% gradient Tris-HCl Ready Gels (Bio-Rad). Samples were run alongside a twofold dilution series of Met-FLAG-BAP (Sigma) from 400 to 6.25 ng. After transfer to nitrocellulose, blots were processed using the One-Step Western Blot kit for mouse (Genscript), according to the instructions of the manufacturer and using M2 anti-FLAG antibody (Sigma) at 1:400 dilution. Blots were then treated with ECL Western Blotting Detection Reagents (RPN2106V1; GE Healthcare) and several exposures on Kodak X-Omat AR, XAR-5 film (Eastman Kodak) were taken for comparison of signal linearity. It was noted that Pierce SuperSignal West Pico Chemiluminescent Substrate was distinctly nonlinear and was therefore not used for these determinations. Exposed films were scanned at 1200 dpi and analyzed using LabWorks software version 4.6 (UVP Inc.) and confirmed using NIH ImageJ software (http://rsb.info.nih.gov/ij/).
Results
Potentiation of IGABA by the classical BDZ DZ was measured (Fig. 1, green traces) in α1β2 receptors (Fig. 1A, without γ2 subunits), α1β2γ2 receptors with stoichiometry fixed by using a 1:1:10 ratio, and in transfections with lower γ2 ratios (Fig. 1B). Potentiation values for receptors with fixed stoichiometry by using high γ2 ratios or by using subunit concatamers ranged from 2.0 to 2.5 (IGABA + DZ/ IGABA − 1), whereas α1β2 receptors showed no potentiation, as expected (Fig. 1A). Receptors formed from transfections of α1β2γ2 in a 1:1:1 ratio showed less potentiation with greater variability (1.26 ± 0.55 for γ2S and 1.20 ± 0.23 for γ2L, mean ± SD), as predicted for a mixture of α1β2 and α1β2γ2 receptors. Moreover, as the γ2S or γ2L ratio was decreased, the mean potentiation dropped further, as more α1β2 receptors were formed. The variability of BDZ potentiation at low ratios is illustrated using α1β2γ2L transfections at 1:1:0.1 (Fig. 1, right).
Figure 1.
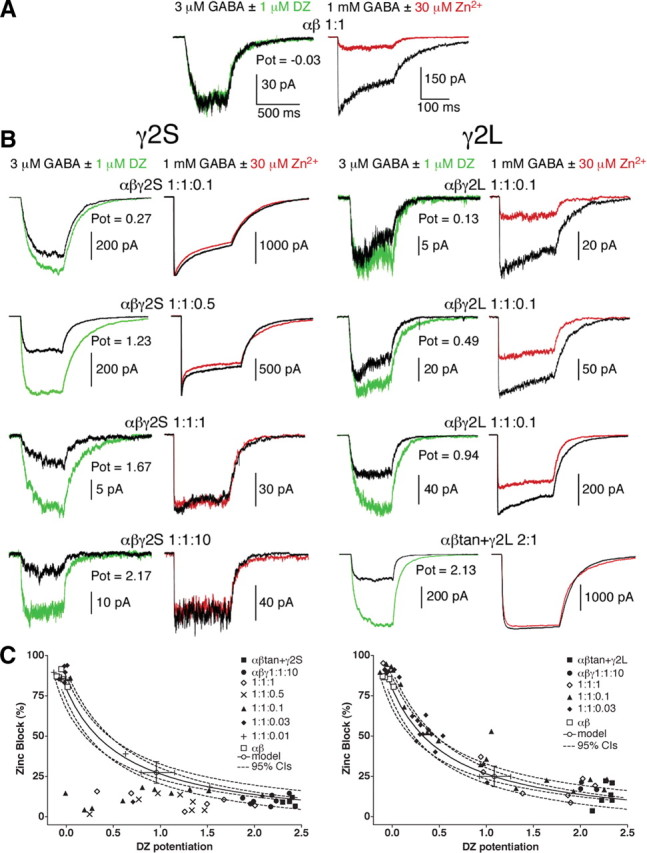
Diazepam potentiation and Zn2+ block for varying γ2S or γ2L transfection ratios. A, HEK293 cells expressing α1 plus β2 subunits. Left, Current traces from 500 ms, 3 μm GABA pulses (black) are superimposed with currents from the same outside-out patch exposed to a coapplication of 3 μm GABA plus 1 μm DZ (green) after equilibration in control solution plus 1 μm DZ. Potentiation (Pot) is reported as (IGABA + DZ/IGABA) − 1. Right, In the same patch, near-maximal current is elicited by 200 ms, 1 mm GABA pulses (black) and blocked by coapplication of 30 μm ZnCl2 (red). Note that α1β2 receptors display significant Zn2+ block but no appreciable DZ potentiation. B, Traces for cells expressing α1 + β2 + γ2S (left) or γ2L (right) at indicated ratios. Note that three γ2L patches are shown from 1:1:0.1 ratio transfections to exhibit variability. Also included are traces for the α1β2γ2L receptors constrained by use of α1–β2 tandem receptors cotransfected with γ2L at a 2:1 ratio. Note that these receptors show similar high potentiation by DZ, minimal blockade by Zn2+, and minimal desensitization in 200 ms pulses (right) at 1 mm GABA, similar to α1β2γ2S receptors constrained by transfection at a 1:1:10 ratio. C, Paired Zn2+ block plotted against DZ potentiation for transfection ratios ranging from 1:1:0.01 to 1:1:10 for α1β2γ2, as well as tandem-constrained αβtan+γ (Boileau et al., 2005) and α1β2. Note that αβ receptors (open squares) show high zinc sensitivity and no potentiation, as do some of the patches from transfections with very low γ2 ratios, indicative of no γ2 subunits incorporated in that patch. The solid curve represents an interpolation between αβ and αβγ DZ potentiation and Zn2+ block values, modeled using EC50, single-channel conductance, and open probability differences between the two receptor types (Boileau et al., 2005). The open circle lies on the model curve and has error bars corresponding to the 95% confidence intervals (CIs) for DZ potentiation and Zn2+ block (dashed lines). Note that many of the Zn2+ block values for transfections with γ2S ratios below 1:1:10 fall beneath the 95% confidence interval (C, left), whereas transfections varying γ2L ratios do not (C, right).
