The intermolecular hydroamination of unactivated alkenes remains an important, unsolved challenge in catalysis.1 Hydroamination has been realized with alkali metal amides,2 lanthanide metallocene complexes,3 or acidic zeolites,4 but these approaches suffer from a number of limitations, most notably poor functional group compatibility. Ru(II),5 Rh(III),6 and Pt(II)7 complexes catalyze the hydroamination of ethylene and, in one case, 1-hexene8 with carboxamides or alkyl or aryl amines, but these transformations require forcing conditions and are of extremely limited scope.9 Although electrophilic gold(I)-10 and platinum(II) triflate11 complexes have been reported to catalyze the intermolecular hydroamination of unactivated alkenes with sulfonamides, these transformations are catalyzed with equal or greater efficiency by Brønsted acids and the metal-catalyzed reactions display behavior consistent with Brønsted acid catalysis.12-14 Given the challenges associated with the intermolecular hydroamination of unactivated alkenes, it is not surprising that the enantioselective intermolecular hydroamination of unactivated alkenes remains unknown.15,16 Here we report the Markovnikov-selective gold(I)-catalyzed hydroamination of ethylene and 1-alkenes with cyclic ureas and the unprecedented enantioselective hydroamination of unactivated 1-alkenes with up to 78% ee.
We have recently reported the room temperature intramolecular hydroamination of γ- and δ-alkenyl ureas catalyzed by a mixture of a gold(I) N-heterocyclic carbene (NHC) complex and AgOTf.17 The mild reaction conditions and the absence of an acid-catalyzed reaction pathway17 pointed to the potential development of a corresponding intermolecular process. However, attempts to realize the hydroamination of ethylene with acyclic ureas catalyzed by gold NHC complexes were uniformly unsuccessful. Conversely, cyclic ureas, employed in combination with a gold o-biphenyl phosphine precatalyst led to efficient hydroamination of ethylene. As an example, treatment of 1-methyl-imidazolidin-2-one (1) (0.4 M) with ethylene (120 psi) and a catalytic 1:1 mixture of (2a)AuCl [2a = P(t-Bu)2o-biphenyl] and AgOTf (5 mol %) in dioxane at 100 °C for 24 h led to isolation of 1-ethyl-3-methyl-imidazolidin-2-one (3) in 99% yield (Table 1, entry 1). In addition to 1, a number of cyclic ureas and 2-oxazolidinone reacted with ethylene at 100 °C to give the corresponding N-ethyl derivatives in good yield (Table 1, entries 5,6,7,10).18
Table 1.
Intermolecular Hydroamination of Alkenes (120 psi) with Cyclic Ureas Catalyzed by a Mixture of (2a)AuCl (5 mol %) and AgOTf (5 mol %) in Dioxane at 100 °C.
| entry | nucleophile | alkene | product | time (h) | yielda |
|---|---|---|---|---|---|
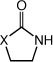
|
|
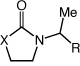
|
|||
| 1 | X = NMe (1) | R = H | 3 | 20 | 99 |
| 2 | R = Me | 62 | 97 | ||
| 3b | R = Et | 69 | 99 | ||
| 4 | R = (CH2)5Me | 24 | 38c | ||
| 5 | X = Nt-Bu | R = H | 40 | 80 | |
| 6 | X = O | R = H | 72 | 75 | |
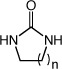
|
|
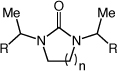
|
|||
| 7 | n = 1 | R = H | 20 | 95 | |
| 8 b | n = 1 | R = Me | 65 | 85 | |
| 9 | n = 1 | R = Et | 68 | 86d | |
| 10 b | n = 2 | R = H | 19 | 98 | |
| 11 | n = 2 | R = Me | 67 | 86 |
Isolated yield of >95% purity.
Catalyst loading = 10 mol %.
GC yield. 1-Octene loading = 10 equiv.
dr = 1:1.
Extension of gold(I)-catalyzed hydroamination to include 1-alkenes was encouraging, but also revealed the limitations of the (2a)AuCl/AgOTf catalyst system. Gold(I)-catalyzed reaction of propene or 1-butene with cyclic ureas at 100 °C led to Markovnikov hydroamination in good yield with high regioselectivity, but extended reaction time and/or higher catalyst loading was required (Table 1, entries 2,3,8,9,11) and the method was ineffective for the hydroamination of 1-octene (Table 1, entry 4).
