Abstract
We have simultaneously measured the expression of postsynaptic γ-aminobutyric acid type A (GABAA) receptor clusters and of presynaptic boutons in neonatal rat hippocampal cultures between days 1 and 30. GABAA receptors were labeled with antibodies recognizing the extracellular domains of β2/3 and γ2 subunits. Boutons were visualized by activity-dependent uptake of the styryl dye FM4-64, or by antibodies against the presynaptic vesicular protein SV2 or the GABA-synthesizing enzyme glutamic acid decarboxylase (GAD). GABAA receptor clusters could be seen in living neurons already 6 h after culturing, much before presynaptic markers could be identified in nerve terminals. The densities of receptor clusters that contained the β2/3 subunits were constant between days 10 and 30 in culture, whereas γ2 subunit-containing clusters fluctuated and reached a maximum on day 20. SV2 and GAD staining could be measured from day 2 onwards. Clustering of GAD in presynaptic terminals and FM4-64 uptake were observed only at day 5 and afterward. SV2 staining and FM4-64 uptake increased in parallel between days 5 and 20 and remained constant thereafter. GAD-stained boutons were fewer than those labeled with other, less specific, presynaptic stains. They reached a maximum on day 20 and fell again toward day 30. Double labeling of GABAA receptors and of presynaptic boutons in neurons during differentiation showed that, even after 30 days in culture, large fractions of GABAA receptor clusters containing β2/3 and/or γ2 subunits remained extrasynaptic.
The type A receptors for γ-aminobutyric acid (GABAA receptors) are ligand-gated ion channels that mediate synaptic inhibition. These chloride channels are heteropentameric structures and can be assembled from a multitude of subunits (α1–6, β1–3, γ1–3, δ, ɛ, π, θ). Some subunit combinations are very common and widely distributed in the nervous system, whereas others are restricted to specific neuronal populations (1–4). The GABAA receptor subunit combinations that are most frequently observed in hippocampal granular and pyramidal cells are α2β2/3γ2 and α1β2/3γ2. The β1 subunit, although less frequent, has a similar distribution as β3. The α1 and the β2 subunits are particularly enriched in interneurons of the hippocampus, where they colocalize with the γ2 subunit (1, 5, 6). In the rat hippocampus, the β2/3 subunits are present at early stages of embryonal development, whereas expression of γ2 increases only after birth to moderate levels (7).
There is increasing evidence for extrasynaptic GABAA receptors in neurons of the adult brain (8–11), and it was recently found (12) that, in hippocampal cultures, only about 50% of GABAA receptor clusters correlated with apposed presynaptic boutons. Furthermore, recent electrophysiological results from brain slices have shown that ion conductances and kinetics of currents through synaptic and extrasynaptic GABA-activated channels are quite different, which may be important for neuronal function in vivo (8, 9).
In the present study, GABAA receptor clusters in living neurons were labeled with antibodies recognizing extracellular epitopes on the β2/3 (12, 13) and γ2 (14) subunits. To find out whether extrasynaptic GABAA receptor densities change during growth and differentiation of the neuronal network, we have quantitatively measured GABAA receptor clusters and their correlation with presynaptic terminals in hippocampal neurons in culture between days 1 and 30. We could show that GABAA receptors are already expressed and clustered in the membrane of neurons before synapses have been formed. Even in well-differentiated neurons after 20–30 days in culture, 50–60% of all clusters remained extrasynaptic.
Materials and Methods
Hippocampal Cell Culture.
Hippocampi were dissected from the brain of neonatal Sprague–Dawley rats (P0–2). Dissociation of cells and culturing procedures have been extensively described before (15).
Antibodies.
Two monoclonal antibodies, one recognizing the synaptic vesicle transporter SV2 (16), the other the 65-kDa isoform of the GABA-synthesizing enzyme glutamic acid decarboxylase (GAD) (17) (both from the Developmental Studies Hybridoma Bank, University of Iowa, Iowa City) were used as markers for synaptic terminals. The affinity-purified rabbit antibodies β3(1–13) (14) and γ2(1–33) (18) were kindly provided by W. Sieghart (University of Vienna, Vienna, Austria) and were used to stain GABAA subunits in living cells (12, 13). The epitopes for both antibodies are located externally in receptors expressed in surface membranes. To test their specificity, human embryonic kidney cells (HEK 293) were transfected with GABAA receptor subunits α1β2γ2 or α1β3γ2 together with green fluorescent protein (GFP) (19). About 60 h after transfection, cells were immunolabeled without or with prior paraformaldehyde fixation by the same method described below.
