Abstract
A surplus of food supply has evoked a worldwide increase in incidence of type 2 diabetes. This trend will have a significant impact on the life span of people living in modern societies. In contrast, reduced calorie intake has significant impact on preventing type 2 diabetes and increasing longevity. Increased production of reactive oxygen species (ROS), resulting in oxidative stress, has long been proposed as a unifying mechanism linking nutrient excess and diabetes. This review describes the updated mechanism by which oxidative stress provoked by nutrient excess contributes to the development of insulin resistance and pancreatic betacell failure. However, despite the promising results in cellular and animal models, major clinical trials have failed to demonstrate beneficial effect of antioxidants on the prevention of type 2 diabetes or the degree of glycemic control in individuals with diabetes. Emerging evidence shows that ROS also function as an insulin-signaling molecule in normal physiology and casts doubt on the potential beneficial effect of antioxidants. The gap between basic research and clinical outcomes heightens the importance for elucidating the precise molecular mechanisms by which cellular redox status affects insulin signaling.
Keywords: Reactive oxygen species, type 2 diabetes, insulin resistance, pancreatic beta-cell
Epidemiology
Type 2 diabetes mellitus has reached epidemic proportions with explosive increase in incidence worldwide over the past few decades. Although type 2 diabetes is more prevalent in developed countries, the increase in incidence seems to be more pronounced in non-European populations, especially those experiencing rapid Westernization [1]. For instance, type 2 diabetes was rare in the Pacific island of Nauru 50 years ago but now it affects almost half of Nauru residents [1]. Similar trends have also been observed in Native American, African Americans, and Asian Indians [1]. The driving force behind this epidemic has been attributed to environmental and behavioral factors, especially nutrient excess [1], as the genetic background remains largely stable over such a short period of time in human evolution. Various epidemiological studies also indicate that obesity is the major risk factor of type 2 diabetes [2]. In contrast, lifestyle modification including diet restriction and physical exercise in highrisk subjects has been shown to reduce progression to diabetes by about 60 % in several large independent prospective studies [3,4]. A recent longterm study in Rhesus monkeys found that animals fed on a calorierestricted diet have fewer age-related diseases including diabetes, cancer, and cardio-vascular diseases than those fed on a regular diet [5]. At the end of the study, 42% of monkeys on a regular diet developed diabetes or pre diabetes. Strikingly, no monkeys on a calorie-restricted diet developed glucose homeostasis abnormalities [5]. Collectively, data from both epidemiological observation and interventional studies strongly indicate that nutrient excess play a central role in the development of type 2 diabetes.
Metabolic staging of type 2 diabetes
Type 2 diabetes is a progressive disorder that begins with peripheral insulin resistance and ends with failure of pancreatic beta-cells. In most cases, peripheral insulin resistance, defined as the attenuated response to insulin in fat tissue, liver, and skeletal muscle, appears long before the development of hyperglycemia [6]. Resistance to insulin in skeletal muscle results in reduced glucose uptake. Resistance to the insulin-mediated suppression of hepatic gluconeogenesis and glycogenolysis increases glucose output from the liver. Resistance to the antilipolytic action of insulin in fat tissue causes increased lipolysis and increased free fatty acid (FFA) flux into circulation. Chronically elevated FFA can induce pancreatic beta-cell death (“lipotoxicity”) [6]. To compensate for peripheral insulin resistance, pancreatic beta-cells increase in mass and secrete more insulin, leading to hyperinsulinemia. However, at some point, beta-cells can no longer compensate for peripheral insulin resistance and plasma glucose levels start to rise. Elevated glucose levels can further damage pancreatic beta-cells (“glucotoxicity”), leading to progressive loss of pancreatic islet beta cells and finally the development of frank hyperglycemia [6].
The insulin-signaling pathway
The insulin receptors carry intrinsic tyrosine kinase activities. Insulin binding to insulin receptor triggers auto-phosphorylation of the receptor. The phosphorylated receptor next phosphorylates the insulin receptor substrate proteins (IRS) on tyrosine residues [7]. The tyrosine phosphorylation activity is a distinguished feature of insulin receptor since tyrosine phosphorylation is infrequent (< 0.03% of total amino acid phosphorylation) in mammalian cells [8]. Phosphorylated IRS recruits a variety of SH2 domain-containing proteins to initiate a complex signaling cascade [7]. One of these proteins, the phosphotidylinisitol-3'-OH kinase (PI3K) mediates most of the metabolic actions of insulin through activating a number of serine/threonine kinases including the Akt /protein kinase B (PKB), atypical protein kinase C (aPKC) -ƛ/ζ, and the mammalian target of rapamycin (mTOR). Akt/PKB stimulates glucose uptake into the cell, promotes glycogen synthesis, suppresses hepatic gluconeogenesis, increase fatty acid and triglycerides synthesis and suppress lipolysis in adipose tissue [7].
Regulation of insulin-signaling pathway
Insulin signaling is regulated at several nodes. The protein tyrosine phosphatases (PTP), especially PTP-1B, dephosphorylate insulin receptor and IRS-1 at tyrosine residues [9]. The tyrosine dephosphorylation of the insulin receptor by PTP -1B is a constitutive process that exerts a negative regulatory tone to the insulin-signaling pathway. The most compelling evidence for the negative regulatory activity of PTP-1B is based on studies showing increased insulin sensitivity in the mice lacking PTP-1B [10,11]. Lipid phos-phatases, including the phosphatase and tensin homologue (PTEN) and the SH2 domain-containing inositol-5-phosphatase (SHIP2), also negatively regulate insulin signaling by tyrosine dephosphorylation of PIP3 to PIP2 [7]. The negative regulatory role of lipid phosphatases in insulin signaling is demonstrated by studies showing that mice lacking PTEN or SHIP2 display increased insulin sensitivity [12-15].
On the other hand, the serine/threonine phos-phorylation of the insulin receptor and IRS proteins provides another key negative regulatory mechanism of insulin signaling [16]. Serine/ threonine phosphorylation of the insulin receptor or IRS blocks insulin signaling by opposing tyrosine phosphorylation [16]. Insulin receptors or IRS protein harbors several potential serine/ threonine phosphorylation sites. Under physiological condition, several “intrinsic” downstream serine/threonine kinases in the insulin pathway such as Akt/PKB, mTOR, and ERK1 phosphorylate serine/threonine residues of the IRS proteins and inhibit insulin signaling as a negative feedback regulation [16].
The origins of reactive oxygen species
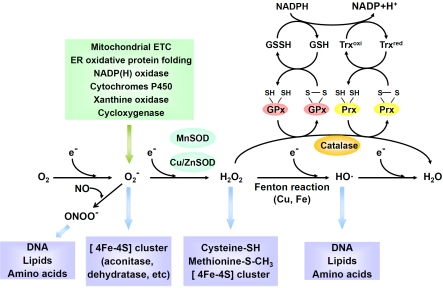
Molecular oxygen (O2) was generally absent until the appearance of photosynthetic organisms (blue-green algae) about 2.5 billion years ago [17]. Photosynthetic organisms convert carbon dioxide (CO2) and water to glucose and O2. In this process, solar energy was transduced into chemical energy of carbon bonds. Water was oxidized to O2 to generate reducing power (hydrogen atoms) required for photosynthesis and O2 was released into the atmosphere as a by-product [17]. The gradual build-up of O2 in the atmosphere drives the evolution of aerobic organisms. About 1.5 billion years ago, eukaryotes containing mitochondria appeared. In mitochondria, glucose is oxidized to CO2 and water. Reducing equivalents generated from oxidation of glucose pass electrons through the mitochondrial electron transfer chain (ETC), building up a proton gradient to drive the phosphorylation of adenosine 5’-diphosphate (ADP) to adenosine 5’-triphosphate (ATP), a process called “oxidative phosphorylation” [17]. The electrons ultimately react with O2 to form H2O, a fully reduced form of O2. In this process, O2 serves as a terminal oxidant (receiver of electrons). This process is highly energy-efficient and is superior to fermentation or respiratory pathways that rely on other terminal oxidants. However, the utilization of O2 as a terminal substrate in oxidative phsophorylation is risky. When electrons pass through mitochondrial ETC, a fraction of them (0.1-0.5%) escape the ETC and combines with O2 prematurely, resulting in the generation of partially reduced product- superoxide (O2-) [18,19]. Superoxides and other partially reduced form of O2 are reactive oxygen species (ROS) that can react with biomolecules and interfere with biological processes (Figure 1).
