Abstract
To study whether natural variation in Arabidopsis thaliana could be used to dissect the genetic basis of responses to herbivory in terms of induced volatile emissions, nine accessions were characterized upon herbivory by biting-chewing Pieris rapae caterpillars or after treatment with the phytohormone jasmonic acid (JA). Analysis of 73 compounds in the headspace showed quantitative differences in the emission rates of several individual compounds among the accessions. Moreover, variation in the emission of volatile compounds after JA treatment was reflected in the behaviour of the parasitoid Diadegma semiclausum when they were offered the headspace volatiles of several combinations of accessions in two-choice experiments. Accessions also differ in transcript levels of genes that are associated with the emission of plant volatiles. The genes BSMT1 and Cyp72A13 could be connected to the emission of methyl salicylate and (E,E)-4,8,12-trimethyltrideca-1,3,7,11-tetraene (TMTT), respectively. Overall, Arabidopsis showed interesting phenotypic variations with respect to the volatile blend emitted in response to herbivory that can be exploited to identify genes and alleles that underlie this important plant trait.
Keywords: Behavioural assay, gene transcript level, herbivory, herbivore-induced volatile, natural variation, qRT-PCR
Introduction
A central issue in ecology is to understand how interactions among individual organisms influence food webs and community dynamics. Therefore, evolutionary ecologists aim to understand how genetic variation affects the fitness of individuals in plant–insect communities. Plants exhibit variation in these traits, which comprises both intra-individual phenotypic plasticity, and genetic polymorphisms among individuals and populations. For example, polymorphism in traits for resistance against herbivory may result from varying selection pressures among populations (Meyers et al., 2005). Induced responses allow plants to be cost-effective and also to diminish the risk that herbivores adapt to plant defences (Agrawal and Karban, 1999; Heil, 2008; Steppuhn and Baldwin, 2008).
In nature, plants are challenged by a wide range of herbivorous insects. Herbivory can severely reduce survivorship and reproduction of native plants, and, in crops, insect herbivore infestation can result in severe yield losses (Schoonhoven et al., 2005). Plants have developed a multitude of defence strategies, including pre-existing physical and chemical barriers, induced defences that are activated upon attack, and tolerance mechanisms. Herbivore-induced plant defence can be subdivided into direct defence and indirect defence. Induced direct defence encompasses the production of anti-digestive proteins or toxic secondary metabolites, such as glucosinolate derivatives, that influence the performance and behaviour of the herbivore (Karban and Baldwin, 1997; Walling, 2000; Schoonhoven et al., 2005). Induced indirect defence comprises the production of, for instance, herbivore-induced plant volatiles (HIPV) that attract natural enemies of the herbivore (Dicke and Hilker, 2003; Dicke and Baldwin, 2010).
Herbivore-induced plant volatiles have been studied in a wide range of plant species and with respect to different questions. This includes the behavioural ecology of plant–herbivore–carnivore interactions, as well as molecular genetics and analytical chemistry of HIPVs (D'Alessandro and Turlings, 2006; Snoeren et al., 2007; Dicke, 2009). Herbivore-induced plant defences are orchestrated by at least three interconnecting signal-transduction pathways, the jasmonic acid (JA), the salicylic acid (SA), and the ethylene pathways (Pieterse and Dicke, 2007; Kazan and Manners, 2008). The induction of HIPVs also depends upon these three signalling pathways (Horiuchi et al., 2001; Kessler and Baldwin, 2002; Van Poecke and Dicke, 2002). The signalling pathways underlying HIPV production have been studied for several plant species, for example, Arabidopsis thaliana, tomato, Lima bean, and tobacco (Dicke et al., 1999; Dicke and Van Poecke, 2002; Kessler and Baldwin, 2002; Ament et al., 2004). Recent molecular genetic tools have been used to generate transgenic genotypes that are altered in certain steps of these signalling pathways leading to HIPVs, which are then studied in the context of the attraction of carnivorous arthropods (Van Poecke and Dicke, 2002; Halitschke and Baldwin, 2003; Kessler et al., 2004; Snoeren et al., 2009). In addition, molecular insight into the biosynthesis of HIPVs has allowed the genetic modification of the emission of these volatile compounds. These modified plants were successfully used to study the behaviour of carnivorous arthropods (Bouwmeester et al., 2003; Kappers et al., 2005; Tholl et al., 2005; Schnee et al., 2006; Loivamäki et al., 2008). In several studies, mutants have been used to unravel signal-transduction and biosynthetic pathways in indirect defence (Van Poecke and Dicke, 2002; Aharoni et al., 2003; Ament et al., 2004; Shiojiri et al., 2006; Snoeren et al., 2009). However, in order to be able to use newly available marker technologies that allow the characterization and positioning of loci that control these types of traits (Lambrix et al., 2001; Kliebenstein et al., 2002), exploring the range of genetic variation for HIPVs is a prerequisite since it defines the boundaries of characterization and positioning these loci.
Arabidopsis is an important model plant to study herbivore-induced plant defence responses (Mitchell-Olds, 2001; Van Poecke and Dicke, 2004; Snoeren et al., 2007). However, to date, functional analysis of genes and the dissection of traits are mostly limited to three Arabidopsis accessions, i.e. the accessions Columbia (Col), Landsberg erecta (Ler), and Wassilewskija (WS). A range of screening techniques have resulted in the functional analysis of many genes, including genes involved in HIPV-production. However, so far, only limited numbers of accessions have been screened, narrowing the explored range of variation in which gene functions can be found. Furthermore, exploring genetic variation among accessions has been limited because most attention has been given to mutants that express qualitative variation and less to quantitative variation as seen more among accessions (Alonso-Blanco et al., 2009).
Phenotypic variation between Arabidopsis accessions is abundant for various traits and enables almost every Arabidopsis accession to be distinguished from others when they are grown together under similar environmental conditions (Alonso-Blanco and Koornneef, 2000; Kliebenstein et al., 2001; Kover and Schaal, 2002; Koornneef et al., 2004). Genetic variation has increasingly been associated with gene transcription in early defence signalling and secondary metabolism (Gao et al., 2008; Steppuhn et al., 2008; Wu et al., 2008), and has been reported for resistance to herbivores (Kusnierczyk et al., 2007; Broekgaarden et al., 2008; Steppuhn et al., 2008). In addition, variation in gene transcription was demonstrated for Arabidopsis accessions treated with the phytohormone methyl jasmonate (Matthes et al., 2008).
To our knowledge, exploring genetic variation in HIPV emission with respect to their role in indirect defence has so far primarily been explored for crop cultivars (Loughrin et al., 1995; Krips et al., 2001; Scutareanu et al., 2003; Hoballah et al., 2004; Bukovinszky et al., 2005; Nissinen et al., 2005; Lou et al., 2006; Kappers et al., 2010). Studying this kind of trait is particularly complex, as the defence mechanism involves many different chemical compounds (Dicke et al., 1990; Turlings et al., 1991; Mattiacci et al., 1995; Dicke and van Loon, 2000; D'Alessandro and Turlings, 2006). Here, the genetic variation in caterpillar-induced indirect defence, i.e. HIPV emission, among nine Arabidopsis accessions originating from different geographic origins is addressed. The objectives of this study were (i) to investigate the variation of volatile emission induced by herbivore feeding or by treatment with jasmonic acid among the accessions, (ii) to study the variation in transcription levels of genes putatively involved in volatile production, and (iii) to assess the effects of genetic variation on the attraction of parasitoid wasps to HIPVs.
Materials and methods
Plant and insect material
Nine Arabidopsis thaliana (L.) Heynh. accessions, either obtained from the European Arabidopsis Stock Centre (http://nasc.nott.ac.uk/) (An-1=N944, C-24=N906, Cvi=N8580, Kond=CS6175, Ler=NW20, the Sendai stock centre in Japan (Kyo-1=JW137) or collected in Sweden by members of the Wageningen Genetics Laboratory (Eri-1=CS22548) were used. Seeds from Col-0 and WS were provided by P Reymond (Lausanne, Switzerland). Seeds were germinated in sandy Arabidopsis soil (Lentse potgrond BV, Lent, The Netherlands), and cultivated in a growth chamber at 21±2 °C, 50–60% relative humidity (RH), and a L8:D16 photoperiod with 80–110 μmol m−2 s−1 photosynthetic photon flux density (PPFD). The soil was heated to 90 °C for at least 2 h prior to sowing of the plants. Two-week-old seedlings were transferred from seed trays to plastic pots (5 cm in diameter) filled with similar soil. Plants were watered twice a week. Six-to-eight-week-old full-grown vegetative plants were used for the experiments. To prevent infestation by sciarid flies, the soil was treated weekly with entomopathogenic nematodes (Steinernema feltiae; Koppert BV, Berkel en Rodenrijs, The Netherlands).
The herbivorous small cabbage white Pieris rapae, was reared on Brussels sprouts plants (Brassica oleracea var. gemmifera, cv. Cyrus) in a growth chamber (16:8 L/D, 20±2 °C, and 70% RH).
The parasitoid wasp Diadegma semiclausum was reared as described in detail by Bukovinszky et al. (2005). Emerging wasps were provided ad libitum with water and honey, and are referred to as ‘naïve’ wasps as they had neither been exposed to plant material nor had an oviposition experience. This parasitoid is known to be attracted to the volatiles that are emitted by P. rapae-infested Arabidopsis Col-0 plants (Loivamäki et al., 2008).
Plant treatment
The emission of volatiles was induced by feeding of P. rapae caterpillars for 24 h. Each plant was infested by placing 20 first-instar P. rapae larvae equally over the fully expanded leaves. In addition, a treatment that mimicked the effect of herbivory was included by spraying with a JA solution in order to check whether variation in induced volatiles is due to differences in leaf tissue consumption by caterpillars. Plants were completely sprayed with a total volume of 5 ml of 1.0 mM (±)-JA (Sigma-Aldrich) aqueous solution containing 0.1% Tween 20 as surfactant.
