Abstract
Toxoplasma gondii is a ubiquitous intracellular parasite which chronically infects 30 to 50% of the human population. While acquired infection is primarily asymptomatic several studies have suggested that such infections may contribute to neurological and psychiatric symptoms. Previous studies in rodents have demonstated that T. gondii infection does not just kill its host, but alters the behavioral repertoire of an infected animal making it more likely that predation with occur completing the parasite life cycle. The aim of the present study was to evaluate the behavioral changes in C57BL/6 mice chronically infected with the avirulent T. gondii (ME49, a type II strain), in a comprehensive test battery. Infected mice demonstrated profound and widespread brain pathology, motor coordination and sensory deficits. In contrast, cognitive function, anxiety levels, social behavior and the motivation to explore novel objects were normal. The observed changes in behavior did not represent “gross” brain damage or dysfunction and were not due to targeted destruction of specific areas of the brain. Such changes point out the subtle interaction of this parasite with its intermediate hosts and are consistent with ideas about increased predation being an outcome of infection.
Keywords: Toxoplasma gondii, latent infection, motor coordination, cognitive tests, social behavior, parasitology
1 INTRODUCTION
The protozoan Toxoplasma gondii is a definitive parasite of cats which has as intermediate hosts all warm blooded animals, including humans [1]. Worldwide, approximately 2 billion people are chronically infected with T. gondii and it has been estimated that T. gondii chronically infects 30 to 50% of the human population [2]. Infection is found throughout the world in the tissues of food animals [2]. While infection with T. gondii has generally thought to be primarily asymptomatic in immune competent humans, recent studies have suggested that infection may contribute to the development of various neurological and psychiatric symptoms [3-10] and that negative outcomes resulting from initial or chronic infection may be under-diagnosed [10-13]. These studies in humans are, however, associative and cannot prove if infection causes the behavior or the behavior increases the risk of infection. There are, however, several lines of evidence in experimental animals that suggest that specific host-parasite interactions influence the behavior of intermediate hosts in important ways and that these behavioral changes increase the likelihood of horizontal transmission [14-16]. Systematic analysis of the behavioral outcomes of infection in intermediate hosts may have implications for understanding and controlling the transmission of the parasite and also for elucidating the host-parasite interactions in regulating the specific outcomes of infection.
Toxoplasma gondii has several life cycle stages. The tachyzoite invades cells, replicates rapidly and results in dissemination of infection. The bradyzoite is found in latent infections as tissue cysts. The sporozoite is found in oocysts shed by cats and is the product of sexual reproduction. Infection can occur in intermediate hosts (including humans) due to the ingestion of tissue cysts, the ingestion of oocysts, or by congenital infection during acute infection in a pregnant host. There has been a clonal expansion of T. gondii lineages with three main lineages in the United States and Europe, Type I, Type II and Type III, which are defined by their ability to form cysts in mice (type II and III) and by their acute virulence (i.e. type I are lethal to mice during acute infection) [17].
Latent infection persists during the lifespan of the intermediate hosts by the formation of cysts in muscle, neurons and glia. In congenitally infected children, T gondii infection causes mental retardation, seizures and loss of vision. Reactivation of latent infection with the transformation of bradyzoites back into tachyzoites can occur in immune suppressed patients, such as individuals with AIDS, and can be fatal [18]. The central nervous system (CNS) is the most common site of such reactivation infections, resulting in Toxoplasmic encephalitis.
Any behavior in an intermediate hosts (i.e. rodents) that influences the likelihood of predation will increase the frequency of transmission to the definitive host (i.e. cats) facilitating completion of the sexual stage of the parasitic life cycle and the potential for recombination and reassortment of genetic loci [9, 14, 15, 19, 20]. Carnivorism of infected intermediate hosts, such as rodents, with subsequent oral-fecal transmission is thought to be the most prevalent route of transmission due to cats [21-23]. Several lines of evidence suggest that specific host-parasite interactions may alter the behavior of infected rodents and increase the risk of predation [14, 15]. Rodents infected with T. gondii [10-12] show reduced fear specifically toward feline predators that does not generalize to non-feline predators [16, 19, 24], which may lead to an augmented rate of predation and multiplication of the parasite through an increased number of life cycles. Chronically infected adult rodents show also show reduced anxiety and risk assessment [25]. Notably, the social withdrawal associated with sickness behavior in most rodents [26] is also not evident in rodents chronically infected with T. gondii [25, 27].
Though several studies demonstrate behavioral changes in rodents infected with T. gondii the precise effects of adult-acquired, chronic latent infection are still unclear. Many studies have primarily examined the behavioral and pathological effects of congenital infection [27, 28], and few studies have included behavioral assays in multiple behavioral domains. Furthermore, the parasite load, anatomical location of parasite stages and behavioral outcomes change during the course of the infection [29, 30] and interpretations of behavioral outcomes in the acute phase of infection are complicated by the high levels of tachyzoites in critical peripheral tissues, such as muscle, heart and lungs. The aim of the present study was to evaluate the behavioral changes in C57BL/6 mice chronically infected with the avirulent T. gondii strain ME49 (a type II strain), in a comprehensive test battery. The mice were tested 7 to 9 weeks after initial infection at a time when chronic infection has developed, as demonstrated by the presence of tissue cysts containing bradzyoties, and acute infection has resolved, as demonstrated by the absence of tachyzoites
2 MATERIALS AND METHODS
2.1 Subjects and infection protocol
Experiments were performed in accordance with the Institutional Animal Care and Use Committee of the Albert Einstein College of Medicine. Eight-week-old C57BL/6 male mice (Jackson Laboratories) were infected with 103 Toxoplasma gondii bradyzoites of the type II ME49 strain diluted in 200 μL of PBS (20 infected mice) and injected intraperitoneally or were injected only with PBS (10 control mice). A total of 10 of the infected mice died within 3 weeks after initial infection during the development of chronic infection. Mice were then housed at 5 animals per cage and behavioral testing began 7 weeks after infection. This time point was chosen for a variety of reasons. First, active parasite levels in critical peripheral organs (i.e. lungs) are absent by this time, making further deaths unlikely in surviving subjects. Second, parasite load in the brain is reasonably stable from this point in chronically infected mice [30]. The colony was documented to be free of a number of standard viral, bacterial, and parasitic agents including Mouse Hepatitis Virus (MHV), Epidemic Diarrhea of Infant Mice (rotavirus, EDIM), Theiler’s Murine Encephalomyelitis Virus (TMEV, GDVII), Mouse Parvovirus (MPV1)(MPV2) (VP2)general (NS1), Minute Virus of Mice (MMV), Mouse Adenovirus I and II (MAD), Ectromelia (Ectro), Lymphocytic Choriomeningitis Virus (LCM), Mycoplasma pulmonis (M. pulmonis), Pneumonia Virus of Mice (PVM), Reovirus (Reo), Sendai Virus (Sendai), Mouse Cytomegalovirus (MCMV), Mouse Norovirus (MNV), Ectoparasites (mites) by microscopic evaluation of fur plucks and Endoparasites (pinworms) by cecal and colon float and anal tape test.