Figure 1.
The cellular origins of reactive oxygen species, their targets, and antioxidant systems. ETC, electron transfer chain; ER, endoplasmic reticulum; NADP(H), nicotinamide adenine dinucleotide phosphate; MnSOD, manganese superoxide dismutase; Cu/ZnSOD, copper/zinc superoxide dismutase; GPx, glutathione peroxidase; Prx, peroxiredoxin; Trx, thioredoxin.
The mitochondrial ETC is the major endogenous source of superoxide. Other sources of endogenous superoxide include protein folding and disulfide bond formation in endoplasmic reticulum (ER) [20], nicotinamide adenine dinucleotide phosphate (NADP[H]) oxidase in the phagosome membrane of phagocytes or in the plasma membrane of nonphagocytes [21] and metabolism of drugs or xenobiotics by cytochrome P450 enzymes.
Antioxidant mechanism
Superoxide is converted spontaneously or by manganese superoxide dismutase (MnSOD) in mitochondria or copperzinc superoxide dismutase (Cu/ZnSOD) in cytosol to hydrogen peroxide (H2O2) [22]. H2O2 can be removed by either catalase or peroxidase. Catalase is mainly expressed in peroxisomes and catalyzes direct decomposition of H2O2 to O2 and water. In contrast, glutathione peroxidase removes H2O2 peroxide by coupling the oxidation of glutathione (GSH), an abundant thiol-containing tripeptide. Thioredoxin peroxidase (peroxiredoxin) removes H2O2 by coupling the oxidation of thioredoxin, a widely distributed polypeptide containing 2 thiol groups. Thioredoxin peroxidase is slower at catalyzing H2O2 but is more abundant in amount than glutathione peroxidase in most tissues [22]. Thioredoxin peroxidase has been shown to be efficient in eliminating low concentration of H2O2 because of their low Km for H2O2. However, when the cell is stimulated with growth factors, thioredoxin peroxidase is inactivated, allowing transient high concentration of H2O2 to accumulate locally [23]. The predisposition of thioredoxin peroxidase to transient inactivation has been proposed as a “floodgate” that permits H2O2 to accumulate in the highly reducing intracellular environment and to act as a signaling molecule [23].
Targets of ROS
Although O2 is strongly oxidative with respect to its fully reduced form, water, it is a rare stable di radical because of the kinetic restriction imposed by its two spin-aligned unpaired electrons. O2 can only react with transition metals or organic radicals with unpaired electron and is a very week oxidant that cannot efficiently oxidize amino acid or nucleic acid [24]. In contrast, its partially reduced products including superoxide, H2O2, and hydroxyl radical (HO·) are more reactive.
Nevertheless, the anionic charge of superoxide inhibits its electrophilic activity toward electron-rich molecules. Therefore, superoxide could only oxidize few biomolecules such as enzymes containing the [4Fe-4S] clusters (aconitase or dehydratase as examples) [24]. The locally positively charged iron atom attracts superoxide electostatically and is particularly susceptible to superoxide damage. H2O2 is also a weak oxidant due to the stable oxygen-oxygen bond that limits its reactivity [24]. H2O2 can oxidize the cysteine (-SH) or methionine residues (-SCH3) of proteins. However, the reaction is very slow unless the cysteine residues are rendered more negatively charged by adjacent positively charged residues to form thiolate anion (-S-) [22]. The thiolate form of cysteine residue is the most nucleophilic amino acid that can react with H2O2. Several protein kinases, phosphatases, and transcription factors with important physiological function contain thiolate residues that can be oxidized reversibly by H2O2. In contrast, hydroxyl radical reacts readily with most biomolecules including lipids, amino acids, and nucleic acids [24]. Theoretically, the damaging effects of H2O2 are mainly due to its conversion to hydroxyl radical by Fenton reaction in the presence of free metal such as copper or iron. Superoxide can also react with another poor oxidant nitric oxide (NO·) to generate a very strong oxidant, peroxyni-trite (ONOO-), that reacts with most bio-molecules [24].
Superoxide and hydroxyl radical are short-lived (estimated intracellular half-life: 10-6 and 10-9 sec) with very low intracellular concentration (10-10 and 10-15 M) [19]. On the other hand, H2O2 is relatively stable (half-life: 10-5 sec) with higher intracellular concentration (10-5 M) [19]. H2O2 is non-polar and can diffuse freely across membrane with a very long diffusion distance (1.5 mm in the presence of 2 mM glutathione) [19]. The relative long diffusion distance of H2O2 and its ability to reversibly oxidize specific protein residues make it a suitable molecule for signal transduction [25].
Molecular mechanism linking nutrient excess to insulin resistance
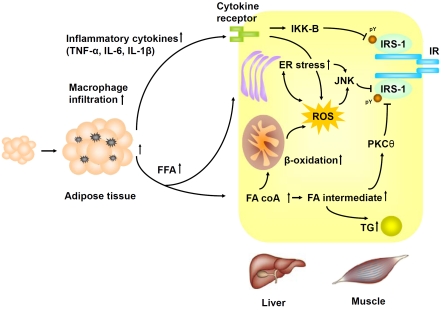
The molecular mechanism by which nutrient excess is linked to insulin resistance is still under debate. One of candidate theories, the “ectopic fat” hypothesis, has gained wide support [26,27]. The hypothesis comes from the observation that both excessive fat tissue, as seen in common obesity, or the inability of store fat, as seen in congenital or acquired lipodystrophy, are associated with insulin resistance [26,27]. Both conditions are associated with increased FFA flux into circulation with ectopic accumulation of fat in the skeletal muscle and liver. Using magnetic resonance spectroscopy, Schulman et al. demonstrated that intracellular accumulation of triacylglycerol (TG) and fatty acids intermediates (e.g., phosphatidic acid, lysophosphatidic acid, diacylglycerol and ceramide) in muscle and liver was associated with insulin resistance in human [28,29]. Fatty acids intermediates have been shown to activate the PKC-θ, a serine/threonine kinase that can phos-phorylate serine residues of IRS and thus attenuate insulin signaling [30-32]. In support of this hypothesis, fat infusion increases in intra-myocellular fatty acids intermediates and induces insulin resistance whereas pharmacological inhibition of lipolysis decreases FFA flux into bloodstream and improves insulin sensitivity in human [33]. Furthermore, pharmacological inhibition of ceramide production or genetic knock-out of PKC-θ prevented fatinduced insulin resistance in mice and human [32, 34].
The “ectopic fat” hypothesis is compatible with the observations that skeletal muscle from insulin-resistant subjects displays reduced mito-chondrial oxidative phosphorylation capacity and reduced expression of genes involved in mitochondrial biogenesis [28, 35-37]. It is proposed that either inherited or acquired (e.g. ageing) mitochondrial dysfunction is the primary event that leads to decreased fatty acid β-oxidation and fat accumulation in skeletal muscle or liver [38].
However, an alternative hypothesis linking chronic nutrient overload to insulin resistance has been recently proposed. When excessive nutrient enters the metabolic pathways, a surplus of reducing equivalents is generated with an increased rate of electron flux through the mitochondrial ETC. The electron leak from respiratory complex I and III of ETC will increase accordingly, leading to increased production of superoxide and subsequently H2O2 from the mitochondria. ROS have been shown to activate the stress-sensitive serine/thronine kinase c-jun N-terminal kinase (JNK), which in turn phos-phorylates IRS at serine residues and thus attenuate insulin signaling [39]. The precise mechanism by which ROS activates JNK remains uncertain. It has been shown that H2O2 can inhibit thioredoxin binding to the apoptosis signal-regulating kinase (ASK1), a member of mitogen-activated protein kinase kinase kinase (MAP3K), by modification of the cysteine residues in the thioredoxin binding domain of ASK1 [40,41]. The unbinding of ASK1 from thiore-doxin leads to the full activation of ASK1, which in turn activates JNK. H2O2 has also been shown to activate JNK through inhibition of MAP kinase phsophatase by cysteine residue oxidation [42].
