Abstract
In addition to regulating reproductive functions in the brain and periphery, estrogen has trophic and neuroprotective functions in the central nervous system (CNS). Estrogen administration has been demonstrated to provide protection in several animal models of CNS disorders, including stroke, brain injury, epilepsy, Parkinson’s disease, Alzheimer’s disease, age-related cognitive decline and multiple sclerosis. Here, we use a model of toxin-induced oligodendrocyte death which results in demyelination, reactive gliosis, recruitment of oligodendrocyte precursor cells and subsequent remyelination to study the potential benefit of 17β-estradiol (E2) administration in male mice. The results indicate that E2 partially ameliorates loss of oligodendrocytes and demyelination in the corpus callosum. This protection is accompanied by a delay in microglia accumulation as well as reduced mRNA expression of the pro-inflammatory cytokine, tumor necrosis factor alpha (TNFα), and insulin-like growth factor-1 (IGF-1). E2 did not significantly alter the accumulation of astrocytes or oligodendrocyte precursor cells, or remyelination. These data obtained from a toxin-induced, T cell-independent model using male mice provide an expanded view of the beneficial effects of estrogen on oligodendrocyte and myelin preservation.
Keywords: Demyelination, myelin, cuprizone, estrogen, E2, 17β-estradiol, oligodendrocyte, microglia, TNFα, tumor necrosis factor alpha
INTRODUCTION
Interest in the use of sex hormones for therapy to treat multiple sclerosis (MS) comes from the observation that disease is partially ameliorated during pregnancy. A large prospective study of MS patients showed a significant decrease in disease relapses during pregnancy, especially the third trimester, compared to the relapse rate in these same women before pregnancy (Confavreux et al. 2003). In addition, the relapse rate significantly increased after delivery, especially in the first 3 months post-partum, prior to returning to pre-pregnancy levels at 9–12 months. This finding suggests a protective role for pregnancy-related factors, one of which could be sex steroids.
The data from animal models of demyelinating disease and in vitro studies provides evidence for a potential benefit of estrogens. Two forms of estrogen, estriol and 17β-estradiol (E2), have been shown to reduce clinical symptoms of experimental autoimmune encephalomyelitis (EAE) (Bebo et al. 2001; Hoffman et al. 2001; Kim et al. 1999). Addition of E2 to rodent primary oligodendrocyte cultures led to increased proliferation of oligodendrocyte precursors and enhanced membrane sheet formation (Ghoumari et al. 2003; Jung-Testas et al. 1992; Marin-Husstege et al. 2004). E2 also prevented toxin and oxygen-mediated death of oligodendrocytes in culture and oxygen-mediated loss of myelin basic protein in neonatal rat white matter (Gerstner et al. 2007; Takao et al. 2004). Furthermore, estrogens have anti-inflammatory effects in CNS models, as reviewed in (Pozzi et al. 2006; Vegeto et al.). E2 inhibits expression of TNFα and several other inflammatory mediators in response to LPS or proinflammatory cytokines in microglial cell cultures (Bruce-Keller et al. 2000; Drew and Chavis 2000; Vegeto et al. 2006; Vegeto et al. 2001), and in the brain (Vegeto et al. 2006). Furthermore, E2 and estrogen receptor ligands administered in mice with EAE prevented axonal loss in the white matter and neuronal pathology in gray matter, highlighting the potential benefit of estrogen in preserving neuronal integrity in demyelinating disease (Morales et al. 2006; Tiwari-Woodruff et al. 2007).
In an effort to determine whether estrogen is capable of influencing demyelinating disease in vivo, we used the cuprizone model of oligodendrocyte death, demyelination and remyelination in male C57BL/6 mice treated with E2 in the form of subcutaneously implanted E2 pellets. We used male mice to avoid estrogen fluctuation during cycling in female mice and because demyelination is well characterized in C57BL/6 males (Hiremath et al. 1998; Matsushima and Morell 2001). Cuprizone causes death of oligodendrocytes, demyelination and infiltration of reactive glia to the demyelinating site, accompanied by oligodendrocyte precursor cells (OPCs) (Hiremath et al. 1998; Mason et al. 2000a; Matsushima and Morell 2001). Remyelination occurs spontaneously by six weeks of treatment, and proceeds rapidly if cuprizone intoxication is discontinued (Arnett et al. 2001; Mason et al. 2001a; Morell et al. 1998). Using this model, we report that E2 is capable of partial amelioration of oligodendrocyte loss and demyelination, but did not alter remyelination following discontinuation of cuprizone. Furthermore, E2 treatment resulted in a delay in microglia accumulation and a reduction in levels of IGF-1 and TNFα mRNA. Thus, a protective effect of E2 in cuprizone-induced demyelination may occur via multiple mechanisms, including enhancement of oligodendrocyte survival and reduction of microglia activation within the lesion.
MATERIALS AND METHODS
Animals and cuprizone treatment
Adult male C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME) and used for experiments at 8 weeks of age. Mice were housed in DLAM facilities under sterile pathogen-free conditions. To induce demyelination, cuprizone (oxalic bis(cyclohexylidenehydrazide)) (Sigma-Aldrich) at a concentration of 0.125% was mixed into ground Purina mouse chow and fed ad libitum for 3 to 5 weeks. We found in a dose titration that 0.125% cuprizone retained the ability to induce demyelination similar to our previous studies using 0.2%; however, this concentration was optimal to assess the efficacy of E2 administration. Remyelination was assessed by returning the mice to a diet of normal chow for one week following 6 weeks of cuprizone administration. Control mice (labeled “no cuprizone”) were fed pelleted Purina chow and were sacrificed at the same time as the 5 week cuprizone group. All animal use was performed in compliance with the NIH Guide for Care and Use of Laboratory Animals and approved by the UNC-CH Institutional Animal Care and Use Committee.
17β-estradiol administration
Continuous release 25 mg 17β-estradiol (E2) or placebo pellets (3×4 mm; Innovative Research of America, Sarasota, FL) were implanted subcutaneously between the shoulder blades by sterile surgical procedure under anesthesia. These pellets are designed to release continuously over a 60 day period, at an approximate rate of 0.42 mg/day. Cuprizone treatment began 5 days after implantation.
Serum 17β-estradiol measurement
Whole blood samples were obtained by cardiac puncture and serum isolated by centrifugation at 2.7g after removal of the blood clot. The serum was stored at −80°C until use. E2 was measured by radioimmunoassay (RIA) using the double antibody estradiol kit (cat# KE2D5, Seimens, Los Angeles, CA) and following the manufacturer’s protocol.
Tissue Preparation
For histology, mice were deeply anesthetized using isoflurane and intracardially perfused with 0.15M phosphate buffer followed by 4% paraformaldehyde (PFA) solution. Brains were removed, post-fixed overnight in PFA, and embedded in paraffin or post-fixed for 4 hours in PFA, followed by 1–2 days in 30% sucrose, and then embedded in Tissue-Tek® O.C.T.™ freezing media (Sakura Finetek, Torrance, CA) and frozen on a bed of dry ice. Five μm paraffin or frozen coronal brain sections were cut at the fornix region of the corpus callosum (approximately Bregma −0.5mm to −0.7mm), corresponding to Figure 37 of The Mouse Brain In Sterotaxic Coordinates (Franklin 2001).
For the isolation of mRNA, corpus callosum tissue was collected from gross coronal cuts at approximately Bregma −0.25mm and −1.25mm. Sagittal cuts were then made through the cingulum, medial to each lateral ventricle, followed by a cut above and below the corpus callosum to remove the majority of cortex and fornix. This block of tissue was immediately submerged in RNAlater solution (cat#AM7020, Applied Biosystems, Foster City, CA) and after overnight 4°C incubation, stored at −80° C until use. The corpus callosum of five brains was combined for each sample.
Luxol Fast Blue – Periodic Acid Schiff’s (LFB-PAS) stain
To examine demyelination and remyelination, paraffin or frozen sections were stained with Luxol fast blue (Sigma, St. Louis, MI), which stains myelin blue, and periodic acid-Schiff (Sigma, St. Louis, MI), which stains demyelinated axons pink. A blinded observer examined the midline corpus callosum (as diagramed previously in Figure 1 of Mason et al. (Mason et al. 2000a) using a didymium filter and 600× magnification. The sections were scored based on the relative ratio of blue or pink fibers detected visibly by the observer, on a scale from 3 (complete myelination equal to an untreated mouse) to 0 (complete demyelination, as seen during peak cuprizone demyelination at week 5).
Immunohistochemistry
The detection of mature oligodendrocytes was performed with antibody to the Pi isoform of glutathione S-transferase (GSTpi) (Biotrin, Newton, MA, no longer available). Paraffin or frozen sections were permeabilized with 0.1% Triton X-100/2% normal goat serum in phosphate-buffered saline (PBS) for 20 minutes at room temperature. Antigen retrieval to better expose the antibody-binding epitope was performed with 0.1% calcium chloride/0.1% trypsin in 0.05M Tris, pH 7.4 for 15 minutes at 37°C. Sections were rinsed in PBS and incubated with anti-GSTpi antibody (1:1000) or isotype control overnight at 4° C.