Caterpillar-feeding
To assess the area of leaf tissue consumed by the caterpillars, five plants of each accession were infested by dividing 20 first-instar P. rapae larvae equally over the fully expanded leaves on three experimental days. Twenty-four hours after infestation individual leaves were cut, taped on paper, and scanned with a Hewlett-Packard scan jet 3570c. Original leaf shapes were reconstructed using drawing software Paint.NET v3.30, Microsoft Corporation. Quantification of consumed leaf tissue area was performed using Winfolia pro 2006a, Regent Instruments (Québec, Canada). A one-way ANOVA with an LSD post-hoc test was used to test whether the consumed leaf area differed between the accessions (SPSS 15.0, Chicago, USA).
Headspace collection and volatile analysis
Dynamic headspace sampling was carried out in a climate room (20±2 °C, 70% RH; L8:D16 photoperiod, and 90–110 μmol photons m−2 s−1 PPFD at canopy height). Twenty-four hours before sampling, the pots were removed, roots and soil were carefully wrapped in aluminium foil, and four plants were placed together in a 2.5 l glass jar. The glass jars were then covered with insect-proof gauze. Just before headspace collection, the gauze was removed and jars were closed with a Viton-lined glass lid having an inlet and outlet. Inlet air was filtered by passing through a stainless steel cartridge (Markes, Llantrisant, UK) filled with 200 mg Tenax TA (20/35 mesh; Grace-Alltech, Deerfield, USA). Volatiles were trapped by sucking air out of the jar at a rate of 100 ml min−1 through a similar cartridge filled with 200 mg Tenax TA. Headspace volatiles for all treatments were collected for 3.5 h. Fresh weights of all rosettes were determined immediately after the experiments. On each experimental day, the headspaces of three or four accessions of each treatment were collected simultaneously. For each accession, 5 (An-1, C-24, Cvi, Eri-1, Kond, Kyo-1, Ler) or 6 (Col-0, WS) replicates for each experimental treatment (control, caterpillar-infested, or JA-treated) were analysed.
Headspace samples were analysed with a Thermo Trace GC Ultra (Thermo Fisher Scientific, Waltham, USA) connected to a Thermo Trace DSQ (Thermo Fisher Scientific, Waltham, USA) quadrupole mass spectrometer. Before desorption of the volatiles, the Tenax cartridges were dry-purged with nitrogen at 30 ml min−1 for 20 min at ambient temperature to remove moisture. Volatiles were desorbed from the cartridges using a thermal desorption system at 250 °C for 3 min (Model Ultra Markes Llantrisant, UK) with a helium flow of 30 ml min−1. Analytes were focused at 0 °C on an electronically-cooled sorbent trap (Unity, Markes, Llantrisant, UK) and were then transferred in splitless mode to the analytical column (Rtx-5ms, 30 m, 0.25 mm i.d., 1.0 μm film thickness, Restek, Bellefonte, USA) by rapid heating of the cold trap to 250 °C. The GC was held at an initial temperature of 40 °C for 3.5 min followed by a linear thermal gradient of 10 °C min−1 to 280 °C and held for 2.5 min with a column flow of 1 ml min−1. The column effluent was ionized by electron impact ionization at 70 eV. Mass spectra were acquired by scanning from 45–400 m/z with a scan rate of 3 scans s−1. Compounds were identified using the deconvolution software AMDIS (version 2.64, NIST, USA) in combination with NIST 98 and Wiley 7th edition spectral libraries, and by comparing their retention indices with those from the literature (Adams, 1995). For quantification, characteristic quantifier ions were selected for 84 compounds (see Supplementary Table S1 at JXB online). MetAlign software (PRI-Rikilt, Wageningen, the Netherlands) was used to remove baseline noise, to align the peaks of all chromatograms of the samples, and to integrate peak areas of quantifier ions. The peak areas of all compounds were corrected for the fresh weight of the leaf rosettes. To visualize differences between accessions and the different treatments, the total volatile profiles were analysed using principal component analysis (PCA, GeneMath XT 2.0). Data were preprocessed by a log10 transformation and subtracting the average value of all treatments.
For a more detailed analysis, emitted quantities of individual volatiles were tested for significant changes between plant treatments using a t test. Individual volatiles were analysed for significant differences between accessions within each of the treatments, using one-way ANOVA followed by a Dunnett T3 post-hoc analysis (SPSS 15.0). Differences in emission of volatile compounds between treatments for each accession were analysed for significance using a one-way ANOVA.
Quantitative RT-PCR analysis
A qRT-PCR analysis was used to screen for differences in expression of JA- or P. rapae-inducible genes involved in volatile production in Arabidopsis. After 24 h of herbivory, leaf material was collected by selecting two almost fully expanded leaves with feeding damage. Caterpillars and their products were removed prior to the collection. For JA- and non-treated plants, similar leaves were collected. Five plants, i.e. ten leaves, were used per replicate. Collected leaf material was immediately flash frozen in liquid nitrogen and stored at –80 °C. As control plants, uninfested plants were used that were sprayed with water and that were otherwise treated in a similar way to the infested plants.
Pooled leaf samples were ground in liquid nitrogen and total RNA was isolated using the RNeasy Plant Mini Kit (Qiagen, Valencia, CA, USA). One μg of total RNA was treated with DNaseI (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. DNA-free total RNA was subsequently converted into cDNA using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA). Gene-specific primers were designed using Beacon Designer 7.0 (Premier Biosoft Int) for seven Arabidopsis genes based on sequences obtained from the TIGR Arabidopsis database. Primer sequences are shown in Supplementary Table S2 at JXB online. Primers were tested for gene specificity by performing melt curve analysis on a MyIQ Single-Color Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). PCR products were sequenced to confirm the amplification of the gene of interest. Sequence results were checked by a BLAST search in the Arabidopsis TIGR database (data not shown).
Quantitative RT-PCR analysis was carried out in optical 96-well plates with a MyIQ Single-Color Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA), using SYBR Green to monitor dsDNA presence. Each reaction contained 10 μl 2× SYBR Green Supermix Reagent (Bio-Rad, Hercules, CA, USA), 10 ng cDNA and 300 nM of the gene-specific primers in a final volume of 20 μl. All qRT-PCR experiments were performed in duplicate. The following PCR program was used for all PCR analyses: 95 °C for 3 min, 40 cycles of 95 °C for 30 s and 60 °C for 45 s. Threshold cycle (Ct) values were calculated using the MyIQ Optical System software (version 2.0, Bio-Rad, Hercules, CA, USA). Subsequently, Ct values were normalized for differences in cDNA synthesis by subtracting the Ct value of the constitutively expressed gene β-actin from the Ct value of the gene of interest. β-Actin is widely used as a reference gene in expression studies and its absolute expression level was not influenced by P. rapae-infestation nor by JA-application in our study (data not shown). Experiments were repeated four times and the differences in normalized gene expression (2–δCt) between treatments were statistically analysed using a one-way ANOVA with an LSD post-hoc test (SPSS 15.0).
Behavioural assays using parasitoid wasps
The effect of JA-induced volatile production on parasitoid behaviour was compared for different accessions in a closed-system Y-tube olfactometer as described by Bukovinszky et al. (2005). In short, filtered air was led through activated charcoal and split into two air streams (4.0 l min−1) that were led through 5.0 l glass jars containing the odour sources, each consisting of four plants. The olfactometer was illuminated with artificial light from above at an intensity of 60±5 μmol m−2 s−1 PPFD. All experiments were conducted in a climatized room (20±2 °C). Naïve, 3–7-d-old Diadegma semiclausum females were individually transferred from a cage into the Y-tube olfactometer using a glass tube. Upon release in the olfactometer, parasitoid behaviour and parasitoid choice for one of the two odour sources was observed. Parasitoids that did not make a choice within 10 min after release or did not choose one of the two arms of the olfactometer within 5 min were considered as non-responding individuals, and were excluded from the statistical analysis. After every three parasitoids tested, the odour sources were interchanged to compensate for any unforeseen asymmetry in the set-up. Parasitoid preference for accessions treated with JA was statistically analysed using a Chi-square test, with the null-hypothesis that parasitoids did not have a preference for any of the two odour sources.
Results
Leaf damage by Pieris rapae
To detect whether there is variation in direct defence between accessions that might explain variation in HIPV-emission, the leaf tissue consumption of P. rapae caterpillars was determined. P. rapae caterpillars consumed 3.5±0.2% (WS) to 7.4±0.5% (C-24) of the total leaf area within 24 h (Table 1). The accessions differed substantially in leaf area (varying from 1935±98 mm2 for C-24 to 4427±179 mm2 for WS; mean ±SE) and therefore the percentage of consumed leaf size was not used but rather the absolute amount of leaf area consumed per plant, which varied from 88±7.5 mm2 (An-1) to 195±10.2 mm2 (Eri-1) (mean ±SE; Table 1). Accession An-1 had the lowest area of leaf tissue consumed per plant compared with all other accessions (ANOVA LSD: P <0.001). Caterpillars consumed significantly more leaf tissue from accessions Eri-1 and Kond compared with accessions An-1, C-24, Col-0, Cvi, Kyo-1, Ler, and WS (Table 1). To take into account that variation of induced volatile emission may be due to the differences in feeding damage, the damage-independent JA-treatment was also included in subsequent experiments.
Table 1.
Leaf damage by Pieris rapae-feeding on plants from nine accessions of Arabidopsis thaliana
| Accession | Mean area remaining per plant (%) ±SE | Mean leaf area consumed |
|
| per plant (mm2) ±SE | |||
| An-1 | 96.4±0.5 | 88±7.5 | a |
| C-24 | 92.6±0.5 | 139±5.2 | ce |
| Col-0 | 94.2±0.4 | 165±7.9 | d |
| Cvi | 95.2±0.5 | 135±8.5 | c |
| Eri | 94.8±0.3 | 195±10.2 | b |
| Kond | 93.6±0.5 | 190±6.9 | b |
| Kyo-1 | 94.8±0.4 | 158±8.6 | de |
| Ler | 94.0±0.4 | 157±6.7 | de |
| WS | 96.5±0.2 | 152±5.3 | cde |
Data show the percentage of leaf that remained after 24 h of infestation, and the leaf area that was consumed by the caterpillars. Mean leaf areas consumed per plant were statistically analysed using a one-way ANOVA followed by LSD post-hoc. Accessions followed by different letters indicate a significant difference (P <0.05).