In support of these findings, high-fat diet has been shown to increase mitochondrial ROS emission and shift the cellular redox environment to a more oxidized state in skeletal muscle of rodents or humans [43-45]. Attenuating mitochondrial ROS emission by antioxidant treatment or over-expression of catalase or MnSOD prevents high fat diet-induced insulin resistance in mice [43]. These data indicate that increased mitochondiral ROS generation by excessive metabolic flux induces insulin resistance. Furthermore, transgenic mice engineered to increase fatty acid β-oxidation develop severe insulin resistance despite being protected from obesity [46]. However, genetically engineered mice with reduced mitochondrial oxidative phos-phorylation capacity were protected from insulin resistance [47]. These observations suggest that reduced mitochondrial oxidative phosphorylation may actually prevent insulin resistance instead of promoting insulin resistance. Recent evidence also demonstrated that mitochondrial dysfunction observed in insulin resistance is not a primary event but is secondary to ROS-induced damage, which can be prevented by antioxidant treatment [44].
In addition to high fat diet-induced insulin resistance, increased mitochondnrial superoxide generation has also been shown to be the underlying mediators of multiple forms of insulin resistance models including inflammation- or glucocorticoid-induced insulin resistance [48,49]. TNF-α or dexamethasone treatment increases mitochondrial superoxide generation and induces insulin resistance whereas antioxidant treatment rescues these effects. Induction of mitochondrial superoxide by chemicals is sufficient to drive cellular insulin resistance, which can also be rescued by antioxidant treatment [48,49].
Another important mechanism linking nutrient excess to insulin resistance is endoplasmic reticulum (ER) stress [50]. The ER is responsible for protein synthesis and proper folding of the proteins. The lumen of ER has a very high concentration of protein (> 100 mg/dl). Physiological stresses that increase the demand of protein synthesis or disrupt protein folding lead to accumulation of unfolded or misfolded protein in the ER, a condition referred as “ER stress” [51]. The eukaryote cells have evolved a set of transcriptional and translational programs, the unfolded protein response (UPR), to cope with ER stress [51]. Several UPR mediators including inositol-requiring enzyme-1 (IRE-1), PKR-like endoplasmic-reticulum kinase (PERK), and activating transcription factor 6 (ATF6), are localized in the ER and are bound by the abundant ER chaperones BiP/glucose-regulated protein 78 (GRP78). In stressful conditions, ER chaperones are sequestered by binding to misfolded protein and UPR mediators are released [51]. These mediators initiate a cascade of reactions to reduce protein translation and increase misfolded protein degradation, chaperone synthesis, and cell apoptosis. IRE-1 can further activate JNK, which in turn phosphorylates IRS at serine residue, leading to attenuated insulin signaling. In support of this notion, both genetic and dietinduced obesity cause ER stress with activation of JNK in mice [50]. Treatment of chemical chaperones or over-expression of BiP/GRP78 alleviate ER stress, restore systemic insulin sensitivity, and resolve fatty liver in obese mice [53,54]. Both genetic knockout of JNK and treatment of cell-permeable JNK -inhibitory peptide improve insulin resistance and ameliorates hyperglycemia in diabetic mice [39,55].
ER stress and oxidative stress are closely interlinked processes [20]. The folding of protein into correct conformation requires the formation of intra-molecular and inter-molecular disulfide bonds, which is an energy-consuming oxidative process. The ER is a unique intracellular environment with oxidizing status. The oxidized glutathione to reduced glutathione ratio (GSSG/ GSH) is 1:1 to 1:3 in the ER, in contrast to the ratio of > 60:1 in the cytosol [56]. During the formation of disulfide bond, two cysteine residues are oxidized and two electrons are released. The released electrons are then transferred to the protein disulfide isomerase (PDI), passed to the ER oxidoreductin (ERO1), and finally react with O2. This process mimics the ETC in mitochondrial respiration. Like mitochondrial respiration, the ER electron transfer during oxidative protein folding is a source of ROS production [20]. It is estimated that up to 25 % of ROS generated in a cell results form oxidative protein folding in the ER [57]. Accumulated ROS will also deplete the reduced glutathione required for protein folding, further exacerbating ER stress. In support of these findings, antioxidant treatment was shown to alleviate ER stress and improve protein secretion both in vitro and in vivo, indicating a close interaction betweenER stress and oxidative stress [58] (Figure 2).
Figure 2.
Molecular mechanism linking nutrient excess to insulin resistance. IR, insulin receptor; IRS-1, insulin-related substrate 1; JNK, c-jun N-terminal kinase; PKC θ, protein kinase C θ; IKK, IκB kinase; FFA, free fatty acid; FA coA, fatty acid co-enzyme A; TG, triglycerides; TNF-α, tumor necrosis factor-α;IL-6, interleukin 6; IL-1β, interleukin 1β; ROS, reactive oxygen species.
Inflammation, ROS and insulin resistance
Chronic lowgrade inflammation is one of the hallmarks of obesity and type 2 diabetes [59]. Biomarkers of inflammation, such as tumor necrosis factor-α (TNF-α) and C-reactive protein (CRP), are elevated in obese subjects [59]. Un-equivocal epidemiological and experimental evidence in animal and human indicate that inflammation is crucial for the development of obesity-induced insulin resistance [59-62]. The major source of inflammation is believed to be the activated macrophages in adipose tissue. It is estimated that about 10 % of the cells in adipose tissue of lean animals or human are composed of macrophages [63,64]. This percentage increased markedly to 40 % in obese counterparts. The infiltrating macrophages in the intra-muscular fat of obese mice are approximately 3 times more than those in lean mice. Inflamed adipose tissue is capable of secreting a variety of pro-inflammatory mediators including TNF-α that induce peripheral insulin resistance.
A central question remained unanswered is how the inflammation is triggered in the adipose tissue. One proposed mechanism is based on the observation that macrophages form ringlike structures surrounding dead adipocytes. As adipose tissue expands during the development of obesity, certain regions become hypoperfused, leading to adipocyte microhypoxia and cell death. Adipocyte hypoxia and death triggers a series of proinflammatory program, which in turn recruit new macrophages [65]. Another proposed mechanism is the activation of inflammatory pathway by oxidative stress and ER stress. Highfat diet induces ER stress in adipo-cytes and activates JNK and IKK that triggers inflammatory response in adipose tissue [66]. High-fat diet has also been shown to increase ROS production in adipose tissue via NADPH oxidase activation in adipocytes [43]. Treatment with NADPH oxidase inhibitor reversed ROS production in adipose tissue and improved systemic insulin resistance [43].
The emerging role of ROS as an insulin-signaling molecule
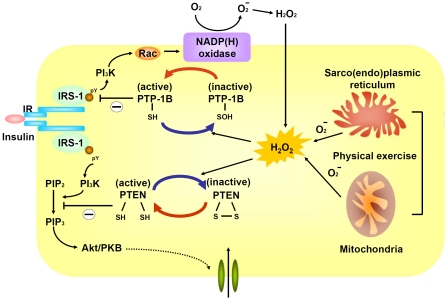
Although excessive oxidative stress is generally linked to the development of insulin resistance, it became apparent in recent years that low-level of H2O2 is actually required for normal insulin signaling [25]. The potential involvement of ROS in normal cellular signaling has been observed for more than 30 years [25]. H2O2 is rapidly and transiently generated in response to various stimuli, including insulin, growth factors, and cytokines. H2O2 itself can exert insulin-mimicking effects and elimination H2O2 attenuates normal insulin signaling. Subsequent studies revealed that insulin receptor is coupled to several types of NADP(H) oxidase at the plasma membrane through small GTP- binding protein in non-phagocytic cells including adipocytes, myocytes, hepatocytes, and vascular cells [21]. Upon insulin stimulation, NADP(H) oxidase generate superoxide, which is then converted to H2O2.