Oligodendrocyte precursor cells were detected with a rabbit antibody to NG2, generously supplied by Dr. W.B. Stallcup (Genomic Institute of Novartis Res. Foundation, CA). Five micrometer frozen sections were fixed in 95% ethanol before being stored at −80 °C. Upon removal from the freezer, sections were post-fixed in cold acetone, rinsed in potassium-phosphate-buffered saline (KPBS), and blocked with 0.1% Triton X-100/5% normal goat serum in KPBS for 1 hour at room temperature. Sections were then incubated with anti-NG2 antibody (1:500 in blocking solution) or isotype control overnight at 4° C.
Microglia/macrophages were detected with biotinylated lectin Ricinus communis agglutin-1 (RCA-1) (Vector Laboratories Inc, Burlingame, CA). Paraffin or frozen sections were unmasked with 0.025% protease, type XIV (Sigma-Aldrich) for 2 minutes at 43° C. Following a brief rinse in PBS, they were blocked with 0.1% Triton X-100/1% bovine serum albumin in PBS for 1 hour at room temperature. Sections were then incubated with RCA-1, 1:500 in blocking solution or blocking solution alone as a control, overnight at 4° C.
Astrocytes were detected with antibody to glial fibrillary acidic protein (GFAP) (Invitrogen). Paraffin sections were rehydrated and unmasked with 0.025% protease, type XIV (Sigma-Aldrich) for 2 minutes at 43° C. Following a brief rinse in PBS they were blocked with 0.1% Triton X-100/2% normal goat serum in PBS for 1 hour at room temperature. Sections were then incubated with anti-GFAP antibody (1:200) or isotype control overnight at 4° C.
Following incubation in the primary detection agent, all immunohistochemistry was completed by first rinsing sections three times in PBS, then incubating for 1 hour at room temp with the appropriate secondary antibody (1:400) conjugated to AlexaFluor (Molecular Probes, Eugene, OR). After rinsing, sections were cover-slipped with Vectashield plus DAPI (Vector Laboratories Inc, Burlingame, CA) to counter stain nuclei.
An Olympus (Melville, NY) BX40 microscope, Olympus DP70 digital camera and ImageProPlus software (Media Cybernetics, Silver Spring, MD) were used to obtain images from two sections per brain. All comparative analyses were focused in the corpus callosum from two fields of view, one on each side of the midline, under 400× magnification. The images of the fluorescent antibody-stained sections were overlayed with DAPI images and immunohistochemically-positive cells which colocalized with a nucleus were quantified per square mm by a blinded observer.
Real-time PCR
Corpus callosum tissue (see above) was pooled for 5 animals per treatment group. RNA was obtained by manual homogenization using Potter-Elvehjem type PTFE pestle and glass tubes (Kontes, Vineland, NJ) in Trizol (Invitrogen, Carlsbad, CA) and cleaned up with RNeasy Mini kit (cat# 74104, Qiagen, Valencia, CA). TaqMan 5′ nuclease real-time PCR assays were performed using an ABI Prism 7500 sequence-detection system (PE Applied Biosystems, Foster City, CA) in the UNC Neuroscience Center Functional Genomics Core Facilty. For IGF-1 analysis the Taqman® Gene Expression Assay system was used (ID# Mm00439559_m1), which includes proprietary sequences (Applied Biosystems, Foster City, CA). For TNFα analysis custom oligonucleotide primer and TMRA™ probe sequences were as follows (Applied Biosystems, Foster City, CA):
TNFα primer-forward: CATCTTCTCAAAATTCGAGTGACAA;
TNFα primer-reverse: CTCCAGCTGCTCCTCCACTT;
TNFα Probe: CCTGTAGCCCACGTCGTAGCAAACCAC
Statistical analysis
All statistical analyses were performed using GraphPad Prism 4.0 (GraphPad Software Inc, La Jolla, CA). Comparisons between placebo and E2 treated mice were analyzed using two-tailed Student’s t test. Differences were considered significant if p ≤ 0.05. Correlation analysis between different biological measures of the E2-treated mice (for instance myelination score and serum E2 levels) were performed using a two-tailed Spearman analysis and considered significant if p ≤ 0.05.
RESULTS
Serum levels of E2 in mice implanted with continuous release hormone pellets
In order to determine the effect of E2 on demyelination and remyelination, male C57BL/6 mice were implanted subcutaneously with continuous release placebo or pellets containing 25 mg E2 designed to release a consistent amount of hormone over 60 days (0.42 mg/day). In order to confirm the effectiveness of the implants, and to rule out an effect of cuprizone on serum E2 levels, we measured E2 in serum at various timepoints following implantation and also, following cuprizone exposure. Thus, blood was collected from a representative sample of E2 and placebo treated mice 4 days following implantation, at 3 and 5 weeks after initiation of cuprizone feeding, and one week following cuprizone removal from the diet. Serum levels of E2 were measured by RIA.
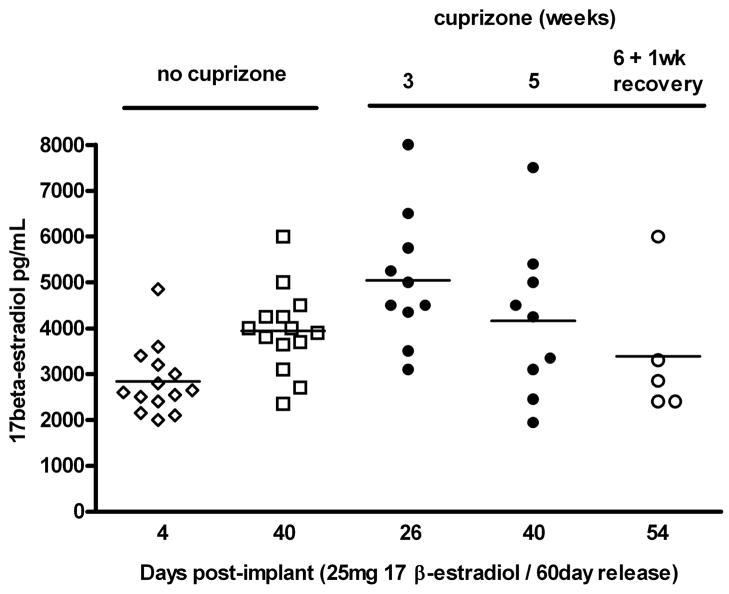
Serum E2 in male mice prior to implantation, or in placebo implanted mice was nearly always below the detection limit of the RIA. When detectable, a range from 5–10pg/mL was found, which is consistent with normal levels in males (data not shown). Four days after E2 implantation, serum E2 rose rapidly and reached an average of about 3000 pg/mL (Figure 1). After 3 or 5 weeks of cuprizone treatment, average serum E2 was 4000 to 5000 pg/mL with similar levels reached in the mice not receiving cuprizone. By the 6 weeks of cuprizone plus 1 week recovery time point, average serum E2 had diminished slightly to 3500 pg/mL. These levels correspond to the lower range of E2 reported for pregnant mice (5000–10,000 pg/mL) (Foster et al. 1983).
Figure 1. Serum levels of E2 achieved using continuous release implants of E2 pellets in male mice.
Serum was collected 4 days after subcutaneous implantation of 25 mg/60 day continuous release E2 pellets (open diamonds), as well as at the time of sacrifice from mice that were not exposed to cuprizone (open squares), or exposed to cuprizone for 3 weeks, 5 weeks (filled circles, or 6 weeks plus 1 week of discontinuation of cuprizone (open circles). Measurement of E2 in the serum was conducted by RIA. Results indicate that serum levels of E2 approached the range reported for pregnant mice, and remained relatively constant during the entire time course.
E2 partially protects against cuprizone-induced demyelination
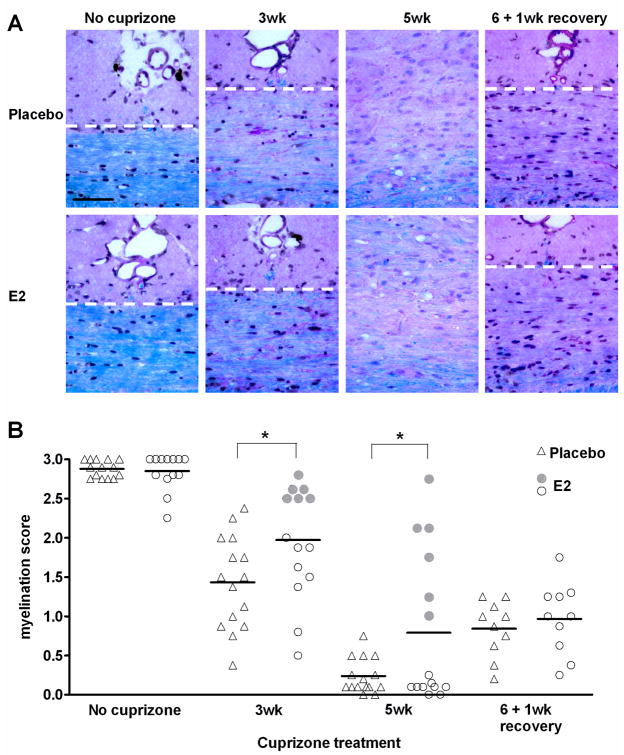
The effect of E2 on demyelination was assessed by exposing mice to cuprizone five days after implantation of placebo or E2 pellets. Mice were analyzed for demyelination after 3 or 5 weeks of cuprizone treatment, when male C57BL/6 are known to be partially or fully demyelinated, respectively (Hiremath et al. 1998). After 3 weeks of cuprizone administration, E2-treated mice are significantly less demyelinated than placebo controls (p < 0.05; Figure 2A and 2B). This effect continues at the 5 week time point in which the E2-treated mice showed significantly less demyelination.