Analysis of headspace volatiles in different accessions
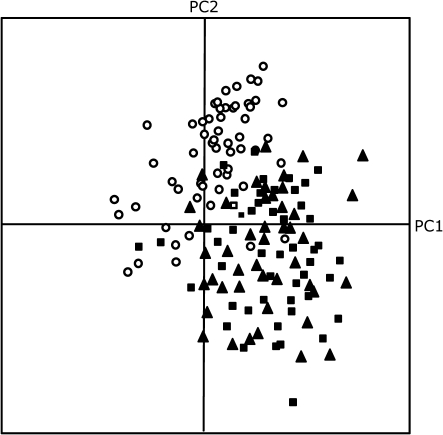
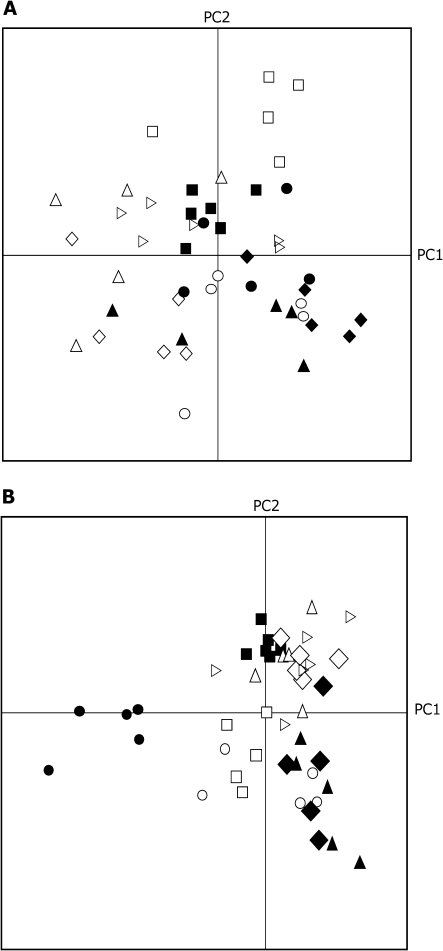
A total of 73 compounds was recorded in the headspace analysis of the nine accessions. First, a principal component analysis (PCA) using the complete data set of 73 volatile compounds was conducted. The first three principal components (PCs) explain 60% of the observed variation. Among all accessions the overall volatile profile clearly changed when plants were induced by P. rapae feeding or by JA treatment compared with non-treated plants (Fig. 1). After omitting batch variation due to different days of headspace sampling, five PCs were extracted that explain 52.5% of the variation for Pieris-treated plants (Fig. 2A), whereas six PCs explain 59.3% of the variation found in JA-treated plants (Fig. 2B). The PCA analysis in both treatments shows that individual samples of each accession cluster together. The relative position of the accessions differed between the P. rapae infestation and JA treatment. For example, after P. rapae feeding, accessions C-24 and Col-0 cluster together (Fig. 2A), which implies that they have a similar HIPV profile, whereas after JA treatment they have a different HIPV profile and hence they are separated in the PCA plot (Fig. 2B). This implies that P. rapae herbivory and JA treatment have different effects on individual volatile compounds. This is also supported by the ANOVA evaluation (α=0.05) for differences in the induction of individual compounds between both treatments (see Supplementary Table S3 at JXB online).
Fig. 1.
Score plot of Principal Component Analysis (PCA) of volatiles emitted by nine Arabidopsis thaliana accessions infested with Pieris rapae (filled squares), treated with JA (filled triangles),or left untreated (control, open circles). The PCA shows the first and second PC.
Fig. 2.
Score plot of Principal Component Analysis (PCA) of volatiles emitted by nine Arabidopsis thaliana accessions infested with Pieris rapae (A) or treated with JA (B). The PCA shows the first and second PC. Open circles, An1; filled circles, C24; open squares, Cvi; filled squares, Col0; open diamonds, Eri; filled diamonds, Kond; open triangles, Ler; filled triangles, Kyo-1; open arrowheads, WS.
To identify differences in the emission of volatile compounds between different accessions after P. rapae feeding or JA treatment, a one-way ANOVA was carried out for each individual compound, followed by a Dunnett T3 post-hoc test. Twenty-eight out of 73 compounds differed significantly between at least two accessions after either treatment (see Supplementary Table S4, and Supplementary Table S5 at JXB online) indicating the presence of natural variation. Compounds belong to various chemical classes, including green leaf volatiles (GLVs) [(E)-2-hexenal, (E)-3-hexenal, (Z)-3-hexenyl acetate, (Z)-2-penten-1-ol, (Z)-3-hexen-1-ol, pentan-2-ol], monoterpenes [(E)-β-ocimene, (Z)-β-ocimene, 3-carene, β-myrcene, α-pinene, α-phellandrene], sesquiterpenes [(E)-nerolidol, β-sesquiphellandrene, β-acoradiene, β-bisabolene, (E)-β-farnesene, (E,E)-α-farnesene, α-humulene], homoterpenes [(E)-4,8-dimethyl-1,3,7-nonatriene (DMNT), (E,E)-4,8,12-trimethyltrideca-1,3,7,11-tetraene (TMTT)], phenylpropanoids/ benzenoids [methyl salicylate (MeSA), ethyl salicylate, indole, benzaldehyde], hexanoic acid, decanal, and geranyl acetone (see Supplementary Table S4 at JXB online).
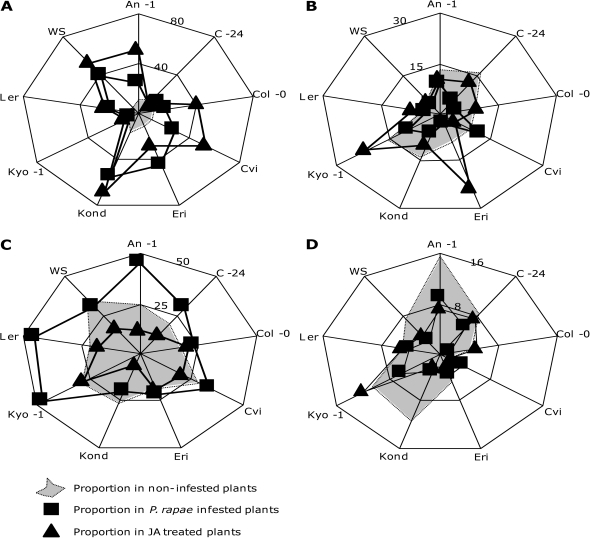
The treatment with JA or caterpillar feeding resulted in a 1.1-fold (P. rapae-infested C-24) to 4.6-fold (JA-treated Kond) increase in the emission rate of volatiles compared with non-treated controls. The composition of the volatile blends, i.e. the relative contribution of compounds that belong to different chemical classes, differed substantially between different accessions (Fig. 3A–D).
Fig. 3.
Proportion of monoterpenoids (A), sesquiterpenoids (B), Green Leaf Volatiles (C), and phenylpropanoids/benzenoids (D) in the total volatile blend of nine Arabidopsis ecotypes infested with Pieris rapae (filled squares) or treated with jasmonic acid (filled triangles). Numbers indicate % of the total blend.
Kond showed the strongest increase in the emission of monoterpenes of all accessions after JA treatment and P. rapae infestation (68% and 54%, respectively). Although P. rapae infestation and JA treatment increased the absolute emission rate of monoterpenes, the contribution of this class of compounds to the total blend was lowest in C-24 and Kyo-1 (11% and 10%, respectively, after JA treatment, 11% and 7%, respectively, after P. rapae infestation; Fig. 3A).
Herbivory increased the absolute emission of sesquiterpenes in most accessions, except for P. rapae-infested C-24 and Col-0 and JA-treated Cvi plants. About one-fifth of the volatile blend of JA-treated plants of accessions Eri-1 (24%) and Kyo-1 (21%) consisted of sesquiterpenes, which is the highest proportion of sesquiterpenes found in the accessions included in this study. By contrast, P. rapae infestation resulted in only 3% (Eri-1) and 9% (Kyo-1) of sesquiterpenes in the total volatile blend. Remarkably, JA treatment failed to increase sesquiterpene emission in the blend of Cvi, whereas P. rapae feeding did increase sesquiterpene emission in Cvi (10%) (Fig. 3B).
P. rapae infestation increased the proportion of GLVs in the total blend in all accessions compared with their respective non-induced controls, except for Kond and WS, although the absolute amount of GLVs did increase in these accessions as well. JA treatment increased GLV emission in all accessions, except An-1, C-24, and Kond, resulting in a relatively lower amount of GLVs emitted by JA-treated plants of these accessions. All accessions showed a higher contribution of GLVs in the blend of P. rapae-infested plants than in JA-treated plants (Fig. 3C), with An-1, Kyo, and Ler as the highest emitters. The highest proportion of GLVs in the blend of JA-treated plants was found in Kyo-1 plants, amounting to more than 25% of the total amount of volatile compounds.
