Abstract
Cytokines such as IL-1α, IL-1β, and IFN-γ have long been implicated in the pathogenesis of autoimmune diabetes, but the mechanisms through which they promote diabetogenesis remain unclear. Here we show that CD4+ T lymphocytes propagated from transgenic nonobese diabetic (NOD) mice expressing the highly diabetogenic, β cell–specific 4.1-T-cell receptor (4.1-TCR) can kill IL-1α–, IL-1β–, and IFN-γ–treated β cells from NOD mice. Untreated NOD β cells and cytokine-treated β cells from Fas-deficient NOD.lpr mice are not targeted by these T cells. Killing of islet cells in vitro was associated with cytokine-induced upregulation of Fas on islet cells and was independent of MHC class II expression. Abrogation of Fas expression in 4.1-TCR–transgenic NOD mice afforded nearly complete protection from diabetes and did not interfere with the development of the transgenic CD4+ T cells or with their ability to cause insulitis. In contrast, abrogation of perforin expression did not affect β cell–specific cytotoxicity or the diabetogenic potential of these T cells. These data demonstrate a novel mechanism of action of IL-1α, IL-1β, and IFN-γ in autoimmune diabetes, whereby these cytokines mark β cells for Fas-dependent lysis by autoreactive CD4+ T cells.
Introduction
Certain organ-specific autoimmune disorders arise when cells of specific tissues become the targets of destruction by autoreactive CD4+ or CD8+ T lymphocytes. The mechanisms through which specific subsets of autoreactive T lymphocytes effect tissue cell damage in autoimmune disorders, however, remain poorly defined. Autoimmune diabetes in nonobese diabetic (NOD) mice is one such disease that results from a complex, T cell–dependent process directed against pancreatic β cells (1).
Numerous studies have observed correlations between expression of proinflammatory cytokines in pancreatic islets of NOD mice (IL-1α, IL-1β, TNF-α, TNF-β, IFN-α, IL-2, IL-12, and IFN-γ) and the presence of destructive insulitis. Some of these proinflammatory cytokines (IL-1α, IL-1β, TNF-α, TNF-β, and IFN-γ) have well-documented cytotoxic or cytostatic effects on pancreatic β cells in vitro, yet studies of transgenic mice expressing some of these cytokines in β cells have shown that they are not directly toxic to β cells in vivo. This suggests that these cytokines participate in diabetogenesis by promoting the recruitment and activation of β cell–toxic T cells, macrophages, and dendritic cells, rather than by killing β cells or impairing their function (2–4).
Adoptive T-cell transfer studies using spleen cells from prediabetic NOD mice have demonstrated that the transfer of diabetes into immunodeficient NOD mice requires both CD4+ and CD8+ T cells (5). Although the mechanisms by which diabetogenic CD4+ and CD8+ T cells collaborate to trigger diabetes remain unclear, it has been proposed that CD8+ cytotoxic T lymphocytes (CTL) effect the initial β-cell insult that triggers the shedding of β-cell autoantigens, the loading of these antigens onto professional antigen-presenting cells (APCs), and the subsequent activation of diabetogenic autoreactive CD4+ T cells (6–10). Adoptive T-cell transfer studies have shown that, once activated, autoreactive CD4+ T cells can effect β-cell destruction and trigger diabetes without the further contribution of CD8+ CTL (5, 11). However, β cells do not normally express MHC class II molecules and therefore cannot directly present autoantigens to autoreactive CD4+ T cells (12). This raises the question of how diabetogenic CD4+ T cells destroy β cells in vivo.
T lymphocytes can kill target cells through 1 of 2 alternative lytic pathways. In the perforin pathway, cell death is caused by the direct effects of perforin and granzymes on the target cell. In the Fas pathway, a T-cell membrane ligand (FasL) that is upregulated when the T-cell receptor (TCR) is engaged binds a target-cell surface receptor (Fas) that induces apoptosis when ligated (13). Studies of NOD mice deficient in either Fas or perforin expression have suggested that destruction of β cells by CTL in spontaneous autoimmune diabetes is a process that depends on both Fas and perforin (14–16). These studies, however, could not distinguish between the roles of these 2 different cytotoxicity pathways in the ability of diabetogenic CD4+ T cells vs. CD8+ T cells to effect β-cell damage, nor could they rule out the possibility that the diabetes resistance of the deficient mice was the result of effects of the perforin or Fas deficiencies on CD4+ or CD8+ T-cell development or function, or some combination of these.
Because CD4+ T cells can differentiate into CTL, and because target cell lysis by CD4+ CTL can be mediated by perforin or Fas, depending in part on whether or not the target cell expresses MHC class II molecules (13, 17, 18), we reasoned that diabetogenic CD4+ T cells might differentiate into CTL in vivo, and thus be able to kill β cells using 1 or both of these lytic pathways. This study was initiated to test this postulate. We investigated whether CD4+ T cells bearing a highly diabetogenic, I-Ag7–restricted, β cell–reactive T-cell receptor (4.1-TCR) (19, 20) differentiate into CTL in 4.1-TCR–transgenic NOD (4.1-NOD) mice, and whether these cells use perforin or Fas pathways (or both) to kill β cells. We show that although 4.1-CD4+ T cells spontaneously differentiate into perforin- and FasL-expressing CTL in vivo, they kill β cells exclusively through the Fas pathway. Surprisingly, the β cell–toxic activity of these T cells not only requires the ability of β cells to express Fas, but can only be effected upon exposure of the β-cell targets to IL-1α, IL-1β, or IFN-γ. These results suggest that, in addition to promoting the recruitment and activation of pathogenic autoreactive T cells, certain cytokines may effect their pro-diabetogenic action by marking MHC class II–deficient β cells for Fas-dependent lysis by autoreactive CD4+ T cells.
Methods
Mice.
4.1-NOD, 8.3-NOD, RAG-2–/– 4.1-NOD, and NOD.PO–/– mice have been described (19–21). NOD/Lt, B6, and B6.MRL-Faslpr mice were produced from stocks purchased from The Jackson Laboratory (Bar Harbor, Maine, USA). 4.1-NOD.PO–/– and RAG-2–/– 4.1-NOD.PO–/– mice were generated by backcrossing the perforin mutation of NOD.PO–/– mice onto 4.1-NOD and RAG-2–/– 4.1-NOD mice, respectively. NOD.lpr+/+ mice were generated by backcrossing the Faslpr gene of B6.MRL-Faslpr mice onto the NOD background for 8 generations, and by intercrossing NOD.lpr+/– mice. 4.1-NOD.lpr+/+ and RAG-2–/– 4.1-NOD.lpr+/+ mice were generated by crossing NOD.lpr+/+ mice with 4.1-NOD and RAG-2–/– 4.1-NOD mice, respectively. Mice were screened for I-Ag7 homozygosity and for inheritance of wild-type and mutated perforin and Fas genes by PCR. Selected 4.1-NOD.lpr+/+ mice were PCR typed for microsatellite polymorphisms linked to Idd2 (D9Mit25), Idd3 (D3Mit95, D3Mit100, D3Mit103), Idd4 (D11Mit115, D11Mit320), Idd5 (D1Mit18, D1Mit46), Idd7 (D7Mit20), Idd8/12 (D14Mit110, D14Mit22), Idd9 (D4Mit59), Idd14 (D13Mit61), and Idd15 (D5Mit48) using fluorochrome-labeled primers, to confirm absence of B6-derived, diabetes-protective alleles at known Idd loci.
Diabetes.
Diabetes was monitored by measuring urine glucose levels with Diastix (Bayer Inc., Etobicoke, Ontario, Canada) twice weekly. Animals were considered diabetic after 2 consecutive readings ≥ 3+ and blood glucose levels above 14 mmol/L.
Cell lines, antibodies, and flow cytometry.
