Abstract
Xenopus laevis is the model of choice for evolutionary, comparative, and developmental studies of immunity, and invaluable research tools including MHC-defined clones, inbred strains, cell lines, and monoclonal antibodies are available for these studies. Recent efforts to use Silurana (Xenopus) tropicalis for genetic analyses have led to the sequencing of the whole genome. Ongoing genome mapping and mutagenesis studies will provide a new dimension to the study of immunity. Here we review what is known about the immune system of X. laevis integrated with available genomic information from S. tropicalis. This review provides compelling evidence for the high degree of similarity and evolutionary conservation between Xenopus and mammalian immune systems. We propose to build a powerful and innovative comparative biomedical model based on modern genetic technologies that takes take advantage of X. laevis and S. tropicalis, as well as the whole Xenopus genus.
Keywords: comparative immunology, developmental immunology, evolution of immunity, genomics
INTRODUCTION
From an evolutionary point of view, Xenopus is one “connecting” taxon that links mammals to vertebrates of more ancient origin (bony and cartilaginous fishes), that shared a common ancestor ~350 MYA (Pough et al., 2002). In addition to its wide use for developmental studies, Xenopus laevis has been, and still is frequently used as the nonmammalian comparative model of choice for immunological studies. Indeed, X. laevis provides a versatile model with which to study ontogeny and phylogeny of humoral and cell-mediated immunity against tumors and pathogens. Features such as the accessibility of early developmental stages free of maternal influence to manipulation and surgery, the transparency of tadpoles, the developmental transition from larva to adult during metamorphosis; and the direct effect of temperature on immune responses in vivo and in vitro are all attractive and useful for fundamental studies of the ontogeny of the immune system.
Vertebrate immune systems are classically categorized into two interconnected types: innate and adaptive immune systems. Innate immunity provides a first line of defense using a wide variety of cells and pathways that target pathogens globally. Effector cells of innate immunity (i.e., macrophages, neutrophils, dendritic cells, and natural killer [NK] cells) eliminate infected cells by phagocytosis or by direct cytotoxicity. Activation of innate immune responses in vertebrates occurs through the interaction of pattern recognition receptors (PRRs) on effector cells with molecules specific to pathogens (pathogen-associated molecular patterns [PAMPs]). The PRRs are germline-encoded and their recognition is necessarily limited (i.e., broad specificity). Engagement of PAMPs by the PRRs initiates biochemical cascades that stimulate effector cells and that induce the release of soluble mediators reacting against different types of pathogens. Innate immunity also includes antimicrobial peptides that are secreted onto the skin, as well as serum proteins (including acute phase proteins) and complement components that are secreted by the liver. Innate immune systems in vertebrates also play a crucial role in initiating adaptive immune responses that are specific to the foreign antigen (reviewed in Janeway, 1992).
The adaptive immune system of vertebrates (defined in details in section 1.2) is characterized by B and T cells expressing surface Ag-specific receptors, which in contrast to germline-encoded innate PRRs, are somatically generated by recombination-activating genes (RAG)-dependent gene rearrangements. These Ag receptors are incredibly diverse in each individual and can recognize Ags that differ even subtly from self at the molecular level. Thus, it is often referred to as an “anticipatory system” because it (theoretically) can be stimulated by any non-self molecule. The vertebrate adaptive immune system is evolutionarily more recent than innate immune systems. It “mysteriously” appeared “as a whole” near the time of the emergence of jawed vertebrates ~500 million years ago (MYA; reviewed in Flajnik and Kasahara, 2001). In jawless fish (e.g., lamprey, hagfish) and some invertebrates (e.g., fruit fly), other types of “adaptive somatic diversification” have arisen as well, apparently by convergence (Pancer et al., 2004, 2005; Watson et al., 2005).
To date, X. laevis remains one of the most comprehensively studied ectothermic vertebrates with respect to its adaptive immune system (reviewed in Du Pasquier et al., 1989), which in adults, is remarkably similar to that of mammals. Importantly, Xenopus is a transitional animal model, being the oldest vertebrate class in which the immunoglobulin (Ig) class switch occurs, but does so in the absence of germinal center formation critical for T cell-dependent B-cell maturation in mammals (Marr et al., 2007; reviewed in Du Pasquier et al., 2000; and Flajnik, 2002). Most of the cell types of hematopoietic origin as defined in mammals are present in Xenopus, including B and T cells, and most of the molecules that define adaptive immunity (e.g., immunoglobulins [Igs], T-cell receptor [TCR], major histocompatibity complex [MHC], RAG, activation-induced cytidine deaminase [AID]) have been characterized. Moreover, studies with X. laevis over several decades have resulted in the generation of many invaluable research tools, including MHC-defined clones and inbred strains of animals, transplantable lymphoid tumor cell lines, monoclonal antibodies (mAbs), and cDNA probes (http://www.urmc.rochester.edu/smd/mbi/xenopus/index.htm).
Recently, rather than the allotetraploid species, X. laevis, the true diploid species, Silurana (Xenopus) tropicalis (reviewed in Kobel and Du Pasquier, 1986), was selected as a model organism for a whole-genome sequencing project and the sequencing assembly is in its final stages. In addition, several expressed sequence tag (EST) projects are targeted from lymphoid tissues (e.g., spleen, thymus) and sequences are deposited in the databases, making it possible to search for genes of interest and discover new genes that have been overlooked. Analysis of the genomic assembly of S. tropicalis reveals that it is rather stable; genetic synteny is well conserved between human and Xenopus genomes, yet Xenopus still maintains the primordial features. Compared with complex teleost fish models (e.g., zebrafish, fugu) in which the genome has been disrupted due to rapid expansion and contraction of species (reviewed in Postlethwait, 2007), the genetic stability of Xenopus clearly reveals that it can become a better model for imunogenetic and developmental immunology studies.
This review presents our current knowledge about the immune system of Xenopus with a particular emphasis on early development and metamorphosis, as well as our bias that by taking advantage of both X. laevis and S. tropicalis one can build a powerful comparative biomedical and immunological model using newly available tools such as transgenesis and genomics.
1. THE ADULT IMMUNE SYSTEM
As reviewed previously, the immune system of X. laevis is fundamentally similar to that of mammals (reviewed in Du Pasquier et al., 1989). Although Xenopus lacks the mammalian equivalent of lymph nodes, it does have a thymus where T cells differentiate and a spleen that represents the main peripheral lymphoid organs where both B and T cells accumulate in the white pulp, especially in the follicular area where IgM+ B cell surrounded by T cells aggregate around a central blood vessel (Marr et al., 2007). Lymphocytes and other leukocytes also accumulate in the periphery of the liver, the kidneys, and along the intestine but without forming the organized lymph nodes or Peyer’s patches of mammals. The extensive studies of both innate and adaptive components of immunity in X. laevis provide the foundation for the analysis of S. tropicalis genomic sequences.
A tool that has been very instrumental for the comparative study of the immune function in Xenopus is unquestionably the generation of inbred MHC homozygous strains such as the F (f/f MHC haplotype; Du Pasquier and Chardonnens, 1975) and J (j/j MHC haplotype; Tochinai and Katagiri, 1975) strains, and of MHC-defined syngeneic clones produced gynogenetically from interspecies crosses (X. laevis × X. gilli; LG) hybrids (Kobel and Du Pasquier, 1975). These clones and inbred strains permit classic adoptive transfer and transplantation manipulations as in mice (reviewed in Robert et al., 2004). As depicted in Figure 1, the availability of MHC-disparate clones as well as clones with identical MHC but differing at minor histocompatibility (H) loci, provides a genetic system that permits studying immune responses in vivo and in vitro against minor H-Ags using skin allografting as well as against transplantable lymphoid tumors (Robert et al., 1995; Goyos et al., 2004). One such tumor, derived from the LG-15 clone (15/0) grows in LG-15 and LG-6 hosts that share the same MHC haplotype (a/c), but is rejected by MHC mismatched (b/d) clones such as LG-3 (see end of section 1.3. for more details on lymphoid tumor lines).
Fig. 1.
Schematic overview of the Xenopus tumor/minor H-Ag transplantation system. Several syngeneic clones produced gynogenetically from X. laevis × X. gilli hybrids have been MHC typed. While some have different MHC haplotypes (e.g., LG-3 [b/d]) and LG6 [a/c]), others share the same MHC haplotype but express different minor H-loci (LG-6 and LG-15). On the left side, skin grafts exchanged among individuals of the same LG-15 isogenetic clone are not rejected (top), whereas skin grafts on individuals of the LG-6 clone that is MHC identical with LG-15 but differs at minor H-loci are rejected significantly more slowly (middle) than are MHC-disparate allografts (bottom). On the right side, the 15/0 tumor is tumorigenic when transplanted in the clone from which it derives (LG-15, top), as well as MHC identical but minor H-Ag–disparate LG-6 clone, but is rejected in the MHC-disparate LG-3 host. For abbreviations, see list.
1.1. Innate Immune System (NK Cells, Leukocytes, Complement, Antimicrobial Peptides)
Based on morphology and Giemsa staining pattern, typical leukocyte types such as neutrophils, basophils, eosinohils, polymorphonuclear (PMN) cells, monocyte and macrophage-like cells, and smaller lymphocytes can be observed in the blood and the peritoneal fluid (Du Pasquier et al., 1985; Hadji-Azimi et al., 1987). Although mAbs recognizing markers specific for particular leukocyte subsets are not available, there are several mAbs identifying surface markers of all leukocytes (Table 1) such as F1F6 (Flajnik et al., 1988), and even leukocytes at early developmental stages such as CD45 (CL-21 mAb; Smith and Turpen, 1991; Barritt and Turpen, 1995), Xl-1 (Ohinata et al., 1989), Xl-2 (Miyanaga et al., 1998), and RC47 (Du Pasquier and Flajnik, 1990).
TABLE 1.
Expression Pattern of Xenopus Lymphocyte Surface Markers Detectable with Currently Available mAbs
| Xenopus markers (mAbs) * | Expression pattern [Molecular weights] | Ref | |
|---|---|---|---|
| B cells | IgM (10A9, 6.16) | Larval and adult B cells. [73 Kd] | 1, 2 |
| IgY (11D5) | Some larval and adult B cells. [66 Kd]. | 1 | |
| IgX (410D9) | Some larval and adult B cells, especially in the gut [80 Kd] |
3, 4 | |
| IgL (1E9, 13B2, 409B8) | Some larval and adult B cells. [30–35 Kd] | 5 | |
| T cells, thymocytes | CD3 epsilon (CD3-2) anti-human | Cross-reacts with Xenopus CD3 epsilon, and coprecipitates the TCR-CD3 complex of T-cells and lymphoid tumor lines. |
6 |
| CD5 (2B1) | Thymocytes (>95%), T-cells and some PMA-activated IgM+ B cells. |
7 | |
| CD8 (AM22, F17) | Larval and adult thymocytes (70–80%) and T-cells (about 20% of splenocytes). [35 Kd] |
8–10 | |
| CTX (X71, 1S9.2) | Larval and adult thymocytes (60–70%); no consistent expression in peripheral lymphocytes. Also expressed in gut epithelial tissue. |
11, 12 | |
| T-cells (XT1) | Most, but not all, larval and adult T-cells; earliest marker of thymocytes. |
13 | |
| T cells (AM15) | Subpopulation of thymocytes and peripheral T cells. [18 Kd] |
8 | |
| MHC | MHC class I (TB17) | Ubiquitous in adult; all lymphopoietic lineages. Not expressed until metamorphosis. [44–45 Kd] |
14 |
| MHC class II (AM20, 14A2) | Thymocytes, B and T-cells (99% of spleen lymphocytes), only B-cells in larvae. [30 Kd] |
8 | |
| Leukocytes | CD45 (CL21) | All leukocytes from early embryonic stage 28. Different size variants expressed by thymocytes and B cells. [180–200 Kd] |
15 |
| NK cells (1F8) | Non-B and non-T, peripheral lymphoid cells [55 Kd]. | 16 | |
| F1F6 | All mature leukocytes and erythrocytes, but not immature cells. [13 Kd] |
17 | |
| RC47 | Leukocyte lineage from very early stage (day 6 post- fertilization). Thymic cortex and medulla (>90% of total thymocytes). |
9 | |
| XL-1 | All leukocytes from early larval stage, ventral blood island stage 35. |
18 | |
| XL-2 | All leukocytes from early embryonic stage 24. [135 Kd] | 19 |
(1) Hsu and Du Pasquier, 1984a; (2) Bleicher and Cohen, 1981 (3) Hsu et al., 1985; (4) Mussmann et al., 1996; (5) Hsu et al., 1991; (6) Göbel et al., 2000; (7) Jürgens et al., 1995; (8) Flajnik et al., 1990; (9) Du Pasquier and Flajnik 1990; (10) Ibrahim et al., 1991; (11) Chrétien et al., 1996; (12) Robert et al., 1997; (13) Nagata, 1985; (14) Flajnik et al., 1991; (15) Barritt and Turpen, 1995; (16) Horton et al., 2000; (17) Flajnik et al., 1988b; (18) Ohinata et al., 1989; (19) Myanaga et al., 1998.
Many of the genes known to be involved in mammalian innate immunity have been identified in X. laevis and S. tropicalis (Table 2). Among them, toll-like receptors (TLR) are one of the innate receptors that recognize PAMPs on pathogens that initiate innate as well as adaptive immune responses (Janeway, 1992). Of interest, in contrast to mammals that have 10 TLRs, a total of 20 different TLR genes, as well as some adaptor proteins, have been identified in the S. tropicalis genome (Fitzgerald et al., 2001; Inoue et al., 2005). All these TLR genes are constitutively expressed in tadpoles and adults (Ishii et al., 2007; Roach et al., 2005), suggesting that the innate immune response through TLR signaling is active throughout life. While most TLRs are evolutionarily conserved due to the strong selection for maintenance of specific PAMP recognition, Xenopus TLR4 (i.e., the receptor responsible of response to the endotoxin lipopolysaccharide [LPS] in mammals; reviewed in Janeway, 1992) seems to be divergent. In this regard, it is interesting to note that Xenopus is poorly responsive to purified LPS (e.g., adult can receive up to 1 mg of LPS without any sign of inflammation or other untoward effects; Bleicher et al., 1983; Marr et al., 2005). Thus, Xenopus carry all the human orthologs and some TLR family members that are expanded in a Xenopus-specific manner (e.g., TLR14).
TABLE 2.
Some Relevant Mammalian Immune Gene Orthologs Identified* in the S. tropicalis Genome
| Types of molecules | Genes | Ref. | ||
|---|---|---|---|---|
| Innate immunity | Leukocyte receptor | NK receptor | NKp30, KIRs, | 1 |
| Toll-like receptor | TLR1, 2, 3, 4, 5, 6, 7, 8, 9 | |||
| Fc receptor | FcR-like, FcR-γ | 1, 3 | ||
| C-type lectin | CLEC | 31 | ||
| Others | SIGLEC, | 31 | ||
| Signaling molecules | NFKB, IKBB, MyD88 TNFα, IL-6 | 4 | ||
| DAP10, DAP12 | OR | |||
| Effector molecules | Cytokines | IL-1β, LTα, LTβ, TNFα, IL-6, IFNα | 5, 6 | |
| Cytotoxic killing | iNOS, granzyme, perforin | 31 | ||
| Anti-bacterial peptide | magainin, xenopsin, caerulein | 31 | ||
| Complement | C1~9, MASP, Bf | 7–10 | ||
| Adaptive immunity | Antigen receptors | Immunoglobulins | IgH (M, D, X, Y, F), IgL (λ, σ, κ) | 11–17 |
| T cell receptors | TCRα, β, γ, δ | 18, 19 | ||
| GOD | Rag 1, 2, AID, TdT | 20–22 | ||
| Antigen presentation | MHC | Class I, Class II, DM, β2M | 6, 23–26 | |
| Immunoproteasome | Psmb8, 9,10 | 6 | ||
| Transporters | Tap1, Tap2 | 6, 27 | ||
| Cathepsins | Cathepsin L | 31 | ||
| Others | Tapasin, calreticulin, calnexin | 6 (Tapasin), 31 | ||
| Accessory molecules | CD4, CD8α, β, BTLA | 28 | ||
| B7 family | CD274, CD276, VTCN1 | 28 | ||
| B7 receptors | CD28, CTLA4 | 28 | ||
| TNFSF family | CD40LG, HVEM | 28 | ||
| TNFRSF family | CD40 | 31 | ||
| Adhesion molecules | MCAM, JAM, ICAM, CD2 | 31 | ||
| Cytokines | IL-2, 3, 4, 5, 7, IFNγ | 29 (IFNγ), 31 | ||
| Effector molecules | IL-6, 17, 21, 23 | 31 | ||
| Signaling molecules | LCK, Fyn, Igα, CD3ε, CD3ζ | 3, 3 |
Orthology sufficiently supported by synteny, sequence similarity and/or other types of characterization (e.g., expression profiles).
(1) Guselnikov et al., 2008; (2) Ishii et al., 2007; (3) Guselnikov et al., 2003; (4) Fitzgerald et al., 2001; (5) Zou et al. 2000; (6) Ohta et al., 2006; (7) Alsenz et al., 1992; (8) Kato et al., 1994; (9) Mo et al., 1996 (10) Endo et al., 1998; (11) Schwager et al., 1988; (12) Schwager et al., 1991b; (13) Zhao et al., 2006; (14) Ohta et al., 2006; (15) Qin et al., 2008; (16) Haire et al., 1989; (17) Amemiya et al., 1989; (18) Chretien et al., 1997; (19) Haire et al., 2002; (20) Greenhalgh et al., 1993; (21) Ichikawa et al., 2006; (22) Lee and Hsu, 1994; (23) Shum et al., 1993; (24) Sato et al., 1993; (25) Liu et al., 2002; (26) Stewart et al., 2005; (27) Ohta et al., 1999; (28) Bernard et al., 2007; (29) Qi and Nie, 2008; (30) Göbel et al., 2000; (31) Ohta and Robert, unpublished
As many other amphibians, the granular glands (also called serous glands) in the dermal layer of the Xenopus skin produce potent antimicrobial peptides (e.g., magainin, xenopsin, caerulein precursor fragments) effective against bacteria and fungi (reviewed in Simmaco et al., 1998; Zasloff, 2002; Rollins-Smith and Conlon, 2005). The variety and remarkable molecular heterogeneity of these antimicrobial peptides that can be secreted in large amount under stress suggest that they provide an important protection of the host against a wide range of pathogens.