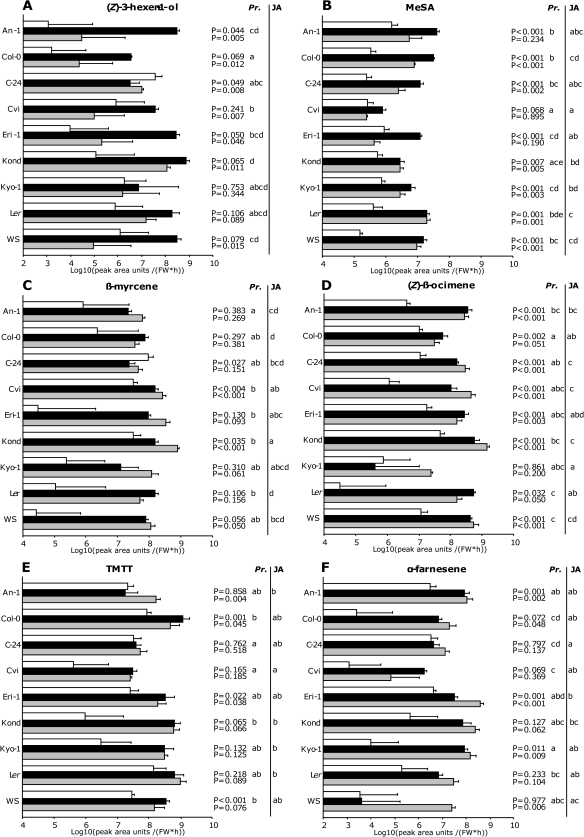
Six volatile compounds that may be related to the genes for which expression levels were analysed in this study are discussed in more detail (Fig. 4). Several GLVs were emitted in larger amounts from P. rapae-infested plants than from non-treated plants (Fig. 4A; see Supplementary Table S6 at JXB online). (Z)-3-hexen-1-ol contributed most to the observed variation in GLVs among the accessions (Fig. 3C). In particular accessions, Kond, that had the highest emission rate of (Z)-3-hexen-1-ol, and Col-0, that showed the lowest emission, contributed to the observed variation among accessions after herbivory (Fig. 4A).
Fig. 4.
Volatile emission [Log10 (peak area units FW−1 h−1)±6+SE)(5–6 replicates) of nine Arabidopsis thaliana accessions infested with Pieris rapae (Pr.) (black bars), treated with JA (grey bars), or left untreated (control bars). Behind bars that represent P. rapae and JA-induced emission of volatiles, P values are given for the induction (t test). Volatile emissions of different accessions followed by different letters, given for both treatments separately, differ significantly (P <0.05) (determined by post-hoc analysis).
All accessions, except Cvi, showed significantly higher MeSA emission after P. rapae-infestation than from uninfested plants (Fig. 4B; see Supplementary Table S6 at JXB online), and after JA treatment all accessions except for An-1, Cvi, and Eri-1 emitted MeSA in significantly higher amounts than non-treated plants (Fig. 4B; see Supplementary Table S6 at JXB online). Accessions varied significantly in the emission rates of MeSA after herbivore damage, particularly accession An-1 emitted more and accession Cvi less than the others (Fig. 4B). After JA treatment, the largest amount of MeSA was emitted by Ler plants while the lowest amount was emitted by accession Cvi (Fig. 4B).
Emission of β-myrcene and (Z)-β-ocimene varied between accessions after P. rapae infestation and JA treatment. Infestation with P. rapae induced an increase in the β-myrcene emission in accessions C-24, Cvi, and Kond, while JA treatment did so in Cvi, Kond, and WS. The emission of (Z)-β-ocimene was induced in most accessions by P. rapae infestation and JA treatment, except in Kyo-1 and in Col-0 after JA treatment (Fig. 4D).
The emission rates of the homoterpene TMTT were significantly higher in P. rapae-infested Col-0, Eri-1, and WS plants and JA-treated An-1, Col-0, and Eri-1 plants than in non-treated plants (Fig. 4E; see Supplementary Table S6 at JXB online). The accessions differed in the emission of TMTT after either treatment (Fig. 4E). After herbivory, Col-0 plants emitted the largest amount of TMTT and Cvi the lowest. Treatment of plants with JA resulted in highest TMTT emission rates in Ler and lowest in Cvi (Fig. 4E).
The emission of (E,E)-α-farnesene was significantly induced after herbivory and JA-treatments in accessions An-1, Col-0, Eri-1, and Kyo-1 (Fig. 4F; see Supplementary Table S6 at JXB online). Herbivory induced the highest emission rate of (E,E)-α-farnesene in An-1 plants while WS plants had the lowest emission rate of (E,E)-α-farnesene after herbivory. JA-treatment resulted in Eri-1 plants emitting most (E,E)-α-farnesene and Cvi plants the lowest amount (Fig. 4F).
Transcriptional changes in different accessions after JA-treatment or P. rapae feeding
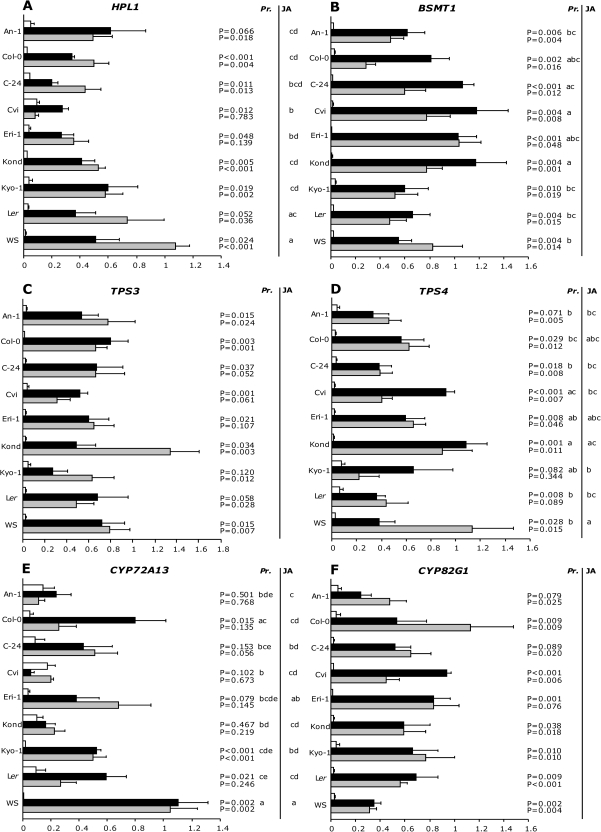
To investigate whether variation in emission of volatiles can be explained by differences in transcription profiles, expression changes of six genes that are involved in the biosynthesis of Arabidopsis HIPVs were monitored for the nine accessions after P. rapae-feeding or JA-treatment (Fig. 5).
Fig. 5.
Expression changes of six Arabidopsis thaliana genes in untreated (white bars), Pieris rapae infested (black bars) or JA-treated (grey bars) plants of nine Arabidopsis thaliana accessions. Bars represent relative expression levels (2–δCt), calculated from four biological replicates (mean +SE). Note that scales on the x-axes are not identical. Behind bars that represent P. rapae and JA-induced transcript levels of genes, P values are given for the induction. Letters represent groups of accessions that did not significantly differ after ANOVA followed by post-hoc analysis, given for each treatment separately.
For HPL1, which encodes a hydroperoxide lyase and is a member of the CYP74B cytochrome P450 family (Bate et al., 1998), transcript levels were induced after JA treatment or herbivore feeding for most accessions. The HPL1 transcript levels did not significantly differ among the accessions after herbivory, whereas JA-treatment resulted in some significant differences in transcript levels between accessions (Fig. 5A).
Transcript levels of BSMT that encode an enzyme that methylates both salicylic acid and benzoic acid (Chen et al., 2003), were significantly induced in all accessions after 24 h of P. rapae feeding or treatment with JA. Significant quantitative differences among the accessions were found for BSMT1 transcript levels after P. rapae treatment but not after JA treatment (Fig. 5B).
The terpene synthase gene TPS3 was selected for transcriptional analysis as this gene is annotated to be an (E)-β-ocimene, (Z)-β-ocimene, and β-myrcene synthase in Arabidopsis (Fäldt et al., 2003). Transcript levels of TPS3 were significantly higher in caterpillar-infested or JA-treated plants compared to control plants in most accessions (Fig. 5C). However, this was not the case for P. rapae-damaged Kyo-1 and Ler as well as for JA-treated C-24, Cvi, and Eri-1 plants. Irrespective of plant treatment, accessions did not differ significantly in induced TPS3 transcript levels (Fig. 5C).
Another terpene synthase, TPS4, encodes a geranyl linalool synthase that catalyses the formation of geranyl linalool, the intermediate in the biosynthesis of TMTT (Herde et al., 2008). TPS4 transcript levels significantly increased after P. rapae infestation or JA treatment in most of the accessions (Fig. 5D). Accessions differed in TPS4 transcript levels after P. rapae infestation or JA treatment. The discrepancy between JA-treatment and P. rapae feeding was highest in Cvi and WS, although the effects were opposite (Fig. 5D).
In addition, two cytochrome P450 genes were included in this analysis that have been postulated to be involved in the conversion of geranyl linalool to TMTT, CYP72A13 (Bruce et al., 2008) and CYP82G1, that both show high co-expression with TPS4 using the Correlated Gene Search Tool (http://prime.psc.riken.jp/?action=coexpression_index) of the RIKEN Plant Science Centre. CYP72A13 transcript levels showed a significant increase after P. rapae infestation in accessions Col-0, Kyo-1, Ler and a marginally insignificant increase in Eri-1, whereas JA treatment resulted in a significant increase in transcript levels in accessions Kyo-1 and WS (Fig. 5E). Transcript levels of CYP82G1 were significantly induced after P. rapae infestation or JA treatment for all of the accessions except for P. rapae-infested An-1 and C-24, and JA-treated Eri-1 (Fig. 5F). There was no significant variation among the accessions after either treatment (Fig. 5F).