The same patches were also tested for Zn2+ block (Fig. 1, red traces). For transfections with the γ2S splice variant (Fig. 1B, left), the vast majority of patches from different transfection ratios, ranging from α1β2γ2 1:1:10 down to 1:1:0.1, demonstrated minimal Zn2+ block. At lower γ2S ratios such as α1β2γ2 1:1:0.03 and 1:1:0.01, the majority of patches showed no potentiation by DZ and maximal Zn2+ block, as if they contained mainly αβ receptors. What is striking is that there were almost no intermediate values for Zn2+ block with γ2S. When the paired DZ and Zn2+ data were plotted together (Fig. 1C), it became clear that, in the majority of patches, even when small DZ potentiation was detected (indicative of a preponderance of α1β2 receptors), the same patch was relatively insensitive to Zn2+ block. Most of the Zn2+-insensitive patches from α1β2γ2 1:1:≤1 transfections fell outside the 95% confidence intervals of a model prediction for a mixture of α1β2 and α1β2γ2 receptors, derived from measurements for EC50, open probability, and single-channel conductances (Boileau et al., 2005). However, DZ potentiation compared with deactivation and concentration–response ratios fit within modeled confidence estimates (supplemental Fig. S1, available at www.jneurosci.org as supplemental material).
In stark contrast, the γ2L subunit showed DZ potentiation and Zn2+ block that fit well with the same model (Fig. 1C, right). When DZ potentiation was high, Zn2+ block was low and vice versa, and intermediate values mimicked those expected for a mixture of α1β2 and α1β2γ2 receptors in a continuum of ratios. These results suggest that γ2S, but not γ2L, subunits somehow confer anomalous Zn2+ pharmacology to the α1β2 receptors in the mixture that should be blocked by Zn2+ but are not. There was no correlation between current size (i.e., expression levels) and zinc blockade. For example, current amplitudes ranged from 23 to 503 pA for α1β2γ2L 1:1:0.1 transfections (coefficient of variation of 0.93) (Fig. 1B), but correlation with zinc block was not significant (R2 = 0.126; data not shown). Similar data were obtained in Xenopus oocyte voltage-clamp experiments (data not shown), lending confidence to the generalizable nature of the phenomenon. The results also demonstrate that reliance on Zn2+ block for estimations of γ2 incorporation into receptors is risky at best for either splice variant (Fig. 1C). Mixtures of α1β2 and α1β2γ2L receptors may be difficult to distinguish from pure α1β2γ2 receptors using zinc alone (e.g., 70% α1β2γ2L receptors would be indistinguishable from 100%). Evidently, currents with even miniscule γ2S can appear to be entirely Zn2+ insensitive, giving a false impression of full γ incorporation.
One documented difference between the two γ2 splice variants is the ability of γ2S to express on the surface of the cell in the absence of α and β subunits, whereas γ2L is retained in intracellular compartments unless coexpressed with α and β subunits (supplemental Fig. S2, available at www.jneurosci.org as supplemental material), presumably in the endoplasmic reticulum (Connolly et al., 1999). Could γ2S subunits express on the surface in the presence of α1 and β2 subunits but not incorporate into α1β2γ2 receptors? To test this, colocalization of α1 subunits to either γ2 splice variant was measured, in α1β2γ2 transfection ratios 1:1:0.1, 1:1:1, and 1:1:10 (Fig. 2). Even in the lowest transfection ratio, γ2S subunits showed significantly less colocalization to α1 subunits than did γ2L subunits (Fig. 2B). In the highest ratio, the difference was overwhelming and shows that, contrary to a previous suggestion (Connolly et al., 1999), γ2S subunits can express on the surface as “rogues” even in the presence of coexpressed α1 and β2 subunits. The converse control, colocalization of α1 to γ2 subunits, showed no differences between splice variants (Fig. 2C). Whether the rogue γ2S subunits are present on the surface as monomers or multimers is presently unknown.
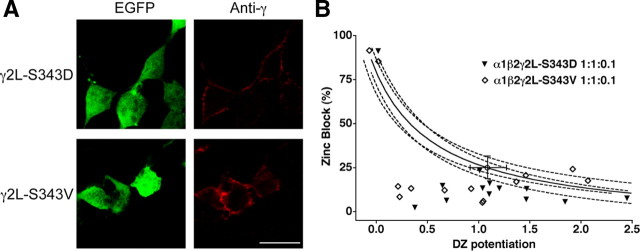
To test further the apparent correlation between surface expression and anomalous Zn2+ block, mutations were introduced into the eight amino acid region that differentiates the two splice variants. Serine at position 343 in the γ2L subunit has been shown to be a target for kinases (Whiting et al., 1990; Moss et al., 1992), and γ2S does not contain this residue. S343 was mutated to nonphosphorylatable valine or to an aspartate as an attempt to add a phosphomimetic side chain (Lin et al., 1998). Both mutations resulted in surface expression of the γ2L subunit when expressed alone, like γ2S (Fig. 3A). Moreover, in α1β2γ2L(mut) transfections at 1:1:0.1 ratios, patches excised from cells expressing either mutant showed low Zn2+ blockade, inconsistent with the paired DZ potentiation measurements from the same patches (Fig. 3B). In other words, the surface expression of γ2L(mut) correlated with the anomalous Zn2+ block and now mimicked γ2S. Because the two types of mutations showed no apparent differences, the aspartate substitution either conferred no phosphomimetic effect and/or the ER retention regulome of the HEK293 cell recognizes a serine or phosphoserine in particular as part of the retention signal.
Figure 3.
Mutations of serine 343 in γ2L subunits allow for rogue surface expression and anomalous Zn2+ blockade. A, Confocal images of cells cotransfected with EGFP and γ2L–S343D (serine to aspartate) or γ2L–S343V (serine to valine) are shown on the left. Scale bar, 20 μm. B, In separate experiments, excised patches from cells transfected with α1β2γ2L–S343D or α1β2γ2L–S343V (both at the transfection ratio 1:1:0.1) were tested for DZ potentiation and Zn2+ blockade, as in Figure 1. Note that both mutations cause γ2L to resemble γ2S.