Labeling of GABAA Receptors on Living Cells and Staining of Active Presynaptic Terminals.
The primary antibody was diluted [2 μg⋅ml−1 for β3(1–13) and 7.8 μg⋅ml−1 for γ2(1–33)] in saline (135 mM NaCl/5 mM KCl/2 mM MgCl2/2 mM CaCl2/10 mM Hepes/30 mM glucose, pH 7.4), supplemented with 5% normal goat serum and applied to the cells for 10 min. To visualize the immunoreactions, IgGs linked to the fluorochrome FITC (Jackson ImmunoResearch) or to Oregon Green 488 (Molecular Probes) were used (1:20 dilution, 10 min). Coverslips with cells were placed into a chamber equipped with platinum electrodes for electrical field stimulation (20-Hz, 1-ms current pulses) and constantly superfused with saline. The chamber was placed on an inverted microscope (Zeiss Axiovert 100 M) integrated in a confocal laser scan microscope (Zeiss LSM 510). To visualize synaptic boutons, the fluorescent styryl membrane probe FM4-64 (10 μM; Molecular Probes) was loaded into synaptic vesicles during excitation. FM4-64 puncta were identified as functional presynaptic boutons when the dye could be released in response to a second period of stimulation (90 s, 20 Hz) (12, 15, 20). FM4-64 was excited by the HeNe laser (543 nm) and its emission was long pass-filtered above 585 nm. Oregon Green and FITC were excited with the argon laser (488 nm) and their emission was filtered between 505 and 530 nm. Differential interference contrast (DIC) images were scanned with either the argon laser (514 nm) or the HeNe laser (543 nm) in transmission mode. Fluorescence images were collected as stacks of three to four optical sections, ca. 1 μm thick.
Double-Labeling Immunocytochemistry.
For double labeling of β2/3 or γ2 subunits together with the presynaptic markers SV2 or GAD, GABAA receptors were labeled first on living cells as described above. Stained cultures were then fixed with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). After 2–4 h, the fixative solution was replaced with 0.05 M TBS, cells were permeabilized with 0.2% Triton X-100 (10 min), and afterward incubated for 20 min with SV2 or GAD antibodies diluted in TBS containing 5% normal goat serum. Tetramethylrhodamine isothiocyanate (TRITC)-labeled anti-rabbit IgGs and FITC-linked anti-mouse IgGs diluted 1:20 in 0.013 M PBS (20 min) were used for visualization. The monoclonal bd-17 antibody (20 μg⋅ml−1; Roche Molecular Biochemicals), which recognized the β3 and β2 subunits, but not the β1 subunit (21), was used as an alternative to β3(1–13). Antibody γ2(1–33) was visualized with Oregon Green-linked IgGs, whereas TRITC-coupled IgGs were used for bd-17. TRITC was excited with the HeNe laser (543) and filtered between 560 and 615 nm.
Quantitative Analysis.
Brightness and contrast of fluorescence images were adjusted so that only punctate fluorescence, but no weak diffuse background labeling was visible. Cell bodies and dendrites that could be clearly recognized in DIC images were selected for quantitative analysis. All labeled receptor clusters and presynaptic structures on the neurites were counted, and neurite length was measured. Receptor clusters on dendrites were defined as extrasynaptic if no neighboring presynaptic markers could be identified, and as synaptic if colocalized with such markers. Densities of receptors and presynaptic structures were normalized to 100 μm dendritic length. Densities of extrasynaptic and synaptic receptors were expressed as fractions of the total cluster numbers on the same dendrite. They were counted on 11–47 dendrites from at least four different cultures per age. ANOVA (post test: Tukey–Kramer) or the Kruskall–Wallis test (post test: Mann–Whitney) were used for statistical analysis, and significance was assumed at P < 0.05.
Results
GABAA Receptors Containing β2/3 Subunits.
To demonstrate the specificity of the polyclonal antibody β3(1−13) to label GABAA receptors, living HEK 293 cells (Fig. 1A) expressing recombinant α1β2γ2 (Fig. 1B) or α1β3γ2 (Fig. 1C) GABAA receptor compositions were decorated with this antibody. Both receptor subtypes were recognized, whereas nontransfected cells were not stained (not shown). This antibody thus crossreacts with the β2 and β3 subunits whose N-terminal sequences (1–13) differ by only two amino acids (22).