2.2 Behavioral assays
Mice were tested behaviorally starting at 7 weeks after infection in the open field, object placement, object recognition, balance beam, grip strength, gait analysis and functional observation battery, in that order.
2.2.1 Functional observation battery
This primary screen was based on the SHIRPA protocol [31]. This is a standard method that provides a quantitative behavioral and functional profile by observational assessment of mice. Assessments were divided into five behavioral domains: general activity/arousal, motor and autonomic, reflexes, sensory and general condition. The details of each observation and list of tests are summarized in table 1. Most observations used an ordinal scale such that 0-1 represented normal or average behavior, negative numbers indicated deficits, hypoactivity or absence of normal behaviors and positive numbers indicate hyper-reactivity or higher incidence of abnormal behavior. Some tests could produce absolute, rather than ordinal values. Activity, for instance was assessed and number of grid crosses in 1 min. Table 1 (results) details these numerous assays and the units of measurement for each assay not employing an the ordinal scale.
Table 1. Functional Observation Battery.
Representative results from a primary screen indicate deficits in several behavioral domains in mice infected with T. gondii. An ordinal scale is used to categorize behaviors with normal defined as 0, hyopo-reactive behaviors or absence of normal behaviors as progressively negative numbers and hyper-reactivity or incidence of abnormal behaviors as positive numbers. Where absolute values could be measured (precise number of rears etc), those values and units are indicated in the table and are used in lieu of the ordinal scale. Probability (p) is based on statistical testing as described in the materials and methods section. NS indicates not significant.
| control | SE | T. gondii | SE | p | |||
|---|---|---|---|---|---|---|---|
| Activity / Arousal | |||||||
| Transfer Arousal (latency to move after transfer, sec) |
4.1 | ± | 0.7 | 3.1 | ± | 1.0 | NS |
| Rears (# in 1 min) | 6.2 | ± | 1.4 | 2.7 | ± | 1.4 | NS |
| Activity (grid crosses in 1 min) |
33.2 | ± | 5.5 | 19.1 | ± | 4.0 | NS |
| Touch escape | 1.2 | ± | 0.2 | 0.7 | ± | 0.2 | NS |
| Positional Passivity | 2.0 | ± | 0.0 | 1.0 | ± | 0.4 | 0.02 |
| Sensorimotor | |||||||
| Pelvic Elevation | 0.0 | ± | 0.0 | −0.7 | ± | 0.3 | 0.02 |
| Gait | −0.6 | ± | 0.2 | −0.8 | ± | 0.3 | NS |
| Tail elevation | 0.0 | ± | 0.0 | −2.0 | ± | 1.6 | 0.04 |
| Eye | 0.0 | ± | 0.0 | −0.7 | ± | 0.7 | 0.009 |
| Ataxia | 0.0 | ± | 0.0 | −1.5 | ± | 0.6 | 0.02 |
| Hind limb use | 0.0 | ± | 0.0 | −0.9 | ± | 0.4 | 0.05 |
| Whisker mobility | 0.0 | ± | 0.0 | −0.4 | ± | 0.2 | 0.05 |
| Grip (latency to fall from a wire in sec) |
24.4 | ± | 4.6 | 2.9 | ± | 3.9 | 0.005 |
| Reflexes | |||||||
| Toe pinch | 1.0 | ± | 0.0 | 1.0 | ± | 0.0 | NS |
| Negative Geotaxis (latency to turn upright after inversion on a grid in sec) |
1.55 | ± | 2.3 | 7.5 | ± | 2.1 | NS |
| Startle | 1.5 | ± | 0.3 | 0.7 | ± | 0.3 | NS |
| Visual Placing | 1.4 | ± | 0.4 | 0.0 | ± | 0.5 | NS |
| Whisker stimulation | 0.0 | ± | 0.0 | −1.0 | ± | 0.3 | 0.005 |
| General condition | |||||||
| Fur | 0.0 | ± | 0.0 | −1.1 | ± | 0.1 | 0.001 |
| Stereotyped Behavior | −0.2 | ± | 0.2 | −1.4 | ± | 0.3 | 0.008 |
| Weight (g) | 26.6 | ± | 1.0 | 17.5 | ± | 0.9 | 0.001 |
2.2.2 Open field
The open field has been extensively validated, ethologically and pharmacologically in both mice and rats [32]. Behavior was recorded manually for 6 min 16 inch square acrylic white box. Exploration was assessed as the number of rears (defined as raising both forepaws off the ground, except when grooming) and general locomotor activity was scored as the number of grid crosses (defined when the animal crossed a grid past the midline of the body).
