Abstract
Walking fruit flies, Drosophila melanogaster, use visual information to orient towards salient objects in their environment, presumably as a search strategy for finding food, shelter or other resources. Less is known, however, about the role of vision or other sensory modalities such as mechanoreception in the evaluation of objects once they have been reached. To study the role of vision and mechanoreception in exploration behavior, we developed a large arena in which we could track individual fruit flies as they walked through either simple or more topologically complex landscapes. When exploring a simple, flat environment lacking three-dimensional objects, flies used visual cues from the distant background to stabilize their walking trajectories. When exploring an arena containing an array of cones, differing in geometry, flies actively oriented towards, climbed onto, and explored the objects, spending most of their time on the tallest, steepest object. A fly's behavioral response to the geometry of an object depended upon the intrinsic properties of each object and not a relative assessment to other nearby objects. Furthermore, the preference was not due to a greater attraction towards tall, steep objects, but rather a change in locomotor behavior once a fly reached and explored the surface. Specifically, flies are much more likely to stop walking for long periods when they are perched on tall, steep objects. Both the vision system and the antennal chordotonal organs (Johnston's organs) provide sufficient information about the geometry of an object to elicit the observed change in locomotor behavior. Only when both these sensory systems were impaired did flies not show the behavioral preference for the tall, steep objects.
Keywords: Drosophila melanogaster, vision, locomotion, search, Johnston's organ, gravity
INTRODUCTION
All motile organisms explore their environment for resources using an array of specialized sensors. Indeed, the need to search effectively for food, mates and shelter in a complex world has undoubtedly been a major selective pressure in the evolution of sensory systems (Nation, 2008). One recurrent theme in the exploratory behavior of animals is the use of different sensory modalities for different stages of a search sequence (Dusenbery, 1992). For example, olfactory or visual cues may guide an animal toward a goal over a long distance whereas tactile and gustatory cues are more important once an animal is in contact with a potential resource.
In part because of its importance as a genetic model organism, much is known about how the fruit fly, Drosophila melanogaster, uses a combination of visual, olfactory and mechanosensory cues while flying in search of food. Like many insects, however, fruit flies search their environment not only while flying, but also while walking. As in flight orientation, walking flies rely on a robust and innate visual-motor behavior termed object fixation to navigate toward conspicuous features within their environment. Horn and Wehner, inspired by Reichardt's work with fixation in flying flies (Reichardt and Poggio, 1975), demonstrated that Drosophila will turn towards, and maintain a course towards, a prominent visual object (Horn, 1978; Horn and Wehher, 1975). As has been demonstrated more recently, flies will maintain a heading toward an object after it disappears (Strauss and Pichler, 1998), or even return to a course toward an object after it disappears and a distracter object is transiently presented (Neuser et al., 2008). If given a choice, flies preferentially approach the closest object, a judgment made using the motion parallax of the object's image on the retina and not expansion cues (Götz, 1994; Schuster et al., 2002). Together, this work demonstrates the saliency of visual objects in the local exploratory behavior of walking Drosophila.
One phenomenological constraint in past research on object fixation in walking flies is that they are typically tested in such a way that they can never actually reach the visual objects to which they orient. Götz and colleagues demonstrated that when choosing between a set of unattainable visual objects, flies show a preference for nearest object, but do not demonstrate any particular innate preference according to features such as size or shape (Götz, 1994). However, it is likely that once an animal reaches and walks onto an object it will use additional sensory cues to assess whether that object warrants further exploration. For example, mechanoreceptors sensitive to gravity or posture could inform an animal about the surface topology of the object it is exploring. Recent studies have shown a role for the Johnston's organs (JO), chordotonal organs located in the antennae of Drosophila, in the detection of gravity in several laboratory assays (Armstrong et al., 2006; Baker et al., 2007; Kamikouchi et al., 2009; Sun et al., 2009), but the role of gravity in ethological aspects of locomotion, such as exploratory behavior, is unknown.
In this study we examined the role of vision and graviperception in shaping the exploratory behavior of freely walking fruit flies. Rather than studying the approach of flies to virtual or unattainable objects, we allowed them to explore a large arena containing actual three-dimensional (3-D) features while we tracked their locomotor behavior using a simple machine vision system. We found that while flies are approaching objects they show little preference for different shapes of visual targets. On reaching the target, however, they demonstrated a clear preference for tall, steep objects. This preference was manifest by much longer residency times on tall, steep objects, which was because of a preponderance of long periods during which they cease walking. Animals lacking either visual information or with impaired gravitational sense still exhibited a preference for tall, steep objects, but animals with both impairments showed no preference. These results demonstrate the role of visual and mechanosensory modalities in the exploratory behavior of Drosophila.
MATERIALS AND METHODS
Flies
All experiments were performed on 3-day-old mated female fruit flies, Drosophila melanogaster Meigen, selected from a laboratory population descended from 200 wild-caught females. The flies were maintained at 25°C and ambient humidity (20–40%) on a 16 h:8 h light:dark cycle. One day before each experimental trial was performed, we anesthetized the flies on a cold plate held at 4°C. The wings of the flies were clipped between the first and second cross-veins, approximately half the length of the wing. If a fly's gravitational sense was to be impaired, it was also done at this time, by immobilizing the joint between the second and third antennal segments with a UV-cured glue (Budick et al., 2007). The flies were allowed to recover with food overnight and then deprived of food, but not water, 10–14 h before the experiments were performed. All experiments were performed during the evening peak in their circadian activity cycle (Shafer et al., 2004). The flies were placed into individual vials with a water source and allowed to acclimate to experimental light levels for at least 30 min prior to experiments. Each fly was used only once, and all trials consisted of a single fly recorded for 10 min.
Walking arena
In order to study the behavior of flies exploring a topologically complex environment we developed a large, free-walking arena. The arena consisted of a 24.5 cm diameter black disk surrounded by a 24.5 cm tall backlit cylindrical panorama of randomized black squares with a 50% filling probability which provided a background visual stimulus (Fig. 1A). As viewed from the center of the arena each square subtended 5 deg. The paper printed with the panorama was backlit by a circular array of eight 35 W halogen lights (Fig. 1B). Flies were maintained within the arena using a thermal barrier, which proved easier to regulate and much more effective than either a water moat or a wall coated with Fluon™ (A.A.R. and M.H.D., unpublished data). Most flies approached the thermal barrier and turned away; rare experiments in which flies did escape over the barrier before the end of the 10 min trial were discarded. Two versions of the arena were used in these experiments simply due to methodological improvements that were made during the course of the study. Arena 1 was equipped with a water heated thermal barrier and a passive cooling system (Fig. 1C) whereas Arena 2 was equipped with an electrically heated thermal barrier and an active cooling system (Fig. 1D). Although both systems worked, the active electrical system is easier to fabricate and permits more precise control of surface temperature. All trials were performed in Arena 2 unless noted otherwise. In all cases in which identical treatments were performed in Arenas 1 and 2, we verified the data were indistinguishable and the results were pooled in subsequent analysis.
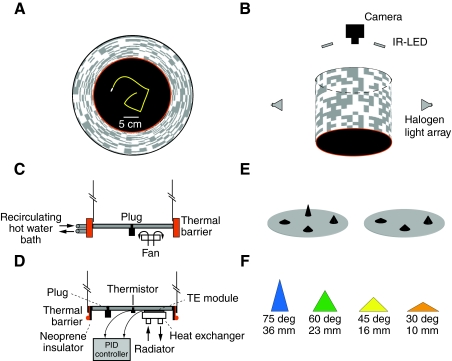
Fig. 1.
Experimental apparatus. (A) Top-down view of the arena with backlit panorama. The thermal barrier is depicted in red. (B) A schematic side view of the fly visualization setup. Near-IR LEDs (light-emitting diodes) mounted with the camera above the arena, and two of the eight halogen lights arranged in a circular array are depicted. (C) A schematic vertical cross section of Arena 1 with passive cooling. Recirculating hot water heats the thermal barrier and four CPU fans cool the walking platform (only one is depicted). (D) A schematic vertical cross section of Arena 2 with active cooling. The thermal barrier is a strip of galvanized steel wrapped in a rope heater and insulated from the walking platform by a layer of neoprene. The walking platform in actively cooled by a PID-controlled array of four thermoelectric modules with water-cooled heat sinks (only one is depicted). (E) The two arrangements of cones in the arena. The arena floor is shown in grey for illustration purposes only; the floor and cones were both painted matte black. (F) The color-code convention used for the cones of equal lateral surface area. The angle between the base and lateral surface, and the height, are noted below each cone.