NK cells are leukocytes of the innate immune system that can kill targets by cell-mediated lysis, especially virus-infected cells and tumors that have down-regulated their surface expression of MHC class I molecules. In addition, through production of cytokines such as interferon-γ, NK cells are involved in the orchestration of both innate and the adaptive immune responses. NK cells have been characterized in X. laevis using a mAb, 1F8 (Horton et al., 2000) that recognizes a 55-kDa surface protein (Horton et al., 2003). 1F8 recognizes a population of large granular non-B, non-T leukocytes that remain present in larvally thymectomized (Tx) T-cell–deficient Xenopus and that kill MHC class I-negative tumor but not MHC-expressing autologous lymphoblast targets (Horton et al., 2000).
In mammals, receptors on NK cells are encoded in two different genetic regions unlinked to the MHC; however these regions are suggested to be the paralogs of the MHC (Du Pasquier, 2000; Teng et al., 2002). Indeed, the linkage of these NK receptors to the MHC has been identified in non-mammalian species such as birds and marsupials; thus, their close linkage is proposed to be primordial (Kaufman et al., 1999; Belov et al., 2006). In the Xenopus MHC, there is a cluster of putative membrane-bound molecules that might function as candidate primordial NK receptors that may have co-evolved with particular MHC class I alleles (Ohta et al,. 2006). There are other NK receptors in the S. tropicalis genome that are not linked to the MHC; these may be orthologous to the mammalian NK receptors (Ohta, unpublished observation). In fact, recent studies of the S. tropicalis genome reveal that receptor systems regulating function of leukocytes and NK cells are rather complex. Indeed, the diploid S. tropicalis genome contains at least 75 genes encoding paired Fc-related receptors (FcR) designated XFLs (Guselnikov et al., 2008). Many homologous genes that are primarily expressed in lymphoid tissues are also found in the allotetraploid X. laevis, and two signaling adapter receptors, FcRγ and TCRζ, have been characterized (Guselnikov et al., 2003). The function of these genes is currently unknown, but their extraordinary diversity and their subdivision into two signaling class of receptors: inhibitors and activators, suggests that they are involved in the regulation of immune function.
The three pathways of the complement system (classical, the lectin, and the alternative) are present in Xenopus (reviewed in Fujita et al., 2004). This system, consisting of more than 30 proteins and receptors, bridges the innate and adaptive immune system and is critical for a potent humoral response. Most genes of the complement are present in the S. tropicalis genome, and many of them have been characterized in X. laevis (Alsenz et al., 1992; Kato et al., 1994; Mo et al., 1996; Endo et al., 1998; reviewed in Fujita et al., 2004) including serum proteins involved in the complement lectin pathways such as ficolins (Kakinuma et al., 2003). Similarly, proinflammatory cytokines (e.g., IL-1, 6, TNFα), chemokines, and the respective receptors genes involved in the activation of innate effector responses are present in the genome of S. tropicalis. However, to date there are very few cytokine/chemokine expression and functional studies in Xenopus. An IL-1β-like factor produced by stimulated peritoneal leukocyte has been reported in X. laevis (Watkins and Cohen, 1987), and more recently, up-regulated expression of IL-1β message upon inflammatory signal such as LPS has been shown (Zou et al., 2008; Marr et al., 2005). Some studies suggest that TGF-β inhibit proliferation of Xenopus splenic blasts induced by a supernatant containing a T-cell growth factor (Haynes and Cohen, 1993a).
1.2. Adaptive Immune System (B cell, T cells, Ag Presenting Cells)
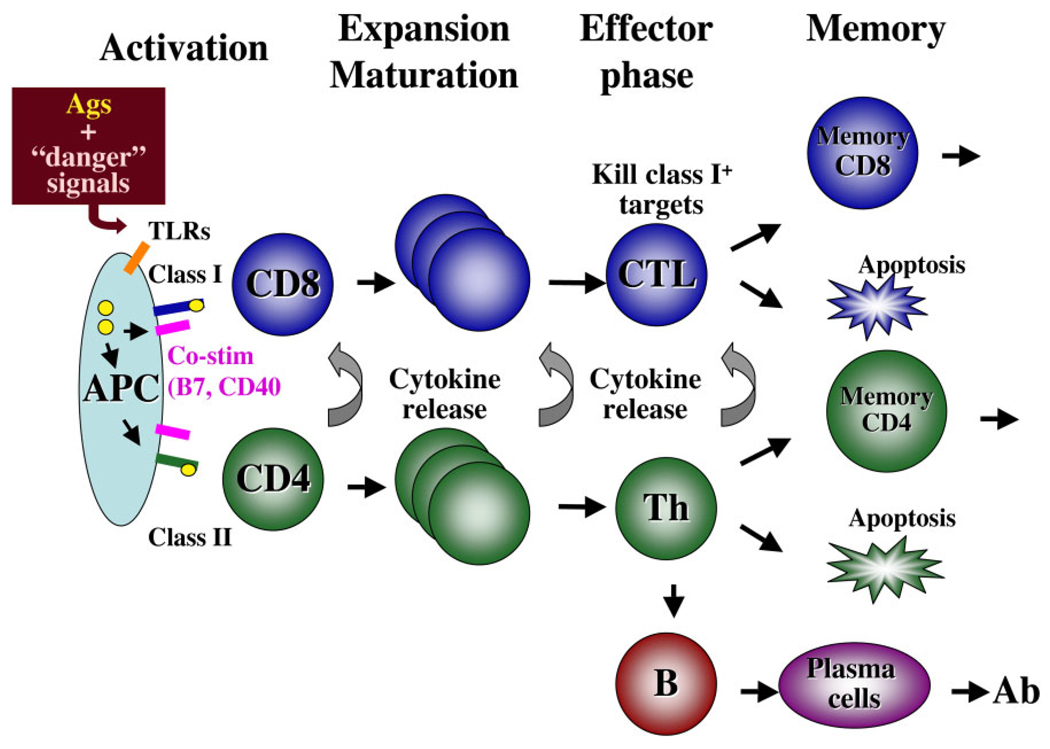
The mammalian adaptive immune system consists of a network of specialized B and T cells expressing surface Ag receptors generated by combinatorial somatic rearrangements that can recognize foreign antigens. The B-cell receptors (BCR) can recognize and bind directly to a particular motif or epitope in a large molecule, whereas TCRs only recognize short peptides that are processed and complexed with MHC molecules on the surface of the specialized or professional antigen presenting cells (APCs) such as dendritic cells, monocytes, and macrophages (Fig. 2). Surface class II molecules loaded with 10 to 30 amino acid-long antigenic peptides are recognized by TCRs on the CD4 helper T cells, whereas surface class I molecules complexed with β2-microglobulin (β2-m) and 8 to 11 amino acid-long antigenic peptides are recognized by the TCR on the surface of cytotoxic CD8 T cells. In addition to TCR signaling, activation of both naive CD4 and CD8 T cells require a second signal from co-stimulatory molecules such as B7 ligands and CD40 provided by APCs interacting with CD28 and CD40L on T cells, respectively. As such APCs bridge the innate and adaptive immune system: through PRRs such as TLRs, APCs initiate innate immune responses by up-regulating costimulatory receptors and releasing proinflammatory cytokines, and through Ag presentation APCs trigger adaptive immune responses (Fig. 2). After their activation, T-cell clones expand and differentiate into cytotoxic CD8 T-cell effectors (CTLs) or CD4 T helper cells. CTLs can kill any cell targets expressing the class I/peptide complex that activated them without costimulation. Various CD4 helper T-cell effector types (e.g., Th1, Th2) are generated that secrete different cytokines critical to help various other immune cells including B cells.
Fig. 2.
Schematic overview of a typical mammalian adaptive immune response. Productive T-cell activation requires two signals from an APC (e.g., dendritic cells, macrophages): the first signal is due to the recognition by the TCR of the MHC molecules complexed with the antigenic peptide (CD4 T cells interact with class II molecules, CD8 T cells with class I molecules), the second signal is provided by costimulatory molecules (e.g., B-7, CD40) that are up-regulated following APC activation by pathogen products or PAMPs binding to PRRs such as TLRs. Activated T cells proliferate and differentiate into cell effectors: CTLs able to kill target expressing the same Ags-class I complex and CD4 T helper cells producing various cytokines that act on the pathogen as well as on other immune cells including CD8 T and B cells. Most T cells die from apoptosis after the response (contraction phase), except a long-lived minor population of memory T cells able to respond faster to a second pathogen exposure. For abbreviations, see list.
Although the Xenopus adaptive immune system is less well characterized than that of mammals, its general pattern is very similar. However, in contrast to mammals where BM is the main site for lymphopoiesis and a reservoir for antibody-secreting B cells, adult Xenopus B cells differentiate mostly in the liver and the spleen (reviewed in Du Pasquier et al., 2000). Furthermore, no accumulation of antibody-secreting B cells in adult Xenopus BM was detected during immune responses to bacteria and virus (Marr et al., 2007). Therefore, even though some lymphopoietic activity is suggested by the expression of RAG (Greenhalgh et al., 1993), the Xenopus BM does not appear to be a major lymphoid organ. The surface BCR is recognized by several mAbs including anti-Ig heavy chain isotypes µ, ν and χ, as well as anti-Ig light chain mAbs (Hsu and Du Pasquier, 1984a; Schwager et al., 1991b; Hsu et al., 1991; Table 1). Expression of IgM and IgX are thymus-independent, whereas the production of IgY is thymus-dependent (Turner and Manning, 1974) and requires collaboration between B and T cells (Blomberg et al., 1980). Majorities of B cells express the IgM isotype and are widely distributed in most tissues. IgX-expressing B cells are a minor population of mostly secretory plasma cells found mainly in the gut epithelium (where they may be involved in mucosal immunity), and to a lesser extent in the spleen and the liver (Mussmann et al., 1996). IgY-producing B cells are found in the liver, spleen, and blood circulation, but not in the intestine (Mussmann et al., 1996). Other B-cell–specific markers are currently lacking although B-cell–specific gene orthologs are present in the S. tropicalis genome (e.g., CD19, CD138, CD180; Otha and Robert, unpublished observations). The Ig gene loci in Xenopus and mammals have a similar organization and usage with somatic combinatorial joining of V, D, and J elements (reviewed in Du Pasquier et al., 2000) that are RAG-dependent (Greenhalgh et al., 1993) and involve terminal deoxynucleotidyl transferase (TdT; Lee and Hsu, 1994) for generation of diversity. Allelic exclusion, which prevents the expression of more than one Ig heavy chain loci, also operates in Xenopus B cells (Du Pasquier and Hsu, 1983; reviewed in Hsu, 1998). Thus Xenopus Ig heavy and Ig light chain repertoires are as diverse as those of mammals.
For a long time, Xenopus was considered to have only the three Ig heavy chain isotypes: IgM, IgX, and IgY (Schwager et al., 1988; Haire et al., 1989; Amemiya et al., 1989). Recently, however, two new isotypes, IgD and IgF, were discovered by in silico analysis of the S. tropicalis genome (Zhao et al., 2006; Ohta et al., 2006). Because of the evolutionary transitional position of Xenopus, the discovery of IgD in Xenopus tied the connection of mammalian IgD to the previously unknown immunoglobulin isotype, IgW in lungfish (Ota et al., 2003) and IgW/X in cartilaginous fish (Kobayashi et al., 1984; Harding et al., 1990; Bernstein et al., 1996; Greenberg et al., 1996), thereby demonstrating that IgD is as old as IgM. Without the genomic database, the discovery of IgD would have not been possible, because of its low level of expression only by naive B cells and its large molecular weight. IgF is the shortest Ig isotype having a hinge exon. The two constant domains of IgF are very similar to those of first and fourth constant domains of IgY, suggesting that IgF was generated by tandem duplication of IgY followed by a loss of internal constant domains. IgX (Mussman et al., 1996) and IgY (Warr et al., 1995) are homologs to the mammalian IgA and IgG (also IgE) isotypes, respectively. Two mammalian orthologous Ig light chain isotypes, λ, κ, (Bengtén et al., 2000; Criscitiello and Flajnik, 2007) have been identified as well as the σ-isotype that is specific to the nonmammalian species, and their genomic organization is similar to that of mammals (Qin et al., 2008).
Class switch recombination (CSR) from IgM to IgY, and somatic hypermutation (SHM) at the Ig locus, occurs during the course of an antibody response in Xenopus (reviewed in Hsu, 1998, Du Pasquier et al., 2000). These two processes typical of affinity maturation involve AID (Ichikawa et al., 2006), but in contrast to mammals, SHM is targeted to G/C nucleotide in Xenopus (Wilson et al., 1992). Although the sequences of the genomic region that serve as substrate for the switch (S region) are divergent between mammals and Xenopus (i.e., Xenopus Sµ is rich in AT and not prone to form R-loops), a Xenopus S region can functionally replace mouse equivalents to mediate CSR in vivo (Zarrin et al., 2004), and Xenopus AID (XlAID) can induce CSR in AID-deficient murine B cells (Ichikawa et al., 2006). Despite the absence of germinal centers in X. laevis, in situ hybridization has detected XlAID mRNA mainly in the spleen, preferentially distributed in follicular B-cell zones. XlAID is markedly up-regulated with different kinetics upon bacterial stimulation and viral infection (Marr et al., 2007). Additionally, during secondary anti-viral response XlAID is also noticeably expressed by PBLs, suggesting that XlAID remains active in a subset of circulating B cells and plasma cells.
Despite these fundamental similarities, affinity maturation in Xenopus is poor when compared with mammals. For example, the affinity of IgY antibody against dinitrophenol (DNP) increases less than 10 times during a humoral response in contrast to more than a 10,000 fold affinity increase in mammals (reviewed in Hsu, 1998). Sequence analysis of the variable domain of Ig heavy chain VH1 gene family during antibody response to DNP has revealed that the rate of somatic mutations is not very different between Xenopus and mice (Wilson et al., 1992). However, the relatively low ratio of replacement to silent mutation in the CDRs (Wilson et al., 1992) suggests that, in Xenopus, the selection mechanism is not optimal. This could be related to the simple organization of the lymphoid organs in Xenopus that lack lymph nodes and germinal centers (Marr et al., 2007; reviewed in Du Pasquier et al., 2000), an observation that correlates with poor affinity maturation. It is important, however, to mention that protective neutralizing antibodies are generated following a secondary infection with the iridovirus Frog virus 3 (FV3), and that immunological memory to FV3 lasts at least 15 months (Maniero et al., 2006). Therefore, when examined in a physiological context involving a natural viral pathogen, antibodies generated by Xenopus appear to provide protective defense against subsequent viral infection even though they are of a weaker affinity than mammalian antibodies.
In contrast to mammalian mature B cells that are generally not phagocytic, peripheral differentiated B cells from teleost fish species and X. laevis are phagocytic and capable of killing ingested microbes. This finding suggests that evolutionarily, B cells and macrophages may share a common origin. That such a primordial phagocytic function has also been demonstrated for Xenopus B cells, offers additional support of this frog as an excellent model of transitional evolution (Li et al., 2006).
T cells are the second main type of lymphocytes. All four types of TCR chains are found in the Xenopus genome, suggesting the presence of γδT cells as well as the conventional αβT cells. Other signaling components of the Xenopus TCR have also been described, including CD3γ and δ (Dzialo and Cooper, 1997; Göbel et al., 2000) and TCRζ (Guselnikov et al., 2003). Co-stimulatory (CD28) and co-inhibitory receptors (CTLA4) that regulate T-cell activation as well as other receptors that include BTLA and ICOS have been identified in Xenopus (Bernard et al., 2007). Most of the B7 family members are also present in the Xenopus (Ohta, manuscript in preparation), suggesting that a similar co-signaling mechanism for T-cell activation by APCs was present before the divergence of amphibian species. So far, the X. laevis TCR repertoire has only been studied for TCRβ (Chretien et al., 1997), α, and γ (Haire et al., 2002).
In mammals, αβT-cell differentiation occurs in the thymus, and is characterized by the successive expression of certain cell surface molecules, including the coreceptors CD4 and CD8 (Anderson et al., 1996). Briefly, thymocyte maturation can be divided into three broad categories based on coreceptor surface expression: (1) an early double negative (DN) CD4−CD8− stage; (2) a predominant double positive (DP) CD4+CD8+ stage; and (3) a mature CD4+ or CD8+ single positive (SP) cell stage (Krangel et al., 2004). Immature DN thymocytes up-regulate the coreceptors following TCRβ locus rearrangement. TCRα chain rearrangement is initiated and TCR αβ heterodimers are expressed on the cell surface at the DP stage. At this point, these thymocytes become eligible for both positive and negative selection. Positive selection ensures that these T cells are able to recognize cognate MHC and hence, is termed the “MHC restriction” phase, whereas negative selection, or “tolerance,” ensures that self-reactive T cells are eliminated. Thus, the mature T-cell repertoire is established in association with self peptide-MHC molecules.
Multiple lines of evidence suggest that thymic education is fundamentally similar in mammals and Xenopus. First, several key factors involved in the regulation of T-cell development are highly conserved and expressed in the thymus in Xenopus (reviewed in Hansen and Zapata, 1998; Rothenberg and Taghon, 2005). These include GATA3 (Zon et al., 1991; Du Pasquier et al., 1995) and Ikaros (Hansen et al., 1997). The thymus dependency of T cells is also conserved between frogs and humans as evidenced by the severe T-cell deficiency that results from thymectomy (Tx) at an early larval stage before stem cell immigration (reviewed in Horton et al., 1998). T-cell–deficient adult X. laevis generated by early larval Tx have dramatically impaired anti-tumor responses (Robert et al., 1997) and skin allograft immunity (reviewed in Horton et al., 1998). In Tx animals, peripheral T cells are absent, whereas the percentage of B cells (Hsu and Du Pasquier, 1984b) and NK cells (Horton et al., 2000) is increased; in vitro responses of Tx splenocytes to phytomitogens or alloantigens are also abrogated (reviewed in Horton et al., 1998). The active role of the thymus in T-cell selection is also supported by studies with chimeras made at embryonic stages between LG clones of different MHC haplotypes. For example, young adults generated by microsurgical fusion of the anterior one-third of a 24-hr-old tail bud embryo containing the thymus anlage with the posterior two-thirds of a MHC-mismatched embryo that contains the anlage of all hematopoietic cells, do not reject skin grafts of the thymus MHC haplotype (Flajnik et al., 1985). Although this tolerance of the thymus haplotype is not absolute (see next section), these data do suggest that there is thymic selection of the T-cell repertoire in Xenopus. In addition, the transcription factor autoimmune regulator (AIRE), that in mammals is required for expression of tissue-specific self-Ags by medullary thymic epithelial cells (reviewed in Mathis and Benoist, 2007), has recently been characterized in X. laevis and S. tropicalis (Saltis et al., 2008). Finally, by using mAbs that recognizes CTX, a thymoctye differentiation pathway has been characterized in vitro in Xenopus. CTX, a homodimeric surface glycoprotein, is a marker of an immature stage that appears equivalent to the mammalian thymocyte double positive stage (Chrétien et al., 1996; Robert et al., 1997; Robert and Cohen, 1998a). In this Xenopus thymocyte developmental series, cells transition from CTX+, CD8+, CD5low, CD45low to more mature CTXneg, CD5bright, CD45bright cells that can be further subdivided into CD8bright and CD8neg (Robert and Cohen, 1999). The CTX gene, first identified in X. laevis, is conserved in S. tropicalis (Du Pasquier et al., 1999), and has subsequently been characterized in other vertebrates (Kong et al., 1998; Chrétien et al., 1998).