2.2.3 Object Recognition and Object Placement Tests
Recognition and spatial memory were assessed in the novel object recognition and placement tasks, respectively essentially as described previously [33]. These tasks utilize the innate tendency of rodents to preferentially explore novel objects and are similar to tests conducted in humans [34, 35]. In the object recognition test mice were placed in the open field and allowed to freely explore two identical, nontoxic objects (e.g., plastic, glass, or ceramic items) and the duration of exploration of each object (in seconds) were recorded during a 3 min (trial 1). After a retention interval of 45 min, mice returned to the same open field for 3 min (trial 2) with one of the familiar objects and a novel object. The time spent exploring both the novel and familiar objects (in seconds) was recorded. Exploration of the objects was defined as any physical contact with an object (whisking, sniffing, rearing on or touching the object). These data can be represented an exploratory preference score (time spent exploring the novel object divided by the total time spent exploring both objects × 100). An exploratory preference score of 50% indicates chance performance, while scores higher than chance reflect intact memory. All the objects have been extensively validated previously to ensure that no intrinsic preferences or aversions exist and that the animals explore all the objects for similar durations (given the same trial duration).
Spatial memory was assessed in a similar way as described above. Mice were allowed to explore two identical objects for 5 min in the arena. High contrast visual cues were placed on the walls of the open field. After a retention interval of 15 min, mice were returned to the open field for 3 min with the objects in different placement within the box. Normal animals preferentially explore the displaced object. As in the object recognition task, an exploratory preference score of 50% indicates chance performance, while scores higher than chance reflect intact memory.
2.2.4 Balance beam
Motor coordination deficits were measured by the number of missteps and the latency to cross a round beam [36]. Before the test, all mice were pre-trained on a wide plank to encourage reliable crossing. The start side was brightly lit and the other site had a small, darkened chamber that contained a palatable food (chocolate cereal) to encourage the mice to cross. Mice were allowed to walk across two times so that they rapidly traversed the plank without reversals or stopping. Immediately after pre-training, animals were assessed on the test beam (2.5-cm diameter, 50-cm long).
2.2.5 Gait analysis
The footprint test was used to compare the gait of control mice with that of T. gondii infected mice. Before the test, mice received several pre-training trials during which they habituated to the runway (65-cm long, 8-cm wide, 15-cm high) to foster reliable crossing of the runway during the test. The start of the runway was brightly lit and the end contained a darkened enclosed chamber with Cocoa Krispies↗ cereal pieces. Immediately after the pre-training sessions, footprints were recorded on a white paper on the runway floor after applying non-toxic tempera paint to the paws. Front paws were painted red and rear paws blue. Mice were then allowed to walk down the runway, and 4 sets of footprints were collected for each animal. The footprint patterns were analyzed using the program Image J (NIH). Stride length was measured from the middle of one rear paw to the middle of the next footprint of the same paw (see fig 3D). Foot drags were counted as a total incidence in the set of 4 tracks, and were identified as smearing either before or after foot placement. The number of missteps was also analyzed as a total incidence in 4 sets of tracks and stringently defined as paw misplacement within an identifiable normal stride. The number of misplacements was likely under-estimated due to these strict criteria.
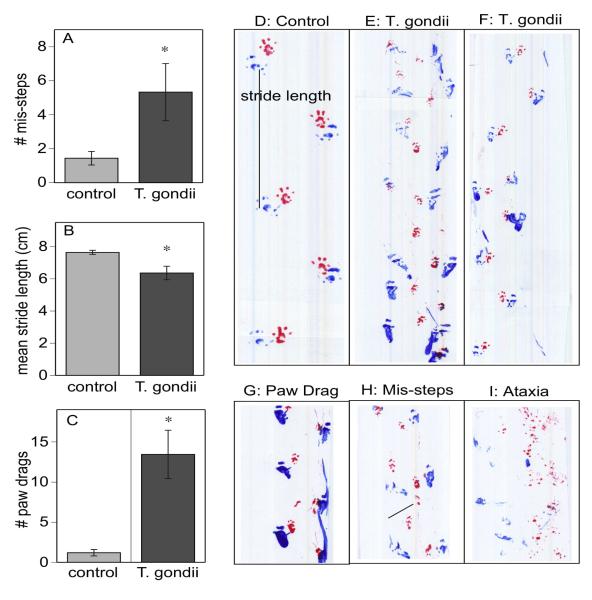
Figure 3.
Gait deficits assessed as the number of mis-steps (A) and the mean stride length (B) and the number of rear paw drags (C). * indicates significant differences between T. gondii infected and control mice, p< 0.05. Representative footprints of a control mouse (D) compared to T. gondii infected mice (E and F) clearly show examples of the shorter and more variable stride pattern, paw misplacements, weaving gait and other abnormalities. Examples of foot drag (G), paw misplacement (H), and gross ataxia (I) from T. gondii infected mice are also illustrated.
2.3 Histopathology
Five controls and ten infected mice were humanely euthanized using CO2 gas after the conclusion of behavioral testing. The brains were carefully removed and fixed in 10% neutral buffered formalin (Fisher Scientific). All brains were sectioned coronally and routinely processed to paraffin blocks. A 5 μm section of brain from each sample was taken at or close to 6 Bregma levels: 1.3 mm; 0 mm; −2.1 mm; −6.2 mm; −7 mm; 8.0 mm. Slides were routinely stained by hematoxylin and eosin and evaluated microscopically by a board certified veterinary pathologist (RSS). Brain samples were evaluated for the severity and distribution of inflammation, the numbers of cysts, tissue destruction/loss, edema, and cell death. Gross examination of muscle tissues demonstrated them to be normal. In our previous studies with T. gondii infection in C57BL/6 mice no pathology was seen in leg muscle tissue.