In Arena 1 (Fig. 1C), the thermal barrier was 0.64 cm high around the platform. It consisted of a cylindrical aluminum walled chamber heated by 55°C recirculating water. The painted aluminum surface facing the arena was ~38°C. An array of four CPU (computer processing unit) fans blowing room air onto the bottom of the acrylic arena floor passively maintained the floor temperature. The surface temperature profile of the arena floor was 24°C at the center and rose gradually to 26°C at a distance of 2 cm from the thermal barrier, beyond which the temperature rose rapidly to 30°C as measured by a thermocouple.
In Arena 2 (Fig. 1D), the thermal barrier was flush with the top surface of the arena floor. It consisted of a 0.2 mm thick, 24 mm wide band of galvanized steel wrapped with a thin electric rope heater (OmegaLux, Stamford, CT, USA), powered by a variable AC transformer (Staco, Dayton, OH, USA) in open loop. The arena floor was insulated from the thermal barrier by a thin strip of neoprene. The arena floor was constructed of a 0.6 cm thick aluminum plate with four circular thermoelectric (TE) modules (TE Technology, Inc., Traverse City, MI, USA) bolted to the underside, each with a water-cooled temperature exchanger. A thermistor, mounted at the center of the underside of the floor, provided input to a proportional integral derivative (PID) controller driving the four TE modules in parallel with a set point of 25°C. The surface temperature of the arena floor varied by less then 1°C as measured by a non-contact infrared thermometer (OmegaScope, Stamford, CT, USA).
Flies were introduced into the arena by placing them into a black vial with a neck that fitted securely into a 3 mm hole in the arena floor. Each fly was allowed to crawl up the vial and out onto the surface of the arena, thereby avoiding the effects of mechanical agitation caused by aspirating flies with a mouth pipette. After the fly entered the arena, the hole was plugged with a stopper that was flush with the arena floor. Flies that did not enter the arena within 1 min were discarded. Of the 191 individual trials attempted for this study with this loading method, only 11 flies (6%) failed to enter the arena by crawling up and out of the black vials. Thus, there is no evidence that our data are biased by inadvertently selecting against flies with weak gravitaxis behavior. In trials using flies that had their antennae manipulated (which do exhibit reduced negative-gravitactic response) we gently tapped the animals into the arena from above. The floor of the arena was washed with detergent and rinsed between each trial.
Fly visualization and tracking
Data were collected using a digital camera mounted 48 cm above the arena floor with a 720 nm high pass optical filter (R72, Hoya Huntington Beach, CA, USA; Fig. 1B). The flies were visualized using near-IR (infrared) light, which reflects well off of the fly's cuticle, and the arena floor was painted matte black to maximize contrast. In Arena 1, we used a camera (Scorpion, Point Grey, Richmond, BC, Canada) with 1600×1200 pixel resolution. Image stacks were collected at 10 frames s−1 and analyzed in real time by a custom software program developed in MATLAB (Mathworks, Waltham, MA, USA). In Arena 2, we used a camera with 1280×1024 pixel resolution (A622F, Basler, Exton, PA, USA). Using this camera, images were collected at 20 frames s−1 and analyzed in real time using Motmot, open source camera software written in Python, using the FlyTrax plug-in (Straw and Dickinson, 2009). Both tracking programs determined the fly's two-dimensional (2-D) position and body orientation with 180 deg ambiguity based on background subtraction. The images of the flies in our movies are approximately ten pixels long and five pixels wide. For each frame, cropped images of a 100×100 pixel region around the fly (used for testing automated algorithms) were saved along with the 2-D coordinates of the fly, body axis angle and a time stamp. A single full resolution image of the arena was also saved. All data were collected in Arena 2 unless otherwise noted.
Empty arena
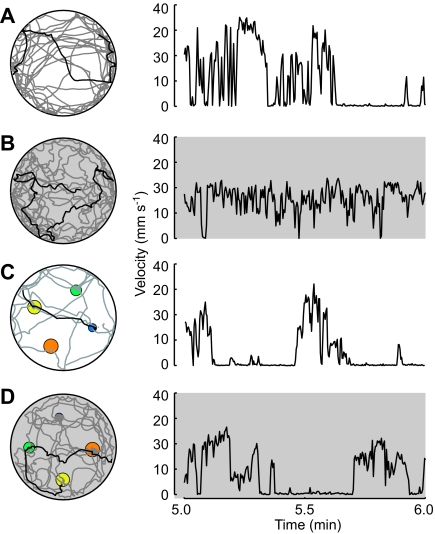
To examine the role of visual input on basic locomotor activity, 66 individual flies were tracked within an empty arena (i.e. void of the conical objects), surrounded by the random checkerboard panorama. Half the flies were tested under lit conditions (450 lux measured at the center of the arena) and half tested in complete darkness. To achieve these conditions, we replaced the translucent cylinder with an opaque black cylinder and all ambient light was eliminated from the room (measured illuminance ≤1 lux). Example trajectories and speed profiles are shown in Fig. 2A,B. We present examples that are representative of the data and have an arena crossing in the fifth minute in order to show the difference in the speed profiles of flies in light versus dark conditions.
Fig. 2.
Example trajectories and corresponding velocity plots. Each 10 min trajectory is plotted in gray with the fifth minute plotted in black. The speed profile for that same period is plotted on the right. Trials run in darkness are shown with a gray background. In trajectories with cones present, the footprint of each cone is indicated according the color scheme in Fig. 1F. Representative traces where chosen for the following cases: (A) empty arena with lights on, (B) empty arena in darkness, (C) four cones with lights on, and (D) four cones in darkness.
Arena with objects
To test the effect of a more complex topology on the flies' exploratory behavior, we placed four right angle cones of equal lateral surface area but of differing heights and slopes in the arena. The geometric dimensions of these cones and the color code that will be used throughout the paper to identify cone type are shown in Fig. 1F. Under these conditions, we performed 45 trials (20 in Arena 1 and 25 in Arena 2). Each object (painted black to match the floor and allow visualization of the flies while they were on the object) was placed in one of four fixed locations, making a square within the arena, but the relative order was randomized between trials (Fig. 1E). The objects were washed with detergent and rinsed between trials. To test whether the assessment of the objects by the flies was absolute or relative, in one set of experiments we removed the tallest, steepest object and arranged the cones in the same grid leaving one spot empty (Fig. 1E) in 24 trials. To test the role of visual input on object exploration we performed another 45 trials in complete darkness (20 in Arena 1 arena and 25 in Arena 2). Example trajectories and speed profiles are shown in Fig. 2C,D. To test the role of gravitational sensation on object exploration we performed 40 trials with flies whose antennae were immobilized at the joint between the second and third segments. Finally, to test the combined effect of the sensory manipulations, we performed 40 trials using flies with immobilized antennae in complete darkness.
Data analysis
The positional and orientation data were recorded in real time but were post-processed using custom software written in Python (www.python.org) and MATLAB (Mathworks, Waltham, MA, USA). All trials were reviewed by examining the stored video record with the tracking data superimposed. Any trials with gross tracking errors (e.g. fly position was lost) were discarded and not included in the enumeration of trial numbers used for analysis. Of 266 trials recorded for this study, only six were discarded for tracking errors.
For each trial with cones present, the locations of the cones were digitized and used to determine the periods of the trial in which a fly was exploring each cone. Because of the cone steepness and the central position of the camera, flies exploring the far side of a cone could have been incorrectly classified as ‘off cone’ with the use of a simple digitization based on the footprint of the cone. To prevent this, the digitized footprint was expanded such that a fly whose center did not appear to be within the footprint of the cone, but was indeed on the cone was correctly classified as ‘on cone’. The assignment of ‘on’ or ‘off’ cone was manually checked against the saved video for each trial.