L1210-Fas+ and L1210-Fas– cells were provided by P. Goldstein (CNRS, Marseille, France). Anti–Lyt-2–phycoerythrin (CD8α-PE) or -FITC (53-6.7), anti–L3T4-FITC (IM7), anti–L3T4-biotin (CD4) (H129.19), anti–Vβ11-FITC (RR3-15), anti-Fas (Jo2, unconjugated and PE-labeled), anti-Kd (SF1-1.1), anti–I-Ag7 (10-3.6), anti–hamster Ig–biotin, and hamster Ig isotype control (anti-TNP) antibodies were purchased from PharMingen (San Diego, California, USA). Streptavidin-PerCP and streptavidin-PE were obtained from Becton Dickinson Canada, Inc. (Oakville, Ontario, Canada). Thymi, spleens, and islet-derived T-cell lines were analyzed by 3-color flow cytometry using a FACScan (Becton Dickinson Canada Inc.).
Analysis of Fas expression on islet cells was done by flow cytometry using untreated and cytokine-treated islet cells from RAG-2–/– NOD mice, which are completely devoid of lymphocytes and therefore do not develop insulitis. Briefly, islets were cultured for 16 hours in DMEM containing 10% heat-inactivated FBS (Life Technologies Inc., Gaithersburg, Maryland, USA), rmIL-1α (100 U/mL; Genzyme Diagnostics, Cambridge, Massachusetts, USA), rmIFN-γ (250 U/mL; Genzyme Diagnostics), or both (10 U/mL and 250 U/mL, respectively). Islets were first dispersed into single cells using an enzyme-free cell-dissociation buffer (Life Technologies Inc.), and were then stained with hamster anti-mouse Fas or anti-TNP hamster Ig isotype control, biotinylated anti-hamster Ig, and PE-conjugated streptavidin.
Apoptosis assays.
Pancreatic islets from RAG-2–/– NOD mice were cultured for 16 hours as above in the presence or absence of rmIL-1α (100 U/mL), rmIFN-γ (250 U/mL), or both (10 U/mL and 250 U/mL, respectively), anti-murine Fas mAb (Jo2; 1 μg/mL), and actinomycin D (30 μg/mL). Islets were then dispersed into single cells as indicated above and were fixed in 2 mL cold 70% ethanol at 4°C for 60 minutes. The cells were washed twice in PBS and resuspended in 250 μL of PBS containing 0.5 mg/mL of RNase Type I-A (Life Technologies Inc.). Then they were stained with 250 μL of 100 μg/mL propidium iodide in PBS and analyzed by flow cytometry to determine the presence of apoptotic nuclei (22).
Generation of spleen- and islet-derived CD4+ T-cell lines and clones.
Spleen cells from 4.1-NOD mice were depleted of CD8+ T cells using magnetic beads coated with anti-CD8 mAb (23). Cells were adjusted to 2 × 104 CD4+ T cells per 100 μL of RPMI-1640 containing 10% FBS. The cells were then stimulated with either irradiated NOD islets (8–10 islets/well) or plate-bound anti-CD3 and anti-CD28 mAbs (10 μg/mL and 1 μg/mL, respectively) for 3–4 days. Cells were expanded in medium containing 0.5 U/mL of rIL-2 (Takeda Co., Osaka, Japan) for 7–14 days. The growing CD4+ cells (> 95% pure) were used as effectors in cytotoxicity assays or as a source of mRNA for RT-PCR. Islet-derived CD4+ T-cell lines were generated from diabetic mice within 1 day of diabetes onset, and were cloned by limiting dilution. The cell lines were phenotyped by flow cytometry and were assayed for serine esterase content as described elsewhere (19, 23). The 8.3-CD8+ CTL clone J2 seen in Figure 1 was derived from CD8+ T cells propagated from islets of a diabetic 8.3-NOD mouse (24).
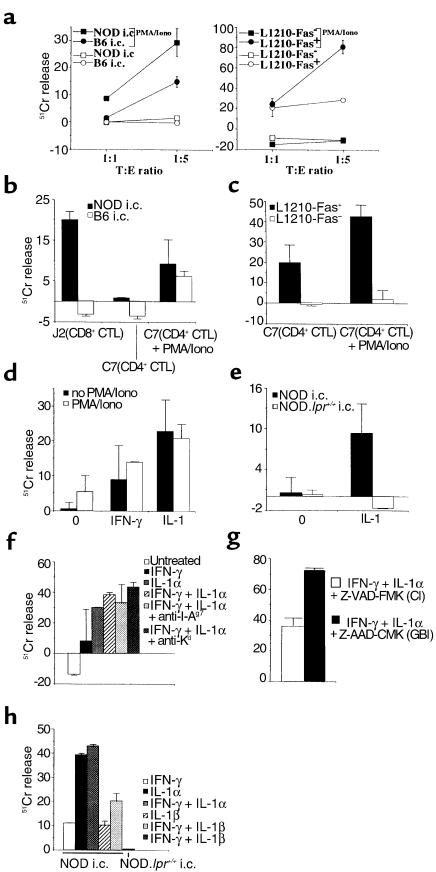
Figure 1.
Cytotoxic activity of 4.1-CD4+ CTL. (a) PMA and ionomycin–treated and untreated 4.1-CD4+ T cells, propagated from pancreatic islets of acutely diabetic 4.1-NOD mice, were tested for cytotoxic activity against NOD and B6 islet cells (i.c.) (left) and L1210-Fas+ and L1210-Fas– fibroblasts (right). (b) Cytotoxic activity of serine esterase–positive 4.1-CD4+ CTL and 8.3-CD8+ CTL clones against NOD and B6 islet cells at a 1:5 target-to-effector (T:E) ratio. (c) Cytolytic activity of a representative 4.1-CD4+ CTL clone against L1210-Fas+ and L1210-Fas– cells at a 1:5 T:E ratio. (d) and (e) Cytolytic activity of in vitro–differentiated 4.1-CD4+ CTL against NOD (d) or NOD.lpr+/+ (e) islet cells cultured overnight in the absence (0) or presence of IFN-γ (250 U/mL) or IL-1α (100 U/mL) at a 1:10 T:E ratio. Although overnight IL-1α treatment caused a significant reduction in islet yield, the spontaneous chromium release by the surviving islet cells was similar to that by untreated or IFN-γ–treated islet cells (< 35%). (f) Cytolytic activity of 4.1-CD4+ CTL against cytokine-treated NOD islet cells (250 U/mL IFN-γ, 100 U/mL IL-1α, or 250 U/mL IFN-γ + 10 U/mL IL-1α) in the presence of blocking concentrations of anti–MHC class II and class I mAbs (at a 1:10 T:E ratio). (g) Cytolytic activity of 4.1-CD4+ CTL against IFN-γ–(250 U/mL) and IL-1α–treated (10 U/mL) NOD islet cells in the presence of a caspase inhibitor (CI) or a control inhibitor (granzyme B inhibitor; GBI). (h) Cytolytic activity of 4.1-CD4+ CTL against cytokine-treated NOD islet cells (250 U/mL IFN-γ, 100 U/mL IL-1α, 100 U/mL IL-1β, or 250 U/mL IFN-γ + 10 U/mL IL-1α or IL-1β). The figures are representative of 2–3 different experiments (mean ± SEM). Iono, ionomycin.
51Cr-release assays.