2.4 Statistical analysis
Data are displayed as means ± SEM. Statistical analysis was performed using JMP (SAS: Cary, NC) using ANOVA for all parametric data (open field, balance, beam, preference scores, gait etc). Welch’s correction was applied to means testing of the gait analysis data, as the variances were not equal. For all ordinal data from the functional observation battery, statistical analysis was performed using an ordinal logistic model.
Two infected animals had severe enough ataxia that it was not possible to accurately or reliably determine strides, and these were excluded from the gait analysis data. These two animals were also unable to rear and explore the objects in the cognitive tests without falling, stumbling and or circling, and were also excluded from analysis in these data.
3 RESULTS
3.1 Pathology
All levels of the brain and brainstem had some minimal to moderate lymphohistiocytic, plasmacytic, and rarely neutrophilic meningoencephalitis with gliosis. The cerebrum was more significantly affected than the brainstem and the lesions, which included glial nodules and rarefaction of brain parenchyma. Lesions in the brain and brainstem were multifocal and random. Intact protozoal cysts (T. gondii.) were present multifocally throughout many sections and were generally intact and unassociated with an inflammatory or cellular reaction. The least affected region tended to be the cerebellar folia, which occasionally had areas of gliosis and white matter loss, but which were generally normal in appearance by hematoxylin and eosin staining.
3.2 Functional Observation Battery
When assessed in a primary function screen [31], mice infected with T. gondii exhibited the most robust differences compared to control mice in sensory, motor and general condition domains (see table 1) . Measures of activity and arousal were generally normal except for significant differences in positional passivity (the struggling response to a tail hold). Most reflexes were grossly normal. In the sensorimotor domain, T. gondii-infected mice exhibited abnormal pelvic elevation, ataxia and poorer grip strength compared to controls. In addition, infected mice had deficits in visual placing, indicating poor visual acuity. Whisker mobility was abnormally low or absent in almost all T. gondii mice and infected mice exhibited a striking lack of orientation response to whisker stimulation. Body weight was lower in T. gondii mice, who also exhibited stereotyped behaviors, including retropulsion, tail dorsoflexion (Straub tail) and circling. When returned to the home cage, social behavior was monitored as normal (immediate exploration of, contact with or return to “huddle” with cage mates). All subjects immediately (under 20 sec) returned to cage mates and thus exhibited normal social behavior as measured by this assay (data not shown) consistent with previous reports of normal social interaction in chronically infected subject [25, 27].
3.3 Motor Coordination
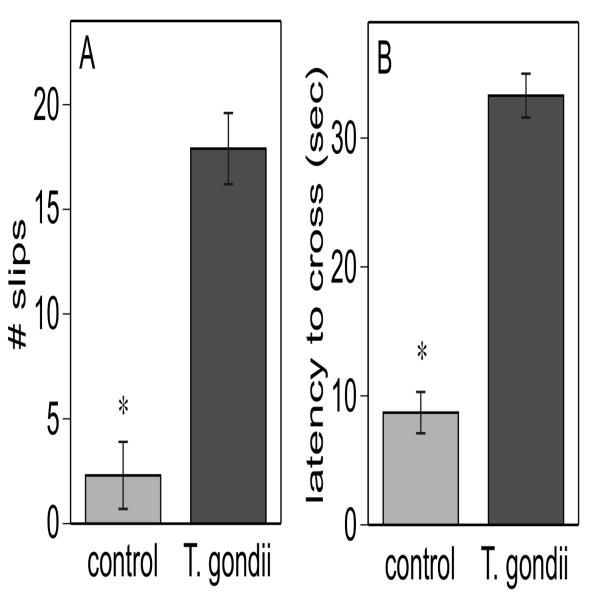
Motor coordination deficits were evident in both the balance beam assay and gait analysis. T. gondii mice had significantly more slips in the balance beam (fig 2A) and a significantly longer latency to cross the beam (fig 2B). It is notable that despite the motor difficulties, all infected animals continued attempting to cross the beam until they reached the end.
Figure 2.
Motor coordination deficits assessed as the number of slips (A) and the time to cross (B) a 120 cm long and 2.5 cm diameter beam. * indicates significant differences between T. gondii infected and control mice, p< 0.001.
Analysis of gait revealed multiple abnormal measures, including higher incidence of mis-steps (putting a paw down in the middle of a stride, fig 3A), shorter stride length (fig 3B), and higher incidence of foot dragging (fig 3C) in the rear paws. The incidence of foot dragging in the front paws was not significantly higher in infected mice compared to controls (data not shown). In addition to differences in absolute measures of stride length, there was also significantly more variability in stride length in T. gondii mice compared to controls.
3.4 Open Field
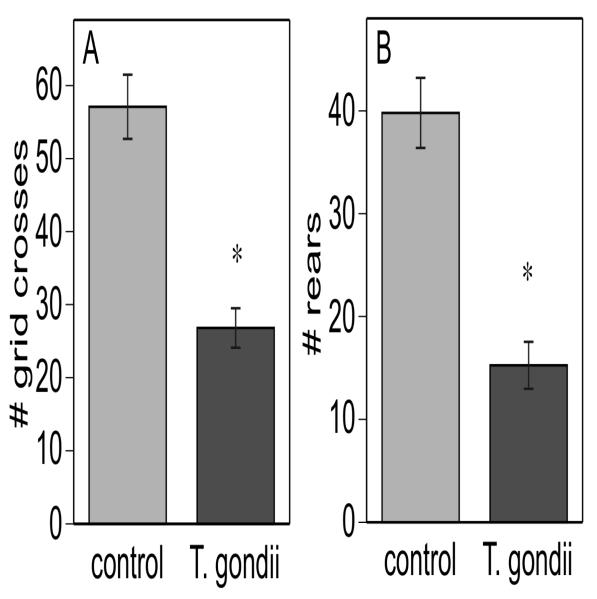
Despite the normal activity/arousal scores in the comparatively short (1 min) test period in the functional observation battery, further open field testing did reveal significant differences in activity (Fig 4A) and exploration (Fig 4B) between T. gondii and control mice. Several infected mice exhibited bouts of circling, which also effectively decreases mean number of grid crosses, whereas none of the control mice exhibited this stereotyped behavior. However, analysis of data without the circling mice included still yielded significant differences between T. gondii and control mice (data not shown). Although infected mice had fewer grid crosses, this was not because of long periods of immobility that reduced the apparent mean activity, but rather infected mice continued exploration for the total test period. Thus the activity difference was likely to be due to speed and coordination of movement and not to longer durations of inactivity. Even the severely ataxic animals continued to ambulate for the entire test period.