In trials without cones present or ‘off’ cone, the 2-D position of the fly was smoothed with a Kalman smoother (Kevin Murphy's Kalman filter MATLAB toolbox) and used to calculate translational speed and total distance traveled. For trials with cones present, we calculated the 3-D position of the fly on the cones using the tracked 2-D positions, a model of the 3-D structure of the arena, and a standard pinhole camera model. The 3-D model of the arena was created from the known geometry of the arena and cones and hand digitization of the cone positions in each trial. The surface of this model was extruded by 1 mm as an approximation for flies' own height above the floor. Through each 2-D fly position on the calibrated image plane of the camera, we projected a ray (from the 3-D location of the pinhole camera model center) and intersected it with the extruded 3-D model of the arena to find the estimated 3-D position of the fly. We calculated the 3-D positions for a second time with a fly height of 2 mm and used the magnitude of the difference between the two z-position data sets as an estimate of the error in the 3-D positions. The 3-D position of the fly was smoothed with a Kalman smoother using the error estimate to assign the uncertainty in the observation data. We evaluated the quality of the 3-D position estimates on the tallest, steepest cone (and the stop–walk assignment described below) by recording simultaneously with a second camera mounted directly over this cone, and found that both the 3-D estimate and stop–walk assignment were accurately determined.
The temporal structure of the flies' locomotor activity can be coarsely modeled as discrete bouts of walking and stopping (Martin, 2004). We manually assigned walks and stops in a subset of data (both ‘on’ and ‘off’ cone) based on the small format images. Using these classifications as ground truth, we defined stops and walks based on velocity (3-D velocity when ‘on’ cone) using a dual threshold: when the velocity was above the high threshold (2.5 mm s−1) the fly was classified as walking and when the velocity was below the low threshold (1 mm s−1) the fly was classified as stopped. When the velocity was between the two thresholds it maintained its previous classification until the second threshold is crossed. This Schmitt trigger avoids rapid changes in classification caused by a single threshold based definition. We also defined the minimum walk duration to be 0.1 s (two frames at 20 frames s−1) to avoid misclassifying as walks the transient center of mass movements associated with grooming. We defined the minimum stop duration to be 0.1 s to avoid incorrectly assigning as stops the brief decrease in translational speed associated with sharp turns and pauses. Using these criteria, we determined the percentage of time each fly spent walking or stopped and the duration of each walk and stop bout, as well as the mean and maximum translational speeds during each walk bout. We set a maximum walking speed threshold of 50 mm s−1 to filter out rare events in which the wing-clipped flies jumped within the arena. ‘On’ cone locomotor activity statistics were only calculated for trials performed in Arena 2, in which we estimated 3-D velocity. Additionally, we used the estimated fly z-positions to determine the height at which each stop was performed when the flies were ‘on’ cone.
The body orientation ambiguity was resolved using a variation of the Viterbi algorithm in which orientation flips and walking rapidly backwards were penalized (Branson et al., 2009), and we then calculated mean angular speed during walking periods. Using a method for estimating position and orientation error based on trajectory segments of constant velocity (Branson et al., 2009), we found the orientation tracking error to be 1.5 degrees for the ‘off’ cone data. As can be seen in supplementary material Movies 1 and 2, the orientation tracking is highly accurate and it is unlikely an expert human could do better.
Statistics
Much of our data were not normally distributed (nor transformable to normal distribution) therefore, throughout the paper we present the distribution of results using box-and-whisker plots in which the central line (colored magenta when on a colored background) indicates the median, the box outlines the interquartile range of the data, and the whiskers encompass the range from minimum to maximum value, excluding any outliers. Outliers (indicated by a small cross) are values that are more than 1.5 times the interquartile range below or above the 25th or 75th percentiles, respectively.
We used various statistical tests in the analysis of our data; depending upon the assumptions of the tests met by the data, we always used the most powerful test possible. If the data were independent and normal, we used a heteroscedastic t-test. If the data were independent but any of the sets being compared were not normal, then we used a Mann–Whitney U-test. In some cases our data were not independent because a fly can only be in one location of the arena at a time. If the data were not independent we used a Wilcoxon signed rank test, and finally if the data had a large number of tied scores we used a Kolmogorov–Smirnov test. Neither the Wilcoxon or Kolmogorov–Smirnov tests require that the data be normal. In all cases where data were being compared multiple times we used a Bonferroni correction for multiple comparisons to adjust the P-value appropriately. All statistical analysis was performed using SPSS (SPSS Inc, Chicago, IL, USA).
To report the results of our significant tests we use a letter code where the groups labeled with the same letter are not significantly different. A group can have more than one label which indicates that it is not significantly different from any of the groups also labeled with any of those letters. For experiments with cones present, we compared the results of the experiments within a trial type, comparing effect of cone type in a given trial condition. Throughout the paper we indicate the results of within trial type hypothesis testing with black lowercase letters. For example, the results of comparing the encounter rates in Fig. 5B are indicated with lowercase letters showing that the blue, green and yellow cones are not significantly different, nor are the yellow and orange cones, e.g. the blue and green cones are significantly different than the orange cone. When multiple trial conditions were tested (such as different sensory manipulations) we also compared the results across trial type, comparing effects of trial conditions on the response to each cone type. We denote the results of across trial type hypothesis testing with uppercase letters (colored to highlight which cone type is being compared). We only compare the same cone type across different trial conditions. For example, the results of comparing the percentage of time spent on the blue cone across trials with different sensory manipulations in Fig. 7 are indicated with uppercase blue letters showing that panels A, B and D are significantly different, but panel C is not significantly different from panel A or B.
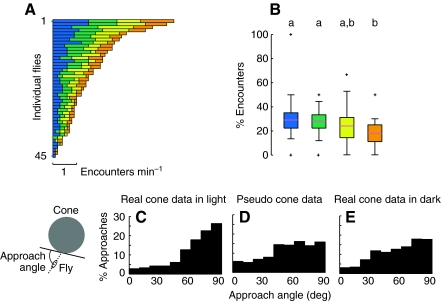
Fig. 5.
Flies encounter objects of differing geometry at similar rates. (A) Horizontal bar plots indicate the encounter rates of each cone type for each fly, ranked according to total encounter rate. (B) Box plots show the percentage of encounters for each cone type (N=45). See Fig. 1F for color code. The Wilcoxon signed rank test for non-independent, non-normal data was used to compare groups (P<0.05 with Bonferroni correction for multiple comparisons). See Fig. 4 for explanation of letter codes for homogeneous groups; crosses denote outliers. (C) The frequency distribution of approach angles to all cone types in the light. (D) The frequency distribution of approach angles to pseudo cones footprints created from the data set in which no cones were present. (E) The frequency distribution of approach angles to all cone types in the dark.
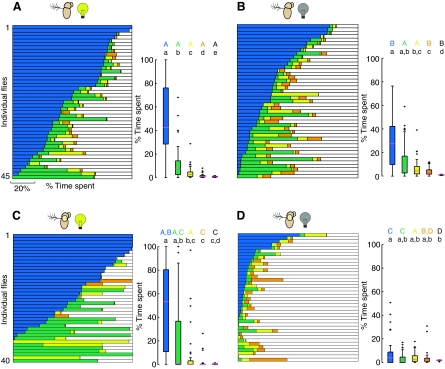
Fig. 7.
Sensory manipulations influence flies' preference for the tallest, steepest cone. Horizontal bar graphs show the percentage of the 10-min trial that each fly spent on each of the four cones and the arena floor (as in Fig. 4). Box plots show distribution of data after dividing by the surface area, which was identical for each cone. (A) Intact flies in the light (N=45). (B) Intact flies in complete darkness (N=45). (C) Flies with antennae immobilized in the light (N=40). (D) Flies with antennae immobilized in complete darkness (N=40). See Fig. 4 for description of statistical analysis and explanation of letter codes for homogenous groups. Crosses denote outliers.