Several unsuccessful chimeric and truncated subunits were constructed to narrow the regions of the γ2S subunit responsible for conferring protection from Zn2+ block to α1β2 receptors (supplemental Fig. S3, available at www.jneurosci.org as supplemental material). For example, coexpression of the γ2S M3–M4 cytoplasmic loop with α1 and β2 subunits was not sufficient to confer protection (data not shown), nor did a chimeric α1/γ2/α1 chimera with the γ2S M3–M4 loop (supplemental Fig. S3E, available at www.jneurosci.org as supplemental material) show Zn2+ insensitivity when expressed with α1 and β2 subunits or with β2 subunits alone. Receptors formed from coexpression of α1, β2, and a previously described chimeric γ2/α1 subunit with γ2 sequence through to the M3 transmembrane region (supplemental Fig. S3D, available at www.jneurosci.org as supplemental material) (Boileau and Czajkowski, 1999) at 1:1:0.5 ratio were variable in Zn2+ block (i.e., not protected), possibly because this chimera did not express as a rogue subunit (data not shown). No truncation mutants (supplemental Fig. S3F–K, available at www.jneurosci.org as supplemental material) expressed on the cell surface (supplemental Fig. S2 shows two examples, available at www.jneurosci.org as supplemental material).
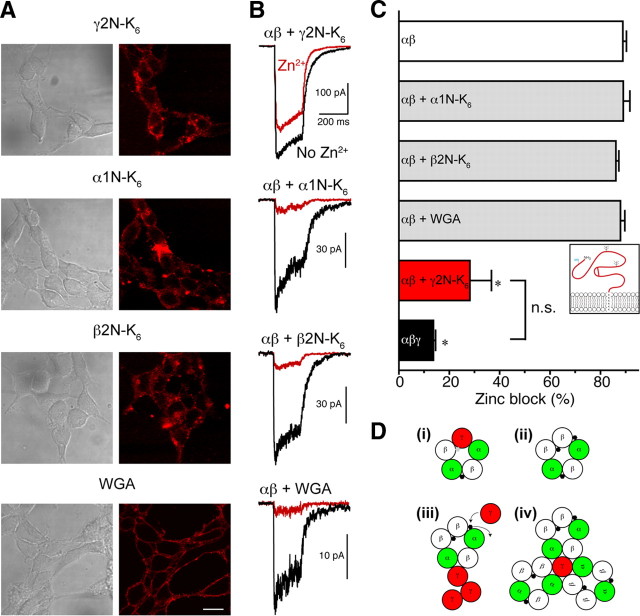
One truncation mutant, consisting of the extracellular N-terminal domain of the γ2 subunit (γ2N), was outfitted with six additional positively charged lysines (supplemental Fig. S3K, available at www.jneurosci.org as supplemental material) to mimic a cell-penetrating peptide (Han et al., 2001). Studies using peptide carriers with fluorescent tags have shown that a substantial portion of the signal remains affixed to the cell surface unless it is cleaved away with proteases (Richard et al., 2003). For the purpose of probing for a direct interaction between the N terminus of the γ2S subunit with α1β2 receptors, sticking to the surface was an exploitable artifact. Confocal images of immunopurified γ2N–K6 applied externally to α1β2-transfected cells for 1 h show that indeed the peptide adheres to the cell surface (Fig. 4A).
Figure 4.
External application of truncated extracellular γ2 subunits reduces Zn2+ block in αβ receptors. A, Confocal images of cells cotransfected with α1 and β2 subunits and treated with immunopurified sticky proteins γ2N–K6, α1N–K6, β2N–K6, or WGA linked to quantum dots. Cells were incubated in PBS for 60 min with immunopurified protein, washed several times, fixed, and treated with anti-FLAG antibody with 655 nm quantum dot-linked 2° antibody for the GABAAR extracellular polypeptides. On the left are transmitted light images, and on the right are the pseudocolored far red images. Scale bar, 20 μm. B, Top, Traces from excised patches showing reduced Zn2+ blockade of α1β2 receptors incubated with the γ2 N-terminal protein γ2N–K6. Traces with or without coapplied Zn2+ are the average of three traces from the same patch. Below are shown traces from α1β2 receptors incubated with control α1N–K6, β2N–K6, or quantum dot-linked WGA, none of which protected the receptors from Zn2+ block. C, Summary of Zn2+ block for α1β2 receptors (white bar), α1β2γ2 receptors (black; pooled α1β2γ2S 1:1:10, α1β2γ2L 1:1:10, αβtan+γ2S and αβtan+γ2L), and α1β2 receptors treated with γ2N–K6 (red bar, average of 200 ng of protein per 12 mm coverslip in a 1 ml well) or controls α1N–K6, β2N–K6, or WGA (gray bars, average of 500 ng of protein per coverslip). *p < 0.001 significance of difference from α1β2 receptors; n.s., α1β2γ2 and α1β2 + γ2N-K6 peptide not significantly different from one another. Inset, Depiction of the sticky γ2N–K6 protein. D, Schematic of possible mechanisms for Zn2+ protective effect of γ2S on α1β2 receptors. i, The α1β2γ2 receptor has one putative external Zn2+ binding site (black hexagon) and one “weak” luminal binding site (gray hexagon). ii, The α1β2 receptor has two identical external Zn2+ binding sites and one internal site that may have higher affinity than in the case of α1β2γ2 receptors. i and ii patterned after Hosie et al. (2003). iii, Rogue γ2S subunits (as lone subunits or multimers of unknown number) may bind to α1β2 receptors and hinder binding of Zn2+ ions. iv, γ2S subunits may cause α1β2 receptors to cluster, thus reducing Zn2+ block of several receptors.
When α1β2 receptors treated with modified γ2N were tested for Zn2+ blockade, protection was prominent (Fig. 4B). Control sticky proteins constructed from the N termini of the α1 subunit (α1N–K6), β2 subunit (β2N–K6), and quantum dot-linked wheat germ agglutinin (WGA) were also able to adhere to the cell surfaces (Fig. 4A) but did not protect α1β2 receptors from Zn2+ block (Fig. 4B). Summary quantitation of these effects are seen in Figure 4C, where Zn2+ block for α1β2 and α1β2γ2 receptors is compared with α1β2 receptors treated with γ2N–K6, α1N–K6, β2N–K6, or WGA. Only γ2N–K6 application protected α1β2 receptors significantly and yielded Zn2+ blockade not significantly different from that for α1β2γ2 receptors. A hexaarginine version of the γ2N was also constructed and tested but did not yield protection as effectively as the hexalysine γ2N, nor did hexaarginine and hexalysine versions of γ2N plus the M1 transmembrane region (supplemental Fig. S3J, available at www.jneurosci.org as supplemental material), for reasons that are not understood (data not shown). Because the γ2N–K6 proteins lack any transmembrane regions to anchor in the membrane or form part of the channel pore (formed by transmembrane region M2), these results strongly suggest that the N terminus of rogue γ2S renders α1β2 receptors insensitive to Zn2+ block, without being incorporated into receptor pentamers themselves. This may occur by either occluding external Zn2+ binding site(s) (Hosie et al., 2003) or causing conformational changes to render Zn2+ ineffective (Fig. 4Diii) (supplemental Fig. S4, available at www.jneurosci.org as supplemental material). The rogue γ2S subunit may act as a lone subunit or multimer (Fig. 4Diii) or may induce clustering of α1β2 receptors, leading to alteration or occlusion of the extracellular Zn2+ binding sites (Fig. 4Div). Regardless of the exact mechanism, it is clear that the γ2S subunit can have an extra-receptor, modulatory role.
