Abstract
Previous studies from this laboratory have demonstrated a critical role of cytosolic phospholipase A2 (cPLA2) and arachidonic acid in angiotensin II (Ang II) AT2 receptor-mediated signal transduction in renal epithelium. In primary proximal tubular epithelial cells exposed to hydrogen peroxide (H2O2), both the selective cPLA2 inhibitors and the cPLA2 antisense oligonucleotides significantly attenuated H2O2-induced arachidonic acid liberation and activation of p38SAPK, ERK1/2, and Akt1. This H2O2-induced kinase activation was significantly attenuated by a Src kinase inhibitor PP2, or by transient transfection of carboxyl-terminal Src kinase (CSK) that maintained Src in the dormant form. Under basal conditions, Src coimmunoprecipitated with epidermal growth factor receptor (EGFR), while H2O2 increased EGFR phosphorylation in the complex. We observed that inhibition of EGFR kinase activity with AG1478 significantly attenuated H2O2-induced p38SAPK and ERK1/2 activation, but did not inhibit Akt1 activation. Furthermore, it seems that p38SAPK is upstream of ERK1/2 and Akt1, since a p38SAPK inhibitor SB203580 significantly blocked H2O2-induced activation of ERK1/2 and Akt1. Interestingly, overexpression of the dominant-negative p38SAPK isoform α inhibited ERK1/2 but not Akt1 activation. Our observations demonstrate that in these nontransformed cells, activation of cPLA2 is a converging point for oxidative stress and Ang II, which share common downstream signaling mechanisms including Src and EGFR. In addition, p38SAPK provides a positive input to both growth and antiapoptotic signaling pathways induced by acute oxidative stress.
Keywords: Proximal tubular epithelial cells, Oxidative stress, Growth factor, Phospholipase A2, Signal transduction, Protein kinase, Free radical
Introduction
Oxidative stress has a major contributory role in the pathophysiology of various forms of renal disease. As a stable reactive oxygen species (ROS), H2O2 has been shown to be the main initiator of proteinuria associated with acute glomerulonephritis caused by anti-glomerular basement membrane antibody, complements, and phorbol ester acetate [1-3]. Exposure of freshly isolated rat renal cortical cells to H2O2 produced significant cell death [4]. H2O2 also increased the transepithelial electrical conductance of cultured MDCK cells, as the result of an increase of the paracellular pathway permeability associated with cytoskeleton reorganization, particularly in the area of cell-to-cell junctions [5]. It is clear that at least a portion of ROS is generated by infiltrated phagocytes in disease states [6,7]; however, the mechanisms of de novo ROS production and oxidative signaling pathways within the renal epithelium have not been clarified.
Oxidative stress has been linked to activation of members of the MAP kinase superfamily in various cell types. ERK1/2 are ubiquitously expressed and generally considered to be responsible for mediating the mitogenic and differential effects of growth factors, while p46/p54SAPK and p38SAPK respond to environmental stress and play an important role in apoptosis [8]. Particularly, p38SAPK mediates Ang II- and ROS-induced hypotrophy which was only partially dependent on ERK activation in vascular smooth muscle cells [9]. Moreover, p38SAPK has been found to inhibit apoptosis, presumably via Akt1 activation by forming a PDK2-like complex upon stimulation [10]. Therefore, we hypothesize that p38SAPK plays an essential role in facilitating survival in renal epithelium in the presence of oxidative stress.
Previous studies from this laboratory and others have shown that Ang II activates ERK1/2 in primary proximal tubular cells. Transactivation of the EGFR tyrosine kinase is an upstream element of shc/grb2/sos-mediated ras activation in our system [11,12]. Recently, we have demonstrated that arachidonic acid and/or its metabolites mediate all downstream effects of Ang II in activation of members of the MAPK, i.e., the ERK1/2, p46/p54SAPK, and p38SAPK [13,14]. Arachidonic acid also induces ROS production in these proximal tubular epithelial cells [15], which provides mechanism for endogenous ROS generation [16]. However, ERK, but not p38SAPK, was suggested to be responsible for Ang II-induced and ROS-mediated hypertrophy in MCT and LLCPK1 cells, cell lines with proximal tubular phenotype [17]. Therefore, the current studies were designed to determine the regulation of oxidative signaling in primary proximal tubular cells based on the hypothesis that arachidonic acid is a common second messenger for oxidative stress and Ang II receptor activation.
Materials and methods
Materials
Cell culture medium, additives, and LipofectAmine for transfection were from Gibco BRL (Gaithersburg, MD). [3H] Arachidonic acid was obtained from Dupont/NEN (Boston, MA). Rabbit polyclonal anti-phospho antibodies recognizing activated p38SAPK, ERK1/2, Akt1, and cPLA2 were from Cell Signaling (Beverly, MA). Polyclonal anti-p38SAPK, anti-ERK1/2, anti-Akt1, anti-p110α, and anti-cPLA2 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The anti-c-Src and anti-EGFR goat polyclonal, anti-ras, anti-vSrc, anti-phosphotyrosine, and anti-phospho-EGFR monoclonal antibodies were purchased from Upstate Biotechnology (Lake Placid, NY). SB203580, PD98059, LY294002, arachidonyltrifluoromethyl ketone (AACOCF3), methyl arachidonyl fluorophosphate (MAFP), oleyloxyethyl phosphorylcholine (OPC), and PP2 were from CalBiochem (San Diego, CA).
Cell culture
Proximal tubule epithelial cells were isolated from New Zealand White rabbits as previous described [15]. They were maintained for 9–14 days in modified DMEM:F12 (1:1) media supplemented with 5% fetal calf serum, 5 μg/ml insulin, 5 μg/ml transferrin, 0.5 μM hydrocortisone, 350 μg/ml l-glutamine, 100 unit/ml penicillin, and 100 μg/ml streptomycin. Subcon-fluent monolayers of first-passage cells were employed for experiments.
Arachidonic acid liberation
Cells grown on 12-well plates to subconfluence were labeled with 0.5 μCi/ml/well [3H]arachidonic acid for 3 h prior to treatment. They were washed 3 times with DMEM medium containing 1 mg/ml fatty acid-free bovine serum albumin to remove free labels. After treatment of cells, the medium was removed and the released arachidonic acid was determined by scintillation counting. Each data point is the average from at least three wells.
Rabbit cPLA2 antisense
The amount of 5 μM of each of the two phosphorothioate modified antisense oligonucleotides was introduced to cells in serum-free medium 24 h prior to experiment, with control cells treated with corresponding sense oligonucleotides. The antisense oligonucleotides were: 5′-GATCTATAAATGACATTTTGGT-3′ and 5′-ATCCGGGGTGTCAAGCATGTCA-3′. They were designed to target the sequences 1–22 and 114–135 that contain the two possible translation starting codons.