At the cellular level, the availability of mAb recognizing the CD8 co-receptor has provided an excellent opportunity to characterize this differentiated T-cell subset in more detail. CD8 T cells co-express XT-1, a pan-T-cell marker (Nagata, 1985), a high level of CD5 (a T-cell marker in Xenopus; Jürgens et al., 1995) and CD45 (Barritt and Turpen, 1995); these markers are not detectable in Tx frogs (Gravenor et al., 1995). Interestingly, a minor population of CD8 T cells co-express the NK cell-associated surface molecules recognized by 1F8 mAb (Rau et al., 2002), suggesting the occurrence in X. laevis as in mammals of CD8 NK-CTLs (Moretta et al., 2003). Biochemical analysis of the TCR/CD3 complex (Göbel et al., 2000) and characterization of TCRβ genes in Xenopus (Chrétien et al., 1997) also revealed features that are similar to mammals. For example, cell-mediated MHC-restricted cytotoxicity against major and minor H-Ags, characterized in vitro with whole splenocytes (Bernard et al., 1979; Watkins et al., 1988), was formally shown to be due to CD8 T cells (Robert et al., 2002). Using immunization against minor H-Ags, cytotoxicity of primed CD8 T cells purified with anti-CD8 mAb was Ag-specific and MHC-restricted, whereas only NK-like killing (e.g., killing of class I-negative tumor but not class I-expressing lymphoblast targets) was detected in CD8-depleted splenocytes. Effector capacity of CD8 T cells was further studied in vivo by developing an adoptive cell transfer in isogenetic clones of X. laevis (Maniero and Robert, 2004). Splenocytes from immunized donors of the same clone but immunized against minor H-Ags were stained with carboxyfluorescein diacetate succinimidyl ester (CFSE) to identify transferred cells as well as the extent of their proliferation in response to exposure of the recipient to the same Ags (reviewed in Robert et al., 2004). Primed anti-LG-15, but not naive CFSE+ T cells accumulated and divided in the spleen of allografted recipients to a greater extent than in those of autografted recipients. Similar accumulation and division occurred when CD8 T cells primed against 15/0 tumor Ags were transferred to an isogenetic recipient bearing the same MHC class I-negative tumor. Furthermore, the transfer of such primed anti-tumor splenocytes into naı¨ve recipients before tumor challenge delayed the appearance of tumors.
With respect to CD8 T-cell effector function, the crucial role of CTLs in Xenopus resistance to viral infection has been demonstrated using FV3, a ranavirus implicated in diseases and mortality of wild and captive amphibian populations (reviewed in Chinchar, 2002). Depletion of CD8 T cells by mAb treatment markedly increases adult susceptibility to FV3 infection (Robert et al., 2005). The anti-FV3 CD8 T cell response was further investigated in vivo using bromodeoxy-uri-dine (BrdU) incorporation to assess lymphocyte proliferative responses by flow cytometry (Morales and Robert, 2007; reviewed in Morales and Robert, 2008a). After primary infection, CD8 T cells significantly proliferated in the spleen and accumulated in infected kidneys (the main site of FV3 infection), from day 6 onward, in parallel with virus clearance. Earlier proliferation and infiltration associated with faster viral clearance were observed during a secondary infection. These results provide evidence of protective Ag-dependent CD8 T cell proliferation, recognition, and memory against a natural pathogen in Xenopus.
Recently, a nonconventional CD8 T-cell subset has been characterized in Xenopus. This subset recognizes and kills thymic lymphoid tumor that do not express classical MHC class I (class Ia). Therefore, by definition, this killing is not MHC classical class Ia-restricted (Goyos et. al., 2004). These CTLs (or CCU-CTL as they have been called) interact with non-classical MHC class Ib gene products (Goyos et al., 2007). Although the 15/0 lymphoid tumor is class Ia-negative, mRNAs for both class Ib (class Ib; Robert et al., 1994, Salter-Cid et al., 1998) and β2m (Stewart et al., 2005; Goyos et al., 2007) are expressed Stable 15/0 tumor transfectants with impaired class Ib expression induced by RNA interference targeting β2m or directly class Ib are more resistant to CD8 T-cell killing and more tumorigenic (Goyos et al., 2007). This finding suggests that class Ib molecules are indeed involved in the recognition of tumor cells by nontypical CD8 cytotoxic T cells. X. laevis possesses at least 20 Xenopus nonclassical class Ib molecules (XNC) genes that have been grouped in 9 subfamilies based on sequence similarity (Flajnik et al., 1993). Two additional novel subfamilies, XNC10 and XNC11, have recently been characterized that are preferentially expressed in thymic lymphoid tumors, thymocytes and T cells (Goyos et al., 2009).
Due to the lack of specific mAbs, little is still known about CD4 T cells other than that a CD4 gene ortholog is present in the S. tropicalis genome, as are cytokines and transcription factors critical for differentiation of Th1 (e.g., T-bet; IFNγ), Th2 (e.g., GATA3; IL-4, 5, 6, 10), Treg (FOXP3; CTLA4), and Th17 (e.g., RORγ; IL-17, 6, 21, 23, TGFβ) cells (Ohta and Robert, unpublished observations). However, typical helper T-cell functions have been identified in X. laevis adults. These include T cell collaboration for B-cell response that is MHC restricted (Blomberg et al., 1980; Flajnik et al., 1985) and proliferative responses in MHC-disparate mixed lymphocyte reactions (MLR; Du Pasquier et al., 1985; Watkins et al., 1988) that are inhibited by anti-class II mAbs (Harding et al., 1993).
Very few studies exist on Xenopus cytokines and chemokines involved in T-cell function. An IL-2–like T-cell growth factors have been functionally characterized in X. laevis (Watkins et al., 1987; Watkins and Cohen, 1987; Haynes and Cohen, 1993b), but IL-2 awaits identification at the molecular level. Recently, the interferon γ gene has been characterized in S. tropicalis, and its expression has been shown to be induced by stimulation of splenocytes with LPS and synthetic double-stranded poly(I:C) (Qi and Nie, 2008).
The Xenopus MHC was identified many years ago (Flajnik and Du Pasquier, 1990; Shum et al., 1993; Sato et al., 1993), and has been extensively studied for functions and polymorphism. The MHC region is also known as the most gene-dense region; in the human genome there are ~250 genes spanning a ~4 Mbp region. A search of the S. tropicalis genome for MHC genes has revealed a total of 122 genes found in 8 genomic scaffolds encompassing ~3.65Mbp. The overall genomic organization is remarkably similar to that of human MHC with some block inversions (Ohta et al., 2006). Yet, the Xenopus MHC demonstrates unique features of which some may be primordial: (1) a single MHC class I gene is tightly linked to the class I processing genes (transporter and immunoproteasome), thus defining the true “class I region,” present in all nonmammalian jawed vertebrates (Nonaka et al., 1997); (2) a strong co-evolution of class I and three other class I processing genes (proteasome subunit β type 8, transporter associated with antigen processing 1 and 2; Namikawa et al., 1995; Ohta et al., 2003; Bos and Waldman, 2006a,b) that can be separated into two ancient (separated 80–100 MYA) allelic lineages. No other species demonstrates such extensive co-evolution in the class I system; (3) maintenance of the two lineages among various Xenopus species; (4) all nonclassical class Ib genes are found outside the MHC region (Courtet et al., 2001) as seen in other nonmammalian species (Juul-Madsen et al., 2000; Belov et al., 2006; Afanassieff et al., 2001); and (5) a cluster of NK-like receptors found in the class III region that may have co-evolved with MHC class I as receptor/ligand relationship (Ohta et al., 2006).
Based on morphological criteria and some markers (e.g., formalin-resistant ATPase activity, MHC class II Ag, and vimentin), putative dendritic cells and Langerhans cells have been described in Xenopus adult skin (Mescher et al., 2007). Peritoneal leukocytes (PLs) also contain putative APCs. Large numbers of PLs are obtained by peritoneal lavage of frogs 3–4 days after elicitation of a cellular exudate by injection with heat-killed E. coli (Marr et al., 2005). These elicited PLs are 50–60% monocucleated cells with multiple pseudopods typical of macrophages. Compared with PLs from untreated frogs, bacteria-elicited PLs display an increased size (i.e., high forward scatter), are enriched for non-lymphocytic leukocytes (e.g., low signal for B- and T-cell markers), and still express, albeit at a lower level, surface MHC class II, and class I. Notably, the Ag-presentation ability of these cells has been demonstrated using the minor H-Ag system provided by LG clones. Adoptive transfer of PLs pulsed with minor H-Ags complexed to the heat shock protein gp96 induces potent CD8 T-cell infiltration and Ag-specific accelerated rejection of minor H-locus disparate skin grafts through an active process involving an endocytic receptor (CD91) expressed at the APCs surface (Robert et al., 2008).
1.3. Immune Responses to Pathogens and Tumors
X. laevis and S. tropicalis have become an important and useful model to study host pathogen interaction (reviewed in Morales and Robert, 2008b). The prevalence of infections and die offs caused by two pathogens: the chytrid fungus Batrachochytrium dendrobatidis (Bd) and ranaviruses (RV, family Iridoviridae) have markedly increased over the past decade. These pathogens are postulated to be among the major causal factors associated with the world-wide amphibian decline (reviewed in Carey et al., 1999; Daszak et al., 2000; Lips et al., 2008). Xenopus is providing a realistic alternative to study anti-fugal and antiviral defenses of natural populations of endangered amphibians (reviewed in Morales and Robert, 2008b). Study of the X. laevis immune response to RVs using FV3 is already well established (Gantress et al., 2003; Robert et al., 2005). Critical involvement of CD8 T cells (Morales and Robert, 2007), and antibodies (Gantress et al., 2003; Maniero et al., 2006) have been well documented. Several amphibian antimicrobial peptides in the skin are reported to inhibit Bd growth (reviewed in Rollins-Smith and Conlon, 2005) as well as frog viruses including FV3 (Chinchar et al., 2004). Studies of immune responses against Bd using microarrays in S. tropicalis, a species that unlike X. laevis is susceptible to this pathogen, suggest a prominent role of innate immune responses including antimicrobial peptides (Ribas and Fischer, unpublished observations); more studies are needed to evaluate the role of adaptive immune responses in controlling Bd infection. In this regard, it could be informative to evaluate the contribution of adaptive immunity to the resistance of X. laevis by depleting B and T cells (e.g., sublethal γ-irradiation, antibody treatments).
A peculiar feature of Xenopus is that like other ectothermic vertebrates, its immune system is affected by temperature (reviewed in Hsu, 1998; Meier, 2003). For example, helminth clearance in X. laevis is slower at 15°C than 25°C (Jackson and Tinsley, 2002). Similarly, minor H-disparate skin grafts are rejected faster at 27°C than at 21°C (Robert et al., 1995) and more slowly than MHC-disparate skin graft at 19°C (Du Pasquier et al., 1975). In addition, switch from IgM to IgY is prevented at 19°C (Wabl and Du Pasquier, 1976). Likewise, in vitro T-cell proliferation in MLR or induced by mitogen (reviewed in Meier, 2003), and of lymphoid thymic tumor cell lines (Robert et al., 1994), occur faster at 27°C (optimum) than at lower temperature (18–25°C). Collectively, these data suggest a selective inhibitory effect of low temperature on T-cell function in Xenopus, and as such could provide a useful model to further explore the influence of temperature on T-cell responses and particularly on MHC presentation and Ag recognition.
X. laevis has also been instrumental in revealing the conservation of immune responses to tumors. It is the only amphibian in which series of true lymphoid tumors (e.g., metastatic proliferation of genetically altered cells, and not other types of deregulated proliferation resulting from wound repair or infection) have been discovered, and cell lines that are transplantable and tumorigenic have been obtained. These lymphoid tumors have opened up new avenues for comparative tumor biology and tumor immunity (reviewed in Robert and Cohen, 1998b; Goyos and Robert, 2009). Four lymphoid tumor lines (e.g., B3B7, ff-2, 15/0 and 15/40) were initially derived from spontaneous thymic lymphoid tumors (Du Pasquier and Robert, 1992; Robert et al., 1994); a similar spontaneous thymic tumor was also reported later independently (Earley et al., 1995). All these tumor cell lines display a unique dual immature T/B phenotype, which, in mammals, is featured only by rare lymphocytic leukemias. All Xenopus lymphoid tumor cell lines express T-cell lineage markers (CD5, CD8, XT-1) and the immature thymocyte marker CTX. They also express RAG1, RAG2, and TdT that are involved in Ig rearrangements, and have undergone extensive Ig heavy and Ig light gene rearrangements, but do not synthesize any Ig protein (Du Pasquier et al., 1995). Interestingly, ff-2 and 15/40 but not B3B7 and 15/0 cell lines express MHC class Ia molecules, whereas they all express nonclassical MHC class Ib and β2m molecules (Robert et al., 1994; Goyos et al., 2007; 2009).
The tumor cell lines differ in their tumorigenicity. The ff-2 tumor cell line that was derived from the partially inbred frog strain homozygous for the f MHC haplotype, is transplantable and tumorigenic in larvae that share at least one MHC haplotype with the tumor, whereas it is rejected by adults of any genetic background (Robert et al., 1995; Robert and Cohen, 1998b). Tumor rejection in adults critically depends on thymic-derived T cells, because it manifests just after metamorphosis in parallel with the T-cell renewal in the thymus and the recovery of T cell effector functions (see section 3.1), it is abrogated by sublethal γ-irradiation that preferentially deplete thymocytes (Robert et al., 1995), and it is impaired in T-cell– deficient Tx animals (Robert et al., 1997). Immune responses to tumor have been further characterized using the 15/0 tumor line, derived from the LG-15 clone, that is very tumorigenic and grows both in larval and adult histocompatible (a/c) hosts. Involvement of NK cells in antitumor response is suggested by the accelerated growth of 15/0 tumor transplanted into animals pretreated with anti-NK cell mAb (Rau et al., 2002) and by NK cell cytotoxicity toward 15/0 tumor targets in vitro (Rau et al., 2002; Goyos et al., 2004). However, evidence strongly supports the fundamental and prominent role of T cells in antitumor responses: First, transplanted 15/0 tumors can develop in T-cell deficient Tx outbreds (Goyos and Robert, 2009), and growth of transplanted 15/0 tumor is accelerated in histocompatible host sublethally γ-irradiated or depleted of CD8 T cells by mAb treatment (Rau et al., 2001); Second, immunization with 15/0 tumor-derived heat shock proteins (e.g., gp96, hsp70) generates potent T-cell responses against 15/0 tumor in vivo (Robert et al., 2001a) that critically involves CD8 T cells (Maniero and Robert, 2004). Gp96 and hsp70 have been shown to chaperone antigenic peptides and to elicit CD8 T cell responses against these Ags as efficiently in Xenopus as in mammals (Robert et al., 2001a, 2002). Because 15/0 tumor is perfectly transplantable in LG-6 recipients that share the same MHC but differ at minor H loci with LG-15 clone (Fig. 1), this system permitted to increase T-cell response by immunizing against both tumor and minor H-Ags (Goyos et al., 2004). Further studies have revealed that T cells involved in antitumor responses in Xenopus include conventional CD8 T cells and the previously discussed unconventional CD8 T cells (CCU-CTLs) that interact with nonclassical MHC class Ib molecules (Goyos et al., 2004, 2007). Parenthetically, these tumor cell lines have also been used to reveal the conserved ability of certain heat shock proteins (gp96, hsp70) to generate potent antitumor responses (Robert et al., 2001a; Maniero and Robert, 2004).
2. EARLY DEVELOPMENT OF THE IMMUNE SYSTEM
Unlike mammals, Xenopus fertilized eggs develop outside the mother in the water where they are exposed to potential pathogens at early developmental stages. The membranes that protect the eggs and the first stages of embryonic development against physical damages also prevent penetration of pathogens. In addition, it has recently been reported that like fish and birds, X. laevis eggs contain maternal antibodies that provide passive protection (Poorten and Kuhn, 2009).
2.1. Organogenesis of the Thymus and Spleen
Like mammals, thymus ontogeny in X. laevis is characterized by successive waves of T-cell precursors moving into the thymus where they expand, differentiate, and leave as mature T cells (reviewed in Hansen and Zapata, 1998). The Xenopus thymus, which arises through an invagination of the dorsal epithelium of the second pharyngeal pouch around day 3 after fertilization (stage [st] 40; Nieuwkoop and Faber, 1967), is colonized by embryonic stem cells a few days later (Tochinai, 1980; Kau and Turpen, 1983; Flajnik et al., 1985). These cells are recognized by immunohistology with the mAb RC47, a surface marker of cells in the leukocyte lineage (Du Pasquier and Flajnik, 1990). By 6–8 days (~ st 48), the cortex–medulla architecture becomes visible with expression of MHC class II molecules in the thymic epithelium (Du Pasquier and Flajnik, 1990) and expression by cortical thymocytes of the surface marker CTX (Robert and Cohen, 1998a). Other T-cell markers, namely CD8, and XT-1 are also detected in the thymus at this stage. Peripheral splenic T cells expressing CD8 and XT1 surface markers are present from st 50 onward (15 days old). The thymus reaches its peak larval size (~1 × 106 lymphocytes) at st 58–63 (Table 3; Du Pasquier and Weiss, 1973; Rollins-Smith et al., 1984).
TABLE 3.