Abundant evidence suggests H2O2 is required for optimal activation of many signaling pathways, especially those mediated through protein phosphorylation [25]. H2O2 is ubiquitous, relatively long-lived, and are readily permeable to membranes. Although H2O2 is generally considered as a weak oxidizing agent that cannot directly oxidize DNA or lipid, it is capable to oxidize certain protein residues. Certain protein residues such as the thiolate (deprotonated) form of cysteine (-S -) are particularly susceptible to the electrophilic attack by H2O2. However, the cysteine residues of most cytosolic proteins are protonated (-SH) due to the low pH environment of cytosol and are resistant to oxidation by H2O2 [20-22]. Only cysteine residues that are more negatively charged by nearby positively charged amino acid can exist as thiolate anions in cytosol. Proteins with susceptible cysteine residues at catalytically active site may become inactive upon oxidation by H2O2.
In insulin signaling or other receptor tyrosine kinase (RTK) pathway, the binding of ligand per se is not sufficient to trigger phosphorylation cascade. Instead, concurrent oxidative inhibition of PTP- 1B, a negative regulator of insulin signaling, is required for phosphorylation propagation [67,68]. All PTPs harbors an essential cysteine residue in the active site motif His-Cys-X-X-Gly-X-X-Arg-Ser/Thr [69]. Given that purified PTP-1B is constitutively active, the reversible oxidation of cysteine residues of PTP-1B into inactive sulfonic acid form (Cys-SOH) offers an important regulatory mechanism of insulin signaling. Similar regulatory mechanism was also observed for PTEN, a lipid phosphatase that dephosphorylates PIP3 to terminate PI3K signaling. The catalytic active Cys-124 in PTEN is oxidized by H2O2 to form disulfide bond with Cys-71 [69].
The physiological role of ROS in insulin signaling is supported by a recent study showing that mice lacking glutathione peroxidase 1 (Gpx1) are resistant to high-fat diet-induced insulin resistance. Gpx1 is a ubiquitous enzyme responsible for elimination of endogenous H2O2, which typically is in micromolar or sub-micromolar range. Mice lacking Gpx1 did not have wide-spread elevated H2O2 and were euglycemic with a regular diet, suggesting the existence of compensatory antioxidant pathways [70]. However, they were protected from the development of insulin resistance under a high-fat diet. Upon insulin stimulation, insulin signaling is more pronounced with more oxidized PTEN in Gpx -/- myotubes as compared with wild-type [70]. In contrast, mice over-expressing Gpx1 were insulin resistant with reduced insulin signaling [71].
The seemingly conflicting role of ROS in insulin signaling may be explained by the degree and context by which ROS is generated. It has been proposed that transient and low-grade oxidative stress be beneficial, whereas sustained oxidative stress may promote insulin resistance [70]. A similar scenario has also been observed for physical exercise-induced enhancement of insulin sensitivity. Exercise-induced improvement in insulin sensitivity was more pronounced in mice lacking Gpx1, which was blocked by antioxidant treatment [70]. In human, physical activity enhances insulin sensitivity and induces expression of antioxidant enzymes in skeletal muscle. However, these beneficial effects were also blocked by antioxidants [72]. In nematode, glucose deprivation promotes ROS formation, induces catalase activity, increases resistance to oxidative stress, and extends life span [73]. Antioxidant treatment prevents the extension of life span. These data suggest that transient lowlevel oxidative stress may enhance stress resistance or enhance insulin sensitivity, a hypothetical concept commonly referred to as “hormessis” (Figure 3).
Figure 3.
Physiological role of hydrogen peroxide in insulin signaling. IR, insulin receptor; IRS-1, insulin-related substrate 1; PI3K, phosphotidylinisitol-3'-OH kinase; NADP(H), nicotinamide adenine dinucleotide phosphate; PTP-1B, protein tyrosine phosphatase 1B; PTEN, phosphatase and tensin homologue; PKB, protein kinase B.
Oxidative stress and beta-cell dysfunction
Glucotoxicity and lipotoxicity are generally considered as the major contributors of beta-cell failure in the developing stage of type 2 diabetes [7]. Abundant evidences demonstrate that chronic exposure to high circulating glucose or FFA increases ROS production and decreases insulin content and glucose-stimulated insulin secretion of beta-cells both in vivo and in vitro [74]. Antioxidant treatment or over-expression of glutathione peroxidase or catalase can reverse these effects [75,76]. Beta-cells express low levels of anti-oxidative enzyme including catalase and glutathione peroxidase, which make them particularly susceptible to oxidative stress [77]. Although the exact mechanism remains uncertain, ROS has been shown to reduce insulin gene expression and insulin secretion of beta-cells, probably through post-translational repression of musculoaponeurotic fibrosarcoma protein A (MafA) and pancreatic duodenal homeobox-1 (PDX-1), two key tran-scriptional factors that bind to insulin gene promoter [78,79]. Superoxide has also been shown to activate uncoupling protein 2 (UCP2), a mito-chondrial protein that increases proton leak, reduces ATP production and metabolic coupling, and negatively regulates glucose-stimulated insulin secretion [80-82]. UCP2 knockout mice are prevented from hyperglycemia-induced loss of glucose responsiveness [82].
However, recent studies reveal that ER stress may be the fundamental mechanism of beta-cell failure under chronic metabolic stress. Pan-creatic beta-cells are heavily loaded endocrine cells. It is estimated that one million proinsulin molecules are synthesized per minute per cell upon glucose stimulation [83]. The synthesis of one proinsulin molecule requires formation of three intra-molecular disulfide bonds, which imposes a high oxidative protein folding demand on beta-cells. Using a transgenic mice model expressing XBP-I and green fluorescent protein fusion protein for in vivo monitoring of ER stress, pancreas has been shown to be the only tissue expressing intense fluorescence in normal condition [84]. In support of this notion, mice deficient for PERK, an UPR transducer responsible for attenuating global protein translation, display reduced beta-cell mass and reduced insulin secretion, and develop neonatal diabetes [85,86]. The phenotype is reminiscent of the Wolcott-Rallison syndrome in human, an autosomal recessive disorder resulting from PERK mutation characterized by severe infantile diabetes [87]. PERK phosphorylates the eukaryotic initiation factor 2 (eIF2α) on Ser 51 and the phosphorylated eIF2α mediates PERK singling to attenuate global protein synthesis. Mice homozygous for mutant eIF2α at Ser 51 have a severe diabetic phenotype with increased oxidative stress, unregulated proinsulin translation, mitochondrial damage, and beta-cell apoptosis [88,89]. Another UPR mediator, the transcription factor C/EBP homologous protein (CHOP) has been shown to trigger beta-cell apoptosis under ER stress. Mice lacking CHOP were protected from ER stress-induced beta-cell apoptosis [90]. Deletion of CHOP preserves beta-cell mass, improves beta-cell function, and reduces oxidative stress in several mouse model of diabetes [91]. Furthermore, over-expression of the ER chaperone, BiP/GRP78, reverses hyperglycemia-induced inhibition of insulin synthesis and secretion [92]. These data indicate UPR and ER chaperones alleviate ER stress in beta-cells and are crucial for the maintenance of beta-cell function and survival under high metabolic burden.
Stress-sensing transcription factors regulate glucose metabolism
In addition to post-translational modification of insulin signaling pathway, oxidative stress also influence glucose metabolism through transcriptional regulation. The FoxO family of Forkhead transcription factor can be activated by oxidative stress through the formation of cysteinethiol disulfide-dependent complex with the p300/CBP acetyltransferase and subsequent acetylation of FoxO [93]. FoxO can also be activated by SIRT1 deacetylase or beta-catenin upon oxidative stress [94,95]. Mammalian FoxOs control various biological functions including cell cycle arrest, antioxidant response, DNA repair, and apoptosis [96]. FoxOs also regulates gluconeogenesis, adipocyte differentiation and beta-cell proliferation [97-99]. Gain-of-function mutation of FoxO1 in mice causes diabetic phenotype arising from increased hepatic glucose output and reduced beta-cell mass. Conversely, deletion of FoxO1 in diabetic mice reverses the diabetic phenotypes [99].