Immunoblot and immunoprecipitation
Cells were serum-deprived for 20-24 h prior to experiments. Following experimental treatments, the cells were washed twice with ice-cold Dubecco's phosphate-buffered saline. They were then lysed on ice with lysis buffer (50 mM Tris, pH 7.2, 1% (vol/vol) Triton X-100, 1 mM Na3VO4, 1 mM EGTA, 0.2 mM phenylmethanesulfonyl fluoride, 25 μg/ml leupeptin, and 10 μg/ml aprotinin). The samples were centrifuged at 14,000g for 10 min. Protein content in the supernatants was determined by the BCA assay according to the manufacturer's instructions (Pierce, Rockford, IL). Fifteen micrograms of total cell lysate protein was subjected to SDS-PAGE and then transferred to a polyvinylidene difluoride membrane by electroblotting at 200 mA for 1.5 h. The membrane was immunoblotted according to Western blot protocol furnished by the manufacturer, and the immunoreactive proteins were detected by enhanced chemiluminescence (ECL) (Amersham Pharmacia Biotech Inc., Piscataway, NJ). All experiments had at least one membrane reprobed with antibodies recognizing nonphosphorylated kinases to confirm equal protein loading. The exposure autoradiograph was analyzed by the OS-Scan image analysis system to obtain the densitometry data. For immuno-precipitation, each cell lysate containing 1 mg protein was mixed with 10 μg antibody and 40 μl protein A + G agarose bead suspension. The bead pellet was washed 3 times and then heated in Laemmli's sample buffer. The supernatant was resolved by 10% SDS-PAGE. The associated protein was detected by immunoblot.
Src GST-SH2 fusion protein and pull-down experiment
Src SH2 sequence (amino acids 140–250) was retrieved from chicken c-Src cDNA plasmid by PCR and cloned into pGEX-2T vector and transformed into BL21 Escherichia coli. The sequence was verified by sequencing both strands at Cleveland Genomic LT with ABI PRISM, Model 377. Standard protocol (Pharmacia) was employed to prepare the GST fusion protein conjugated with glutathione Sepharose 4B beads. The preparation was washed and resuspended to 1:1 slurry in PBS containing 0.1% azide, 0.1% bovine serum albumin. Twenty microliters GST fusion protein slurry was mixed with 1 mg cell lysate prepared in SDS/RIPA buffer (150 mM NaCl, 50 mM Tris-HCl, pH 7.5, 0.25% sodium deoxycholate, 0.1% Nonidet P-40, 100 μM NaVO4,1 mM NaF, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 25 μg/ml leupeptin, 0.1% SDS) at 4°C for 2 h. The samples were centrifuged and washed for 3 times in lysis buffer to remove nonspecific bound protein. The final pellets were boiled in Laemmli's sample buffer and resolved by 8% SDS-PAGE. The associated EGF receptor was detected by anti-EGF receptor immunoblot.
cDNA transient transfection
Cells grown on 6-well plates were transfected using LipofectAmine 2000 with 2 μg/well of CSK plasmid or p38SAPK α (TGY → AGF) plasmid or control plasmid for mock transfection. These cells were employed 48–72 h following transfection.
Data analysis
The obtained densitometry arbitrary units varied from blot to blot depending on the exposure time of ECL. Therefore, data were converted to percentage of the resting value (set as 100%). The basal effect of a putative inhibitor was first compared with resting value, and then the effect on H2O2-stimulated kinase activation was calculated as (change of kinase activity in the presence of inhibitor/change of kinase activity in the absence of inhibitor) × 100. The significance was determined by the paired two-tailed Student t test, with p ≤ 0.05 designated as significant. The effects of putative inhibitors or antisense on arachidonic acid release were calculated as above, except that the raw data are the mean of released radioactive counts (percentage of total) from three wells.
Results
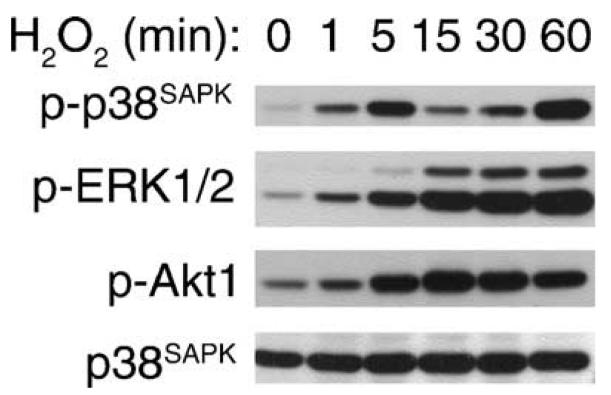
We used H2O2 as the model molecule to initiate intracellular oxidative stress, as this molecule mediates renal pathologies and is readily permeable to the plasma membrane. We have previously employed immune complex kinase assay and demonstrated in primary proximal tubular epithelial cells that H2O2 dose dependently activates p46/p54SAPK [15]. In the current studies, activation of p38SAPK, ERK1/2, and Akt1 was determined by immunoblot with anti-phospho-p38SAPK (Thr180/Tyr182), anti-phospho-ERK1/2 (Thr202/Tyr204), or anti-phospho-Akt1 (Ser473) antibodies which recognize the activated enzymes. Therefore, we refer to the phosphorylation as enzyme activation in the text. We have chosen 0.5 mM H2O2 to stimulate cells according to a dose-dependent activation of Akt1 in preliminary studies (not shown). As shown in Fig. 1, when cells were exposed to 0.5 mM H2O2, the activation of p38SAPK peaked at 5 min (8.9 ± 5-fold) and subsided, which was followed by a second activation that peaked at 60 min. At 2 and 4 h, p38SAPK activation was 118 ± 37 and 51 ± 16% of the 60 min value, respectively. The activation of ERK1, ERK2, and Akt1 was significant at 5 min (2.5 ± 0-fold, 2.3 ± 0-fold, and 7.8 ± 2-fold, respectively) and was sustained for 1 h. During this time frame, there was no change of protein expression level (bottom panel) for p38SAPK.
Fig. 1.
Kinetics of kinase activation: 0.5 mM H2O2 was added to quiescent cells for the time period indicated. Fifteen micrograms of each cell lysate was resolved by 10% SDS-PAGE. Activation of p38SAPK, ERK1/2, and Akt1 was detected by immunoblotting with anti-phospho antibodies that recognize the active forms of the kinases. p38SAPK protein level and equal protein loading are shown at the bottom.