Figure 2. Demyelination and remyelination in placebo and E2-treated mice.
A. Representative images of LFB-PAS-stained midline corpus callosum sections. The corpus callosum is the area below the dashed white lines (except the 5wk images, in which the entire image encompasses corpus callosum). Scale bar represents 50 micrometers.
B. Myelination scores obtained by blind-scoring of midline corpus callosum sections stained with LFB-PAS. A score of 3 reflects normal myelination in an untreated mouse, whereas a score of 0 reflects the absence of myelin. Individual data points (triangle and round symbols) and mean values (horizontal bars) are plotted for 10–15 animals per group at each time point. * p < 0.05. At the 3 and 5 week time points, E2-treated mice showing the greatest protection from demyelination are indicated with filled circles so that they may be followed in subsequent figures of this manuscript..
A visual analysis of the individual myelin scores during demyelination indicates that E2 was effective at attenuating demyelination in some, but not all mice receiving it. To determine if these “responders” represent a sub-population, we marked all data points from them with filled circles (Figure 2B), and tracked them throughout the study. At the five week time point, there is a clear difference between mice that are almost fully demyelinated (open circles) and those which had myelin scores greater than any of the placebo-treated mice (filled circles). Although variability in myelin scores of placebo- treated mice at the 3 week time point is greater than at 5 week, there is still a population of E2-treated mice with higher myelin scores than mice receiving placebo. The reason for the variability in the protective effect of E2 is unclear. We speculate it could be a reflection of the amount of cuprizone ingested. However, potential variation in exposre to cuprizone could not be confirmed as mass spectrometry of serum samples was unable to detect cuprizone. Spearman correlation analysis indicated that demyelination did not correlate with the level of serum E2 (p > 0.05), suggesting that varability in E2 levels was not responsible for variability in the potency of E2 protection.
As expected, after 1 week of recovery from cuprizone, the placebo-treated mice began to remyelinate substantially. However, the E2-treated mice displayed an average myelin score very similar to their 5 week time point (Figure 2B). This suggested that remyelination may be partly delayed in the E2-treated mice.
E2 preserves oligodendrocytes
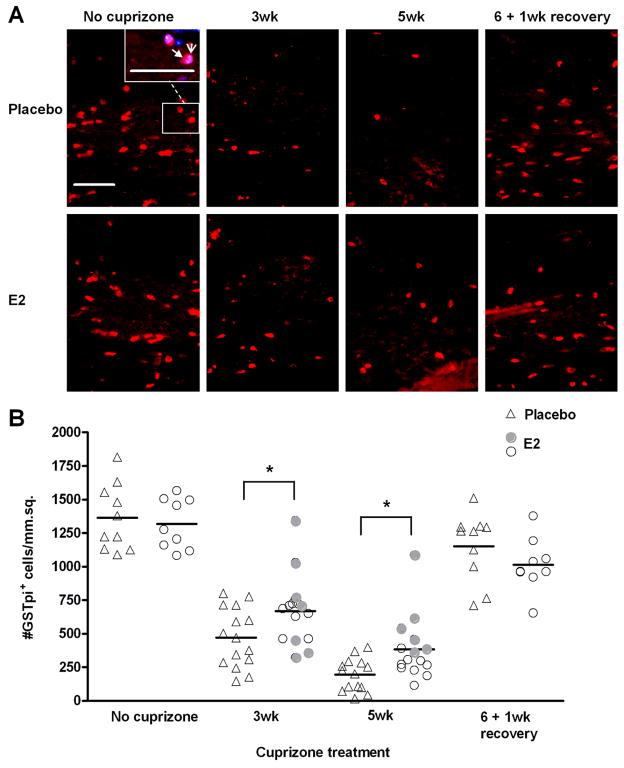
In addition to myelin, the number of oligodendrocytes was examined in corpus callosum sections by immunohistochemistry to detect the marker GSTpi, which allows visualization of mature oligodendrocyte cell bodies (Figure 3A). Equal numbers of oligodendrocytes were detected in E2-treated and placebo mice that did not receive cuprizone (Figure 3B). Similar to myelin staining in histological sections in Figure 2B, E2-treated mice displayed preservation of oligodendrocytes during demeylination, at both the 3 and 5 week time points. Compared to placebo controls, there was nearly twice the number of surviving mature oligodendrocytes in E2-treated mice at week 5 (p< 0.05; Figure 3A and 3B). This suggests that E2 at least partially protects mature oligodendrocytes from cuprizone toxicity.
Figure 3. Cuprizone-induced loss of oligodendrocytes and subsequent repopulation during recovery in placebo and E2-treated mice.
A. Representative images of GSTpi+ mature oligodendrocytes in the corpus callosum of placebo and E2-treated mice during cuprizone administration and recovery. The inset in the no cuprizone, placebo image shows an example of a GSTpi-positive cell (red, filled arrowhead) overlayed with DAPI nuclei counterstain (blue, open arrowhead). DAPI overlayed images were used for quantification. Scale bar represents 50 micrometers.
B. Quantification of GSTpi+ mature oligodendrocytes. Individual data points and mean bars are plotted for 10–15 animals per group at each time point. * p < 0.05. At the 3 and 5 week time points, E2-treated mice that exhibited the greatest protection from demyelination (Figure 2B) are indicated with filled circles.
As stated previously, the E2-treated group of mice at week 5 in Figure 2B could be segregated into E2 non-responders and E2 responders, the latter showing protection from demyelination. E2 responders also showed a tendency for higher numbers of oligodendrocytes, indicative of oligodendrocyte preservation (Figure 3B), particularly at the 5 week time point. Thus, E2 treatment inhibits demyelination and provides a protective effect for mature oligodendrocytes.
The number of mature oligodendrocytes during remyelination was also assessed in E2-treated and placebo mice. One week after removal of cuprizone from the diet, oligodendrocyte numbers were almost completely restored to levels observed in control mice not receiving cuprizone. Although the number of mature oligodendrocytes appears lower in E2-treated mice than placebo controls, the difference was not statistically significant (Figure 3B).
E2 does not affect the number of oligodendrocyte precursor cells in demyelinating/remyelinating lesions of treated mice
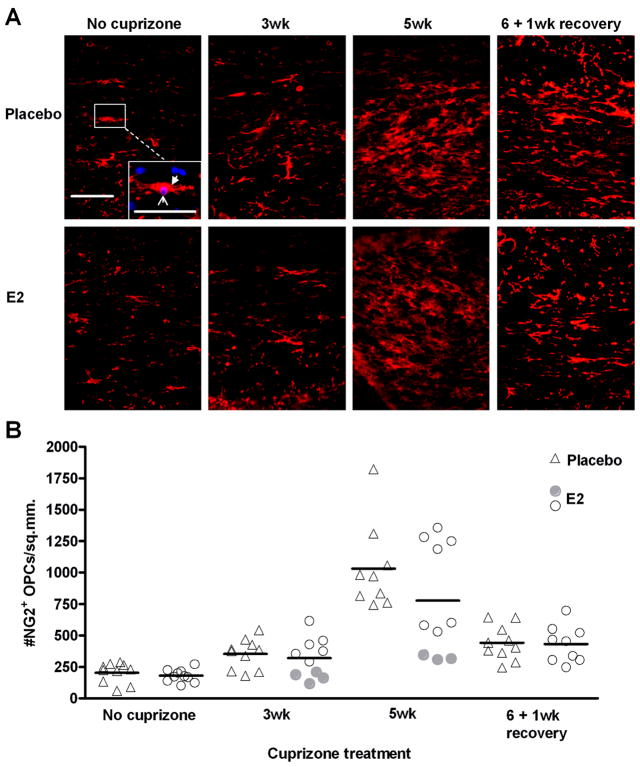
In vitro studies indicate that E2 can promote proliferation of oligodendrocytes (Marin-Husstege et al. 2004). During exposure to cuprizone, oligodendrocyte precursor cells (OPCs) proliferate and accumulate in the demyelinated corpus callosum (Mason et al. 2000a). Therefore, we hypothesized that numbers of OPCs would be greater in demyelinated lesions of E2-treated mice. OPCs were detected by immunohistochemistry to the proteoglycan NG2. It is well established that NG2-positive cells generate oligodendrocytes, however it has recently been demonstrated that they can also generate protoplasmic astrocytes in the gray matter, and potentially a small population of neurons in certain brain regions (reviewd in (Nishiyama et al. 2009)). The suitability of NG2 as an OPC marker in the corpus callosum is supported by findings from a genetic fate-mapping study using NG2-Cre-transgenic mice which showed that astrocytes of the white matter were not generated from NG2 cells (Zhu et al. 2008). Furthermore, we have perfomed co-immunolabelling studies with NG2 and the astrocyte marker GFAP and have not observed any co-localization (data not shown). Contrary to our expectations, there was no statistically significant difference in numbers of OPCs in the lesions of E2-treated mice compared to placebo, at any of the time points (Figure 4). However, fewer OPCs accumulated in the corpus callosum of E2 responders (filled circles) when E2 was most effective at reducing demyelination. Spearman correlation analysis indicates a significant correlation between myelin score and OPC numbers at both 3wk (p = 0.0011) and 5wk (p = 0.0072) in E2-treated mice. During remyelinaton, one week after removal of cuprizone from the diet, OPC numbers are reduced, consistent with differentiation to mature oligodendrocytes, and there is no difference between placebo and E2-treated mice (Figure 4). Thus, although we observed a correlation between reduced demyelination and fewer OPCs within the E2 group, OPC numbers were not statistically different when compared to the placebo group.