Summary of the Main Developmental Steps of the Xenopus Immune System
| Devel. stages (days) | Liver | Thymus | Spleen | GALT |
|---|---|---|---|---|
| 40 (d3) | Thymic epithelium buds from 2nd visceral pouch |
Absent | Few scattered CD3ε Expressing leukocytes |
|
| 46 (d4) | Lymphopoiesis in peripheral layer, Ig (µ)and sterile TCRβ RAG, AID |
Epithelium, no precursors |
Spleen anlage mesenchymal thickening in the mesogastrium |
No B cells |
| 47 (d4–5) | Lymphopoiesis, and B cell development in absence of Ag |
Colonization by Lymphopoietic precursors from post- VBI (~100 cells) RC47+ |
Blood cells (No Lymphopoiesis) |
|
| 48 (d6–7) | Cortex-meddula, full TCRβ mRNA, CD3ε CD8+ Thym., class II+ epithelial cells |
|||
| 49 (d10–13) | IgL rearrangements | First CTX+ thymocyte, more CD8+ |
Spleen B cells (~200) and 1st detect Ab responses |
|
| 50 (d15) | Ongoing thymocyte differentiation (3×104 cells) |
|||
| 56 (d38) | Ongoing thymocyte differentiation (9×105 cells) |
Detectable T cell responses | ||
| 58 (d44) | Max. size of the thymus (1–2×106 cells) |
Max. larval T cell response (1 × 106 cells) |
||
| Adult (>d60) | Adult-type leukocytes | Thymus move near tympanum New adult–type thymocyte differentiation |
Adult T cell responses (1–2 × 107 cells) |
|
| Adult (> 1 yr) | Thymus progressively filled by fat tissues |
(1–2 × 107 cells) | Many IgM+ and IgX+ B cells, as well as T cells (CD8+ and CD8-) |
The spleen anlage forms around 4–5 days postfertilization (st 47) as a mesenchymal thickening in the meso-gastrium but displays only blood cells involved in hematopoiesis; at approximately 12–14 days postfertilization (st 49), lymphocytes accumulate in the spleen, a time that corresponds to the first detectable Ab response (reviewed in Hsu, 1998; Du Pasquier et al., 2000). Note that in larva, lymphopoiesis takes place mainly in the liver, although Ig gene rearrangements presumably occur in the spleen (reviewed in Du Pasquier et al., 2000). BM is absent in tadpoles, and in contrast to adult the larval gut does not contain any surface IgM+ or IgX+ B cells (Mussmann et al., 1996; Du Pasquier et al., 2000). The spleen increases in size and lymphocyte number in parallel with the tadpole’s growth and reaches its maximum size at st 58 at the beginning of metamorphosis with up to 1–2 107 lymphocytes; this developmental time point coincides with maximal T-cell responses (Table 3).
2.2. Lymphocytes (B and T Cells)
RAG activity in the liver is detectable as early as 3 days after fertilization (Mussman et al., 1998), and rearrangement of the Ig heavy chain starts on day 5 (reviewed in Du Pasquier et al., 2000). However, mature competent B cells that have also rearranged IgL genes and express a complete B-cell receptor are not detected until 8 to 10 days postfertilization or st 49 (Hadji-Azimi et al., 1982; Mussmann et al., 1998). The immune repertoire at this early stage is subject to particular constraints, because it has to be built rapidly (larvae hatch 2 days after fertilization and must be immunocompetent) and a very few cells are available (~200 IgM+ B cells on days 12 after fertilization). These early differentiation events occur without the need for Ag selection and before the development of the spleen. Larvae generate an Ig repertoire with a VH usage similar to that of adults but not as heterogeneous. Moreover, larval Ig rearrangements lack N diversity (Schwager et al., 1991a; reviewed in Du Pasquier et al., 2000), which in adults is generated by terminal deoxynucleotidyl transferase (TdT; Lee and Hsu, 1994). This resulted in a shorter and less diverse CDR3 region (reviewed in Du Pasquier et al., 2000). Nevertheless, somatic hypermutation in larvae and adults is not significantly different from what has been observed in mammals (Wilson et al., 1992). Interestingly, AID mRNA is also detected very early in the liver before the detection of Ig light chain full rearrangement, which suggests that this enzyme may have other functions in early Xenopus development (Marr et al., 2007). By radioimmunoassay with specific mAbs, IgM protein is first detectable in the larval peritoneal fluid around day 12 (st 49) and IgY around day 15 (st 50) postfertilization (Hsu and Du Pasquier, 1984b). Around day 20 of development (st 52), antibody responses consisting of mainly IgM but also thymus-dependent IgY can be elicited against various Ags with similar kinetics to those of adult (Du Pasquier and Wabl, 1978; Hsu and Du Pasquier, 1984b). The weak IgY response likely results from difficulty in class switching and possibly a poor T-cell help, because it can be improved by injection of adult splenocytes (Hsu and Du Pasquier, 1984b) or by raising tadpoles at higher temperature (e.g., 27°C rather than 21°C, reviewed in Du Pasquier et al., 2000).
Very little is known about the generation of the TCR repertoire in larvae. Coincident with T-cell differentiation in the thymus, full-length TCRβ transcripts are detected 6 to 7 days postfertilization (st 48; Meier, 2003). Using CTX as a putative marker of the Xenopus equivalent of the mammalian double positive stage (i.e., immature stage) of thymocytes, a differentiation pathway similar to that of Xenopus adults has been characterized in vitro in larvae (Robert et al, 2001b). By immunohistology, cells expressing CD3ε have been detected from day 6 postfertilization in the thymus, liver, spleen, esophagus, skin, stomach, and the lining of the gut (Göbel et al., 2000; Meier, 2003). Because in mammals this signaling molecule is expressed by NK, γ/δ and α/β CD8 T cells, it is too premature to conclude for the presence of a possible extrathymic T cell development in Xenopus.
2.3. MHC Gene Expression in Larvae
A striking peculiarity of Xenopus larvae is the very limited surface expression of MHC class Ia molecules in most tissues until the onset of metamorphosis (review in Flajnik et al., 1987). This includes the thymus where class I expression would be required for CD8 T-cell selection. In fact, neither MHC class Ia nor LMP7 mRNAs can be detected in the thymus until 2 months postmetamorphosis (Flajnik et al., 1986; Salter-Cid et al., 1998); this finding strongly suggests an absence of MHC class I education during larval life. Class I surface expression becomes detectable at a low level on a minor fraction of splenocytes at premetamorphic stage (st 56–58), and increases during metamorphosis independent of thyroid hormones (Rollins-Smith et al., 1997a,b). Although larvae are immunocompetent and have CD8 T cells, it is unknown how (or if) larval CD8 T cell are educated and restricted without class Ia (Flajnik et al., 1986; Flajnik, 2002) and whether they are capable of cytotoxicity. Larval CD8 T cells should, however, be able to possess some type of cytotoxic activity because they can reject MHC incompatible skin grafts (Barlow and Cohen, 1983; reviewed in Cohen et al., 1985). In contrast to humans where impairment of class I expression results in severe immunodeficiency and/or death, Xenopus larvae are immunocompetent in the absence of class I expression. MHC class II Ag expression during larval life is restricted to thymic epithelium centrally and to B cells and accessory cells in the periphery (Du Pasquier and Flajnik, 1990; Rollins-Smith and Blair, 1990). T-cell proliferation induced by MLR, a typical helper T-cell assay, can be detected between MHC-disparate larval lymphocytes from stage 55–56 (Du Pasquier and Weiss, 1973; Du Pasquier et al., 1979).
2.4. Leukocytes
Although the Xenopus adaptive immune system develops relatively rapidly compared with mammals, it still takes 12 days postfertilization for differentiated B and T cells to emerge in the periphery of the feeding tadpole (Table 3). Before this time, the embryo must rely on innate type of myeloid cells or nonlymphocyte leukocytes for defense (reviewed in Hansen and Zapata, 1998). In Xenopus, primitive myeloid cells arise very early just after neurulation (st 20, 22–23 hr post-fertilization) from the anterior ventral blood island (aVBI), an embryonic region equivalent to the mammalian yolk sac, whereas erythroid and lymphoid blood cell lineages originate from the adjacent posterior ventral blood island (pVBI; Ciau-Uitz et al., 2000; Kau and Turpen, 1983; Maeno et al., 1985). Grafting experiments and fluorescence time-lapse video microscopy revealed that a population of early primitive myeloid cells migrate all over the embryo and constitute the first blood cells to differentiate in the embryo (Costa et al., 2008). Of interest, these so-called myelocytes are readily recruited to embryonic wounds or local exposure to bacteria, well before the establishment of a functional vasculature that first develops around 2 days after fertilization (st 35; Levine et al., 2003). Therefore, these cells could provide a transitory innate type of immune defense. In addition, spib, an ETS transcription factor, is involved in the differentiation and migration of myeloid cells expressed at the primitive myeloid precursor stage (Costa et al., 2008). Two other molecular markers that typify early myelocytes, a peroxidase family member (XPOX2) and Ly-6/uPAR-Related Protein (XLURP-1) have been identified (Smith et al., 2002; Tashiro et al., 2006). At the protein level, a leukocyte-specific mAb, XL-1, has similarly identified a widely dispersed population of nonlymphoid leukocytes in X. laevis tadpoles before the onset of cardiovascular circulation at the tail bud stage (Ohinata et al., 1989). Another mAb, XL-2, also recognizes an even earlier type of leukocyte at st 26 (Miyanaga et al., 1998); it is quite possible that these two mAbs recognize the same cell population (Tashiro et al., 2006). Although these different results are consistent with the establishment, during early development, of a rudimentary or transitory innate type of immune cell effector of the myeloid lineage, the heterogeneity and fate of these myelocytes at later stages of development, as well as their mode of action remain to be determined.
The liver, which is colonized by stem cells after blood circulation is established, becomes the site of hematopoiesis (formation of erythrocytes, leukocytes, and B cells) in larvae (Chen and Turpen, 1995). The different leukocyte types found in the adult are also present in the blood circulation and peritoneal fluid (e.g., neutrophils, basophils, eosinophils, PMNs, monocytes, macrophages, and lymphocytes). Larval macrophages, however, are morphologically distinct (Hsu pers. communication), do not express MHC class I molecules and respond differently to bacterial stimulation (Marr et al., 2005). Finally, no NK cells have been detected with the anti-NK cell 1F8 mAb until the time of metamorphosis 7–8 weeks postfertilization (st 58), and no in vitro NK killing activity can be detected with st 58 larval splenocytes (Horton et al., 2003). However, it remains to be determined whether a fraction of CD3ε-expressing cells (see section 2.2; Göbel et al., 2000; Meier, 2003) found in various larval tissues could be a larval type of NK cells.
3. REMODELING OF THE IMMUNE SYSTEM DURING METAMORPHOSIS
3.1. Thymus Histolysis, Differentiation of New Precursors, and the T-Cell Repertoire
The metamorphosing tadpole undergoes a remarkable series of physiological changes that includes an increase in glucocorticoids (Rollins-Smith et al., 1997b) and a profound reduction in the numbers of thymocytes and splenic lymphocytes (Du Pasquier et al., 1989). The thymus involutes during metamorphosis, losing 50 to 90% of its lymphocytes (Du Pasquier and Weiss, 1973; Rollins-Smith et al., 1984; reviewed in Du Pasquier et al., 1989) and translocates toward the tympanum. This thymocyte loss is followed by a second wave of stem cell immigration and a second histogenesis (Turpen and Smith, 1989; Bechtold et al., 1992). T-cell renewal during metamorphosis involves the pituitary gland as suggested by the impairment of thymus development and T-cell expansion in adults that were hypophy-sectomized at larval stages (Rollins-Smith et al., 2000).
The embryonic and metamorphic periods of thymocyte differentiation take place in different environments; many new proteins are expressed during metamorphosis that could be considered antigenic by the larval immune system (reviewed in Flajnik et al., 1987). The emerging adult lymphocytes, therefore, must be subjected to a new wave of negative selection by the adult “self,” resulting in a new balance of self-tolerance. This ability to become self-tolerant to adult Ags has been modeled by experiments revealing that allotolerance to adult skin graft from minor H-Ags mismatched donor can be induced just before (DiMarzo and Cohen, 1982a,b) and during metamorphic climax (Chardonnens and Du Pasquier, 1973; Du Pasquier and Chardonnens, 1975). Tolerance was measured by the permanent survival of a second-set skin graft from the same H-Ag mismatched donor that is grafted after metamorphosis. However, even though allotolerance is established, lymphocyte infiltration in the grafted skin and proliferative responses of the recipient splenocytes cocultured with irradiated splenocytes from the skin donor are detected. This finding suggests that some T cells recognizing minor H-Ag are still present in the recipient and can be activated to proliferate (Horton et al., 1993). This phenomenon, called “split” tolerance was first described for transplanted thymic epithelium in frogs, birds and mammals (Houssaint and Flajnik, 1990), and was shown to be induced in Xenopus larvae by non-thymic tissues such as skin (reviewed in Cohen et al., 1985). “Split” tolerance is considered to be a nondeletional type of tolerance involving MHC class II molecules (reviewed in Houssaint and Flajnik, 1990). Larvally-induced allotolerance is thymusdependent (Barlow and Cohen, 1983; Arnall and Horton, 1986, 1987). Moreover, it does not depend upon deletion of alloreactive cells because it can be adoptively transferred (Nakamura et al., 1987) and its maintenance can be broken by cyclophosphamide treatment (Horton et al., 1989). In contemporary terms, “split” tolerance in Xenopus is likely to reflect a type of anergy and/or involve regulatory T cells (Treg; reviewed in Sakaguchi et al., 2008). On the other hand, adults may lose tolerance to certain larval Ags, because MHC class II proliferative responses of adult T cells against larval skin Ags have been reported (reviewed in Izutsu et al., 2000).
3.2. Down-regulation of Immune Functions
In parallel with the major loss of thymocytes in the thymus, T-cell functions become severely impaired during metamorphosis. For example MLR is impaired from st 58–59 up to approximately 4 to 6 months after metamorphosis completion (Du Pasquier et al., 1979), as well as proliferation induced by mitogens such as PHA (Fig. 3; Rollins-Smith et al., 1984). There is also a marked decrease of lymphocyte numbers in the liver and spleen during this period (Du Pasquier and Weiss, 1973). Tolerance to minor H-Ag–disparate skin grafts that occurs already at larval stages is also observed during metamorphosis (Chardonnens and Du Pasquier, 1973; Du Pasquier and Chardonnens, 1975; reviewed in Du Pasquier et al., 1989). Another indication of the relative impairment of immune defense comes from experimental transplantation of the lymphoid tumor line ff-2 (Robert et al., 1994). Whereas, inbred f/f adults reject this tumor, larvae as well as young adults up to 6 months post-metamorphosis are susceptible to tumor growth (Robert et al., 1995). The possibility of obtaining partial protection by immunization at larval stage would suggest that the susceptibility of young postmetamorphic animals is the result of an immune regulatory mechanism rather than a deletion of immune cells (Robert et al., 1995). Consistent with this possibility is the ability of lymphocytes from metamorphosing animals transferred into iso-genetic adult recipients to inhibit the rejection of a minor H Ag-disparate skin graft (Du Pasquier and Bernard, 1980). Moreover, thymectomy performed at late premetamorphic developmental stage abrogates the ability to induce larval allotolerance of skin grafts (Barlow and Cohen, 1983). Although NK cells are detected from the beginning of metamorphosis with the mAb 1F8, significant NK killing activity in vitro can only be detected in adults of approximately 1 year of age.
Fig. 3.
Schematic overview summarizing the major developmental steps of the Xenopus immune system. For abbreviations, see list.
3.3. B Cells and Immunoglobulin Repertoire Switch
In contrast to the dramatic loss of thymocytes and consequently, peripheral T cells, not much is known about the fate of larval B cells during metamorphosis. Pre-B cells, defined as surface IgM negative/cytoplasmic IgM+, have been reported to decline sharply in the liver at st 58 and reappear from st 60 onward both in the liver and in the spleen that becomes the main site of B-cell differentiation in the adult (Hadji-Azimi et al., 1990). It is also clear that, at the molecular level, the Ig repertoire in larvae and adults is different (Mussmann et al., 1998); this finding suggests that a new adult type B cell population differentiates at some developmental time point (Chen and Turpen, 1995). Tadpoles and adults of the same clone were used to compare the antibody response to DNP by isoelectric focusing. Results showed that immunized larvae produce quite different and less heterogeneous anti-DNP spectrotypes than those of adults (Du Pasquier et al., 1979; Hsu and Du Pasquier, 1984b). Furthermore, inhibition of metamorphosis by goitrogen prevents the appearance of the adult-type anti-DNP antibody pattern (Hsu and Du Pasquier, 1992). Therefore, it appears that, as for T cells, a second wave of B cells expressing a new antibody repertoire differentiate after metamorphosis.
3.4. Differential Gene Expression of MHC and Other Immune Genes
MHC class I and class II genes are differentially regulated during metamorphosis. MHC class I molecules are first detected on erythrocytes and a minor splenocyte population at the beginning of metamorphosis (Flajnik et al., 1986; Flajnik and Du Pasquier, 1988; Rollins-Smith et al., 1997a). After metamorphosis, however, class II molecule is constitutively expressed on virtually all thymocytes and mature peripheral T as well as B cells (Du Pasquier and Flajnik, 1990; Rollins-Smith and Blair, 1990). As in the case of the antibody repertoire, the change in MHC class II expression is not initiated if metamorphosis is blocked by goitrogens (Rollins-Smith and Blair, 1990), whereas inhibition of metamorphosis has little effect on the onset of MHC class I expression on splenocytes and erythrocytes (Rollins-Smith et al., 1997a). It is noteworthy that the developmentally regulated acquisition of MHC class I molecules during metamorphosis and the ease with which one can experimentally manipulate embryos and larvae before expression of MHC class I and other adult-specific Ags, allows one to address questions about MHC restriction, autoimmunity, and the development of self-tolerance that cannot be easily studied in other animal models.
Studies with the 1F8 anti-NK cell mAb (Horton et al., 2003) have revealed that a small population of splenic NK cells emerges in metamorphosing larvae 1 week after the first appearance of surface class Ia. This observation is interesting, because in mammals, host MHC class I molecules regulates NK cell activity and tolerance through interaction with NK inhibitory receptors (Yoon et al., 2007). Another potentially interesting finding is that an Ag expressed in the tail appears to be recognized during metamorphosis and is involved in the tail resorption (Izutsu and Maeno, 2005; reviewed in Izutsu et al., 2000).
3.5. Persistence of Immunological Memory
Despite the drastic remodeling during metamorphosis, multiple evidences indicate that long-lived immunological memory persists through metamorphosis. MHC-disparate skin grafts are rejected faster by adults that were primed by a first skin graft of the same MHC donor at larval stages (Barlow and Cohen, 1983). Priming with DNP at larval stage also results in an accelerated antibody response in adult that involves the persistence of the larval Ig spectrotypes (Hsu and Du Pasquier, 1984b). In another system using the ff-2 thymic lymphoid tumor, priming with ff-2 irradiated tumor cells at larval stages interferes with the growth of the same tumor transplanted at postmetamorphic stages (Robert et al., 1995). Furthermore, immunization of larvae with the irradiated ff-2 tumor that only expresses minor H-Ag differences, leads to specific accelerated skin graft rejection in adults (Robert et al., 1997). Finally, thymectomy at late larval stages (st 57–58) impairs the emergence of adult T cells, which suggests that a small population of larval T cells survive metamorphosis (Rollins-Smith et al., 1996).