As is often observed in the literature at low to intermediate γ2S ratios (e.g., 1:1:1 and 1:1:0.5), many patches showed a very fast desensitization component (Fig. 5A–C), concomitant with a trend toward faster rise times (Fig. 5D,E). The fastest component of desensitization in α1β2 receptors is 21 ± 4 ms (mean ± SD), whereas in pure populations of α1β2γ2 receptors (α1β2γ2 at 1:1:10, αβtan+γ2, α-β-α+γ-β concatamers), the fastest time constant is on the order of 1 s (Boileau et al., 2005; Ericksen and Boileau, 2007). However, in mixed α1β2/α1β2γ2 populations, the fastest time constant component is ∼7 ms (Fig. 5C), representing a new “ultrafast” component more rapid than either α1β2 or α1β2γ2 receptors. Comparable fast time constants could be seen with α1β2γ2L when transfected at low ratios that yield a mixed α1β2/α1β2γ2L population, suggesting that this effect is not attributable to rogue γ2S subunits. These data suggest rather that mixtures or clusters of α1β2γ2 with α1β2 receptors change the characteristics of both receptor subtypes compared with a pure population of either type (Fig. 5F). A fast time constant component can also be observed in acutely dissociated neurons (Kapur et al., 1999), suggesting the possibility of mixed receptor types in vivo. The 10–90% rise times for α1β2γ2L transfections are fairly uniform in mixed α1β2/α1β2γ2L populations (∼1 ms) (Fig. 5D), suggesting that clustering of α1β2 with α1β2γ2 receptors affects receptor kinetics, coincident with the appearance of the ultrafast desensitization component. An example of two αβ receptors clustered with two αβγ receptors is depicted in Figure 5Fiii.
Figure 5.
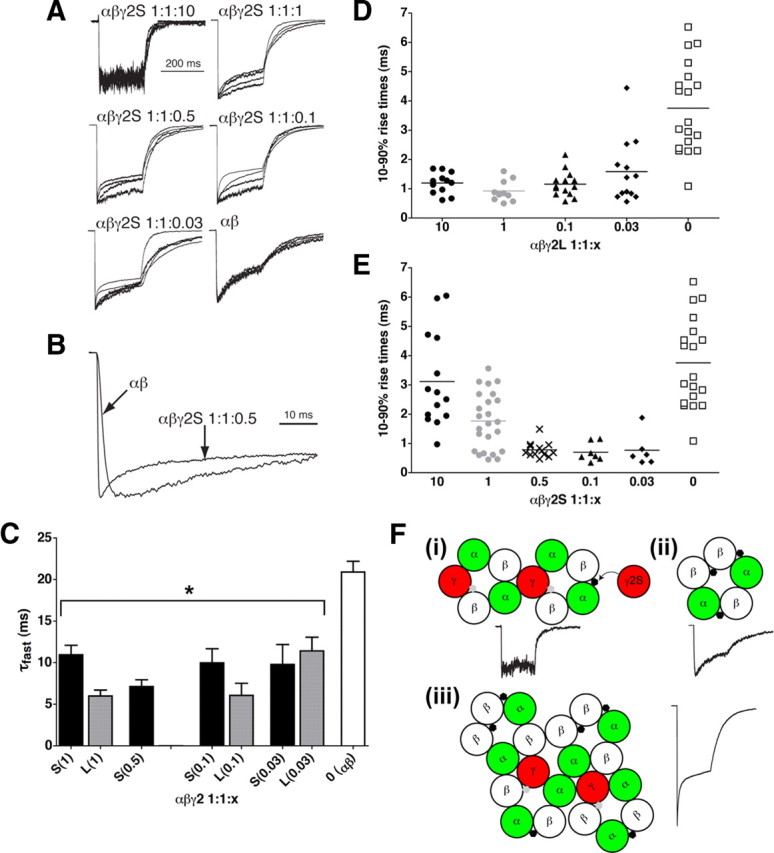
Mixtures of α1β2 and α1β2γ2 receptors exhibit faster kinetics than either pure population but are altered by rogue γ2S subunits. A, Overlaid current traces from four patches each, from α1β2γ2S 1:1:10, 1:1:1, 1:1:0.5, 1:1:0.1, 1:1:0.03, and 1:1:0 (α1β2) transfections, in response to 200 ms pulses of 1 mm GABA. B, Expanded trace comparing fast desensitization in α1β2 receptors with α1β2γ2S 1:1:0.5. Note that the α1β2γ2 1:1:0.5 current is not only faster to desensitize but also faster to rise to peak. C, Plot of fast desensitization time constants (τfast) transfected with α1 and β2 subunits, with varying ratios of γ2S or γ2L subunits, wherein a fast component could be detected. Data are mean ± SEM for n = 17, 6, 13, 8, 3, 6, 7, and 10 patches (left to right). *p < 0.01 compared with α1β2 patches. D, Scatter plot of 10–90% rise times for the cells transfected with α1 and β2 subunits, with varying ratios of γ2L subunits. Note that ratios over a 100-fold range (1:1:0.1 to 1:1:10) give similar rise times for γ2L. E, Scatter plot of 10–90% rise times for αβγ2S transfections in varying ratios. Note that, as γ2S ratio is increased above 0.5, rise times are slowed. F, Models depicting fast desensitization differences between receptors and mixtures. i, α1β2γ2 receptors, possibly clustered, with trace showing no fast desensitization. In the case of γ2S only, rogue γ2S subunits may bind at the α/β interface to cause a slowing of the rise time (compare D with E at 1:1:1 and 1:1:10). ii, α1β2 receptor with fast desensitization. iii, Mixtures of αβ and αβγ receptors may cluster and change kinetic profiles to allow for the ultrafast (7 ms) desensitization time constant. Traces were culled from Figure 1.