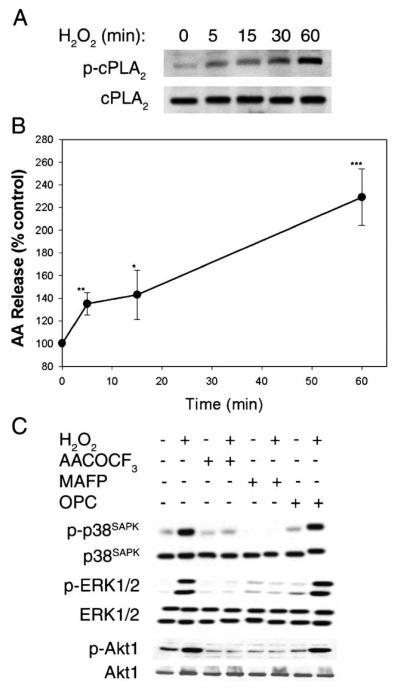
Our previous studies demonstrated that cPLA2 activation is critical for Ang II-induced MAP kinase activation in these cells. We decided to investigate if cPLA2 serves as a converging point of oxidative stress- and growth hormone-induced signaling. We observed that H2O2 time dependently induced cPLA2 activation, by phosphorylation at Ser505[18], in these proximal tubular cells (Fig. 2A). We then measured H2O2–induced arachidonic acid liberation from [3H]arachidonic acid-labeled cells. A significant 135 ± 6% increase was observed within 5 min, and reached 230 ± 15% of the control level at 60 min (Fig. 2B). Our experiments have revealed that the presence of 3 mM EGTA or pretreatment of cells with a nonspecific PLA2 inhibitor mepacrine significantly blocked above H2O2-induced arachidonic acid liberation (not shown). We then pretreated cells with two structurally unrelated specific cPLA2 inhibitors arachidonyl trifluoromethyl ketone and methyl arachidonyl fluorophosphate [19-21]. Both inhibitors blocked H2O2-induced p38SAPK, ERK1/2, and Akt1 activation (Fig. 2C). AACOCF3 also blocked H2O2-induced arachidonic acid release by 66–80% in two independent experiments (data not shown). In contrast, H2O2-induced activation of kinases was not inhibited by oleyloxyethyl phosphorylcholine, an inhibitor of secretory PLA2 (Fig. 2C).
Fig. 2.
(A) Time course of H2O2-induced cPLA2 activation: 0.5 mM H2O2 was added to quiescent cells for the time period indicated. Fifteen micrograms of each cell lysate was resolved by 7.5% SDS-PAGE. Activation of cPLA2 was detected by immunoblotting with anti-phospho-cPLA2 antibody (top panel). The same blot was stripped and reprobed with control anti-cPLA2 antibody showing equal loading (lower panel). (B) Time course of H2O2-induced arachidonic acid release. Cells were labeled with [3H]arachidonic acid and were treated with 0.5 mM H2O2 for the time period indicated. Aliquots of medium were counted in the scintillation fluid and calculated as a percentage of radioactivity released from cells exposed to medium alone for the same length of time. Arachidonic acid release was calculated from at least 3 determinations of two similar experiments (*p < 0.05, **p < 0.01, ***p < 0.001). (C) Effects of PLA2 inhibitors on H2O2-induced p38SAPK, ERK1/2, and Akt1 phosphorylation. Quiescent cells were incubated with or without 10 μM AACOCF3,10 μM MAFP, or 10 μM OPC for 30 min followed by exposure to 0.5 mM H2O2 for 5 min. The cell lysates were subjected to immunoblot analysis as described in Fig. 1 legend. The same blot was stripped and reprobed with control anti-p38SAPK, anti-ERK1/2, and anti-Akt1 antibodies showing equal loading (lower panels).
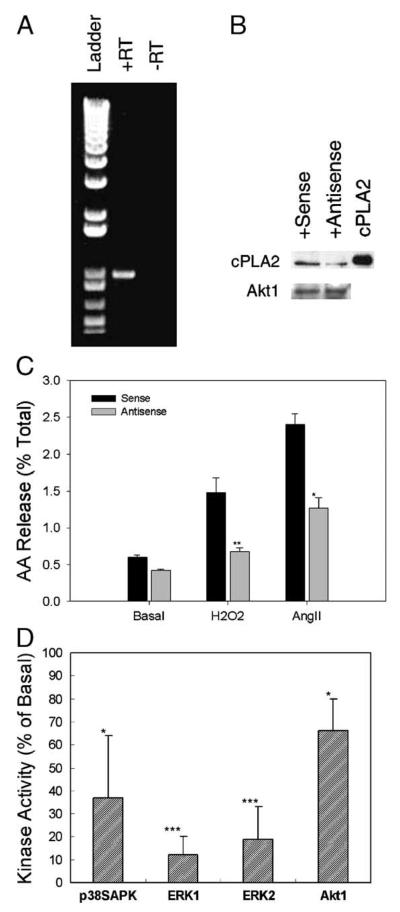
To further investigate the role of cPLA2 in the signal transduction, we cloned the full-length rabbit cPLA2 cDNA (GenBank Accession No. AF204923) by RT-PCR of total RNA extracted from heart tissue of New Zealand White rabbit. We verified the presence of cPLA2 in rabbit renal epithelial cells. As shown in Fig. 3A, a 999-bp fragment was amplified from renal epithelial cell total RNA employing the primers designed with the heart tissue cPLA2 sequence. With introduction of the cPLA2 antisense oligonucleotides that target the translation starting sites of cPLA2, we observed a decreased expression of cPLA2 protein by 50% as compared to cells treated with sense oligonucleotides (Fig. 3B). As expected, expression of another protein, Akt1, which is also involved in the H2O2 signaling, was not affected by this antisense treatment. The antisense treatment attenuated arachidonic acid release induced by H2O2 and Ang II to 23 ± 7 and 31 ± 22%, respectively, as compared to those treated with sense oligonucleotides (Fig. 3C). Furthermore, introduction of the cPLA2 antisense oligonucleotides significantly attenuated activation of p38SAPK, ERK1/2, and Akt1 to 37 ± 27, 12 ± 8 (ERK1), 19 ± 14 (ERK2), and 66 ± 14%, respectively, as compared to levels in cells exposed to sense oligonucleotides (Fig. 3D). Therefore, we have demonstrated that H2O2-induced kinase activation is mediated by a cPLA2-dependent release of arachidonic acid in these cells. It indicates that oxidative stress (represented by H2O2) and growth hormones (represented by Ang II) share a common signaling mechanism in these cells.
Fig. 3.