Figure 4. Accumulation of OPCs during demyelination and remyelination in placebo and E2-treated mice.
A. Representative images of NG2+ OPCs in the corpus callosum of placebo and E2-treated mice during cuprizone administration and recovery. The inset in the no cuprizone, placebo image shows an example of an NG2-positive cell (red, filled arrowhead) overlayed with DAPI nuclei counterstain (blue, open arrowhead). Images overlayed with DAPI were used for quantification. Scale bar represents 50 micrometers.
B. Quantification of NG2+ OPCs. Individual data points and mean bars are plotted for 8–10 animals per group at each time point. There are no statistically significant differences between placebo and E2-treated mice at any time point. At the 3 and 5 week time points, E2-treated mice that exhibited the greatest protection from demyelination (Figure 2B) are indicated with filled circles.
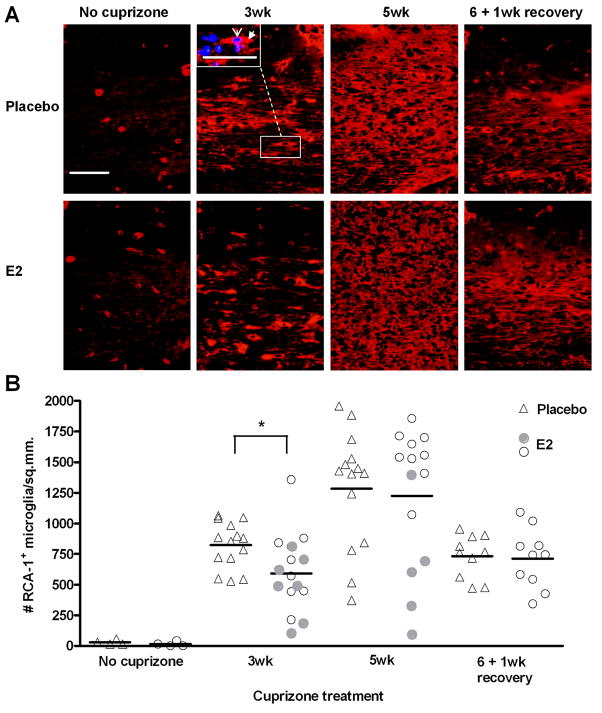
Accumulation of microglia/macrophages is delayed in E2-treated mice
Microglia, and to a lesser extent macrophage, activation and accumulation in demyelinated lesions is a hallmark feature in cuprizone-induced demyelination (Hiremath 2008; Hiremath et al. 1998; McMahon et al. 2002). These cells phagocytize myelin debris as well as produce a variety of cytokines and other molecules which may both exacerbate cuprizone-induced demyelination, and promote the repair process (Arnett et al. 2001; Arnett et al. 2002; Iocca et al. 2008; Irvine and Blakemore 2006; Mason et al. 2001b; Pasquini et al. 2007; Plant et al. 2005). Here, histochemical staining was performed with the lectin RCA-1, a marker for microglia/macrophages, in the corpus callosum during the time course of demyelination and remyelination. Very few microglia were present in mice that have not been exposed to cuprizone, and this was not altered by E2 treatment (Figure 5). After three weeks of cuprizone treatment, there were significantly fewer microglia in the demyelinated lesions of E2-treated versus placebo-treated mice. However, by 5 weeks of cuprizone intoxication, there were similar numbers of microglia present in placebo and E2-treated mice, despite the decreased demyelination in E2-treated mice at this time point. An analysis of the E2 responders (filled circles) indicated no clear pattern at the 3 week time point. However, there is a clear correlation between fewer microglia and less demyelination at 5 weeks (Spearman correlation analysis; p = 0.0048). During remyelination, the microglia population was diminished to a similar extent in both placebo and E2-treated mice (Figure 5).
Figure 5. Accumulation of microglia during demyelination and remyelination in placebo and E2-treated mice.
A. Representative images of RCA-1+ microglia/macrophages in the corpus callosum of placebo and E2-treated mice during cuprizone administration and recovery. The inset in the 3wk cuprizone, placebo image shows an example of an RCA-1-positive cell (red, filled arrowhead) overlayed with DAPI nuclei counterstain (blue, open arrowhead). DAPI-overlayed images were used for quantification. Scale bar represents 50 micrometers.
B. Quantification of RCA-1+ microglia/macrophages. Individual data points and mean bars are plotted for 10–15 animals per group at each time point. * p < 0.05. At the 3 and 5 week time points, E2-treated mice that exhibited the greatest protection from demyelination (Figure 2B) are indicated with filled circles.
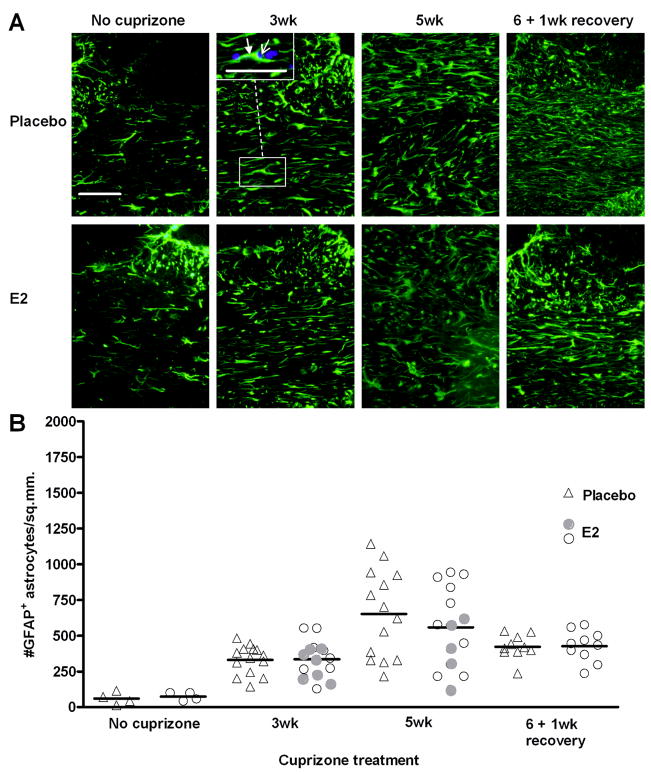
E2 does not affect the number of astrocytes during demyelination or remyelination
Astrocytes respond to demyelination and also secrete cytokines and growth factors that participate in cuprizone-induced demyelination and remyelination (Komoly et al. 1992; Mason et al. 2000a; Mason et al. 2000b; Plant et al. 2005; Plant et al. 2007; Selvaraju et al. 2004). Similar to microglia, immunostaining with the astrocyte marker GFAP was performed to examine whether E2 affects the numbers of these cells during demyelination and remyelination. In the absence of cuprizone administration, there are few astrocytes present in the corpus callosum (Figure 6). During demyelination, the astrocyte population increased similarly in placebo and E2-treated mice at week 3 and week 5 (Figure 6). Furthermore, there appears to be no obvious correlation with protection from demyelination (filled circles) and astrocyte number at 3 weeks; however, at the 5 week time point, the E2 responders showed decreased astrocyte accumulation. E2 had no effect on astrocyte numbers in the corpus callosum during remyelination (Figure 6B).
Figure 6. Accumulation of astrocytes during demyelination and remyelination in placebo and E2-treated mice.
A. Representative images of GFAP+ astrocytes in the corpus callosum of placebo and E2-treated mice during cuprizone administration and recovery. The inset in the 3wk cuprizone, placebo image shows an example of a GFAP-positive cell (green, filled arrowhead) overlayed with DAPI nuclei counterstain (blue, open arrowhead). DAPI-overlayed images were used for quantification. Scale bar represents 50 micrometers.
B. Quantification of GFAP+ astrocytes. Individual data points and mean bars are plotted for 10–15 animals per group at each time point. There are no statistically significant differences between placebo and E2-treated mice at any time point. At the 3 and 5 week time points, E2-treated mice that exhibited the greatest protection from demyelination (Figure 2B) are indicated with filled circles.