A second kinetic phenomenon that does not appear to depend on clustering, but rather on γ2S expression, was also observed. In the case of increased transfection ratio of γ2S in particular (1:1:1, 1:1:10), the rise time becomes slower (Fig. 5E). Parsimony suggests that rogue γ2S binding to α1β2γ2S receptors might lengthen their rise times (compare 1:1:1 and 1:1:10 α1β2γ2L rise times in Fig. 5D with α1β2γ2S in Fig. 5E), such as by steric hindrance of the GABA binding site(s) or allosteric changes, similar to the possible mechanism of protection from Zn2+ blockade (Fig. 5Fi). Alternative explanations for this difference in rise times include the following possibilities: (1) γ2S-containing receptors normally have slower rise times than γ2L-containing receptors without the need to invoke rogue subunits, and the faster rise times at lower transfection ratios are a common function of α1β2/α1β2γ2 clustering independent of splice variant (Fig. 5Fiii); (2) the additional γ2S subunits that are expressed but not incorporated may bind to intracellular proteins that affect α1β2γ2 binding, gating, or desensitization mechanisms, or (3) a combination thereof. However, rise times for α1β2 receptors were unaffected by γ2 N-terminal peptides (data not shown), suggesting that more of the γ2 subunit than the extracellular portion would be needed to slow rise times for αβ receptors, if indeed it can. Possible effects of rogue γ on αβ receptors in native tissue awaits additional study, because the existence of this receptor combination in vivo is still a fairly recent discovery (Mortensen and Smart, 2006). Effects of γ2S rogue subunits on mixed clusters (as in Fig. 5Fiii) that display the fast desensitization component and fast rise times (perhaps representing the synaptic configuration) (Boileau et al., 2005) could be studied using concatamer pentamers coexpressed with rogue γ2S.
Discussion
In the present study, it has been demonstrated that the ability of the short γ2 variant to express independently on the cell surface correlates with changes in coexpressed GABAARs in both pharmacology (zinc blockade) and fast receptor kinetics. However, what might be the physiological role of the modulatory effect of the rogue γ2S subunit? At this juncture, there are some speculations that can be broached. In early development, brain slice recordings show GABAARs to be less BDZ sensitive, coincident with lower expression of γ2 subunits (Brooks-Kayal et al., 1998). In γ2S-enriched dentate gyrus, Kapur and Macdonald (1999) observed that, at rat postnatal day 45 (P45) to P52, dentate granule cells had normal (high) diazepam potentiation and normal (low) zinc inhibition. However, at P7–P14, although zinc inhibition was still low, BDZ potentiation was vastly reduced. This could result from fewer fully incorporated αβγ receptors, with a few γ2S rogues conferring protection from zinc to αβ receptors.
Developmental differences in zinc inhibition could be related to receptor clustering. Clustering of γ2-containing synaptic receptors become evident by P20 in rats (Scotti and Reuter, 2001). However, in hippocampal neurons, Zn2+ sensitivity is relatively low at P7–P14. We can speculate that, because the γ2S variant dominates in that region, it might protect nascent neurons from diffuse, nonvesicularized Zn2+ ions (Valente et al., 2002). It is interesting that very young rats (P10) cannot be made epileptic with pilocarpine (Zhang et al., 2004), perhaps because the higher expression level of the γ2S subunit protects young neurons from deleterious overstimulation. Pathogenic conditions such as neonatal stress keep GABAARs in an immature declustered state with fewer γ2 subunits in the mixture (Hsu et al., 2003), and microtubule depolymerizing drug treatment in hippocampal neurons showed declustering of GABAARs concomitant with a loss of γ2 subunit-containing receptors as assessed by the BDZ sensitivity (Petrini et al., 2004). This declustering also led to faster rise times and faster desensitization of miniature IPSCs, reminiscent of the changes in α1β2γ2S kinetics with decreased γ2S ratio (Fig. 5A,D).
Several papers have suggested the existence of αβ receptors in vivo under normal (Brickley et al., 1999; Yeung et al., 2003) or γ2 knock-out (Günther et al., 1995) conditions, and a recent study has described αβ receptors extrasynaptically (Mortensen and Smart, 2006). In the latter study, Zn2+ block of αβ receptors was detectable, but, in a brain region in which γ2S subunits predominate or in the synapses in which γ2 subunits tend to cluster (Alldred et al., 2005; Christie et al., 2006), the present study would predict that αβ receptors would be difficult if not impossible to detect using Zn2+ pharmacology alone (Fig. 1). Even in on-cell patch recordings, an α1β2/α1β2γ2 mixture might be easy to miss because α1β2 receptors desensitize to a much greater extent, tend to stand silent (desensitized) under constant agonist exposure, and have a main conductance (∼15 pS) close to the main subconductance state of α1β2γ2 receptors (∼21 pS) (Boileau et al., 2005). Therefore, BDZ potentiation, which is thoroughly γ subunit dependent, appears to be a more reliable test for receptor mixtures. For example, in γ2S-rich posterior pituitary nerve terminals, Zhang and Jackson (1993) observed only small potentiation by the full agonist BDZ chlordiazepoxide on subsaturating GABA currents in membrane patches, indicating that not every receptor incorporates γ2 in this region. Meanwhile, the minimal blockade by 100 μm Zn2+ (24 ± 6%) in the same cells (Zhang and Jackson, 1995) would suggest that most or all of the receptors have γ2, if that were the only criterion used. The lower BDZ potentiation is likely not attributable to the presence of native α4 or α6 subunits, because these have been shown to exhibit higher sensitivity to Zn2+ block (Knoflach et al., 1996).
One may also speculate about the involvement of γ2 slice variants in the formation or prevention of epileptiform activity. Certain epileptiform mutations in the γ2 gene (Macdonald et al., 2004; Hirose, 2006) can cause a reduction in γ2 expression, sometimes from assembly impairments (Sancar and Czajkowski, 2004; Hales et al., 2005; Kang et al., 2006; Eugène et al., 2007). Animal models of epileptogenesis and adult human epileptic brain have hinted at reduced γ2 expression along with increased expression of BDZ-insensitive α4 and δ subunits (Brooks-Kayal et al., 1998; Coulter, 2001), both of which form receptors more zinc sensitive than α1β2γ2 (Saxena and Macdonald, 1994; Knoflach et al., 1996). The effects on BDZ and zinc modulation of GABAARs during induction of status epilepticus were tested previously in rat (Kapur and Macdonald, 1997). The authors observed a rapid reduction in BZD sensitivity (on the order of minutes), and, although there was a shift in IC50 for Zn2+ blockade, the relative sensitivity in the 30 μm range remained rather low. Again, a possible involvement of γ2S would be worth exploring with what we know now. Whether rogue γ2S subunits can also protect these receptor subtypes is an intriguing possibility. It will also be interesting to test for differences in Zn2+ protection using disease state assays (e.g., kindling or other epileptogenic treatment) in transgenic mice with γ2S or γ2L knocked into the γ2 knock-out background (Wick et al., 2000), to determine whether inhibition or behavior differ for the splice variants under stressed conditions.