(A) Amplification of cPLA2 by RT-PCR. Total RNA was extracted using a Qiagen kit and was reverse-transcribed (RT) with random hexamer primers. The primers used for PCR were 5′-GGAGATCACATTGATGGATGC-3′ (forward) and 5′-TCTTCCTCCATTGTGGAACC-3′ (reverse). (B) Protein expression. The cPLA2 protein expression was detected by immunoblot of 15 μg lysate from sense or antisense oligonucleotide-treated cells employing anti-mouse cPLA2 antisera. Mouse cPLA2 was employed as standard. Membrane of above blot was stripped followed by immunoblotting with anti-Akt1 antibody. (C) Effects of cPLA2 antisense on H2O2- and Ang II-induced arachidonic acid release. Cells treated with sense or antisense oligonucleotides were labeled with [3H]arachidonic acid and treated with 0.5 mM H2O2 or 1 μM Ang II for 5 min. Arachidonic acid release was determined as described in Fig. 2B legend and the results are presented as the percentage of released arachidonic acid of total label. The mean of two experiments is shown with 3 replicates in each experiment. (D) Effects of cPLA2 antisense on H2O2-induced kinase activation. Cells treated with cPLA2 sense or antisense oligonucleotides were exposed to 0.5 mM H2O2 for 5 min. Kinase activities were determined as described in Fig. 1 legend (*p < 0.05, **p < 0.01, ***p < 0.001).
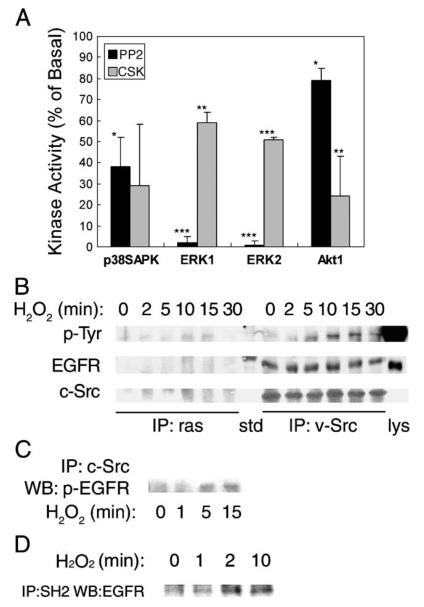
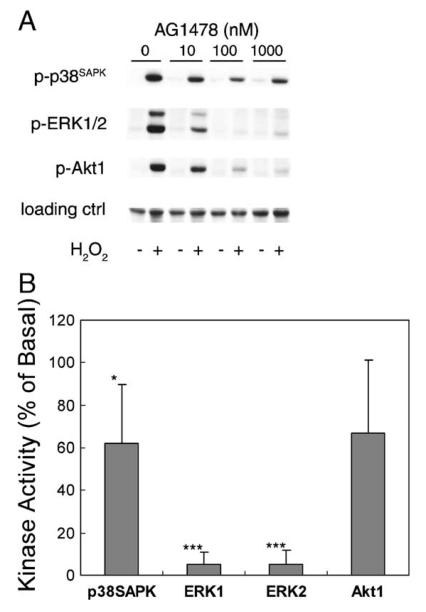
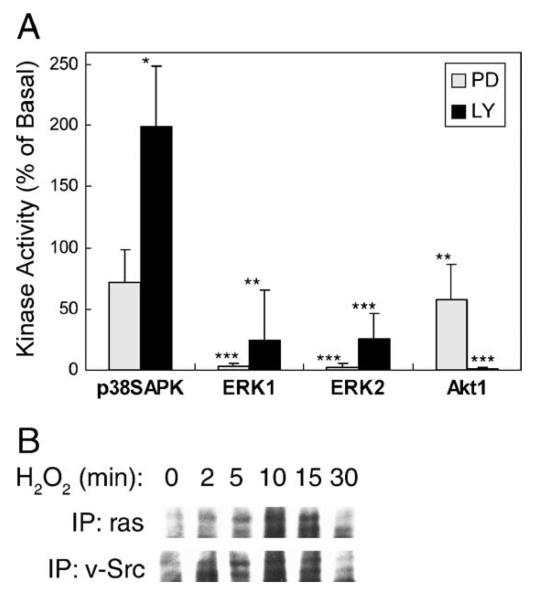
We have previously demonstrated that arachidonic acid mimics Ang II in activation of Src tyrosine kinase, induction of Src and EGFR association via SH2 domain, transactivation of the EGFR tyrosine kinase, activation of small GTPase ras, and activation of downstream ERK1/2 [11,12,14,22]. Experiments were designed to test whether Src and EGFR tyrosine kinases are responsible for mediating H2O2-induced signaling in these epithelial cells. As shown in Fig. 4A, a specific Src-family kinase inhibitor PP2 significantly attenuated p38SAPK activation to 38 ± 14%, blocked ERK1/2 activation to 2 ± 3 and 1 ± 2%, and decreased Akt1 activation to 79 ± 6%, at a submaximum dose of 5 μM (effective range 1–50 μM, not shown). Alternatively, transient transfection of CSK phosphorylates Tyr529 in Src C-terminus and keeps the Src SH2 domain intramolecularly bond [22,23]. This manipulation also significantly attenuated H2O2-induced ERK1/2 and Akt1 activation to 59 ± 5, 51 ± 1, and 24 ± 19%, respectively. Immunoprecipitation with anti-v-Src antibody and blotting with anti-total EGFR antibody indicated a constitutive association of Src and EGFR in these cells (Fig. 4B, middle panel). However, H2O2 induced tyrosine phosphorylation of the Src-associated EGFR, which peaked at 10 min (Fig. 4B, top panel). In parallel, H2O2 induced the association of phosphorylated EGFR with ras, suggesting induction of downstream ERK1/2 signaling as seen with Ang II stimulation. The induction of Src-phospho-EGFR association was confirmed with Western blot analysis using anti-phospho-EGFR antibody (Fig. 4B), as well as with in vitro pull-down experiments employing immobilized Src-SH2 protein (Fig. 4C). Further, an EGFR kinase inhibitor AG1478 [23] dose dependently attenuated H2O2–induced kinase activation (Fig. 5A). But AG1478 was obviously more effective in blocking ERK1/2 activation (5 ± 6 and 5 ± 7%, respectively) than p38SAPK (62 ± 28%) and Akt1 (67 ± 37%) activation (Fig. 5B). Taken together, our data confirmed the existence of Src/EGFR-dependent ERK1/2 pathway induced by acute oxidative stress.
Fig. 4.
Assessment of the role of Src. (A) Cells pretreated with 5 μM PP2 for 30 min or transfected with Csk plasmid were stimulated with 0.5 mM H2O2 for 5 min. Kinase activities were determined as described in Fig. 1 legend (*p < 0.05, **p < 0.01, ***p < 0.001). (B) Cells were lysed following treatment with 0.5 mM H2O2 for the time period indicated. One milligram total protein was immunoprecipitated with either anti-ras (left) or anti-v-Src (right) monoclonal antibody followed by blotting with (a) anti-phosphotyrosine antibody (shown are bands that resolve at 180 kDa); (b) anti-EGF receptor antibody; and (c) anti-c-Src antibody; std, molecular weight standard; Lys, lysate of EGF-treated A431 cell as positive control. (C) Cells were lysed following treatment with 0.5 mM H2O2 for 0-15 min. Lysates containing 1 mg total protein were immunoprecipitated with anti-c-Src goat antibody followed by blotting with anti-phospho-EGFR monoclonal antibody. (D) Association of the SH2 domain of Src with the EGF receptor. Cells were lysed following treatment with 0.5 mM H2O2 for the time period indicated. Cell lysates were mixed with GST-SH2 fusion protein conjugated with agarose beads. The pull-down products were resolved by SDS-PAGE and immunoblotted with anti-EGF receptor antibody.
