Abstract
Brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF) are neurotrophic factors that are critical for the growth, survival, and differentiation of developing neurons. These neurotrophic factors also play important roles in the survival and function of adult neurons, learning and memory, and synaptic plasticity. Since the mid 1990s, investigators have studied the role of BDNF and GDNF in the behavioral effects of abused drugs and in the neuroadaptations induced by repeated exposure to drugs in the mesocorticolimbic dopamine system. Here, we review rodent studies on the role of BDNF and GDNF in drug reward, as assessed in the drug self-administration and the conditioned place preference procedures, and in drug relapse, as assessed in extinction and reinstatement procedures. Our main conclusion is that whether BDNF or GDNF would facilitate or inhibit drug-taking behaviors is dependent on the drug type, brain site, the addiction phase (initiation, maintenance, or abstinence/relapse), and the time interval between site-specific BDNF or GDNF injections and the reward- and relapse-related behavioral assessments.
Keywords: addiction, alcohol, cocaine, craving, drug self-administration, extinction, heroin, incubation, mesocorticolimbic dopamine system, neuroadaptations, relapse, reinstatement
1. Introduction
Drug addiction is characterized by compulsive drug use despite adverse consequences and high relapse rates during abstinence periods (Hunt et al., 1971; Wikler, 1973). Results from neurobiological studies in laboratory animals demonstrate that neuronal activity in the mesocorticolimbic dopamine system mediates drug reward (Pierce and Kumaresan, 2006; Wise and Rompre, 1989) and relapse to drug seeking (See, 2002; Shalev et al., 2002); this dopamine system comprises of cell bodies in VTA (ventral tegmental area) that project to several brain areas, including the medial prefrontal cortex (mPFC), nucleus accumbens, amygdala, bed nucleus of stria terminalis (BNST), and hippocampus (Fallon and Moore, 1978; Ungerstedt, 1971). A current popular hypothesis is that compulsive drug seeking and long-term relapse vulnerability are due to drug-induced neuroadaptations in the mesocorticolimbic dopamine system and glutamatergic corticolimbic circuitry in which the dopamine projections are embedded (Kalivas and Volkow, 2005; Nestler, 2001b; Wolf, 1998).
This drug-induced neuroadaptation hypothesis has inspired studies on the role of cellular events and signaling cascades that underlie synaptic plasticity (Box 1) processes of learning and memory in the behavioral effects of drugs (Nestler, 2001a; Thomas et al., 2008; Wolf et al., 2004). Within this framework, since the mid 1990s (Altar et al., 1992; Berhow et al., 1995; Berhow et al., 1996; Martin-Iverson et al., 1994), investigators have explored the effect of exposure to abused drugs on the expression and function of BDNF and GDNF in the mesocorticolimbic dopamine system, and the effect of manipulating their function on the behavioral effects of drugs (Bolanos and Nestler, 2004; Carnicella and Ron, 2009; McGinty et al., 2009; Pierce and Bari, 2001; Ron and Janak, 2005; Russo et al., 2009a).
Box 1. Glossary of terms.
CONDITIONED PLACE PREFERENCE (CPP)
A classical conditioning (Pavlovian) procedure used to study the rewarding effects of unconditioned stimuli (e.g., food, drugs). During training, one area of a test chamber is associated with a stimulus and another area is not. During testing, in the absence of the stimulus, the subject is allowed to choose between the two areas. An increase in preference for the stimulus-paired area serves as a measure of its Pavlovian rewarding effects (Bardo and Bevins, 2000; Mucha et al., 1982).
INCUBATION OF COCAINE CRAVING
A hypothetical motivational process inferred from the findings of time-dependent increases in cue-induced cocaine seeking after withdrawal from cocaine self-administration in rats (Grimm et al., 2001; Lu et al., 2004b). In studies on the role of BDNF in incubation of cocaine craving, cue-induced cocaine seeking was assessed in extinction tests in which rats were exposed to contextual cues previously associated with drug availability and lever-presses lead to contingent presentations of a discrete tone-light compound cue previously paired with cocaine injections. cocaine craving refers to an affective state that can be induced in human cocaine users by acute exposure to cocaine, cocaine-associated cues, or stress.
DRUG SELF-ADMINISTRATION
A procedure in which laboratory animals perform a voluntary, or operant, response (e.g., lever press, nose-poke) to obtain a drug. The premise of this procedure is that drugs (or non-drug rewards) control behavior by functioning as positive reinforcers (Schuster and Thompson, 1969; Weeks, 1962). A stimulus is defined as a positive reinforcer in operant conditioning if its presentation following a response increases or maintains the likelihood of the response.
PSYCHOMOTOR SENSITIZATION
A term that often refers to the progressive increase in locomotor activity or stereotypy with repeated drug (e.g., cocaine) administration (Post and Kopanda, 1976). Typically, psychomotor sensitization studies include two phases: an initial phase (often referred to as ‘development of psychomotor sensitization’ phase) in which laboratory subjects are injected repeatedly with drugs over days, and a subsequent test for ‘expression of psychomotor sensitization’ during which the subjects are injected acutely with drugs at different withdrawal days after the end of the ‘development’ phase and sensitized locomotor activity or stereotypy is assessed (Vanderschuren and Kalivas, 2000).
LONG-TERM POTENTIATION (LTP)
A form of synaptic plasticity defined by a persistent increase in synaptic strength. It is often induced by an experimenter-delivered train of synaptic stimuli that produces a strong postsynaptic depolarization lasting up to several seconds (Bliss and Lomo, 1973).
REINSTATEMENT PROCEDURE (MODEL)
An animal model of relapse to drug use. In the operant conditioning version, animals are trained to respond for drug infusions (or oral solutions in the case of alcohol), typically by pressing a lever; then, following extinction of the responding, non-reinforced pressing on the drug-associated lever is induced by drug priming injections (de Wit and Stewart, 1981; Self et al., 1996; Spealman et al., 1999), drug cues (Crombag et al., 2008; Meil and See, 1996; Weiss et al., 2000), or stressors (Lu et al., 2003; Shaham et al., 2000). In the classical conditioned version, CPP is induced by a drug, extinguished, and then induced again by drug priming injections (Mueller and Stewart, 2000) or stressors (Wang et al., 2006).
RELAPSE
A term used to describe the resumption of drug-taking behavior during periods of self-imposed or forced abstinence in humans (Wikler, 1973).
SYNAPTIC PLASTICITY
A term that refers to activity-dependent, direct or indirect modifications of the strength of synaptic transmission at preexisting synapses (Citri and Malenka, 2008).
BDNF and GDNF are well known for their role in growth, survival, and differentiation of developing neurons (Bespalov and Saarma, 2007; Chao, 2003; Chao et al., 2006). Over the last two decades, results from many studies have implicated BDNF and GDNF in the survival and function of adult dopamine neurons, learning and memory, and synaptic plasticity (Airaksinen and Saarma, 2002; Andressoo and Saarma, 2008; Bekinschtein et al., 2008; Chiocco et al., 2007; Lu et al., 2008; Poo, 2001). Here, we review studies on the role of BDNF and GDNF in drug reward, as assessed in the conditioned-place preference (CPP, Box 1) (Mucha et al., 1982) and the drug self-administration (Schuster and Thompson, 1969; Weeks, 1962) procedures, and in drug relapse as assessed in the reinstatement procedure (Epstein et al., 2006; Shaham et al., 2003; Stewart and de Wit, 1987). We also review studies on the role of BDNF and GDNF in incubation of cocaine craving (Grimm et al., 2001; Lu et al., 2004b).
The focus of our review is the role of BDNF and GDNF in drug reward and relapse. Thus, we do not discuss results on the role of GDNF and BDNF in the acute effects of drugs on locomotor activity (Airavaara et al., 2004; Airavaara et al., 2007; Gerlai et al., 2001; Martin-Iverson et al., 1994) or drug psychomotor sensitization (for reviews see Carnicella and Ron, 2009; Niwa et al., 2007a; Pierce and Bari, 2001). In Tables S1 and S2 (supplementary online material) we summarize results on the effect of exposure to different regimens of different drugs on BDNF and GDNF expression in different components of the mesocorticolimbic dopamine system. In keeping with the focus of our review, with a few exceptions these results are not discussed, because in many of these studies, investigators did not attempt to connect the drug-induced expression changes of BDNF and GDNF to behavioral measures of drug reward or relapse.
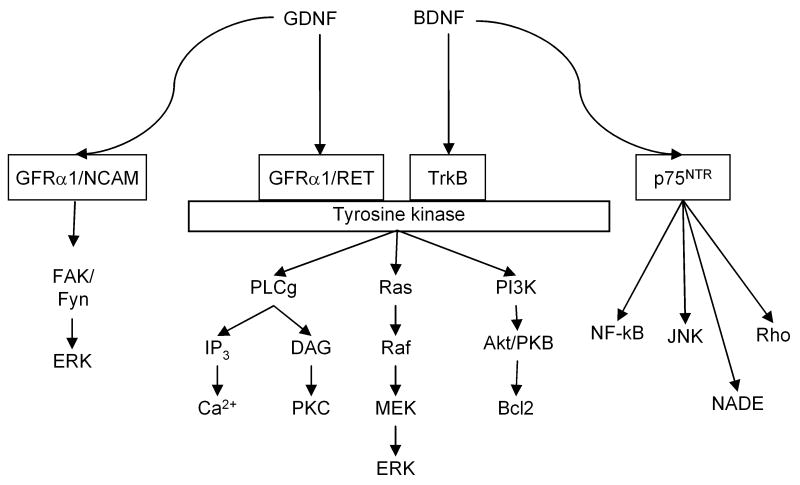
Our review complements previous reviews on the role of neurotrophic factors in psychomotor sensitization (Pierce and Bari, 2001) and signaling pathways underlying their role in drug-induced changes in synaptic plasticity and behavior (Bolanos and Nestler, 2004; Russo et al., 2009a), and GDNF's role in alcohol-taking behavior (Carnicella and Ron, 2009; Ron and Janak, 2005), and methamphetamine sensitization and reward (Niwa et al., 2007b). In Box 1, we provide a glossary of terms that appear in the text in capital letters and in Box 2, we provide a general overview of BDNF and GDNF signaling mechanisms.
Box 2. Overview of the molecular biology of BDNF and GDNF signal transduction mechanisms.
BDNF belongs to the family of neutotrophins. The cellular actions of BDNF are mediated through TrkB tyrosine kinase receptor and by p75NTR (Chao, 2003). Binding of dimeric BDNF causes dimerization of TrkB receptor and autophosphorylation of intracellular tyrosine residues. Activation of TrkB receptor leads to signaling cascades involving activation of Ras/ERK pathway, PI3K and Phospholipase Cγ (PLC-γ) (Roux and Barker, 2002). The Ras pathway regulates neuronal survival and differentiation through downstream signaling that includes c-RAF/B-Raf/ERK1/ERK2. BDNF signaling through PI3K plays an important role in survival of neurons and the downstream signaling includes serine/threonine kinases 3-phosphoinositide-dependent kinase-1 (PDK1) (Vanhaesebroeck and Alessi, 2000) and Akt (Jones et al., 1991). Akt activated by PDK1 in turn activates substrates involved in neuronal survival such as Bcl-2, Caspase-9, IκB kinase glycogen synthase kinase-3, and Forkhead family members (Roux and Barker, 2002). PLC-γ activation leads to increased levels of inositol triphosphate (IP3) and diacylglycerol (DAG) (Vetter et al., 1991). IP3 increases cytoplasmic Ca2+ concentrations and DAG activates PKCδ (Corbit et al., 1999). p75NTR receptor signaling is involved in cell survival, neurogenesis, cell cycle effects, and apoptosis during developmental cell death and after nervous system injury (Roux and Barker, 2002). Pro-apoptotic p75NTR triggered cell death has been observed during stress, inflammation and injury conditions (Chao et al., 2006). p75NTR receptor signaling cascades include JNK (c-Jun N-terminal kinase), NF-κB (nuclear factor κB), NADE and RhoA (Roux and Barker, 2002).