RESULTS
Visual input modulates locomotor behavior
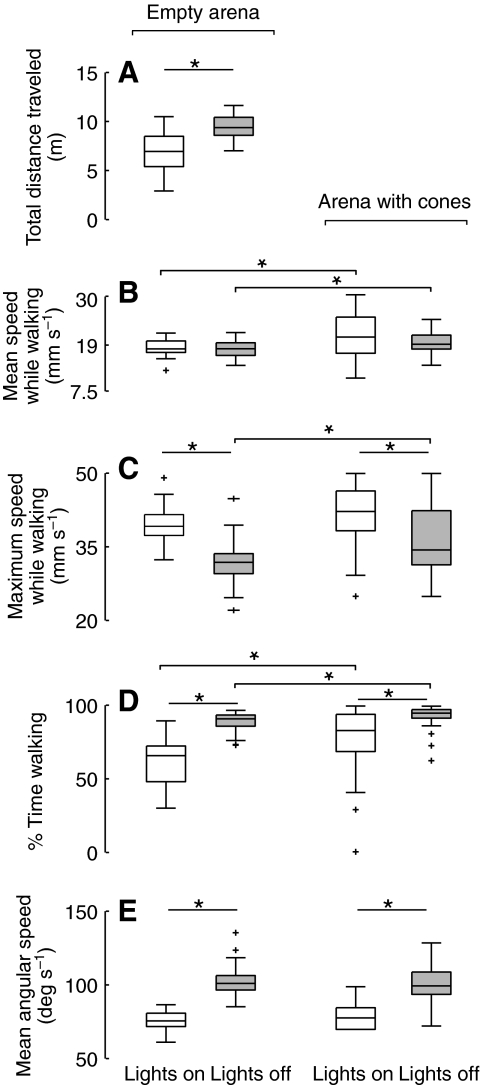
To test how visual input or the lack thereof affects locomotor behavior, we tracked individual starved flies as they explored the large free-walking arena for 10 min (N=66). Half the trials were performed in complete darkness (except for the near-IR light used by the tracking system). Sample trajectories and translation speed profiles are shown in Fig. 2. Using the tracked x–y position and body orientation of the fly, we calculated basic statistics of walking behavior (Fig. 3). Without visual cues (i.e. in the dark) flies traveled a longer total distance, not because they traveled at a higher mean speed, but because they spent more of their time walking (Fig. 3A,B,D). In lit arenas, flies reached higher maximal speeds but spent less time walking (Fig. 3C,D). The trajectories of flies in lit arenas appeared straighter than the trajectories of flies in dark arenas (Fig. 2), an observation that was confirmed by comparing the mean angular speed of the flies under the two conditions (Fig. 3E).
Fig. 3.
Visual information influences the basic statistics of walking. The two leftmost box plots in each panel show data for flies exploring an empty arena in the light (white, N=33) and in the dark (gray, N=33). The two rightmost box plots show data from flies exploring the floor of the arena with cones present in the light (white, N=45) and in darkness (gray, N=45). (A) The total distance traveled by individual flies during a 10 min trial. (B) The mean speed while the flies were walking. (C) The maximum speed calculated while the flies were walking. (D) The percentage of time the flies spent in the walking state, normalized for the total time spent on the floor of the arena when cones were present. (E) The mean angular speed calculated while the flies were walking. Statistically comparisons were made using heteroscedastic two-sample t-tests unless the data were not normally distributed in which case the Mann–Whitney U-test was used (C,D). Asterisks indicate significantly different distributions (P<0.05 with Bonferroni correction) between the indicated pairs of data; crosses denote outliers.
The differences in basic locomotor behaviors due to visual input were for the most part conserved in flies (N=90) exploring the floor of an arena with 3-D objects present (Fig. 3B–E). In the presence of cones, flies spent more of their time walking while on the arena floor (Fig. 3D), and walked at a higher mean speed (Fig. 3B) than they did when the cones were absent. Curiously, this cone-dependent change in behavior was present even in conditions of darkness when the flies could not see the cones. This result suggests that some mechanical effect of encountering a cone stimulates general locomotor activity with a time constant that lasts longer than a fly's immediate interaction with the object.
Flies spend more time on tallest, steepest cone
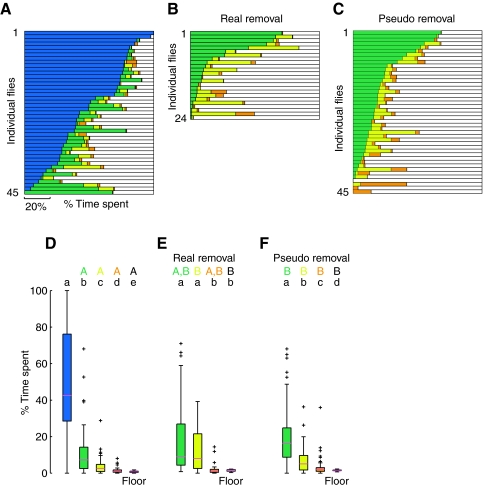
To determine how a topologically complex environment influences the exploratory behavior of flies, we tracked individual flies for 10 min in an arena with four cones of equal lateral surface area but differing height and slope (Fig. 1F). As illustrated by the trajectory in Fig. 2C and supplementary material Movie 1, the presence of the cones qualitatively altered the overall exploratory behavior in the arena. Flies appear to orient towards cones from a distance and, once encountered, climb on top of them. To test if particular cones were more attractive than others, we measured the percentage of the 10 min trial that the flies spent on each cone, as well as the arena floor (Fig. 4). For simplicity, we will often refer to the cones by the color codes indicated in Fig. 1F. Thus, the blue cone is the tallest, steepest cone; the green cone is the next tallest, steepest cone, etc. It is important to note, however, that these colors are simply a code for cone shape; the actual color of the cones was black in all experiments. From inspection of Fig. 4A, it is clear that the flies spent much more time on the tallest, steepest cone (blue). As shown in Fig. 4D, the time spent on the tallest, steepest cone was significantly larger than for all other cones and even larger than the time spent on the arena floor when it is normalized for area. This strong, differential response to the tallest, steepest cone is not consistent with what would be expected from a random walk exploration of the arena surface as the cones all had identical surface area.
Fig. 4.
Flies spend more time on tallest, steepest cone. Color-coded (see Fig. 1F) horizontal bar graphs show the percentage of the 10 min trial that each fly spent on the four cones and the floor of the arena (white). The data are ranked by the time spent on the blue (A) or green (B,C) cone. (A) Data for trials with all four cones present (N=45). (B) Data from trials in which the tallest, steepest (blue) cone was removed from the arena (N=24). (C) ‘Pseudo removal’ data created by scaling the data from (A) after excluding visits to the blue cone (see text for details; N=45). (D–F) The distributions of the data in A, B, and C respectively, are shown after normalizing for area of the surfaces being explored. The results of statistical tests are indicated with a letter code; groups labeled with the same letter are not statistically different and a group can have more than one label, indicating that group(s) with any of the same letter are not significantly different (for more details see methods). Across trial statistical tests compare a given cone type across experimental conditions and the results are denoted with uppercase letters of the color indicating the cone type being compared (color code from Fig. 1F and uppercase black letter=arena floor). Comparisons were made using a Mann–Whitney U-test with Bonferroni correction, P<0.05. Within trial statistical tests compare the different cone types in a given experimental condition and homogenous groups are denoted with lowercase black letters. Comparisons were made using Wilcoxon's signed-ranks test with Bonferroni correction, P<0.05.
Flies make absolute judgment of cone geometry
Flies might spend more time on the tallest, steepest cone because of some absolute sensory cue they perceive about this object or, alternatively, they might make a relative assessment by comparing it with other objects in the arena. To test whether the flies' preference for the tallest, steepest cone was absolute or relative, we removed the blue cone (Fig. 1E) and repeated the experiments. When the blue cone was absent, the flies did not spend significantly more time on the green cone than they did when the blue was present (Fig. 4B,E). These results suggest that the flies' response to the slope and height of each cone is absolute, and is not made by relative comparison. However, when the blue cone was absent, flies did spend slightly more time on the remaining cones, as evidenced by expanded interquartile ranges for the green and yellow cones in Fig. 4E. Such a bias is expected because, without the blue cone present, the flies had more time to encounter and explore the other three cones in the arena. To take this effect into account we created ‘pseudo removal’ data from the results of the original four-cone experiments by excluding all segments spent on the blue cone and scaling the remaining time to be 100% (Fig. 4C,F). The results of the pseudo removal experiment were not significantly different from those in the real removal experiment (Fig. 4E,F), supporting our conclusion that flies' preference is mediated by an absolute measurement of cone geometry.