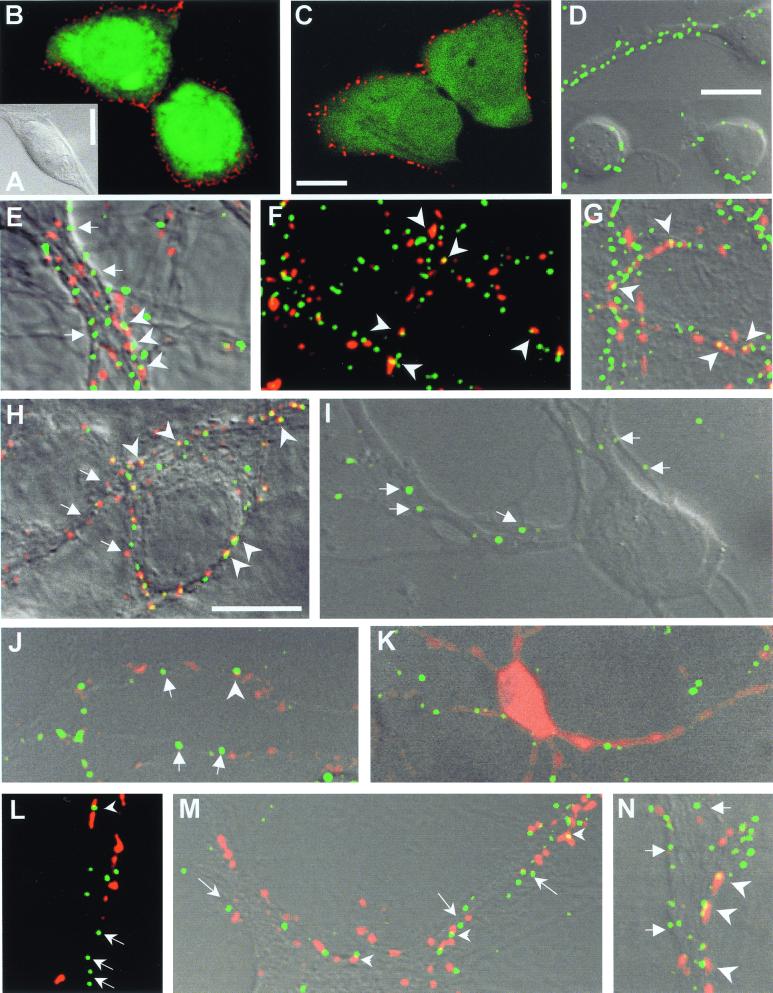
Figure 1.
HEK cells (A) transfected with recombinant α1β2γ2 (B) or α1β3γ2 (C) GABAA receptors. In living cells, the antibody recognized both β2- and β3-containing receptors. They appeared as clusters on the cell surface (red). Expression of cotransfected GFP accounts for the cytoplasmic green fluorescence. (D) Live stain for β2/3 subunits 6 h after plating (green label on DIC image). (E–G) β2/3-containing receptors in 20-day-old cultures. Synaptic terminals (red label) were either loaded in living cultures with FM4-64 (E), or labeled with antibodies against the synaptic vesicle marker SV2 (F) or against the GABA-synthesizing enzyme GAD (G). Arrowheads point to some doubly stained, synaptically located β2/3 clusters, and arrows to extrasynaptic receptors. (Bars equal 10 μm; bar in C also applies to B, bar in D also to E–G.) (H) Double labeling of β2/3 (with bd-17 antibody, red) and γ2 subunits (green) in living 20-day-old cultures. Arrowheads point to GABAA clusters, where the β2/3 and γ2 subunits colocalize (yellow spots). β2/3 clusters appear more numerous (arrows) than γ2 clusters. (I) On the second day, γ2-labeled neurites (green) failed to take up FM4-64 during stimulation, but SV2 (red) labeling (J) was detectable. (K) GAD was homogeneously distributed on day 2 in all processes of GABAergic neurons (red). γ2-immunoreactive GABAA clusters (green) and FM4-64 (L), SV2 (M), and GAD (N) labeled punctate synaptic terminals (all red) have increased by 20 days of culture. Arrows point to extrasynaptic γ2 clusters, whereas arrowheads indicate synaptic γ2 clusters. (Bars equal 10 μm; the bar in H applies for H–J and L–M .)