L1210-Fas+ and L1210-Fas– cells were labeled with [51Cr]sodium chromate (NEN Life Science Products Inc., Boston, Massachusetts, USA) for 2 hours at 37°C. After being washed twice with RPMI-1640, cells were resuspended at 105 cells/mL in complete media and then seeded at 104 cells/100 μL per well. Pancreatic islet cells were prepared from NOD/Lt mice, B6 mice, and NOD.lpr+/+ mice (5–10 weeks old), labeled with [51Cr]sodium chromate for 2 hours at 37°C, and then seeded at 104 cells/well. Some experiments used islet cells derived from islets that had been preincubated overnight with rmIL-1α (100 U/mL), rmIFN-γ (250 U/mL; Genzyme Diagnostics), rmIL-1β (100 U/mL), rmTNF-α (50 ng/mL; R&D Systems Inc., Minneapolis, Minnesota, USA), rmIL-1α + rmIFN-γ (10 U/mL and 250 U/mL, respectively), or rmIL-1β + rmIFN-γ (10 U/mL and 250 U/mL, respectively) to increase their susceptibility to Fas-mediated cytotoxicity (25–29). Effector cells (islet-activated splenic CD4+ T cells or islet-derived CD4+ T-cell clones; 100 μL) were added to each well in duplicate at different target-to-effector ratios. For some experiments, the CD4+ T-cell effectors were preactivated with PMA (2 μg/mL) and ionomycin (40 ng/mL) for 6 hours. In other experiments, the cytotoxicity assays were done in the presence of 10 μg/mL anti–MHC class I or anti–MHC class II mAbs (anti-Kd and anti–I-Ag7, respectively), or 2 μg/mL granzyme B inhibitor or caspase inhibitor (Z-AAD-CMK and Z-VAD-FMK, respectively; Oncogene Research Products, Cambridge, Massachusetts, USA). Plain medium or 1% Triton X-100 was added to sets of target cells treated under the same conditions as the experimental cultures, to examine spontaneous and total cell lysis, respectively. The plates were incubated at 37°C for 8 hours, and the supernatants were collected for determination of specific 51Cr release (23).
Proliferation assays.
Naive, CD8+ T cell–depleted splenic CD4+ T cells (2 × 104) were incubated in triplicate with γ-irradiated (30 Gy) islet cells (105/well) for 3 days at 37°C in 5% CO2. Cultures were pulsed with 1 μCi of [3H]thymidine during the last 18 hours of culture and then harvested and counted.
Cytokine secretion.
Naive, CD8+ T cell–depleted CD4+ T cells (2 × 104 cells/well) were incubated with γ-irradiated NOD islet cells (105/well) in 96-well plates for 48 hours at 37°C. The supernatants (100 μL/well) were assayed for IL-2, IL-4, and IFN-γ content by ELISA using commercially available kits (Genzyme Diagnostics).
Adoptive T-cell transfer.
Naïve 4.1-CD4+ T cells from 4.1-NOD or 4.1-NOD.PO–/– mice were activated with plate-bound anti-CD3 and anti-CD28 mAbs (10 μg/mL and 1 μg/mL, respectively) for 3–4 days, expanded in medium containing 0.5 U/mL rIL-2 for an additional 4–5 days, and injected into the tail veins of 6- to 9-week-old NOD.scid (Fas+) or RAG-2–/– NOD.lpr+/+ mice (Fas–) (4 × 106 to 5 × 106 cells/mouse). Recipient mice were followed for diabetes development for at least 3 months after T-cell transfer.
Histopathology.
Pancreata were fixed in formalin and then embedded in paraffin. They were then sectioned at 4.5 μm, stained with hematoxylin and eosin, and examined for inflammation. The degree of insulitis was evaluated by scoring 15–30 islets per mouse using previously described criteria (23).
Statistical analyses.
Statistical analyses were performed using the Mann-Whitney U test, the χ2 test, and Fisher’s exact test.
Results
Diabetogenic CD4+ T cells differentiate into CTL in vivo and kill β cells via the Fas pathway.
Because 4.1-CD4+ T cells are highly diabetogenic, even in the absence of endogenous T cells (19, 20), we reasoned that they might differentiate into CTL in vivo. Studies of CD4+ T-cell clones propagated from pancreatic islets of acutely diabetic 4.1-NOD mice indeed revealed that a significant percentage of these T cells (33/63 clones, or 52%) contained cytotoxic granules (as determined by measuring the clones’ serine esterase content) and expressed perforin mRNA (data not shown). Subsequent cytotoxicity assays revealed that these islet-derived CD4+ T-cell lines (and clones) could lyse fibroblasts expressing high levels of Fas (L1210-Fas+), but could not lyse Fas-deficient fibroblasts (L1210-Fas–), indicating that these lines and clones expressed FasL as well (Figure 1a). These T cells, however, were unable to kill freshly isolated syngeneic and allogeneic islet cells (Figure 1a). We reasoned that, because of the following 3 reasons, these T cells might actually be able to kill β cells through Fas, but only transiently after activation: (a) the cytolytic activity of 4.1-CD4+ CTL against L1210-Fas+ cells was rather low; (b) T cells quickly downregulate FasL from the surface upon detachment from APCs (30); and (c) β cells express very low levels of Fas (25, 28). To investigate this hypothesis, we stimulated 4.1-CD4+ CTL with a combination of PMA and ionomycin to upregulate FasL on their surface. We then retested the cells’ ability to kill L1210-Fas+ cells, L1210-Fas– cells, and islet cells. As shown in Figure 1a, 4.1-CD4+ CTL activated with PMA and ionomycin were capable of killing L1210-Fas+ (but not L1210-Fas–) fibroblasts and both syngeneic (NOD; H-2g7) and allogeneic (C57BL/6; H-2b) islet cells. To determine whether Fas-dependent destruction of β cells by 4.1-CD4+ CTL involved a cognate interaction between the CTL and β cells, we compared the β cell–cytolytic activity of 4.1-CD4+ T-cell clones with that of CD8+ CTL clones generated from NOD mice expressing another highly diabetogenic, but MHC class I–restricted (H-2Kd–restricted), β cell–reactive TCR (8.3-NOD mice) (20). Because 8.3-CD8+ CTL clones kill β cells via the Fas pathway by engaging antigen–MHC complexes directly on the β-cell surface (21), they could specifically kill syngeneic (but not allogeneic) islet cells in the absence of treatment with PMA and ionomycin (Figure 1b). In contrast, 4.1-CD4+ CTL clones were only able to lyse islet cells after treatment with PMA and ionomycin (Figure 1b). The islet-cytotoxic activity of the 4.1-CD4+ CTL clones was not restricted by MHC class II molecules expressed on islet cells, having targeted both syngeneic and allogeneic islet cells (Figure 1b) and L1210-Fas+ fibroblasts (Figure 1c).
Because stimulation of T cells with PMA and ionomycin in vitro may not mimic the processes that activate the lytic machinery of diabetogenic CD4+ CTL in vivo, we tested the role of Fas in T cell–mediated destruction of β cells by using a more physiological in vitro system. We did this by investigating whether Fas-dependent destruction of β cells by 4.1-CD4+ T cells could be promoted by 2 diabetogenic cytokines known to upregulate Fas expression on β cells (IFN-γ and IL-1α) (25–28). Interestingly, 4.1-CD4+ CTL killed IFN-γ– or IL-1α–treated, Fas-competent NOD β cells quite efficiently, but were unable to kill IL-1α–treated β cells from Fas-deficient NOD.lpr+/+ mice (Figure 1, d and e).