Flies encounter cones with equal frequency
The flies may have spent more time on the tallest, steepest cone because they oriented towards it more frequently (i.e. it was more attractive from afar), or because, once encountered, they tended to spend more time on it before returning to the arena floor. In order to test between these two alternatives, we calculated the flies' rate of encountering each object. Fig. 5A shows the individual encounter rates for each fly to each cone, ranked by total number of encounters from highest to lowest. Although there was a large range of encounter rates across individuals, when we examine the percentage of encounters for each cone type, it is clear that the population encountered each cone type with equal probability. The one exception was the shortest, broadest cone (orange), which the flies encountered at a slightly lower rate than the blue and green cones (Fig. 5B). These results do not indicate whether the flies encountered the cones by chance, as in a random walk. However, as can be seen by comparing locomotor trajectories in the presence and absence of cones (Fig. 2), the presence of the objects in the arena strongly structured the flies' exploratory behavior. Subjectively at least, it appeared as if the flies often walked towards the cones. To quantify this effect, we examined the flies' body orientation relative to the tangent of the circumference of the cone footprint in the frame before they encountered each cone. There is a clear peak in the histogram of the absolute value of approach angle near 90 deg (Fig. 5C), suggesting that flies made a directed approach to the cones rather than randomly encountering them. We compared this to ‘pseudo cone’ data, in which we analyzed fly trajectories from trials with no objects in the arena as if there had been cones present. These control data show no distinct peak in approach angle as in the case with real cones present (Fig. 5D). Furthermore, we examined the distribution of approach angles when there were real cones present, but under dark conditions (Fig. 5E). These approach angle data resemble our ‘pseudo cone’ condition, supporting the conclusion that the flies orient toward the cones using visual cues. These analyses, together with the results showing that flies exhibit little preference in encounter rate (Fig. 5B), suggest that flies actively orient towards objects, but do not demonstrate preference based on the geometry of the objects on their retina. The slightly lower encounter rate with the orange cone suggests, however, that there may be a lower limit for detection of this cone from a distance. Given that the percentage of encounters did not vary across the three taller, steeper cones, any difference in object attractiveness during approach cannot underlie the preference for the tallest, steepest cone.
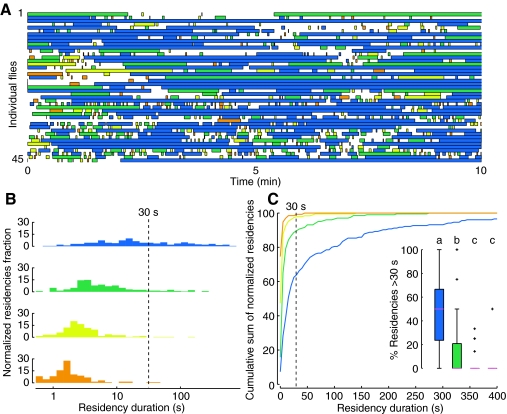
Increased residency time on tallest, steepest cone
After ruling out encounter rate as the cause of the flies' preference to spend time on the tallest, steepest cone, we next examined how long the flies remained on each object once they had reached it. Fig. 6 shows time series plots indicating each fly's location throughout the trial and the residency times on each cone type. From the data in Fig. 6, it is clear that the flies' visits to the blue cone were much longer than their visits to any of the other cones, and longer even than most periods of exploring the arena floor (Fig. 6A). We have plotted the normalized population distributions of residency durations on each cone type in two ways. First, we plotted the normalized histograms of the log of residency time for all flies on each cone type in Fig. 6B, which shows that the flies' distribution of residencies on the blue cone were shifted towards higher values. Second, we plotted the cumulative sum of the population data for each cone type in Fig. 6C, which shows a larger portion of long duration residencies on the blue cone than any other cone type. The inset in Fig. 6C shows the fraction of each individual fly's residencies that were longer than 30 s for each cone type. The results show a preponderance of long residency times on the tallest, steepest cone (blue). Although 30 s was a somewhat arbitrary choice, the relationship holds over a range of thresholds for long residency.
Fig. 6.
Flies exhibit long residency times on the tallest, steepest cone. (A) Each row represents the time series data of a single fly (N=45). The color (see Fig. 1F) indicates the identity of the cone the fly resides on and white spaces indicate periods spent on the arena floor. (B) Normalized frequency distribution of log of the residency durations by all flies on each cone type from data plotted in A. (C) Cumulative sums of the normalized frequency distribution of all residency durations by all flies. The inset shows the distribution of the percentage of individual flies' residency times longer than 30 s. Statistical comparisons were made using a Kolmogorov–Smirnov two-sample test (P<0.05, with Bonferroni correction); crosses denote outliers. See Fig. 4 for explanation of letter codes for homogenous groups.
The results of Fig. 3 suggest the flies perform an absolute rather than comparative measure of cone geometry. To further rule out a role for short-term memory in the assessment of cones, we also examined the residency durations on a given cone type parsed according to the previous cone visited. As shown in supplementary material Fig. S1, the type of cone visited immediately before had no effect on the distribution of residency times, indicating that dwell time on a particular cone does not depend on the prior history of cone visits.
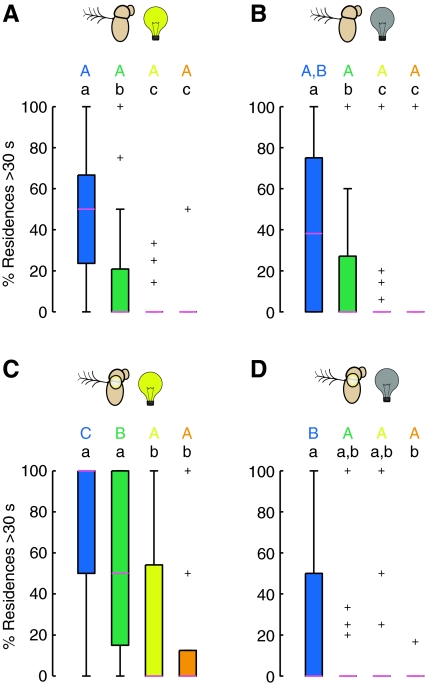
Sensory modalities involved in preference for tallest, steepest cone
Together, the results in Fig. 6 show that the flies, once they reached the tallest, steepest cone, remained on it for longer than the other cones. To investigate what sensory modalities underlie this preference, we repeated the experiments on flies with deficits in their visual and gravitational senses. We impaired vision simply by running trials in complete darkness (flies could still be seen by the near-IR-sensitive tracking camera because of 850 nm lighting), and we impaired gravitational sense by gluing the joint between the second and third antennal segments, a manipulation that disrupts the function of the Johnston's organ (Budick et al., 2007; Duistermars et al., 2009; Sun et al., 2009). It is important to note that the flies with immobilized antennae exhibit robust locomotor behavior during exploration as measured by our basic statistics (compare supplementary material Fig. S2 with Fig. 3). Fig. 7 shows the percentage of time spent on each cone type arranged according to a Punnett square of the two sensory ablations. Intact flies in the light exhibited the normal behavior, as seen in Fig. 4 (Fig. 7A). Interestingly, flies with either sensory manipulation (visual, Fig. 7B; mechanosensory, Fig. 7C) showed fairly typical responses to cone geometry, whereas flies with impairments of both visual and gravitational senses exhibited a greatly diminished preference for the blue cone (Fig. 7D and supplementary material Movie 2). These results suggest that either vision or antennal mechanosensory modalities alone provide cues sufficient to establish a fly's preference for the tallest, steepest cone. Only with both modalities compromised were the flies unable to assess the properties of the tallest, steepest cone and thus unable to exhibit a preference. Because we could not assume a priori that the same behavioral change (increased residency time) underlies the behavior of flies that had undergone sensory manipulations, we examined the cone residency durations for these flies. Fig. 8 shows that flies with either single sensory manipulation exhibited long duration residencies on the blue cone. However, the flies with gravitational sensation impairment but intact vision (Fig. 8C) showed significantly stronger responses to the two tallest, steepest cones (blue and green) compared with intact flies in the light (Fig. 8A), not distinguishing between these cones, and exhibited a larger range of responses to all the cones. This may indicate that the visual mechanism used to assess the quality of a cone is less accurate than the mechanisms using the gravitational sense.
Fig. 8.