Continued optimization of gold(I)-catalyzed intermolecular hydroamination (Table S1) revealed that employment of AgSbF6 as co-catalyst in combination with either (2a)AuCl or (2b)AuCl [2b = 2-di-t-butylphosphino-1,1′-binaphthyl] in dioxane led to efficient hydroamination of ethylene and 1-alkenes with 1 (Table 2). Ethylene reacted with 1 under surprisingly mild conditions (60 psi, 60 °C, 24 h) to form 3 in 99% isolated yield (Table 2, entry 1). Likewise, gold(I)-catalyzed reaction of 1 with propene, 1-butene, or 1-octene was complete within 24 h to form the corresponding Markovnikov hydroamination products in >95% yield as a single regioisomers (Table 2, entries 2-4).18 Gold(I)-catalyzed intermolecular hydroamination was also effective for 1-alkenes that contained a distal hydroxyl, benzyloxy, carboxylic acid, or carboxylic ester moiety (Table 2, entries 5-8). Styrene, isobutylene, and norbornene also underwent gold-catalyzed hydroamination with 1, albeit with diminished efficiency (Table 2, entries 9-11). Unstrained internal alkenes and α-substituted 1-alkenes failed to undergo efficient gold(I)-catalyzed intermolecular hydroamination under these conditions.
Table 2.
Intermolecular Hydroamination of Alkenes with 1 (0.4 M) Catalyzed by a Mixture of (L)AuCl (5 mol %) (L = 2a, 2b) and AgSbF6 (5 mol %) in Dioxane at 100 °C.
| entry | alkene | alkene amt | product | L | time (h) | yield (%)a |
|---|---|---|---|---|---|---|
|
|
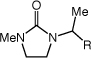
|
|||||
| 1 | R = H | 60 psi b | 3 | 2b | 24 | 99 |
| 2 | R = Me | 120 psi | 2b | 24 | 98 | |
| 3 | R = Et | 120 psi | 2b | 24 | 96 | |
| 4 | R = (CH2)5CH3 | 10 equiv | 2b | 24 | 96 | |
| 5 | R = (CH2)3OH | 10 equiv | 2b | 24 | 98 | |
| 6 | R = (CH2)3OBn | 10 equiv | 2b | 40 | 95 | |
| 7 | R = (CH2)2CO2H | 10 equiv | 2a | 40 | 98 | |
| 8 | R = (CH2)4CO2Et | 10 equiv | 2a | 40 | 90 | |
| 9 | R = Ph | 15 equiv | 2b | 36 | 75 | |
| 10 |
|
120 psic |
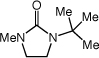
|
2a | 48 | 72 |
| 11 |
|
20 equivc |
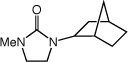
|
2b | 70 | 86d |
Yield of isolated, regiochemically pure material of >95% chemical purity.
Reaction temperature = 60 °C.
Catalyst loading = 10 mol %.
Single diastereomer formed, relative configuration not determined.
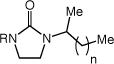
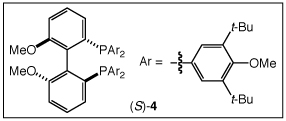
The efficient and highly regioselective hydroamination of unactivated 1-alkenes catalyzed by gold(I) phosphine complexes supported the feasibility of enantioselective intermolecular hydroamination. Indeed, a screen of enantiomerically pure bis(gold) phosphine complexes and achiral silver salts (Tables S2 and S3) led to identification of [(S)-4](AuCl)2 [(S)-4 = (S)-3,5-t-Bu-4-MeO-MeOBIPHEP] in combination with AgOTf as an effective catalyst system for the enantioselective hydroamination of 1-alkenes with imidazolidin-2-ones (Table 3).19 For example, reaction of 1 with 1-octene (60 equiv) catalyzed by a mixture of [(S)-4](AuCl)2 (2.5 mol %) and AgOTf (5 mol %) in m-xylene at 100 °C for 48 h led to the isolation of 1-methyl-3-(octan-2-yl)imidazolidin-2-one in 86% yield with 76% ee (Table 3, entry 1);18 lower octene loading led to diminished enantioselectivity. A number of 1-substituted imidazolidin-2-ones reacted with 1-alkenes in the presence of [(S)-4](AuCl)2/AgOTf to form the corresponding Markovnikov hydroamination products in good yield with 71-78% ee (Table 3, entries 2-6).
Table 3.