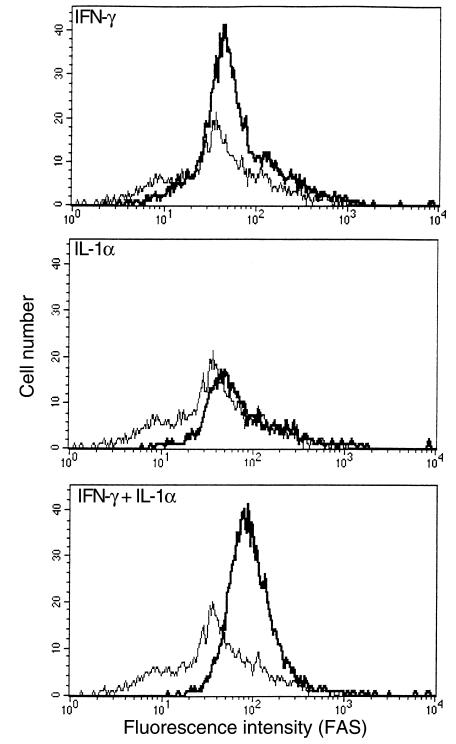
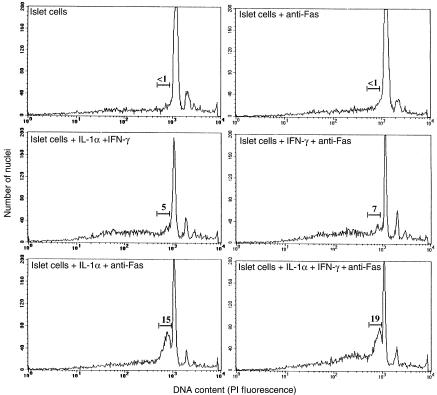
Subsequent flow cytometric analyses of islet cells treated with IFN-γ (250 U/mL), IL-1α (100 U/mL), or both (250 U/mL and 10 U/mL, respectively) showed that the relative ability of these cytokines to prime islet cells for 4.1-CTL lysis correlated with their ability to upregulate Fas expression on the islet-cell surface. Islet cells treated with both IFN-γ and IL-1α expressed the highest levels of Fas (Figure 2), followed by those treated with IL-1α and finally IFN-γ−treated cells. Islet cells treated with both IFN-γ and IL-1α were also more susceptible to 4.1-CD4+ CTL–induced lysis (Figure 1f) and anti-Fas mAb–induced apoptosis (Figure 3) than were islet cells treated with 10-fold higher concentrations of IL-1α, suggesting that these cytokines synergize with each other at marking β cells for Fas-mediated lysis by 4.1-CD4+ CTL. We determined that susceptibility of these IFN-γ– and IL-1α–treated islet cells to 4.1-CD4+ CTL–mediated cytotoxicity was not restricted by MHC class I or class II molecules on islet cells, because the cytotoxicity could not be blocked with anti–I-Ag7 or anti-Kd mAbs (Figure 1f). The role of Fas in the ability of 4.1-CD4+ CTL to kill cytokine-treated islet cells was further supported by the observation that treatment of cytokine-stimulated islet cells with a caspase inhibitor significantly reduced their susceptibility to 4.1-CD4+ CTL–mediated lysis compared with cytokine-stimulated islet cells treated with a control inhibitor (granzyme B inhibitor) (Figure 1g). Lastly, experiments with NOD and NOD.lpr+/+ islet cells stimulated with IL-1β alone or IL-1β together with IFN-γ revealed that IL-1β is also capable of rendering islet cells susceptible to the cytotoxic activity of 4.1-CTL, and that this effect requires the ability of the islet cells to express Fas (Figure 1h).
Figure 2.
IFN-γ and IL-1α upregulate the expression of Fas on NOD islets. Islet cells from RAG-2–/– NOD mice were cultured for 16 hours in the presence of IFN-γ (250 U/mL), IL-1α (100 U/mL), or IFN-γ (250 U/mL) + IL-1α (10 U/mL). Islets were then dispersed into single cells and stained with hamster anti-mouse Fas (thick line) or anti-TNP hamster Ig isotype control (thin line), plus biotinylated anti-hamster Ig and PE-conjugated streptavidin.
Figure 3.
Susceptibility of cytokine-treated islet cells to Fas-induced apoptosis. Islets from RAG-2–/– NOD mice were cultured for 16 hours as above in the presence or absence of IL-1α, IFN-γ, IL-1α + IFN-γ, and anti-murine Fas mAb. Islets were then dispersed into single cells, fixed in ethanol, stained with propidium iodide (PI), and analyzed by flow cytometry to determine the presence of apoptotic nuclei. The numbers above the bars indicate the percentage of cells with hypodiploid DNA content (early apoptotic cells).
Taken together, these results indicate that (a) 4.1-CD4+ T cells spontaneously differentiate into CTL; (b) the β cell–toxic activity of 4.1-CD4+ CTL does not involve a cognate interaction with β cells; and (c) IL-1α, IL-1β, and IFN-γ prime β cells for Fas-dependent destruction by 4.1-CD4+ CTL.
Phenotype and function of 4.1-CD4+ T cells from perforin-deficient 4.1-NOD mice.
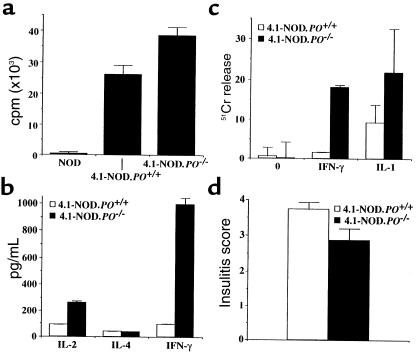
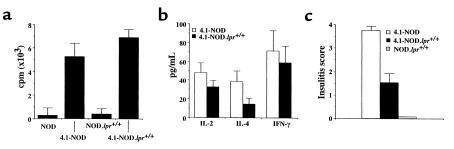
The above results, however, did not rule out the involvement of perforin in the β cell–toxic activity of 4.1-CD4+ CTL in vivo. To investigate this possibility, we produced perforin-deficient 4.1-NOD (4.1-NOD.PO–/–) mice. We first compared the fate and functional activity of transgenic CD4+ T cells in 4.1-NOD.PO–/– and 4.1-NOD.PO+/– mice. Cytofluorometric studies indicated that 4.1-NOD.PO–/– mice and 4.1-NOD.PO+/– mice had virtually identical numbers of the different thymocyte and splenocyte subpopulations (data not shown). Surprisingly, peripheral 4.1-CD4+ T cells from 4.1-NOD.PO–/– mice proliferated even more, and secreted greater amounts of IFN-γ (P < 0.0001) and IL-2 (P < 0.05) than did CD4+ T cells from 4.1-NOD.PO+/+ mice in response to irradiated NOD islet cells (Figure 4, a and b). These responses were I-Ag7 restricted, β-cell specific, and 4.1-TCR driven: they did not occur when using B6 islet cells (I-Ab) or NOD splenocytes as a source of antigen, or when using nontransgenic CD4+ T cells as responders (Figure 4a and data not shown). Cytotoxicity assays using NOD islet-differentiated 4.1-CD4+ CTL revealed that perforin-deficient 4.1-CD4+ CTL could lyse cytokine-stimulated NOD islet cells in vitro at least as efficiently as could perforin-competent 4.1-CD4+ CTL. Perforin-deficient CTL actually killed IFN-γ–treated islet cells significantly better than did perforin-competent CTL (P < 0.05; Figure 4c). Lastly, histopathological analyses of pancreata from mice 9–12 weeks old revealed that perforin-deficient 4.1-CD4+ T cells were as efficient at causing insulitis as perforin-competent 4.1-CD4+ T cells (Figure 4d). These results indicated that 4.1-NOD.PO–/– mice export β cell–hyperreactive CD4+ T cells to the periphery, and that these T cells can respond to and kill NOD islet cells in a perforin-independent manner. We know that the hyperreactivity of 4.1-CD4+ T cells in 4.1-NOD.PO–/– mice is not a peculiarity of 4.1-TCR–bearing T cells because it was also seen in 8.3-NOD.PO–/– mice (21).
Figure 4.
Functional activity of 4.1-CD4+ T cells from 4.1-NOD.PO–/– mice. (a) Proliferation of CD4+ T cells from NOD/Lt, 4.1-NOD.PO+/+, and 4.1-NOD.PO–/– mice in response to NOD islet cells. (b) Cytokine secretion by splenic CD4+ T cells from NOD/Lt, 4.1-NOD.PO+/+, and 4.1-NOD.PO–/– mice in response to stimulation with NOD islet cells. (c) Cytolytic activity of in vitro–differentiated 4.1-CD4+ CTL from 4.1-NOD.PO–/– mice against NOD islet cells cultured overnight in the absence (0) or presence of IFN-γ or IL-1α. (d) Insulitis scores of nondiabetic 4.1-NOD.PO+/+ and 4.1-NOD.PO–/– mice (n = 3–6 mice/group; 9–12 weeks old; 15–30 islets/mouse). Data are shown as mean ± SEM.
Spontaneous diabetes in oligoclonal and monoclonal 4.1-NOD.PO–/– mice.