Another possible protecting protein is the recently described GABAAR ε subunit. Data from one study with limiting ε coexpressed with α1 and β1 might be reinterpreted as the ε subunit conferring Zn2+ protection in a manner similar to γ2S (Thompson et al., 2002). The obvious follow-up question—do ε subunits express on the surface by themselves?—has very recently been answered in the affirmative (Jones and Henderson, 2007) in a study that also sheds some light on the functional variability seen in heterologously expressed ε subunits.
Trafficking and assembly of GABAARs regulates the subtypes of inhibitory GABAergic signaling in the brain. An intriguing line of inquiry arising from this study would be to determine the differences in the trafficking of rogue γ2S subunits versus those γ2S subunits that incorporate into normal synaptic and extrasynaptic GABAARs. Recent studies of GABAAR trafficking proteins show that, in mice knock-outs of PRIP (phospholipase C-related catalytically inactivated proteins that bind to GABARAP), diazepam function was reduced, possibly as a result of disassembly of αβγ receptors (Kanematsu et al., 2007). PRIP associates not only with the β subunit but also with GABARAP (GABAA receptor-associated protein), an accessory protein that binds to and enhances γ2 subunit incorporation and/or stability (Boileau et al., 2005). One current model of interaction is that γ2 subunit binding and incorporation is facilitated by the quaternary interactions of β subunit to PRIP, PRIP to GABARAP, and in turn to the γ2 subunit (Kanematsu et al., 2007). Does this scheme differ for the two splice variants? Do GABARAP or PRIP-bound GABARAP bind to rogue γ2S subunits, and, if so, are they then held in close proximity to fully formed receptors by indirect association with β subunits? Or do rogues disregard all affiliations on their route to the surface and bind to fully formed receptors via translocation, followed by extracellular protein–protein interactions? A recent paper combining molecular modeling with experimental data on the high-affinity Zn2+ binding site in the pore of α1β2 receptors (partially reconstituted in α1β2γ2S receptors by mutation of the γ2S subunit) suggests a possible role for membrane-spanning residues in rogue protection as well (Trudell et al., 2008). Trafficking and binding mechanisms notwithstanding, whether other Cys-loop receptor subunits can go rogue and alter their familial receptors in vivo remains to be determined. Rogue subunits add another level of intricacy to the exquisite control of neuronal signaling by nature.
Footnotes
This work was funded by National Institutes of Health Grants MH66406 (A.J.B., C.C.) and NS34727 (C.C.). We thank the W. M. Keck Laboratory for Biological Imaging for help with confocal images, Drs. Mathew V. Jones, Eugenia M. C. Jones (University of Wisconsin-Madison, Madison, WI), Xue Han (Massachusetts Institute of Technology/Harvard University, Cambridge, MA), and Robert L. Macdonald (Vanderbilt University, Nashville, TN) for helpful discussions, Dr. Srinivasan Venkatachalan for editing assistance, and Dr. Chenlei Leng (National University of Singapore, Statistics Department, Singapore) for help with statistical analysis.
References
- Akabas MH. GABAA receptor structure-function studies: a reexamination in light of new acetylcholine receptor structures. Int Rev Neurobiol. 2004;62:1–43. doi: 10.1016/S0074-7742(04)62001-0. [DOI] [PubMed] [Google Scholar]
- Alldred MJ, Mulder-Rosi J, Lingenfelter SE, Chen G, Lüscher B. Distinct γ2 subunit domains mediate clustering and synaptic function of postsynaptic GABAA receptors and gephyrin. J Neurosci. 2005;25:594–603. doi: 10.1523/JNEUROSCI.4011-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann SW, Baur R, Sigel E. Forced subunit assembly in α1β2γ2 GABAA receptors. Insight into the absolute arrangement. J Biol Chem. 2002;277:46020–46025. doi: 10.1074/jbc.M207663200. [DOI] [PubMed] [Google Scholar]
- Baur R, Minier F, Sigel E. A GABA(A) receptor of defined subunit composition and positioning: concatenation of five subunits. FEBS Lett. 2006;580:1616–1620. doi: 10.1016/j.febslet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- Boileau AJ, Czajkowski C. Identification of transduction elements for benzodiazepine modulation of the GABAA receptor: three residues are required for allosteric coupling. J Neurosci. 1999;19:10213–10220. doi: 10.1523/JNEUROSCI.19-23-10213.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau AJ, Baur R, Sharkey LM, Sigel E, Czajkowski C. The relative amount of cRNA coding for gamma2 subunits affects stimulation by benzodiazepines in GABAA receptors expressed in Xenopus oocytes. Neuropharmacology. 2002;43:695–700. doi: 10.1016/s0028-3908(02)00036-9. [DOI] [PubMed] [Google Scholar]
- Boileau AJ, Pearce RA, Czajkowski C. Tandem subunits effectively constrain GABAA receptor stoichiometry and recapitulate receptor kinetics but are insensitive to GABAA receptor-associated protein. J Neurosci. 2005;25:11219–11230. doi: 10.1523/JNEUROSCI.3751-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Single-channel properties of synaptic and extrasynaptic GABAA receptors suggest differential targeting of receptor subtypes. J Neurosci. 1999;19:2960–2973. doi: 10.1523/JNEUROSCI.19-08-02960.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Jin H, Price M, Dichter MA. Developmental expression of GABAA receptor subunit mRNAs in individual hippocampal neurons in vitro and in vivo. J Neurochem. 1998;70:1017–1028. doi: 10.1046/j.1471-4159.1998.70031017.x. [DOI] [PubMed] [Google Scholar]