Fig. 5.
Assessment of the role of EGFR. (A) Cells were exposed to 0–1000 nM AG1478 for 30 min prior to stimulation with 0.5 mM H2O2 for 5 min. (B) Cells pretreated with 100 nM AG1478 for 30 min were stimulated with 0.5 mM H2O2 for 5 min. Kinase activities were detected as described in Fig. 1 legend (*p < 0.05, **p < 0.01, ***p < 0.001).
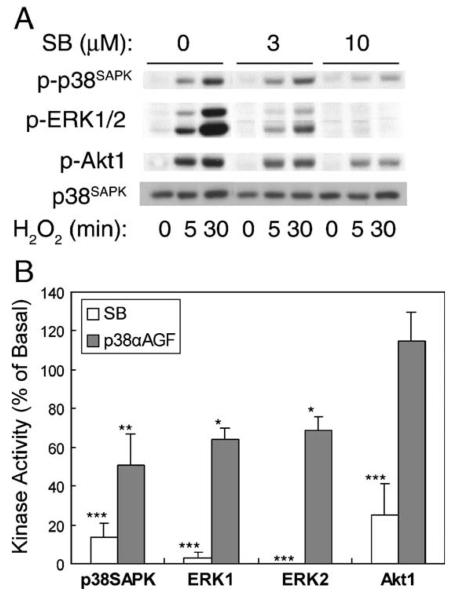
The pyridinyl imidazole compound SB203580 has been reported to inhibit p38SAPK isoforms [24]. Pretreatment of renal proximal tubular cells with SB203580 dose dependently inhibited H2O2-induced early and sustained p38SAPK activation at 5 and 30 min, respectively, while ERK1/2 and Akt1 activation was attenuated as well (Fig. 6A). Fig. 6B shows that 10 μM SB203580 pretreatment decreased 5 min H2O2 stimulation of p38SAPK to 14 ± 7% as compared to values in the absence of the inhibitor, while activation of ERK1/2 and Akt1 was decreased to 3 ± 3, 0 ± 0, and 25 ± 16%, respectively. In addition, we employed the dominant-negative p38SAPK α (TGY → AGF) plasmid to transiently transfect the cells. Interestingly, expression of the dominant-negative p38SAPKαAGF only significantly attenuated p38SAPK and ERK1/2 activation following a 30 min H2O2 exposure (51 ± 16, 64 ± 6, and 69 ± 7%, respectively). Akt1 activation was not affected. With these data, we conclude that p38SAPK differentially regulates the ERK1/2 and Akt1 pathways in renal epithelial cells.
Fig. 6.
Assessment of the role of p38SAPK. (A) Cells were incubated with 0–10 μM SB203580 for 20 min prior to stimulation with 0.5 mM H2O2 for 5 and 30 min, respectively. (B) Cells were pretreated with 10 μM SB203580 followed by stimulation with H2O2 for 5 min, or transfected with the dominant-negative p38αAGF plasmid followed by stimulation with H2O2 for 30 min. Kinase activities were determined as described in Fig. 1 legend (*p < 0.05, **p < 0.01, ***p < 0.001).
The relationship between the ERK1/2 and Akt1 pathways was next examined. As shown in Fig. 7A, the specific ERK1/2 pathway inhibitor PD98059 did not affect p38SAPK activation (72 ± 27%), while it completely blocked ERK1/2 activation (3 ± 2 and 2 ± 3%, respectively). PD98059 also attenuated Akt1 activity to 58 ± 28%. On the other hand, the specific inhibitor of the PI3-kinase/Akt1 pathway LY20094 completely blocked Akt1 activation (1 ± 1%) and decreased ERK1/2 activation to 24 ± 41 and 25 ± 22%, respectively. By contrast, LY20094 significantly potentiated p38SAPK activation to 199 ± 50%. With immunoprecipitation employing anti-ras antibody, we observed that H2O2 stimulated association of the PI3-kinase subunit p110α with ras, which peaked at 10 min. The association of p110α with Src was also found increased in a similar pattern (Fig. 7B).
Fig. 7.
Assessment of the crosstalk between the pathways. (A) Cells were incubated with 30 μM PD98059 or 50 μM LY294002 for 20 min prior to stimulation with 0.5 mM H2O2 for 5 min. Kinase activities were detected as described in Fig. 1 legend (*p < 0.05, **p < 0.01, ***p < 0.001). (B) Cells were lysed following treatment with 0.5 mM H2O2 for the time period indicated. One milligram total protein was immunoprecipitated with either anti-ras or anti-v-Src monoclonal antibody followed by immunoblotting with anti-p110α antibody. Two protein bands were seen in the blots and in positive control lysate (not shown).
Discussion
The current work establishes a signal transduction pathway whereby H2O2 induced activation of cPLA2, Src, EGFR, and p38SAPK that link two downstream kinases, i.e., ERK1/2 and Akt1 in kidney epithelial cells. The conclusion that cPLA2 is a key upstream enzyme which mediates the oxidative signaling is based on observations that (1) cPLA2 is activated by H2O2; (2) specific cPLA2 inhibitors (AACOCF3 and MAFP) and the cPLA2 antisense oligonucleotides blocked H2O2-induced arachidonic acid release and activation of all kinases in the signaling pathway; and (3) other PLA2 family members such as the secretory PLA2 play a negligible role as we could not detect their existence in these cells by immunoblot, and inhibitors such as OPC [25] and sPLA2-IIA Inhibitor I (CalBiochem) [26] were not effective (L.D. Alexander, unpublished observations). To our knowledge this is the first study in nontransformed cells to show that oxidative stress induces cPLA2 activation and shares a common signaling mechanism of Ang II.
Arachidonic acid is a polyunsaturated fatty acid with significant importance in membrane structural integrity and function [27]. It is also a regulator of oxidative status documented by its role in activation of NADPH oxidase to generate ROS in the epithelial cells used herein and other cell types [15,28,29]. Recent studies demonstrate that exposure of these renal epithelial cells to exogenous arachidonic acid and/or its metabolites differentially activate ERK1/2, p46/p54SAPK, and p38SAPK [13]. On the other hand, both ERK2 and p38SAPK have been shown to activate cPLA2 in various cell systems [18,30,31]. Therefore, our work provides a potential mechanism for feed forward of the oxidative signaling at multiple levels. That is, a combined effect of arachidonic acid and H2O2 could yield significant damage to the kidney.