GDNF is a secreted protein (Lin et al., 1993), which plays an important role in vivo in maintenance and survival of adult dopamine neurons (Boger et al., 2006; Granholm et al., 2000; Hoffer et al., 1994; Pascual et al., 2008; Tomac et al., 1995). GDNF is one of the GDNF-family ligands (GFLs) and is part of the TGF-beta super-family (Bespalov and Saarma, 2007). GDNF is a basic and dimeric molecule and it acts through the receptor tyrosine kinase Ret, which is also activated by other members of the GDNF family (Durbec et al., 1996; Jing et al., 1996; Treanor et al., 1996; Trupp et al., 1996). GDNF binds first to its corresponding GDNF family receptor-α1 (GFRα1). Homodimeric GDNF binds to either monomeric or dimeric GFRα1; the complex brings two RET molecules together, and tyrosine residues in the intracellular domains of the RET proteins are autophosphorylated (Bespalov and Saarma, 2007). GFRα1 and RET are also expressed at high levels in the VTA and substantia nigra (Golden et al., 1998; Trupp et al., 1997). RET activation is induced by the GDNF/GFRα1-complex and tyrosine residues in the intracellular domains of the RET proteins are autophosphorylated (Sariola and Saarma, 2003). RET activation is involved in neuronal survival, differentiation and proliferation, neurite outgrowth, and synaptic plasticity (Sariola and Saarma, 2003). Activation of RET leads to activation of a series of intracellular signaling cascades, including ERK, phosphoinositide 3-kinase (PI3K), and Phospholipase Cγ (PLC-γ) (Fisher et al., 2001; Fukuda et al., 2002; Sariola and Saarma, 2003). In addition, protein kinase A dependent Ser696 is involved in GDNF-induced RET activation (Fukuda et al., 2002). Independent of RET activation, GDNF also activates a neural cell adhesion molecule (NCAM) (Paratcha et al., 2003). The neural cell adhesion molecule (NCAM) functions as an alternative signaling receptor for GDNF (Paratcha et al., 2003; Paratcha and Ledda, 2008). Midbrain dopamine neurons express NCAM (Chao et al., 2003). NCAM is involved in several developmental processes, including neurite outgrowth and cell migration (Povlsen et al., 2008; Schmid and Maness, 2008). Loss of NCAM function leads to impairments in spatial learning and reduction in long-term potentiation (LTP) (Cremer et al., 1994; Luthl et al., 1994). In vivo, the RET-independent signaling of GDNF is supported by the findings that GFRα1 is more widely expressed in the central nervous system than RET (Golden et al., 1998; Trupp et al., 1997). Studies with cells lacking RET have shown that GDNF, in the presence of GFRα1, binds to NCAM, and causes activation of Fyn and focal adhesion kinase (FAK) (Paratcha et al., 2003). The presence of GFRα1 downregulates NCAM-NCAM interaction, but promotes GDNF binding to NCAM (Paratcha et al., 2003). As GDNF, GFRα1 and NCAM are involved in synaptic plasticity and mutant mice lacking these molecules have learning deficits, it has been hypothesized that GDNF, GFRα1 and NCAM function in cooperation to promote trans-synaptic adhesion (Paratcha and Ledda, 2008).
2. BDNF
Horger et al. (1999) provided one of the most dramatic illustrations of the role of accumbens BDNF in cocaine's behavioral effects. It is well-established that systemic or accumbens injections of psychostimulant drugs potentiate the rat's response to cues previously paired with non-drug rewards (Robbins, 1975; Taylor and Robbins, 1984). Horger et al. (1999) showed that chronic delivery of BDNF into the accumbens (via minipumps) profoundly increased systemic cocaine-induced potentiation of responding for a conditioned cue previously paired with water in thirsty rats. This effect lasted up to 5 weeks after cessation of BDNF administration. This study was the inspiration for subsequent work on the role of BDNF in drug self-administration, reinstatement, and incubation of drug craving. In Table 1 we summarize results from studies described below on the role of BDNF on drug reward and relapse, as assessed in animal models.
Table 1.
Effect of experimental manipulations of BDNF function on drug-taking behavior.
| Drug | Experimental procedure | Behavioral measure | Brain area | Reference |
|---|---|---|---|---|
| Cocaine | BDNF knockout | CPP ▼ | Not applicable | (Hall et al., 2003) |
| Lentivirus-BDNF (over-expression) | CPP expression ▲ | NAc | (Bahi et al., 2008) | |
| CPP extinction responding ▲ | ||||
| CPP priming reinstatement ▲ | ||||
| BDNF knockdown | CPP ▼ | VTA, NAc | (Graham et al., 2009) | |
| TrkB knockdown | CPP ▼ | NAc | ||
| SA Acquisition ─ | NAc | |||
| SA drug intake ▼ | NAc | |||
| BDNF injection | SA drug intake and PR ▲ | NAc shell | (Graham et al., 2007) | |
| Extinction responding ▲ | ||||
| Cue reinstatement ▲ | ||||
| Priming reinstatement ▲ | ||||
| Footshock reinstatement ▲ | ||||
| Anti-BDNF (monoclonal antibody) injection | SA drug intake and PR ▼ | NAc shell | ||
| Extinction responding ▼ | ||||
| Cue reinstatement ─ | ||||
| Priming reinstatement ▼ | ||||
| Stress reinstatement ▼ | ||||
| BDNF knockdown (mice) | SA drug intake and PR ▼ | NAc | ||
| BDNF injection | Extinction responding ▲ | VTA | (Lu et al., 2004a) | |
| BDNF injection | Extinction responding ▼ | mPFC | (Berglind et al., 2007) | |
| Cue reinstatement ▼ | ||||
| Priming reinstatement ▼ | ||||
| Morphine | BDNF injection | CPP ─ | VTA | (Vargas-Perez et al., 2009) |
| Amphetamine | K-252a (a non-selective Trk inhibitor) | CPP ▼ | DG/CA3 | (Shen et al., 2006) |
| Alcohol | BDNF overexpression (via systemic administration of Tat-RACK1) | Ethanol consumption ▼ | Not applicable | (Jeanblanc et al., 2006) |
| BDNF knockout | Ethanol consumption ▲ | Not applicable | (Hensler et al., 2003) | |
| BDNF knockout | CPP ▲ | Not applicable | (McGough et al., 2004) | |
| SA ▲ | ||||
| BDNF injection | SA ▼ | DLS, DMS | (Jeanblanc et al., 2009) | |
| viral-mediated siRNA (decreases BDNF mRNA expression | SA ▲ | DLS (no effect in DMS) | ||
Abbreviations: SA: self-administration; CPP: conditioned place preference; NAc: nucleus accumbens; VTA: ventral tegmental area; DG: dentate gyrus; CA3, hippocampal CA3 field; PR: progressive ratio; DLS: dorsolateral striatum; DMS: dorsomedial striatum. Symbols: ▲, increase; ▼, decrease; ─, no effect.
2.A. Conditioned place preference
Cocaine and amphetamine
Hall et al. (2003) reported that the cocaine dose-response curve in BDNF heterozygote knockout mice in the CPP procedure is shifted to the right, suggesting decreases in cocaine reward. The interpretation of these data, however, is not straightforward. First, a main limitation of this study (and other constitutive knockout studies described below) is that when a gene is deleted or partially deleted throughout development, it is unknown whether the observed changes in behavior are reflective of a given gene's normal role in behavior or some unknown compensatory changes that result from the gene deletion (Routtenberg, 1996). Second, CPP is a contextual learning task and there is evidence that hippocampal BDNF plays a role in contextual learning (Tyler et al., 2002). Thus, decreased cocaine CPP in heterozygote BDNF knockout mice may be due learning deficits. This possibility is unlikely, because while the heterozygote BDNF knockout mice showed impaired CPP with 10 mg/kg of cocaine, they demonstrated normal CPP with 20 mg/kg cocaine (Hall et al., 2003). Third, in the absence of data demonstrating lack of decreases in CPP induced by non-drug rewards, a potential interpretation of the data of Hall et al. (2003) is that decreased cocaine CPP in heterozygote BDNF knockout mice reflects a more general reward deficit.
Bahi et al. (2008) studied the role of accumbens BDNF and TrkB in cocaine-induced CPP and locomotor activity using lentiviral gene delivery and siRNA (small (short) interfering RNA) procedures to increase and decrease gene expression, respectively. Lentiviral gene delivery and siRNA procedures were used in the locomotor activity study (not described here) while lentiviral gene delivery to over-express accumbens BDNF and TrkB was used in the CPP study. Their main finding was that BDNF and TrkB accumbens over-expression increased cocaine CPP (5 or 20 mg/kg). These findings (and other findings from this report, see below) are compelling but in interpreting them, as well as results from other studies described below on the effect of exogenous or viral delivery of BDNF, several issues should be considered. The first is that the demonstration that strong over-expression (or activation) of a given molecule increases drug reward (or other behaviors) may not necessarily reflect an endogenous role of the molecule in reward. This is because viral over-expression typically increases gene expression to a greater degree than that induced by drug (or cue) exposure (for a discussion of this issue see Harvey et al., 2007). The second issue is the anatomical specificity of Bahi et al. (2008) findings, which were derived from a study in which the authors used a large injection volume (4 μL); in studies using intracranial injections into the accumbens and other brain areas the injection volume is typically about 0.25 to 0.5 μL per site (Wise and Hoffman, 1992). While intracranially injected viruses do not spread as much as small molecule drugs, when foreign substances are locally injected, they can diffuse away from the injection site and act on nearby areas (Wise and Hoffman, 1992). Additionally, when cannulae penetrate the ventricles (as is often the case with accumbens injections) injected drugs can diffuse into the ventricular system and act at distal sites (Johnson and Epstein, 1975).
Graham et al. (2009) provided additional evidence for a role of accumbens (and VTA) BDNF and TrkB in cocaine CPP. They used an adeno-associated virus (AAV) approach to decrease BDNF and TrkB expression in accumbens and VTA and found that decreased BDNF expression in both brain areas decreases cocaine (10 mg/kg) CPP while decreased TrkB expression in the accumbens but not VTA decreased this CPP.
Shen et al. (2006) provided potential evidence for a role of BDNF in amphetamine CPP. They first showed that amphetamine CPP is associated with increased expression of TrkB in hippocampus (CA3/dentate gyrus) and accumbens shell. These authors then showed that amphetamine CPP is blocked by CA3/DG injections of K-252a, a non-selective Trk inhibitor (Shen et al., 2006). In the context of hippocampus BDNF's role in amphetamine CPP, a question for future research is whether the effect of K-252a would be mimicked by a manipulation that more selectively interferes with BDNF signaling (e.g., an anti-BDNF monoclonal antibody Graham et al., 2007), but not the signaling of other neurotrophic factors that act on other Trk receptors.
Morphine
Recent evidence implicates mesocorticolimbic BDNF in morphine's rewarding effects in the CPP procedure (Vargas-Perez et al., 2009). In this study, the authors explored the role of VTA BDNF in the shift from a dopamine-independent to a dopamine-dependent morphine CPP that occurs when rats are repeatedly exposed to heroin (to induce opiate dependence) and then withdrawn from the drug (Bechara et al., 1998). In their studies, Van Der Kooy and colleagues demonstrated that morphine CPP in previously drug-naïve rats is not blocked by the neuroleptic blocker flupenthixol (i.e., dopamine independent). In contrast, in heroin-exposed and withdrawn rats, morphine CPP is blocked by flupenthixol (i.e., dopamine-dependent) (Bechara et al., 1998; Laviolette et al., 2004). Vargas-Perez et al. (2009) first demonstrated that repeated heroin exposure (0.5 mg/kg/d for 8 d) increased BDNF protein and mRNA expression in the VTA. They then demonstrated that in previously drug-naïve rats a single BDNF VTA injection caused a shift from a dopamine-independent to a dopamine-dependent morphine CPP, similar to that observed in the dependent-withdrawn heroin-exposed rats. Subsequently, the authors demonstrated that BDNF VTA injections also cause a switch in VTA GABA-A receptors from inhibitory to excitatory signaling, similar to that previously observed in dependent-withdrawn heroin-exposed rats (Laviolette et al., 2004). A potential interpretation issue in this study (and other studies where exogenous BDNF is injected into the brain) is that BDNF can undergo retrograde and anterograde transport from the injection site and act in distal brain areas to control behavior (Altar and DiStefano, 1998). However, based on previous work of van der Kooy and colleagues where they identified the VTA as the critical site in the switch from dopamine-independent to dopamine-dependent morphine CPP (Laviolette et al., 2004), it is unlikely that BDNF effects in sites distal to the VTA can account for the results of Vargas-Perez (2009). Finally, it should be noted Vargas-Perez (2009) finding that repeated exposure to heroin, a mu opiate receptor agonist, increased BDNF expression in VTA is somewhat surprising. This is because investigators previously reported that chronic morphine (also a mu opiate receptor agonist) exposure either has no effect on VTA BDNF expression (Numan et al., 1998) or decreases this expression (Chu et al., 2007), and that morphine exposure also decreases BDNF-dependent intracellular signaling and cell morphology in VTA (Russo et al., 2007; Sklair-Tavron et al., 1996).
2.B. Drug self-administration
Cocaine
Results from two excellent studies of Graham et al. (2007; 2009) indicate that accumbens BDNF and TrkB play an important role in the rewarding effects of cocaine, as assessed in the drug self-administration procedure. In the first study, they trained rats to self-administer cocaine and then injected BDNF or anti-BDNF antibody into the accumbens shell of rats immediately after cocaine-self administration training for 5 days (Graham et al., 2007). Three to seven days after the last injection, BDNF-exposed rats self-administered more cocaine over a range of cocaine doses (an upward shift in the dose-response curve), suggesting increases in cocaine's rewarding effects. In contrast, exposure to anti-BDNF had a modest effect in the opposite direction. Injections of BDNF, but not anti-BDNF, also potently increased the rats' motivation to work for cocaine when the response requirement for each successive injection exponentially increased under a progressive ratio reinforcement schedule (Richardson and Roberts, 1996). Graham et al. (2007) also locally injected an AAV vector to knockdown local BDNF expression in mice accumbens shell. This manipulation caused a modest downward shift in the dose-response curve, suggesting a decrease in cocaine's reward. In the second study, Graham et al. (2009) used the AAV approach to decrease accumbens TrkB expression in mice and found that this manipulation decreased cocaine self-administration under a fixed-ratio 1 (FR1) reinforcement schedule over a range of unit doses (63 μg/kg to 500 μg/kg). The same manipulation had no effect on acquisition of cocaine self-administration or on lever responding for sucrose, indicating that performance deficits cannot account for the decreases in cocaine self-administration.