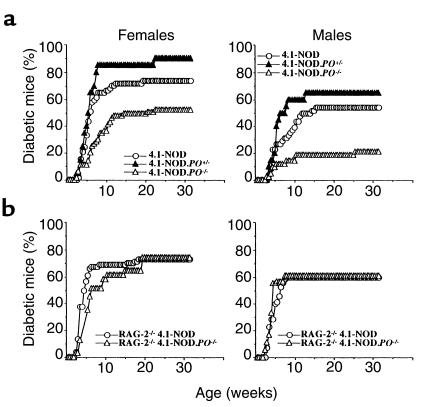
We next compared the incidence of diabetes in 4.1-NOD.PO+/+, 4.1-NOD.PO+/–, and 4.1-NOD.PO–/– mice. As shown in Figure 5a, both female and male 4.1-NOD.PO–/– mice developed diabetes less frequently (P < 0.003) and slightly later than did 4.1-NOD.PO+/– and 4.1-NOD.PO+/+ mice. 4.1-NOD.PO+/– mice developed a slightly increased incidence of diabetes when compared with 4.1-NOD.PO+/+ mice, but these differences were not statistically significant. These results suggested that perforin was somehow involved in the destruction of β cells in 4.1-NOD mice. Because nontransgenic NOD.PO–/– mice are diabetes resistant (14, 21), we considered the possibility that β-cell destruction in 4.1-NOD mice was effected by both transgenic 4.1-CD4+ T cells (via Fas) and CD8+ T cells expressing endogenous TCRs (via perforin). To investigate this, we compared the natural history of diabetes in RAG-2–/– 4.1-NOD.PO–/– mice and RAG-2–/– 4.1-NOD.PO+/+ mice, neither of which can rearrange endogenous TCRs and which therefore bear a monoclonal TCR repertoire completely devoid of CD8+ T cells. As shown in Figure 5b, both female and male RAG-2–/– 4.1-NOD.PO–/– mice developed diabetes essentially like RAG-2–/– 4.1-NOD.PO+/+ mice did. These data demonstrate that although perforin-expressing T cells are involved in the natural history of diabetes in 4.1-NOD mice, 4.1-CD4+ CTL do not require perforin to kill β cells.
Figure 5.
Spontaneous diabetes in oligoclonal and monoclonal 4.1-NOD, 4.1-NOD.PO+/–, and 4.1-NOD.PO–/– mice. (a) Oligoclonal mice. Data correspond to 57 female and 49 male 4.1-NOD mice, 21 female and 20 male 4.1-NOD.PO+/– mice, and 63 female and 42 male 4.1-NOD.PO–/– mice. The incidence of diabetes in 4.1-NOD.PO–/– mice was significantly lower than in 4.1-NOD mice (P < 0.02). (b) Monoclonal mice. Data correspond to 74 female and 70 male RAG-2–/– 4.1-NOD mice, and 31 female and 18 male RAG-2–/– 4.1-NOD.PO–/– mice.
Oligoclonal and monoclonal 4.1-NOD.lpr+/+ mice develop insulitis but not diabetes.
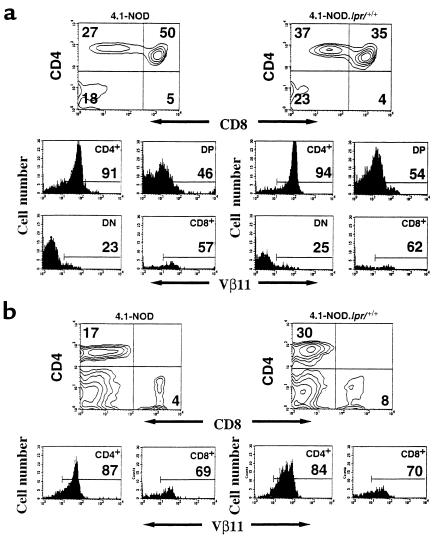
Our next set of experiments were conducted to determine whether the β cell–toxic activity of 4.1-CD4+ CTL in vivo was effected via Fas, as the in vitro experiments had suggested. To that end, we produced and studied Fas-deficient 4.1-NOD.lpr+/+ mice. Representative mice were found to be homozygous for NOD diabetes-susceptibility alleles at Idd1, Idd2, Idd3, Idd4, Idd5, Idd7, Idd8/Idd12, Idd9, Idd14, and Idd15 loci. Three-color cytofluorometric studies suggested that 4.1-CD4+ T cells matured normally in 4.1-NOD.lpr+/+ mice; as shown in Figure 6, 4.1-NOD.lpr+/+ and 4.1-NOD mice had similar percentages of Vβ11+CD4+CD8+ and Vβ11+CD4+CD8– thymocytes, and Vβ11+CD4+ splenocytes. We next compared the proliferative and cytokine secretion activities of naive splenic CD4+ T cells from 4.1-NOD.lpr+/+ and 4.1-NOD mice in response to irradiated NOD islet cells. As shown in Figure 7, a and b, the CD4+ T cells of 4.1-NOD.lpr+/+ mice proliferated as well as, and secreted as much IFN-γ and IL-2 as the CD4+ T cells of 4.1-NOD mice did.
Figure 6.
Flow cytometric profiles of thymocytes and splenocytes from 4.1-NOD and 4.1-NOD.lpr+/+ mice. CD4, CD8, and Vβ11 profiles of thymocytes (a) and splenic cells (b) from 4.1-NOD and 4.1-NOD.lpr+/+ mice (n = 4–8 mice/group, 8–14 weeks old). Upper panels show CD4 vs. CD8 contour plots of cells stained with anti-CD8–PE, anti-Vβ11–FITC, and anti-CD4–biotin plus streptavidin-PerCP. The lower panels show the Vβ11 fluorescence histograms of each T-cell subset after electronic gating. Numbers indicate the average percentage of cells (upper panels) or number of Vβ11+ cells (lower panels) in each subset. DP, double-positive cells; DN, double-negative cells.
Figure 7.
Functional activity of 4.1-CD4+ T cells from 4.1-NOD.lpr+/+ mice. (a) Proliferation of CD4+ T cells from NOD/Lt, NOD.lpr+/+, 4.1-NOD, and 4.1-NOD.lpr+/+ mice in response to NOD islet cells. (b) Cytokine secretion by splenic CD4+ T cells from 4.1-NOD and 4.1-NOD.lpr+/+ mice in response to NOD islet cells. (c) Insulitis scores of nondiabetic NOD.lpr+/+, 4.1-NOD, and 4.1-NOD.lpr+/+ mice (n = 3–6 mice/group; 8–12 weeks old; 15–30 islets/mouse). Data are shown as mean ± SEM.
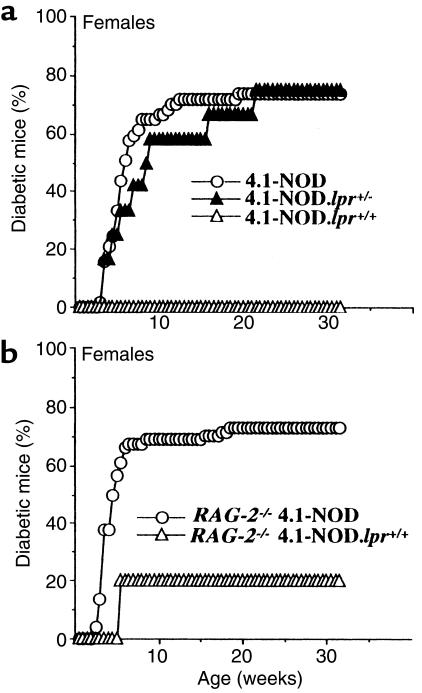
We then compared the natural history of insulitis and diabetes in 4.1-NOD, 4.1-NOD.lpr+/–, and 4.1-NOD.lpr+/+ mice. Unlike female 4.1-NOD.lpr+/– mice, which displayed an incidence and average age at onset of diabetes similar to those of 4.1-NOD mice, female 4.1-NOD.lpr+/+ mice were completely resistant to diabetes (Figure 8a). These observations were also true for male mice (none of 10 mice developed diabetes) (not shown). Unlike nontransgenic NOD.lpr+/+ mice, however, 4.1-NOD.lpr+/+ mice developed a moderate yet significant degree of insulitis (P < 0.0001 vs. NOD.lpr+/+ mice; Figure 7c). Therefore, Fas expression is not required for 4.1-CD4+ T cells to initiate insulitis, but insulitic 4.1-CD4+ T cells cannot destroy β cells lacking Fas.