In living cultured hippocampal cells, GABAA receptors decorated with the antibody β3(1–13) could already be detected 6 h after plating, mainly on those cells that had started to extend neurites (Fig. 1D). The immunoreactive puncta most likely represent GABAA receptors aggregated in clusters of irregular shapes and different sizes. Their optical appearance was similar to that of 0.2-μm fluorescent beads used for calibration under identical conditions (not shown). Such clusters were also observed in cultured hippocampal cells (12) and motoneurons (13) after fixation, suggesting that they did not appear because of antibody-induced crosslinking of receptors. Living cells became excitable only after day 2 in culture (see next section). Thus, at early ages when receptor clusters could already be seen, nerve terminals could not be loaded with FM4-64 to estimate how many of such clusters were associated with functional presynaptic boutons. Older cells were first decorated with β3(1–13) antibody and then electrically stimulated to load FM4-64 into synaptic vesicles. However, even on day 20, many extrasynaptic receptor clusters were still observed, not only with FM4-64 (Fig. 1E) but also with other presynaptic markers, such as the synaptic vesicle protein SV2, or the GABA-synthesizing enzyme GAD (Fig. 1 F and G) Quantitative estimates showed that the densities of β2/3-containing GABAA clusters along dendrites did not change with age of cultures between days 10 and 30 (Table 1). The number of boutons measured with all presynaptic markers, however, increased significantly during this period, reaching a maximum between days 10 and 20. GAD-containing terminals showed a significantly lower density than those labeled with FM4-64 or SV2 (Table 1, see also Fig. 3E).
Table 1.
Numbers of presynaptic (FM4-64, SV2, GAD) and postsynaptic (β2/3) puncta per 100 μm of neurite length
| Group | No./100 μm
|
||
|---|---|---|---|
| Day 10 | Day 20 | Day 30 | |
| FM4–64 boutons | 28.89 ± 2.51 | 43.62 ± 2.44* | 42.41 ± 2.95** |
| β2/3 clusters | 42.85 ± 1.80 (277) | 48.18 ± 2.37 (368) | 45.73 ± 6.26 (346) |
| SV2 boutons | 31.73 ± 4.10 | 38.87 ± 1.47** | 39.63 ± 3.27*** |
| β2/3 clusters | 49.21 ± 4.30 (405) | 48.16 ± 2.16 (430) | 51.64 ± 5.55 (187) |
| GAD boutons | 17.15 ± 1.69† | 29.68 ± 1.88*† | 25.98 ± 2.54***† |
| β2/3 clusters | 49.14 ± 2.47 (683) | 48.48 ± 2.09 (834) | 50.01 ± 3.80 (187) |
Values represent the total densities of puncta and are the means ± SEM of the counts in 11–29 dendrites per group. Figures in parentheses represent the numbers of β2/3 immunoreactive clusters counted in each group with presynaptic markers FM4-64, SV2, or GAD. *, P < 0.001; **, P < 0.01; ***, P < 0.05 vs. density at day 10;
, P < 0.001 vs. FM bouton density and P < 0.01 vs. SV2 bouton density.
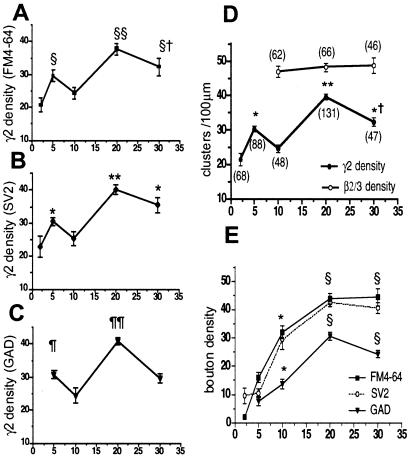
Figure 3.
(A–C) Densities of γ2 subunit-containing GABAA receptor clusters vary with age. γ2 clusters were counted in three independent sets of cells with double labelings (n = 15–47 dendrites per time point). §§, P < 0.0001 vs. days 2 and 10, P < 0.01 vs. day 5; §†, P < 0.001 vs. day 2; P < 0.05 vs. day 10; §, P < 0.001 vs. day 2. **, P < 0.0001 vs. days 2–10; *, P < 0.01 day 5 vs. day 2, and day 30 vs. days 2 and 10. ¶¶, P < 0.0001 vs. days 5, 10, and 30; ¶, P < 0.05 vs. day 10. (D) Values of densities of γ2 clusters in A–C were pooled and plotted together with pooled densities of β2/3 clusters. Numbers in parentheses represent the pooled numbers of dendrites. γ2 density varies with age in culture and peaks on day 20 (**, P < 0.0001 vs. days 2, 10, and 30; *†, P < 0.0001 vs. days 2 and 10; *, P < 0.001 vs. days 2 and 10), whereas β2/3 clusters remain constant between days 10 and 30. GABAA receptors containing the γ2 subunit were significantly less than β2/3-labeled receptors (P < 0.001). (E) Densities of presynaptic boutons increased with age of cells in culture. Presynaptic labelings (FM4-64 and SV2) were low between 2 and 5 days, but all presynaptic boutons, including those labeled with GAD antibody, doubled between 5 and 10 days in vitro, and reached maximal values on day 20. Each time point is the mean ± SEM of the counts in 15–47 dendrites; (*, P < 0.001 vs. day 2 or 5 for FM4-64 and SV2; P < 0.05 for GAD. §, P < 0.001 vs. days 2, 5, 10). The density of GAD-labeled boutons declined significantly between days 20 and 30 (P < 0.05). GAD-positive boutons were significantly less than FM4-64- or SV2-labeled ones at the same age (P < 0.001).