Alcohol
In an initial study, Hensler et al. (2003) reported increased home-cage alcohol (3 to 20%) consumption in heterozygote BDNF knockout mice. These mice demonstrated normal intake of oral saccharin or quinine, suggesting that the changes in alcohol intake are not due to taste differences between the wild-type and the heterozygote knockout mice. The authors interpret their data to suggest increased pharmacological and rewarding effects of alcohol in the heterozygote knockout mice. However, several issues should be considered in the interpretation of home-cage drug consumption results from genetically modified mice. First, as discussed above when a gene is deleted or partially deleted throughout development, it is unknown whether the observed changes in behavior are reflective of a given gene's normal role in behavior or some unknown compensatory changes that result from the gene deletion. Second, genetic differences in alcohol consumption may be due to differences in drug metabolism in the genetically modified mice, an issue that was not addressed by Hensler et al. (2003). Third, increased drug intake may reflect a decrease in the drug rewarding effects (Yokel, 1987). While this possibility is rarely considered in the alcohol field, Olive et al. (2003) reported that genetic deletion of corticotropin-releasing factor (CRF) results in a phenotype that consumes twice as much alcohol as wild-type mice but, unlike the wild-type, the CRF-deficient mice were insensitive to the rewarding effects of alcohol in the CPP procedure and the locomotor stimulant effects of alcohol.
In an impressive series of studies, Dorit Ron and colleagues reported data that are consistent with the notion that activation of BDNF signaling pathways in the dorsal striatum decreases alcohol intake and reward. McGough et al. (2004) reported that acute alcohol injections (2 mg/kg) increased BDNF expression in hippocampus and dorsal striatum but not PFC. They also reported increased alcohol CPP and home-cage intake in BDNF heterozygote knockout mice. These CPP data, together with the alcohol intake data, suggest that increased alcohol intake in the heterozygote BDNF knockout mice likely reflects increased alcohol reward. Another main finding in McGough et al. (2004) study is that BDNF effects on alcohol intake are mediated by the scaffolding protein, RACK1 (Yaka et al., 2003). In a subsequent study, Jeanblanc et al. (2006) reported that the inhibitory effect of BDNF-TrkB signaling (via activation of RACK1) on both home-cage alcohol intake and operant alcohol self-administration involves dopamine D3-receptor activation in the dorsal striatum. Additionally, Logrip et al. (2008) used a striatal slice preparation to perform mechanistic experiments that their results suggest that the inhibitory effect of BDNF on alcohol intake also involves activation of the extracellular-regulated kinase (ERK) signaling pathway and the endogenous opioid dynorphin. A question raised by Logrip et al. (2008) study is whether this molecular mechanism identified in striatal slices exposed to alcohol is also operating in the dorsal striatum of the intact rat or mouse. In this regard, missing pieces of evidence to support Logrip et al. (2008) dorsal striatum cellular model (p. 2400) is that local injections of BDNF, kappa opioid receptor agonists, and ERK antagonists decrease alcohol intake.
Most recently, Jeanblanc et al. (2009) provided initial critical evidence to support the model of Logrip et al. (2008). They reported that alcohol self-administration increases BDNF expression in the dorsolateral striatum and that locally decreasing endogenous BDNF by viral-mediated siRNA increases alcohol self-administration. Additionally, they reported that local BDNF injections (3 h before the test sessions) profoundly decrease alcohol self-administration. Jeanblanc et al. (2009) also studied the role of BDNF in the dorsomedial striatum. They reported that alcohol self-administration increases BDNF expression in the dorsomedial striatum but to a lesser degree than in the dorsolateral striatum. Additionally, while dorsomedial striatum BDNF injections mimicked the effect of dorsolateral striatum injections on alcohol self-administration, this was not the case with the viral-mediated siRNA manipulation to decrease endogenous BDNF expression. Taken together, the authors' findings indicate that alcohol-induced activation of endogenous BDNF in the dorsolateral striatum results in inhibition of alcohol self-administration.
2.C. Extinction, reinstatement, and incubation
Cocaine
Results from several studies indicate that mesocorticolimbic BDNF plays an important role in cocaine craving and relapse, as assessed in rat models. In an initial study on the mechanisms of incubation of cocaine craving, we reported that the time-dependent increase in cue-induced cocaine seeking after withdrawal (incubation) is associated with time-dependent increases in BDNF protein expression in VTA, accumbens, and amygdala (Grimm et al., 2003). Based on these findings, we injected BDNF acutely into VTA and assessed cue-induced cocaine seeking in extinction tests after 3, 10 or 30 withdrawal days (Lu et al., 2004a). We found that these BDNF injections enhanced responding for cocaine cues for up to 30 d after cessation of cocaine self-administration, an effect that was reversed by the MEK inhibitor U0126, which blocks ERK activity (phosphorylation). The finding of reversal of BDNF effects by U0126 is consistent with results from previous studies on the role of ERK signaling in BDNF physiological effects (Poo, 2001). We also found that acute VTA BDNF injections 2 h before testing had no effect on cue-induced cocaine seeking (Lu et al., 2004a), suggesting that BDNF effects on responding to cues involve stable long-term synaptic alterations rather than acute effects on synaptic transmission or short-term plasticity.
Graham et al. (2007) reported that 5-daily accumbens BDNF injections during training, which increased cocaine self-administration (see section 2.B.), also potently increase extinction responding and subsequent reinstatement of cocaine seeking induced by cocaine priming injections, discrete cues previously paired with cocaine injections, or a footshock stressor. In contrast, accumbens injections of anti-BDNF antibody during training had effects in the opposite direction. The recent findings of Bahi et al. (2008) are in agreement with those of Graham et al. (2007). They reported that lentiviral gene delivery to over-express accumbens BDNF and TrkB (see section 2.B) increases resistance to extinction of previously acquired cocaine CPP (suggesting enhanced responding to cocaine cues) and also increases cocaine-priming-induced reinstatement of the extinguished cocaine CPP.
In apparent contrast with the findings of Graham et al. (2007) and Bahi et al. (2008), Berglind et al. (2007) reported that mPFC BDNF injections, which increased BDNF levels in accumbens a day later [presumably via anterograde transport (Altar et al., 1997)], decrease extinction responding after 1 or 6 withdrawal days, and also decrease discrete-cue- and cocaine-priming-induced reinstatement after 6 withdrawal days. These behavioral findings were replicated by Berglind et al. (2009) in a study in which they showed that the mPFC BDNF injections also prevented cocaine self-administration-induced reduction in basal extracellular glutamate, as well as cocaine priming-induced increases in accumbens extracellular glutamate levels. Based on previous studies on the role of accumbens glutamate transmission in cocaine-priming-induced reinstatement (Baker et al., 2003; Cornish and Kalivas, 2000), the authors suggested that these effects of mPFC BDNF injections mediate the inhibitory effect of this manipulation on cocaine seeking (McGinty et al., 2009).
The reasons for the potentially discrepant findings between Berglind et al. (2007) results and those of Graham et al. (2007) and Bahi et al. (2008) results are unknown. One possibility is that the release of BDNF into the accumbens following mPFC injections in the study by Berglind et al. (2007) more closely mimic spatially localized, activity-dependent release of BDNF relative to the pharmacological or viral manipulations made by Graham et al. (2007) and Bahi et al. (2008) directly into the accumbens. Furthermore, a methodological issue in these three studies, and in our study (Lu et al., 2004a), is that it is unknown whether the effect of exogenous injections of local high doses of BDNF mimics cocaine-induced neuroplasticity of endogenous BDNF systems (Grimm et al., 2003). In this regard, however, the data of Graham et al. (2007) of opposite effects of accumbens injections of BDNF versus anti-BDNF antibody and the AAV vector manipulation to knockdown BDNF expression are very important for interpreting BDNF's roles in cocaine-seeking behaviors. This is because the anti-BDNF antibody and the viral vector interfere with the ability of endogenous BDNF to regulate these behaviors, indicating a role of endogenous BDNF in cocaine seeking.
2.D. Conclusions
Drug reward
The data reviewed above indicate that BDNF within the mesocorticolimbic dopamine system is a positive modulator of psychostimulant and opiate reward. In the VTA, BDNF-TrkB transmission contributes to cocaine and morphine CPP (Graham et al., 2009; Vargas-Perez et al., 2009). In the accumbens, BDNF-TrkB transmission contributes to both cocaine CPP and cocaine self-administration (Bahi et al., 2008; Graham et al., 2007; Graham et al., 2009), In the hippocampus, BDNF-TrkB transmission contributes to amphetamine CPP (Shen et al., 2006). In contrast, published data that BDNF within the mesocorticolimbic dopamine system contributes to alcohol reward do not exist. The data reviewed, however, suggest that central BDNF likely serves as a negative modulator of alcohol reward (Hensler et al., 2003; McGough et al., 2004) and that the critical brain site is the dorsal striatum (Jeanblanc et al., 2006; Jeanblanc et al., 2009; Logrip et al., 2008). A question for future research is the reasons for the opposite modulatory effects of BDNF on psychostimulant and opiate reward versus alcohol reward.
One possibility is that this is due to the opposite effects of BDNF on reward in the mesoaccumbens versus the nigrostriatal pathway; as mentioned above, BDNF signaling is associated with increased cocaine reward in the accumbens and decreased alcohol reward in the dorsal striatum. This possibility is unlikely for two reasons. First, in BDNF heterozygote knockout mice, alcohol reward is increased and cocaine reward is decreased (Hall et al., 2003; Hensler et al., 2003). Second, Graham et al. (2007) reported that the same BDNF injection procedure that in the accumbens increased cocaine self-administration did not have an opposite effect in the dorsal striatum where BDNF injections were merely ineffective. A more likely possibility for the drug-specific opposite role of BDNF is that differences in BDNF modulation of psychostimulant and opiate reward versus alcohol reward reflect differences in the neurobiological substrates underlying the rewarding effects of these drugs. In this regard, while there is strong evidence that the mesocorticolimbic dopamine system is critical for psychostimulant reward (Pierce and Kumaresan, 2006; Roberts et al., 1980; Wise and Rompre, 1989), this is not the case for alcohol reward. Results from several studies demonstrate normal alcohol intake after mesocorticolimbic dopamine lesions or dopamine receptor blockade (Amit and Brown, 1982; Goodwin et al., 1996; Rassnick et al., 1993).
Drug relapse
Activation of BDNF-TrkB signaling in both the VTA and accumbens strongly potentiates extinction responding, and reinstatement induced by cocaine priming, cocaine cue, and intermittent footshock stress (Graham et al., 2007; Lu et al., 2004a). An important characteristic of this effect is that it is time-dependent: the effect of acute BDNF injections on cocaine seeking begins to manifest several days after the injections and last for several weeks. In contrast, at least in the VTA, acute BDNF injections 2 h prior to an extinction test for cue-induced cocaine seeking are ineffective (Lu et al., 2004a). The enhancing effect of activation of BDNF-TrkB signaling in the accumbens on extinction responding and reinstatement by cocaine priming is also observed in the CPP procedure (Bahi et al., 2008). In contrast to the VTA and accumbens, mPFC BDNF injections decrease cocaine seeking, potentially via the effect of these injections on glutamate transmission in the accumbens (Berglind et al., 2007; Berglind et al., 2009). An adequate explanation for this region-specific effect of BDNF injections on cocaine seeking does not exist and this state of affairs is particularly surprising, because the major source of accumbens BDNF is from the mPFC (Altar et al., 1997).
Finally, the observation that the time-dependent increases in cue-induced cocaine seeking (incubation) are associated with time-dependent increases in BDNF in mesocorticolimbic areas (Grimm et al., 2003), that acute BDNF VTA and accumbens injections cause long-lasting potentiation of cocaine seeking (Graham et al., 2007; Lu et al., 2004a), and that inhibition of accumbens BDNF signaling decreases cocaine seeking (Graham et al., 2007), support the notion that cocaine-induced BDNF-mediated long-term neuroplasticity contributes to cocaine craving and relapse (Thomas et al., 2008). Based on the data of Pu et al. (2006) that VTA BDNF contributes to cocaine-induced long-term potentiation (LTP) in VTA neurons, it is tempting to speculate that cocaine-induced BDNF-mediated synaptic plasticity in VTA dopamine neurons causes enhanced responsiveness of these neurons to drugs, drug cues, or stress. In this regard, VTA neuronal activity is critical for relapse to drug seeking induced by drug priming, drug cues, or intermittent footshock stress (Bossert et al., 2004; Stewart, 1984; Wang et al., 2005).
3. GDNF
Over the last several decade investigators have assessed the role of GDNF in drug reward and relapse, as assessed in animal models. The results from these studies are summarized in Table 2 and are discussed below.
Table 2.