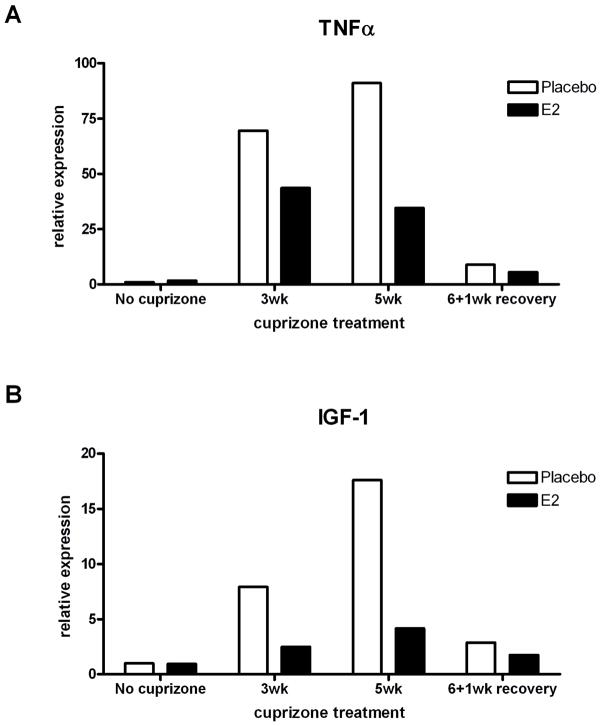
TNFα and IGF-1 mRNA expression is diminished in E2-treated mice during demyelination
In an effort to identify potential mechanisms of E2 protection against cuprizone-induced demyelination and oligodendrocyte loss, we measured the expression of specific for two candidate mediators, TNFα and IGF-1 by real-time PCR. Due to the small amount of tissue from isolated corpus callosum and variability in E2-induced protection from demyelination (Figure 2B), corpus callosi of 5 mice per group were pooled for RNA analysis. TNFα is an inflammatory cytokine that has been associated both with exacerbation of demyelinating disease (Arnett et al. 2001; Ito et al. 2001; Probert et al. 1995) as well as having an important beneficial role in remyelination (Arnett et al. 2001). Furthermore, estrogen is known to reduce TNFα expression in models of CNS injury/inflammation (Lin et al. 2009; Matejuk et al. 2001; Vegeto et al. 2006). Therefore, we sought to determine whether TNFα expression was altered by E2 administration during cuprizone-induced demyelination or remyelination.
Previous studies have shown that TNFα is upregulated during cuprizone-induced demyelination, and is primarily expressed by microglia, and occasionally by astrocytes (Arnett et al. 2001). As expected, placebo-treated mice showed an increase in TNFα mRNA during demyelination (70-fold compared to no cuprizone placebo at 3 weeks and 90-fold compared to no cuprizone placebo at 5 weeks of cuprizone treatment (Figure 7A). During remyelination, TNFα mRNA levels were reduced, which corresponds to the reduction in microglia and astrocytes in both groups at this time point (Figure 5B). Interestingly, E2 treatment inhibited TNFα induction during demyelination by approximately 33% at 3 weeks and 66% at 5 weeks of cuprizone treatment compared to placebo. This reduction in TNFα induction occurs at the same time points that higher numbers of surviving mature oligodendrocytes are observed in E2-treated mice (Figure 3).
Figure 7. Expression of TNFα and IGF-1 mRNA during demyelination and remyelination in placebo and E2 treated mice.
A. Real-time PCR analysis of TNFα mRNA. Results show the expected upregulation during demyelination in placebo-treated mice, which is reduced by approximately half in E2-treated mice. All samples are normalized to the no cuprizone placebo group.
B. Real-time PCR analysis of IGF-1 mRNA. Results show the expected upregulation during demyelination in placebo-treated mice, which is dramatically reduced in E2-treated mice. All samples are normalized to the no cuprizone placebo group.
The second factor we measured is IGF-1, a growth factor expressed by most astrocytes and by a subpopulation of microglia during cuprizone-induced demyelination (Mason et al. 2001b). IGF-1 has also been demonstrated to promote oligodendrocyte survival and remyelination (Mason et al. 2003; Mason et al. 2000b). Furthermore, there is evidence for cross-talk between estrogen and IGF-1 effects in the CNS (Cardona-Gomez et al. 2001; Mendez et al. 2003; Mendez et al. 2005). Therefore, we hypothesized that increased survival of oligodendrocytes would be concomitant with increased expression of IGF-1. The data indicate that IGF-1 mRNA expression increases during demyelination in placebo-treated mice (Figure 7B) and decreases during remyelination, similar to previous results in unmanipulated mice subjected to cuprizone intoxication (Mason et al. 2000a). However, when mice were exposed to E2 administration, IGF-1 mRNA was substantially attenuated at both 3 and 5 weeks of demyelination (Figure 7B). Levels of IGF-1 receptor mRNA were not altered by E2 administration (data not shown). This suppression of IGF-1, in addition to the suppression of TNFa, may indicate an overall suppression of astrocytes and microglia by E2 administration.
DISCUSSION
The sex hormone E2 has been demonstrated to reduce neuronal loss and demyelination in several models of CNS injury (Behl and Moosmann 2002; Hoffman et al. 2006; Pozzi et al. 2006; Tiwari-Woodruff et al. 2007), as well as prevent oligodendrocyte cell death and promote proliferation of oligodendrocyte precursors in vitro (Gerstner et al. 2007; Marin-Husstege et al. 2004; Takao et al. 2004). In this study, we used a toxin model of primary oligodendrocyte death to evaluate the role of E2 in CNS demyelination and remyelination. We report that E2 administration to male mice partially ameliorated corpus callosum demyelination and reduced mature oligodendrocyte loss. This protection was accompanied by a delay in microglia accumulation in the demyelinating lesion as well as reduced IGF-1 and TNFα expression. However, there was no statistically significant effect on the numbers of oligodendrocyte precursors or astrocytes that infiltrated the lesion. In addition, E2 did not appear to alter remyelination.
The two general mechanisms by which E2 may attenuate demyelination include a direct anti-apoptotic effect on mature oligodendrocytes, or an anti-inflammatory mechanism acting on microglia and astrocytes. Both of these scenarios are plausible, given that E2 has been demonstrated to prevent death of oligodendrocytes and neurons in vivo (Dubal et al. 1998; Garcia-Segura et al. 1999; Gerstner et al. 2007; Quesada and Micevych 2004; Wen et al. 2004) as well as inhibit glial activation and inflammation in the CNS (Barreto et al. 2007; Matejuk et al. 2001; Vegeto et al. 2003). Therefore, a combination of direct protection of mature oligodendrocytes by E2 and reduced noxious products from microglia such as TNFα may contribute to reduced demyelination.
Quantification of microglia and astrocytes during cuprizone-induced demyelination and remyelination indicated that, overall, E2 delayed microglia accumulation, but had no effect on astrocyte numbers in the corpus callosum. However, an analysis of the mice that showed the greatest protection from demyelination by E2 indicated that there was a reduction in both of these cell types, especially at the 5 week time point (Figures 5B and 6B, filled circles). In addition to a delay in microglia accumulation at week 3 (Figure 5B), TNFα mRNA was reduced in E2-treated mice (Figure 7A). If one assumes a similar reduction in protein levels, this attenuation in a microglial response may be at least partially responsible for the protective effect of E2, consistent with the delay in demyelination observed in TNFα-deficient mice (Arnett et al. 2001).
Another potential mechanism to explain the increased survival of mature oligodendrocytes in E2 treated mice (Figure 3) is a direct inhibition of oligodendrocyte apoptosis. E2 has been shown to attenuate hyperoxia-induced apoptotic death of primary oligodendrocytes in vitro through downregulation of proapoptotic mediators (Gerstner et al. 2007). Future studies to measure expression of pro- and anti-apoptotic mediators localized to mature oligodendrocytes may provide important insights into the mechanism of E2 protection in cuprizone-induced demyelination.
Although E2 has been shown to increase proliferation of oligodendrocyte precursors in vitro (Jung-Testas et al. 1992; Marin-Husstege et al. 2004), we did not observe an increase in the numbers of OPCs in mice treated with E2 compared to placebo. In fact, E2-treated mice exhibiting the most protection from demyelination also exhibited fewer OPCs in the lesion compared to any of the other mice (Figure 4B, filled circles). The most likely interpretation for this observation is that fewer OPCs were recruited into the lesion, because there was less damage to the mature oligodendrocytes, and hence, less need for repair. This phenomenon has been reported in IGF-1 transgenic mice (Mason et al. 2000b) and nNOS−/− mice (Linares et al. 2006), in which there was very little loss of mature oligodendrocytes in the demyelinating lesion, and subsequently very little accumulation of OPCs. Alternatively, the reduction of TNFα in E2-treated mice may be at least partly responsible for diminished OPC numbers, given that cuprizone-treated TNFα-deficient mice display a significant reduction in accumulation and proliferation of OPCs during demyelination (Arnett et al. 2001). A third possible explaination is that E2 may induce differentiation of OPCs, thus leading to the observation of fewer OPCs and greater numbers of mature oligos at 5 weeks of cuprizone exposure in a subset of the E2 treated mice (Figures 3B and 4B, filled circles). In summary, unlike in vitro studies, E2 does not appear to increase OPC numbers in demyelinated lesions.