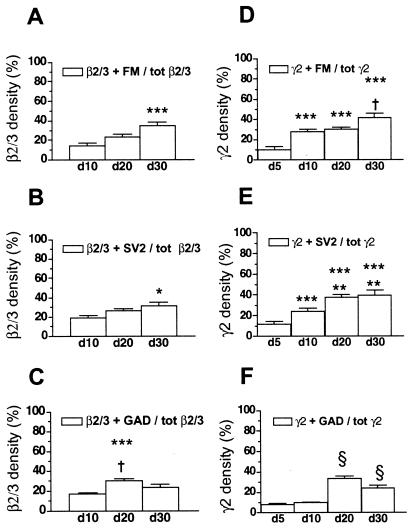
Fig. 2 A–C shows plots of synaptic and extrasynaptic β2/3-containing clusters in cultures of different ages. The relative density of clusters associated with FM4-64-loaded presynaptic boutons increased significantly between days 10 and 30 (Fig. 2A). Similar ratios between synaptic and extrasynaptic clusters were obtained in fixed cells with SV2 antibody as an alternative presynaptic marker (Fig. 2B). Both markers, however, do not distinguish between excitatory and inhibitory synapses. Colocalization of β2/3 clusters with GABA-specific, GAD-positive synaptic terminals, was highest on day 20 and dropped again toward day 30 (Fig. 2C). This behavior depended on a 12.5% loss of GAD-stained synapses between days 20 and 30 (Table 1). At those ages, only about 50% (day 20, 51.9 ± 3.5%; day 30, 47.3 ± 5.7%) of these terminals were adjacent to GABAA receptors containing β2/3 subunits.
Figure 2.
(A–C) Relative densities of β2/3 subunit-containing clusters with presynaptic contacts. Synaptically located receptors increase with increasing age of the cultures. However, the majority of all β2/3 clusters are lacking presynaptic partners even in well-differentiated cultures. Colocalizations with FM4-64 or SV2-positive synapses are maximal at day 30 (A and B). The highest density of synaptic β2/3 clusters at GABAergic terminals was measured on day 20 (C). *, P < 0.05; ***, P < 0.001 vs. day 10. †, P < 0.05 vs. day 30. (D–F) Relative densities of γ2 subunit-containing clusters with presynaptic contacts. Synaptic receptor clusters containing γ2 increase with increasing age of the cultures, although the majority of the clusters remain without a presynaptic partner, even in mature cultures on day 30. With GAD-stained synapses, the density is highest on day 20 (F). ***, P < 0.0001 vs. day 5; †, P < 0.002 vs. day 10 and P < 0.05 vs. day 20; **, P < 0.02 vs. day 10; §, P < 0.0001 vs. days 5 and 10.
These data indicate that β2/3 immunoreactive GABAA receptors clusters occur very early in dendritic membranes of cultured hippocampal neurons and that their densities remain constant between days 10 and 30. Although the fraction of clusters adjacent to presynaptic boutons increased with ongoing synaptogenesis, at least 50% of GABAA receptor clusters containing β2/3 subunits remained extrasynaptic, even in 30-day-old cultures.
GABAA Receptors Containing γ2 Subunit.
The β3(1–13) antibody does not differentiate between GABAA receptor compositions without or with γ subunits. Although both subunits are expressed in neuronal membranes (2, 3), the β2/3 subunits are three times more frequent at extrasynaptic than at synaptic sites (11), whereas γ2 subunits seem to be mainly targeted to complete synapses (9, 10). However, differences in kinetics of currents through synaptic and extrasynaptic GABAA receptors seem to depend on different receptor compositions with α rather than γ subunits (8). If receptors containing the γ2 subunit are preferentially targeted to synapses, more γ2 than β2/3 labeled clusters should be observed adjacent to presynaptic boutons. Therefore, we have measured at different stages of development the densities of synaptic and extrasynaptic receptor clusters containing γ2 subunits. For this, we have used the antibody γ2(1–33) that recognizes an extracellular epitope of the γ2 subunit in living cells (18). We also estimated the densities of GABAA receptors containing both γ2 and β2/3 subunits by additional staining with the β2/3-specific monoclonal antibody bd-17 (21) in living 20-day-old cultures. Fig. 1H shows that the two populations of GABAA receptors partially overlap. About 40% percent of all visible clusters contained both γ2 and β2/3 subunits, whereas 35% represented β2/3 and 25% γ2 clusters only. When related to the total number of β2/3 clusters, 53.4 ± 4.5% also contained the γ2 subunits. Similarly, 60.7 ± 5.1% of all γ2-labeled puncta colocalized with the β2/3 subunits. Thus, about half of the clusters tagged by β2/3 or γ2 antibody in our cultures may represent αxβ2/3γ2 GABAA receptor compositions. The receptor clusters where only γ2 subunits were labeled could be associated with β1 subunit (1, 6) that is not recognized by the antibodies (21). Those clusters where only β2/3 was labeled could possibly be associated with other γ subunit isoforms (2, 9).