Effect of experimental manipulations of GDNF function on drug-taking behavior.
| Drug | Experimental procedure | Behavioral measure | Brain area | Reference |
|---|---|---|---|---|
| Cocaine | GDNF cDNA-AAV injection | Extinction responding ▲ | VTA | (Lu et al., 2009) |
| GDNF injection | Extinction responding ▲ | VTA | ||
| Anti-GDNF injection | Extinction responding ▼ | VTA | ||
| A human astrocyte-like cell line that produces and excretes GDNF | SA drug intake (initiation) ▼ | Striatum and NAc | (Green-Sadan et al., 2003) | |
| GDNF chronic delivery (minipump) | Striatum-NAc border | |||
| GDNF-conjugated nanoparticles injection | SA drug intake (initiation) ▼ | Striatum | (Green-Sadan et al., 2005) | |
| SA dose response ▼ | ||||
| GDNF chronic delivery (minipump) | CPP ▼ | VTA | (Messer et al., 2000) | |
| Anti-GDNF chronic delivery (minipump) | CPP ▲ | VTA | ||
| GDNF knockout | CPP ▲ | Not applicable | (Messer et al., 2000) (Niwa et al., 2007b) | |
| Morphine | GDNF knockout | CPP ▲ | Not applicable | (Niwa et al., 2007b) |
| GDNF knockout | CPP ─ | Not applicable | (Airavaara et al., 2007) | |
| CPP maintenance ▼ | ||||
| Methampheta mine | GDNF knockout | CPP ▼ | Not applicable | (Niwa et al., 2007b) |
| GDNF knockout | SA intake and PR ▲ | Not applicable | (Yan et al., 2007) | |
| Cue reinstatement ▲ | ||||
| Priming reinstatement ▲ | ||||
| Alcohol | GDNF injection | SA ▼ | VTA | (Carnicella et al., 2008) |
| SA Reacquisition ▼ | (He et al., 2005) | |||
| Escalated home-cage intake ▼ | (Carnicella et al., 2009c) | |||
| Anti-GDNF injection | SA ─ | VTA | (He et al., 2005) | |
| GDNF knockout | CPP ▲ | Not applicable | (Carnicella et al., 2009b) | |
| Home-cage intake ─ | ||||
| Home-cage intake after deprivation ▲ | ||||
Abbreviations: SA: self-administration; CPP: conditioned place preference; NAc: nucleus accumbens; VTA: ventral tegmental area; PR: progressive ratio. Symbols: ▲, increase; ▼, decrease; ─, no effect.
3.A. Conditioned place preference
Cocaine and methamphetamine
In an initial study on the role of GDNF in cocaine CPP, Messer et al. (2000) reported that heterozygote GDNF knockout mice demonstrated increased sensitivity to cocaine CPP: the minimal effective dose in the knockout mice and the wild-type mice was 5 and 10 mg/kg, respectively. Additionally, they reported that chronic delivery of GDNF via minipumps into the VTA decreased cocaine CPP while local chronic delivery of GDNF neutralizing antibody increased cocaine-induced CPP. Messer et al. (2000) also reported that cocaine exposure decreased the phosphorylation of Ret (the protein kinase mediating GDNF signaling) in the VTA. They interpreted their results to suggest that cocaine exposure decreases GDNF signaling in the VTA, which causes increased sensitivity to subsequent drug exposure. An issue to consider with this interpretation is that a large body of research indicates that exogenous administration of GDNF into midbrain dopamine cell body areas (substantia nigra and VTA) facilitates both local and striatal dopamine-mediated synaptic transmission and increases spontaneous and psychostimulant-induced locomotor activity (Bourque and Trudeau, 2000; Hebert et al., 1996; Hebert and Gerhardt, 1997; Hudson et al., 1995; Martin et al., 1996). Thus, based on these previous findings and the literature on the role of midbrain dopamine in drug reward (Wise, 2004) it is surprising that a manipulation that typically increases VTA dopamine transmission (GDNF administration) decreases cocaine CPP. However, Messer et al. (2000) reported that under their experimental conditions, GDNF VTA injections reversed cocaine-induced increases in the local expression of tyrosine hydroxylase (TH, the rate-limiting enzyme for dopamine synthesis), and the glutamate receptor subunit NMDAR1, which its activation increases dopamine release in terminal areas (Westerink et al., 1996). These effects of GDNF should lead to reversal of cocaine-induced increases in dopamine transmission and consequently to decreased cocaine CPP.
The role of GDNF in methamphetamine CPP was assessed in several studies by Nabeshima and colleagues (Niwa et al., 2007b). They reported that a low dose of methamphetamine (0.3 mg/kg) caused CPP in GDNF heterozygote mice but not in wild-type mice (Niwa et al., 2007c). Additionally, they reported that repeated Leu-Ile exposure, which leads to increased GDNF mRNA expression in the NAc, decreases methamphetamine CPP (Niwa et al., 2007c). Leu-Ile is a hydrophobic di-peptide that up-regulates GDNF expression by activating signal transdcution through the heat shock protein 90/Akt/cyclic adenosine 3′, 5′-monophosphate response element binding protein (Cen et al., 2006). The brain sites involved in the effects of Leu-Ile on methamphetamine CPP are unknown. Additionally, there are interpretation issues with these studies, including the use of conventional knockout mice (see discussion of this issue in Section 2), and the fact that it cannot be ruled out that the effect of Leu-Ile are mediated by non-GDNF mechanisms. Thus, in the absence of additional studies in which GDNF function is directly manipulated the observation that Leu-Ile increases GDNF expression and decrease methamphetamine CPP does not necessary implies that increased GDNF expression causes decreased drug CPP.
Morphine
The results of the studies on the role of GDNF on morphine CPP are mixed. Niwa et al. (2007a) reported enhanced morphine (3 mg/kg) CPP in heterozygote GDNF knockout mice. In contrast, Airavaara et al. (2007) reported that morphine (5 or 10 mg/kg) CPP was similar in heterozygote GDNF knockout mice and wild-type mice. They also reported shorter retention of the expression of morphine CPP after training in the knockout mice, an effect potentially related to subtle learning deficits in these mice (Gerlai et al., 2001). The reasons for the different results of Niwa et al. (2007a) versus Airavaara et al. (2007) may be due to the different drug doses and the use of unbalanced (morphine injections in the non-preferred side in Niwa et al. study) versus balanced (morphine injections in both non-preferred and the preferred side in Airavaara et al. study) CPP procedures. Finally, Niwa et al. (2007a) reported that as in the case of methamphetamine CPP (see above) repeated Leu-Ile exposure, which leads to increased GDNF mRNA expression in the NAc, decreases morphine CPP (Niwa et al., 2007c). As discussed above, the degree to which these findings implicate GDNF in the behavioral effects of Lue-Ile is unknown.
Alcohol
In a recent study, Carnicella et al. (2009b) reported that GDNF or GDNF family receptor-α1 (GFRα1, Fig. 1 and Box 2) heterozygote mice demonstrate stronger alcohol (1.8 g/kg, i.p) CPP than wild-type mice. The heterozygote mice did not differ from the wild-type mice in their response to the locomotor stimulating effects of alcohol.
Figure 1.
General overview of BDNF and GDNF signaling mechanisms. GDNF binds to GFRα1 and activates Ret tyrosine kinase receptor. Alternatively, GDNF can activate the neural adhesion molecule (NCAM) signaling pathway. BDNF physiological effects are mediated through the signaling cascades of the trkB and the p75 neurotrophin receptor.
3.B. Drug self-administration
Cocaine and methamphetamine
Gal Yadid and colleagues reported that experimental manipulations that increase GDNF expression in the dorsal striatum and NAc (injections/minipump delivery targeted both brain areas) decreased the acquisition of intravenous cocaine self-administration under limited access conditions (1 h per day) (Green-Sadan et al., 2003; Green-Sadan et al., 2005). In the first study, the experimental manipulations were striatal transplantation of simian virus-40 glial cell that produce GDNF or chronic delivery of GDNF via minipumps (Green-Sadan et al., 2003). In the second study, the experimental manipulation was striatal injections of GDNF-conjugated nanoparticles (Green-Sadan et al., 2005). However, interpretation of these data in reference to striatal GDNF's role in cocaine reward is not straightforward. Green-Sadan et al. (2003; 2005) used a fixed ratio 1 (FR1) reinforcement schedule and a cocaine dose (1 mg/kg) that is on the descending limb of the cocaine dose-response curve. Under these conditions, dopamine receptor agonists typically decrease cocaine-reinforced responding while dopamine receptor antagonists increase responding (Yokel, 1987). Thus, decreased cocaine self-administration after increasing striatal GDNF levels may reflect a leftward-shift in the dose-response-curve, or increases in cocaine rewarding effects. Another interpretation issue in the studies of Green-Sadan et al. (2003; 2005) is the site of action of GDNF, because these authors used a very high infusion volume (8 μL) and implanted cannulae in very close vicinity to the lateral ventricles (1.6 lateral to bregma).
Nabeshima and colleagues assessed GDNF's role in methamphetamine self-administration by using heterozygote GDNF knockout mice (Yan et al., 2007). They reported that, in these mice, the dose-response curve for self-administered methamphetamine is shifted upward and to the left and that responding on progressive-ratio reinforcement schedule is higher, suggesting enhanced methamphetamine reward in GDNF heterozygote knockout mice. It is unknown, however, how to interpret these data in reference to GDNF's normal role in psychostimulant reward, because of potential compensatory developmental changes. In this regard, a compensatory change that may be relevant to enhanced methamphetamine reward in GDNF heterozygote mice is increased striatal dopamine levels (Airavaara et al., 2004; Airavaara et al., 2007).
Alcohol
In several comprehensive studies, Dorit Ron and colleagues have explored the role of GDNF in operant alcohol self-administration and in alcohol home-cage consumption. Carnicella et al. (2009b) reported that over a range of alcohol concentrations (2.5 to 20%) GDNF and GFRα1 heterozygote mice and wild-type mice did not differ in their home-cage alcohol intake or preference (alcohol versus water) during 4-h daily sessions. However, both types of heterozygote mice consumed higher amounts of alcohol when it became available after 7 days of abstinence. Thus, under the authors' experimental conditions the alcohol deprivation effect--the increased in alcohol consumption after a period of abstinence (Sinclair and Senter, 1967; Sinclair, 1972)--was only observed in GDNF and GFRα1 heterozygote mice. In control experiments, Carnicella et al. (2009b) demonstrated that saccharin or quinine intake was similar in GDNF and GFRα1 heterozygote mice and wild-type mice. These data suggest that GDNF is a negative modulator of alcohol consumption. However, an interpretation issue with these data is that the typical alcohol deprivation effect (Le and Shaham, 2002) was only observed in the heterozygote mice but not in the wild-type mice, a finding that questions the findings generality.
In three studies Ron and colleagues demonstrated that the VTA is a critical site for the negative modulatory effect of GDNF on alcohol self-administration and home-cage alcohol consumption (Carnicella and Ron, 2009). In the first study, He et al. (2005) reported that VTA GDNF injections (5 μg/μl) 10 min before an ethanol self-administration session decreased limited access (1 h) operant alcohol self-administration. Additionally, they reported that the drug ibogaine, which decreases alcohol self-administration after systemic or VTA injections, increases GDNF VTA expression and that chronic delivery via minipumps of anti-GDNF monoclonal antibodies into the VTA reverses ibogaine's systemic effect on alcohol self-administration. However, a question that remained unresolved from this study is the role of endogenous GDNF in VTA in alcohol self-administration, because 14 days of chronic delivery of anti-GDNF monoclonal antibodies into the VTA had no effect on alcohol self-administration.
In the second study, Carnicella et al. (2008) reported that VTA, but not substantia nigra, GDNF injections (5 or 10 μg/μl) decrease operant alcohol self-administration, and reacquisition of the operant response for alcohol after extinction of the drug-reinforced responding. This acute inhibitory effect of GDNF occurred when GDNF was injected into the VTA either 10 min or 3 h prior to the self-administration sessions. The authors also reported that acute VTA GDNF injections increase ERK activity (phosphorylation) and that injections of U0126, which inhibits ERK activity, reverse the inhibitory effect of GDNF on alcohol self-administration. In contrast, VTA injections of PI3K inhibitor wortmannin or the PLC inhibitor U73122 did not reverse the effect of GDNF on alcohol self-administration. These data suggest that inhibition of alcohol self-administration by GDNF is mediated by the ERK signaling pathway, but not the PLC or the PI3K pathways.
In the third study, Carnicella et al. (2009c) assessed the effect of VTA GDNF injections on alcohol intake in a home-cage alcohol drinking procedure in which repeated periods of deprivation lead to escalated alcohol intake (Wise, 1973). They reported that VTA injections of GDNF (10 μg) 10 min before the alcohol intake sessions (24 h) reduced escalated alcohol consumption in the alcohol escalation model developed by Wise (1973). This effect was most pronounced in the first 30 min of the sessions. Finally, Carnicella et al. (2009a) recently reported evidence suggesting that, as in the case of ibogaine (see above) the inhibitory effect of the D2-family receptor agonist cabergoline on alcohol intake is likely mediated by GDNF signaling in the VTA.
3.C. Extinction, reinstatement, and incubation
Cocaine and methamphetamine
In a comprehensive study, Yan et al. (2007) assessed methamphetamine self-administration (data discussed above), extinction responding, and reinstatement induced by discrete infusion cues, and methamphetamine priming injections in GDNF heterozygote knockout mice and wild-type mice. The drug priming test (0.2 to 3 mg/kg) was performed once after extinction, and the cue tests were performed after initial extinction training, and 3 and 6 months after extinction; before each late cue test the rats underwent several extinction sessions. The authors reported that both the priming- and cue-induced reinstatement were significantly more pronounced in the GDNF heterozygote knockout mice than in the wild-type mice.