Figure 4.
Open field behavior in a 6 min test period indicates that T. gondii infected mice have lower levels of general locomotor activity (A: grid crosses) and exploratory activity (B: number of rears) and the number of rear paw drags. * indicates significant differences between T. gondii infected and control mice, p< 0.005.
The decreased rearing in infected mice is consistent with the almost total lack of whisking and lack of whisker orientation response demonstrated in the functional observation battery. Thus, though the animals are ambulating, they are doing so without the exploration (rearing, sniffing, whisking) that normally occurs in the open field.
3.5 Cognitive Function
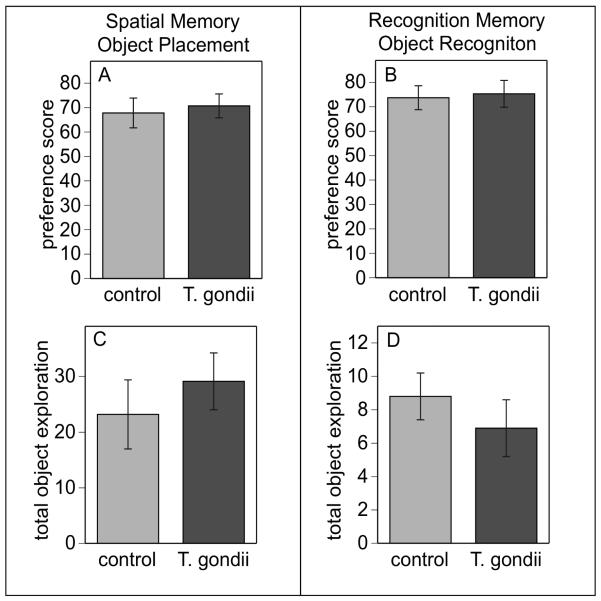
Notably, despite the profound deficits in sensorimotor function, infected mice had comparatively normal cognitive function. Infected mice had normal spatial memory (fig 5A) and normal levels of novel object exploration (fig 5C) in the object placement test after a 15 min retention interval and normal recognition memory (fig 5B) and object exploration (5D) in the object recognition assay after a 45 min retention interval.
Figure 5.
Cognitive deficits were not evident in T. gondii infected mice (Toxo) in either spatial memory (A) or in recognition memory (B) in the novel object recognition and object placement tasks. Extent of object exploration (sec) was also normal during the trial 1 of each task (D and E) in both T. gondii infected and control mice
4 DISCUSSION
The protozoan parasite T. gondii remains as a chronic, latent brain infection throughout the life of the host and may affect host behavior after the acute phase of infection [37]. The decreased activity and exploration during first exposure to the open field reported here (fig 4) is consistent with previous studies in mice with chronic latent infection [29, 38], but in contrast to studies reporting higher activity levels in congenitally infected mice or mice in acute phases of infection [39, 40]. The lack of rearing and whisking would be consistent with host-parasite interactions that increase the risk of predation, as these (and olfactory) behaviors are a primary means of gathering information for a nocturnal species with poor visual acuity.
The motor coordination deficits reported here are also largely consistent with previous reports [37, 41, 42] and with the decreased levels of dopamine and other catecholamines reported in the brains of infected mice [43]. Cysts were found throughout the brain consistent with previous studies [24], however, we did not appreciate an increase in cyst density in the amygdalar structures [24]. There were pathologic changes in the cerebellum and in all cortical regions that regulate sensorimotor function. It is possible that skeletal muscle pathology [44] is a contributing factor to the deficits we demonstrate in the balance beam (fig 2) and the gait analysis (fig 3). However, myopathy alone would not explain the lack of whisking and the fact that foot dragging was specific to the hind limbs, nor the high incidence of stereotypies, such as circling, which are more likely to be of central nervous system origin. In previous studies we had not seen significant muscle pathology, nor has this been seen by other investigators [24, 37]. In either case, the lack of motor coordination, including decreased stride length, indicative a slower speed of locomotion, would obviously be consistent with the risk of predation and with motor coordination deficits purported to occur in humans [10-12].
Although we found normal cognition (fig 5), some previous studies have demonstrated cognitive deficits in mice after infection with T gondii [45-47]. However, in these studies spatial learning was altered in a maze assay while memory was found to be intact [45, 46]. Rodents have also been demonstrated to lose their fear of cat urine (cat kariomones), which is a change in an innate hard-wired behavior unrelated to learning or memory [24,48] and to have a decrease in learned fear response [48]. The object recognition and placement tasks essentially only assess memory, so these data are not necessarily inconsistent. In addition, lower locomotor activity and poor motor coordination in infected mice may confound interpretations in maze assays where latency to make a correct choice is one of the primary measures and where lower levels of maze exploration prevents learning. It is thus difficult to distinguish between performance deficits and slower rates of learning. Furthermore, these studies require food deprivation or restriction. Given the significant differences in body weight between infected and control mice, there may confounding effects due to food deprivation effects on stamina or metabolism in infected mice evaluated using a maze assay. There is also a possibility that the cognitive tests used here were simply not sensitive enough or appropriate to detect cognitive deficits should these exist in infected mice. However, we have previously detected cognitive deficits at even shorter retention intervals using these tasks in subjects with no visible pathology [49] or with focal pathology [50] and in various other rodents models . It is also remarkable, given the gross sensorimotor deficits, the lack of normal exploratory activity and the profound and widespread central nervous system pathology evident in infected mice that they should have normal spatial and recognition memory at any retention interval or would in fact perform the task at all. This reinforces our observations of the lack of normal sickness behaviors in these mice, including lethargy, reduced response to novel objects and social withdrawal typical of sick or distressed rodents.