Consequences of variation in volatile profiles for parasitoid attraction
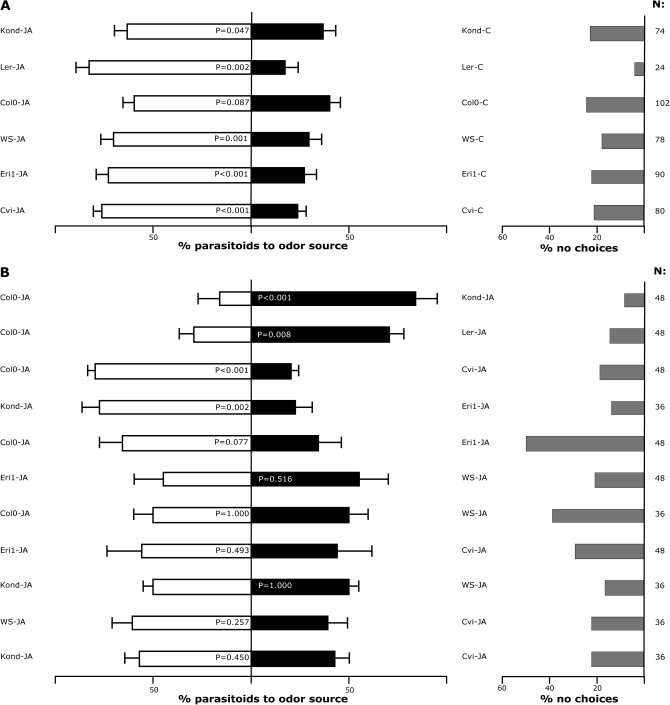
To investigate whether induced volatile emission of the different accessions affects the searching behaviour of the parasitoid wasp D. semiclausum, Y-tube olfactometer experiments were carried out. Due to logistic limitations, six out of the nine accessions used in this study were randomly selected for the behavioural assays. D. semiclausum is known to respond to P. rapae-induced Arabidopsis volatiles (Loivamäki et al., 2008). To exclude variation in herbivore-induced emission due to differences in leaf damage, JA was chosen for volatile induction.
The volatiles emitted by accessions Cvi, Eri-1, Kond, Ler, and WS plants treated with JA attracted more D. semiclausum wasps than volatiles from non-treated control plants of the same accession (Fig. 6A). For Col-0, parasitoids did not discriminate between JA-treated and control plants, although there was a tendency of attraction towards the JA-treated plants (P=0.087; Fig. 6A); a recent study in our laboratory shows that JA-treated Col-0 plants significantly attract the parasitoid D. semiclausum (Snoeren et al., 2009).
Fig. 6.
Responses of naïve Diadegma semiclausum females to the volatiles of two sets of Arabidopsis thaliana accessions, as assessed in a Y-tube olfactometer. All plants were treated with 1 mM aqueous solution JA solution. Each bar represents the percentage of choices for each of the two odour sources as determined in 3–4 replicate experiments; if JA-treated accessions were compared (B) 12 parasitoids were tested on each replicate day, and six parasitoids when JA and non-treated treated plants were compared (A): error bars indicate SE. The higher part of the figure (A) depicts the percentage of choices for all tested wasps used as controls for the separate JA-treated accession comparisons. Grey bars indicate the percentage of no choice in each experiment; total number of tested parasitoids are given next to these bars (χ2 test, P values).
In two-choice experiments, JA-treated plants from different accessions were tested against each other (Fig. 6B). The JA-induced volatile blend of accession Kond was more attractive than that of Col-0 and Eri-1, but it was as attractive as the blend of JA-treated WS and Cvi plants. Parasitoids preferred JA-induced volatiles of Col-0 when tested against Cvi, but Col-0 volatiles were less preferred than volatiles of JA-treated Ler plants (Fig. 6B). Parasitoids tended to prefer volatiles of Col-0 plants to Eri-1 plants (P=0.077; Fig. 6B). Volatiles of JA-treated WS plants were as attractive as volatiles from JA-treated Kond, Cvi, Col-0, and Eri-1 plants. JA-induced Cvi volatiles were also as attractive as JA-induced Eri-1 and Kond volatiles (Fig. 6B).
Discussion
Functional analysis of Arabidopsis genes is largely based on mutants that are selected in forward and reverse genetic studies. Alternatively, a complementary source of genetic variation is available, i.e. the naturally occurring variation among different accessions. The use of genetic methods developed to map quantitative trait loci, in combination with the resources available for molecular biology in Arabidopsis, allow this natural variation to be exploited up to the molecular level. Thus, the systematic exploitation of naturally occurring variation provides a valuable complementary resource for the functional analysis of the Arabidopsis genome (Atwell et al., 2010). The results presented here represent an extensive study of the genetic variation in an indirect defence mechanism, i.e. HIPV emission, of nine Arabidopsis accessions originating from different geographic origins.
Indirect plant defence has been studied in many plant species (Arimura et al., 2005; Dicke, 2009). To date, intraspecific variation of HIPV emission has primarily been explored for crop varieties. In some of the systems it was demonstrated that variation in emission of HIPVs among cultivars also influences the behaviour of carnivorous arthropods (Krips et al., 2001; Hoballah et al., 2002; Bukovinszky et al., 2005; Lou et al., 2006). It is demonstrated here that the nine different Arabidopsis accessions (An-1, C-24, Col-0, Cvi, Eri-1, Kond, Kyo-1, Ler, and WS) emit different odour blends in response to feeding damage by P. rapae caterpillars or JA treatment. Furthermore, the parasitoid D. semiclausum discriminated between the JA-induced volatile blends of some of the accessions.
The first line of defence that herbivores encounter upon contact with the plant, i.e. epicuticular wax loads, trichomes or glucosinolate levels, was already proven to be subject to genetic variation in Arabidopsis (Larkin et al., 1996; Rashotte et al., 1997; Luo and Oppenheimer, 1999; Lambrix et al., 2001; Kliebenstein et al., 2002; Reymond et al., 2004). These direct lines of defence might explain the observed variation in leaf consumption of Arabidopsis by insect herbivores (Mauricio, 1998; Agrawal et al., 2002; Mewis et al., 2005), and this may correspond with our findings of variation in leaf consumption. To exclude that the variation in volatile induction was caused by this variation in leaf tissue consumption, a herbivory-mimicking treatment was included. Given that P. rapae caterpillars mainly induce the jasmonate pathway in Arabidopsis (De Vos et al., 2005), JA was applied to induce plant volatile emission.
Variation in HIPV emission occurred for various compounds of different chemical classes, including GLVs, terpenoids, and phenolic compounds. The HIPV blends of some accessions strongly varied, particularly those of An-1, Col-0, C-24, and Cvi, after P. rapae feeding. By contrast, the HIPV blends emitted by Eri-1, Kyo-1, Ler, and WS plants were more similar, and the number of compounds that were significantly induced after herbivory or JA treatment among these accessions was relatively small (see Supplementary Table S7 at JXB online). Most of the variation between the accessions is quantitative, i.e. all accessions emit the same compounds, but in various amounts, resulting in different compositions of the volatile blend. By contrast, the HIPV blend of accession Cvi was quite distinct compared with the blends of other accessions as it had a low emission of sequiterpenes and some of the sesquiterpenes, such as α-cuparene and β-bisabolene, were not detectable or only present in very small amounts. In addition to the findings of Tholl et al. (2005), who reported that the transcript levels of At5g23960 in inflorescences was comparable in both Cvi and Col-0, sesquiterpenes were detected that have been reported as products of the terpene synthase encoded by At5g23960 (Tholl et al., 2005) such as α-humulene and (E)-β-caryophyllene in the headspace of P. rapae-infested or JA-treated leaves of Cvi.
Up to now, most studies investigating herbivore-induced defence traits such as volatile production in Arabidopsis were done with one of the three laboratory accessions Col-0, Ler, or WS. Significant differences were observed in HIPV emission between the accessions Col-0, Ler, and WS after P. rapae herbivory or JA treatment. Nevertheless, variation in HIPV emission between these three accessions is rather moderate when compared with the variation that is present among all nine accessions studied (Fig. 2). Thus, when comparing these three accessions, with respect to the behaviour of parasitoids, for example, it should be noted that these accessions do not completely represent the width of genetic variation in induced volatile formation existing in Arabidopsis thaliana. Therefore, the study presented here for nine Arabidopsis accessions is a considerable contribution, as it compares the HIPV emission of the three above-mentioned accessions together with six other accessions and its effects on the behaviour of carnivorous natural enemies.
The variation found between the induced headspace volatiles of, for example, Col-0 and WS plants suggests that foraging behaviour of carnivorous arthropods may be influenced by these differences. However, naïve D. semiclausum females did not discriminate between JA-treated Col-0 and WS plants. This suggests that (E)-β-ocimene, (Z)-β-ocimene, and DMNT, which represent the main differences between the two accessions, are not of high importance for this parasitoid during the host location process (see Supplementary Table S5 at JXB online). An attempt was made to identify which individual compounds in the headspace of JA-treated plants influence the attraction of parasitoid wasps while searching for hosts. Despite the observed differences between accessions, it was not possible to allocate individual compounds to explain parasitoid behaviour. The complex variation in odour blends is likely to interfere with drawing conclusions about the contribution of individual compounds. It is more likely that the contribution of individual compounds to the total headspace composition of the plant after induction is crucial for the parasitoid (De Boer et al., 2004; Mumm and Hilker, 2005). For instance, TMTT is not attractive to the predatory mite Phytoseiulus persimilis when offered as a single compound but when added to a complex herbivore-induced volatile blend it does affect the predator's behaviour (De Boer et al., 2004). Moreover, quantitative aspects have not been included. Possibly, the discrimination between blends is affected by absolute emission rates rather than only the relative emission rates.
To study the underlying molecular level of volatile emission, transcriptional changes of genes involved in volatile biosynthesis were examined. At present, only a few genes have been functionally associated with the production of specific volatile compounds in Arabidopsis (Bate et al., 1998; Chen et al., 2003; Fäldt et al., 2003). For several other genes a function has been proposed in volatile production, such as TPS4 and CYP72A13 (Bruce et al., 2008; Herde et al., 2008). Variation in transcript levels of genes potentially involved in the biosynthesis of herbivore-inducible volatiles has already been demonstrated (Arimura et al., 2004; De Vos et al., 2005; Gomez et al., 2005). These studies used a single hybrid or plant line. However, variation was looked for in stress-induced transcript levels of six genes that are putatively involved in the biosynthesis of volatile compounds among nine Arabidopsis accessions. Differences in transcript levels among the accessions were found for BSMT1, TPS4, CYP72A13, and HPL1 after herbivory and/or JA-treatment. By contrast, transcript levels of TPS3 and CYP82G1 did not vary among accessions after caterpillar feeding or JA treatment.