Sensory manipulations influence residency times. The percentage of residency durations that were longer than 30 s under four experimental conditions. See Fig. 1F for color code. (A) Intact flies in the light (N=45). (B) Intact flies in complete darkness (N=45). (C) Flies with antennae immobilized in the light (N=40). (D) Flies with antennae immobilized in complete darkness (N=40). Statistically significant differences within and across trials were determined using the Kolmogorov–Smirnov two-sample test (P<0.05, with Bonferroni correction); crosses denote outliers. See Fig. 4 for explanation of letter codes for homogenous groups.
Alteration of locomotor pattern during object exploration
After having demonstrated that flies can use either visual or mechanosensory cues to assess the geometry of the objects they are exploring, we next wanted to determine how flies use this information to alter their locomotor behavior. The flies' exploratory behavior in the arena can be modeled as periods of walking and stopping. We applied our behavioral definition of walks and stops to the flies' trajectories (see Methods) and quantified the percentage of time stopped on each surface of the arena (all four cones and the floor; Fig. 9). Because we assigned all frames of each trajectory as either walks or stops, the percentage of time walking is the inverse of the stop data and is not shown. The flies with unimpaired vision and intact gravity sensation (Fig. 9A) were stopped for the majority of the time they were on the blue cone. This was also true of the flies with single sensory impairments, (visual, Fig. 9B; gravitational, Fig. 9C). Conversely, flies with both visual and antennal mechanosensory impairments (Fig. 9D) spent significantly less of their time on the blue cone in a stopped state. Intact flies in the light and the single sensory ablation flies all spent less time stopped on the yellow and orange cones than the blue cones. By contrast, the flies with both visual and gravitational sense impairments could not distinguish between any of the cone types as measured by their time stopped. Flies of all types consistently spent the majority of their time on the arena floor walking rather than stopped. The intact flies' locomotor pattern in the light shifted to a higher percentage of time stopped when residing on the cones, with the largest shift on the blue cone. Thus, this increase in percentage of time stopped is likely responsible for the large percentage of time spent on the tallest, steepest cone.
Fig. 9.
Sensory manipulations affect the percentage of time spent stopped on cones. Color-coded (see Fig. 1F) box plots indicate the percentage of time stopped on each surface of the arena, with white indicating the arena floor. (A) Intact flies in the light (N=25). (B) Intact flies in complete darkness (N=25). (C) Flies with antennae immobilized in the light (N=40). (D) Flies with antennae immobilized in complete darkness (N=40). Statistically significant differences within and across trials were determined using the Mann–Whitney U-test (P<0.05, with Bonferroni correction); crosses denote outliers. See Fig. 4 for explanation of letter codes for homogenous groups.
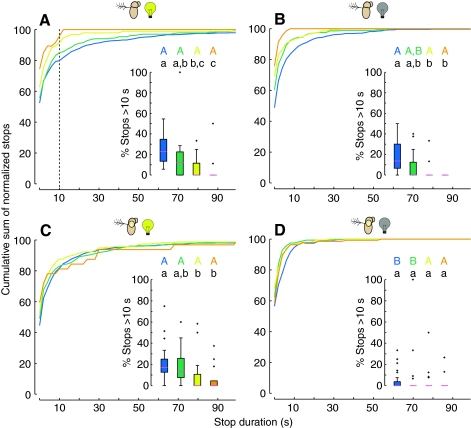
The frequency of stops did not change in a systematic way according to cone type (A.A.R. and M.H.D., unpublished data), however the duration of stops did vary according to the type of cone the fly was exploring. The cumulative sums of the percentage of stop durations for all stops by all flies are presented in Fig. 10, with the portion of each individual fly's distribution of stop durations that was longer than 10 s shown in the inset. Fig. 10A shows that intact flies in the light performed a larger percentage of long stops on the blue cone than they did on the yellow and orange cones. Flies with single sensory impairments, visual (Fig. 10B) and antennal mechanosensory (Fig. 10C), still exhibited significantly more long stops while exploring the blue cone than the yellow and orange cones. Their responses to the cones were not significantly different than those of the intact flies in the light. By contrast, flies with impairments in both their visual and gravitational senses (Fig. 10D) performed few long stops on any of the cone types, indicating their inability to sense cues that would allow them to assess a cone's geometry.
Fig. 10.
Sensory manipulations influence distributions of stop durations. Each panel shows cumulative sums of the normalized distribution of stop durations, with insets showing the percentage of individual flies' stop durations that were longer than 10 s (see Fig. 6C). See Fig. 1F for color code. (A) Intact flies in the light (N=25). (B) Intact flies in complete darkness (N=25). (C) Flies with antennae immobilized in the light (N=40). (D) Flies with antennae immobilized in complete darkness (N=40). Statistically significant differences within and across trials were determined using the Kolmogorov–Smirnov two-sample test (P<0.05, with Bonferroni correction); crosses denote outliers. See Fig. 4 for explanation of letter codes for homogenous groups.
Flies perform stops at the top of tallest, steepest cone
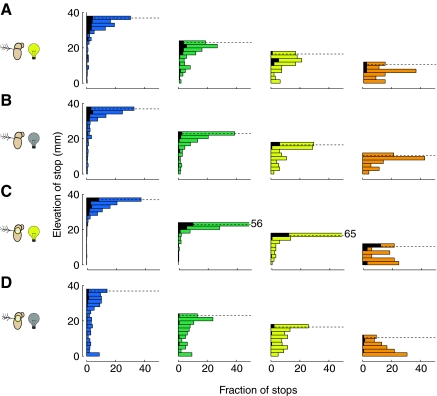
Having determined that the assessment of cone geometry plays a role in structuring locomotor behavior, we were interested in where the flies stopped on the cones. The colored histograms in Fig. 11 show the fraction of stops performed by the flies on each cone at a given elevation, and the elevations of long stops (defined in Fig. 10) are shown by the superimposed black histograms. The intact flies in the light clearly had a preference for stopping at the top of the tallest, steepest (blue) cone, and the long stops were also primarily at the top of the cone (Fig. 11A). These flies also performed the majority of their stops at the top of the green and yellow cones but not the orange cone. Flies with single sensory manipulations (Fig. 11B, visual; Fig. 11C, mechanosensory) also performed the majority of their stops at the top of the blue, green and yellow cones, but not the orange cone. This indicates that flies using either visual or antennal mechanosensory information can still localize the top of the cones. The flies lacking both visual or antennal mechanosensory information show a less pronounced preference for stopping at high elevations.
Fig. 11.
Flies tend to stop at the top of the cones. Horizontal bar graphs show the fraction of all stops (colored) and long stops (black) that were performed at a given elevation. Each column represents the stops on a given cone type, color code as in Fig. 1F. The dashed black line in each column is the height of the top of that cone; stop elevations can be taller than the height of the cone because we included the flies' body height (1 mm) in our 3-D model. Each row is a different sensory condition: (A) intact flies in the light (N=25), (B) intact flies in complete darkness (N=25), (C) flies with antennae immobilized in the light (N=40) and (D) flies with antennae immobilized in complete darkness (N=40). In C, the top bin of the green and yellow histograms is truncated at 50% for presentation purposes; the real values are 56% (green) and 65% (yellow).
DISCUSSION
We developed a large arena to study the locomotor behavior of walking Drosophila in both simple and topologically more complex environments. The role of vision in structuring locomotor behavior was apparent even when 3-D objects were absent from the arena (but a surrounding visual panorama was present). Flies exploring an empty, dark arena spent more time walking, traveled a greater distance, and followed more convoluted paths than flies exploring a lit arena (Figs 2, 3). While exploring an environment containing a set of cones, flies spent substantially more time on the tallest, steepest cone even though all cones had the same lateral surface area (Fig. 4). Cone removal experiments suggest that the flies assess object geometry via some absolute measure and not by comparison with other objects (Fig. 4). The increased time spent on the tallest, steepest cone is the result of longer residency times once the object is encountered (Fig. 6) and not a greater frequency of approaches (Fig. 5). The increased residency times are in turn explained by a shift in the distribution of stop durations towards long stopping intervals (Fig. 10). These long stops occur at the top of the cone (Fig. 11). Experiments conducted in complete darkness and with flies whose antennal mechanosensory function was impaired indicated that flies can use either visual or mechanosensory cues to assess cone geometry (Figs 7, 8, 9 and 10). Only if both modalities are impaired do the flies demonstrate no preference for tall, steep objects (Figs 7, 8, 9 and 10).