In a recent study, we assessed the role of VTA GDNF in incubation of cocaine craving in rats that were trained to self-administered cocaine for 6 h/d for 10 days and tested for cue-induced cocaine seeking in extinction tests after 3-4, 10-11, or 30 days of withdrawal from the drug (Lu et al., 2009). We found that VTA injections of an AAV vector containing rat GDNF cDNA (but not red fluorescent protein) on withdrawal day 1 increased cue-induced cocaine-seeking on withdrawal days 11 and 31. Additionally, VTA, but not substantia nigra, GDNF injections (12.5 μg/side) immediately after the last cocaine self-administration session increased cue-induced drug seeking on withdrawal days 3 and 10; this effect was reversed by local inhibition of ERK activity by U0126. Finally, interfering with VTA GDNF function by chronic delivery of anti-GDNF monoclonal neutralizing antibodies via minipumps during withdrawal days 1-14 prevented the time-dependent increases in cue-induced cocaine-seeking on withdrawal days 11 and 31. We interpret these data to indicate that during the first weeks of withdrawal from cocaine self-administration GDNF-dependent neuroadaptations in midbrain VTA neurons play an important role in the development of incubation of cocaine craving.
It is unlikely that our results are due to non-specific effects of GDNF VTA on spontaneous locomotor activity (Gash et al., 1998), because our experimental manipulations had had no effect on inactive lever responding, a putative measure of nonspecific increases in spontaneous activity and/or response generalization (Shalev et al., 2002). Additionally, our VTA GDNF injections had no effect on spontaneous locomotor activity in a nondrug context (Lu et al., 2009). It is also unlikely that GDNF diffusion to nearby brain areas or axonal transport to distal brain areas can account for our data. We found that GDNF injections into the substantia nigra had no effect on cue-induced cocaine seeking and that our viral GDNF delivery was localized to the VTA and was not transported to substantia nigra or the NAc (Lu et al., 2009).
In reconciling the differences between the knockout mice data of Yan et al. (2007) and our data two main issues should be considered. First, the constituent GDNF knockout manipulation changes GDNF function in the entire brain (and periphery) and affects multiple systems in addition to the VTA neurons that we studied. Second, as mentioned above, compensatory changes in GDNF heterozygote mice, including increased striatal dopamine levels (Airavaara et al., 2004; Airavaara et al., 2007), may alter drug-seeking behaviors independent of the normal role GDNF in these behaviors.
3.D. Conclusions
Drug reward
The data reviewed above lead to the surprising conclusion that GDNF--a neurotrophic factor that is critical for the survival and function of midbrain dopamine neurons (Airaksinen and Saarma, 2002) that are known to be involved in drug reward (Wise, 2004)--is a negative modulator of psychostimulant and alcohol reward. GDNF heterozygote knockout mice demonstrate increased alcohol intake (Carnicella et al., 2009b; Hensler et al., 2003), cocaine CPP (Messer et al., 2000), methamphetamine CPP and self-administration (Niwa et al., 2007c; Yan et al., 2007), and morphine CPP (Niwa et al., 2007a), but see (Airavaara et al., 2007). A critical site for the inhibitory effect of GDNF on drug reward is the VTA where local GDNF injections decrease cocaine CPP (Messer et al., 2000), alcohol self-administration (Carnicella et al., 2008; He et al., 2005), and alcohol home-cage consumption (Carnicella et al., 2009c). The studies of Ron and colleagues indicate that the ERK signaling pathway plays a critical role in the inhibitory effect of GDNF on alcohol self-administration and intake (Carnicella and Ron, 2009). There is also less definitive evidence for a role of the striatum in GDNF effects on cocaine self-administration. Yadid and colleagues reported that increased striatal GDNF expression by transplantation of simian virus-40 glial cell that produce GDNF, chronic delivery of GDNF via minipumps, and GDNF-conjugated nanoparticles led to decreased cocaine intake (Green-Sadan et al., 2003; Green-Sadan et al., 2005). However, as discussed above, because a single high cocaine dose was used in these studies it is unknown whether decreased cocaine intake by increasing striatal GDNF expression reflects an increase or a decrease in cocaine rewarding effects.
Drug relapse
The data on the role of GDNF in drug relapse, as assessed in rodent models, is mixed. Yan et al. (2007) reported that the magnitude of cue- and methamphetamine-priming-induced reinstatement is stronger in GDNF heterozygote knockout mice than in wild-type mice. In contrast, we found that VTA GDNF injections or local viral over-expression of GDNF strongly potentiate the time-dependent increases in cue-induced cocaine seeking (incubation of cocaine craving) while chronic delivery of anti-GDNF monoclonal antibodies into the VTA has an opposite effect.
A question that deserves discussion is the reasons of the different effects of manipulations of GDNF on the initiation of cocaine self-administration (Green-Sadan et al., 2003; Green-Sadan et al., 2005) and cocaine CPP (Messer et al., 2000) versus cue-induced cocaine seeking after withdrawal (incubation of craving). In reconciling these differences several issues should be considered. One issue is that Messer et al. (2000) and Green-Sadan et al. (2003; 2005) assessed the role of mesolimbic GDNF in the initial rewarding effects of cocaine, as assessed by the CPP procedure or by a limited-access (1-h) drug self-administration procedure, respectively. In these studies rats were exposed to small amounts of daily cocaine (1.25-10 mg/kg, i.p. or ∼12 mg/kg, i.v) and GDNF function was manipulated before and during drug exposure. In contrast, we assessed VTA GDNF's role in cue-induced cocaine seeking in rats given extended access to cocaine (6 h/d) that leads to substantially larger daily cocaine intake (∼45 mg/kg, i.v.) (Lu et al., 2009); we also manipulated GDNF function after withdrawal from cocaine rather than prior to or during exposure to cocaine. In this regard, there is evidence for differences in the mechanisms underlying the acute initial rewarding effects of cocaine versus those underlying cue-induced drug seeking after withdrawal from the drug (Kalivas and Volkow, 2005; Shaham et al., 2003; Shalev et al., 2002). Additionally, extended daily cocaine exposure leads to drug-taking patterns and drug-induced neuroadaptations that are not observed under conditions of limited access (Ahmed et al., 2005; Bozarth and Wise, 1985; Mutschler et al., 2000; Vanderschuren and Everitt, 2004).
Finally, an issue to consider in reconciling the different behavioral effects of VTA GDNF on incubation of cocaine craving versus alcohol self-administration is the time of GDNF injections in reference to the behavioral assessments. Thus, in the studies of Ron and colleagues (Carnicella et al., 2008; He et al., 2005), acute effects of GDNF were studies as GDNF was injected 10 min to 3 h prior to the test sessions. In contrast, in our study (Lu et al., 2009), delayed effects of GDNF were studied as the GDNF was injected acutely 3 to 10 days prior to the test sessions. Thus, while there are many other differences among these studies one possibility for their different results is that acute and delayed GDNF injections have opposite effects on drug-taking behaviors. In this regard, as RET receptors are also located on VTA GABAergic neurons (Sarabi et al., 2001), a potential mechanism for the inhibitory effect of acute GDNF injections on drug-taking behavior is activation of inhibitory GABAergic neurons. In contrast, the delayed effects of GDNF would consist of increased midbrain and striatal dopamine transmission (Hebert et al., 1996; Hebert and Gerhardt, 1997; Hudson et al., 1995; Martin et al., 1996), leading to increased responding to cocaine cues in our incubation studies.
4. Concluding remarks
We reviewed results from studies on the role of BDNF and GDNF in drug reward and relapse, as assessed in rat models. Our main conclusion is that whether BDNF or GDNF would facilitate or inhibit drug-taking behaviors is dependent on the drug type, the brain site, the addiction phase (initiation, maintenance, or abstinence/relapse), and the time interval between site-specific BDNF or GDNF injections and the reward- and relapse-related behavioral assessments. This is an unexpected conclusion, because both neurotrophic factors provide trophic support to midbrain dopamine neurons (Airaksinen and Saarma, 2002; Chao, 2003), which play a critical role in drug reward (Wise, 2004; Wise, 2009) and relapse (Bossert et al., 2005; Schmidt et al., 2005; Self, 2004; Weiss, 2005). Nonetheless, divergent and often opposite results were obtained after manipulations of BDNF and GDNF signaling and function in midbrain dopamine neurons and their terminal projection regions.
In the case of alcohol, activating BDNF or GDNF signaling consistently decreases alcohol self-administration and alcohol intake, but the brain sites involved in these effects are different: the VTA for GDNF and the dorsal striatum for BDNF (Carnicella and Ron, 2009; Jeanblanc et al., 2009; Logrip et al., 2008). Interestingly, in both cases activation of the ERK pathway plays a critical role in the effect of the neurotrophic factors on alcohol self-administration and intake (Carnicella et al., 2008; Logrip et al., 2008). In the case of cocaine, an unexpected potential conclusion is that VTA BDNF and GDNF have opposite effects on the initial cocaine rewarding effects: facilitation by BDNF (Bahi et al., 2008; Graham et al., 2007; Graham et al., 2009) and inhibition by GDNF (Messer et al., 2000). On the other hand, at least in wild-type rats, after withdrawal of self-administered cocaine, BDNF and GDNF actions in the VTA both potentiate cocaine seeking via activation of the ERK pathway (Lu et al., 2004a; Lu et al., 2009). However, in terminal mesocorticolimbic dopamine regions BDNF's actions in the accumbens potentiate cocaine seeking (Graham et al., 2007), while BDNF actions in the mPFC have opposite effects (McGinty et al., 2009). As mentioned above, these opposite effects of BDNF are particularly surprising, because the mPFC is the major source of NAc BDNF (Altar et al., 1997).
Despite many years of research, a key question that for the most part has remained unanswered is what role endogenous BDNF and GDNF in mesocorticolimbic dopamine areas (and other brain areas) play in drug reward and relapse. This is a largely open question, because in only a few cases (Graham et al., 2007; Graham et al., 2009; Jeanblanc et al., 2009; Lu et al., 2009; Messer et al., 2000) conclusions are based on studies in which the experimental manipulations (e.g., anti-BDNF or anti-GDNF blocking antibodies, or viral-mediated site-specific decreases in BDNF or TrkB expression) targeted endogenous BDNF or GDNF. Most of the studies reviewed above involved either constituent knockout gene deletion that can lead to compensatory developmental changes or exogenous administration of BDNF or GDNF that may or may not mimic the normal or drug-induced physiological effects of the neurotrophic factors.
Another unresolved question is whether BDNF and GDNF signaling is a viable target for medication development for drug addiction, as suggested by several authors (Carnicella and Ron, 2009; Graham et al., 2007; Niwa et al., 2007b). In our view, however, it is unlikely that this approach would lead to effective treatments for drug addiction, especially with systemic drug administration and for addicts who use more than one drug (e.g., heroin + cocaine or nicotine + alcohol). As the effects of BDNF and GDNF administration on drug-taking behavior is drug-specific, brain-site specific, and time-dependent, it is unlikely that a single small molecule that targets TrkB, RET or other BDNF or GDNF signal transduction mechanisms can serve as an effective medication for the treatment of drug addiction.
Finally, as both BDNF and GDNF are known to be involved in synaptic and structural plasticity (Chao, 2003; Poo, 2001), a question for future research is what role these neurotrophic factors play in drug-induced synaptic plasticity changes that potentially contribute to drug-taking behaviors. These include, among others, cocaine-induced LTP and LTD in VTA and accumbens (Jones and Bonci, 2005; Mameli et al., 2009; Thomas et al., 2001), drug-induced experience-dependent structural plasticity of dendrites and dendritic spines in mesocorticolimbic dopamine areas (Robinson and Kolb, 1997; Robinson and Kolb, 2004; Russo et al., 2009b; Shen et al., 2009), drug- and cue-induced activation of the ERK signaling pathway in the accumbens and amygdala (Girault et al., 2007; Lu et al., 2006), and long-lasting drug-induced changes in glutamate receptor expression (Conrad et al., 2008; Wolf et al., 2004) and synaptic and non-synaptic transmission in the accumbens (Baker et al., 2003; Kalivas, 2004).
Supplementary Material
Figure 2.
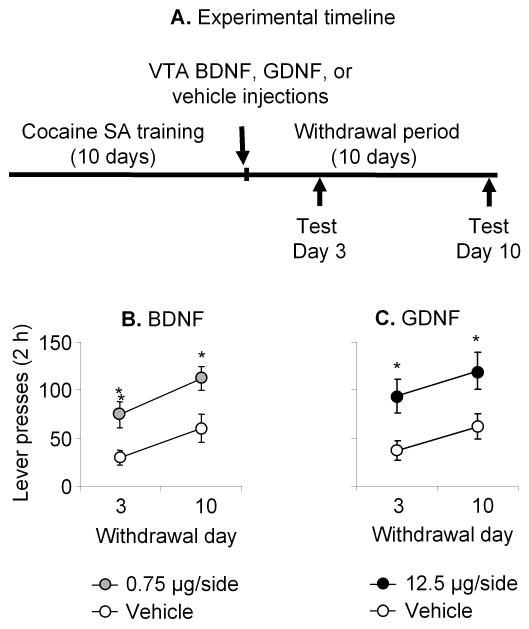
Ventral tegmental area (VTA) injections of BDNF or GDNF potentiate cue-induced cocaine seeking after withdrawal from the drug. Data are mean±SEM responses per 2 h on the previously active lever during extinction tests for cue-induced cocaine-seeking that were performed on withdrawal days 3 and 10. During the test sessions, cocaine was not available and lever presses resulted in the delivery of the tone-light cue previously paired with cocaine injections. Vehicle, BDNF or GDNF was injected bilaterally into the VTA 1-2 h after the last training session. (A) Timeline of the experiment, (B-C) VTA vehicle, BDNF, or GDNF injections. * Different from Vehicle condition, p< 0.05. SA, self-administration. Data were redrawn from Lu et al. (2004a; 2009).