Another example is p53, a pivotal tumor suppressor involved in apoptosis, cell senescence, and DNA repair [100]. p53 is primarily induced by genotoxic stress but also by oxidative stress. A recent study shows that either genotoxic stress or oxidative stress in adipose tissue triggers the activation of p53, increases pro-inflammatory gene expression, and promotes senescence-like changes of adipocytes. Inhibition of p53 ameliorates the senescence-like change, decreases inflammatory cytokines and improves insulin sensitivity [101]. Conversely, over-expression of p53 induces inflammatory response and insulin resistance in adipose tissue [101]. These examples indicate oxidative stress may influence glucose metabolism through complex transcriptional regulation.
Implication for therapy
Despite the promising results of antioxidant treatment on glucose homeostasis in animals and the strong inverse associations between dietary antioxidant intake and risk of developing type 2 diabetes in observational studies, several large randomized controlled trials (RCT) failed to support a beneficial effect of anti-oxidant supplements (vitamin E, beta-carotene, and vitamin C) on the prevention of type 2 diabetes (Table 1). In the Women’s Health Study involving 38,167 healthy US women, supplement of vitamin E provided no benefit on prevention of type 2 diabetes over 10-year follow-up [102]. In the Women’s Antioxidant Cardiovascular Study involving 6,574 non-diabetic women with high cardiovascular risk, supplement of vitamin C, vitamin E, or beta-carotene also did not offer benefit on prevention of type 2 diabetes after over 9.2 years [103]. In the Physician Health Study recruiting 22,071 healthy US physicians, supplements of beta-carotene for an average of 12 years had no effect on the prevention of type 2 diabetes [104]. In the Aalpha-Tocopherol, Beta-Carotene Cancer Prevention Study, vitamin A plus beta-carotene for 12.5 years did not prevent type 2 diabetes in 29,379 male smokers [105].
Table 1.
Major randomized placebo-control trials investigating the effects of antioxidant supplement on prevention of diabetes or glucose homeostasis
| Study | Study Population | Duration (Years) | Antioxidants (daily dose) | Endpoint | Results |
|---|---|---|---|---|---|
| Women Health Study | 38,716 healthy US women | 10 | Vitamin E (α-tocopherol 600 IU) | Incident diabetes | No effect |
| Women’s Antioxidant Cardiovascular Study | 6,574 non-diabetic US women at high risk of cardiovascular disease | 9.2 | Vitamin E (α-tocopherol 300 IU), vitamin C (500 mg), and beta-carotene (25 mg) | Incident diabetes | No effect |
| Physician Health Study | 22,071 healthy US male physician | 12 | Beta-carotene (25 mg) | Incident diabetes | No effect |
| Aalpha-Tocopherol, Beta-Carotene Cancer Prevention Study | 27,379 non-diabetic male Finnish smokers | 12.5 | Vitamin E(α-tocopherol 50 mg) | and Beta-carotene (20 mg) | Incident diabetes |
| Supplementation with Antioxidant Vitamins and Minerals study | 3,146 non-diabetic French | 7.5 | Vitamin C (120mg), Vitamin E (30mg) Beta-carotene (6mg) Selenium (100μg), and Zn (20mg) | Fasting glucose | No effect |
Multiple small clinical trials investigating the effect of antioxidant vitamins, alpha-lipoic acids, and taurine on glycemic control in diabetic patients have yielded inconsistent results. Although some trials reported promising results on glycemic control [106-109], most trials reported neutral effects [110-115]. In the Supplementation with Antioxidant Vitamin and Minerals study involving 3,146 non-diabetic subjects, daily supplement with vitamin C, vitamin E, beta -carotene, selenium, and zinc for 7.5 years had no effect on fasting plasma glucose [116]. These neutral effects of antioxidants on glucose homeostasis are consistent with their neutral or even harmful effects on cardiovascular disease or cancer prevention [117-125]. Vitamin E supplement increased risk of heart failure [119] and beta-carotene appeared to increase cancer incidence and mortality in smokers [123]. In a meta-analysis pooling 68 randomized trials, vitamin A, beta-carotene, and vitamin E supplement are associated with increased mortality whereas vitamin C and selenium had no effects on mortality [125].
The overwhelming failure of antioxidants in clinical trial in contrast to their promising effects in animal or observation studies remained an open question. Understanding how specific ROS and antioxidants act in normal physiology may be critical when attempting to elucidate this paradox. Antioxidants are divided into two broad classes referred as preventive antioxidants and chain-breaking antioxidants. Preventive antioxidants such as catalase or peroxidase reduce the initiation of radical chain reaction, whereas chain-breaking antioxidants such as vitamin E interfere with radical chain propagation by trapping radicals. However, scavenging of radicals generates a secondary radical. The antioxidant activity of a chain-breaking antioxidant depends on the production of a resonance-stabilized and less reactive radical.
An antioxidant is considered protective only if the product is less reactive than the initial damaged molecule. The enzymatic conversion of two election oxidants by enzymatic antioxidants such as catalase or peroxidase fulfills this criterion. Vitamin E is converted tocopheroxyl radical with low reactivity that needs to be removed by a secondary antioxidant such as ascorbic acid (vitamin C). If the removal of tocopheroxyl radical by a secondary antioxidant is delayed, it may promote lipid peroxidation. Vitamin C is an excellent antioxidant because ascorbyl radical is usually converted disproportionately to ascorbic acid and dehydroascorbate [19]. Beta-carotene exhibits good radical trapping activity in low partial oxygen pressure. However, at higher oxygen pressure, beta-carotene shows an autolytic pro-oxidant effect, which may explain its carcinogenic potential in the lung [126]. Therefore, conventional chain-breaking antioxidants may become pro-oxidant under certain circumstances. The development of small-molecule catalase or peroxidase mimetics may provide new hope into the field of antioxidant medicine.