We have also demonstrated that E2-treatment attenuated demyelination-induced upregulation of IGF-1 expression. IGF-1 is a survival factor for oligodendrocytes (Barres et al. 1993; Mason et al. 2000b; Ye and D’Ercole 1999) and promotes remyelination (Mason et al. 2003). There is evidence for cross-talk between the actions of estradiol and IGF-1 in several neural events, including survival of developing neurons, neuronal differentiation, synaptic plasticity, female sexual behavior, adult neurogenesis, and neuroprotection (reviewed in (Cardona-Gomez et al. 2001; Mendez et al. 2003; Mendez et al. 2005)). Therefore, we had originally hypothesized that E2 administration may mediate a protective effect through the IGF-1 signalling pathway. In contrast, we found that E2 administration resulted in a diminished production of IGF-1 mRNA during demyelination. We were not able to assess IGF-1 protein levels from the corpus callosum of mice with available antibodies for ELISA assay or Western blots. In the literature, the effects of E2 on the expression of IGF-1 appear mixed, with a few reports that it results in increased IGF-1 expression in various tissues (Michels et al. 1993; Murphy et al. 1987; Shingo and Kito 2003) and others reporting a reduction (Borski et al. 1996; Durrer et al. 2007). We and others have previously showed IGF-1 is primarily produced by microglia and astrocytes (Mason et al. 2000; Komoly et al. 1992). The reduction of IGF-1 mRNA expression during cuprizone-induced demyelination in our study may be secondary to reduced activation of microglia and astrocytes, indicated by reduced TNFα expression (Figure 7A). In any case, the protective effect of E2 does not appear to require upregulation of IGF-1 mRNA.
Expression of both estrogen receptor-alpha (ERα) and estrogen receptor-beta (ERβ) has been demonstrated by a variety of methods in brain cells. Neurons and astrocytes can express either ERα or ERβ in vivo (Garcia-Segura et al. 1999; Milner et al. 2001; Milner et al. 2005). ERβ is co-localized on oligodendrocytes and the myelin sheath in vivo (Zhang et al. 2004); and ERα has been demonstrated in microglia in vivo (Sierra et al. 2008). Experiments in our cuprizone intoxication model to measure mRNA levels of ERα and ERβ during demyelination showed no significant change compared to the no cuprizone control (data not shown). We did not detect ERα by immunohistochemical staining in the corpus callosum, although positive cells were found in regions of the brain known to express ERα such as the ventromedial hypothalamic nucleus and arcuate nucleus (Merchenthaler et al. 2004; Mitra et al. 2003). We were unable to detect the presence of ERβ due to nonspecific staining by available commercial antibodies. Thus, is it not clear whether either or both ER type is required for the E2-mediated protection from cuprizone-induced demyelination. Preliminary studies in our laboratory with ERα- and ERβ-deficient mice indicate that ERα but not ERβ may be necessary for the protective effect of E2 administration. This is partially consistent with findings in the EAE model, in which the use of ERα or ERβ-deficient mice as well as selective agonists to each receptor, revealed that the anti-inflammatory effects and the reduction in clinical symptoms by E2 administration was mediated by ERα (Elloso et al. 2005; Garidou et al. 2004; Polanczyk et al. 2004). Interestingly, these studies revealed that the E2 effect was not mediated through ERa expression on T cells (Polanczyk et al. 2004), or any other bone marrow derived cells (Garidou et al. 2004), indicating that E2 action on brain cells may be the critical factor. The cuprizone model, which does not involve T cell mediated autoimmunity, may thus be an excellent model to continue studying the mechanism involed in E2 mediated protection in demyelination pathology. Relatively little attention has been paid to effects of E2 in oligodendrocytes in EAE studies. Here, we have demonstrated that E2 can reduce demyelination and prevent the loss of oligodendrocytes in a toxin model of demyelination (Figures 2, 3).
Recently, it was demonstrated that a combination of E2 and progesterone was successful in reducing cuprizone-induced demyelination, although neither was effective when administered alone (Acs et al. 2009). In our study, we used a higher dose of E2, and also a lower dose of cuprizone than reported by Acs et al., perhaps allowing the moderate protection of E2 to become apparent. Interestingly, this group found that the combined E2 and progestrerone therapy resulted in an increase in microglia and astrocytes, as well as IGF-1 expression. We interpret these findings to suggest that a combination of E2 and progesterone leads to accumulation of gliosis, while E2 administration alone leads to decreased gliosis. However, both treatments provide partial amelioration from demyelination.
In conclusion, we have demonstrated that E2 administration to male mice provides partial protection from oligodendrocyte loss and demyelination. This protection may be due to a combination of a delay in microglia accumulation and a reduction in TNFα mRNA expression. This work adds to and expands the body of work indicating that estrogen can be beneficial therapy for CNS diseases such as multiple sclerosis and provides a basis for future experiments designed to delineate the mechanism of E2 protection.
Acknowledgments
This work was supported by NIAID AI51770 and grants from the National Multiple Sclerosis Society; RG3898 and collaborative research center grant CA1053-A. J.P-Y.T. was supported by NMSS 1785; and W.G. by NMSS PP1568 and the Nancy Davis Foundation.
We thank Dr. Arlene Bridges, Director of the ADME Mass Spectrometry Center at UNC-Chapel Hill for the analyses of cuprizone in serum samples.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acs P, Kipp M, Norkute A, Johann S, Clarner T, Braun A, Berente Z, Komoly S, Beyer C. 17beta-estradiol and progesterone prevent cuprizone provoked demyelination of corpus callosum in male mice. Glia. 2009;57(8):807–814. doi: 10.1002/glia.20806. [DOI] [PubMed] [Google Scholar]
- Arnett HA, Hellendall RP, Matsushima GK, Suzuki K, Laubach VE, Sherman P, Ting JP. The protective role of nitric oxide in a neurotoxicant-induced demyelinating model. J Immunol. 2002;168(1):427–433. doi: 10.4049/jimmunol.168.1.427. [DOI] [PubMed] [Google Scholar]
- Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci. 2001;4(11):1116–1122. doi: 10.1038/nn738. [DOI] [PubMed] [Google Scholar]
- Barres BA, Jacobson MD, Schmid R, Sendtner M, Raff MC. Does oligodendrocyte survival depend on axons? Curr Biol. 1993;3(8):489–497. doi: 10.1016/0960-9822(93)90039-q. [DOI] [PubMed] [Google Scholar]
- Barreto G, Veiga S, Azcoitia I, Garcia-Segura LM, Garcia-Ovejero D. Testosterone decreases reactive astroglia and reactive microglia after brain injury in male rats: role of its metabolites, oestradiol and dihydrotestosterone. Eur J Neurosci. 2007;25(10):3039–3046. doi: 10.1111/j.1460-9568.2007.05563.x. [DOI] [PubMed] [Google Scholar]
- Bebo BF, Jr, Fyfe-Johnson A, Adlard K, Beam AG, Vandenbark AA, Offner H. Low-dose estrogen therapy ameliorates experimental autoimmune encephalomyelitis in two different inbred mouse strains. J Immunol. 2001;166(3):2080–2089. doi: 10.4049/jimmunol.166.3.2080. [DOI] [PubMed] [Google Scholar]
- Behl C, Moosmann B. Antioxidant neuroprotection in Alzheimer’s disease as preventive and therapeutic approach. Free Radic Biol Med. 2002;33(2):182–191. doi: 10.1016/s0891-5849(02)00883-3. [DOI] [PubMed] [Google Scholar]
- Borski RJ, Tsai W, DeMott-Friberg R, Barkan AL. Regulation of somatic growth and the somatotropic axis by gonadal steroids: primary effect on insulin-like growth factor I gene expression and secretion. Endocrinology. 1996;137(8):3253–3259. doi: 10.1210/endo.137.8.8754747. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Keeling JL, Keller JN, Huang FF, Camondola S, Mattson MP. Antiinflammatory effects of estrogen on microglial activation. Endocrinology. 2000;141(10):3646–3656. doi: 10.1210/endo.141.10.7693. [DOI] [PubMed] [Google Scholar]
- Cardona-Gomez GP, Mendez P, DonCarlos LL, Azcoitia I, Garcia-Segura LM. Interactions of estrogens and insulin-like growth factor-I in the brain: implications for neuroprotection. Brain Res Brain Res Rev. 2001;37(1–3):320–334. doi: 10.1016/s0165-0173(01)00137-0. [DOI] [PubMed] [Google Scholar]