- Christie SB, Li RW, Miralles CP, Yang BY, De Blas AL. Clustered and non-clustered GABAA receptors in cultured hippocampal neurons. Mol Cell Neurosci. 2006;31:1–14. doi: 10.1016/j.mcn.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Connolly CN, Uren JM, Thomas P, Gorrie GH, Gibson A, Smart TG, Moss SJ. Subcellular localization and endocytosis of homomeric gamma2 subunit splice variants of gamma-aminobutyric acid type A receptors. Mol Cell Neurosci. 1999;13:259–271. doi: 10.1006/mcne.1999.0746. [DOI] [PubMed] [Google Scholar]
- Coulter DA. Epilepsy-associated plasticity in gamma-aminobutyric acid receptor expression, function, and inhibitory synaptic properties. Int Rev Neurobiol. 2001;45:237–252. doi: 10.1016/s0074-7742(01)45013-6. [DOI] [PubMed] [Google Scholar]
- Ericksen SS, Boileau AJ. Tandem couture: cys-loop receptor concatamer insights and caveats. Mol Neurobiol. 2007;35:113–128. [PMC free article] [PubMed] [Google Scholar]
- Eugène E, Depienne C, Baulac S, Baulac M, Fritschy JM, Le Guern E, Miles R, Poncer JC. GABAA receptor γ2 subunit mutations linked to human epileptic syndromes differentially affect phasic and tonic inhibition. J Neurosci. 2007;27:14108–14116. doi: 10.1523/JNEUROSCI.2618-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günther U, Benson J, Benke D, Fritschy JM, Reyes G, Knoflach F, Crestani F, Aguzzi A, Arigoni M, Lang Y. Benzodiazepine-insensitive mice generated by targeted disruption of the gamma 2 subunit gene of gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci U S A. 1995;92:7749–7753. doi: 10.1073/pnas.92.17.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez A, Khan ZU, De Blas AL. Immunocytochemical localization of γ2 short and γ2 long subunits of the GABAA receptor in the rat brain. J Neurosci. 1994;14:7168–7179. doi: 10.1523/JNEUROSCI.14-11-07168.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez A, Khan ZU, Miralles CP, De Blas AL. Altered expression of gamma 2L and gamma 2S GABAA receptor subunits in the aging rat brain. Brain Res Mol Brain Res. 1996;35:91–102. doi: 10.1016/0169-328x(95)00187-w. [DOI] [PubMed] [Google Scholar]
- Hales TG, Tang H, Bollan KA, Johnson SJ, King DP, McDonald NA, Cheng A, Connolly CN. The epilepsy mutation, gamma2(R43Q) disrupts a highly conserved inter-subunit contact site, perturbing the biogenesis of GABAA receptors. Mol Cell Neurosci. 2005;29:120–127. doi: 10.1016/j.mcn.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Han K, Jeon MJ, Kim SH, Ki D, Bahn JH, Lee KS, Park J, Choi SY. Efficient intracellular delivery of an exogenous protein GFP with genetically fused basic oligopeptides. Mol Cells. 2001;12:267–271. [PubMed] [Google Scholar]
- Hevers W, Lüddens H. The diversity of GABAA receptors. Pharmacological and electrophysiological properties of GABAA channel subtypes. Mol Neurobiol. 1998;18:35–86. doi: 10.1007/BF02741459. [DOI] [PubMed] [Google Scholar]
- Hirose S. A new paradigm of channelopathy in epilepsy syndromes: intracellular trafficking abnormality of channel molecules. Epilepsy Res. 2006;70(Suppl 1):S206–S217. doi: 10.1016/j.eplepsyres.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Horenstein J, Wagner DA, Czajkowski C, Akabas MH. Protein mobility and GABA-induced conformational changes in GABAA receptor pore-lining M2 segment. Nat Neurosci. 2001;4:477–485. doi: 10.1038/87425. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Dunne EL, Harvey RJ, Smart TG. Zinc-mediated inhibition of GABAA receptors: discrete binding sites underlie subtype specificity. Nat Neurosci. 2003;6:362–369. doi: 10.1038/nn1030. [DOI] [PubMed] [Google Scholar]
- Hsu FC, Zhang GJ, Raol YS, Valentino RJ, Coulter DA, Brooks-Kayal AR. Repeated neonatal handling with maternal separation permanently alters hippocampal GABAA receptors and behavioral stress responses. Proc Natl Acad Sci U S A. 2003;100:12213–12218. doi: 10.1073/pnas.2131679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntsman MM, Tran BV, Potkin SG, Bunney WE, Jr, Jones EG. Altered ratios of alternatively spliced long and short gamma2 subunit mRNAs of the gamma-amino butyrate type A receptor in prefrontal cortex of schizophrenics. Proc Natl Acad Sci U S A. 1998;95:15066–15071. doi: 10.1073/pnas.95.25.15066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BL, Henderson LP. Trafficking and potential assembly patterns of epsilon-containing GABAA receptors. J Neurochem. 2007;103:1258–1271. doi: 10.1111/j.1471-4159.2007.04833.x. [DOI] [PubMed] [Google Scholar]
- Kanematsu T, Mizokami A, Watanabe K, Hirata M. Regulation of GABAA-receptor surface expression with special reference to the involvement of GABARAP (GABAA receptor-associated protein) and PRIP (phospholipase C-related, but catalytically inactive protein) J Pharmacol Sci. 2007;104:285–292. doi: 10.1254/jphs.cp0070063. [DOI] [PubMed] [Google Scholar]
- Kang JQ, Shen W, Macdonald RL. Why does fever trigger febrile seizures? GABAA receptor γ2 subunit mutations associated with idiopathic generalized epilepsies have temperature-dependent trafficking deficiencies. J Neurosci. 2006;26:2590–2597. doi: 10.1523/JNEUROSCI.4243-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur J, Macdonald RL. Rapid seizure-induced reduction of benzodiazepine and Zn2+ sensitivity of hippocampal dentate granule cell GABAA receptors. J Neurosci. 1997;17:7532–7540. doi: 10.1523/JNEUROSCI.17-19-07532.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur J, Macdonald RL. Postnatal development of hippocampal dentate granule cell gamma-aminobutyric acidA receptor pharmacological properties. Mol Pharmacol. 1999;55:444–452. [PubMed] [Google Scholar]
- Kapur J, Haas KF, Macdonald RL. Physiological properties of GABAA receptors from acutely dissociated rat dentate granule cells. J Neurophysiol. 1999;81:2464–2471. doi: 10.1152/jn.1999.81.5.2464. [DOI] [PubMed] [Google Scholar]