Enantioselective Intermolecular Hydroamination of 1-Alkenes (60 equiv) with Imidazolidin-2-ones Catalyzed by a Mixture of [(S)-4](AuCl)2 (2.5 mol %) and AgOTf (5 mol %) in m-Xylene at 100 °C for 48 h.
| entry | nucleophile | alkene | product | yield (%)a | ee (%)b |
|---|---|---|---|---|---|
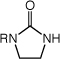
|
|
|
|||
| 1 | R = Me (1) | n = 5 | 86 | 76 | |
| 2 | R = Ph | n = 5 | 80 | 71 | |
| 3 | R = 4-C6H4F | n = 5 | 81 | 74 | |
| 4 | R = t-Bu | n = 5 | 89 | 78 | |
| 5 | R = Me (1) | n = 7 | 83 | 74 | |
| 6 | R = Me (1) | n = 9 | 76 | 75 | |
|
|||||
Yield of isolated, regiochemically pure material of >95% chemical purity.
Enantiopurity determined by HPLC analysis employing chiral stationary phase.
In summary, we have developed a mild and efficient gold(I)-catalyzed protocol for the intermolecular hydroamination of ethylene and unactivated 1-alkenes with cyclic ureas, which proceeds at or below 100 °C with high Markovnikov regioselectivity. We have extended this methodology to include the unprecedented enantioselective hydroamination of unactivated 1-alkenes in good yield with up to 78% ee. We continue to work toward the development of more general and more efficient methods for the intermolecular enantioselective hydroamination of unactivated alkenes.
Supplementary Material
Acknowledgment
is made to the NSF (CHE-0555425), NIH (GM-080422), and Johnson&Johnson for support of this research and to the NCBC (2008-IDG-1010) for support of the Duke University NMR facility.
Footnotes
Supporting Information Available: Experimental procedures, spectroscopic data, and scans of NMR spectra (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.(a) Müller TE, Hultzsch KC, Yus M, Foubelo F, Tada M. Chem. Rev. 2008;108:3795. doi: 10.1021/cr0306788. [DOI] [PubMed] [Google Scholar]; (b) Widenhoefer RA, Han X. Eur. J. Org. Chem. 2006:4555. [Google Scholar]; (c) Pohlki F, Doye S. Chem. Soc. Rev. 2003;32:104. doi: 10.1039/b200386b. [DOI] [PubMed] [Google Scholar]; (d) Hong S, Marks TJ. Acc. Chem. Res. 2004;37:673. doi: 10.1021/ar040051r. [DOI] [PubMed] [Google Scholar]
- 2.(a) Khedkar V, Tillack A, Benisch C, Melder J-P, Beller M. J. Mol. Catal. 2005;241:175. [Google Scholar]; (b) Lehmkuhl H, Reinehr D. J. Organomet. Chem. 1973;55:215. [Google Scholar]
- 3.(a) Ryu J-S, Li GY, Marks TJ. J. Am. Chem. Soc. 2003;125:12584. doi: 10.1021/ja035867m. [DOI] [PubMed] [Google Scholar]; (b) Li Y, Marks TJ. Organometallics. 1996;15:3770. [Google Scholar]
- 4.(a) Deeba M, Ford ME, Johnson TA. J. Chem. Soc. Chem. Commun. 1987:562. [Google Scholar]; (b) Mizuno N, Tabata M, Uematsu T, Iwamoto M. J. Catal. 1994;146:249. [Google Scholar]; (c) Hoelderich WF. Catal. Today. 2000;62:115. [Google Scholar]
- 5.Yi CS, Yun SY. Org. Lett. 2005;7:2181. doi: 10.1021/ol050524+. [DOI] [PubMed] [Google Scholar]
- 6.(a) Baudequin C, Brunet J-J, Rodriguez-Zubiri M. Organometallics. 2007;26:5264. [Google Scholar]; (b) Diamond SE, Szalkiewicz A, Mares F. J. Am. Chem. Soc. 1979;101:490. [Google Scholar]; (c) Coulson DR. Tetrahedron Lett. 1971;12:429. [Google Scholar]
- 7.(a) Brunet J-J, Cadena M, Chu NC, Diallo O, Jacob K, Mothes E. Organometallics. 2004;23:1264. [Google Scholar]; (b) Wang X, Widenhoefer RA. Organometallics. 2004;23:1649. [Google Scholar]
- 8.(a) Brunet J-J, Chu NC, Diallo O. Organometallics. 2005;24:3104. [Google Scholar]; (b) Brunet J-J, Chu N-C, Rodriguez-Zubiri M. Eur. J. Inorg. Chem. 2007:4711. [Google Scholar]
- 9.For recent examples of the late transition metal-catalyzed intermolecular hydroamination of vinyl arenes, conjugated dienes, and strained alkenes see: Shaffer AR, Schmidt JAR. Organometallics. 2008;27:1259. Sakai N, Ridder A, Hartwig JF. J. Am. Chem. Soc. 2006;128:8134. doi: 10.1021/ja061349a. Johns AM, Liu Z, Hartwig JF. Angew. Chem. Int. Ed. 2007;46:7259. doi: 10.1002/anie.200701899. Brouwer C, He C. Angew. Chem. Int. Ed. 2006;45:1744. doi: 10.1002/anie.200504495. Kovács G, Ujaque G, Lledós A. J. Am. Chem. Soc. 2008;130:853. doi: 10.1021/ja073578i. Utsunomiya M, Hartwig JF. J. Am. Chem. Soc. 2003;125:14286. doi: 10.1021/ja0375535. Utsunomiya M, Hartwig JF. J. Am. Chem. Soc. 2004;126:2702. doi: 10.1021/ja031542u.