The Src family of nonreceptor tyrosine kinases have been reported to be activated by ROS in various cell types [32,33]. Environmental stress, including ROS, has also been shown to induce MAP kinase pathway activation via activation of receptor tyrosine kinases [34,35]. A number of mechanisms have been proposed for ROS-induced EGFR activation [36]. In some cell lines, Src is responsible for direct activation of the EGFR through phosphorylating essential tyrosine residues on EGFR [37,38]. In renal epithelium, Src tyrosine kinase activation and transactivation of EGFR were found to activate the ERK1/2 pathway in responding to Ang II or arachidonic acid stimulation [11,14]. A physical association of Src and EGFR was observed in these cells under basal conditions. However, H2O2 induces Src-bond EGFR phosphorylation, that may be via new formation through the Src SH2 domain. It seems that Src-dependent oxidative signaling in these cells is complex. Inhibition of Src activation with PP2 or CSK transient transfection significantly decreased kinase activation, however, with different profiles. PP2 was more efficient in ERK1/2 inhibition and CSK was more efficient in Akt1 inhibition (Fig. 4A). It suggests that the Src and EGFR kinase activities are essential for ERK1/2 activation, while Akt1 might be regulated by conformational change of Src or perhaps Src association with proximal Akt1 effectors, rather than being an EGFR kinase target.
It is interesting that H2O2 induces p38SAPK activation with different kinetics (Fig. 1). At present we are unsure whether the biphasic activation of p38SAPK is a result of activation of two different isoforms of p38SAPK or a consequence of simultaneous activation of protein phosphatases that dephosphorylates and inactivates p38SAPK after the immediate activation phase. Protein tyrosine phosphatase 2C has been reported to directly interact with p38SAPK [39], and MKP-1 is another potential candidate [40]. It is worth noting that the p38SAPK isoforms have been reported to differentially regulate cell functions such as cardiac myocyte hypertrophy and apoptosis [41]. In addition, a p38SAPKα activation mechanism that depends on interaction with transforming growth factor-β-activated protein kinase 1, rather than MEK, has been elucidated [42]. In human kidney, β and α isoforms of the p38SAPK were identified by Northern blot [43,44]. Indeed, we have amplified two p38SAPK -related fragments from the rabbit epithelial cells employing RT-PCR (data not shown). The current data favor activation of distinct p38SAPK isoforms for the following reasons: (1) although SB203580 blocked both ERK1/2 and Akt1 activation which suggests that both kinases are regulated by p38SAPK, the dominant-negative p38α construct blocked the delayed phase of p38SAPK and the ERK1/2 pathway rather than the Akt1 pathway; (2) inhibition of the EGF receptor kinase with AG1478 or the Src kinase with PP2 blocked ERK1/2 activation profoundly, but had moderate effect on Akt1 activation. As a matter of fact, in all our experiments, Akt1 inhibition would not be obvious if p38SAPK inhibition were not deep or complete. Therefore, we propose that the two activation phases of p38SAPK may have distinct physiological roles. Although the current study focused more on the acute phase of oxidative stress, it is important to further study the mechanism of p38SAPK activation starting with cloning the p38SAPK isoforms.
There are apparent interactions of the ERK1/2 and Akt1 pathways because the inhibitors of either pathway were partially effective on each other. We realize that what we observed here could be cell type specific. For example, in opossum kidney proximal tubular cells, both kinases mediate the proliferating effect of lysophosphatidic acid without converging to each other [45]. However, PI3-kinase-dependent ERK activation has been shown to mediate insulin-induced protein synthesis in renal epithelial cells[46]. At the upstream level, ras-dependent activation of PI3-kinase has been elucidated both in vivo and in vitro [47,48]. H2O2 induces association of the p110α subunit of PI3-kinase with ras and Src in the renal epithelial cells. It is then possible that Src, EGFR, ras, and PI3-kinase form a dynamic protein complex that is regulated by acute oxidative input. These data also reveal the complexity of the signaling pathways and suggest, again, regulation at multiple levels.
Thus in summary, our work provides the novel observation that in renal epithelial cells, cPLA2 activation and arachidonic acid release are critical mediators of oxidative stress signaling in a manner similar to Ang II AT2 receptor signaling. The current studies also demonstrated that p38SAPK is an essential oxidative mediator, as it transduces the signal to at least two downstream pathways, i.e., ERK1/2 and Akt1. It extends our previous observations wherein arachidonic acid-dependent ROS generation via NADPH oxidase was responsible for activation of p46/p54SAPK in these same kidney epithelial cells [15]. Together, the data provide a signaling paradigm for ROS-induced renal pathology.
Acknowledgments
This work was supported by American Heart Association Mid-America Research Consortium Beginning Grant-in-Aid Award 9806215 and National Heart, Lung, and Blood Institute Grants HL41618 and HL04363. We thank Nnennaya Nkemere for technical assistance. We thank Dr. Roger Davis for providing p38SAPK plasmid and Dr. Sarah Courtnadge for CSK plasmid.