- Knoflach F, Benke D, Wang Y, Scheurer L, Lüddens H, Hamilton BJ, Carter DB, Mohler H, Benson JA. Pharmacological modulation of the diazepam-insensitive recombinant gamma-aminobutyric acidA receptors alpha 4 beta 2 gamma 2 and alpha 6 beta 2 gamma 2. Mol Pharmacol. 1996;50:1253–1261. [PubMed] [Google Scholar]
- Lin R, Heylbroeck C, Pitha PM, Hiscott J. Virus-dependent phosphorylation of the IRF-3 transcription factor regulates nuclear translocation, transactivation potential, and proteasome-mediated degradation. Mol Cell Biol. 1998;18:2986–2996. doi: 10.1128/mcb.18.5.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald RL, Gallagher MJ, Feng HJ, Kang J. GABAA receptor epilepsy mutations. Biochem Pharmacol. 2004;68:1497–1506. doi: 10.1016/j.bcp.2004.07.029. [DOI] [PubMed] [Google Scholar]
- Miralles CP, Gutiérrez A, Khan ZU, Vitorica J, De Blas AL. Differential expression of the short and long forms of the gamma 2 subunit of the GABAA/benzodiazepine receptors. Brain Res Mol Brain Res. 1994;24:129–139. doi: 10.1016/0169-328x(94)90124-4. [DOI] [PubMed] [Google Scholar]
- Mohler H, Knoflach F, Paysan J, Motejlek K, Benke D, Lüscher B, Fritschy JM. Heterogeneity of GABAA-receptors: cell-specific expression, pharmacology, and regulation. Neurochem Res. 1995;20:631–636. doi: 10.1007/BF01694546. [DOI] [PubMed] [Google Scholar]
- Mortensen M, Smart TG. Extrasynaptic αβ subunit GABAA receptors on rat hippocampal pyramidal neurons. J Physiol. 2006;577:841–856. doi: 10.1113/jphysiol.2006.117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss SJ, Doherty CA, Huganir RL. Identification of the cAMP-dependent protein kinase and protein kinase C phosphorylation sites within the major intracellular domains of the beta 1, gamma 2S, and gamma 2L subunits of the gamma-aminobutyric acid type A receptor. J Biol Chem. 1992;267:14470–14476. [PubMed] [Google Scholar]
- Petrini EM, Marchionni I, Zacchi P, Sieghart W, Cherubini E. Clustering of extrasynaptic GABAA receptors modulates tonic inhibition in cultured hippocampal neurons. J Biol Chem. 2004;279:45833–45843. doi: 10.1074/jbc.M407229200. [DOI] [PubMed] [Google Scholar]
- Richard JP, Melikov K, Vives E, Ramos C, Verbeure B, Gait MJ, Chernomordik LV, Lebleu B. Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. J Biol Chem. 2003;278:585–590. doi: 10.1074/jbc.M209548200. [DOI] [PubMed] [Google Scholar]
- Sancar F, Czajkowski C. A GABAA receptor mutation linked to human epilepsy (γ2R43Q) impairs cell surface expression of αβγ receptors. J Biol Chem. 2004;279:47034–47039. doi: 10.1074/jbc.M403388200. [DOI] [PubMed] [Google Scholar]
- Saxena NC, Macdonald RL. Assembly of GABAA receptor subunits: role of the delta subunit. J Neurosci. 1994;14:7077–7086. doi: 10.1523/JNEUROSCI.14-11-07077.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotti AL, Reuter H. Synaptic and extrasynaptic gamma-aminobutyric acid type A receptor clusters in rat hippocampal cultures during development. Proc Natl Acad Sci U S A. 2001;98:3489–3494. doi: 10.1073/pnas.061028798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SA, Bonnert TP, Cagetti E, Whiting PJ, Wafford KA. Overexpression of the GABAA receptor epsilon subunit results in insensitivity to anaesthetics. Neuropharmacology. 2002;43:662–668. doi: 10.1016/s0028-3908(02)00162-4. [DOI] [PubMed] [Google Scholar]
- Trudell JR, Yue ME, Bertaccini EJ, Jenkins A, Harrison NL. Molecular modeling and mutagenesis reveals a tetradentate binding site for Zn2+ in GABAA αβ receptors and provides a structural basis for the modulating effect of the gamma subunit. J Chem Inf Model. 2008;48:344–349. doi: 10.1021/ci700324a. [DOI] [PubMed] [Google Scholar]
- Valente T, Auladell C, Pérez-Clausell J. Postnatal development of zinc-rich terminal fields in the brain of the rat. Exp Neurol. 2002;174:215–229. doi: 10.1006/exnr.2002.7876. [DOI] [PubMed] [Google Scholar]
- Wang JB, Burt DR. Differential expression of two forms of GABAA receptor gamma 2-subunit in mice. Brain Res Bull. 1991;27:731–735. doi: 10.1016/0361-9230(91)90054-n. [DOI] [PubMed] [Google Scholar]
- Whiting P, McKernan RM, Iversen LL. Another mechanism for creating diversity in gamma-aminobutyrate type A receptors: RNA splicing directs expression of two forms of gamma 2 phosphorylation site. Proc Natl Acad Sci U S A. 1990;87:9966–9970. doi: 10.1073/pnas.87.24.9966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick MJ, Radcliffe RA, Bowers BJ, Mascia MP, Lüscher B, Harris RA, Wehner JM. Behavioural changes produced by transgenic overexpression of gamma2L and gamma2S subunits of the GABAA receptor. Eur J Neurosci. 2000;12:2634–2638. doi: 10.1046/j.1460-9568.2000.00160.x. [DOI] [PubMed] [Google Scholar]
- Yeung JY, Canning KJ, Zhu G, Pennefather P, MacDonald JF, Orser BA. Tonically activated GABAA receptors in hippocampal neurons are high-affinity, low-conductance sensors for extracellular GABA. Mol Pharmacol. 2003;63:2–8. doi: 10.1124/mol.63.1.2. [DOI] [PubMed] [Google Scholar]
- Zhang G, Raol YH, Hsu FC, Coulter DA, Brooks-Kayal AR. Effects of status epilepticus on hippocampal GABAA receptors are age-dependent. Neuroscience. 2004;125:299–303. doi: 10.1016/j.neuroscience.2004.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SJ, Jackson MB. GABA-activated chloride channels in secretory nerve endings. Science. 1993;259:531–534. doi: 10.1126/science.8380942. [DOI] [PubMed] [Google Scholar]
- Zhang SJ, Jackson MB. Properties of the GABAA receptor of rat posterior pituitary nerve terminals. J Neurophysiol. 1995;73:1135–1144. doi: 10.1152/jn.1995.73.3.1135. [DOI] [PubMed] [Google Scholar]