We deliberately designed these experiments using objects that control for lateral surface area, and as a consequence two potentially salient features of geometry, slope and height, were positively correlated. Thus, in none of our experiments could we distinguish between the flies' response to slope and height. It is clearly of interest to determine which of these two features of object geometry are encoded by the visual-mediated and mechanosensory-mediated mechanisms. We are currently carrying out experiments along these lines, but such analyses are beyond the scope of the study described here.
Visual stimuli influence the statistics of locomotor behavior
This work corroborates earlier studies showing that salient visual information can structure the locomotor behavior of walking fruit flies (Figs 2, 3) (Bulthoff et al., 1982; Götz, 1980; Götz, 1994; Götz and Wenking, 1973; Horn, 1978; Neuser et al., 2008; Schuster et al., 2002; Strauss and Pichler, 1998; Strauss et al., 1997). Furthermore, we have shown that the presence or absence of visual stimuli can change fundamental characteristics of walking behavior such as maximal translational speed, walking bout duration, and mean angular speed (Fig. 3). The observed changes in the statistics of walking behavior are probably the result of visual reflexes, such as object fixation and both rotatory and translatory optomotor responses (Götz, 1975; Götz, 1980; Götz and Wenking, 1973; Kalmus, 1964; Katsov and Clandinin, 2008; Zhu et al., 2009). Indeed, all animals depend on external cues in order to maintain a straight course over a significant time or distance (Dusenbery, 1992), and even humans depend on visual and auditory cues to walk straight (Schaeffer, 1928).
Object fixation
Whereas the visual environment we used in our experiments was much more complicated than those used in earlier experiments of object fixation in walking flies, our results confirm certain components of those earlier studies. Walking flies used vision to orient towards 3-D objects (Fig. 5), as observed in earlier experiments with virtual or unreachable visual objects (Götz, 1980; Horn, 1978; Horn and Wehher, 1975). Götz and colleagues also described a curious behavioral phenomenon when a fly is presented with two equally attractive, but unreachable, objects on opposite sides of an arena – an experiment known as ‘Buridan's paradigm’. Under such conditions, flies tend to walk back and forth between the two objects indefinitely even if the objects are of different size and shape (Bulthoff et al., 1982; Götz, 1980; Strauss and Pichler, 1998). This has been explained as an alteration between fixation and antifixation of objects and may facilitate efficient search among multiple visual targets (Götz, 1989; Götz, 1994). Although we observed a similar indifference to object geometry during the approach phase of exploratory behavior (Fig. 5), we did not observe a regular and sustained alteration of approach to different objects, perhaps because in our arena the flies could actually reach the objects and explore them, thereby breaking the cycle of fixation and antifixation that is required for Buridan's paradigm.
Preference for tall, steep objects
Our experiments demonstrate that hungry Drosophila exhibit a preference for tall, steep objects and that they assess object geometry using either visual cues, mechanosensory cues, or a combination of the two. However, we discovered this preference in a laboratory setting using hungry flies whose wings had been clipped, and it is therefore not immediately clear what selective pressure in a natural environment would lead to this innate and robust behavior. We speculate that the strongest drive on these hungry flies would be to find food and that the preference for tall, steep objects is somehow related to a food search strategy. Our experimental arena contained no source of attractive odor, which hungry flies would otherwise use to search for food (Bell, 1991). One possible ethological interpretation of our results is that hungry flies prefer high perches because by moving above the ground-air boundary layer they increase the likelihood of encountering an odor plume from a distant food source. In this scenario, the long stop periods represent pauses in which the flies are waiting for the chance encounter of an attractive odor. Another possibility, which is not mutually exclusive, is that the long stop periods on a steep slope represent a predator avoidance strategy. Flies might be avoiding the open field of the arena floor because they would be more vulnerable when walking or stopping on open ground rather then when perched on a vertical object. Yet another possibility is that steep surfaces or high elevations may represent good take-off locations, and anecdotally we have observed that flies appear much more likely to jump from the surface of a steep cone than from the arena floor. Although it is tempting to interpret such jumps as attempted flight initiations, it is very probable that wing clipping – a manipulation that was necessary for our experiments – alters the behavioral state of the flies. It is noteworthy that for the most part the wing-clipped flies did not persistently try to escape from the arena by jumping, even though such flies will perform escape jumps in responses to looming stimuli with the same probability as intact flies (Card and Dickinson, 2008).
Sensory modalities involved in cone assessment
Although our results implicate both vision and the mechanosensory function of the antennae in the flies' ability to assess the geometry of 3-D objects, such conclusions must be made with caution. Although several recent studies suggest that the JO is used in gravitational sensing in Drosophila (Armstrong et al., 2006; Baker et al., 2007; Kamikouchi et al., 2009; Sun et al., 2009), insects are known to have other mechanosensory systems capable of measuring gravity and posture (Beckingham et al., 2005). Thus, we cannot assume that immobilizing the antennal joint removes all cues about the flies' orientation in the gravitational field. Moreover, by immobilizing the joint we have likely compromised the function of the entire JO, which is also known to function in audition (Eberl et al., 2000) and wind detection (Yorozu et al., 2009). Another problem is that by removing all visible light we eliminate sensory input to both the compound eyes and the ocelli.
Despite the caveats with our sensory manipulations, it is nevertheless informative that together these two relatively simple sensory manipulations do appear to be sufficient to eliminate the flies' preference for tall, steep objects (Figs 7, 8, 9 and 10). Our experiments on intact flies in the dark suggest that the flies are able to sense the slope or height of a given cone using the antennal mechanosensory system. Recent work has shown that some JO neurons are responsive to steady-state deflections of the third antennal segment relative the second (Kamikouchi et al., 2009), as well as body rotations designed to simulate gravity (Sun et al., 2009). Furthermore, the increased likelihood of long stops on tall, steep objects is similar to a recently described behavior in which flies cease walking in response to air currents (Yorozu et al., 2009). This behavior is mediated by a subset of mechanoreceptor neurons within the JO, which are also thought to underlie gravity sensing (Kamikouchi et al., 2009). Motivated by these two recent studies, we attempted to test flies in which this subset of JO neurons were ablated by ectopic expression of ricin A, but the results were confounded by additional effects on locomotor behavior (A.A.R. and M.H.D., unpublished data). We also note that although the JO is well-suited to perform an instantaneous assessment of surface slope, it is also possible that the fly uses its gravitational sense in combination with an idiothetic step counter, such as that proposed for the desert ant Cataglyphus (Wittlinger et al., 2006), to perform vertical path integration, thus providing an estimate of object height.
Flies with visual cues available, but with the JO immobilized, also showed a preference for the tallest, steepest cone. There are many mechanisms by which flies might employ visual cues to assess the object geometry. Once atop the cones, flies might estimate height by actively peering to provide motion parallax cues. Drosophila do use motion parallax cues to estimate the distance to objects as they approach (Schuster et al., 2002), and locusts use active peering to judge the distance to objects before they jump (Kral, 2003; Wallace, 1959). Another possibility, suggested by the studies showing that bees are able to integrate optic flow to estimate distance flown (Srinivasan et al., 2000), is that flies might also use optic flow to measure the distance traveled up the surface of an object – a form of path integration using visual information rather than ideothetic cues. To accurately measure height, such a behavior would require some JO-independent measure of gravity or body posture. Another vision-based mechanism is that flies might use their compound eyes or ocelli to determine body orientation relative to the local horizon and thus estimate the steepness of the surface they are exploring. Whether the compound eyes or the ocelli are involved, it is interesting to note that the flies with vision intact but their JOs impaired exhibit a decreased ability to distinguish between the blue and green cones in our experiments, suggesting that the vision-based means of assessing cone geometry is less precise than the mechanosensory-based mechanism.