- 10.(a) Zhang J, Yang C-G, He C. J. Am. Chem. Soc. 2006;128:1798. doi: 10.1021/ja053864z. [DOI] [PubMed] [Google Scholar]; (b) Giner X, Nájera C. Org. Lett. 2008;10:2919. doi: 10.1021/ol801104w. [DOI] [PubMed] [Google Scholar]; (c) Liu X-Y, Li C-H, Che C-M. Org. Lett. 2006;8:2707. doi: 10.1021/ol060719x. [DOI] [PubMed] [Google Scholar]
- 11.Karshtedt D, Bell AT, Tilley TD. J. Am. Chem. Soc. 2005;127:12640. doi: 10.1021/ja052836d. [DOI] [PubMed] [Google Scholar]
- 12.A recent study supports a Brønsted acid-catalyzed mechanism for the platinum(II)-catalyzed hydroamination of alkenes with sulfonamides. McBee JL, Bell AT, Tilley TD. J. Am. Chem. Soc. 2008;130:16562. doi: 10.1021/ja8030104.
- 13.(a) Rosenfeld DC, Shekhar S, Takemiya A, Utsunomiya M, Hartwig JF. Org. Lett. 2006;8:4179. doi: 10.1021/ol061174+. [DOI] [PubMed] [Google Scholar]; (b) Li Z, Zhang J, Brouwer C, Yang C-G, Reich NW, He C. Org. Lett. 2006;8:4175. doi: 10.1021/ol0610035. [DOI] [PubMed] [Google Scholar]; (c) Marcseková K, Doye S. Synthesis. 2007:145. [Google Scholar]; (d) Anderson LL, Arnold J, Bergman RG. J. Am. Chem. Soc. 2005;127:14542. doi: 10.1021/ja053700i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Notable behavior includes the lack of activity toward ethylene and the formation of 2- and 3-N-octyl sulfonamides from the hydroamination of 1-octene.10a,13a
- 15.(a) Aillaud I, Collin J, Hannedouche J, Schulz E. Dalton Trans. 2007:5105. doi: 10.1039/b711126f. [DOI] [PubMed] [Google Scholar]; (b) Hultzsch KC. Org. Biomol. Chem. 2005;3:1819. doi: 10.1039/b418521h. [DOI] [PubMed] [Google Scholar]; (c) Hultzsch KC. Adv. Synth. Catal. 2005;347:367. [Google Scholar]
- 16.The intramolecular enantioselective hydroamination of unactivated alkenes15 and the intermolecular enantioselective hydroamination of vinyl arenes,16a conjugated dienes,16b and norbornene16c,d have been demonstrated. Kawatsura M, Hartwig JF. J. Am. Chem. Soc. 2000;122:9546. Löber O, Kawatsura M, Hartwig JF. J. Am. Chem. Soc. 2001;123:4366. doi: 10.1021/ja005881o. Dorta R, Egli P, Zürcher F, Togni A. J. Am. Chem. Soc. 1997;119:10857. Zhou J, Hartwig JF. J. Am. Chem. Soc. 2008;130:12220. doi: 10.1021/ja803523z.
- 17.Bender CF, Widenhoefer RA. Org. Lett. 2006;8:5303. doi: 10.1021/ol062107i. [DOI] [PubMed] [Google Scholar]
- 18.Control experiments ruled out silver- or acid-catalyzed pathways for hydroamination of alkenes with 1 (see Supporting Information).
- 19.For examples of the enantioselective functionalization of C–C bonds with chiral bis(gold)phosphine complexes see: Widenhoefer RA. Chem. Eur. J. 2008;14:5382. doi: 10.1002/chem.200800219. Bongers N, Krause N. Angew. Chem. Int. Ed. 2008;47:2178. doi: 10.1002/anie.200704729.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