About 6 h after plating, γ2 clusters were only seen in cells that had already extended neurites, but not in those that still lacked these extensions (not shown). On day 2, most neurons showed numerous branched neurites, all decorated with sparse γ2-positive puncta (Fig. 1I). To find out why neurons at this age did not take up FM4-64 during field stimulation, we checked their excitability by measuring changes in the intracellular calcium concentration with the calcium-sensitive dye Fluo-3-AM (20). The membrane potential of cells in culture is low (less than −50 mV) at this age, and sodium currents are largely inactivated (not shown). Therefore, in contrast to older cultures, day 2 neurons did not respond to electrical stimulation with a rise in intracellular calcium (not shown). However, individual SV2 immunoreactive puncta were detectable at this age (Fig. 1J), whereas GAD immunoreactivity still appeared to be distributed rather homogeneously in cell bodies and neurites (Fig. 1K). Colocalization of both presynaptic markers with γ2 clusters was rare at that stage (Fig. 1 J and K). We found that, in cultures from day 5 onwards, patchy accumulation of GAD in axonal swellings coincided with the appearance of stimulation induced uptake of FM4-64. This argues for the formation of functional synapses at this age. On day 20, the densities of such presynaptic boutons and of γ2 clusters had both increased, although there still was a large surplus of receptor clusters unrelated to presynaptic markers (Fig. 1 L–N).
As for β2/3 (Fig. 2 A–C), changes in colocalization of γ2-containing GABAA receptor clusters with presynaptic markers were statistically analyzed as function of culture age (Fig. 2 D–F). Five days after plating, receptors colocalizing with FM4-64 or SV2 represented on average only about 10% of the total γ2-containing clusters. Thereafter, this number increased until day 30, when about 40% of all receptor clusters colocalized with presynaptic boutons (Fig. 2 D and E). With GAD as a presynaptic marker, a similar colocalization density was obtained on day 20, but it fell again significantly toward day 30 (Fig. 2F). This is similar to the changes observed with the colocalization of β2/F3 clusters and GAD-containing terminals. A significant difference between colocalizations of γ2 and β2/3 clusters with presynaptic markers was observed only with FM4-64 on day 10 (γ2 + FM 27.4 ± 2.9% vs. β2/3 + FM 14.8 ± 2.8%, P < 0.005) and day 20 (30.3 ± 1.8% vs. 23.1 ± 2.6%, P < 0.05) (cf. Fig. 2 A and D).
Time-dependent changes in the total densities of γ2-immunoreactive GABAA receptor clusters and of presynaptic markers on neurites were also plotted independently of their respective colocalizations (Fig. 3 A–C). When measured together with each individual presynaptic marker, γ2 cluster densities were lowest on day 2, then a first peak was measured on day 5, and, after a transient fall on day 10, cluster densities rose again to a maximum on day 20. Thereafter, they declined to significantly lower values on day 30. The densities of γ2 subunits on day 20 appeared to be similar to those previously reported for cultured cortical and hippocampal neurons of about the same age (23). Because these variations in γ2-containing cluster densities were observed with all presynaptic markers in randomly chosen cultures, the data were pooled and compared with the pooled densities of β2/3 clusters listed in Table 1 (Fig. 3D). GABAA receptor clusters containing the β2/3 subunits remained constant between days 10 and 30 and were at all times significantly higher than those with γ2 subunits. The densities of FM4-64 and SV2-stained presynaptic boutons rose along identified dendrites by about 4-fold between days 5 and 20 and remained constant thereafter, whereas the number of GAD-stained boutons rose to a maximum on day 20 and fell again significantly until day 30 (Fig. 3E). As expected, GAD-stained GABAergic bouton densities were at all times lower than those with GABA-unspecific markers FM4-64 and SV2.