Abbreviations
- H2O2
hydrogen peroxide
- ROS
reactive oxygen species
- Ang II
angiotensin II
- cPLA2
cytosolic phospholipase A2
- EGFR
epidermal growth factor receptor
- SAPK
stress-activated protein kinase
- CSK
Src carboxyl-terminal kinase
- SDS-PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- AACOCF3
arachidonyltrifluoromethyl ketone
- MAFP
methyl arachidonyl fluorophosphates
- OPC
oleyloxyethyl phosphorylcholine
References
- 1.Rehan A, Johnson KJ, Wiggins RC, Kunkel RG, Ward PA. Evidence for the role of oxygen radicals in acute nephrotoxic nephritis. Lab. Invest. 1984;51:396–403. [PubMed] [Google Scholar]
- 2.Rehan A, Johnson KJ, Kunkel RG, Wiggins RC. Role of oxygen radicals in phorbol myristate acetate-induced glomerular injury. Kidney Int. 1985;27:503–511. doi: 10.1038/ki.1985.39. [DOI] [PubMed] [Google Scholar]
- 3.Kerjaschki D, Neale TJ. Molecular mechanisms of glomerular injury in rat experimental membranous nephropathy (Heymann nephritis) J. Am. Soc. Nephrol. 1996;7:2518–2526. doi: 10.1681/ASN.V7122518. editorial. [DOI] [PubMed] [Google Scholar]
- 4.Keane WF, Welch R, Gekker G, Peterson PK. Mechanism of Escherichia coli alpha-hemolysin-induced injury to isolated renal tubular cells. Am. J. Pathol. 1987;126:350–357. [PMC free article] [PubMed] [Google Scholar]
- 5.Welsh MJ, Shasby DM, Husted RM. Oxidants increase paracellular permeability in a cultured epithelial cell line. J. Clin. Invest. 1985;76:1155–1168. doi: 10.1172/JCI112071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauer V, Bauer F. Reactive oxygen species as mediators of tissue protection and injury. Gen. Physiol. Biophys. 1999;18:7–14. [PubMed] [Google Scholar]
- 7.Clark RA. Activation of the neutrophil respiratory burst oxidase. J. Infect. Dis. 1999;179(Suppl 2):S309–S317. doi: 10.1086/513849. [DOI] [PubMed] [Google Scholar]
- 8.Woodgett JR, Avruch J, Kyriakis J. The stress activated protein kinase pathway. Cancer Surv. 1996;27:127–138. [PubMed] [Google Scholar]
- 9.Ushio-Fukai M, Alexander RW, Akers M, Griendling KK. p38 Mitogen-activated protein kinase is a critical component of the redox-sensitive signaling pathways activated by angiotensin II. Role in vascular smooth muscle cell hypertrophy. J. Biol. Chem. 1998;273:15022–15029. doi: 10.1074/jbc.273.24.15022. [DOI] [PubMed] [Google Scholar]
- 10.Rane MJ, Coxon PY, Powell DW, Webster R, Klein JB, Pierce W, Peipei P, McLeish KR. p38 kinase-dependent MAPKAPK-2 activation functions as 3-phosphoinositide-dependent kinase-2 for Akt in human neutrophils. J. Biol. Chem. 2001;276:3517–3523. doi: 10.1074/jbc.M005953200. [DOI] [PubMed] [Google Scholar]
- 11.Dulin NO, Sorokin A, Douglas JG. Arachidonate-induced tyrosine phosphorylation of epidermal growth factor receptor and Shc-Grb2-Sos association. Hypertension. 1998;32:1089–1093. doi: 10.1161/01.hyp.32.6.1089. [DOI] [PubMed] [Google Scholar]
- 12.Jiao H, Cui XL, Torti M, Chang CH, Alexander LD, Lapetina EG, Douglas JG. Arachidonic acid mediates angiotensin II effects on p21ras in renal proximal tubular cells via the tyrosine kinase-Shc-Grb2-Sos pathway. Proc. Natl. Acad. Sci. USA. 1998;95:7417–7421. doi: 10.1073/pnas.95.13.7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexander LD, Cui XL, Falck JR, Douglas JG. Arachidonic acid directly activates members of the MAPK superfamily in rabbit proximal tubule cells. Kidney Int. 2001;59:2039–2053. doi: 10.1046/j.1523-1755.2001.00718.x. [DOI] [PubMed] [Google Scholar]
- 14.Aleander LD, Ding Y, Alagarsamy S, Cui XL, Douglas JG. Arachidonic acid induces ERK activation via Src SH2-domain association with the epidermal growth factor receptor. Kidney Int. doi: 10.1038/sj.ki.5000363. in press. [DOI] [PubMed] [Google Scholar]
- 15.Cui XL, Douglas JG. Arachidonic acid activates c-jun N-terminal kinase through NADPH oxidase in rabbit proximal tubular epithelial cells. Proc. Natl. Acad. Sci. USA. 1997;94:3771–3776. doi: 10.1073/pnas.94.8.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li WG, Miller FJ, Jr., Zhang HJ, Spitz DR, Oberley LW, Weintraub NL. H(2)O(2)-induced O(2) production by a nonphagocytic NAD(P)H oxidase causes oxidant injury. J. Biol. Chem. 2001;276:29251–29256. doi: 10.1074/jbc.M102124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hannken T, Schroeder R, Zahner G, Stahl RAK, Wolf G. Reactive oxygen species stimulate p44/42 mitogen-activated protein kinase and induce p27kip1: role in angiotensin II-mediated hypertrophy of proximal tubular cells. J. Am. Soc. Nephrol. 2000;11:1387–1397. doi: 10.1681/ASN.V1181387. [DOI] [PubMed] [Google Scholar]
- 18.Borsch-Haubold AG, Bartoli F, Asselin J, Dudler T, Kramer RM, Apitz-Castro R, Watson SP, Gelb MH. Identification of the phosphorylation sites of cytosolic phospholipase A2 in agonist-stimulated human platelets and HeLa cells. J. Biol. Chem. 1998;273:4449–4458. doi: 10.1074/jbc.273.8.4449. [DOI] [PubMed] [Google Scholar]
- 19.Street IP, Lin HK, Laliberte F, Ghomashchi F, Wang Z, Perrier H, Tremblay NM, Huang Z, Weech PK, Gelb MH. Slow- and tight-binding inhibitors of the 85-kDa human phospholipase A2. Biochemistry. 1993;32:5935–5940. doi: 10.1021/bi00074a003. [DOI] [PubMed] [Google Scholar]
- 20.Woo CH, Eom YW, Yoo MH, You HJ, Han HJ, Song WK, Yoo YJ, Chun JS, Kim JH. Tumor necrosis factor-alpha generates reactive oxygen species via a cytosolic phospholipase A2-linked cascade. J. Biol. Chem. 2000;275:32357–32362. doi: 10.1074/jbc.M005638200. [DOI] [PubMed] [Google Scholar]
- 21.Balsinde J, Dennis EA. Distinct roles in signal transduction for each of the phospholipase A2 enzymes present in P388D1 macrophages. J. Biol. Chem. 1996;271:6758–6765. doi: 10.1074/jbc.271.12.6758. [DOI] [PubMed] [Google Scholar]
- 22.Dulin NO, Alexander LD, Harwalkar S, Falck JR, Douglas JG. Phospholipase A2-mediated activation of mitogen-activated protein kinase by angiotensin II. Proc. Natl. Acad. Sci. USA. 1998;95:8098–8102. doi: 10.1073/pnas.95.14.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osherov N, Levitzki A. Epidermal-growth-factor-dependent activation of the src-family kinases. Eur. J. Biochem. 1994;225:1047–1053. doi: 10.1111/j.1432-1033.1994.1047b.x. [DOI] [PubMed] [Google Scholar]