References
- 1.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–7. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 2.Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, Willett WC. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345:790–7. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- 3.Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M. Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–50. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 4.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;46:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–4. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saltiel AR. New perspectives into the molecular pathogenesis and treatment of type 2 diabetes. Cell. 2001;104:517–29. doi: 10.1016/s0092-8674(01)00239-2. [DOI] [PubMed] [Google Scholar]
- 7.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 8.Fantl WJ, Johnson DE, Williams LT. Signalling by receptor tyrosine kinases. Annu Rev Biochem. 1993;62:453–81. doi: 10.1146/annurev.bi.62.070193.002321. [DOI] [PubMed] [Google Scholar]
- 9.Meng TC, Buckley DA, Galic S, Tiganis T, Tonks NK. Regulation of insulin signaling through reversible oxidation of the protein-tyrosine phosphatases TC45 and PTP1B. J Biol Chem. 2004;279:37716–25. doi: 10.1074/jbc.M404606200. [DOI] [PubMed] [Google Scholar]
- 10.Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC, Ramachandran C, Gresser MJ, Tremblay ML, Kennedy BP. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283:1544–8. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- 11.Klaman LD, Boss O, Peroni OD, Kim JK, Martino JL, Zabolotny JM, Moghal N, Lubkin M, Kim YB, Sharpe AH, Stricker-Krongrad A, Shulman GI, Neel BG, Kahn BB. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol Cell Biol. 2000;20:5479–89. doi: 10.1128/mcb.20.15.5479-5489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurlawalla-Martinez C, Stiles B, Wang Y, Devaskar SU, Kahn BB, Wu H. Insulin hypersensitivity and resistance to streptozotocin-induced diabetes in mice lacking PTEN in adipose tissue. Mol Cell Biol. 2005;25:2498–510. doi: 10.1128/MCB.25.6.2498-2510.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wijesekara N, Konrad D, Eweida M, Jefferies C, Liadis N, Giacca A, Crackower M, Suzuki A, Mak TW, Kahn CR, Klip A, Woo M. Muscle-specific Pten deletion protects against insulin resistance and diabetes. Mol Cell Biol. 2005;25:1135–45. doi: 10.1128/MCB.25.3.1135-1145.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clément S, Krause U, Desmedt F, Tanti JF, Behrends J, Pesesse X, Sasaki T, Penninger J, Doherty M, Malaisse W, Dumont JE, Le Marchand-Brustel Y, Erneux C, Hue L, Schurmans S. The lipid phosphatase SHIP2 controls insulin sensitivity. Nature. 2001;409:92–7. doi: 10.1038/35051094. [DOI] [PubMed] [Google Scholar]
- 15.Sleeman MW, Wortley KE, Lai KM, Gowen LC, Kintner J, Kline WO, Garcia K, Stitt TN, Yancopoulos GD, Wiegand SJ, Glass DJ. Absence of the lipid phosphatase SHIP2 confers resistance to dietary obesity. Nat Med. 2005;11:199–205. doi: 10.1038/nm1178. [DOI] [PubMed] [Google Scholar]
- 16.Zick Y. Ser/Thr phosphorylation of IRS proteins: a molecular basis for insulin resistance. Sci STKE. 2005;268:pe4. doi: 10.1126/stke.2682005pe4. [DOI] [PubMed] [Google Scholar]
- 17.Semenza GL. Life with oxygen. Science. 2007;318:62–4. doi: 10.1126/science.1147949. [DOI] [PubMed] [Google Scholar]
- 18.Imlay JA. Cellular defenses against superoxide and hydrogen peroxide. Annu Rev Biochem. 2008;77:755–76. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol. 2008;4:278–86. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- 20.Riemer J, Bulleid N, Herrmann JM. Disulfide formation in the ER and mitochondria: two solutions to a common process. Science. 2009;324:1284–7. doi: 10.1126/science.1170653. [DOI] [PubMed] [Google Scholar]
- 21.Ushio-Fukai M. Localizing NADPH oxidase-derived ROS. Sci STKE. 2006;349:re8. doi: 10.1126/stke.3492006re8. [DOI] [PubMed] [Google Scholar]
- 22.Bindoli A, Fukuto JM, Forman HJ. Thiol chemistry in peroxidase catalysis and redox signaling. Antioxid Redox Signal. 2008;10:1549–64. doi: 10.1089/ars.2008.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woo HA, Yim SH, Shin DH, Kang D, Yu DY, Rhee SG. Inactivation of peroxiredoxin I by phosphorylation allows localized H2O2 accumulation for cell signaling. Cell. 2010;140:517–28. doi: 10.1016/j.cell.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Imlay JA. Pathways of oxidative damage. Annu Rev Microbiol. 2003;57:395–418. doi: 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- 25.D'Autréaux B, Toledano MB. ROS as signaling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813–24. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 26.Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87:507–20. doi: 10.1152/physrev.00024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garg A. Acquired and inherited lipodystrophies. N Engl J Med. 2004;350:1220–34. doi: 10.1056/NEJMra025261. [DOI] [PubMed] [Google Scholar]
- 28.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–71. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–2. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Griffin ME, Marcucci MJ, Cline GW, Bell K, Barucci N, Lee D, Goodyear LJ, Kraegen EW, White MF, Shulman GI. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 1999;48:1270–4. doi: 10.2337/diabetes.48.6.1270. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Soos TJ, Li X, Wu J, Degennaro M, Sun X, Littman DR, Birnbaum MJ, Polakiewicz RD. Protein kinase C Theta inhibits insulin signaling by phosphorylating IRS1 at Ser(1101) J Biol Chem. 2004;279:45304–7. doi: 10.1074/jbc.C400186200. [DOI] [PubMed] [Google Scholar]
- 32.Kim JK, Fillmore JJ, Sunshine MJ, Albrecht B, Higashimori T, Kim DW, Liu ZX, Soos TJ, Cline GW, O'Brien WR, Littman DR, Shulman GI. PKC-theta knockout mice are protected from fatinduced insulin resistance. J Clin Invest. 2004;114:823–7. doi: 10.1172/JCI22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002;51:2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 34.Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167–79. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–73. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 36.Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, Pypaert M, Shulman GI. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin resistant offspring of type 2 diabetic parents. J Clin Invest. 2005;115:3587–93. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A. 2003;100:8466–71. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–7. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 39.Hirosumi J, Tuncman G, Chang L, Görgün CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–6. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 40.Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–4. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 41.Imoto K, Kukidome D, N5ishikawa T, Matsuhisa T, Sonoda K, Fujisawa K, Yano M, Motoshima H, Taguchi T, Tsuruzoe K, Matsumura T, Ichijo H, Araki E. Impact of mitochondrial reactive oxygen species and apoptosis signal-regulating kinase 1 on insulin signaling. Diabetes. 2006;55:1197–204. doi: 10.2337/db05-1187. [DOI] [PubMed] [Google Scholar]
- 42.Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–61. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 43.Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, 3rd, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009 doi: 10.1172/JCI37048. Feb 2. pii: 37048. doi: 10.1172/JCI37048. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonnard C, Durand A, Peyrol S, Chanseaume E, Chauvin MA, Morio B, Vidal H, Rieusset J. Mito-chondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest. 2008;118:789–800. doi: 10.1172/JCI32601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–61. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Finck BN, Bernal-Mizrachi C, Han DH, Coleman T, Sambandam N, LaRiviere LL, Holloszy JO, Semenkovich CF, Kelly DP. A potential link between muscle peroxisome proliferator- activated receptoralpha signaling and obesity-related diabetes. Cell Metab. 2005;1:133–44. doi: 10.1016/j.cmet.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 47.Pospisilik JA, Knauf C, Joza N, Benit P, Orthofer M, Cani PD, Ebersberger I, Nakashima T, Sarao R, Neely G, Esterbauer H, Kozlov A, Kahn CR, Kroemer G, Rustin P, Burcelin R, Penninger JM. Targeted deletion of AIF decreases mitochondrial oxidative phosphorylation and protects from obesity and diabetes. Cell. 2007;131:476–91. doi: 10.1016/j.cell.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 48.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–8. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 49.Hoehn KL, Salmon AB, Hohnen-Behrens C, Turner N, Hoy AJ, Maghzal GJ, Stocker R, Van Remmen H, Kraegen EW, Cooney GJ, Richardson AR, James DE. Insulin resistance is a cellular antioxidant defense mechanism. Proc Natl Acad Sci U S A. 2009;106:17787–92. doi: 10.1073/pnas.0902380106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–61. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 51.Ron D, Walter P. Signal integration in the endo-plasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–29. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 52.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–6. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 53.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–40. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, Ferré P, Foufelle F. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009;119:1201–15. doi: 10.1172/JCI37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaneto H, Nakatani Y, Miyatsuka T, Kawamori D, Matsuoka TA, Matsuhisa M, Kajimoto Y, Ichijo H, Yamasaki Y, Hori M. Possible novel therapy for diabetes with cell-permeable JNK-inhibitory peptide. Nat Med. 2004;10:1128–32. doi: 10.1038/nm1111. [DOI] [PubMed] [Google Scholar]