Overall, these studies demonstrate the complex and subtle interactions that have occurred in the evolution of the relationship of T. gondii with its intermediate hosts. The parasite does not just kill its host, but alters the behavioral repertoire of an infected animal making it more likely that predation with occur completing the parasite life cycle facilitating the transmission of genetic information to subsequent generations of parasites. The changes in behavior are specific and do not represent “gross” brain damage or dysfunction, but display specificity. This specificity, however, is not due to targeted destruction of specific areas of the brain, but a subtle dysregulation of brain function. The mechanisms by which T. gondii alters behavior deserve further investigation and may involve mimicking of neurotransmitters, or alternations or nitric oxide levels in the brain resulting in the modulation of signaling pathways.
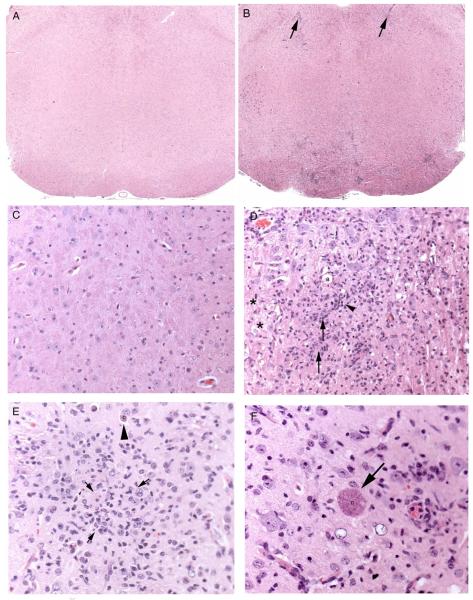
Figure 1.
Histological findings in brain stained with hematoxylin and eosin. (A) Normal brainstem (25x magnification). (B) Toxoplasma treated mouse brainstem. There is extensive lymphocytic perivascular inflammation (arrows) and focally extensive inflammation at the base of the brainstem (boxed area) (25x magnification). (C) Normal brainstem (200x magnification). (D) T. gondii infected mouse brainstem. There are nodules of glial cells (arrows), rarefaction of white matter (*), infiltrates of lymphocytes (arrow heads), and axonal swelling (a). (200x magnification). (E) T. gondii infected mouse cortex. There are glial cell aggregates (surrounded by arrows) and a protozoal cyst (large arrowhead) (200x magnification). (F) T. gondii infected mouse brainstem. There is a single large protozoal cyst (arrow) with no apparent inflammatory reaction (200x magnification).
Acknowledgements
This work was supported by NIH Grants AI39454 (LMW) and NIH D43TW007129 (MA) and NCI P30CA013330 to RS
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5 REFERENCES
- [1].Dubey JP. History of the discovery of the life cycle of Toxoplasma gondii. International Journal for Parasitology. 2009;39:877–882. doi: 10.1016/j.ijpara.2009.01.005. [DOI] [PubMed] [Google Scholar]
- [2].Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000;30:1217–1258. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Henriquez SA, Brett R, Alexander J, Pratt J, Roberts CW. Neuropsychiatric disease and Toxoplasma gondii infection. Neuroimmunomodulation. 2009;16:122–133. doi: 10.1159/000180267. [DOI] [PubMed] [Google Scholar]
- [4].Yolken RH, Dickerson FB, Torrey E. Fuller. Toxoplasma and schizophrenia. Parasite Immunol. 2009;31:706–715. doi: 10.1111/j.1365-3024.2009.01131.x. [DOI] [PubMed] [Google Scholar]
- [5].Carruthers VB, Suzuki Y. Effects of Toxoplasma gondii infection on the brain. Schizophr Bull. 2007;33:745–751. doi: 10.1093/schbul/sbm008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Flegr J. Effects of toxoplasma on human behavior. Schizophr Bull. 2007;33:757–760. doi: 10.1093/schbul/sbl074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Webster JP, Lamberton PH, Donnelly CA, Torrey EF. Parasites as causative agents of human affective disorders? The impact of anti-psychotic, mood-stabilizer and anti-parasite medication on Toxoplasma gondii’s ability to alter host behaviour. Proc Biol Sci. 2006;273:1023–1030. doi: 10.1098/rspb.2005.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Prandota J. Autism spectrum disorders may be due to cerebral toxoplasmosis associated with chronic neuroinflammation causing persistent hypercytokinemia that resulted in an increased lipid peroxidation, oxidative stress, and depressed metabolism of endogenous and exogenous substances. Research in Autism Spectrum Disorders In Press. 2009. Corrected Proof.