In conclusion, during synaptogenesis, the number of GABAA receptor clusters associated with presynaptic terminals increased. However, independently of their subunit composition, a majority of GABAA receptor clusters on the surface of hippocampal neurons was lacking a presynaptic counterpart, even after 30 days in culture.
Discussion
The present study was designed with two goals. First, we wanted to find out whether immunocytochemically identified GABAA receptor clusters could be detected in dendrites independently of the presence of functional presynaptic boutons. Second, we were interested in the time course of formation of synaptic and extrasynaptic GABAA receptor clusters during differentiation of cultured hippocampal neurons.
In a previous study with 15- to 19-day-old hippocampal cultures, only about 50% of β2/3 subunit-containing GABAA receptor clusters visualized on living neurons were associated with functional presynaptic terminals (12). We have now extended these studies by also using an antibody that recognizes an extracellular epitope of the γ2 subunit.
We found that, already a few hours after plating, both β2/3 and γ2 subunit clusters could be detected in neurons that had started to extend neurites. Colocalization of these clusters with SV2-immunoreactive synaptic vesicles seemed to appear only around day 2, although the cells were not yet excitable at this stage of development. This is in agreement with data showing that synaptic vesicles are among the first structural elements that accumulate in axonal varicosities during synaptogenesis (24, 25). In our cultures, maturation of the exocytotic machinery occurred between days 2 and 5. Correspondingly, excitation-dependent loading of synaptic vesicles with FM4-64 dye was observed on day 5. During this time, the distribution of GAD also changed from a diffuse to a punctate presynaptic pattern. Our results thus clearly demonstrate the expression of dendritic clusters of β2/3- or γ2-containing GABAA receptors before the formation of functional presynaptic nerve terminals. However, this does not exclude the possibility of tonic activation of extrasynaptic GABAA receptors by spontaneous GABA release from vesicles outside the terminals (26, 27). In cultured cortical neurons, GABAA receptor currents could be evoked as early as 1 day after plating, but they did not correlate with the presence of functional synapses either (28). In those cells, like in ours, synaptogenesis became prominent after day 4 of culturing.
Our extensive use of presynaptic markers revealed that, even in cells kept in culture for 30 days, less than 50% of all GABAA receptor clusters containing β2/3 subunits colocalized with presynaptic nerve terminals. Because these are predominant subunits in the hippocampus (1, 2, 29), they provide a rather good estimate of all GABAA receptor clusters. However, our data additionally showed a developmental increase of synaptic vs. extrasynaptic GABAA receptor clusters. This seems to depend less on the expression of specific receptors, but rather on the formation of functional presynaptic boutons (cf. Table 1 and Fig. 3E). A similar age-dependent increase of synaptic vs. extrasynaptic GABAA receptor clusters was found for γ2-containing clusters, but we were unable to consistently demonstrate a preferential synaptic targeting of γ2-positive receptors in our cultures.
Large numbers of extrasynaptic GABAA receptors are not only a feature of cultured neurons. With outside-out patch-clamp recordings, differences in synaptic and extrasynaptic GABAA receptors were characterized in hippocampal (8, 30) and cerebellar slices (9–11). Based on differences in decay times of synaptic and extrasynaptic currents, Banks and Pearce (8) have estimated the contribution of extrasynaptic receptors in hippocampal CA1 pyramidal cells to the total GABA-evoked current responses as more than 60%. These functional results agree remarkably well with our immunocytochemical data in a mixed population of hippocampal cells.
In conclusion, our results provide clear evidence for the expression of GABAA receptor clusters in cultured hippocampal neurons before the occurrence of functional presynaptic nerve terminals. They also show that the numbers of receptor clusters associated with presynaptic terminals increase during differentiation and growth of the cells in culture. But even after 30 days in culture, large fractions of GABAA receptor complexes containing β2/3 or γ2 subunits were not apposed to presynaptic boutons.
Acknowledgments
We thank Prof. W. Sieghart (Vienna) for his generous gift of antibodies. We thank Charlotte Becker for her unfailing preparation of the hippocampal cultures. This work was supported by Swiss National Science Foundation Grant 31-45093.95 (to H.R.).