- 56.Sitia R, Molteni SN. Stress, protein (mis)folding, and signaling: the redox connection. Sci STKE. 2004;239:pe27. doi: 10.1126/stke.2392004pe27. [DOI] [PubMed] [Google Scholar]
- 57.Tu BP, Weissman JS. Oxidative protein folding in eukaryotes: mechanisms and consequences. J Cell Biol. 2004;164:341–6. doi: 10.1083/jcb.200311055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Malhotra JD, Miao H, Zhang K, Wolfson A, Pennathur S, Pipe SW, Kaufman RJ. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci USA. 2008;105:18525–30. doi: 10.1073/pnas.0809677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest. 2008;118:2992–3002. doi: 10.1172/JCI34260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–8. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 61.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–90. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity-and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–7. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 63.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, 2nd, DeFuria J, Jick Z, Greenberg AS, Obin MS. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56:2910–8. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 66.Guo W, Wong S, Xie W, Lei T, Luo Z. Palmitate modulates intracellular signaling, induces endoplasmic reticulum stress, and causes apoptosis in mouse 3T3-L1 and rat primary preadi-pocytes. Am J Physiol Endocrinol Metab. 2007;293:E576–86. doi: 10.1152/ajpendo.00523.2006. [DOI] [PubMed] [Google Scholar]
- 67.Mahadev K, Zilbering A, Zhu L, Goldstein BJ. Insulin-stimulated hydrogen peroxide reversibly inhibits protein-tyrosine phosphatase 1b in vivo and enhances the early insulin action cascade. J Biol Chem. 2001;276:21938–42. doi: 10.1074/jbc.C100109200. [DOI] [PubMed] [Google Scholar]
- 68.Salmeen A, Andersen JN, Myers MP, Meng TC, Hinks JA, Tonks NK, Barford D. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature. 2003;423:769–73. doi: 10.1038/nature01680. [DOI] [PubMed] [Google Scholar]
- 69.Tonks NK. Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Biol. 2006;11:833–46. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- 70.Loh K, Deng H, Fukushima A, Cai X, Boivin B, Galic S, Bruce C, Shields BJ, Skiba B, Ooms LM, Stepto N, Wu B, Mitchell CA, Tonks NK, Watt MJ, Febbraio MA, Crack PJ, Andrikopoulos S, Tiganis T. Reactive oxygen species enhance insulin sensitivity. Cell Metab. 2009;10:260–72. doi: 10.1016/j.cmet.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McClung JP, Roneker CA, Mu W, Lisk DJ, Langlais P, Liu F, Lei XG. Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proc Natl Acad Sci USA. 2004;101:8852–7. doi: 10.1073/pnas.0308096101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ristow M, Zarse K, Oberbach A, Klöting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Blüher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106:8665–70. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–93. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 74.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29:351–66. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gurgul E, Lortz S, Tiedge M, Jörns A, Lenzen S. Mitochondrial catalase overexpression protects insulin-producing cells against toxicity of reactive oxygen species and proinflammatory cytokines. Diabetes. 2004;53:2271–80. doi: 10.2337/diabetes.53.9.2271. [DOI] [PubMed] [Google Scholar]
- 76.Tanaka Y, Tran PO, Harmon J, Robertson RP. A role for glutathione peroxidase in protecting pancreatic beta cells against oxidative stress in a model of glucose toxicity. Proc Natl Acad Sci USA. 2002;99:12363–8. doi: 10.1073/pnas.192445199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tiedge M, Lortz S, Drinkgern J, Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 1997;46:1733–42. doi: 10.2337/diab.46.11.1733. [DOI] [PubMed] [Google Scholar]
- 78.Harmon JS, Stein R, Robertson RP. Oxidative stress-mediated, post-translational loss of MafA protein as a contributing mechanism to loss of insulin gene expression in glucotoxic beta cells. J Biol Chem. 2005;280:11107–13. doi: 10.1074/jbc.M410345200. [DOI] [PubMed] [Google Scholar]
- 79.Olson LK, Sharma A, Peshavaria M, Wright CV, Towle HC, Rodertson RP, Stein R. Reduction of insulin gene transcription in HIT-T15 beta cells chronically exposed to a supraphysiologic glucose concentration is associated with loss of STF-1 transcription factor expression. Proc Natl Acad Sci USA. 1995;92:9127–31. doi: 10.1073/pnas.92.20.9127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, Harper JA, Roebuck SJ, Morrison A, Pickering S, Clapham JC, Brand MD. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002;415:96–9. doi: 10.1038/415096a. [DOI] [PubMed] [Google Scholar]
- 81.Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, Grujic D, Hagen T, Vidal-Puig AJ, Boss O, Kim YB, Zheng XX, Wheeler MB, Shulman GI, Chan CB, Lowell BB. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell. 2001;105:745–55. doi: 10.1016/s0092-8674(01)00378-6. [DOI] [PubMed] [Google Scholar]
- 82.Krauss S, Zhang CY, Scorrano L, Dalgaard LT, St-Pierre J, Grey ST, Lowell BB. Superoxide-mediated activation of uncoupling protein 2 causes pancreatic beta cell dysfunction. J Clin Invest. 2003;112:1831–42. doi: 10.1172/JCI19774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Van Lommel L, Janssens K, Quintens R, Tsukamoto K, Vander Mierde D, Lemaire K, Denef C, Jonas JC, Martens G, Pipeleers D, Schuit FC. Probe-independent and direct quantification of insulin mRNA and growth hormone mRNA in enriched cell preparations. Diabetes. 2006;12:3214–20. doi: 10.2337/db06-0774. [DOI] [PubMed] [Google Scholar]
- 84.Iwawaki T, Akai R, Kohno K, Miura M. A transgenic mouse model for monitoring endoplasmic reticulum stress. Nat Med. 2004;10:98–102. doi: 10.1038/nm970. [DOI] [PubMed] [Google Scholar]
- 85.Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in perk-/- mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7:1153–63. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 86.Zhang W, Feng D, Li Y, Iida K, McGrath B, Cavener DR. PERK EIF2AK3 control of pancreatic beta cell differentiation and proliferation is required for postnatal glucose homeostasis. Cell Metab. 2006;4:491–7. doi: 10.1016/j.cmet.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 87.Delépine M, Nicolino M, Barrett T, Golamaully M, Lathrop GM, Julier C. EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat Genet. 2000;25:406–9. doi: 10.1038/78085. [DOI] [PubMed] [Google Scholar]
- 88.Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7:1165–76. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 89.Back SH, Scheuner D, Han J, Song B, Ribick M, Wang J, Gildersleeve RD, Pennathur S, Kaufman RJ. Translation attenuation through eIF2alpha phosphorylation prevents oxidative stress and maintains the differentiated state in beta cells. Cell Metab. 2009;10:13–26. doi: 10.1016/j.cmet.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Oyadomari S, Koizumi A, Takeda K, Gotoh T, Akira S, Araki E, Mori M. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest. 2002;109:525–32. doi: 10.1172/JCI14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest. 2008;118:3378–89. doi: 10.1172/JCI34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang L, Lai E, Teodoro T, Volchuk A. GRP78, but Not Protein-disulfide Isomerase, Partially Reverses Hyperglycemia-induced Inhibition of Insulin Synthesis and Secretion in Pancreatic ﹛beta﹜-Cells. J Biol Chem. 2009;284:5289–98. doi: 10.1074/jbc.M805477200. [DOI] [PubMed] [Google Scholar]