- [9].da Silva RC, Langoni H. Toxoplasma gondii: host-parasite interaction and behavior manipulation. Parasitol Res. 2009;105:893–898. doi: 10.1007/s00436-009-1526-6. [DOI] [PubMed] [Google Scholar]
- [10].Havlicek J, Gasova ZG, Smith AP, Zvara K, Flegr J. Decrease of psychomotor performance in subjects with latent ‘asymptomatic’ toxoplasmosis. Parasitology. 2001;122:515–520. doi: 10.1017/s0031182001007624. [DOI] [PubMed] [Google Scholar]
- [11].Flegr J, Klose J, Novotna M, Berenreitterova M, Havlicek J. Increased incidence of traffic accidents in Toxoplasma-infected military drivers and protective effect RhD molecule revealed by a large-scale prospective cohort study. BMC Infect Dis. 2009;9:72. doi: 10.1186/1471-2334-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yereli K, Balcioglu IC, Ozbilgin A. Is Toxoplasma gondii a potential risk for traffic accidents in Turkey? Forensic Sci Int. 2006;163:34–37. doi: 10.1016/j.forsciint.2005.11.002. [DOI] [PubMed] [Google Scholar]
- [13].Arendt G, Hefter H, Figge C, Neuen-Jakob E, Nelles HW, Elsing C, Freund HJ. Two cases of cerebral toxoplasmosis in AIDS patients mimicking HIV-related dementia. J Neurol. 1991;238 doi: 10.1007/BF00314650. [DOI] [PubMed] [Google Scholar]
- [14].Lyte M, Gaykema RPA, Goehler LE, Moselio S. Encyclopedia of Microbiology. Academic Press; Oxford: 2009. Behavior Modification of Host by Microbes; pp. 121–127. [Google Scholar]
- [15].Webster JP. Rats, cats, people and parasites: the impact of latent toxoplasmosis on behaviour. Microbes and Infection. 2001;3:1037–1045. doi: 10.1016/s1286-4579(01)01459-9. [DOI] [PubMed] [Google Scholar]
- [16].Lamberton PH, Donnelly CA, Webster JP. Specificity of the Toxoplasma gondii-altered behaviour to definitive versus non-definitive host predation risk. Parasitology. 2008;135:1143–1150. doi: 10.1017/S0031182008004666. [DOI] [PubMed] [Google Scholar]
- [17].Howe DK, Honore S, Derouin F, Sibley LD. Determination of genotypes of Toxoplasma gondii strains isolated from patients with toxoplasmosis. J Clin Microbiol. 1997;35:1411–1414. doi: 10.1128/jcm.35.6.1411-1414.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dubey JP. Strategies to reduce transmission of Toxoplasma gondii to animals and humans. Veterinary Parasitology. 1996;64:65–70. doi: 10.1016/0304-4017(96)00961-2. [DOI] [PubMed] [Google Scholar]
- [19].Berdoy M, Webster JP, Macdonald DW. Fatal attraction in rats infected with Toxoplasma gondii. Proc Biol Sci. 2000;267:1591–1594. doi: 10.1098/rspb.2000.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Webster JP. The effect of Toxoplasma gondii on animal behavior: playing cat and mouse. Schizophr Bull. 2007;33:752–756. doi: 10.1093/schbul/sbl073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Afonso E, Lemoine M, Poulle M-L, Ravat M-C, Romand S, Thulliez P, Villena I, Aubert D, Rabilloud M, Riche B, Gilot-Fromont E. Spatial distribution of soil contamination by Toxoplasma gondii in relation to cat defecation behaviour in an urban area. International Journal for Parasitology. 2008;38:1017–1023. doi: 10.1016/j.ijpara.2008.01.004. [DOI] [PubMed] [Google Scholar]
- [22].Afonso E, Thulliez P, Gilot-Fromont E. Transmission of Toxoplasma gondii in an urban population of domestic cats (Felis catus) International Journal for Parasitology. 2006;36:1373–1382. doi: 10.1016/j.ijpara.2006.07.010. [DOI] [PubMed] [Google Scholar]
- [23].Dubey JP. Comparative infectivity of oocysts and bradyzoites of Toxoplasma gondii for intermediate (mice) and definitive (cats) hosts. Veterinary Parasitology. 2006;140:69–75. doi: 10.1016/j.vetpar.2006.03.018. [DOI] [PubMed] [Google Scholar]
- [24].Vyas A, Kim SK, Giacomini N, Boothroyd JC, Sapolsky RM. Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors. Proc Natl Acad Sci U S A. 2007;104:6442–6447. doi: 10.1073/pnas.0608310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gonzalez LE, Rojnik B, Urrea F, Urdaneta H, Petrosino P, Colasante C, Pino S, Hernandez L. Toxoplasma gondii infection lower anxiety as measured in the plus-maze and social interaction tests in rats: A behavioral analysis. Behavioural Brain Research. 2007;177:70–79. doi: 10.1016/j.bbr.2006.11.012. [DOI] [PubMed] [Google Scholar]
- [26].Dantzer R. Cytokine, sickness behavior, and depression. Immunol Allergy Clin North Am. 2009;29:247–264. doi: 10.1016/j.iac.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Arnott MA, Cassella JP, Aitken PP, Hay J. Social interactions of mice with congenital Toxoplasma infection. Ann Trop Med Parasitol. 1990;84:149–156. doi: 10.1080/00034983.1990.11812448. [DOI] [PubMed] [Google Scholar]
- [28].Hay J, Hair DM, Graham DI. Localization of brain damage in mice following Toxoplasma infection. Ann Trop Med Parasitol. 1984;78:657–659. doi: 10.1080/00034983.1984.11811878. [DOI] [PubMed] [Google Scholar]
- [29].Hrda S, Votypka J, Kodym P, Flegr J. Transient nature of Toxoplasma gondii-induced behavioral changes in mice. J Parasitol. 2000;86:657–663. doi: 10.1645/0022-3395(2000)086[0657:TNOTGI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- [30].Derouin F, Garin YJF. Toxoplasma gondii: Blood and tissue kinetics during acute and chronic infections in mice. Experimental Parasitology. 1991;73:460–468. doi: 10.1016/0014-4894(91)90070-d. [DOI] [PubMed] [Google Scholar]