- Confavreux C, Vukusic S, Adeleine P. Early clinical predictors and progression of irreversible disability in multiple sclerosis: an amnesic process. Brain. 2003;126(Pt 4):770–782. doi: 10.1093/brain/awg081. [DOI] [PubMed] [Google Scholar]
- Drew PD, Chavis JA. Female sex steroids: effects upon microglial cell activation. J Neuroimmunol. 2000;111(1–2):77–85. doi: 10.1016/s0165-5728(00)00386-6. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Kashon ML, Pettigrew LC, Ren JM, Finklestein SP, Rau SW, Wise PM. Estradiol protects against ischemic injury. J Cereb Blood Flow Metab. 1998;18(11):1253–1258. doi: 10.1097/00004647-199811000-00012. [DOI] [PubMed] [Google Scholar]
- Durrer S, Ehnes C, Fuetsch M, Maerkel K, Schlumpf M, Lichtensteiger W. Estrogen sensitivity of target genes and expression of nuclear receptor co-regulators in rat prostate after pre- and postnatal exposure to the ultraviolet filter 4-methylbenzylidene camphor. Environ Health Perspect. 2007;115(Suppl 1):42–50. doi: 10.1289/ehp.9134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elloso MM, Phiel K, Henderson RA, Harris HA, Adelman SJ. Suppression of experimental autoimmune encephalomyelitis using estrogen receptor-selective ligands. J Endocrinol. 2005;185(2):243–252. doi: 10.1677/joe.1.06063. [DOI] [PubMed] [Google Scholar]
- Foster HL, Small JD, Fox JG. Normative Biology, Immunology and Husbandry of Laboratory Rodents. Orlando: Academic Press; 1983. [Google Scholar]
- Franklin GPaKBJ. the Mouse Brain in Stereotaxic Coordinates. 2001. [Google Scholar]
- Garcia-Segura LM, Wozniak A, Azcoitia I, Rodriguez JR, Hutchison RE, Hutchison JB. Aromatase expression by astrocytes after brain injury: implications for local estrogen formation in brain repair. Neuroscience. 1999;89(2):567–578. doi: 10.1016/s0306-4522(98)00340-6. [DOI] [PubMed] [Google Scholar]
- Garidou L, Laffont S, Douin-Echinard V, Coureau C, Krust A, Chambon P, Guery JC. Estrogen receptor alpha signaling in inflammatory leukocytes is dispensable for 17beta-estradiol-mediated inhibition of experimental autoimmune encephalomyelitis. J Immunol. 2004;173(4):2435–2442. doi: 10.4049/jimmunol.173.4.2435. [DOI] [PubMed] [Google Scholar]
- Gerstner B, Sifringer M, Dzietko M, Schuller A, Lee J, Simons S, Obladen M, Volpe JJ, Rosenberg PA, Felderhoff-Mueser U. Estradiol attenuates hyperoxia-induced cell death in the developing white matter. Ann Neurol. 2007;61(6):562–573. doi: 10.1002/ana.21118. [DOI] [PubMed] [Google Scholar]
- Ghoumari AM, Ibanez C, El-Etr M, Leclerc P, Eychenne B, O’Malley BW, Baulieu EE, Schumacher M. Progesterone and its metabolites increase myelin basic protein expression in organotypic slice cultures of rat cerebellum. J Neurochem. 2003;86(4):848–859. doi: 10.1046/j.1471-4159.2003.01881.x. [DOI] [PubMed] [Google Scholar]
- Hiremath MM, Chen VS, Suzuki K, Ting JP-Y, Matsushima GK. MHC class II exacerbates demyelination in vivo independently of T cells. Journal of Neuroimmunology. 2008;203(1):23–22. doi: 10.1016/j.jneuroim.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiremath MM, Saito Y, Knapp GW, Ting JP, Suzuki K, Matsushima GK. Microglial/macrophage accumulation during cuprizone-induced demyelination in C57BL/6 mice. J Neuroimmunol. 1998;92(1–2):38–49. doi: 10.1016/s0165-5728(98)00168-4. [DOI] [PubMed] [Google Scholar]
- Hoffman GE, Le WW, Murphy AZ, Koski CL. Divergent effects of ovarian steroids on neuronal survival during experimental allergic encephalitis in Lewis rats. Exp Neurol. 2001;171(2):272–284. doi: 10.1006/exnr.2001.7783. [DOI] [PubMed] [Google Scholar]
- Hoffman GE, Merchenthaler I, Zup SL. Neuroprotection by ovarian hormones in animal models of neurological disease. Endocrine. 2006;29(2):217–231. doi: 10.1385/ENDO:29:2:217. [DOI] [PubMed] [Google Scholar]
- Iocca HA, Plant SR, Wang Y, Runkel L, O’Connor BP, Lundsmith ET, Hahm K, van Deventer HW, Burkly LC, Ting JP. TNF superfamily member TWEAK exacerbates inflammation and demyelination in the cuprizone-induced model. J Neuroimmunol. 2008;194(1–2):97–106. doi: 10.1016/j.jneuroim.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Irvine KA, Blakemore WF. Age increases axon loss associated with primary demyelination in cuprizone-induced demyelination in C57BL/6 mice. J Neuroimmunol. 2006;175(1–2):69–76. doi: 10.1016/j.jneuroim.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Ito A, Bebo BF, Jr, Matejuk A, Zamora A, Silverman M, Fyfe-Johnson A, Offner H. Estrogen treatment down-regulates TNF-alpha production and reduces the severity of experimental autoimmune encephalomyelitis in cytokine knockout mice. J Immunol. 2001;167(1):542–552. doi: 10.4049/jimmunol.167.1.542. [DOI] [PubMed] [Google Scholar]
- Jung-Testas I, Renoir M, Bugnard H, Greene GL, Baulieu EE. Demonstration of steroid hormone receptors and steroid action in primary cultures of rat glial cells. J Steroid Biochem Mol Biol. 1992;41(3–8):621–631. doi: 10.1016/0960-0760(92)90394-x. [DOI] [PubMed] [Google Scholar]
- Jung-Testas I, Schumacher M, Robel P, Baulieu EE. Actions of steroid hormones- and growth factors on glial cells of the central and peripheral nervous system. J Steroid Biochem Mol Biol. 1994;48(1):145–154. doi: 10.1016/0960-0760(94)90261-5. [DOI] [PubMed] [Google Scholar]
- Kim S, Liva SM, Dalal MA, Verity MA, Voskuhl RR. Estriol ameliorates autoimmune demyelinating disease: implications for multiple sclerosis. Neurology. 1999;52(6):1230–1238. doi: 10.1212/wnl.52.6.1230. [DOI] [PubMed] [Google Scholar]
- Komoly S, Hudson LD, Webster HD, Bondy CA. Insulin-like growth factor I gene expression is induced in astrocytes during experimental demyelination. Proc Natl Acad Sci U S A. 1992;89(5):1894–1898. doi: 10.1073/pnas.89.5.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CL, Dumont AS, Su YF, Tsai YJ, Huang JH, Chang KP, Howng SL, Kwan AL, Kassell NF, Kao CH. Attenuation of cerebral vasospasm and secondary injury by 17beta-estradiol following experimental subarachnoid hemorrhage. J Neurosurg. 2009;110(3):457–461. doi: 10.3171/2008.6.17622. [DOI] [PubMed] [Google Scholar]
- Linares D, Taconis M, Mana P, Correcha M, Fordham S, Staykova M, Willenborg DO. Neuronal nitric oxide synthase plays a key role in CNS demyelination. J Neurosci. 2006;26(49):12672–12681. doi: 10.1523/JNEUROSCI.0294-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Husstege M, Muggironi M, Raban D, Skoff RP, Casaccia-Bonnefil P. Oligodendrocyte progenitor proliferation and maturation is differentially regulated by male and female sex steroid hormones. Dev Neurosci. 2004;26(2–4):245–254. doi: 10.1159/000082141. [DOI] [PubMed] [Google Scholar]
- Mason JL, Jones JJ, Taniike M, Morell P, Suzuki K, Matsushima GK. Mature oligodendrocyte apoptosis precedes IGF-1 production and oligodendrocyte progenitor accumulation and differentiation during demyelination/remyelination. J Neurosci Res. 2000a;61(3):251–262. doi: 10.1002/1097-4547(20000801)61:3<251::AID-JNR3>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Mason JL, Langaman C, Morell P, Suzuki K, Matsushima GK. Episodic demyelination and subsequent remyelination within the murine central nervous system: changes in axonal calibre. Neuropathol Appl Neurobiol. 2001a;27(1):50–58. doi: 10.1046/j.0305-1846.2001.00301.x. [DOI] [PubMed] [Google Scholar]
- Mason JL, Suzuki K, Chaplin DD, Matsushima GK. Interleukin-1beta promotes repair of the CNS. J Neurosci. 2001b;21(18):7046–7052. doi: 10.1523/JNEUROSCI.21-18-07046.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JL, Xuan S, Dragatsis I, Efstratiadis A, Goldman JE. Insulin-like growth factor (IGF) signaling through type 1 IGF receptor plays an important role in remyelination. J Neurosci. 2003;23(20):7710–7718. doi: 10.1523/JNEUROSCI.23-20-07710.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JL, Ye P, Suzuki K, D’Ercole AJ, Matsushima GK. Insulin-like growth factor-1 inhibits mature oligodendrocyte apoptosis during primary demyelination. J Neurosci. 2000b;20(15):5703–5708. doi: 10.1523/JNEUROSCI.20-15-05703.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matejuk A, Adlard K, Zamora A, Silverman M, Vandenbark AA, Offner H. 17 beta-estradiol inhibits cytokine, chemokine, and chemokine receptor mRNA expression in the central nervous system of female mice with experimental autoimmune encephalomyelitis. J Neurosci Res. 2001;65(6):529–542. doi: 10.1002/jnr.1183. [DOI] [PubMed] [Google Scholar]