Figure 8.
Spontaneous diabetes in oligoclonal and monoclonal 4.1-NOD, 4.1-NOD.lpr+/–, and 4.1-NOD.lpr+/+ female mice. (a) Oligoclonal mice. Data correspond to 57 female 4.1-NOD mice, 12 female 4.1-NOD.lpr+/– mice, and 11 female 4.1-NOD.lpr+/+ mice. The incidence of diabetes in female 4.1-NOD.lpr+/+ mice was significantly lower than in 4.1-NOD and 4.1-NOD.lpr+/– mice (P < 0.0001). (b) Monoclonal mice. Data correspond to 74 female RAG-2–/– 4.1-NOD mice and 7 female RAG-2–/– 4.1-NOD.lpr+/+ mice. The incidence of diabetes in the latter mice was significantly lower than in the former (P < 0.003).
Because nontransgenic NOD.lpr+/+ mice exhibit severe defects in lymphocyte homeostasis, including massive lymphadenopathy, accumulation of CD4–CD8–B220+ T cells, and constitutive upregulation of FasL on T cells (15), it was possible that the diabetes resistance of 4.1-NOD.lpr+/+ mice was an artifact of these phenotypic abnormalities. Cytometric studies indicated that 4.1-NOD.lpr+/+ mice also displayed these abnormalities, although they were not as severe as in NOD.lpr+/+ mice. For example, 4.1-NOD.lpr+/+ mice did not develop lymphadenopathy, and the percentage of splenic CD4–CD8–B220+ T cells in these mice was about half that seen in NOD.lpr+/+ mice (40 ± 15% vs. 17 ± 11%). Nevertheless, it was important that we confirm that the diabetes resistance of 4.1-NOD.lpr+/+ mice was not mediated by the presence of abnormally developed or immature lymphocytes. To do this, we followed the natural history of diabetes in female RAG-2–/– 4.1-NOD.lpr+/+ mice, which produce 4.1-CD4+ T cells but lack CD4–CD8–B220+ T cells (data not shown). As shown in Figure 8b, only 1 of 7 female RAG-2–/– 4.1-NOD.lpr+/+ mice (14%) developed diabetes, compared with 56 of 74 female RAG-2–/– 4.1-NOD mice (76%) (P < 0.003). The complete absence of diabetes in RAG-2+/+ 4.1-NOD.lpr+/+ mice (but not RAG-2–/– 4.1-NOD.lpr+/+ mice) might be due to the presence of some abnormally developed lymphocytes in the former. To further confirm that 4.1-CD4+ CTL required the presence of Fas on β cells to kill them in vivo, we investigated the ability of perforin-deficient and perforin-competent 4.1-CD4+ CTL to transfer diabetes into immunodeficient NOD mice that were able or unable to express Fas, respectively. Five out of 5 Fas-competent mice injected with perforin-deficient 4.1-CD4+ CTL developed diabetes within 10 days after injection (at 8, 8, 9, 9, and 9 days). In contrast, 3 of 3 Fas-deficient mice that were injected with perforin-competent 4.1-CD4+ CTL remained euglycemic for at least 3 months after injection (P < 0.04). When taken together, these results conclusively demonstrate that 4.1-CD4+ T cells cannot destroy Fas-deficient β cells in vivo.
Discussion
It has been shown previously that NOD.lpr+/+ mice develop neither diabetes nor insulitis, suggesting a dominant role for Fas in diabetogenesis (15, 16). However, because diabetogenesis in NOD mice is a complex cellular process that is based on a delicate balance between CD4+ and CD8+ T cells and is highly susceptible to bystander suppression, we and others have reasoned that the diabetes resistance of NOD.lpr+/+ mice might result from effects of Fas deficiency on CD4+ and CD8+ T-cell development and function, rather than from the absence of Fas on β cells (21, 31). In this report, we have studied the roles of Fas and perforin in the ability of a highly diabetogenic, CD8+ T cell–independent, I-Ag7–restricted, and β cell–specific TCR to kill β cells in vitro and to trigger diabetogenesis in transgenic NOD (4.1-NOD) mice bearing an oligoclonal or monoclonal TCR repertoire (19, 20). We have shown that 4.1-CD4+ CTL destroy cytokine-treated β cells exclusively via Fas and that, like NOD.lpr+/+ mice, RAG-2–/– 4.1-NOD.lpr+/+ mice are highly resistant to diabetes development despite the fact that they do not develop lymphadenopathy, lack CD4–CD8–B220+ T cells, and develop insulitis. Taken together, our data provide conclusive evidence that diabetogenic CD4+ T cells spontaneously differentiate into CTL capable of destroying β cells through a Fas-dependent mechanism that is potentiated by cytokines implicated in the pathogenesis of autoimmune diabetes (IL-1α, IL-1β, and IFN-γ) (2–4, 25–27).
It has been known for quite some time that β cell–reactive CD4+ T-cell clones can transfer diabetes into NOD.scid mice (5, 11) and that CD4+ T cell–mediated β-cell damage in diabetes is restricted by MHC molecules expressed on APCs, but not on the target β cells (32, 33). These observations, which are compatible with the fact that β cells do not normally express MHC class II molecules, suggested that CD4+ T cell–mediated damage to β cells is most likely effected by secreted T-cell factors (i.e., cytokines) rather than by cell-mediated cytolysis (32, 33). This interpretation, however, was at odds with the results of studies of transgenic mice expressing pathogenic cytokines (or dominant-negative cytokine receptors) in β cells, including IFN-γ (34, 35), IFN-α (36), TNF-α (37), and TNF-β (38). These studies suggested that most cytokines having a β cell–toxic effect in vitro are not directly toxic to β cells in vivo. Because some of the cytokines implicated in diabetogenesis can upregulate the expression of Fas on multiple cell types, including β cells, and because Fas- or FasL-deficient mice are resistant to certain autoimmune disorders, including diabetes (15, 16, 29, 39–41), we reasoned that some of these cytokines might upregulate Fas on tissue target cells and therefore mark them for lysis by pathogenic autoreactive CD4+ T cells capable of differentiating into CTL. Our observation that 4.1-CD4+ CTL can kill IL-1α–, IL-1β–, and IFN-γ–treated β cells in an MHC-independent manner, but not untreated β cells or cytokine-treated Fas-deficient β cells, provides direct evidence in support of this hypothesis. Indeed, in vitro–differentiated 4.1-CD4+ CTL were highly diabetogenic when transferred into immunodeficient NOD mice (even when the T cells could not express perforin), but not when transferred into immunodeficient NOD.lpr+/+ mice. Furthermore, 4.1-CD4+ T cells from Fas-deficient 4.1-NOD.lpr+/+ mice expressed perforin (our unpublished data) and spontaneously homed to pancreatic islets in vivo, but were unable to cause autoimmune diabetes in mice whose β cells lacked the ability to express Fas. These data also provide 1 possible explanation for the observation that TCR-transgenic and nontransgenic NOD mice selectively depleted of macrophages — which are major producers of prodiabetogenic cytokines such as IL-1α, IL-1β, and TNF — do not develop diabetes (42, 43). We do not yet know which of the cytokines we have studied (IL-1α, IL-1β, and IFN-γ) prime NOD β cells for Fas-dependent lysis in vivo. Experiments in 4.1-NOD mice expressing transgenic dominant-negative cytokine receptors in β cells may provide some clues. However, the ability of multiple cytokines to sensitize β cells for 4.1-CD4+ CTL damage is likely to make interpretation of the data obtained in these transgenic systems difficult. In fact, TNF-α, which has also been shown to upregulate Fas on some cell types (29), also sensitized NOD islet cells to 4.1-CD4+ CTL lysis (an effect quantitatively similar to that of IL-1β; our unpublished data).