Acknowledgments
This work was supported by the National Basic Research Program of China (2009CB522004) and the Natural Science Foundation of China (No: 30725016) and the Intramural Program of NIDA, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH, Lutjens R, van der Stap LD, Lekic D, Romano-Spica V, Morales M, Koob GF, Repunte-Canonigo V, Sanna PP. Gene expression evidence for remodeling of lateral hypothalamic circuitry in cocaine addiction. Proc Natl Acad Sci U S A. 2005;102:11533–8. doi: 10.1073/pnas.0504438102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–94. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- Airavaara M, Planken A, Gaddnas H, Piepponen TP, Saarma M, Ahtee L. Increased extracellular dopamine concentrations and FosB/DeltaFosB expression in striatal brain areas of heterozygous GDNF knockout mice. Eur J Neurosci. 2004;20:2336–44. doi: 10.1111/j.1460-9568.2004.03700.x. [DOI] [PubMed] [Google Scholar]
- Airavaara M, Tuomainen H, Piepponen TP, Saarma M, Ahtee L. Effects of repeated morphine on locomotion, place preference and dopamine in heterozygous glial cell line-derived neurotrophic factor knockout mice. Genes Brain Behav. 2007;6:287–98. doi: 10.1111/j.1601-183X.2006.00260.x. [DOI] [PubMed] [Google Scholar]
- Altar CA, Boylan CB, Jackson C, Hershenson S, Miller J, Wiegand SJ, Lindsay RM, Hyman C. Brain-derived neurotrophic factor augments rotational behavior and nigrostriatal dopamine turnover in vivo. Proc Natl Acad Sci U S A. 1992;89:11347–11351. doi: 10.1073/pnas.89.23.11347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsay RM, Wiegand SJ. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–60. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- Altar CA, DiStefano PS. Neurotrophin trafficking by anterograde transport. Trends Neurosci. 1998;21:433–7. doi: 10.1016/s0166-2236(98)01273-9. [DOI] [PubMed] [Google Scholar]
- Amit Z, Brown ZW. Actions of drugs of abuse on brain reward systems: a reconsideration with specific attention to alcohol. Pharmacol Biochem Behav. 1982;17:233–238. doi: 10.1016/0091-3057(82)90075-2. [DOI] [PubMed] [Google Scholar]
- Andressoo JO, Saarma M. Signalling mechanisms underlying development and maintenance of dopamine neurons. Curr Opin Neurobiol. 2008;18:297–306. doi: 10.1016/j.conb.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Bahi A, Boyer F, Dreyer JL. Role of accumbens BDNF and TrkB in cocaine-induced psychomotor sensitization, conditioned-place preference, and reinstatement in rats. Psychopharmacology (Berl) 2008;199:169–82. doi: 10.1007/s00213-008-1164-1. [DOI] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Bechara A, Nader K, van der Kooy D. A two-separate-motivational-systems hypothesis of opioid addiction. Pharmacol Biochem Behav. 1998;59:1–17. doi: 10.1016/s0091-3057(97)00047-6. [DOI] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Izquierdo I, Medina JH. BDNF and memory formation and storage. Neuroscientist. 2008;14:147–56. doi: 10.1177/1073858407305850. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, See RE, Fuchs RA, Ghee SM, Whitfield TW, Jr, Miller SW, McGinty JF. A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats. Eur J Neurosci. 2007;26:757–66. doi: 10.1111/j.1460-9568.2007.05692.x. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, Whitfield TW, Jr, LaLumiere RT, Kalivas PW, McGinty JF. A single intra-PFC infusion of BDNF prevents cocaine-induced alterations in extracellular glutamate within the nucleus accumbens. J Neurosci. 2009;29:3715–9. doi: 10.1523/JNEUROSCI.5457-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berhow MT, Russell DS, Terwilliger RZ, Beitner-Johnson D, Self DW, Lindsay RM, Nestler EJ. Influence of neurotrophic factors on morphine- and cocaine-induced biochemical changes in the mesolimbic dopamine system. Neuroscience. 1995;68:969–79. doi: 10.1016/0306-4522(95)00207-y. [DOI] [PubMed] [Google Scholar]
- Berhow MT, Hiroi N, Kobierski LA, Hyman SE, Nestler EJ. Influence of cocaine on the JAK-STAT pathway in the mesolimbic dopamine system. J Neurosci. 1996;16:8019–26. doi: 10.1523/JNEUROSCI.16-24-08019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bespalov MM, Saarma M. GDNF family receptor complexes are emerging drug targets. Trends Pharmacol Sci. 2007;28:68–74. doi: 10.1016/j.tips.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–56. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boger HA, Middaugh LD, Huang P, Zaman V, Smith AC, Hoffer BJ, Tomac AC, Granholm AC. A partial GDNF depletion leads to earlier age-related deterioration of motor function and tyrosine hydroxylase expression in the substantia nigra. Exp Neurol. 2006;202:336–47. doi: 10.1016/j.expneurol.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Bolanos CA, Nestler EJ. Neurotrophic mechanisms in drug addiction. Neuromolecular Med. 2004;5:69–83. doi: 10.1385/NMM:5:1:069. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Liu SY, Lu L, Shaham Y. A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J Neurosci. 2004;24:10726–10730. doi: 10.1523/JNEUROSCI.3207-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: An update and clinical implications. Eur J Pharmacol. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Bourque MJ, Trudeau LE. GDNF enhances the synaptic efficacy of dopaminergic neurons in culture. Eur J Neurosci. 2000;12:3172–3180. doi: 10.1046/j.1460-9568.2000.00219.x. [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. Toxicity associated with long-term intravenous heroin and cocaine self-administration in the rat. Jama. 1985;254:81–3. [PubMed] [Google Scholar]
- Carnicella S, Kharazia V, Jeanblanc J, Janak PH, Ron D. GDNF is a fast-acting potent inhibitor of alcohol consumption and relapse. Proc Natl Acad Sci U S A. 2008;105:8114–9. doi: 10.1073/pnas.0711755105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Ahmadiantehrani S, He DY, Nielsen CK, Bartlett SE, Janak PH, Ron D. Cabergoline decreases alcohol drinking and seeking behaviors via glial cell line-derived neurotrophic factor. Biol Psychiatry. 2009a;66:146–53. doi: 10.1016/j.biopsych.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Ahmadiantehrani S, Janak PH, Ron D. GDNF is an endogenous negative regulator of ethanol-mediated reward and of ethanol consumption after a period of abstinence. Alcohol Clin Exp Res. 2009b;33:1012–24. doi: 10.1111/j.1530-0277.2009.00922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Amamoto R, Ron D. Excessive alcohol consumption is blocked by glial cell line-derived neurotrophic factor. Alcohol. 2009c;43:35–43. doi: 10.1016/j.alcohol.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Ron D. GDNF--a potential target to treat addiction. Pharmacol Ther. 2009;122:9–18. doi: 10.1016/j.pharmthera.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cen X, Nitta A, Ohya S, Zhao Y, Ozawa N, Mouri A, Ibi D, Wang L, Suzuki M, Saito K, Ito Y, Kawagoe T, Noda Y, Ito Y, Furukawa S, Nabeshima T. An analog of a dipeptide-like structure of FK506 increases glial cell line-derived neurotrophic factor expression through cAMP response element-binding protein activated by heat shock protein 90/Akt signaling pathway. J Neurosci. 2006;26:3335–44. doi: 10.1523/JNEUROSCI.5010-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao CC, Ma YL, Chu KY, Lee EH. Integrin alphav and NCAM mediate the effects of GDNF on DA neuron survival, outgrowth, DA turnover and motor activity in rats. Neurobiol Aging. 2003;24:105–16. doi: 10.1016/s0197-4580(02)00047-7. [DOI] [PubMed] [Google Scholar]
- Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- Chao MV, Rajagopal R, Lee FS. Neurotrophin signalling in health and disease. Clin Sci (Lond) 2006;110:167–73. doi: 10.1042/CS20050163. [DOI] [PubMed] [Google Scholar]
- Chiocco MJ, Harvey BK, Wang Y, Hoffer BJ. Neurotrophic factors for the treatment of Parkinson's disease. Parkinsonism Relat Disord. 2007;13(Suppl 3):S321–8. doi: 10.1016/S1353-8020(08)70024-5. [DOI] [PubMed] [Google Scholar]
- Chu NN, Zuo YF, Meng L, Lee DY, Han JS, Cui CL. Peripheral electrical stimulation reversed the cell size reduction and increased BDNF level in the ventral tegmental area in chronic morphine-treated rats. Brain Res. 2007;1182:90–8. doi: 10.1016/j.brainres.2007.08.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008;33:18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit KC, Foster DA, Rosner MR. Protein kinase Cdelta mediates neurogenic but not mitogenic activation of mitogen-activated protein kinase in neuronal cells. Mol Cell Biol. 1999;19:4209–18. doi: 10.1128/mcb.19.6.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer H, Lange R, Christoph A, Plomann M, Vopper G, Roes J, Brown R, Baldwin S, Kraemer P, Scheff S, et al. Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature. 1994;367:455–9. doi: 10.1038/367455a0. [DOI] [PubMed] [Google Scholar]
- Crombag H, Bossert JM, Koya E, Shaham Y. Context-induced relapse to drug seeking: a review. Trans R Soc Lond B: Biol Sci. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology. 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Durbec PL, Larsson-Blomberg LB, Schuchardt A, Costantini F, Pachnis V. Common origin and developmental dependence on c-ret of subsets of enteric and sympathetic neuroblasts. Development. 1996;122:349–58. doi: 10.1242/dev.122.1.349. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology. 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JH, Moore RY. Catecholamine innervation of the basal forebrain. IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol. 1978;180:545–580. doi: 10.1002/cne.901800310. [DOI] [PubMed] [Google Scholar]
- Fisher CE, Michael L, Barnett MW, Davies JA. Erk MAP kinase regulates branching morphogenesis in the developing mouse kidney. Development. 2001;128:4329–38. doi: 10.1242/dev.128.21.4329. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Kiuchi K, Takahashi M. Novel mechanism of regulation of Rac activity and lamellipodia formation by RET tyrosine kinase. J Biol Chem. 2002;277:19114–21. doi: 10.1074/jbc.M200643200. [DOI] [PubMed] [Google Scholar]
- Gash DM, Gerhardt GA, Hoffer BJ. Effects of glial cell line-derived neurotrophic factor on the nigrostriatal dopamine system in rodents and nonhuman primates. Adv Pharmacol. 1998;42:911–5. doi: 10.1016/s1054-3589(08)60895-9. [DOI] [PubMed] [Google Scholar]
- Gerlai R, McNamara A, Choi-Lundberg DL, Armanini M, Ross J, Powell-Braxton L, Phillips HS. Impaired water maze learning performance without altered dopaminergic function in mice heterozygous for the GDNF mutation. Eur J Neurosci. 2001;14:1153–63. doi: 10.1046/j.0953-816x.2001.01724.x. [DOI] [PubMed] [Google Scholar]
- Girault JA, Valjent E, Caboche J, Herve D. ERK2: a logical AND gate critical for drug-induced plasticity? Curr Opin Pharmacol. 2007;7:77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Golden JP, Baloh RH, Kotzbauer PT, Lampe PA, Osborne PA, Milbrandt J, Johnson EM., Jr Expression of neurturin, GDNF, and their receptors in the adult mouse. CNS J Comp Neurol. 1998;398:139–50. doi: 10.1002/(sici)1096-9861(19980817)398:1<139::aid-cne9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Goodwin FL, Koechling UM, Smith BR, Amit Z. Lack of effect of dopamine D2 blockade on ethanol intake in selected and unselected strains of rats. Alcohol. 1996;13:273–279. doi: 10.1016/0741-8329(95)02055-1. [DOI] [PubMed] [Google Scholar]
- Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10:1029–37. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- Graham DL, Krishnan V, Larson EB, Graham A, Edwards S, Bachtell RK, Simmons D, Gent LM, Berton O, Bolanos CA, DiLeone RJ, Parada LF, Nestler EJ, Self DW. Tropomyosin-related kinase B in the mesolimbic dopamine system: region-specific effects on cocaine reward. Biol Psychiatry. 2009;65:696–701. doi: 10.1016/j.biopsych.2008.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm AC, Reyland M, Albeck D, Sanders L, Gerhardt G, Hoernig G, Shen L, Westphal H, Hoffer B. Glial cell line-derived neurotrophic factor is essential for postnatal survival of midbrain dopamine neurons. J Neurosci. 2000;20:3182–90. doi: 10.1523/JNEUROSCI.20-09-03182.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green-Sadan T, Kinor N, Roth-Deri I, Geffen-Aricha R, Schindler CJ, Yadid G. Transplantation of glial cell line-derived neurotrophic factor-expressing cells into the striatum and nucleus accumbens attenuates acquisition of cocaine self-administration in rats. Eur J Neurosci. 2003;18:2093–8. doi: 10.1046/j.1460-9568.2003.02943.x. [DOI] [PubMed] [Google Scholar]