4. NEW TOOLS TO STUDY COMPARATIVE IMMUNOLOGY IN XENOPUS
The past and ongoing work in X. laevis and the new genomic information, together with the application of contemporary genetic technologies (e.g., whole genome mutagenesis, mapping, and transgenesis) developed in S. tropicalis, add to the potential of the genus Xenopus to become a very powerful nonmammalian biomedical model. Rather than arguing for choosing one Xenopus species versus another and limit further studies, we are proposing to use not only the two species X. laevis and S. tropicalis, but also other polyploid Xenopus species, and profit from this synergy to build a comprehensive model for the future study of many important aspects of immunity that includes but is not restricted to evolution, development, genomic regulation, host pathogen interaction, tumor immunity, autoimmunity, and immune pathology.
4.1. Immune Genes in Xenopus Polyploid Species
Nearly 40 years ago, the esteemed geneticist Susumu Ohno proposed that two rounds of genome-wide duplication occurred in a short evolutionary time period in early vertebrate evolution ~550 MYA (‘2R hypothesis’). He further proposed that creating new genes by genome duplication, followed by subfunctionalization is a quick and easy way to produce vast numbers of new genes (Ohno, 1970). The human genome sequence has revealed that there are more than 1,000 regions in which homologous genes are linked in a similar order (McLysaght et al., 2002). These so-called “paralogous” regions can be explained by genome duplication. The MHC and HOX regions are the best examples of such duplication (McLysaght et al., 2002). However, in most cases, genome duplication had occurred a long time ago, and underwent extensive genetic modifications (e.g., gene reshuffling, translocation, deletion, subfunc-tionalization). Hence, it is difficult to track genome evolution of the duplicated genes (Wolfe, 2001).
The genus Xenopus exists as a series of polyploid species ranging from 2n (diploid) to 12n (dodecaploid; Table 4) with no major reorganization of the chromosomes. These polyploid Xenopus species that arose by whole genome-wide duplication provide us with a series of relatively recently derived taxa (1–30 MYA reviewed in Kobel et al., 1996). The number of CTX genes can be used as a marker for the ploidy level (Du Pasquier et al., 1999). S. tropicalis is the only diploid Xenopus species possessing 20 chromosomes, whereas X. laevis with 36 chromosomes, most likely arose by allotetraploidization from its diploid ancestor. The comparison of S. tropicalis and X. laevis genomes provides an ideal model for examining the fate of duplicated genes because the divergence time between the two species is recent and genetic modifications still seem to be ongoing (Sammut et al., 2002; reviewed in Evans, 2008). Recent analysis using genes in the databases estimated that 25–50% of genes are retained as a double-copy in X. laevis after whole genome duplication, and it is proposed that slowly evolving genes are more likely to be retained as subfunctionalized paralogues, whereas fast evolving genes are more likely subjected to purifying selection (i.e., gene loss of one of the duplicates; Hellsten et al., 2007; Sémon and Wolfe, 2008). Indeed genes involved in Ag-specific adaptive immunity, which generally evolve faster to deal with ever-changing pathogens, are often diploidized in the tetraploid Xenopus. This rapid diploidization has been best studied for the MHC (Sato et al., 1993; Shum et al., 1993; Kato et al., 1994), RAG (Evans et al., 2005, Evans, 2007), and the Ig heavy chain (Courtet et al., 2001). In the dodecaploid animal, a maximum of four MHC class I genes were detected, having only two loci (Sammut et al., 2002). In these gene loci, accumulated mutations and deletions of parts of the genes were seen in one of the duplicated genes, presumably in the process of gene silencing. It is thought that diploidization is a mechanism to maintain a relatively low number of MHC genes to balance positive and negative selection, thereby generating a sufficiently heterogeneous T-cell repertoire for efficient T-cell responses (Flajnik et al., 1999). It is worth mentioning that, during the gene silencing process, some interacting genes or coordinating genes remained genetically linked, but some genes were active as paralogous loci (Evans, 2007; Ohta et al., 2003).
TABLE 4.
List of Existing Xenopus Species Ordered According to Their Evolutionary Origin and Ploidy
| MYA | Ploidy (Chr. No.) |
Xenopus species |
|---|---|---|
| 2n (20) | S. tropicalis | |
| S. epitropicalis | ||
| X. laevis | ||
| X. gilli | ||
| 4n | X. muelleri | |
| (36) | X. borearlis | |
| X. clivii | ||
| X. fraseri | ||
| X. largeni | ||
| X. pygmaeus | ||
| X. vestitus | ||
| X. amieti | ||
| 8n | X. andrei | |
| (72) | X. wittei | |
| X. boumbaensis | ||
| X. itombwensis | ||
| 12n | X. ruwenzoriensis | |
| (102) | X. longipes | |
| X. cf. boumbaensis |
Reviewed in Evans (2008). Note: We omitted the additional putative Xenopus species that were recently described from the table (reviewed in Evans 2008)
There is accumulating evidence that Ohno’s proposed two rounds of genome duplications were instrumental in the emergence of the adaptive immune system (Kasahara et al., 1996; Kasahara, 1998; Ohno, 1999; Flajnik and Kasahara, 2001; McLysaght et al., 2002; Gu et al., 2002), which is only found in jawed vertebrates (Flajnik, 1998, 2002; Flajnik et al., 1999). Therefore, studies of the fate of duplicated immune genes after whole genome duplication using Xenopus polyploidy species will help to understand how the mammalian adaptive immune system emerged as well as to provide insight into the mechanism by which genes remain linked to carry out optimal coordinated functions. Although there are other cases of polyploidy in vertebrates (Hordvik, 1998), Xenopus provides the only clear-cut and evolutionarily recent example of a distinct series of polyploid species (reviewed in Evans, 2007).
4.2. Transition From X. laevis to S. tropicalis to Study Comparative Immunology
Although very little is known about the S. tropicalis immune system, information that has already been obtained from the whole genome sequence strongly suggests that most features of its immune system are likely to be similar to those of X. laevis. The concerted effort to develop S. tropicalis for comparative developmental and biomedical studies opens new and exciting possibilities for immunological studies but this effort will also require the investment of time, work, and resources for generating the appropriate tools. We suggest below the most important steps for further developing the S. tropicalis system.
4.3. Generation of New MHC-Defined Xenopus Lines and Monoclonal Antibodies
As illustrated in this review, the availability of Xenopus-specific mAb recognizing surface makers as well as important immune molecules are invaluable for extending the potential of molecular and genetic studies. Presently, the way to study various leukocyte populations is very limited in S. tropicalis owing to the lack of mAbs. Most X. laevis-specific mAbs do not cross-react with S. tropicalis, which is not surprising because most immunologically relevant genes evolve rapidly, perhaps as a result of the “arms race” between host and pathogen. However, the high level of synteny between the S. tropicalis and mammalian genomes has been very useful in identifying immune genes. In addition, the availability of ESTs clones from S. tropicalis should considerably facilitate the task of generating new mAbs by recombinant assisted technologies. The benefit of such reagents will not be restricted to immunological studies, because mAbs specific for immunologically important proteins can also serve as markers for developmental studies. For example, mAbs against CTX can serve as early marker of thymus development, and anti-RAG for early development of liver.
Another crucial immunological tool would be development of MHC-defined inbred lines. Although, S. tropicalis so called Nigerian and Ivory Coast TGA inbred strains are now available (http://tropicalis.berkeley.edu/home/request_frogs/frogs.html), they have not been MHC typed, and will not be sufficient to provide inbred strains with different MHC haplotypes unless congenic strains or additional inbred stains are produced. It would be very important for the future development of S. tropicalis as an immunological as well as biomedical model to derive such MHC-defined inbred strains. In addition to fundamental immunology, many human diseases have important factors associated with the MHC (e.g., lupus or type II diabetes, increased susceptibility to cancer). Many host resistance factors to viral infections are associated with particular MHC haplotypes (reviewed in Taylor, 2004; Nolan et al., 2006), and similar susceptibility associated with the MHC homozygous J but not the F strain of X. laevis is documented (Gantress et al., 2003).
4.4. Genome Wide Mutagenesis and Transgenesis to Study Novel Immune Functions
The S. tropicalis genome project has provided us with an invaluable amount of new information. In addition, linkage analyses using microsatellite and chromosomal mapping (http://tropmap.biology.uh.edu) adds the syntenic relationships among the genes of interest. Our prediction is that the evolutionarily conserved genes might have vital roles in fundamental immune function, whereas novel genes found only in particular species but speculated to be in the ancestral linkage, could impart new insights into the mammalian immune system. Therefore, examining the novel genes in nonmammalian models such as Xenopus is very likely to reveal the functions of their human homologues having unknown or poorly described functions (e.g., toll-like receptor). Once candidate genes are identified and preliminary analysis (expression pattern, etc.) is conducted, mutagenesis will further help to reveal the phenotype of the deficient gene. Another powerful outcome expected from the genome-wide mutagenesis is the identification of immunologically relevant genes and the isolation of S. tropicalis with corresponding functional deficiency of this gene. Among potentially interesting genes to focus on, one can consider FOXP3 that would affect regulatory T cells (Treg) and tolerance during metamorphosis, or AIRE that would also affect central tolerance in thymus and tolerance during metamorphosis.
A last aspect that would merit consideration is the generation of S. tropicalis transgenic lines expressing reporter transgenes under the control of immune promoters. In addition to the conventional Restriction Enzyme Mediated Integration (REMI) technique for Xenopus (Kroll and Amaya, 1996), several new transgenic techniques, including the φC31 integrase (Allen and Weeks, 2005), the Sleeping Beauty (SB) transposon (Sinzelle et al., 2006), and the meganuclease methods (Pan et al., 2006; Ogino et al., 2006), have been described. Although the reliability and suitability of these different techniques still awaits a full evaluation (Chesnau et al., 2008), they clearly extend the possibilities of reverse genetics in Xenopus, especially with regard to immunology (Robert et al., 2009). The availability of full genomic sequences and large insert genomic clones in bacterial artificial chromosome library would greatly facilitate identification and manipulation of essential promoter regions under specific regulation. Genes of potential interest to follow are those differentially expressed during early development and metamorphosis such as MHC class Ia.
4.5. Concluding Remarks
The Xenopus genus as a whole has the potential to provide a unique model system of comparative biomedical and immunological research for the 21st century. With some investment, this model system would combine modern molecular, genetic, and genomic technologies at the cellular, organismic, and species levels. The progress of genetic and genomic studies with S. tropicalis can be complemented and synergized by the existing tools and knowledge accumulated from X. laevis, as well as by the multi-genome dimension offered by the other naturally polyploid Xenopus species. The benefit for fundamental and applied biomedical science of such a comprehensive nonmammalian animal model is likely to exceed, by far, the efforts and investments in its development.
ACKNOWLEDGMENTS
We thank Drs. N. Cohen and M. Flajnik for critically reading the manuscript. Y.O. was funded by the NIH and J.R. was funded by the NIH and NSF.
Grant sponsor: NIH; Grant number: AI27877; Grant number: R01-CA-108982-02; Grant number: R24-AI-059830; Grant sponsor: NSF; Grant number: MCB-0445509.
ABBREVIATIONS
- AID
activation-induced deaminase
- Ag
Antigen
- APC
antigen presenting cell
- β2m
beta-2 microglobulin
- BM
bone marrow
- DNP
dinitrophenol
- EST
expressed sequence tag
- GALT
gut-associated lymphoid tissue
- H-antigen
histocompatibility antigen
- Ig
immunoglobulin
- mAB
monoclonal antibody
- MHC
major histocompatibility complex
- MYA
million years ago
- MLR
mixed lymphocyte reaction
- NK cells
natural killer cells
- PAMPs
pathogen-associated molecular patterns
- PRRs
pattern recognition receptors
- PMN
polymorphonuclear
- RAG
recombination-activating gene
- TdT
terminal deoxynucleotidyl transferase
- TCR
T-cell receptor
- Tx
Thymectomize
REFERENCES
- Afanassieff M, Goto RM, Ha J, Sherman MA, Zhong L, Auffray C, Coudert F, Zoorob R, Miller MM. At least one class I gene in restriction fragment pattern-Y (Rfp-Y), the second MHC gene cluster in the chicken, is transcribed, polymorphic, and shows divergent specialization in antigen binding region. J Immunol. 2001;166:3324–3333. doi: 10.4049/jimmunol.166.5.3324. [DOI] [PubMed] [Google Scholar]
- Allen BG, Weeks DL. Transgenic Xenopus laevis embryos can be generated using phiC31 integrase. Nat Methods. 2005;2:975–979. doi: 10.1038/nmeth814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsenz J, Avila D, Huemer HP, Esparza I, Becherer JD, Kinoshita T, Wang Y, Oppermann S, Lambris JD. Phylogeny of the third component of complement, C3: analysis of the conservation of human CR1, CR2, H, and B binding sites, concanavalin A binding sites, and thiolester bond in the C3 from different species. Dev Comp Immunol. 1992;16:63–76. doi: 10.1016/0145-305x(92)90052-e. [DOI] [PubMed] [Google Scholar]
- Amemiya CT, Haire RN, Litman GW. Nucleotide sequence of a cDNA encoding a third distinct Xenopus immunoglobulin heavy chain isotype. Nucleic Acids Res. 1989;17:5388. doi: 10.1093/nar/17.13.5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson G, Moore NC, Owen JT, Jenkinson EJ. Cellular interactions in thymocyte development. Annu Rev Immunol. 1996;14:73–99. doi: 10.1146/annurev.immunol.14.1.73. [DOI] [PubMed] [Google Scholar]
- Arnall JC, Horton JD. Impaired rejection of minor-histocompatibility-antigen-disparate skin grafts and acquisition of tolerance to thymus donor antigens in allothymus-implanted, thymectomized Xenopus. Transplantation. 1986;41:766–776. doi: 10.1097/00007890-198606000-00020. [DOI] [PubMed] [Google Scholar]
- Arnall JC, Horton JD. In vivo studies on allotolerance perimetamorphically induced in control and thymectomized Xenopus. Immunology. 1987;62:315–319. [PMC free article] [PubMed] [Google Scholar]
- Barlow EH, Cohen N. The thymus dependency of transplantation allotolerance in the metamorphosing frog, Xenopus laevis. Transplantation. 1983;35:612–619. doi: 10.1097/00007890-198306000-00018. [DOI] [PubMed] [Google Scholar]
- Barritt LC, Turpen JB. Characterization of lineage restricted forms of a Xenopus CD45 homologue. Dev Comp Immunol. 1995;19:525–536. doi: 10.1016/0145-305x(95)00031-n. [DOI] [PubMed] [Google Scholar]
- Bechtold TE, Smith PB, Turpen JB. Differential stem cell contributions to thymocyte succession during development of Xenopus laevis. J Immunol. 1992;148:2975–2982. [PubMed] [Google Scholar]
- Belov K, Deakin JE, Papenfuss AT, Baker ML, Melman SD, Siddle HV, Gouin N, Goode DL, Sargeant TJ, Robinson MD, Wakefield MJ, Mahony S, Cross JG, Benos PV, Samollow PB, Speed TP, Graves JA, Miller RD. Reconstructing an ancestral mammalian immune super-complex from a marsupial major histocompatibility complex. PLoS Biol. 2006;4:e46. doi: 10.1371/journal.pbio.0040046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtén E, Wilson M, Miller N, Clem LW, Pilström L, Warr GW. Immunoglobulin isotypes: structure, function, and genetics. Curr Top Microbiol Immunol. 2000;248 doi: 10.1007/978-3-642-59674-2_9. 189-182. [DOI] [PubMed] [Google Scholar]
- Bernard C, Bordmann G, Blomberg B, Du Pasquier L. Immunogenetic studies on the cell-mediated cytotoxicity in the clawed toad Xenopus laevis. Immunogenetics. 1979;9:443–454. [Google Scholar]