- Matsushima GK, Morell P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol. 2001;11(1):107–116. doi: 10.1111/j.1750-3639.2001.tb00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon EJ, Suzuki K, Matsushima GK. Peripheral macrophage recruitment in cuprizone-induced CNS demyelination despite an intact blood-brain barrier. J Neuroimmunol. 2002;130(1–2):32–45. doi: 10.1016/s0165-5728(02)00205-9. [DOI] [PubMed] [Google Scholar]
- Mendez P, Azcoitia I, Garcia-Segura LM. Estrogen receptor alpha forms estrogen-dependent multimolecular complexes with insulin-like growth factor receptor and phosphatidylinositol 3-kinase in the adult rat brain. Brain Res Mol Brain Res. 2003;112(1–2):170–176. doi: 10.1016/s0169-328x(03)00088-3. [DOI] [PubMed] [Google Scholar]
- Mendez P, Cardona-Gomez GP, Garcia-Segura LM. Interactions of insulin-like growth factor-I and estrogen in the brain. Adv Exp Med Biol. 2005;567:285–303. doi: 10.1007/0-387-26274-1_12. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Lane MV, Numan S, Dellovade TL. Distribution of estrogen receptor alpha and beta in the mouse central nervous system: in vivo autoradiographic and immunocytochemical analyses. J Comp Neurol. 2004;473(2):270–291. doi: 10.1002/cne.20128. [DOI] [PubMed] [Google Scholar]
- Michels KM, Lee WH, Seltzer A, Saavedra JM, Bondy CA. Up-regulation of pituitary [125I]insulin-like growth factor-I (IGF-I) binding and IGF binding protein-2 and IGF-I gene expression by estrogen. Endocrinology. 1993;132(1):23–29. doi: 10.1210/endo.132.1.7678216. [DOI] [PubMed] [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J Comp Neurol. 2005;491(2):81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429(3):355–371. [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144(5):2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Morales LB, Loo KK, Liu HB, Peterson C, Tiwari-Woodruff S, Voskuhl RR. Treatment with an estrogen receptor alpha ligand is neuroprotective in experimental autoimmune encephalomyelitis. J Neurosci. 2006;26(25):6823–6833. doi: 10.1523/JNEUROSCI.0453-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morell P, Barrett CV, Mason JL, Toews AD, Hostettler JD, Knapp GW, Matsushima GK. Gene expression in brain during cuprizone-induced demyelination and remyelination. Mol Cell Neurosci. 1998;12(4–5):220–227. doi: 10.1006/mcne.1998.0715. [DOI] [PubMed] [Google Scholar]
- Murphy LJ, Murphy LC, Friesen HG. Estrogen induces insulin-like growth factor-I expression in the rat uterus. Mol Endocrinol. 1987;1(7):445–450. doi: 10.1210/mend-1-7-445. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10(1):9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- Pasquini LA, Calatayud CA, Bertone Una AL, Millet V, Pasquini JM, Soto EF. The neurotoxic effect of cuprizone on oligodendrocytes depends on the presence of pro-inflammatory cytokines secreted by microglia. Neurochem Res. 2007;32(2):279–292. doi: 10.1007/s11064-006-9165-0. [DOI] [PubMed] [Google Scholar]
- Plant SR, Arnett HA, Ting JP. Astroglial-derived lymphotoxin-alpha exacerbates inflammation and demyelination, but not remyelination. Glia. 2005;49(1):1–14. doi: 10.1002/glia.20089. [DOI] [PubMed] [Google Scholar]
- Plant SR, Iocca HA, Wang Y, Thrash JC, O’Connor BP, Arnett HA, Fu YX, Carson MJ, Ting JP. Lymphotoxin beta receptor (Lt betaR): dual roles in demyelination and remyelination and successful therapeutic intervention using Lt betaR-Ig protein. J Neurosci. 2007;27(28):7429–7437. doi: 10.1523/JNEUROSCI.1307-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk MJ, Jones RE, Subramanian S, Afentoulis M, Rich C, Zakroczymski M, Cooke P, Vandenbark AA, Offner H. T lymphocytes do not directly mediate the protective effect of estrogen on experimental autoimmune encephalomyelitis. Am J Pathol. 2004;165(6):2069–2077. doi: 10.1016/S0002-9440(10)63257-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi S, Benedusi V, Maggi A, Vegeto E. Estrogen action in neuroprotection and brain inflammation. Ann N Y Acad Sci. 2006;1089:302–323. doi: 10.1196/annals.1386.035. [DOI] [PubMed] [Google Scholar]
- Probert L, Akassoglou K, Pasparakis M, Kontogeorgos G, Kollias G. Spontaneous inflammatory demyelinating disease in transgenic mice showing central nervous system-specific expression of tumor necrosis factor alpha. Proc Natl Acad Sci U S A. 1995;92(24):11294–11298. doi: 10.1073/pnas.92.24.11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada A, Micevych PE. Estrogen interacts with the IGF-1 system to protect nigrostriatal dopamine and maintain motoric behavior after 6-hydroxdopamine lesions. J Neurosci Res. 2004;75(1):107–116. doi: 10.1002/jnr.10833. [DOI] [PubMed] [Google Scholar]
- Selvaraju R, Bernasconi L, Losberger C, Graber P, Kadi L, Avellana-Adalid V, Picard-Riera N, Van Evercooren AB, Cirillo R, Kosco-Vilbois M, Feger G, Papoian R, Boschert U. Osteopontin is upregulated during in vivo demyelination and remyelination and enhances myelin formation in vitro. Mol Cell Neurosci. 2004;25(4):707–721. doi: 10.1016/j.mcn.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Shingo AS, Kito S. Estrogen induces insulin-like growth factor-1 mRNA expression in the immortalized hippocampal cell: determination by quantitative real-time polymerase chain reaction. Neurochem Res. 2003;28(9):1379–1383. doi: 10.1023/a:1024900616704. [DOI] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore A, Milner TA, McEwen BS, Bulloch K. Steroid hormone receptor expression and function in microglia. Glia. 2008;56(6):659–674. doi: 10.1002/glia.20644. [DOI] [PubMed] [Google Scholar]
- Takao T, Flint N, Lee L, Ying X, Merrill J, Chandross KJ. 17beta-estradiol protects oligodendrocytes from cytotoxicity induced cell death. J Neurochem. 2004;89(3):660–673. doi: 10.1111/j.1471-4159.2004.02370.x. [DOI] [PubMed] [Google Scholar]
- Tiwari-Woodruff S, Morales LB, Lee R, Voskuhl RR. Differential neuroprotective and antiinflammatory effects of estrogen receptor (ER)alpha and ERbeta ligand treatment. Proc Natl Acad Sci U S A. 2007;104(37):14813–14818. doi: 10.1073/pnas.0703783104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegeto E, Belcredito S, Etteri S, Ghisletti S, Brusadelli A, Meda C, Krust A, Dupont S, Ciana P, Chambon P, Maggi A. Estrogen receptor-alpha mediates the brain antiinflammatory activity of estradiol. Proc Natl Acad Sci U S A. 2003;100(16):9614–9619. doi: 10.1073/pnas.1531957100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegeto E, Belcredito S, Ghisletti S, Meda C, Etteri S, Maggi A. The endogenous estrogen status regulates microglia reactivity in animal models of neuroinflammation. Endocrinology. 2006;147(5):2263–2272. doi: 10.1210/en.2005-1330. [DOI] [PubMed] [Google Scholar]
- Vegeto E, Benedusi V, Maggi A. Estrogen anti-inflammatory activity in brain: a therapeutic opportunity for menopause and neurodegenerative diseases. Front Neuroendocrinol. 2008;29(4):507–519. doi: 10.1016/j.yfrne.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegeto E, Bonincontro C, Pollio G, Sala A, Viappiani S, Nardi F, Brusadelli A, Viviani B, Ciana P, Maggi A. Estrogen prevents the lipopolysaccharide-induced inflammatory response in microglia. J Neurosci. 2001;21(6):1809–1818. doi: 10.1523/JNEUROSCI.21-06-01809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y, Yang S, Liu R, Perez E, Yi KD, Koulen P, Simpkins JW. Estrogen attenuates nuclear factor-kappa B activation induced by transient cerebral ischemia. Brain Res. 2004;1008(2):147–154. doi: 10.1016/j.brainres.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Ye P, D’Ercole AJ. Insulin-like growth factor I protects oligodendrocytes from tumor necrosis factor-alpha-induced injury. Endocrinology. 1999;140(7):3063–3072. doi: 10.1210/endo.140.7.6754. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Cerghet M, Mullins C, Williamson M, Bessert D, Skoff R. Comparison of in vivo and in vitro subcellular localization of estrogen receptors alpha and beta in oligodendrocytes. J Neurochem. 2004;89(3):674–684. doi: 10.1111/j.1471-4159.2004.02388.x. [DOI] [PubMed] [Google Scholar]
- Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135(1):145–157. doi: 10.1017/S1740925X09000015. [DOI] [PubMed] [Google Scholar]