- [31].Rogers DC, Fisher EM, Brown SD, Peters J, Hunter AJ, Martin JE. Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mamm Genome. 1997;8:711–713. doi: 10.1007/s003359900551. [DOI] [PubMed] [Google Scholar]
- [32].Wilson RC, Vacek T, Lanier DL, Dewsbury DA. Open-field behavior in muroid rodents. Behav Biol. 1976;17:495–506. doi: 10.1016/s0091-6773(76)90901-9. [DOI] [PubMed] [Google Scholar]
- [33].Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp Brain Res. 1997;113:509–519. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- [34].Kessels RP, Haan E.H. de, Kappelle LJ, Postma A. Selective impairments in spatial memory after ischaemic stroke. J Clin Exp Neuropsychol. 2002;24:115–129. doi: 10.1076/jcen.24.1.115.967. [DOI] [PubMed] [Google Scholar]
- [35].Salame P, Burglen F, Danion JM. Differential disruptions of working memory components in schizophrenia in an object-location binding task using the suppression paradigm. J Int Neuropsychol Soc. 2006;12:510–518. doi: 10.1017/s1355617706060668. [DOI] [PubMed] [Google Scholar]
- [36].Stanley JL, Lincoln RJ, Brown TA, McDonald LM, Dawson GR, Reynolds DS. The mouse beam walking assay offers improved sensitivity over the mouse rotarod in determining motor coordination deficits induced by benzodiazepines. J Psychopharmacol. 2005;19:221–227. doi: 10.1177/0269881105051524. [DOI] [PubMed] [Google Scholar]
- [37].Hermes G, Ajioka JW, Kelly KA, Mui E, Roberts F, Kasza K, Mayr T, Kirisits MJ, Wollmann R, Ferguson DJ, Roberts CW, Hwang JH, Trendler T, Kennan RP, Suzuki Y, Reardon C, Hickey WF, Chen L, McLeod R. Neurological and behavioral abnormalities,ventricular dilatation, altered cellular functions, inflammation, and neuronal injury in brains of mice due to common, persistent, parasitic infection. J Neuroinflammation. 2008;5:48. doi: 10.1186/1742-2094-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Skallova A, Kodym P, Frynta D, Flegr J. The role of dopamine in Toxoplasma-induced behavioural alterations in mice: an ethological and ethopharmacological study. Parasitology. 2006;133:525–535. doi: 10.1017/S0031182006000886. [DOI] [PubMed] [Google Scholar]
- [39].Hay J, Hutchison WM, Aitken PP. Congenital Toxoplasma infection and response to novelty in mice. Ann Trop Med Parasitol. 1983;77:437–439. doi: 10.1080/00034983.1983.11811733. [DOI] [PubMed] [Google Scholar]
- [40].Hay J, Hutchison WM, Aitken PP, Graham DI. The effect of congenital and adult-acquired Toxoplasma infections on activity and responsiveness to novel stimulation in mice. Ann Trop Med Parasitol. 1983;77:483–495. doi: 10.1080/00034983.1983.11811741. [DOI] [PubMed] [Google Scholar]
- [41].Hay J, Aitken PP, Hutchison WM, Graham DI. The effect of congenital and adult-acquired Toxoplasma infections on the motor performance of mice. Ann Trop Med Parasitol. 1983;77:261–277. doi: 10.1080/00034983.1983.11811707. [DOI] [PubMed] [Google Scholar]
- [42].Hutchison WM, Aitken PP, Wells BW. Chronic Toxoplasma infections and motor performance in the mouse. Ann Trop Med Parasitol. 1980;74:507–510. doi: 10.1080/00034983.1980.11687376. [DOI] [PubMed] [Google Scholar]
- [43].Stibbs HH. Changes in brain concentrations of catecholamines and indoleamines in Toxoplasma gondii infected mice. Ann Trop Med Parasitol. 1985;79:153–157. doi: 10.1080/00034983.1985.11811902. [DOI] [PubMed] [Google Scholar]
- [44].Tonino P, Finol HJ, Marquez A. Skeletal muscle pathology in mice experimentally infected with Toxoplasma gondii. J Submicrosc Cytol Pathol. 1996;28:521–526. [PubMed] [Google Scholar]
- [45].Witting PA. Learning capacity and memory of normal andToxoplasma-infected laboratory rats and mice. Parasitology Research. 1979;61:29–51. doi: 10.1007/BF00927085. [DOI] [PubMed] [Google Scholar]
- [46].Piekarski G. Behavioral alterations caused by parasitic infection in case of latent toxoplasma infection. Zentralbl Bakteriol Mikrobiol Hyg A. 1981;250:403–406. [PubMed] [Google Scholar]
- [47].Hodkova H, Kodym P, Flegr J. Poorer results of mice with latent toxoplasmosis in learning tests: impaired learning processes or the novelty discrimination mechanism? Parasitology. 2007;134:1329–1337. doi: 10.1017/S0031182007002673. [DOI] [PubMed] [Google Scholar]
- [48].Vyas A, Kim SK, Sapolsky RM. The effects of Toxoplasma infeciton on rodent behavior are dependent on dose of the stimulus. Neuroscience. 2007;148:342–348. doi: 10.1016/j.neuroscience.2007.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Li Y, Vijayanathan V, Gulinello ME, Cole PD. Systemic methotrexate induces spatial memory deficits and depletes cerebrospinal fluid folate in rats. Pharmacol Biochem Behav. 2009;94:454–463. doi: 10.1016/j.pbb.2009.10.008. [DOI] [PubMed] [Google Scholar]
- [50].Gulinello M, Lebesgue D, Jover-Mengual T, Zukin RS, Etgen AM. Acute and chronic estradiol treatments reduce memory deficits induced by transient global ischemia in female rats. Horm Behav. 2006;49:246–260. doi: 10.1016/j.yhbeh.2005.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]