These results raise several additional questions. Does the site of activation or the nature of the target antigen (or both) dictate whether naive MHC class II–restricted autoreactive CD4+ T cells will differentiate into highly diabetogenic CTL that kill via Fas in vivo? Is pathogenicity a function of the ability of CD4+ T cells to differentiate into Fas-killing CTL? Fas-mediated killing has the potential to affect innocent bystanders (13, 17, 18), so why do diabetogenic CD4+ T cells spare other endocrine islet cells? We do not have answers to all these questions, but we do have some clues. Although we do not yet know the molecular nature of their target autoantigen, naive 4.1-CD4+ T cells proliferate in response to islet cells from immunodeficient NOD mice in the absence of exogenous APCs (44). This suggests that 4.1-CD4+ T cells may undergo activation in vivo by engaging β-cell autoantigen–I-Ag7 complexes on intra-islet resident APCs with high affinity or avidity. This ability of the CD4+ T cell to undergo strong activation in situ is likely to endow the activated CD4+ CTL with the ability to lyse Fas-expressing β cells that are marked for death by cytokines before downregulating FasL from the T-cell surface. This view is supported by recent evidence indicating that costimulatory molecules on professional APCs are necessary for stimulation of CD4+ CTL to kill bystander targets (45). This view is also consistent with the facts that islet cells express very low levels of Fas (25, 28) and that the islet-associated 4.1-CD4+ T cells from 4.1-NOD mice can kill β cells only under conditions capable of upregulating Fas on β cells and FasL on T cells. The nature of the target autoantigen may be another critical factor in this process, because Fas-mediated cytotoxicity by some CTL can be triggered independently of perforin-mediated cytotoxicity, depending on the structure of the triggering antigenic peptide–MHC complex (46). The fact that diabetogenic CD4+ T cells spare α cells and other endocrine islet cells cannot be taken as an argument against a role for Fas in β-cell lysis by CD4+ T cells; these other islet cells may be resistant to Fas-mediated cell death. In fact, IL-1 has been shown to selectively induce Fas expression on β cells but not on other endocrine cells (26, 27), and α cells express FasL, but not Fas (47).
In conclusion, we have shown that CD4+ T cells expressing a highly diabetogenic TCR spontaneously differentiate into CTL in vivo and kill β cells via Fas. It is noteworthy that Fas also plays a critical role in the ability of a CD8+ CTL specificity that is putatively involved in the initiation of spontaneous diabetes in NOD mice to kill β cells in vitro and in vivo (21, 24). On the basis of these findings, we propose that diabetogenesis in NOD mice is initiated by CD8+ and CD4+ CTL clonotypes capable of lysing β cells exclusively via Fas, and is later amplified by clonotypes that can kill via other death effector pathways. This would explain (a) why NOD.lpr+/+ mice do not develop insulitis (15, 16); (b) why Fas-deficient islet grafts are readily destroyed in spontaneously diabetic NOD mice (31) or after adoptive transfer of splenic T cells from NOD or NOD mice transgenic for the BDC-2.5 TCR (48); and (c) why perforin-deficient NOD mice develop severe insulitis and yet remain resistant to diabetes (14, 21).
Acknowledgments
We thank P. Goldstein and T. Utsugi for providing reagents, and Y. Yang for critical review of the manuscript. We also thank D. Serreze for advice on Idd typing, S. Culp and K. Rouleau for technical assistance, L. Bryant for flow cytometry, and H. Kominek for editorial assistance. This work was supported by the Juvenile Diabetes Foundation International (JDFI) and the Medical Research Council of Canada. A. Amrani is supported by fellowships from the Alberta Heritage Foundation for Medical Research (AHFMR) and the JDFI. J. Verdaguer was supported by a fellowship from the Canadian Diabetes Association. P. Santamaria is a Senior Scholar of the AHFMR.
References
- 1.Delovitch T, Singh B. The nonobese diabetic mouse as a model of autoimmune diabetes: immune disregulation gets the NOD. Immunity. 1997;7:727–738. doi: 10.1016/s1074-7613(00)80392-1. [DOI] [PubMed] [Google Scholar]
- 2.Lee M-S, Sarvetnick N. Transgenes in autoimmunity. Curr Opin Immunol. 1992;4:723–727. doi: 10.1016/0952-7915(92)90052-g. [DOI] [PubMed] [Google Scholar]
- 3.Gianani R, Sarvetnick N. Viruses, cytokines, antigens and autoimmunity. Proc Natl Acad Sci USA. 1996;93:2257–2259. doi: 10.1073/pnas.93.6.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabinovitch A. Cytokines in the pathogenesis of type 1 diabetes. Can J Diabetes Care. 1999;23:40–51. [Google Scholar]
- 5.Christianson S, Shultz L, Leiter E. Adoptive transfer of diabetes into immunodeficient NOD-scid/scid mice. Relative contributions of CD4+ and CD8+ T-cells from diabetic versus prediabetic NOD.NON-thy-1a donors. Diabetes. 1993;42:44–55. doi: 10.2337/diab.42.1.44. [DOI] [PubMed] [Google Scholar]
- 6.Katz J, Benoist C, Mathis D. Major histocompatibility complex class I molecules are required for the generation of insulitis in non-obese diabetic mice. Eur J Immunol. 1993;23:3358–3360. doi: 10.1002/eji.1830231244. [DOI] [PubMed] [Google Scholar]
- 7.Wicker L, et al. β2-microglobulin-deficient NOD mice do not develop insulitis or diabetes. Diabetes. 1994;43:500–504. doi: 10.2337/diab.43.3.500. [DOI] [PubMed] [Google Scholar]
- 8.Serreze D, Leiter E, Christianson G, Greiner D, Roopenian D. Major histocompatibility complex class I-deficient NOD.β1mnull mice are diabetes and insulitis resistant. Diabetes. 1994;43:505–508. doi: 10.2337/diab.43.3.505. [DOI] [PubMed] [Google Scholar]
- 9.Kay T, Parker J, Stephens L, Thomas H, Allison J. RIP-β2-microglobulin transgene expression restores insulitis, but not diabetes, in β2-microglobulinnull nonobese diabetic mice. J Immunol. 1996;157:3688–3693. [PubMed] [Google Scholar]
- 10.Serreze D, et al. Initiation of autoimmune diabetes in NOD/Lt mice is MHC class I-dependent. J Immunol. 1997;157:3978–3986. [PubMed] [Google Scholar]
- 11.Peterson J, Haskins K. Transfer of diabetes in the NOD-scid mouse by CD4 T cell clones. Differential requirement for CD8 T cells. Diabetes. 1996;45:328–336. doi: 10.2337/diab.45.3.328. [DOI] [PubMed] [Google Scholar]
- 12.McInerney M, Rath S, Janeway C. Exclusive expression of MHC class II proteins on CD45+ cells in pancreatic islets of NOD mice. Diabetes. 1991;40:648–651. doi: 10.2337/diab.40.5.648. [DOI] [PubMed] [Google Scholar]
- 13.Kagi D, Ledermann B, Burki K, Zinkernagel R, Hengartner H. Lymphocyte-mediated cytotoxicity in vitro and in vivo: mechanisms and significance. Immunol Rev. 1995;146:95–115. doi: 10.1111/j.1600-065x.1995.tb00686.x. [DOI] [PubMed] [Google Scholar]
- 14.Kagi D, et al. Reduced incidence and delayed onset of diabetes in perforin-deficient nonobese diabetic mice. J Exp Med. 1997;186:989–997. doi: 10.1084/jem.186.7.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Itoh N, et al. Requirement of Fas for the development of autoimmune diabetes in nonobese diabetic mice. J Exp Med. 1997;186:613–618. doi: 10.1084/jem.186.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chervonsky A, et al. The role of Fas in autoimmune diabetes. Cell. 1997;89:17–24. doi: 10.1016/s0092-8674(00)80178-6. [DOI] [PubMed] [Google Scholar]