- Green-Sadan T, Kuttner Y, Lublin-Tennenbaum T, Kinor N, Boguslavsky Y, Margel S, Yadid G. Glial cell line-derived neurotrophic factor-conjugated nanoparticles suppress acquisition of cocaine self-administration in rats. Exp Neurol. 2005;194:97–105. doi: 10.1016/j.expneurol.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–7. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FS, Drgonova J, Goeb M, Uhl GR. Reduced behavioral effects of cocaine in heterozygous brain-derived neurotrophic factor (BDNF) knockout mice. Neuropsychopharmacology. 2003;28:1485–90. doi: 10.1038/sj.npp.1300192. [DOI] [PubMed] [Google Scholar]
- Harvey BK, Hope BT, Shaham Y. Tolerance to opiate reward: role of midbrain IRS2-Akt pathway. Nat Neurosci. 2007;10:9–10. doi: 10.1038/nn0107-9. [DOI] [PubMed] [Google Scholar]
- He DY, McGough NN, Ravindranathan A, Jeanblanc J, Logrip ML, Phamluong K, Janak PH, Ron D. Glial cell line-derived neurotrophic factor mediates the desirable actions of the anti-addiction drug ibogaine against alcohol consumption. J Neurosci. 2005;25:619–28. doi: 10.1523/JNEUROSCI.3959-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert MA, Van Horne CG, Hoffer BJ, Gerhardt GA. Functional effects of GDNF in normal rat striatum: presynaptic studies using in vivo electrochemistry and microdialysis. J Pharmacol Exp Ther. 1996;279:1181–1190. [PubMed] [Google Scholar]
- Hebert MA, Gerhardt GA. Behavioral and neurochemical effects of intranigral administration of glial cell line-derived neurotrophic factor on aged Fischer 344 rats. J Pharmacol Exp Ther. 1997;282:760–768. [PubMed] [Google Scholar]
- Hensler JG, Ladenheim EE, Lyons WE. Ethanol consumption and serotonin-1A (5-HT1A) receptor function in heterozygous BDNF (+/-) mice. J Neurochem. 2003;85:1139–47. doi: 10.1046/j.1471-4159.2003.01748.x. [DOI] [PubMed] [Google Scholar]
- Hoffer BJ, Hoffman A, Bowenkamp K, Huettl P, Hudson J, Martin D, Lin LF, Gerhardt GA. Glial cell line-derived neurotrophic factor reverses toxin-induced injury to midbrain dopaminergic neurons in vivo. Neurosci Lett. 1994;182:107–11. doi: 10.1016/0304-3940(94)90218-6. [DOI] [PubMed] [Google Scholar]
- Horger BA, Iyasere CA, Berhow MT, Messer CJ, Nestler EJ, Taylor JR. Enhancement of locomotor activity and conditioned reward to cocaine by brain-derived neurotrophic factor. J Neurosci. 1999;19:4110–22. doi: 10.1523/JNEUROSCI.19-10-04110.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson J, Granholm AC, Gerhardt GA, Henry MA, Hoffman A, Biddle P, Leela NS, Mackerlova L, Lile JD, Collins F, et al. Glial cell line-derived neurotrophic factor augments midbrain dopaminergic circuits in vivo. Brain Res Bull. 1995;36:425–432. doi: 10.1016/0361-9230(94)00224-o. [DOI] [PubMed] [Google Scholar]
- Hunt WA, Barnett LW, Branch LG. Relapse rates in addiciton programs. J Clin Psychol. 1971;27:455–456. doi: 10.1002/1097-4679(197110)27:4<455::aid-jclp2270270412>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Jeanblanc J, He DY, McGough NN, Logrip ML, Phamluong K, Janak PH, Ron D. The dopamine D3 receptor is part of a homeostatic pathway regulating ethanol consumption. J Neurosci. 2006;26:1457–64. doi: 10.1523/JNEUROSCI.3786-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanblanc J, He DY, Carnicella S, Kharazia V, Janak PH, Ron D. Endogenous BDNF in the dorsolateral striatum gates alcohol drinking. J Neurosci. 2009;29:13494–502. doi: 10.1523/JNEUROSCI.2243-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing S, Wen D, Yu Y, Holst PL, Luo Y, Fang M, Tamir R, Antonio L, Hu Z, Cupples R, Louis JC, Hu S, Altrock BW, Fox GM. GDNF-induced activation of the ret protein tyrosine kinase is mediated by GDNFR-alpha, a novel receptor for GDNF. Cell. 1996;85:1113–24. doi: 10.1016/s0092-8674(00)81311-2. [DOI] [PubMed] [Google Scholar]
- Johnson AK, Epstein AN. The cerebral ventricles as the avenue for the dipsogenic action of intracranial angiotensin. Brain Res. 1975;86:399–418. doi: 10.1016/0006-8993(75)90891-4. [DOI] [PubMed] [Google Scholar]
- Jones PF, Jakubowicz T, Pitossi FJ, Maurer F, Hemmings BA. Molecular cloning and identification of a serine/threonine protein kinase of the second-messenger subfamily. Proc Natl Acad Sci U S A. 1991;88:4171–5. doi: 10.1073/pnas.88.10.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Bonci A. Synaptic plasticity and drug addiction. Curr Opin Pharmacol. 2005;5:20–5. doi: 10.1016/j.coph.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Glutamate systems in cocaine addiction. Curr Opin Pharmacol. 2004;4:23–29. doi: 10.1016/j.coph.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Gallegos RA, Henriksen SJ, van der Kooy D. Opiate state controls bi-directional reward signaling via GABAA receptors in the ventral tegmental area. Nat Neurosci. 2004;7:160–9. doi: 10.1038/nn1182. [DOI] [PubMed] [Google Scholar]
- Le A, Shaham Y. Neurobiology of relapse to alcohol in rats. Pharmacol Ther. 2002;94:137–156. doi: 10.1016/s0163-7258(02)00200-0. [DOI] [PubMed] [Google Scholar]
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–2. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Janak PH, Ron D. Dynorphin is a downstream effector of striatal BDNF regulation of ethanol intake. FASEB J. 2008;22:2393–404. doi: 10.1096/fj.07-099135. [DOI] [PubMed] [Google Scholar]
- Lu L, Shepard JD, Scott Hall F, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neurosci Biobehav Rev. 2003;27:457–491. doi: 10.1016/s0149-7634(03)00073-3. [DOI] [PubMed] [Google Scholar]
- Lu L, Dempsey J, Liu SY, Bossert JM, Shaham Y. A single infusion of brain-derived neurotrophic factor into the ventral tegmental area induces long-lasting potentiation of cocaine seeking after withdrawal. J Neurosci. 2004a;24:1604–11. doi: 10.1523/JNEUROSCI.5124-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004b;47(Suppl 1):214–26. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Lu L, Koya E, Zhai H, Hope BT, Shaham Y. Role of ERK in cocaine addiction. Trends Neurosci. 2006;29:695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Lu L, Wang X, Wu P, Xu C, Zhao M, Morales M, Harvey BK, Hoffer BJ, Shaham Y. Role of ventral tegmental area glial cell line-derived neurotrophic factor in incubation of cocaine craving. Biol Psychiatry. 2009;66:137–45. doi: 10.1016/j.biopsych.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Christian K, Lu B. BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol Learn Mem. 2008;89:312–23. doi: 10.1016/j.nlm.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthl A, Laurent JP, Figurov A, Muller D, Schachner M. Hippocampal long-term potentiation and neural cell adhesion molecules L1 and NCAM. Nature. 1994;372:777–9. doi: 10.1038/372777a0. [DOI] [PubMed] [Google Scholar]
- Mameli M, Halbout B, Creton C, Engblom D, Parkitna JR, Spanagel R, Luscher C. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat Neurosci. 2009;12:1036–41. doi: 10.1038/nn.2367. [DOI] [PubMed] [Google Scholar]
- Martin-Iverson MT, Todd KG, Altar CA. Brain-derived neurotrophic factor and neurotrophin-3 activate striatal dopamine and serotonin metabolism and related behaviors: interactions with amphetamine. J Neurosci. 1994;14:1262–1670. doi: 10.1523/JNEUROSCI.14-03-01262.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D, Miller G, Cullen T, Fischer N, Dix D, Russell D. Intranigral or intrastriatal injections of GDNF: effects on monoamine levels and behavior in rats. Eur J Pharmacol. 1996;317:247–256. doi: 10.1016/s0014-2999(96)00756-x. [DOI] [PubMed] [Google Scholar]
- McGinty JF, Whitfield TW, Jr, Berglind WJ. Brain-derived neurotrophic factor and addiction. Brain Res. 2009 doi: 10.1016/j.brainres.2009.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGough NN, He DY, Logrip ML, Jeanblanc J, Phamluong K, Luong K, Kharazia V, Janak PH, Ron D. RACK1 and brain-derived neurotrophic factor: a homeostatic pathway that regulates alcohol addiction. J Neurosci. 2004;24:10542–52. doi: 10.1523/JNEUROSCI.3714-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meil WM, See RE. Conditioned cued recovery of responding following prolonged withdrawal from self-administered cocaine in rats: an animal model of relapse. Behav Pharmacol. 1996;7:754–763. [PubMed] [Google Scholar]
- Messer CJ, Eisch AJ, Carlezon WA, Jr, Whisler K, Shen L, Wolf DH, Westphal H, Collins F, Russell DS, Nestler EJ. Role for GDNF in biochemical and behavioral adaptations to drugs of abuse. Neuron. 2000;26:247–57. doi: 10.1016/s0896-6273(00)81154-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucha RF, van der Kooy D, O'Shaughnessy M, Bucenieks P. Drug reinforcement studied by the use of place conditioning in rat. Brain Res. 1982;243:91–105. doi: 10.1016/0006-8993(82)91123-4. [DOI] [PubMed] [Google Scholar]
- Mueller D, Stewart J. Cocaine-induced conditioned place preference: reinstatement by priming injections of cocaine after extinction. Behav Brain Res. 2000;115:39–47. doi: 10.1016/s0166-4328(00)00239-4. [DOI] [PubMed] [Google Scholar]
- Mutschler NH, Miczek KA, Hammer RP., Jr Reduction of zif268 messenger RNA expression during prolonged withdrawal following “binge” cocaine self-administration in rats. Neuroscience. 2000;100:531–8. doi: 10.1016/s0306-4522(00)00298-0. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001a;2:119–28. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular neurobiology of addiction. Am J Addict. 2001b;10:201–17. doi: 10.1080/105504901750532094. [DOI] [PubMed] [Google Scholar]
- Niwa M, Nitta A, Shen L, Noda Y, Nabeshima T. Involvement of glial cell line-derived neurotrophic factor in inhibitory effects of a hydrophobic dipeptide Leu-Ile on morphine-induced sensitization and rewarding effects. Behav Brain Res. 2007a;179:167–71. doi: 10.1016/j.bbr.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Niwa M, Nitta A, Yamada K, Nabeshima T. The roles of glial cell line-derived neurotrophic factor, tumor necrosis factor-alpha, and an inducer of these factors in drug dependence. J Pharmacol Sci. 2007b;104:116–21. doi: 10.1254/jphs.cp0070017. [DOI] [PubMed] [Google Scholar]
- Niwa M, Nitta A, Yamada Y, Nakajima A, Saito K, Seishima M, Shen L, Noda Y, Furukawa S, Nabeshima T. An inducer for glial cell line-derived neurotrophic factor and tumor necrosis factor-alpha protects against methamphetamine-induced rewarding effects and sensitization. Biol Psychiatry. 2007c;61:890–901. doi: 10.1016/j.biopsych.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Numan S, Lane-Ladd SB, Zhang L, Lundgren KH, Russell DS, Seroogy KB, Nestler EJ. Differential regulation of neurotrophin and trk receptor mRNAs in catecholaminergic nuclei during chronic opiate treatment and withdrawal. J Neurosci. 1998;18:10700–8. doi: 10.1523/JNEUROSCI.18-24-10700.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, Mehmert KK, Koenig HN, Camarini R, Kim JA, Nannini MA, Ou CJ, Hodge CW. A role for corticotropin releasing factor (CRF) in ethanol consumption, sensitivity, and reward as revealed by CRF-deficient mice. Psychopharmacology. 2003;165:181–187. doi: 10.1007/s00213-002-1248-2. [DOI] [PubMed] [Google Scholar]
- Paratcha G, Ledda F, Ibanez CF. The neural cell adhesion molecule NCAM is an alternative signaling receptor for GDNF family ligands. Cell. 2003;113:867–79. doi: 10.1016/s0092-8674(03)00435-5. [DOI] [PubMed] [Google Scholar]
- Paratcha G, Ledda F. GDNF and GFRalpha: a versatile molecular complex for developing neurons. Trends Neurosci. 2008;31:384–91. doi: 10.1016/j.tins.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Pascual A, Hidalgo-Figueroa M, Piruat JI, Pintado CO, Gomez-Diaz R, Lopez-Barneo J. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat Neurosci. 2008;11:755–61. doi: 10.1038/nn.2136. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Bari AA. The role of neurotrophic factors in psychostimulant-induced behavioral and neuronal plasticity. Rev Neurosci. 2001;12:95–110. doi: 10.1515/revneuro.2001.12.2.95. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Post RM, Kopanda RT. Cocaine, kindling, and psychosis. Am J Psychiat. 1976;133:627–634. doi: 10.1176/ajp.133.6.627. [DOI] [PubMed] [Google Scholar]