In this work, we have focused on describing flies' preference for tall, steep objects, the underlying change in locomotor statistics responsible for this preference, and the sensory modalities used for the assessment of object geometry. Our research has identified an innate behavior in which sensory information from the visual system and the antennae are used to regulate locomotion in the context of the exploratory behavior of hungry flies. In the future, it will be of interest to determine the functional role of this behavior in the animal's natural history, as well as elucidate the underlying neural mechanisms.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Kristin Branson for her discussions of behavioral analysis, as well as Martin Peek and Neil Halelamien for their help developing the first version of the arena and tracking software. This work was supported by grants from the National Institutes of Health (R01DA022777-04; A.R.), and the National Science Foundation (0623527) to M.H.D. Deposited in PMC for release after 12 months.
Footnotes
Supplementary material available online at http://jeb.biologists.org/cgi/content/full/213/14/2494/DC1
REFERENCES
- Armstrong J. D., Texada M. J., Munjaal R., Baker D. A., Beckingham K. M. (2006). Gravitaxis in Drosophila melanogaster: a forward genetic screen. Genes, Brain Behav. 5, 222-239 [DOI] [PubMed] [Google Scholar]
- Baker D. A., Beckingham K. M., Armstrong J. D. (2007). Functional dissection of the neural substrates for gravitaxic maze behavior in Drosophila melanogaster. J. Comp. Neurol. 501, 756-764 [DOI] [PubMed] [Google Scholar]
- Beckingham K. M., Texada M. J., Baker D. A., Munjaal R., Armstrong J. D. (2005). Genetics of graviperception in animals. Adv. Genet. 55, 105-145 [DOI] [PubMed] [Google Scholar]
- Bell W. J. (1991). Searching Behaviour: the Behavioural Ecology of Finding Resources New York: Chapman and Hall; [Google Scholar]
- Branson K., Robie A. A., Bender J., Perona P., Dickinson M. H. (2009). High-throughput ethomics in large groups of Drosophila. Nat. Methods 6, 451-457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budick S. A., Reiser M. B., Dickinson M. H. (2007). The role of visual and mechanosensory cues in structuring forward flight in Drosophila melanogaster. J. Exp. Biol. 210, 4092-4103 [DOI] [PubMed] [Google Scholar]
- Bulthoff H., Götz K. G., Herre M. (1982). Recurrent inversion of visual orientation in the walking fly, Drosophila melanogaster. J. Comp. Physiol. 148, 471-481 [Google Scholar]
- Card G., Dickinson M. (2008). Performance trade-offs in the flight initiation of Drosophila. J. Exp. Biol. 211, 341-353 [DOI] [PubMed] [Google Scholar]
- Duistermars B. J., Chow D. M., Frye M. A. (2009). Flies require bilateral sensory input to track odor gradients in flight. Curr. Biol. 19, 1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusenbery D. (1992). Sensory Ecology: How Organisms Acquire and Respond to Information New York: W. H. Freeman and Company; [Google Scholar]
- Eberl D. F., Hardy R. W., Kernan M. J. (2000). Genetically similar transduction mechanisms for touch and hearing in Drosophila. J. Neurosci. 20, 5981-5988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz K. G. (1975). Optomotor equilibrium of Drosophila navigation system. J. Comp. Physiol. 99, 187-210 [Google Scholar]
- Götz K. G. (1980). Visual guidance in Drosophila. In Development and Neurobiology of Drosophila (ed. Siddiqi Q., Babu P., Hall L., Hall J.), pp. 391-407 New York: Plenum Press; [Google Scholar]
- Götz K. G. (1989). Search and choice in Drosophila. In Neurobiology of Sensory Systems (ed. Singh R. N., Strausfeld N. J.), pp. 139-153 New York: Plenum Publishing Corporation; [Google Scholar]
- Götz K. G. (1994). Exploratory strategies in Drosophila. In Neural Basis of Behavioral Adaptations (ed. Schildberger K., Elsner N.), pp. 47-59 Stuttgart, Germany: G. Fischer; [Google Scholar]
- Götz K. G., Wenking H. (1973). Visual control of locomotion in walking fruitfly Drosophila. J. Comp. Physiol. 85, 235-266 [Google Scholar]
- Horn E. (1978). The mechanism of object fixation and its relation to spontaneous pattern preferences in Drosophila melanogaster. Biol. Cybern. 31, 145-158 [DOI] [PubMed] [Google Scholar]
- Horn E., Wehher R. (1975). Mechanism of visual-pattern fixation in walking fly, Drosophila melanogaster. J. Comp. Physiol. 101, 39-56 [Google Scholar]
- Kalmus H. (1964). Animals as mathematicans. Nature 202, 1156-1160 [DOI] [PubMed] [Google Scholar]
- Kamikouchi A., Inagaki H. K., Effertz T., Hendrich O., Fiala A., Göpfert M. C., Ito K. (2009). The neural basis of Drosophila gravity-sensing and hearing. Nature 458, 165-171 [DOI] [PubMed] [Google Scholar]
- Katsov A. Y., Clandinin T. R. (2008). Motion processing streams in Drosophila are behaviorally specialized. Neuron 59, 322-335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral K. (2003). Behavioural-analytical studies of the role of head movements in depth perception in insects, birds and mammals. Behav. Process. 64, 1-12 [DOI] [PubMed] [Google Scholar]
- Martin J.-R. (2004). A portrait of locomotor behaviour in Drosophila determined by a video-tracking paradigm. Behav. Process. 67, 207-219 [DOI] [PubMed] [Google Scholar]
- Nation J. L. (2008). Insect Physiology and Biochemistry Boca Raton: CRC Press; [Google Scholar]
- Neuser K., Triphan T., Mronz M., Poeck B., Strauss R. (2008). Analysis of a spatial orientation memory in Drosophila. Nature 453, 1244-1248 [DOI] [PubMed] [Google Scholar]
- Reichardt W., Poggio T. (1975). Theory of pattern induced flight orientation of fly Musca domestica. 2. Biol. Cybern. 18, 69-80 [DOI] [PubMed] [Google Scholar]
- Schaeffer A. (1928). Spiral movement in man. J. Morphol. 45, 293-398 [Google Scholar]
- Schuster S., Strauss R., Götz K. G. (2002). Virtual-reality techniques resolve the visual cues used by fruit flies to evaluate object distances. Curr. Biol. 12, 1591-1594 [DOI] [PubMed] [Google Scholar]
- Shafer O., Levine J., Truman J., Hall J. (2004). Flies by night: effects of changing day length on Drosophila's circadian clock. Curr. Biol. 14, 424-432 [DOI] [PubMed] [Google Scholar]
- Srinivasan M. V., Zhang S., Altwein M., Tautz J. (2000). Honeybee navigation: nature and calibration of the “Odometer”. Science 287, 851-853 [DOI] [PubMed] [Google Scholar]
- Strauss R., Pichler J. (1998). Persistence of orientation toward a temporarily invisible landmark in Drosophila melanogaster. J. Comp. Physiol. A 182, 411-423 [DOI] [PubMed] [Google Scholar]
- Strauss R., Schuster S., Götz K. G. (1997). Processing of artificial visual feedback in the walking fruit fly Drosophila melanogaster. J. Exp. Biol. 200, 1281-1296 [DOI] [PubMed] [Google Scholar]
- Straw A. D., Dickinson M. H. (2009). Motmot, an open-source toolkit for realtime video acquisition and analysis. Source Code for Biology and Medicine 4, 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Liu L., Ben-Shahar Y., Jacobs J. S., Eberl D. F., Welsh M. J. (2009). TRPA channels distinguish gravity sensing from hearing in Johnston's organ. Proc. Natl. Acad. Sci. USA 106, 13606-13611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace G. K. (1959). Visual scanning in the desert locust Schistocerca gregaria Forskål. J. Exp. Biol. 36, 512-525 [Google Scholar]
- Wittlinger M., Wehher R., Wolf H. (2006). The ant odometer: stepping on stilts and stumps. Science 312, 1965-1967 [DOI] [PubMed] [Google Scholar]
- Yorozu S., Wong A., Fischer B. J., Dankert H., Kernan M. J., Kamikouchi A., Ito K., Anderson D. J. (2009). Distinct sensory representations of wind and near-field sound in the Drosophila brain. Nature 458, 201-205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Nern A., Zipursky S. L., Frye M. A. (2009). Peripheral visual circuits functionally segregate motion and phototaxis behaviors in the fly. Curr. Biol. 19, 613-619 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.