All accessions, except Cvi, showed an induced emission of MeSA after P. rapae feeding, and also transcript levels of BSMT1 were significantly higher in all accessions including Cvi after induction when compared with control plants. Overall, the data show that the emission of MeSA is reflected in the BSMT1 transcript levels.
Accessions An-1, Col-0, Eri-1, and Kond showed an induced emission of (Z)-3-hexen-1-ol after herbivore feeding. The GLV (Z)-3-hexen-1-ol is one of the compounds shown to result from HPL1 activity (Bate et al., 1998). Transcript levels of HPL1 were induced by P. rapae feeding in most accessions compared with non-treated controls. By contrast, HPL1 transcript levels were not induced in An-1 and Ler after herbivory, whereas the emission of (Z)-3-hexen-1-ol significantly increased in these accessions after P. rapae feeding (Fig. 4A). Therefore, our study does not fully support the earlier findings that only HPL1 transcript abundance correlates with the emission of GLVs (Duan et al., 2005). Moreover, since HPL1 expression and (Z)-3-hexen-1-ol emission were observed in Col-0 plants, our data do not confirm the study by Duan et al. (2005) who reported that a 10-nucleotide deletion in HPL1 results in a loss of function in Col-0 plants. Interestingly, the involvement of HPL1 in the biosynthesis of GLVs was originally shown for the accession ‘Columbia’ (Bate and Rothstein, 1998), which in fact was Col-0 (S Rothstein, personal communication).
Herbivory induced the emission of TMTT in Col-0, Eri-1, and WS, but not in the other accessions. Yet, none of the three accessions had significantly higher TPS4 transcript levels after either treatment than the other accessions. TPS4 has been shown to encode a geranyl linalool synthase. Geranyl linalool is an intermediate in the formation of TMTT (Herde et al., 2008). Furthermore, for accessions with a high TPS4 transcript level after herbivore feeding or JA-treatment, like Cvi and Kond, no significantly induced TMTT emission was observed. The results suggest that TPS4 is not the only regulatory step in TMTT formation or that TMTT emission is not directly correlated with TPS4 transcript level, which is in contrast to Herde et al. (2008). So far it is not known which enzymes convert geranyl linalool into TMTT. Using the co-expression database from RIKEN, it was found that TPS4 expression correlates with the expression of CYP82G1 (r=0.639). As Bruce et al. (2008) postulated a possible involvement of CYP72A13 in TMTT formation, transcript levels of this gene were also monitored after herbivory and JA-application. Indeed, Col-0 and WS, which exhibited a higher emission rate of TMTT compared with other accessions, also showed higher transcript levels of CYP72A13. This finding supports an involvement of CYP72A13 in the conversion of geranyl linalool into TMTT. The other cytochrome P450 gene, CYP82G1, also showed a high correlation with TPS4 transcript levels and was previously shown to be induced in Arabidopsis by several micro-organisms and insects, including P. rapae (De Vos et al., 2005). In most accessions CYP82G1 was induced by P. rapae infestation and JA treatment, but no differences were found between accessions. Thus, although TMTT emission could not be linked directly to either CYP72A13 or CYP82G1 transcription, the results suggest that it is worthwhile to study this in more depth, for example, by analysing the (induced) TMTT emission of knock-out mutants of these genes.
TPS3 has been shown to encode a terpene synthase involved in the biosynthesis of (E)-β-ocimene, (Z)-β-ocimene, and β-myrcene in Arabidopsis (Fäldt et al., 2003). Although TPS3 transcript levels showed no variation between accessions, the herbivore-induced amounts of β-myrcene, (E)-β-ocimene, and (Z)-β-ocimene significantly varied among the accessions. This discrepancy may be explained by differences in substrate availability among the accessions or the presence of another TPS involved in the biosynthesis of one of these monoterpenes.
Chemical ecology addresses the effects of chemical information on interactions within a plant–insect community. The natural variation in herbivore-induced plant volatiles has been explored here between nine accessions of Arabidopsis thaliana obtained from different geographical origins. In addition, it has been investigated whether this variation is also reflected in the transcript levels of the genes that are associated with the formation of some of these volatiles. Genotypic variation has been demonstrated in indirect defence traits, both at the metabolomic level and the transcriptomic level. This enables the use of newly available marker technologies that allow the characterization and positioning of loci that control these types of traits. It would be very interesting to screen recombinant inbred line (RIL) populations of those accessions that have very distinct volatile patterns for their individual volatile compounds and subsequently to perform (expression) quantitative trait locus (eQTL) analysis. These RIL-populations would allow clarification of genetic regulation of HIPV- formation. Finally, it was shown that the genetic variation in induced volatile blends also has consequences for the interactions with members of the third trophic level, i.e. the attraction of carnivorous parasitoids.
Supplementary data
Supplementary data can be found at JXB online.
Supplementary Table S1. Characteristics of the identified induced volatile compounds in the headspace of Arabidopsis thaliana leaves after Pieris rapae herbivory or jasmonic acid treatment.
Supplementary Table S2. Sequences of Arabidopsis thaliana-derived primers used in quantitative Real Time-PCR analyses.
Supplementary Table S3. Results of one-way ANOVA for each compound on differences between JA and Pieris rapae treatment.
Supplementary Table S4. Volatile compounds that are emitted in significantly different rates among nine accessions of Arabidopsis thaliana treated with JA, infested with 20 first instar larvae of Pieris rapae or left untreated (control).
Supplementary Table S5. Significant differences in headspace among accessions after either treatment or left non-treated.
Supplementary Table S6. Results of t tests for each volatile compound on differences between non-treated plants and P. rapae-infested plants, and non-treated plants and JA-treated plants.
Supplementary Table S7. Total number of compounds that are significantly different between accessions
Supplementary Material
Acknowledgments
The authors gratefully acknowledge Leo Koopman, Frans van Aggelen, and André Gidding for culturing the insects, Dick Vreugdenhil and Phillip Reymond for providing us with the Arabidopsis seeds, Suzanne Bulder for technical assistance with behavioural assays, Peter Vredenbregt for technical assistance with RNA isolations, Roel Wagenaar (NIOO) for explaining Winfolia-software, and Erik Poelman for statistical assistance regarding volatile data. The study was supported by a VICI grant (nr 865.03.002) from the Earth and Life Sciences Foundation (ALW) (TALS, RM, and MD), by a Technology Foundation Grant (STW, nr 5479) (IFK), which are both subsidized by the Netherlands Organization for Scientific Research (NWO), by the European Commission contract MC-RTN-CT-2003-504720 ‘ISONET’ (RM), and by the Dutch Ministry of Agriculture, Nature and Food Quality (CB).
References
- Adams RP. Identification of essential oil components by gas chromatography/mass spectrometry. Illinois: Allured Publishing Corporation; 1995. [Google Scholar]
- Agrawal AA, Conner JK, Johnson MTJ, Wallsgrove R. Ecological genetics of an induced plant defense against herbivores: additive genetic variance and costs of phenotypic plasticity. Evolution. 2002;56:2206–2213. doi: 10.1111/j.0014-3820.2002.tb00145.x. [DOI] [PubMed] [Google Scholar]
- Agrawal AA, Karban R. Why induced defenses may be favored over constitutive strategies in plants. In: Tollrian R, Harvell CD, editors. The ecology and evolution of inducible defenses. Princeton, New Jersey: Princeton University Press; 1999. pp. 45–61. [Google Scholar]
- Aharoni A, Giri AP, Deuerlein S, Griepink F, de Kogel WJ, Verstappen FWA, Verhoeven HA, Jongsma MA, Schwab W, Bouwmeester HJ. Terpenoid metabolism in wild-type and transgenic Arabidopsis plants. The Plant Cell. 2003;15:2866–2884. doi: 10.1105/tpc.016253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Blanco C, Aarts MGM, Bentsink L, Keurentjes JJB, Reymond M, Vreugdenhil D, Koornneef M. What has natural variation taught us about plant development, physiology, and adaptation? The Plant Cell. 2009;21:1877–1896. doi: 10.1105/tpc.109.068114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Blanco C, Koornneef M. Naturally occurring variation in Arabidopsis: An underexploited resource for plant genetics. Trends in Plant Science. 2000;5:22–29. doi: 10.1016/s1360-1385(99)01510-1. [DOI] [PubMed] [Google Scholar]
- Ament K, Kant MR, Sabelis MW, Haring MA, Schuurink RC. Jasmonic acid is a key regulator of spider mite-induced volatile terpenoid and methyl salicylate emission in tomato. Plant Physiology. 2004;135:2025–2037. doi: 10.1104/pp.104.048694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura G, Kost C, Boland W. Herbivore-induced, indirect plant defences. Biochimica et Biophysica Acta. 2005;1734:91–111. doi: 10.1016/j.bbalip.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Arimura GI, Huber DPW, Bohlmann J. Forest tent caterpillars (Malacosoma disstria) induce local and systemic diurnal emissions of terpenoid volatiles in hybrid poplar (Populus trichocarpa×deltoides): cDNA cloning, functional characterization, and patterns of gene expression of (-)-germacrene D synthase, PtdTPS1. The Plant Journal. 2004;37:603–616. doi: 10.1111/j.1365-313x.2003.01987.x. [DOI] [PubMed] [Google Scholar]