- Povlsen GK, Berezin V, Bock E. Neural cell adhesion molecule-180-mediated homophilic binding induces epidermal growth factor receptor (EGFR) down-regulation and uncouples the inhibitory function of EGFR in neurite outgrowth. J Neurochem. 2008;104:624–39. doi: 10.1111/j.1471-4159.2007.05033.x. [DOI] [PubMed] [Google Scholar]
- Pu L, Liu QS, Poo MM. BDNF-dependent synaptic sensitization in midbrain dopamine neurons after cocaine withdrawal. Nat Neurosci. 2006;9:605–7. doi: 10.1038/nn1687. [DOI] [PubMed] [Google Scholar]
- Rassnick S, Stinus L, Koob GF. The effects of 6-hydroxydopamine lesions of the nucleus accumbens and the mesolimbic dopamine system on oral self-administration of ethanol in the rat. Brain Res. 1993;623:16–24. doi: 10.1016/0006-8993(93)90004-7. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The potentiation of conditioned reinforcement by psychomotor stimulant drugs: a test of Hill's hypothesis. Psychopharmacologia. 1975;45:103–114. [Google Scholar]
- Roberts DC, Koob GF, Klonoff P, Fibiger HC. Extinction and recovery of cocaine self-administration following 6-hydroxydopamine lesions of the nucleus accumbens. Pharmacol Biochem Behav. 1980;12:781–787. doi: 10.1016/0091-3057(80)90166-5. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci. 1997;17:8491–7. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Ron D, Janak PH. GDNF and addiction. Rev Neurosci. 2005;16:277–85. doi: 10.1515/revneuro.2005.16.4.277. [DOI] [PubMed] [Google Scholar]
- Routtenberg A. Reverse piedpiperase: is the knockout mouse leading neuroscientists to a watery end? Trends Neurosci. 1996;19:471–2. doi: 10.1016/S0166-2236(96)20051-7. [DOI] [PubMed] [Google Scholar]
- Roux PP, Barker PA. Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol. 2002;67:203–33. doi: 10.1016/s0301-0082(02)00016-3. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Bolanos CA, Theobald DE, DeCarolis NA, Renthal W, Kumar A, Winstanley CA, Renthal NE, Wiley MD, Self DW, Russell DS, Neve RL, Eisch AJ, Nestler EJ. IRS2-Akt pathway in midbrain dopamine neurons regulates behavioral and cellular responses to opiates. Nat Neurosci. 2007;10:93–9. doi: 10.1038/nn1812. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Mazei-Robison MS, Ables JL, Nestler EJ. Neurotrophic factors and structural plasticity in addiction. Neuropharmacology. 2009a;56(Suppl 1):73–82. doi: 10.1016/j.neuropharm.2008.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Wilkinson MB, Mazei-Robison MS, Dietz DM, Maze I, Krishnan V, Renthal W, Graham A, Birnbaum SG, Green TA, Robison B, Lesselyong A, Perrotti LI, Bolanos CA, Kumar A, Clark MS, Neumaier JF, Neve RL, Bhakar AL, Barker PA, Nestler EJ. Nuclear factor kappa B signaling regulates neuronal morphology and cocaine reward. J Neurosci. 2009b;29:3529–37. doi: 10.1523/JNEUROSCI.6173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarabi A, Hoffer BJ, Olson L, Morales M. GFRalpha-1 mRNA in dopaminergic and nondopaminergic neurons in the substantia nigra and ventral tegmental area. J Comp Neurol. 2001;441:106–17. doi: 10.1002/cne.1400. [DOI] [PubMed] [Google Scholar]
- Sariola H, Saarma M. Novel functions and signalling pathways for GDNF. J Cell Sci. 2003;116:3855–62. doi: 10.1242/jcs.00786. [DOI] [PubMed] [Google Scholar]
- Schmid RS, Maness PF. L1 and NCAM adhesion molecules as signaling coreceptors in neuronal migration and process outgrowth. Curr Opin Neurobiol. 2008;18:245–50. doi: 10.1016/j.conb.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Anderson SM, Famous KR, Kumaresan V, Pierce RC. Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. Eur J Pharmacol. 2005;526:65–76. doi: 10.1016/j.ejphar.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Schuster CR, Thompson T. Self administration of and behavioral dependence on drugs. Annu Rev Pharmacol. 1969;9:483–502. doi: 10.1146/annurev.pa.09.040169.002411. [DOI] [PubMed] [Google Scholar]
- See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav. 2002;71:517–529. doi: 10.1016/s0091-3057(01)00682-7. [DOI] [PubMed] [Google Scholar]
- Self DW, Barnhart WJ, Lehman DA, Nestler EJ. Opposite modulation of cocaine-seeking behavior by D1- and D2-like dopamine receptor agonists. Science. 1996;271:1586–9. doi: 10.1126/science.271.5255.1586. [DOI] [PubMed] [Google Scholar]
- Self DW. Regulation of drug-taking and -seeking behaviors by neuroadaptations in the mesolimbic dopamine system. Neuropharmacology. 2004;47(Suppl 1):242–255. doi: 10.1016/j.neuropharm.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Shen F, Meredith GE, Napier TC. Amphetamine-induced place preference and conditioned motor sensitization requires activation of tyrosine kinase receptors in the hippocampus. J Neurosci. 2006;26:11041–51. doi: 10.1523/JNEUROSCI.2898-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HW, Toda S, Moussawi K, Bouknight A, Zahm DS, Kalivas PW. Altered dendritic spine plasticity in cocaine-withdrawn rats. J Neurosci. 2009;29:2876–84. doi: 10.1523/JNEUROSCI.5638-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair JD, Senter RJ. Increased preference for ethanol in rats following alcohol deprivation. Psychonom Sci. 1967;8:11–16. [Google Scholar]
- Sinclair JD. The alcohol-deprivation effect. Influence of various factors. Q J Stud Alcohol. 1972;33:769–782. [PubMed] [Google Scholar]
- Sklair-Tavron L, Shi WX, Lane SB, Harris HW, Bunney BS, Nestler EJ. Chronic morphine induces visible changes in the morphology of mesolimbic dopamine neurons. Proc Natl Acad Sci U S A. 1996;93:11202–7. doi: 10.1073/pnas.93.20.11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spealman RD, Barrett-Larimore RL, Rowlett JK, Platt DM, Khroyan TV. Pharmacological and environmental determinants of relapse to cocaine-seeking behavior. Pharmacol Biochem Behav. 1999;64:327–336. doi: 10.1016/s0091-3057(99)00049-0. [DOI] [PubMed] [Google Scholar]
- Stewart J. Reinstatement of heroin and cocaine self-administration behavior in the rat by intracerebral application of morphine in the ventral tegmental area. Pharmacol Biochem Behav. 1984;20:917–923. doi: 10.1016/0091-3057(84)90017-0. [DOI] [PubMed] [Google Scholar]
- Stewart J, de Wit H. Reinstatement of drug-taking behavior as a method of assessing incentive motivational properties of drugs. In: Bozarth MA, editor. Methods of assessing the reinforcing properties of abused drugs. Vol. Springer-Verlag; New York: 1987. pp. 211–227. [Google Scholar]
- Taylor JR, Robbins TW. Enhanced behavioural control by conditioned reinforcers following microinjections of d-amphetamine into the nucleus accumbens. Psychopharmacology. 1984;84:405–412. doi: 10.1007/BF00555222. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci. 2001;4:1217–23. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol. 2008;154:327–42. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomac A, Widenfalk J, Lin LF, Kohno T, Ebendal T, Hoffer BJ, Olson L. Retrograde axonal transport of glial cell line-derived neurotrophic factor in the adult nigrostriatal system suggests a trophic role in the adult. Proc Natl Acad Sci U S A. 1995;92:8274–8. doi: 10.1073/pnas.92.18.8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treanor JJ, Goodman L, de Sauvage F, Stone DM, Poulsen KT, Beck CD, Gray C, Armanini MP, Pollock RA, Hefti F, Phillips HS, Goddard A, Moore MW, Buj-Bello A, Davies AM, Asai N, Takahashi M, Vandlen R, Henderson CE, Rosenthal A. Characterization of a multicomponent receptor for GDNF. Nature. 1996;382:80–3. doi: 10.1038/382080a0. [DOI] [PubMed] [Google Scholar]
- Trupp M, Arenas E, Fainzilber M, Nilsson AS, Sieber BA, Grigoriou M, Kilkenny C, Salazar-Grueso E, Pachnis V, Arumae U, Sariola H, Saarma M, Ibánez C. Functional receptor for GDNF encoded by the c-ret proto-oncogene. Nature. 1996;381:785–9. doi: 10.1038/381785a0. [DOI] [PubMed] [Google Scholar]
- Trupp M, Belluardo N, Funakoshi H, Ibanez CF. Complementary and overlapping expression of glial cell line-derived neurotrophic factor (GDNF), c-ret proto-oncogene, and GDNF receptor-alpha indicates multiple mechanisms of trophic actions in the adult rat. CNS J Neurosci. 1997;17:3554–67. doi: 10.1523/JNEUROSCI.17-10-03554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Alonso M, Bramham CR, Pozzo-Miller LD. From acquisition to consolidation: on the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn Mem. 2002;9:224–37. doi: 10.1101/lm.51202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerstedt U. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiol Scand. 1971;(Suppl 367):1–48. doi: 10.1111/j.1365-201x.1971.tb10998.x. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Alessi DR. The PI3K-PDK1 connection: more than just a road to PKB. Biochem J. 2000;346(Pt 3):561–76. [PMC free article] [PubMed] [Google Scholar]
- Vargas-Perez H, Kee RT, Walton CH, Hansen DM, Razavi R, Clarke L, Bufalino MR, Allison DW, Steffensen SC, van der Kooy D. Ventral tegmental area BDNF induces an opiate-dependent-like reward state in naive rats. Science. 2009;324:1732–4. doi: 10.1126/science.1168501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter ML, Martin-Zanca D, Parada LF, Bishop JM, Kaplan DR. Nerve growth factor rapidly stimulates tyrosine phosphorylation of phospholipase C-gamma 1 by a kinase activity associated with the product of the trk protooncogene. Proc Natl Acad Sci U S A. 1991;88:5650–4. doi: 10.1073/pnas.88.13.5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Fang Q, Liu Z, Lu L. Region-specific effects of brain corticotropin-releasing factor receptor type 1 blockade on footshock-stress- or drug-priming-induced reinstatement of morphine conditioned place preference in rats. Psychopharmacology (Berl) 2006;185:19–28. doi: 10.1007/s00213-005-0262-6. [DOI] [PubMed] [Google Scholar]
- Weeks JR. Experimental morphine addiction: Method for automatic intravenous injections in unrestrained rats. Science. 1962;143:143–144. doi: 10.1126/science.138.3537.143. [DOI] [PubMed] [Google Scholar]
- Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smit DL, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci (USA) 2000;97:4321–4326. doi: 10.1073/pnas.97.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F. Neurobiology of craving, conditioned reward and relapse. Curr Opin Pharmacol. 2005;5:9–19. doi: 10.1016/j.coph.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Westerink BH, Kwint HF, deVries JB. The pharmacology of mesolimbic dopamine neurons: a dual-probe microdialysis study in the ventral tegmental area and nucleus accumbens of the rat brain. J Neurosci. 1996;16:2605–2611. doi: 10.1523/JNEUROSCI.16-08-02605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikler A. Dynamics of drug dependence, implication of a conditioning theory for research and treatment. Arch Gen Psychiatry. 1973;28:611–616. doi: 10.1001/archpsyc.1973.01750350005001. [DOI] [PubMed] [Google Scholar]
- Wise RA. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia. 1973;29:203–10. doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Wise RA, Hoffman DC. Localization of drug reward mechanisms by intracranial injections. Synapse. 1992;10:247–63. doi: 10.1002/syn.890100307. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–94. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wise RA. Roles for nigrostriatal--not just mesocorticolimbic--dopamine in reward and addiction. Trends Neurosci. 2009;32:517–24. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Sun X, Mangiavacchi S, Chao SZ. Psychomotor stimulants and neuronal plasticity. Neuropharmacology. 2004;47(Suppl 1):61–79. doi: 10.1016/j.neuropharm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Yaka R, He DY, Phamluong K, Ron D. Pituitary adenylate cyclase-activating polypeptide (PACAP(1-38)) enhances N-methyl-D-aspartate receptor function and brain-derived neurotrophic factor expression via RACK1. J Biol Chem. 2003;278:9630–8. doi: 10.1074/jbc.M209141200. [DOI] [PubMed] [Google Scholar]
- Yan Y, Yamada K, Niwa M, Nagai T, Nitta A, Nabeshima T. Enduring vulnerability to reinstatement of methamphetamine-seeking behavior in glial-cell-line-derived neurotrophic factor mutant mice. Faseb J. 2007;21:1994–2004. doi: 10.1096/fj.06-7772com. [DOI] [PubMed] [Google Scholar]
- Yokel RA. Intravenous self-administration: Response rates, the effects of pharmacological challenges, and drug preference. In: Bozarth MA, editor. Methods of assessing the reinforcing properties of abused drugs. Vol. Springer-Verlag; New York: 1987. pp. 1–34. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.