- Bernard D, Hansen JD, Du Pasquier L, Lefranc MP, Benmansour A, Boudinot P. Costimulatory receptors in jawed vertebrates: conserved CD28, odd CTLA4 and multiple BTLAs. Dev Comp Immunol. 2007;31:255–271. doi: 10.1016/j.dci.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Bernstein RM, Schluter SF, Shen S, Marchalonis JJ. A new high molecular weight immunoglobulin class from the carcharhine shark:implications for the properties of the primordial immunoglobulin. Proc Natl Acad Sci U S A. 1996;93:3289–3293. doi: 10.1073/pnas.93.8.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleicher PA, Cohen N. Monoclonal anti-IgM can separate T cell from B cell proliferative responses in the frog, Xenopus laevis. J Immunol. 1981;127:1549–1555. [PubMed] [Google Scholar]
- Bleicher PA, Rollins-Smith LA, Jacobs DM, Cohen N. Mitogenic responses of frog lymphocytes to crude and purified preparations of bacterial lipopolysaccharide (LPS) Dev Comp Immunol. 1983;7:483–496. doi: 10.1016/0145-305x(83)90033-2. [DOI] [PubMed] [Google Scholar]
- Blomberg B, Bernard CC, Du Pasquier L. In vitro evidence for T-B lymphocyte collaboration in the clawed toad, Xenopus. Eur J Immunol. 1980;10:869–876. doi: 10.1002/eji.1830101112. [DOI] [PubMed] [Google Scholar]
- Bos DH, Waldman B. Evolution by recombination and transspecies polymorphism in the MHC class I gene of Xenopus laevis. Mol Biol Evol. 2006a;23:137–143. doi: 10.1093/molbev/msj016. [DOI] [PubMed] [Google Scholar]
- Bos DH, Waldman B. Polymorphism, natural selection, and structural modeling of class Ia MHC in the African clawed frog (Xenopus laevis) Immunogenetics. 2006b;58:433–442. doi: 10.1007/s00251-006-0114-5. [DOI] [PubMed] [Google Scholar]
- Carey C, Cohen N, Rollins-Smith L. Amphibian declines: an immunological perspective. Dev Comp Immunol. 1999;23:459–472. doi: 10.1016/s0145-305x(99)00028-2. [DOI] [PubMed] [Google Scholar]
- Chardonnens X, Du Pasquier L. Induction of skin allograft tolerance during metamorphosis of the toad Xenopus laevis: a possible model for studying generation of self tolerance to histocompatibility antigens. Eur J Immunol. 1973;3:569–573. doi: 10.1002/eji.1830030909. [DOI] [PubMed] [Google Scholar]
- Chen XD, Turpen JB. Intraembryonic origin of hepatic hematopoiesis in Xenopus laevis. J Immunol. 1995;154:2557–2567. [PubMed] [Google Scholar]
- Chesneau A, Sachs LM, Chai N, Chen Y, Du Pasquier L, Loeber J, Pollet N, Reilly M, Weeks DL, Bronchain OJ. Transgenesis procedures in Xenopus. Biol Cell. 2008;100:503–521. doi: 10.1042/BC20070148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchar VG. Ranaviruses (Family Iridoviridae): emerging cold-blooded killers. Arch Virol. 2002;147:447–470. doi: 10.1007/s007050200000. [DOI] [PubMed] [Google Scholar]
- Chinchar VG, Bryan L, Silphadaung U, Noga E, Wade D, Rollins-Smith L. Inactivation of viruses infecting ectothermic animals by amphibian and piscine antimicrobial peptides. Virology. 2004;323:268–275. doi: 10.1016/j.virol.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Chrétien I, Robert J, Marcuz A, Garcia-Sanch J, Courtet M, Du Pasquier L. Specific expression on cortical thymocytes of CTX a new polymorphic IgSF member. Eur J Immunol. 1996;26:780–791. doi: 10.1002/eji.1830260409. [DOI] [PubMed] [Google Scholar]
- Chretien I, Marcuz A, Fellah J, Charlemagne J, Du Pasquier L. The T cell receptor beta genes of Xenopus. Eur J Immunol. 1997;27:763–771. doi: 10.1002/eji.1830270327. [DOI] [PubMed] [Google Scholar]
- Chrétien I, Marcuz A, Courtet M, Katevuo K, Vainio O, Heath JK, White SJ, Du Pasquier L. CTX, a Xenopus thymocyte receptor, defines a molecular family conserved throughout vertebrates. Eur J Immunol. 1998;28:4094–4104. doi: 10.1002/(SICI)1521-4141(199812)28:12<4094::AID-IMMU4094>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Ciau-Uitz A, Walmsley M, Patient R. Distinct origins of adult and embryonic blood in Xenopus. Cell. 2000;102:787–796. doi: 10.1016/s0092-8674(00)00067-2. [DOI] [PubMed] [Google Scholar]
- Cohen N, DiMarzo S, Rollins-Smith L, Barlow E, Vanderschmidt-Parson S. The ontogeny of allo-tolerance in larval Xenopus. In: Balls M, Bownes ME, editors. Metamorphosis. Oxford, England: Oxford Univiversity Press; 1985. pp. 388–419. [Google Scholar]
- Costa RM, Soto X, Chen Y, Zorn AM, Amaya E. spib is required for primitive myeloid development in Xenopus. Blood. 2008;112:2287–2296. doi: 10.1182/blood-2008-04-150268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtet M, Flajnik M, Du Pasquier L. Major histocompatibility complex and immunoglobulin loci visualized by in situ hybridization on Xenopus chromosomes. Dev Comp Immunol. 2001;25:149–157. doi: 10.1016/s0145-305x(00)00045-8. [DOI] [PubMed] [Google Scholar]
- Criscitiello MF, Flajnik MF. Four primordial immunoglobulin light chain isotypes, including λ and κ, identified in the most primitive living jawed vertebrates. Eur J Immunol. 2007;37:2683–2694. doi: 10.1002/eji.200737263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daszak P, Cunningham AA, Hyatt AD. Emerging infectious diseases of wildlife—threats to biodiversity and human health. Science. 2000;287:443–449. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- DiMarzo SJ, Cohen N. Immunogenetic aspects of in vivo allotolerance induction during the ontogeny of Xenopus laevis. Immunogenetics. 1982a;16:103–116. doi: 10.1007/BF00364398. [DOI] [PubMed] [Google Scholar]
- DiMarzo SJ, Cohen N. An in vivo study of the ontogeny of alloreactivity in the frog, Xenopus laevis. Immunology. 1982b;45:39–48. [PMC free article] [PubMed] [Google Scholar]
- Du Pasquier L. Relationships among the genes encoding MHC molecules and the specific antigen receptors. In: Du Pasquier L, Kasahawa M, editors. MHC evolution, structure and function. Tokyo: Springer-Verlag; 2000. pp. 53–65. [Google Scholar]
- Du Pasquier L, Bernard CA. Active suppression of allogeneic histocompatibility reactions during metamorphosis of the clawed toad Xenopus. Differentiation. 1980;16:1–7. doi: 10.1111/j.1432-0436.1980.tb01052.x. [DOI] [PubMed] [Google Scholar]
- Du Pasquier L, Chardonnens X. Genetic aspects of the tolerance to allografts induced at metamorphosis in the toad Xenopus laevis. Immunogenetics. 1975;2:431–440. [Google Scholar]
- Du Pasquier L, Flajnik MF. Expression of MHC class II antigens during Xenopus development. Dev Immunol. 1990;1:85–95. doi: 10.1155/1990/67913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Pasquier L, Hsu E. Immunoglobulin expression in diploid and polyploid interspecies hybrid of Xenopus: evidence for allelic exclusion. Eur J Immunol. 1983;13:585–590. doi: 10.1002/eji.1830130714. [DOI] [PubMed] [Google Scholar]
- Du Pasquier L, Robert J. In vitro growth of thymic tumor cell lines from Xenopus. Dev Immunol. 1992;2:295–307. doi: 10.1155/1992/41823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Pasquier L, Wabl MR. Antibody diversity in amphibians: inheritance of isoelectric focusing antibody patterns in isogenic frogs. Eur J Immunol. 1978;8:428–433. doi: 10.1002/eji.1830080611. [DOI] [PubMed] [Google Scholar]
- Du Pasquier L, Weiss N. The thymus during the ontogeny of the toad Xenopus laevis: growth, membrane-bound immunoglobulins and mixed lymphocyte reaction. Eur J Immunol. 1973;3:773–777. doi: 10.1002/eji.1830031207. [DOI] [PubMed] [Google Scholar]
- Du Pasquier L, Chardonnens X, Miggiano VC. A major histocompatibility complex in the toad Xenopus laevis. Immunogenetics. 1975;1:482–494. [Google Scholar]
- Du Pasquier L, Blomberg B, Bernard CC. Ontogeny of immunity in amphibians: changes in antibody repertoires and appearance of adult major histocompatibility antigens in Xenopus. Eur J Immunol. 1979;9:900–906. doi: 10.1002/eji.1830091112. [DOI] [PubMed] [Google Scholar]
- Du Pasquier L, Flajnik MF, Guiet C, Hsu E. Methods used to study the immune system of Xenopus (Amphibia, Anura) Immunol Methods. 1985;3:425–465. [Google Scholar]
- Du Pasquier L, Schwager J, Flajnik MF. The immune system of Xenopus. Annu Rev Immunol. 1989;7:251–275. doi: 10.1146/annurev.iy.07.040189.001343. [DOI] [PubMed] [Google Scholar]
- Du Pasquier L, Courtet M, Robert J. A Xenopus lymphoid tumor cell line with complete Ig genes rearrangements and T-cell characteristics. Mol Immunol. 1995;32:583–593. doi: 10.1016/0161-5890(95)00002-v. [DOI] [PubMed] [Google Scholar]
- Du Pasquier L, Courtet M, Chretien I. Duplication and MHC linkage of the CTX family of genes in Xenopus and in mammals. Eur J Immunol. 1999;29:1729–1739. doi: 10.1002/(SICI)1521-4141(199905)29:05<1729::AID-IMMU1729>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Du Pasquier L, Robert J, Courtet M, Mussmann R. B-cell development in the amphibian Xenopus. Immunol Rev. 2000;175:201–213. doi: 10.1111/j.1600-065x.2000.imr017501.x. [DOI] [PubMed] [Google Scholar]
- Dzialo RC, Cooper MD. An amphibian CD3 homologue of the mammalian CD3 gamma and delta genes. Eur J Immunol. 1997;27:1640–1647. doi: 10.1002/eji.1830270708. [DOI] [PubMed] [Google Scholar]
- Endo Y, Takahashi M, Nakao M, Saiga H, Sekine H, Matsushita M, Nonaka M, Fujita T. Two lineages of mannose-binding lectin-associated serine protease (MASP) in vertebrates. J Immunol. 1998;161:4924–4930. [PubMed] [Google Scholar]
- Evans BJ. Ancestry influences the fate of duplicated genes millions of years after polyploidization of clawed frogs (Xenopus) Genetics. 2007;176:1119–1130. doi: 10.1534/genetics.106.069690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans BJ. Genome evolution and speciation genetics of clawed frogs (Xenopus and Silurana) Front Biosci. 2008;13:4687–4706. doi: 10.2741/3033. [DOI] [PubMed] [Google Scholar]
- Evans BJ, Kelley DB, Melnick DJ, Cannatella DC. Evolution of RAG-1 in polyploid clawed frogs. Mol Biol Evol. 2005;22:1193–1207. doi: 10.1093/molbev/msi104. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KA, Palsson-McDermott EM, Bowie AG, Jefferies CA, Mansell AS, Brady G, Brint E, Dunne A, Gray P, Harte MT, McMurray D, Smith DE, Sims JE, Bird TA, O’Neill LA. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature. 2001;413:78–83. doi: 10.1038/35092578. [DOI] [PubMed] [Google Scholar]
- Flajnik MF. Churchill and the immune system of ectothermic vertebrates. Immunol Rev. 1998;166:5–14. doi: 10.1111/j.1600-065x.1998.tb01248.x. [DOI] [PubMed] [Google Scholar]
- Flajnik MF. Comparative analyses of immunoglobulin genes: surprises and portents. Nat Rev Immunol. 2002;2:688–698. doi: 10.1038/nri889. [DOI] [PubMed] [Google Scholar]
- Flajnik MF, Du Pasquier L. MHC class I antigens as surface markers of adult erythrocytes during the metamorphosis of Xenopus. Dev Biol. 1988;128:198–206. doi: 10.1016/0012-1606(88)90282-5. [DOI] [PubMed] [Google Scholar]
- Flajnik MF, Du Pasquier L. The major histocompatibility complex of frogs. Immunol Rev. 1990;113:47–63. doi: 10.1111/j.1600-065x.1990.tb00036.x. [DOI] [PubMed] [Google Scholar]
- Flajnik MF, Kasahara M. Comparative genomics of the MHC: glimpses into the evolution of the adaptive immune system. Immunity. 2001;15:351–362. doi: 10.1016/s1074-7613(01)00198-4. [DOI] [PubMed] [Google Scholar]
- Flajnik MF, Du Pasquier L, Cohen N. Immune responses of thymus/lymphocyte embryonic chimeras. Studies on tolerance and MHC restriction in Xenopus. Eur J Immunol. 1985;15:540–547. doi: 10.1002/eji.1830150603. [DOI] [PubMed] [Google Scholar]
- Flajnik MF, Kaufman JF, Hsu E, Manes M, Parisot R, Du Pasquier L. Major histocompatibility complex-encoded class I molecules are absent in immunologically competent Xenopus before metamorphosis. J Immunol. 1986;137:3891–3899. [PubMed] [Google Scholar]
- Flajnik MF, Hsu E, Kaufman JF, Du Pasquier L. Changes in the immune system during metamorphosis of Xenopus. Immunol Today. 1987;8:58–64. doi: 10.1016/0167-5699(87)90240-4. [DOI] [PubMed] [Google Scholar]
- Flajnik MF, Hsu E, Kaufman JF, Du Pasquier L. Biochemistry, tissue distribution and ontogeny of surface molecules detected on Xenopus hemopoietic cells. In: Miyasaka M, Trnka Z, editors. Differentiation antigens on lymphohemopoietic tissues. New York: Dekker; 1988. pp. 387–419. [Google Scholar]
- Flajnik MF, Ferrone S, Cohen N, Du Pasquier L. Evolution of the MHC: antigenicity and unusual tissue distribution of Xenopus (frog) class II molecules. Mol Immunol. 1990;27:451–462. doi: 10.1016/0161-5890(90)90170-5. [DOI] [PubMed] [Google Scholar]
- Flajnik MF, Taylor E, Canel C, Gross-berger D, Du Pasquier L. Reagents specific for MHC I antigens of Xenopus. Am Zool. 1991;31:580–591. [Google Scholar]
- Flajnik MF, Kasahara M, Shum BP, Salter-Cid L, Taylor E, Du Pasquier L. A novel type of class I gene organization in vertebrates: a large family of non-MHC-linked class I genes is expressed at the RNA level in the amphibian Xenopus. EMBO J. 1993;12:4385–4396. doi: 10.1002/j.1460-2075.1993.tb06123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flajnik MF, Ohta Y, Namikawa-Yamada C, Nonaka M. Insight into the primordial MHC from studies of ectothermic vertebrates. Immunol Rev. 1999;167:59–67. doi: 10.1111/j.1600-065x.1999.tb01382.x. [DOI] [PubMed] [Google Scholar]
- Fujita T, Endo Y, Nonaka M. Primitive complement system—recognition and activation. Mol Immunol. 2004;41:103–311. doi: 10.1016/j.molimm.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Gantress J, Bell A, Maniero G, Chinchar GN, Cohen N, Robert J. Xenopus, a model to study immune response to iridovirus. Virology. 2003;311:254–262. doi: 10.1016/s0042-6822(03)00151-x. [DOI] [PubMed] [Google Scholar]
- Göbel TW, Meier EL, Du Pasquier L. Biochemical analysis of the Xenopus laevis TCR/CD3 complex supports the “stepwise evolution” model. Eur J Immunol. 2000;30:2775–2781. doi: 10.1002/1521-4141(200010)30:10<2775::AID-IMMU2775>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Goyos A, Robert J. Tumorigenesis and anti-tumor immune response in Xenopus laevis. In: The frog Xenopus as a model to study evolutionary, developmental and biomedical immunology. Front Biosci. 2009;14:167–176. doi: 10.2741/3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyos A, Cohen N, Gantress J, Robert J. Anti-tumor MHC class Ia-unrestricted CD8 T cell cytotoxicity elicited by the heat shock protein gp96. Eur J Immunol. 2004;34:2449–2458. doi: 10.1002/eji.200425105. [DOI] [PubMed] [Google Scholar]
- Goyos A, Guselnikov S, Chida AS, Sniderhan LF, Maggirwar SB, Nedelkovska H, Robert J. Involvement of nonclassical MHC class Ib molecules in heat shock protein-mediated anti-tumor responses. Eur J Immunol. 2007;37:1494–1501. doi: 10.1002/eji.200636570. [DOI] [PubMed] [Google Scholar]
- Goyos A, Ohta Y, Guselnikov S, Robert J. Novel nonclassical MHC class Ib genes associated with CD8 T cell development and thymic tumors. Mol Immunol. 2009 doi: 10.1016/j.molimm.2009.01.016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravenor I, Horton TL, Ritchie P, Flint E, Horton JD. Ontogeny and thymus-dependence of T cell surface antigens in Xenopus: flow cytometric studies on monoclonal antibody-stained thymus and spleen. Dev Comp Immunol. 1995;19:507–523. doi: 10.1016/0145-305x(95)00030-w. [DOI] [PubMed] [Google Scholar]
- Greenberg AS, Hughes AL, Guo J, Avila D, McKinney EC, Flajnik MF. A novel “chimeric” antibody class in cartilaginous fish: IgM may not be the primordial immunoglobulin. Eur J Immunol. 1996;26:1123–1129. doi: 10.1002/eji.1830260525. [DOI] [PubMed] [Google Scholar]
- Greenhalgh P, Olesen CE, Steiner LA. Characterization and expression of recombination activating genes (RAG-1 and RAG-2) in Xenopus laevis. J Immunol. 1993;151:3100–3110. [PubMed] [Google Scholar]
- Gu X, Wang Y, Gu J. Age distribution of human gene families shows significant roles of both large- and small-scale duplications in vertebrate evolution. Nat Genet. 2002;31:205–209. doi: 10.1038/ng902. [DOI] [PubMed] [Google Scholar]
- Guselnikov SV, Bell A, Najakshin AM, Robert J, Taranin AV. Signaling FcRgamma and TCRzeta subunit homologs in the amphibian Xenopus laevis. Dev Comp Immunol. 2003;27:727–733. doi: 10.1016/s0145-305x(03)00055-7. [DOI] [PubMed] [Google Scholar]
- Guselnikov SV, Ramanayake T, Yerilova A, Mechetina LV, Najakshin AM, Robert J, Taranin AV. The Xenopus FcR-family demonstrates continually high diversification of paired receptors in vertebrate evolution. BMC Evol Biol. 2008;8:148. doi: 10.1186/1471-2148-8-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadji-Azimi I, Schwager J, Thiebaud C. B-lymphocyte differentiation in Xenopus laevis larvae. Dev Biol. 1982;90:253–258. doi: 10.1016/0012-1606(82)90374-8. [DOI] [PubMed] [Google Scholar]
- Hadji-Azimi I, Coosemans V, Canicatti C. Atlas of adult Xenopus laevis lae-vis hematology. Dev Comp Immunol. 1987;11:807–874. doi: 10.1016/0145-305x(87)90068-1. [DOI] [PubMed] [Google Scholar]
- Hadji-Azimi I, Coosemans V, Canicatti C. B-lymphocyte populations in Xenopus laevis. Dev Comp Immunol. 1990;14:69–84. doi: 10.1016/0145-305x(90)90009-4. [DOI] [PubMed] [Google Scholar]
- Haire RN, Shamblott MJ, Amemiya CT, Litman GW. A second Xenopus immunoglobulin heavy chain constant region isotype gene. Nucleic Acids Res. 1989;17:1776. doi: 10.1093/nar/17.4.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haire RN, Kitzan Haindfield MK, Turpen JB, Litman GW. Structure and diversity of T-lymphocyte antigen receptors alpha and gamma in Xenopus. Immunogenetics. 2002;54:431–438. doi: 10.1007/s00251-002-0474-4. [DOI] [PubMed] [Google Scholar]