- Atwell S, Huang YS, Vilhjálmsson BJ, et al. Genome-wide association study of 107 phenotypes in a common set of Arabidopsis thaliana inbred lines. Nature. 2010 doi: 10.1038/nature08800. 10.1038/nature08800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bate NJ, Rothstein SJ. C-6-volatiles derived from the lipoxygenase pathway induce a subset of defense-related genes. The Plant Journal. 1998;16:561–569. doi: 10.1046/j.1365-313x.1998.00324.x. [DOI] [PubMed] [Google Scholar]
- Bate NJ, Sivasankar S, Moxon C, Riley JMC, Thompson JE, Rothstein SJ. Molecular characterization of an Arabidopsis gene encoding hydroperoxide lyase, a cytochrome P-450 that is wound inducible. Plant Physiology. 1998;117:1393–1400. doi: 10.1104/pp.117.4.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwmeester HJ, Kappers IF, Verstappen FWA, Aharoni A, Luckerhoff LLP, Lücker J, Jongsma MA, Dicke M. Exploring multi-trophic plant–herbivore interactions for new crop protection methods. International Congress of Crop Science and Technology, 10–12 November 2003. Glasgow: British Crop Protection Council, Alton, UK 1123–1134; 2003. [Google Scholar]
- Broekgaarden C, Poelman EH, Steenhuis G, Voorrips RE, Dicke M, Vosman B. Responses of Brassica oleracea cultivars to infestation by the aphid Brevicoryne brassicae: an ecological and molecular approach. Plant, Cell and Environment. 2008;31:1592–1605. doi: 10.1111/j.1365-3040.2008.01871.x. [DOI] [PubMed] [Google Scholar]
- Bruce TJA, Matthes MC, Chamberlain K, Woodcock CM, Mohib A, Webster B, Smart LE, Birkett MA, Pickett JA, Napier JA. cis-Jasmone induces Arabidopsis genes that affect the chemical ecology of multitrophic interactions with aphids and their parasitoids. Proceedings of the National Academy of Sciences, USA. 2008;105:4553–4558. doi: 10.1073/pnas.0710305105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukovinszky T, Gols R, Posthumus MA, Vet LEM, van Lenteren JC. Variation in plant volatiles and attraction of the parasitoid Diadegma semiclausum (Hellen) Journal of Chemical Ecology. 2005;31:461–480. doi: 10.1007/s10886-005-2019-4. [DOI] [PubMed] [Google Scholar]
- Chen F, D'Auria JC, Tholl D, Ross JR, Gershenzon J, Noel JP, Pichersky E. An Arabidopsis thaliana gene for methylsalicylate biosynthesis, identified by a biochemical genomics approach, has a role in defense. The Plant Journal. 2003;36:577–588. doi: 10.1046/j.1365-313x.2003.01902.x. [DOI] [PubMed] [Google Scholar]
- D'Alessandro M, Turlings TCJ. Advances and challenges in the identification of volatiles that mediate interactions among plants and arthropods. Analyst. 2006;131:24–32. doi: 10.1039/b507589k. [DOI] [PubMed] [Google Scholar]
- De Boer JG, Posthumus MA, Dicke M. Identification of volatiles that are used in discrimination between plants infested with prey or nonprey herbivores by a predatory mite. Journal of Chemical Ecology. 2004;30:2215–2230. doi: 10.1023/b:joec.0000048784.79031.5e. [DOI] [PubMed] [Google Scholar]
- De Vos M, Van Oosten VR, Van Poecke RMP, et al. Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Molecular Plant–Microbe Interactions. 2005;18:923–937. doi: 10.1094/MPMI-18-0923. [DOI] [PubMed] [Google Scholar]
- Dicke M. Behavioural and community ecology of plants that cry for help. Plant, Cell and Environment. 2009;32:654–665. doi: 10.1111/j.1365-3040.2008.01913.x. [DOI] [PubMed] [Google Scholar]
- Dicke M, Baldwin IT. The evolutionary context for herbivore-induced plant volatiles: beyond the cry for help. Trends in Plant Science. 2010;15:167–175. doi: 10.1016/j.tplants.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Dicke M, Gols R, Ludeking D, Posthumus MA. Jasmonic acid and herbivory differentially induce carnivore-attracting plant volatiles in lima bean plants. Journal of Chemical Ecology. 1999;25:1907–1922. [Google Scholar]
- Dicke M, Hilker M. Induced plant defences: from molecular biology to evolutionary ecology. Basic and Applied Ecology. 2003;4:3–14. [Google Scholar]
- Dicke M, Van Beek TA, Posthumus MA, Ben Dom N, Van Bokhoven H, De Groot AE. Isolation and identification of volatile kairomone that affects acarine predator–prey interactions. Involvement of host plant in its production. Journal of Chemical Ecology. 1990;16:381–396. doi: 10.1007/BF01021772. [DOI] [PubMed] [Google Scholar]
- Dicke M, van Loon JJA. Multitrophic effects of herbivore-induced plant volatiles in an evolutionary context. Entomologia Experimentalis et Applicata. 2000;97:237–249. [Google Scholar]
- Dicke M, Van Poecke RMP. Signaling in plant–insect interactions: signal transduction in direct and indirect plant defence. In: Scheel D, Wasternack C, editors. Plant signal transduction. Oxford, UK: Oxford University Press; 2002. pp. 289–316. [Google Scholar]
- Duan H, Huang MY, Palacio K, Schuler MA. Variations in CYP74B2 (hydroperoxide lyase) gene expression differentially affect hexenal signaling in the Columbia and Landsberg erecta ecotypes of Arabidopsis. Plant Physiology. 2005;139:1529–1544. doi: 10.1104/pp.105.067249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fäldt J, Arimura G, Gershenzon J, Takabayashi J, Bohlmann J. Functional identification of AtTPS03 as (E)-beta-ocimene synthase: a monoterpene synthase catalyzing jasmonate- and wound-induced volatile formation in Arabidopsis thaliana. Planta. 2003;216:745–751. doi: 10.1007/s00425-002-0924-0. [DOI] [PubMed] [Google Scholar]
- Gao LL, Klingler JP, Anderson JP, Edwards OR, Singh KB. Characterization of pea aphid resistance in. Medicago truncatula. Plant Physiology. 2008;146:996–1009. doi: 10.1104/pp.107.111971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez SK, Cox MM, Bede JC, Inoue K, Alborn HT, Tumlinson JH, Korth KL. Lepidopteran herbivory and oral factors induce transcripts encoding novel terpene synthases in Medicago truncatula. Archives of Insect Biochemistry and Physiology. 2005;58:114–127. doi: 10.1002/arch.20037. [DOI] [PubMed] [Google Scholar]
- Halitschke R, Baldwin IT. Antisense LOX expression increases herbivore performance by decreasing defense responses and inhibiting growth-related transcriptional reorganization in Nicotiana attenuata. The Plant Journal. 2003;36:794–807. doi: 10.1046/j.1365-313x.2003.01921.x. [DOI] [PubMed] [Google Scholar]
- Heil M. Indirect defence via tritrophic interactions. New Phytologist. 2008;178:41–61. doi: 10.1111/j.1469-8137.2007.02330.x. [DOI] [PubMed] [Google Scholar]
- Herde M, Gärtner K, Köllner TG, Fode B, Boland W, Gershenzon J, Gatz C, Tholl D. Identification and regulation of TPS04/GES, an Arabidopsis geranyllinalool synthase catalyzing the first step in the formation of the insect-induced volatile C16-homoterpene TMTT. The Plant Cell. 2008;20:1152–1168. doi: 10.1105/tpc.106.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoballah ME, Kollner TG, Degenhardt J, Turlings TCJ. Costs of induced volatile production in maize. Oikos. 2004;105:168–180. [Google Scholar]
- Hoballah MEF, Tamo C, Turlings TCJ. Differential attractiveness of induced odors emitted by eight maize varieties for the parasitoid Cotesia marginiventris: is quality or quantity important? Journal of Chemical Ecology. 2002;28:951–968. doi: 10.1023/a:1015253600083. [DOI] [PubMed] [Google Scholar]
- Horiuchi J, Arimura G, Ozawa R, Shimoda T, Takabayashi J, Nishioka T. Exogenous ACC enhances volatiles production mediated by jasmonic acid in lima bean leaves. FEBS Letters. 2001;509:332–336. doi: 10.1016/s0014-5793(01)03194-5. [DOI] [PubMed] [Google Scholar]
- Kappers IF, Aharoni A, van Herpen T, Luckerhoff LLP, Dicke M, Bouwmeester HJ. Genetic engineering of terpenoid metabolism attracts, bodyguards to Arabidopsis. Science. 2005;309:2070–2072. doi: 10.1126/science.1116232. [DOI] [PubMed] [Google Scholar]
- Kappers IF, Verstappen FWA, Luckerhoff LLP, Bouwmeester HJ, Dicke M. Genetic variation in jasmonic acid- and spider mite-induced plant volatile emission of cucumber accessions and attraction of the predator Phytoseiulus persimilis. Journal of Chemical Ecology. 2010 doi: 10.1007/s10886-010-9782-6. 10.1007/s10886-010-9782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karban R, Baldwin IT. Induced responses to herbivory. Chicago: The University of Chicago Press Ltd; 1997. [Google Scholar]
- Kazan K, Manners JM. Jasmonate signaling: toward an integrated view. Plant Physiology. 2008;146:1459–1468. doi: 10.1104/pp.107.115717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT. Plant responses to insect herbivory: the emerging molecular analysis. Annual Review of Plant Biology. 2002;53:299–328. doi: 10.1146/annurev.arplant.53.100301.135207. [DOI] [PubMed] [Google Scholar]
- Kessler A, Halitschke R, Baldwin IT. Silencing the jasmonate cascade: induced plant defenses and insect populations. Science. 2004;305:665–668. doi: 10.1126/science.1096931. [DOI] [PubMed] [Google Scholar]