- 93.Dansen TB, Smits LM, van Triest MH, de Keizer PL, van Leenen D, Koerkamp MG, Szypowska A, Meppelink A, Brenkman AB, Yodoi J, Holstege FC, Burgering BM. Redox-sensitive cysteines bridge p300/CBP-mediated acetylation and FoxO4 activity. Nat Chem Biol. 2009;5:664–72. doi: 10.1038/nchembio.194. [DOI] [PubMed] [Google Scholar]
- 94.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–5. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 95.Essers MA, de Vries-Smits LM, Barker N, Polderman PE, Burgering BM, Korswagen HC. Functional interaction between beta-catenin and FOXO in oxidative stress signaling. Science. 2005;308:1181–4. doi: 10.1126/science.1109083. [DOI] [PubMed] [Google Scholar]
- 96.van der Horst A, Burgering BM. Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol Cell Biol. 2007;8:440–50. doi: 10.1038/nrm2190. [DOI] [PubMed] [Google Scholar]
- 97.Kim JJ, Li P, Huntley J, Chang JP, Arden KC, Olefsky JM. FoxO1 haploinsufficiency protects against highfat diet-induced insulin resistance with enhanced peroxisome proliferator-activated receptor gamma activation in adipose tissue. Diabetes. 2009;58:1275–82. doi: 10.2337/db08-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kitamura YI, Kitamura T, Kruse JP, Raum JC, Stein R, Gu W, Accili D. FoxO1 protects against pancreatic beta cell failure through NeuroD and MafA induction. Cell Metab. 2005;2:153–63. doi: 10.1016/j.cmet.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 99.Nakae J, Biggs WH, 3rd, Kitamura T, Cavenee WK, Wright CV, Arden KC, Accili D. Regulation of insulin action and pancreatic beta-cell function by mutated alleles of the gene encoding fork-head transcription factor Foxo1. Nat Genet. 2002;32:245–53. doi: 10.1038/ng890. [DOI] [PubMed] [Google Scholar]
- 100.Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8:275–83. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- 101.Minamino T, Orimo M, Shimizu I, Kunieda T, Yokoyama M, Ito T, Nojima A, Nabetani A, Oike Y, Matsubara H, Ishikawa F, Komuro I. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat Med. 2009;15:1082–7. doi: 10.1038/nm.2014. [DOI] [PubMed] [Google Scholar]
- 102.Liu S, Lee IM, Song Y, Van Denburgh M, Cook NR, Manson JE, Buring JE. Vitamin E and risk of type 2 diabetes in the women's health study randomized controlled trial. Diabetes. 2006;55:2856–62. doi: 10.2337/db06-0456. [DOI] [PubMed] [Google Scholar]
- 103.Song Y, Cook NR, Albert CM, Van Denburgh M, Manson JE. Effects of vitamins C and E and beta-carotene on the risk of type 2 diabetes in women at high risk of cardiovascular disease: a randomized controlled trial. Am J Clin Nutr. 2009;2:429–37. doi: 10.3945/ajcn.2009.27491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu S, Ajani U, Chae C, Hennekens C, Buring JE, Manson JE. Long-term beta-carotene supplementation and risk of type 2 diabetes mellitus: a randomized controlled trial. JAMA. 1999;282:1073–5. doi: 10.1001/jama.282.11.1073. [DOI] [PubMed] [Google Scholar]
- 105.Kataja-Tuomola M, Sundell JR, Männistö S, Virtanen MJ, Kontto J, Albanes D, Virtamo J. Effect of alpha-tocopherol and beta-carotene supplementation on the incidence of type 2 diabetes. Diabetologia. 2008;51:47–53. doi: 10.1007/s00125-007-0864-0. [DOI] [PubMed] [Google Scholar]
- 106.Jain SK, McVie R, Jaramillo JJ, Palmer M, Smith T. Effect of modest vitamin E supplementation on blood glycated hemoglobin and triglyceride levels and red cell indices in type I diabetic patients. J Am Coll Nutr. 1996;15:458–61. doi: 10.1080/07315724.1996.10718624. [DOI] [PubMed] [Google Scholar]
- 107.Ceriello A, Giugliano D, Quatraro A, Donzella C, Dipalo G, Lefebvre PJ. Vitamin E reduction of protein glycosylation in diabetes. New prospect for prevention of diabetic complications? Diabetes Care. 1991;14:68–72. doi: 10.2337/diacare.14.1.68. [DOI] [PubMed] [Google Scholar]
- 108.Paolisso G, D'Amore A, Galzerano D, Balbi V, Giugliano D, Varricchio M, D'Onofrio F. Daily vitamin E supplements improve metabolic control but not insulin secretion in elderly type II diabetic patients. Diabetes Care. 1993;16:1433–7. doi: 10.2337/diacare.16.11.1433. [DOI] [PubMed] [Google Scholar]
- 109.Xiao C, Giacca A, Lewis GF. Oral taurine but not N-acetylcysteine ameliorates NEFA-induced impairment in insulin sensitivity and beta cell function in obese and overweight, non-diabetic men. Diabetologia. 2008;51:139–46. doi: 10.1007/s00125-007-0859-x. [DOI] [PubMed] [Google Scholar]
- 110.Gómez-Pérez FJ, Valles-Sanchez VE, López-Alvarenga JC, Choza-Romero R, Ibarra Pascuali JJ, González Orellana R, Pérez Ortiz OB, Rodríguez Padilla EG, Aguilar Salinas CA, Rull JA. Vitamin E modifies neither fructosamine nor HbA1c levels in poorly controlled diabetes. Rev Invest Clin. 1996;48:421–4. [PubMed] [Google Scholar]
- 111.Engelen W, Keenoy BM, Vertommen J, De Leeuw I. Effects of long-term supplementation with moderate pharmacologic doses of vitamin E are saturable and reversible in patients with type 1 diabetes. Am J Clin Nutr. 2000;72:1142–9. doi: 10.1093/ajcn/72.5.1142. [DOI] [PubMed] [Google Scholar]
- 112.Boshtam M, Rafiei M, Golshadi ID, Ani M, Shirani Z, Rostamshirazi M. Long term effects of oral vitamin E supplement in type II diabetic patients. Int J Vitam Nutr Res. 2005;75:341–6. doi: 10.1024/0300-9831.75.5.341. [DOI] [PubMed] [Google Scholar]
- 113.McSorley PT, Bell PM, Young IS, Atkinson AB, Sheridan B, Fee JP, McCance DR. Endothelial function, insulin action and cardiovascular risk factors in young healthy adult offspring of parents with Type 2 diabetes: effect of vitamin E in a randomized doubleblind, controlled clinical trial. Diabet Med. 2005;22:703–10. doi: 10.1111/j.1464-5491.2005.01506.x. [DOI] [PubMed] [Google Scholar]
- 114.Manning PJ, Sutherland WH, Walker RJ, Williams SM, De Jong SA, Ryalls AR, Berry EA. Effect of high-dose vitamin E on insulin resistance and associated parameters in overweight subjects. Diabetes Care. 2004;27:2166–71. doi: 10.2337/diacare.27.9.2166. [DOI] [PubMed] [Google Scholar]
- 115.Crinò A, Schiaffini R, Manfrini S, Mesturino C, Visalli N, Beretta Anguissola G, Suraci C, Pitocco D, Spera S, Corbi S, Matteoli MC, Patera IP, Manca Bitti ML, Bizzarri C, Pozzilli P, IMDIAB group A randomized trial of nicotinamide and vitamin E in children with recent onset type 1 diabetes (MDIAB IX) Eur J Endocrinol. 2004;150:719–24. doi: 10.1530/eje.0.1500719. [DOI] [PubMed] [Google Scholar]
- 116.Czernichow S, Couthouis A, Bertrais S, Vergnaud AC, Dauchet L, Galan P, Hercberg S. Anti-oxidant supplementation does not affect fasting plasma glucose in the Supplementation with Antioxidant Vitamins and Minerals [SU.VI.MAX) study in France: association with dietary intake and plasma concentrations. Am J Clin Nutr. 2006;84:395–9. doi: 10.1093/ajcn/84.1.394. [DOI] [PubMed] [Google Scholar]
- 117.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:23–33. [Google Scholar]
- 118.de Gaetano G. Collaborative Group of the Primary Prevention Project. Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Lancet. 2001;357:89–95. doi: 10.1016/s0140-6736(00)03539-x. [DOI] [PubMed] [Google Scholar]
- 119.Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, Ross C, Arnold A, Sleight P, Probstfield J, Dagenais GR. HOPE and HOPE-TOO Trial Investigators. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 2005;293:1338–47. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 120.Lonn E, Yusuf S, Hoogwerf B, Pogue J, Yi Q, Zinman B, Bosch J, Dagenais G, Mann JF, Gerstein HC, HOPE Study. MICRO-HOPE Study Effects of vitamin E on cardiovascular and microvascular outcomes in high-risk patients with diabetes: results of the HOPE study and MICRO-HOPE substudy. Diabetes Care. 2002;25:1919–27. doi: 10.2337/diacare.25.11.1919. [DOI] [PubMed] [Google Scholar]
- 121.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Out-comes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:154–60. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 122.Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Glynn RJ, Gaziano JM. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2008;300:2123–33. doi: 10.1001/jama.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group, editor. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330:1029–35. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 124.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Valanis B, Williams JH, Barnhart S, Hammar S. N Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. Engl J Med. 1996;334:1150–5. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 125.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–57. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 126.Burton GW, Ingold KU. Beta-Carotene: an unusual type of lipid antioxidant. Science. 1984;224:569–73. doi: 10.1126/science.6710156. [DOI] [PubMed] [Google Scholar]