- 24.Cuenda A, Rouse J, Doza YN, Meier R, Cohen P, Gallagher TF, Young PR, Lee JC. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- 25.Magolda RL. Novel synthesis of potent site-specific phospholipase A2 inhibitors. In: Bailey JM, editor. Prostaglandins, Leukotrienes, and Lipoxins: Biochemistry, Mechanism of Action, and Clinical Applications. Plenum; New York: 1985. pp. 669–676. [Google Scholar]
- 26.Church WB, Inglis AS, Tseng A, Duell R, Lei PW, Bryant KJ, Scott KF. A novel approach to the design of inhibitors of human secreted phospholipase A2 based on native peptide inhibition. J. Biol. Chem. 2001;276:33156–33164. doi: 10.1074/jbc.M101272200. [DOI] [PubMed] [Google Scholar]
- 27.Piomelli D. Arachidonic acid in cell signaling. Curr. Opin. Cell Biol. 1993;5:274–280. doi: 10.1016/0955-0674(93)90116-8. [DOI] [PubMed] [Google Scholar]
- 28.Curnutte JT, Kuver R, Scott PJ. Activation of neutrophil NADPH oxidase in a cell-free system. Partial purification of components and characterization of the activation process. J. Biol. Chem. 1987;262:5563–5569. [PubMed] [Google Scholar]
- 29.Shiose A, Sumimoto H. Arachidonic acid and phosphorylation synergistically induce a conformational change of p47phox to activate the phagocyte NADPH oxidase. J. Biol. Chem. 2000;275:13793–13801. doi: 10.1074/jbc.275.18.13793. [DOI] [PubMed] [Google Scholar]
- 30.Hazan-Halevy I, Levy R. Activation of cytosolic phospholipase A2 by opsonized zymosan in human neutrophils requires both ERK and p38 MAP-kinase. Adv. Exp. Med. Biol. 2000;479:115–123. doi: 10.1007/0-306-46831-X_10. [DOI] [PubMed] [Google Scholar]
- 31.Kramer RM, Roberts EF, Um SL, Borsch-Haubold AG, Watson SP, Fisher MJ, Jakubowski JA. p38 mitogen-activated protein kinase phosphorylates cytosolic phospholipase A2 (cPLA2) in thrombin-stimulated platelets. Evidence that proline-directed phosphorylation is not required for mobilization of arachidonic acid by cPLA2. J. Biol. Chem. 1996;271:27723–27729. doi: 10.1074/jbc.271.44.27723. [DOI] [PubMed] [Google Scholar]
- 32.Abe J, Takahashi M, Ishida M, Lee JD, Berk BC. c-Src is required for oxidative stress-mediated activation of big mitogen-activated protein kinase 1. J. Biol. Chem. 1997;272:20389–20394. doi: 10.1074/jbc.272.33.20389. [DOI] [PubMed] [Google Scholar]
- 33.Aikawa R, Komuro I, Yamazaki T, Zou Y, Kudoh S, Tanaka M, Shiojima I, Hiroi Y, Yazaki Y. Oxidative stress activates extracellular signal-regulated kinases through Src and Ras in cultured cardiac myocytes of neonatal rats. J. Clin. Invest. 1997;100:1813–1821. doi: 10.1172/JCI119709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kudoh S, Komuro I, Hiroi Y, Zou Y, Harada K, Sugaya T, Takekoshi N, Murakami K, Kadowaki T, Yazaki Y. Mechanical stretch induces hypertrophic responses in cardiac myocytes of angiotensin II type 1a receptor knockout mice. J. Biol. Chem. 1998;273:24037–24043. doi: 10.1074/jbc.273.37.24037. [DOI] [PubMed] [Google Scholar]
- 35.Wan Y, Belt A, Wang Z, Voorhees J, Fisher G. Transmodulation of epidermal growth factor receptor mediates IL-1 beta-induced MMP-1 expression in cultured human keratinocytes. Int. J. Mol. Med. 2001;7:329–334. [PubMed] [Google Scholar]
- 36.Lee SR, Kwon KS, Kim SR, Rhee SG. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J. Biol. Chem. 1998;273:15366–15372. doi: 10.1074/jbc.273.25.15366. [DOI] [PubMed] [Google Scholar]
- 37.Wilson LK, Parsons SJ. Enhanced EGF mitogenic response is associated with enhanced tyrosine phosphorylation of specific cellular proteins in fibroblasts overexpressing c-src. Oncogene. 1990;5:1471–1480. [PubMed] [Google Scholar]
- 38.Stover DR, Becker M, Liebetanz J, Lydon NB. Src phosphorylation of the epidermal growth factor receptor at novel sites mediates receptor interaction with Src and P85 alpha. J. Biol. Chem. 1995;270:15591–15597. doi: 10.1074/jbc.270.26.15591. [DOI] [PubMed] [Google Scholar]
- 39.Takekawa M, Maeda T, Saito H. Protein phosphatase 2Calpha inhibits the human stress-responsive p38 and JNK MAPK pathways. EMBO J. 1998;17:4744–4752. doi: 10.1093/emboj/17.16.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mendelson KG, Contois LR, Tevosian SG, Davis RJ, Paulson KE. Independent regulation of JNK/p38 mitogen-activated protein kinases by metabolic oxidative stress in the liver. Proc. Natl. Acad. Sci. USA. 1996;93:12908–12913. doi: 10.1073/pnas.93.23.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Huang S, Sah VP, Ross J, Jr., Brown JH, Han J, Chien KR. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J. Biol. Chem. 1998;273:2161–2168. doi: 10.1074/jbc.273.4.2161. [DOI] [PubMed] [Google Scholar]
- 42.Ge B, Gram H, Di Padova F, Huang B, New L, Ulevitch RJ, Luo Y, Han J. MAPKK-independent activation of p38alpha mediated by TAB1-dependent autophosphorylation of p38alpha. Science. 2002;295:1291–1294. doi: 10.1126/science.1067289. [DOI] [PubMed] [Google Scholar]
- 43.Lee JC, Kumar S, Griswold DE, Underwood DC, Votta BJ, Adams JL. Inhibition of p38 MAP kinase as a therapeutic strategy. Immunopharmacology. 2000;47:185–201. doi: 10.1016/s0162-3109(00)00206-x. [DOI] [PubMed] [Google Scholar]
- 44.Wang XS, Diener K, Manthey CL, Wang S, Rosenzweig B, Bray J, Delaney J, Cole CN, Chan-Hui PY, Mantlo N, Lichenstein HS, Zukowski M, Yao Z. Molecular cloning and characterization of a novel p38 mitogen-activated protein kinase. J. Biol. Chem. 1997;272:23668–23674. doi: 10.1074/jbc.272.38.23668. [DOI] [PubMed] [Google Scholar]
- 45.Dixon RJ, Brunskill NJ. Lysophosphatidic acid-induced proliferation in opossum kidney proximal tubular cells: role of PI 3-kinase and ERK. Kidney Int. 1999;56:2064–2075. doi: 10.1046/j.1523-1755.1999.00797.x. [DOI] [PubMed] [Google Scholar]
- 46.Bhandari BK, Feliers D, Duraisamy S, Stewart JL, Gingras AC, Abboud HE, Choudhury GG, Sonenberg N, Kasinath BS. Insulin regulation of protein translation repressor 4E-BP1, an eIF4E-binding protein, in renal epithelial cells. Kidney Int. 2001;59:866–875. doi: 10.1046/j.1523-1755.2001.059003866.x. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ, Waterfield MD, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez-Viciana P, Warne PH, Vanhaesebroeck B, Waterfield MD, Downward J. Activation of phosphoinositide 3-kinase by interaction with Ras and by point mutation. EMBO J. 1996;15:2442–2451. [PMC free article] [PubMed] [Google Scholar]