Abbreviations
- GABAA
γ-aminobutyric acid type A
- GAD
glutamic acid decarboxylase
- GFP
green fluorescent protein
- DIC
differential interference contrast
References
- 1.Fritschy J M, Möhler H. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- 2.MacDonald R L, Olsen R W. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- 3.Rabow L E, Russek S J, Farb D H. Synapse. 1995;21:189–274. doi: 10.1002/syn.890210302. [DOI] [PubMed] [Google Scholar]
- 4.Sieghart W. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- 5.Gao B, Fritschy J M. Eur J Neurosci. 1994;6:837–853. doi: 10.1111/j.1460-9568.1994.tb00994.x. [DOI] [PubMed] [Google Scholar]
- 6.Sperck G, Schwarzer C, Tsunashima K, Fuchs K, Sieghart W. Neuroscience. 1997;80:987–1000. doi: 10.1016/s0306-4522(97)00146-2. [DOI] [PubMed] [Google Scholar]
- 7.Laurie D J, Wisden W, Seeburg P H. J Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banks M I, Pearce R E. J Neurosci. 2000;20:937–948. doi: 10.1523/JNEUROSCI.20-03-00937.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brickley S G, Cull-Candy S G, Farrant M. J Neurosci. 1999;19:2960–2973. doi: 10.1523/JNEUROSCI.19-08-02960.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nusser Z, Sieghart W, Somogyi P. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nusser Z, Roberts J D B, Baude A, Richards J G, Somogyi P. J Neurosci. 1995;15:2948–2960. doi: 10.1523/JNEUROSCI.15-04-02948.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kannenberg K, Sieghart W, Reuter H. Eur J Neurosci. 1999;11:1256–1264. doi: 10.1046/j.1460-9568.1999.00533.x. [DOI] [PubMed] [Google Scholar]
- 13.Lévi S, Chesnoy-Marchais D, Sieghart W, Triller A. J Neurosci. 1999;19:7434–7449. doi: 10.1523/JNEUROSCI.19-17-07434.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tretter V, Ehya N, Fuchs K, Sieghart W. J Neurosci. 1997;17:2728–2737. doi: 10.1523/JNEUROSCI.17-08-02728.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scotti A L, Chatton J Y, Reuter H. Phil Trans R Soc London B. 1999;354:357–364. doi: 10.1098/rstb.1999.0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feany M B, Lee S, Edwards R H, Buckley K M. Cell. 1992;70:861–867. doi: 10.1016/0092-8674(92)90319-8. [DOI] [PubMed] [Google Scholar]
- 17.Chang Y C, Gottlieb D I. J Neurosci. 1988;8:2123–2130. doi: 10.1523/JNEUROSCI.08-06-02123.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ebert V, Scholze P, Fuchs K, Sieghart W. Neurochem Int. 1999;34:453–463. doi: 10.1016/s0197-0186(99)00039-x. [DOI] [PubMed] [Google Scholar]
- 19.Buhr A, Schaerer M T, Baur R, Sigel E. Mol Pharmacol. 1997;52:676–682. doi: 10.1124/mol.52.4.676. [DOI] [PubMed] [Google Scholar]
- 20.Ryan T A, Reuter H, Smith S J. Nature (London) 1997;388:478–482. doi: 10.1038/41335. [DOI] [PubMed] [Google Scholar]
- 21.Ewert M, Shivers B D, Luddens H, Möhler H, Seeburg P H. J Cell Biol. 1990;110:2043–2048. doi: 10.1083/jcb.110.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ymer S, Schofield P R, Draguhn A, Werner P, Koehler M, Seeburg P H. EMBO J. 1989;8:1665–1670. doi: 10.1002/j.1460-2075.1989.tb03557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Essrich C, Lorez M, Benson J A, Fritschy J M, Lüscher B. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- 24.Ahmari S E, Buchanan J A, Smith S J. Nat Neurosci. 2000;3:445–451. doi: 10.1038/74814. [DOI] [PubMed] [Google Scholar]
- 25.Fletscher T L, De Camilli P, Banker G. J Neurosci. 1994;14:6695–6706. doi: 10.1523/JNEUROSCI.14-11-06695.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brickley S G, Cull-Candy S G, Farrant M. J Physiol (London) 1996;497:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao X-B, van den Pol A N. J Physiol (London) 2000;523:629–637. doi: 10.1111/j.1469-7793.2000.t01-1-00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hutcheon B, Morley P, Poulter M O. J Physiol (London) 2000;522:3–17. doi: 10.1111/j.1469-7793.2000.t01-5-00003.xm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Killisch I, Dotti C G, Laurie D L, Lüddens H, Seeburg P H. Neuron. 1991;7:927–936. doi: 10.1016/0896-6273(91)90338-z. [DOI] [PubMed] [Google Scholar]
- 30.Edwards F A, Konnerth A, Sakmann B. J Physiol (London) 1990;430:213–249. doi: 10.1113/jphysiol.1990.sp018289. [DOI] [PMC free article] [PubMed] [Google Scholar]