- Kliebenstein DJ, Figuth A, Mitchell-Olds T. Genetic architecture of plastic methyl jasmonate responses in Arabidopsis thaliana. Genetics. 2002;161:1685–1696. doi: 10.1093/genetics/161.4.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliebenstein DJ, Kroymann J, Brown P, Figuth A, Pedersen D, Gershenzon J, Mitchell-Olds T. Genetic control of natural variation in Arabidopsis glucosinolate accumulation. Plant Physiology. 2001;126:811–825. doi: 10.1104/pp.126.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M, Alonso-Blanco C, Vreugdenhil D. Naturally occurring genetic variation in Arabidopsis thaliana. Annual Review of Plant Biology. 2004;55:141–172. doi: 10.1146/annurev.arplant.55.031903.141605. [DOI] [PubMed] [Google Scholar]
- Kover PX, Schaal BA. Genetic variation for disease resistance and tolerance among Arabidopsis thaliana accessions. Proceedings of the National Academy of Sciences, USA. 2002;99:11270–11274. doi: 10.1073/pnas.102288999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krips OE, Willems PEL, Gols R, Posthumus MA, Gort G, Dicke M. Comparison of cultivars of ornamental crop Gerbera jamesonii on production of spider mite-induced volatiles, and their attractiveness to the predator Phytoseiulus persimilis. Journal of Chemical Ecology. 2001;27:1355–1372. doi: 10.1023/a:1010313209119. [DOI] [PubMed] [Google Scholar]
- Kusnierczyk A, Winge P, Midelfart H, Armbruster WS, Rossiter JT, Bones AM. Transcriptional responses of Arabidopsis thaliana ecotypes with different glucosinolate profiles after attack by polyphagous Myzus persicae and oligophagous Brevicoryne brassicae. Journal of Experimental Botany. 2007;58:2537–2552. doi: 10.1093/jxb/erm043. [DOI] [PubMed] [Google Scholar]
- Lambrix V, Reichelt M, Mitchell-Olds T, Kliebenstein DJ, Gershenzon J. The Arabidopsis epithiospecifier protein promotes the hydrolysis of glucosinolates to nitriles and influences Trichoplusia ni herbivory. The Plant Cell. 2001;13:2793–2807. doi: 10.1105/tpc.010261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin JC, Young N, Prigge M, Marks MD. The control of trichome spacing and number in Arabidopsis. Development. 1996;122:997–1005. doi: 10.1242/dev.122.3.997. [DOI] [PubMed] [Google Scholar]
- Loivamäki M, Mumm R, Dicke M, Schnitzler J- P. Isoprene interferes with the attraction of bodyguards by herbaceous plants. Proceedings of the National Academy of Sciences, USA. 2008;105:17430–17435. doi: 10.1073/pnas.0804488105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou YG, Hua XY, Turlings TCJ, Cheng JA, Chen XX, Ye GY. Differences in induced volatile emissions among rice varieties result in differential attraction and parasitism of Nilaparvata lugens eggs by the parasitoid Anagrus nilaparvatae in the field. Journal of Chemical Ecology. 2006;32:2375–2387. doi: 10.1007/s10886-006-9151-7. [DOI] [PubMed] [Google Scholar]
- Loughrin JH, Manukian A, Heath RR, Tumlinson JH. Volatiles emitted by different cotton varieties damaged by feeding beet armyworm larvae. Journal of Chemical Ecology. 1995;21:1217–1227. doi: 10.1007/BF02228321. [DOI] [PubMed] [Google Scholar]
- Luo D, Oppenheimer DG. Genetic control of trichome branch number in Arabidopsis: The roles of the FURCA loci. Development. 1999;126:5547–5557. doi: 10.1242/dev.126.24.5547. [DOI] [PubMed] [Google Scholar]
- Matthes MC, Pickett JA, Napier JA. Natural variation in responsiveness of Arabidopsis thaliana to methyl jasmonate is developmentally regulated. Planta. 2008;228:1021–1028. doi: 10.1007/s00425-008-0804-3. [DOI] [PubMed] [Google Scholar]
- Mattiacci L, Dicke M, Posthumus MA. beta-Glucosidase: an elicitor of herbivore-induced plant odor that attracts host-searching parasitic wasps. Proceedings of the National Academy of Sciences, USA. 1995;92:2036–2040. doi: 10.1073/pnas.92.6.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauricio R. Costs of resistance to natural enemies in field populations of the annual plant Arabidopsis thaliana. American Naturalist. 1998;151:20–28. doi: 10.1086/286099. [DOI] [PubMed] [Google Scholar]
- Mewis I, Appel HM, Hom A, Raina R, Schultz JC. Major signaling pathways modulate Arabidopsis glucosinolate accumulation and response to both phloem-feeding and chewing insects. Plant Physiology. 2005;138:1149–1162. doi: 10.1104/pp.104.053389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers BC, Kaushik S, Nandety RS. Evolving disease resistance genes. Current Opinion in Plant Biology. 2005;8:129–134. doi: 10.1016/j.pbi.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Mitchell-Olds T. Arabidopsis thaliana and its wild relatives: a model system for ecology and evolution. Trends in Ecology and Evolution. 2001;16:693–700. [Google Scholar]
- Mumm R, Hilker M. The significance of background odour for an egg parasitoid to detect plants with host eggs. Chemical Senses. 2005;30:337–343. doi: 10.1093/chemse/bji028. [DOI] [PubMed] [Google Scholar]
- Nissinen A, Ibrahim M, Kainulainen P, Tiilikkala K, Holopainen JK. Influence of carrot psyllid (Trioza apicalis) feeding or exogenous limonene or methyl jasmonate treatment on composition of carrot (Daucus carota) leaf essential oil and headspace volatiles. Journal of Agricultural and Food Chemistry. 2005;53:8631–8638. doi: 10.1021/jf0511897. [DOI] [PubMed] [Google Scholar]
- Pieterse CMJ, Dicke M. Plant interactions with microbes and insects: from molecular mechanisms to ecology. Trends in Plant Science. 2007;12:564–569. doi: 10.1016/j.tplants.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Rashotte AM, Jenks MA, Nguyen TD, Feldmann KA. Epicuticular wax variation in ecotypes of Arabidopsis thaliana. Phytochemistry. 1997;45:251–255. doi: 10.1016/s0031-9422(96)00792-3. [DOI] [PubMed] [Google Scholar]
- Reymond P, Bodenhausen N, Van Poecke RMP, Krishnamurthy V, Dicke M, Farmer EE. A conserved transcript pattern in response to a specialist and a generalist herbivore. The Plant Cell. 2004;16:3132–3147. doi: 10.1105/tpc.104.026120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnee C, Kollner TG, Held M, Turlings TCJ, Gershenzon J, Degenhardt J. The products of a single maize sesquiterpene synthase form a volatile defense signal that attracts natural enemies of maize herbivores. Proceedings of the National Academy of Sciences, USA. 2006;103:1129–1134. doi: 10.1073/pnas.0508027103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoonhoven LM, Van Loon JJA, Dicke M. Insect–plant biology. Oxford: Oxford University Press; 2005. [Google Scholar]
- Scutareanu P, Bruin J, Posthumus MA, Drukker B. Constitutive and herbivore-induced volatiles in pear, alder and hawthorn trees. Chemoecology. 2003;13:63–74. [Google Scholar]
- Shiojiri K, Kishimoto K, Ozawa R, Kugimiya S, Urashimo S, Arimura G, Horiuchi J, Nishioka T, Matsui K, Takabayashi J. Changing green leaf volatile biosynthesis in plants: an approach for improving plant resistance against both herbivores and pathogens. Proceedings of the National Academy of Sciences, USA. 2006;103:16672–16676. doi: 10.1073/pnas.0607780103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoeren TAL, De Jong PW, Dicke M. Ecogenomic approach to the role of herbivore-induced plant volatiles in community ecology. Journal of Ecology. 2007;95:17–26. [Google Scholar]
- Snoeren TAL, Van Poecke RMP, Dicke M. Multidisciplinary approach to unravelling the relative contribution of different oxylipins in indirect defense of Arabidopsis thaliana. Journal of Chemical Ecology. 2009;35:1021–1031. doi: 10.1007/s10886-009-9696-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steppuhn A, Baldwin IT. Induced defenses and the cost-benefit paradigm. In: Schaller A, editor. Induced plant resistance to herbivory. Stuttgart: Springer Science Business Media BV; 2008. pp. 61–83. [Google Scholar]
- Steppuhn A, Schuman MC, Baldwin IT. Silencing jasmonate signalling and jasmonate-mediated defences reveals different survival strategies between two Nicotiana attenuata accessions. Molecular Ecology. 2008;17:3717–3732. doi: 10.1111/j.1365-294X.2008.03862.x. [DOI] [PubMed] [Google Scholar]
- Tholl D, Chen F, Petri J, Gershenzon J, Pichersky E. Two sesquiterpene synthases are responsible for the complex mixture of sesquiterpenes emitted from Arabidopsis flowers. The Plant Journal. 2005;42:757–771. doi: 10.1111/j.1365-313X.2005.02417.x. [DOI] [PubMed] [Google Scholar]
- Turlings TCJ, Tumlinson JH, Heath RR, Proveaux AT, Doolittle RE. Isolation and identification of allelochemicals that attract the larval parasitoid, Cotesia marginiventris (Cresson), to the microhabitat of one of its hosts. Journal of Chemical Ecology. 1991;17:2235–2251. doi: 10.1007/BF00988004. [DOI] [PubMed] [Google Scholar]
- Van Poecke RMP, Dicke M. Induced parasitoid attraction by Arabidopsis thaliana: involvement of the octadecanoid and the salicylic acid pathway. Journal of Experimental Botany. 2002;53:1793–1799. doi: 10.1093/jxb/erf022. [DOI] [PubMed] [Google Scholar]
- Van Poecke RMP, Dicke M. Indirect defence of plants against herbivores: using Arabidopsis thaliana as a model plant. Plant Biology. 2004;6:387–401. doi: 10.1055/s-2004-820887. [DOI] [PubMed] [Google Scholar]
- Walling LL. The myriad plant responses to herbivores. Journal of Plant Growth Regulation. 2000;19:195–216. doi: 10.1007/s003440000026. [DOI] [PubMed] [Google Scholar]
- Wu JQ, Hettenhausen C, Schuman MC, Baldwin IT. A comparison of two Nicotiana attenuata accessions reveals large differences in signaling induced by oral secretions of the specialist herbivore. Manduca sexta. Plant Physiology. 2008;146:927–939. doi: 10.1104/pp.107.114785. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.