- Hansen JD, Zapata AG. Lymphocyte development in fish and amphibians. Immunol Rev. 1998;166:199–220. doi: 10.1111/j.1600-065x.1998.tb01264.x. [DOI] [PubMed] [Google Scholar]
- Hansen JD, Strassburger P, Du Pasquier L. Conservation of a master hematopoietic switch gene during vertebrate evolution: isolation and characterization of Ikaros from teleost and amphibian species. Eur J Immunol. 1997;27:3049–3058. doi: 10.1002/eji.1830271143. [DOI] [PubMed] [Google Scholar]
- Harding FA, Amemiya CT, Litman RT, Cohen N, Litman GW. Two distinct immunoglobulin heavy chain isotypes in a primitive, cartilaginous fish, Raja erinacea. Nucleic Acids Res. 1990;18:6369–6376. doi: 10.1093/nar/18.21.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding FA, Flajnik MF, Cohen N. MHC restriction of T cell proliferative responses in Xenopus. Dev Comp Immunol. 1993;17:425–437. doi: 10.1016/0145-305x(93)90034-n. [DOI] [PubMed] [Google Scholar]
- Haynes L, Cohen N. Transforming growth factor beta (TGF beta) is produced by and influences the proliferative response of Xenopus laevis lymphocytes. Dev Immunol. 1993a;3:223–230. doi: 10.1155/1993/63626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes L, Cohen N. Further characterization of an interleukin-2-like cytokine produced by Xenopus Laevis T lymphocytes. Dev Immunol. 1993b;3:231–238. doi: 10.1155/1993/68197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellsten U, Khokha MK, Grammer TC, Harland RM, Richardson P, Rokhsar DS. Accelerated gene evolution and subfunctionalization in the pseudotet-raploid frog Xenopus laevis. BMC Biol. 2007;5:31. doi: 10.1186/1741-7007-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hordvik I. The impact of ancestral polyploidy on antibody heterogeneity in salmonid fishes. Immunol Rev. 1998;166:153–157. doi: 10.1111/j.1600-065x.1998.tb01260.x. [DOI] [PubMed] [Google Scholar]
- Horton JD, Horton TL, Varley CA, Ruben LN. Attempts to break perimetamorphically induced skin graft tolerance by treatment of Xenopus with cyclophosphamide and interleukin-2. Transplantation. 1989;47:883–887. doi: 10.1097/00007890-198905000-00027. [DOI] [PubMed] [Google Scholar]
- Horton JD, Horton TL, Ritchie P. Incomplete tolerance induced in Xenopus by larval tissue allografting: evidence from immunohistology and mixed leucocyte culture. Dev Comp Immunol. 1993;17:249–262. doi: 10.1016/0145-305x(93)90044-q. [DOI] [PubMed] [Google Scholar]
- Horton JD, Horton TL, Dzialo R, Gravenor I, Minter R, Ritchie P, Gartland L, Watson M, Cooper M. T-cell and natural killer cell development in thymectomized Xenopus. Immunol Rev. 1998;166:245–258. doi: 10.1111/j.1600-065x.1998.tb01267.x. [DOI] [PubMed] [Google Scholar]
- Horton TL, Minter R, Stewart R, Ritchie P, Watson MD, Horton JD. Xenopus NK cells identified by novel monoclonal antibodies. Eur J Immuol. 2000;30:604–613. doi: 10.1002/1521-4141(200002)30:2<604::AID-IMMU604>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Horton T, Stewart R, Cohen N, Rau L, Ritchie P, Watson M, Robert J, Horton J. Ontogeny of Xenopus NK cells in the absence of MHC class I antigens. Dev Comp Immunol. 2003;27:715–726. doi: 10.1016/s0145-305x(03)00040-5. [DOI] [PubMed] [Google Scholar]
- Houssaint E, Flajnik M. The role of thymic epithelium in the acquisition of tolerance. Immunol Today. 1990;11:357–360. doi: 10.1016/0167-5699(90)90141-u. [DOI] [PubMed] [Google Scholar]
- Hsu E. Mutation, selection, and memory in B lymphocytes of exothermic vertebrates. Immunol Rev. 1998;162:25–36. doi: 10.1111/j.1600-065x.1998.tb01426.x. [DOI] [PubMed] [Google Scholar]
- Hsu E, Du Pasquier L. Studies in Xenopus immunoglobulins using monoclonal antibodies. Mol Immunol. 1984a;21:257–270. doi: 10.1016/0161-5890(84)90096-8. [DOI] [PubMed] [Google Scholar]
- Hsu E, Du Pasquier L. Ontogeny of the immune system in Xenopus. I. Larval immune response. Differentiation. 1984b;28:109–115. [Google Scholar]
- Hsu E, Flajnik MF, Du Pasquier L. A third immunoglobulin class in amphibians. J Immunol. 1985;135:1998–2004. [PubMed] [Google Scholar]
- Hsu E, Lefkovits I, Flajnik M, Du Pasquier L. Light chain heterogeneity in the amphibian Xenopus. Mol Immunol. 1991;28:985–994. doi: 10.1016/0161-5890(91)90184-l. [DOI] [PubMed] [Google Scholar]
- Ibrahim B, Gartland LA, Kishimoto T, Dzialo R, Kubagawa H, Bucy RP, Cooper MD. Analysis of T cell development in Xenopus. Fed Proc. 1991;5:7651. [Google Scholar]
- Ichikawa HT, Sowden MP, Torelli AT, Bachl J, Huang P, Dance GS, Marr SH, Robert J, Wedekind JE, Smith HC, Bottaro A. Structural phylogenetic analysis of activation-induced deaminase function. J Immunol. 2006;177:355–361. doi: 10.4049/jimmunol.177.1.355. [DOI] [PubMed] [Google Scholar]
- Inoue J, Yagi S, Ishikawa K, Azuma S, Ikawa S, Semba K. Identification and characterization of Xenopus laevis homologs of mammalian TRAF6 and its binding protein TIFA. Gene. 2005;358:53–59. doi: 10.1016/j.gene.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Ishii A, Kawasaki M, Matsumoto M, Tochinai S, Seya T. Phylogenetic and expression analysis of amphibian Xenopus toll-like receptors. Immunogenetics. 2007;59:281–293. doi: 10.1007/s00251-007-0193-y. [DOI] [PubMed] [Google Scholar]
- Izutsu Y, Maéno M. Analyses of immune responses to ontogeny-specific antigens using an inbred strain of Xenopus laevis (J strain) Methods Mol Med. 2005;105:149–158. doi: 10.1385/1-59259-826-9:149. [DOI] [PubMed] [Google Scholar]
- Izutsu Y, Tochinai S, Iwabuchi K, Onoè K. Larval antigen molecules recognized by adult immune cells of inbred Xenopus laevis: two pathways for recognition by adult splenic T cells. Dev Biol. 2000;221:365–374. doi: 10.1006/dbio.2000.9681. [DOI] [PubMed] [Google Scholar]
- Jackson JA, Tinsley RC. Effects of environmental temperature on the susceptibility of Xenopus laevis and X. wittei (Anura) to Protopolystoma xenopodis (Monogenea) Parasitol Res. 2002;88:632–638. doi: 10.1007/s00436-002-0629-0. [DOI] [PubMed] [Google Scholar]
- Janeway CA., Jr The immune system evolved to discriminate infectious non-self from noninfectious self. Immunol Today. 1992;13:11–16. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- Jürgens JB, Gartland LA, Kishimoto T, Du Pasquier L, Horton JD, Cooper MD. Identification of a candidate CD5 homologue in the amphibian Xenopus laevis. J Immunol. 1995;155:4218–4223. [PubMed] [Google Scholar]
- Juul-Madsen HR, Dalgaard TS, Guldbrandtsen B, Salomonsen J. A polymorphic major histocompatibility complex class II-like locus maps outside of both the chicken B-system and Rfp-Y-system. Eur J Immunogenet. 2000;27:63–71. doi: 10.1046/j.1365-2370.2000.00200.x. [DOI] [PubMed] [Google Scholar]
- Kakinuma Y, Endo Y, Takahashi M, Nakata M, Matsushita M, Takenoshita S, Fujita T. Molecular cloning and characterization of novel ficolins from Xenopus laevis. Immunogenetics. 2003;55:29–37. doi: 10.1007/s00251-003-0552-2. [DOI] [PubMed] [Google Scholar]
- Kasahara M. What do the paralogous regions in the genome tell us about the origin of the adaptive immune system? Immunol Rev. 1998;166:159–175. doi: 10.1111/j.1600-065x.1998.tb01261.x. [DOI] [PubMed] [Google Scholar]
- Kasahara M, Hayashi M, Tanaka K, Inoko H, Sugaya K, Ikemura T, Ishibashi T. Chromosomal localization of the proteasome subunit Z subunit reveals an ancient chromosomal duplication involving the major histocompatibility complex. Proc Natl Acad Sci U S A. 1996;93:9096–9101. doi: 10.1073/pnas.93.17.9096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Salter-Cid L, Flajnik MF, Kasahara M, Namikawa C, Sasaki M, Nonaka M. Isolation of the Xenopus complement factor B complementary DNA and linkage of the gene to the frog MHC. J Immunol. 1994;15310:4546–4554. [PubMed] [Google Scholar]
- Kau CL, Turpen JB. Dual contribution of embryonic ventral blood island and dorsal lateral plate mesoderm during ontogeny of hematopoietic cells in Xenopus laevis. J Immunol. 1983;131:2262–2269. [PubMed] [Google Scholar]
- Kaufman J, Milne S, Göbel TW, Walker BA, Jacob JP, Auffray C, Zoorob R, Beck S. The chicken B locus is a minimal essential major histocompatibility complex. Nature. 1999;401:923–925. doi: 10.1038/44856. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Tomonaga S, Kajii T. A second class of immunoglobulin other than IgM present in the serum of a cartilaginous fish, the skate, Raja kenojei: isolation and characterization. Mol Immunol. 1984;21:397–404. doi: 10.1016/0161-5890(84)90037-3. [DOI] [PubMed] [Google Scholar]
- Kobel HR, Du Pasquier L. Production of large clones of histocompatible, fully identical clawed toads (Xenopus) Immunogenetics. 1975;2:7–91. [Google Scholar]
- Kobel HR, Du Pasquier L. Genetics of polyploid Xenopus. Trends Genet. 1986;12:310–314. [Google Scholar]
- Kobel HR, Loumont C, Tinsley RC. Xenopus species and ecology. 2. The extant species. In: Tinsley RC, Kobel HR, editors. The biology of Xenopus. Oxford, England: Clarendon; 1996. pp. 9–31. [Google Scholar]
- Kong F, Chen CH, Cooper MD. Thymic function can be accurately monitored by the level of recent T cell emigrants in the circulation. Immunity. 1998;8:97–104. doi: 10.1016/s1074-7613(00)80462-8. [DOI] [PubMed] [Google Scholar]
- Krangel MS, Carabana J, Abbarategui I, Schlimgen R, Hawwari A. Enforcing order within a complex locus: current perspectives on the control of V(D)J recombination at the murine T-cell receptor a/d locus. Immunol Rev. 2004;200:224–232. doi: 10.1111/j.0105-2896.2004.00155.x. [DOI] [PubMed] [Google Scholar]
- Kroll KL, Amaya E. Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development. 1996;122:3173–3183. doi: 10.1242/dev.122.10.3173. [DOI] [PubMed] [Google Scholar]
- Lee A, Hsu E. Isolation and characterization of the Xenopus terminal deoxynucleotidyl transferase. J Immunol. 1994;152:4500–4507. [PubMed] [Google Scholar]
- Levine AJ, Munoz-Sanjuan I, Bell E, North AJ, Brivanlou AH. Fluorescent labeling of endothelial cells allows in vivo, continuous characterization of the vascular development of Xenopus laevis. Dev Biol. 2003;254:50–67. doi: 10.1016/s0012-1606(02)00029-5. [DOI] [PubMed] [Google Scholar]
- Li J, Barreda DR, Zhang YA, Boshra H, Gelman AE, Lapatra S, Tort L, Sunyer JO. B lymphocytes from early vertebrates have potent phagocytic and microbicidal abilities. Nat Immunol. 2006;7:1116–1124. doi: 10.1038/ni1389. [DOI] [PubMed] [Google Scholar]
- Lips KR, Diffendorfer J, Mendelson JR, Sears MW. Riding the wave: reconciling the roles of disease and climate change in amphibian declines. Plos Biol. 2008;6:E72. doi: 10.1371/journal.pbio.0060072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Kasahara M, Rumfelt LL, Flajnik MF. Xenopus class II A genes: studies of genetics, polymorphism, and expression. Dev Comp Immunol. 2002;26:735–750. doi: 10.1016/s0145-305x(02)00034-4. [DOI] [PubMed] [Google Scholar]
- Maeno M, Tochinai S, Katagiri C. Differential participation of ventral and dorsolateral mesoderms in the hemopoiesis of Xenopus, as revealed in diploid-triploid or interspecific chimeras. Dev Biol. 1985;110:503–508. doi: 10.1016/0012-1606(85)90108-3. [DOI] [PubMed] [Google Scholar]
- Maniero GD, Robert J. Phylogenetic conservation of gp96-mediated antigen-specific cellular immunity: new evidence from adoptive cell transfer in Xenopus. Transplantation. 2004;78:1415–1421. doi: 10.1097/01.tp.0000140846.73210.91. [DOI] [PubMed] [Google Scholar]
- Maniero GD, Morales H, Gantress J, Robert J. Generation of a long-lasting, protective, and neutralizing antibody response to the ranavirus FV3 by the frog Xenopus. Dev Comp Immunol. 2006;30:649–657. doi: 10.1016/j.dci.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Marr S, Goyos A, Gantress J, Maniero GD, Robert J. CD91 up-regulates upon immune stimulation in Xenopus adult but not larval peritoneal leukocytes. Immunogenetics. 2005;56:735–742. doi: 10.1007/s00251-004-0736-4. [DOI] [PubMed] [Google Scholar]
- Marr S, Morales H, Bottaro A, Cooper M, Flajnik M, Robert J. Evolutionary analysis of activation-induced cytosine deaminase (AID) in Xenopus ontogeny and during an Immune Response. J Immunol. 2007;179:6783–6789. doi: 10.4049/jimmunol.179.10.6783. [DOI] [PubMed] [Google Scholar]
- Mathis D, Benoist C. A decade of AIRE. Nat Rev Immunol. 2007;7:645–650. doi: 10.1038/nri2136. [DOI] [PubMed] [Google Scholar]
- McLysaght A, Hokamp K, Wolfe KH. Extensive genomic duplication during early chordate evolution. Nat Genet. 2002;31:200–203. doi: 10.1038/ng884. [DOI] [PubMed] [Google Scholar]
- Meier EL. T cell development and the diversification and selection of the Xenopus T cell recpetor beta chain repertoire. Thesis University of Alberta CA; 2003. p. 336. [Google Scholar]
- Mescher AL, Wolf WL, Moseman EA, Hartman B, Harrison C, Nguyen E, Neff AW. Cells of cutaneous immunity in Xenopus: studies during larval development and limb regeneration. Dev Comp Immunol. 2007;31:383–393. doi: 10.1016/j.dci.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Miyanaga Y, Shiurba R, Nagata S, Pfeiffer CJ, Asashima M. Induction of blood cells in Xenopus embryo explants. Dev Genes Evol. 1998;207 doi: 10.1007/s004270050132. 417-226. [DOI] [PubMed] [Google Scholar]
- Mo R, Kato Y, Nonaka M, Nakayama K, Takahashi M. Fourth component of Xenopus laevis complement: cDNA cloning and linkage analysis of the frog MHC. Immunogenetics. 1996;43:360–369. doi: 10.1007/BF02199804. [DOI] [PubMed] [Google Scholar]
- Morales HD, Robert J. In vivo characterization of primary and secondary anti- ranavirus CD8 T cell responses in Xenopus laevis. J Virol. 2007;81:2240–2248. doi: 10.1128/JVI.01104-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales H, Robert J. In vivo and in vitro techniques for comparative study of antiviral T-cell responses in the amphibian Xenopus. Biol Proced Online. 2008a;10:1–8. doi: 10.1251/bpo137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales HD, Robert J. Nature conservation: global, environmental and economic issues. Hauppauge, NY: Nova Publishers; 2008b. Relationships between the state of immune defenses and the increased prevalence of viral and fungal infectious diseases in wild and cultured amphibian populations. [Google Scholar]
- Moretta A, Romagnani C, Pietra G, Moretta A, Mingari M. NK-CTLs, anovel HLA-E-restricted T-cell subset. Trends Immunol. 2003;24:136–143. doi: 10.1016/s1471-4906(03)00031-0. [DOI] [PubMed] [Google Scholar]
- Mussmann R, Du Pasquier L, Hsu E. Is Xenopus IgX an analog of IgA? Eur J Immunol. 1996;26:2823–2830. doi: 10.1002/eji.1830261205. [DOI] [PubMed] [Google Scholar]
- Mussmann R, Courtet M, Du Pasquier L. Development of the early B cell population in Xenopus. Eur J Immunol. 1998;28:2947–2959. doi: 10.1002/(SICI)1521-4141(199809)28:09<2947::AID-IMMU2947>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Nagata S. A cell surface marker of thymus dependent lymphocytes in Xenopus laevis is identified by mouse monoclonal antibody. Eur J Immunol. 1985;15:837. doi: 10.1002/eji.1830150818. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Maéno M, Tochinai S, Katagiri C. Tolerance induced by grafting semi-allogeneic adult skin to larval Xenopus laevis: possible involvement of specific suppressor cell activity. Differentiation. 1987;35:108–114. doi: 10.1111/j.1432-0436.1987.tb00157.x. [DOI] [PubMed] [Google Scholar]
- Namikawa C, Salter-Cid L, Flajnik MF, Kato Y, Nonaka M, Sasaki M. Isolation of Xenopus LMP-7 homologues. Striking allelic diversity and linkage to MHC. J Immunol. 1995;155:1964–1971. [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal tables of Xenopus laevis (Daudin) Amsterdam: North-Holland; 1967. [Google Scholar]
- Nolan D, Gaudieri S, Mallal S. Host genetics and viral infections: immunology taught by viruses, virology taught by the immune system. Curr Opin Immunol. 2006;18:413–421. doi: 10.1016/j.coi.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Nonaka M, Namikawa C, Kato Y, Sasaki M, Salter-Cid L, Flajnik MF. Major histocompatibility complex gene mapping in the amphibian Xenopus implies a primordial organization. Proc Natl Acad Sci U S A. 1997;94:5789–5791. doi: 10.1073/pnas.94.11.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino H, McConnell WB, Grainger RM. Highly efficient transgenesis in Xenopus tropicalis using I-SceI meganuclease. Mech Dev. 2006;123:103–113. doi: 10.1016/j.mod.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Ohinata H, Tochinai S, Katagiri C. Ontogeny and tissue distribution of leukocyte-common antigen bearing cells during early development of Xenopus laevis. Development. 1989;107:445–452. doi: 10.1242/dev.107.3.445. [DOI] [PubMed] [Google Scholar]
- Ohno S. Evolution by gene duplication. Berlin: Springer-Verlag; 1970. [Google Scholar]
- Ohno S. Gene duplication and the uniqueness of vertebrate genomes circa 1970–1999. Semin Cell Dev Biol. 1999;10:517–522. doi: 10.1006/scdb.1999.0332. [DOI] [PubMed] [Google Scholar]