- 17.Hahn S, Geri R, Erb P. Mechanism and biological significance of CD4-mediated cytotoxicity. Immunol Rev. 1995;146:57–79. doi: 10.1111/j.1600-065x.1995.tb00684.x. [DOI] [PubMed] [Google Scholar]
- 18.Williams NS, Engelhard V. Identification of a population of CD4+ CTL that utilizes a perforin rather than a Fas ligand dependent cytotoxic mechanism. J Immunol. 1996;156:153–159. [PubMed] [Google Scholar]
- 19.Schmidt D, Verdaguer J, Averill N, Santamaria P. A mechanism for the major histocompatibility complex-linked resistance to autoimmunity. J Exp Med. 1997;186:1059–1075. doi: 10.1084/jem.186.7.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verdaguer J, et al. Spontaneous autoimmune diabetes in monoclonal T cell nonobese diabetic mice. J Exp Med. 1997;186:1663–1676. doi: 10.1084/jem.186.10.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amrani A, et al. Perforin-independent beta cell destruction by diabetogenic CD8+ T lymphocytes in transgenic nonobese diabetic mice. J Clin Invest. 1999;103:1201–1209. doi: 10.1172/JCI6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicoletti I, Migliorati G, Pagliacci M, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 23.Verdaguer J, et al. Acceleration of spontaneous diabetes in TCR beta-transgenic nonobese diabetic mice by β cell-cytotoxic CD8+ T cells expressing identical endogenous TCRα chains. J Immunol. 1996;157:4726–4735. [PubMed] [Google Scholar]
- 24.Anderson B, Park BJ, Verdaguer J, Amrani A, Santamaria P. Prevalent CD8+ T cell response against one peptide/MHC complex in autoimmune diabetes. Proc Natl Acad Sci USA. 1999;96:9311–9316. doi: 10.1073/pnas.96.16.9311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamada K, Takane-Gyotoku N, Ichikawa F, Inada C, Nokada K. Mouse islet cell lysis mediated by interleukin-1-induced Fas. Diabetologia. 1996;39:1306–1312. doi: 10.1007/s001250050574. [DOI] [PubMed] [Google Scholar]
- 26.Stassi G, et al. Nitric oxide primes pancreatic beta cells for Fas-mediated destruction in insulin-dependent diabetes mellitus. J Exp Med. 1997;186:1193–1199. doi: 10.1084/jem.186.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heitmeier M, Scarim A, Corbett J. IFN-γ increases the sensitivity of islets of Langerhans for inducible nitric oxide synthase expression induced by interleukin 1. J Biol Chem. 1997;272:13697–13705. doi: 10.1074/jbc.272.21.13697. [DOI] [PubMed] [Google Scholar]
- 28.Thomas HE, Darwiche R, Corbett JA, Kay TW. Evidence that beta cell death in the nonobese diabetic mouse is Fas independent. J Immunol. 1999;163:1562–1569. [PubMed] [Google Scholar]
- 29.Bretz JD, Arscott PL, Myc A, Baker JR., Jr Inflammatory cytokine regulation of Fas-mediated apoptosis in thyroid follicular cells. J Biol Chem. 1999;274:25433–25438. doi: 10.1074/jbc.274.36.25433. [DOI] [PubMed] [Google Scholar]
- 30.Clark W, et al. Molecular pathways of CTL-mediated cytotoxicity. Immunol Rev. 1995;146:33–44. doi: 10.1111/j.1600-065x.1995.tb00682.x. [DOI] [PubMed] [Google Scholar]
- 31.Allison J, Strasser A. Mechanisms of beta cell death in diabetes: a minor role for CD95. Proc Natl Acad Sci USA. 1998;95:13818–13822. doi: 10.1073/pnas.95.23.13818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Pontesilli O, Gill RG, Rosa FGL, Lafferty KJ. The role of CD4+ and CD8+ T cells in the destruction of islet grafts by spontaneously diabetic mice. Proc Natl Acad Sci USA. 1991;88:527–531. doi: 10.1073/pnas.88.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lo D, et al. Antigen-presenting cells in adoptively transferred and spontaneous autoimmune diabetes. Eur J Immunol. 1993;23:1693–1698. doi: 10.1002/eji.1830230744. [DOI] [PubMed] [Google Scholar]
- 34.Sarvetnick N, et al. Loss of pancreatic islet tolerance induced by beta cell-expression of interferon-gamma. Nature. 1990;346:844–847. doi: 10.1038/346844a0. [DOI] [PubMed] [Google Scholar]
- 35.Thomas H, Parker J, Schreiber R, Kay T. INF-γ action on panreatic beta cells causes class I MHC upregulation but not diabetes. J Clin Invest. 1998;102:1249–1257. doi: 10.1172/JCI2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stewart T, et al. Induction of type I diabetes by interferon-alpha in transgenic mice. Science. 1993;260:1942–1946. doi: 10.1126/science.8100367. [DOI] [PubMed] [Google Scholar]
- 37.Higuchi Y, et al. Expression of a tumor necrosis factor-α transgene in murine pancreatic beta cells results in severe and permanent insulitis without evolution towards diabetes. J Exp Med. 1992;176:1719–1726. doi: 10.1084/jem.176.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Picarella D, Kratz A, Li C, Ruddle N, Flavell R. Insulitis in transgenic mice expressing tumor necrosis factor beta (lymphotoxin) in the pancreas. Proc Natl Acad Sci USA. 1992;89:10036–10041. doi: 10.1073/pnas.89.21.10036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sabelko K, Kelly K, Nahm M, Cross A, Russell J. Fas and Fas ligand enhance the pathogenesis of experimental allergic encephalomyelitis, but are not essential for immune privilege in the central nervous system. J Immunol. 1997;159:3096–3099. [PubMed] [Google Scholar]
- 40.Waldner H, Sobel R, Howard E, Kuchroo V. Fas- and FasL-deficient mice are resistant to induction of autoimmune encephalomyelitis. J Immunol. 1997;159:3100–3103. [PubMed] [Google Scholar]
- 41.Thilenius A, Sabelko-Downes K, Russell J. The role of the antigen-presenting cell in Fas-mediated direct and bystander killing: potential in vivo function of Fas in experimental allergic encephalomyelitis. J Immunol. 1999;162:643–650. [PubMed] [Google Scholar]
- 42.Jun H-S, Santamaria P, Lim H-W, Zhang M, Yoon J-W. Absolute requirement of macrophages for the development and activation of β-cell cytotoxic CD8+ T-cells in T-cell receptor transgenic NOD mice. Diabetes. 1999;48:34–42. doi: 10.2337/diabetes.48.1.34. [DOI] [PubMed] [Google Scholar]
- 43.Jun H, Yoon C, Zbytnuik L, Rooijen NV, Yoon J. The role of macrophages in T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Exp Med. 1999;189:347–358. doi: 10.1084/jem.189.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt D, Amrani A, Verdaguer J, Bou S, Santamaria P. Autoantigen-independent deletion of diabetogenic CD4+ thymocytes by protective MHC class II molecules. J Immunol. 1999;162:4627–4636. [PubMed] [Google Scholar]
- 45.Wang R, Rogers A, Ratliff T, Russell J. CD95-dependent bystander lysis caused by CD4+ T helper 1 effectors. J Immunol. 1996;157:2961–2969. [PubMed] [Google Scholar]
- 46.Cao W, Tykodi S, Esser M, Braciale V, Braciale T. Partial activation of CD8+ T cells by a self-derived peptide. Nature. 1995;378:295–298. doi: 10.1038/378295a0. [DOI] [PubMed] [Google Scholar]
- 47.Signore A, et al. CD95 and CD95-ligand expression in endocrine pancreas of NOD, NOR and BALB/c mice. Diabetologia. 1997;40:1476–1479. doi: 10.1007/s001250050852. [DOI] [PubMed] [Google Scholar]
- 48.Pakala S, Chivetta M, Kelly C, Katz J. In autoimmune diabetes the transition from benign to pernicious insulitis requires an inslet cell response to tumor necrosis factor α. J Exp Med. 1999;189:1053–1062. doi: 10.1084/jem.189.7.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]