- Ohta Y, Flajnik M. IgD, like IgM, is a primordial immunoglobulin class perpetuated in most jawed vertebrates. Proc Natl Acad Sci U S A. 2006;103:10723–10728. doi: 10.1073/pnas.0601407103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta Y, Powis SJ, Coadwell WJ, Haliniewski DE, Liu Y, Li H, Flajnik MF. Identification and genetic mapping of Xenopus TAP2 genes. Immunogenetics. 1999;49:171–182. doi: 10.1007/s002510050478. [DOI] [PubMed] [Google Scholar]
- Ohta Y, Powis SJ, Lohr RL, Nonaka M, Pasquier LD, Flajnik MF. Two highly divergent ancient allelic lineages of the transporter associated with antigen processing (TAP) gene in Xenopus: further evidence for co-evolution among MHC class I region genes. Eur J Immunol. 2003;33:3017–3027. doi: 10.1002/eji.200324207. [DOI] [PubMed] [Google Scholar]
- Ohta Y, Goetz W, Hossain MZ, Nonaka M, Flajnik MF. Ancestral organization of the MHC revealed in the amphibian Xenopus. J Immunol. 2006;176:3674–3685. doi: 10.4049/jimmunol.176.6.3674. [DOI] [PubMed] [Google Scholar]
- Ota T, Rast JP, Litman GW, Amemiya CT. Lineage-restricted retention of a primitive immunoglobulin heavy chain isotype within the Dipnoi reveals an evolutionary paradox. Proc Natl Acad Sci U S A. 2003;100:2501–2506. doi: 10.1073/pnas.0538029100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan FC, Chen Y, Loeber J, Henningfeld K, Pieler T. I-SceI meganuclease-mediated transgenesis in Xenopus. Dev Dyn. 2006;235:247–252. doi: 10.1002/dvdy.20608. [DOI] [PubMed] [Google Scholar]
- Pancer Z, Amemiya CT, Ehrhardt GR, Ceitlin J, Gartland GL, Cooper MD. Somatic diversification of variable lymphocyte receptors in the agnathan sea lamprey. Nature. 2004;430:174–180. doi: 10.1038/nature02740. [DOI] [PubMed] [Google Scholar]
- Pancer Z, Saha NR, Kasamatsu J, Suzuki T, Amemiya CT, Kasahara M, Cooper MD. Variable lymphocyte receptors in hagfish. Proc Natl Acad Sci U S A. 2005;102:9224–9229. doi: 10.1073/pnas.0503792102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorten TJ, Kuhn RE. Maternal transfer of antibodies to eggs in Xenopus laevis. Dev Comp Immunol. 2009;33:171–175. doi: 10.1016/j.dci.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Postlethwait JH. The zebrafish genome in context: ohnologs gone missing. J Exp Zoolog B Mol Dev Evol. 2007;308:563–577. doi: 10.1002/jez.b.21137. [DOI] [PubMed] [Google Scholar]
- Pough FH, Janis CM, Heiser JB. Vertebrate life. Upper Saddle River, NJ: Prntice-Hall, Inc.; 2002. Origin and radiation of tetrapods and modifications for life on land; pp. 178–220. [Google Scholar]
- Qi ZT, Nie P. Comparative study and expression analysis of the interferon gamma gene locus cytokines in Xenopus tropicalis. Immunogenetics. 2008;60:699–710. doi: 10.1007/s00251-008-0326-y. [DOI] [PubMed] [Google Scholar]
- Qin T, Ren L, Hu X, Guo Y, Fei J, Zhu Q, Butler JE, Wu C, Li N, Hammarstrom L, Zhao Y. Genomic organization of the immunoglobulin light chain gene loci in Xenopus tropicalis: evolutionary implications. Dev Comp Immunol. 2008;32:156–165. doi: 10.1016/j.dci.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Rau L, Cohen N, Robert J. MHC-restricted and -unrestricted CD8 T cells: an evolutionary perspective. Transplantation. 2001;72:1830–1835. doi: 10.1097/00007890-200112150-00020. [DOI] [PubMed] [Google Scholar]
- Rau L, Gantress J, Bell A, Horton T, Stewart R, Cohen N, Horton J, Robert J. Identification and characterization of Xenopus CD8 T cells expressing an NK-associated molecule. Eur J Immunol. 2002;32:1574–1583. doi: 10.1002/1521-4141(200206)32:6<1574::AID-IMMU1574>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Roach JC, Glusman G, Rowen L, Kaur A, Purcell MK, Smith KD, Hood LE, Aderem A. The evolution of vertebrate Toll-like receptors. Proc Natl Acad Sci U S A. 2005;102:9577–9582. doi: 10.1073/pnas.0502272102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert J, Cohen N. Ontogeny of CTX expression in Xenopus. Dev Comp Immunol. 1998a;12:605–612. doi: 10.1016/s0145-305x(98)00028-7. [DOI] [PubMed] [Google Scholar]
- Robert J, Cohen N. Evolution of immune surveillance and tumor immunity: studies in Xenopus. Immunol Rev. 1998b;166:231–243. doi: 10.1111/j.1600-065x.1998.tb01266.x. [DOI] [PubMed] [Google Scholar]
- Robert J, Cohen N. Induction of thymocyte differentiation and proliferation in vitro by PMA/ionophore correlates with a down-regulation of CTX expression in Xenopus. Int Immunol. 1999;11:499–508. [Google Scholar]
- Robert J, Guiet C, Du Pasquier L. Lymphoid tumors of Xenopus laevis with different capacities for growth in larvae and adults. Dev Immunol. 1994;3:297–307. doi: 10.1155/1994/37392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert J, Guiet C, Du Pasquier L. Ontogeny of the alloimmune response against transplanted tumor in Xenopus laevis. Differentiation. 1995;59:135–146. doi: 10.1046/j.1432-0436.1995.5930135.x. [DOI] [PubMed] [Google Scholar]
- Robert J, Guiet C, Cohen N, Du Pasquier L. Effects of thymectomy and tolerance induction on tumor immunity in adult Xenopus laevis. Int J Cancer. 1997;70:330–334. doi: 10.1002/(sici)1097-0215(19970127)70:3<330::aid-ijc14>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Robert J, Ménoret A, Basu S, Cohen N, Srivastava PR. Phylogenetic conservation of the molecular and immunological properties of the chaperones gp96 and hsp70. Eur J Immunol. 2001a;31:186–195. doi: 10.1002/1521-4141(200101)31:1<186::AID-IMMU186>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Robert J, Sung M, Cohen N. Ontogenic and phylogenic conservation of the intra-thymic T-cell differentiation pathway. Dev Comp Immunol. 2001b;25:323–328. doi: 10.1016/s0145-305x(00)00066-5. [DOI] [PubMed] [Google Scholar]
- Robert J, Gantress J, Rau L, Bell A, Cohen N. Minor histocompatibility antigen-specific MHC-restricted CD8 T-cell responses elicited by heat shock proteins. J Immunol. 2002;168:1697–1703. doi: 10.4049/jimmunol.168.4.1697. [DOI] [PubMed] [Google Scholar]
- Robert J, Maniero G, Cohen N, Gantress J. Xenopus as an model system to study evolution of HSP-immune system interactions. In: Srivastava P, editor. Methods: a companion to methods in enzymology (HSP-immune system interactions) Vol. 32. Philadelphia: Academic Press; 2004. pp. 42–53. [DOI] [PubMed] [Google Scholar]
- Robert J, Morales H, Buck W, Cohen N, Marr S, Gantress J. Histopatho-genesis and immune responses to FV3 virus infection in Xenopus. Virology. 2005;332:667–675. doi: 10.1016/j.virol.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Robert J, Ramanayake T, Maniero G, Morales H, Chida S. Phylogenetic conservation of gp96 ability to interact with CD91 and facilitate antigen cross-presentation. J Immunol. 2008;180:3176–3182. doi: 10.4049/jimmunol.180.5.3176. [DOI] [PubMed] [Google Scholar]
- Robert J, Goyos A, Nedelkovska H. Xenopus, a unique comparative model to explore the role of certain heat shock proteins and nonclassical MHC class Ib gene products in immune surveillance. Immunol Res. 2009 doi: 10.1007/s12026-009-8094-9. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins-Smith LA, Blair P. Expression of class II major histocompatibility complex antigens on adult T cells in Xenopus is metamorphosis-dependent. Dev Immunol. 1990;1:97–104. doi: 10.1155/1990/25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins-Smith LA, Conlon JM. Antimicrobial peptide defenses against chytridiomycosis, an emerging infectious disease of amphibian populations. Dev Comp Immunol. 2005;29:589–598. doi: 10.1016/j.dci.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Rollins-Smith LA, Parsons SC, Cohen N. During frog ontogeny, PHA and Con A responsiveness of splenocytes precedes that of thymocytes. Immunology. 1984;52:491–500. [PMC free article] [PubMed] [Google Scholar]
- Rollins-Smith LA, Needham DA, Davis AT, Blair PJ. Late thymectomy in Xenopus tadpoles reveals a population of T cells that persists through metamorphosis. Dev Comp Immunol. 1996;20:165–174. doi: 10.1016/0145-305x(96)00018-3. [DOI] [PubMed] [Google Scholar]
- Rollins-Smith LA, Flajnik MF, Blair PJ, Davis AT, Green WF. Involvement of thyroid hormones in the expression of MHC class I antigens during ontogeny in Xenopus. Dev Immunol. 1997a;5:133–144. doi: 10.1155/1997/38464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins-Smith LA, Barker KS, Davis AT. Involvement of glucocorticoids in the reorganization of the amphibian immune system at metamorphosis. Dev Immunol. 1997b;5:145–152. doi: 10.1155/1997/84841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins-Smith LA, Davis AT, Reinert LK. Pituitary involvement in T cell renewal during development and metamorphosis of Xenopus laevis. Brain Behav Immun. 2000;14:185–197. doi: 10.1006/brbi.1999.0569. [DOI] [PubMed] [Google Scholar]
- Rothenberg EV, Taghon T. Molecular Genetics of T cell Development. Annu Rev Immunol. 2005;23:601–649. doi: 10.1146/annurev.immunol.23.021704.115737. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Salter-Cid L, Nonaka M, Flajnik MF. Expression of MHC class Ia and class Ib during ontogeny: high expression in epithelia and coregulation of class Ia and lmp7 genes. J Immunol. 1998;160:2853–2862. [PubMed] [Google Scholar]
- Saltis M, Criscitiello MF, Ohta Y, Keefe M, Trede NS, Goitsuka R, Flajnik MF. Evolutionarily conserved and divergent regions of the autoimmune regulator (Aire) gene: a comparative analysis. Immunogenetics. 2008;60:105–114. doi: 10.1007/s00251-007-0268-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammut B, Marcuz A, Pasquier LD. The fate of duplicated major histocompatibility complex class Ia genes in a dodecaploid amphibian, Xenopus ruwenzoriensis. Eur J Immunol. 2002;32:1593–1604. doi: 10.1002/1521-4141(200206)32:6<1593::AID-IMMU1593>3.0.CO;2-6. Corrected and republished in: Eur J Immunol 32:2698–2709. [DOI] [PubMed] [Google Scholar]
- Sato K, Flajnik MF, Du Pasquier L, Katagiri M, Kasahara M. Evolution of the MHC: isolation of class II beta-chain cDNA clones from the amphibian Xenopus laevis. J Immunol. 1993;150:2831–2843. [PubMed] [Google Scholar]
- Schwager J, Mikoryak CA, Steiner LA. Amino acid sequence of heavy chain from Xenopus laevis IgM deduced from cDNA sequence: implications for evolution of immunoglobulin domains. Proc Natl Acad Sci U S A. 1988;85:2245–2249. doi: 10.1073/pnas.85.7.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwager J, Bu¨rckert N, Courtet M, Du Pasquier L. The ontogeny of diversification at the immunoglobulin heavy chain locus in Xenopus. EMBO J. 1991a;10:2461–2470. doi: 10.1002/j.1460-2075.1991.tb07785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwager J, Bürckert N, Schwager M, Wilson M. Evolution of immunoglobulin light chain genes: analysis of Xenopus IgL isotypes and their contribution to antibody diversity. EMBO J. 1991b;10:505–511. doi: 10.1002/j.1460-2075.1991.tb07976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sémon M, Wolfe KH. Preferential subfunctionalization of slow-evolving genes after allopolyploidization in Xenopus laevis. Proc Natl Acad Sci U S A. 2008;105:8333–8338. doi: 10.1073/pnas.0708705105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum BP, Avila D, Du Pasquier L, Kasahara M, Flajnik MF. Isolation of a classical MHC class I cDNA from an amphibian. Evidence for only one class I locus in the Xenopus MHC. J Immunol. 1993;151:5376–5386. [PubMed] [Google Scholar]
- Simmaco M, Mignogna G, Barra D. Antimicrobial peptides from amphibian skin: what do they tell us? Biopolymers. 1998;47:435–450. doi: 10.1002/(SICI)1097-0282(1998)47:6<435::AID-BIP3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Sinzelle L, Vallin J, Coen L, Chesneau A, Pasquier DD, Pollet N, Demeneix B, Mazabraud A. Generation of transgenic Xenopus laevis using the Sleeping Beauty transposon system. Transgenic Res. 2006;15:751–760. doi: 10.1007/s11248-006-9014-6. [DOI] [PubMed] [Google Scholar]
- Smith PB, Turpen JB. Expression of a leukocyte-specific antigen during ontogeny in Xenopus laevis. Dev Immunol. 1991;1:295–307. doi: 10.1155/1991/60923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SJ, Kotecha S, Towers N, Latinkic BV, Mohun TJ. XPOX2-peroxidase expression and the XLURP-1 promoter reveal the site of embryonic myeloid cell development in Xenopus. Mech Dev. 2002;117:173–186. doi: 10.1016/s0925-4773(02)00200-9. [DOI] [PubMed] [Google Scholar]
- Stewart R, Ohta Y, Minter RR, Gibbons T, Horton TL, Ritchie P, Horton JD, Flajnik MF, Watson MD. Cloning and characterization of Xenopus beta2-mi-croglobulin. Dev Comp Immunol. 2005;29:723–732. doi: 10.1016/j.dci.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Tashiro S, Sedohara A, Asashima M, Izutsu Y, Maéno M. Characterization of myeloid cells derived from the anterior ventral mesoderm in the Xenopus laevis embryo. Dev Growth Differ. 2006;48:499–512. doi: 10.1111/j.1440-169X.2006.00885.x. [DOI] [PubMed] [Google Scholar]
- Taylor RL., Jr Major histocompatibility (B) complex control of responses against Rous sarcomas. Poult Sci. 2004;83:638–649. doi: 10.1093/ps/83.4.638. [DOI] [PubMed] [Google Scholar]
- Teng MS, Stephens R, Du Pasquier L, Freeman T, Lindquist JA, Trowsdale J. A human TAPBP (TAPASIN)-related gene, TAPBP-R. Eur J Immunol. 2002;32:1059–1068. doi: 10.1002/1521-4141(200204)32:4<1059::AID-IMMU1059>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Tochinai S. Direct observation of cell migration into Xenopus thymus rudiments through mesenchyme. Dev Comp Immunol. 1980;4:273–282. doi: 10.1016/s0145-305x(80)80031-0. [DOI] [PubMed] [Google Scholar]
- Tochinai S, Katagiri CH. Complete abrogation of immune responses to skin allografts and rabbit erythrocytes in the early thymectomized Xenopus. Dev Growth Differ. 1975;17:283–294. doi: 10.1111/j.1440-169X.1975.00383.x. [DOI] [PubMed] [Google Scholar]
- Turner RJ, Manning MJ. Thymic dependence of amphibian antibody responses. Eur J Immunol. 1974;4:343–346. doi: 10.1002/eji.1830040506. [DOI] [PubMed] [Google Scholar]
- Turpen JB, Smith PB. Precursor immigration and thymocyte succession during larval development and metamorphosis in Xenopus. J Immunol. 1989;142:41–47. [PubMed] [Google Scholar]
- Wabl MR, Du Pasquier L. Antibody patterns in genetically identical frogs. Nature. 1976;264:642–644. doi: 10.1038/264642a0. [DOI] [PubMed] [Google Scholar]
- Warr GW, Magor KE, Higgins DA. IgY: clues to the origins of modern antibodies. Immunol Today. 1995;16:392–398. doi: 10.1016/0167-5699(95)80008-5. [DOI] [PubMed] [Google Scholar]
- Watkins D, Cohen N. Mitogen activated Xenopus laevis lymphocytes produce a T cell growth factor. Immunology. 1987;62:119–125. [PMC free article] [PubMed] [Google Scholar]
- Watkins D, Parsons SV, Cohen N. A factor with interleukin-1 activity is produced by peritoneal exudate cells from the frog, Xenopus laevis. Immunology. 1987;62:669–673. [PMC free article] [PubMed] [Google Scholar]
- Watkins D, Harding F, Cohen N. In vitro proliferation and cytotoxic responses against Xenopus minor histocompatibility antigens. Transplantation. 1988;45:499–502. doi: 10.1097/00007890-198802000-00054. [DOI] [PubMed] [Google Scholar]
- Watson FL, Püttmann-Holgado R, Thomas F, Lamar DL, Hughes M, Kondo M, Rebel VI, Schmucker D. Extensive diversity of Ig-superfamily proteins in the immune system of insects. Science. 2005;309 doi: 10.1126/science.1116887. 1874-1778. [DOI] [PubMed] [Google Scholar]
- Wilson M, Hsu E, Marcuz A, Courtet M, Du Pasquier L, Steinberg C. What limits affinity maturation of antibodies in Xenopus—the rate of somatic mutation or the ability to select mutants? EMBO J. 1992;11:4337–4347. doi: 10.1002/j.1460-2075.1992.tb05533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe KH. Yesterday’s polyploids and the mystery of diploidization. Nat Rev Genet. 2001;2:333–341. doi: 10.1038/35072009. [DOI] [PubMed] [Google Scholar]
- Yoon SR, Chung JW, Choi I. Development of natural killer cells from hematopoietic stem cells. Mol Cells. 2007;24:1–8. [PubMed] [Google Scholar]
- Zarrin AA, Alt FW, Chaudhuri J, Stokes N, Kaushal D, Du Pasquier L, Tian M. An evolutionarily conserved target motif for immunoglobulin class-switch recombination. Nat Immunol. 2004;5:1275–1281. doi: 10.1038/ni1137. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Pan-Hammarstro¨m Q, Yu S, Wertz N, Zhang X, Li N, Butler JE, Hammarström L. Identification of IgF, a hinge-region-containing Ig class, and IgD in Xenopus tropicalis. Proc Natl Acad Sci U S A. 2006;103:12087–12092. doi: 10.1073/pnas.0600291103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- Zon LI, Mather C, Burgess S, Bolce ME, Harland RM, Orkin SH. Expression of GATA-binding proteins during embryonic development in Xenopus laevis. Proc Natl Acad Sci U S A. 1991;88:10642–10666. doi: 10.1073/pnas.88.23.10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Bird S, Minter R, Horton J, Cunningham C, Secombes CJ. Molecular cloning of the gene for interleukin-1beta from Xenopus laevis and analysis of expression in vivo and in vitro. Immunogenetics. 2000;51:332–338. doi: 10.1007/s002510050627. [DOI] [PubMed] [Google Scholar]