Abstract
Alveolar macrophages and BDMCs undergo sequential biochemical changes during the chronic inflammatory response to chemically induced lung carcinogenesis in mice. Herein, we examine two chronic lung inflammation models—repeated exposure to BHT and infection with Mycobacterium tuberculosis—to establish whether similar macrophage phenotype changes occur in non-neoplastic pulmonary disease. Exposure to BHT or M. tuberculosis results in pulmonary inflammation characterized by an influx of macrophages, followed by systemic effects on the BM and other organs. In both models, pulmonary IFN-γ and IL-4 production coincided with altered polarization of alveolar macrophages. Soon after BHT administration or M. tuberculosis infection, IFN-γ content in BALF increased, and BAL macrophages became classically (M1) polarized, as characterized by increased expression of iNOS. As inflammation progressed in both models, the amount of BALF IFN-γ content and BAL macrophage iNOS expression decreased, and BALF IL-4 content and macrophage arginase I expression rose, indicating alternative/M2 polarization. Macrophages present in M. tuberculosis-induced granulomas remained M1-polarized, implying that these two pulmonary macrophage populations, alveolar and granuloma-associated, are exposed to different activating cytokines. BDMCs from BHT-treated mice displayed polarization profiles similar to alveolar macrophages, but BDMCs in M. tuberculosis-infected mice did not become polarized. Thus, only alveolar macrophages in these two models of chronic lung disease exhibit a similar progression of polarization changes; polarization of BDMCs was specific to BHT-induced pulmonary inflammation, and polarization of granuloma macrophages was specific to the M. tuberculosis infection.
Keywords: tuberculosis, IFN-γ, IL-4, butylated hydroxytoluene
Introduction
Chronic pulmonary diseases can persist for months or years because of an inability to remove the inciting stimulus and/or to repair cellular damage [1]. Currently, 35 million Americans each year suffer from chronic pulmonary disease, which constitutes the third leading cause of mortality in the United States [2]. Macrophages are key to chronic inflammatory pathology, and their behavior is coordinated largely via cytokine signaling. Macrophages exhibit diverse biochemical properties that influence pathobiology with classical/M1 and alternative/M2 polarization representing phenotypic extremes [3, 4]. Temporal changes in the polarization of macrophages during chronic pulmonary diseases help regulate tissue repair [5,6,7,8]. Classical/M1 polarization induced by IFN-γ or LPS is characterized by high expression of iNOS and consequent NO production, secretion of proinflammatory cytokines IL-1 and IL-12, and enhanced phagocytosis [3, 4, 9, 10]. Alternative/M2 polarization of macrophages elicited by IL-4 and IL-13 results in high arginase I and mannose receptor expression, anti-inflammatory cytokine IL-10 production, and polyamine production that aids wound repair [3, 9,10,11,12]. In vitro, these activation states are plastic in that macrophages can switch back and forth between them in a phenotypic continuum, depending on the cytokines present in the extracellular milieu [3].
In a model of chronic pulmonary inflammation, mice are exposed to at least four weekly injections of BHT. Mice exposed to a single BHT injection undergo reversible pulmonary injury characterized in part by alveolar type I cell necrosis and the hyperproliferation of type II cells, which then differentiate into type I cells as a compensatory response to type I cell death [13,14,15,16,17,18]. Repeated exposure to BHT causes little lung damage but induces extensive macrophage infiltration in certain susceptible mouse strains [17], providing a valuable chronic pulmonary inflammation model [19, 20]. Although pathologies associated with acute BHT treatment have been studied extensively, those associated with chronic BHT dosing, other than pulmonary inflammation and subsequent lung tumor promotion, have not been fully characterized.
In a second mouse chronic inflammation model, mice inhaling Mycobacterium tuberculosis bacilli develop TB, which if left untreated, persists throughout life [2]. This immune-mediated tissue destruction occurs on a background of slowly progressive bacterial growth [21]. Alveolar macrophages, along with mast cells, comprise a first line of immune defense, establishing an immune response upon encountering M. tuberculosis bacilli [22]. In addition to lung inflammation and the formation of lung tubercles, pulmonary infection with M. tuberculosis has systemic consequences. The bacilli infect other organs (i.e., liver and spleen), increase peripheral blood monocytes, and induce anemia [23]. Infection by routes of administration other than inhalation yields a similar disease progression [24]. M. tuberculosis-infected mice undergo a pulmonary pathology dominated by macrophages that is similar to the human disease and provides a model in which environmental and genetic events can be controlled.
We demonstrated recently that BDMCs as well as tumor-associated macrophages were alternatively polarized as chemically induced benign tumors in A/J mice developed [8]. Herein, we use two macrophage-dominant models of chronic pulmonary inflammation to determine whether similar phenotypic changes in macrophages and BM cells accompany non-neoplastic chronic pulmonary diseases.
MATERIALS AND METHODS
BHT model of chronic pulmonary inflammation
A/J mice (males, 4–6 weeks of age) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Mice were housed on hardwood bedding with 12 h light/dark cycles and fed standard rodent diet (Harlan Teklad, Madison, WI, USA) at the Center of Laboratory Animal Care in the University of Colorado Denver (Aurora, CO, USA). Animal protocols involving BHT treatment or cytokine administration were approved by the Animal Care and Use Committee of the University of Colorado at Denver. A/J mice (6–8 weeks old, five animals/group) were injected i.p., with freshly prepared BHT (Sigma Chemical Co., St. Louis, MO, USA) at an initial dose of 100 mg/kg i.p., followed by three weekly 200 mg/kg injections; control mice received four weekly corn oil (Mazola) vehicle injections [20]. The initial BHT treatment causes transient lung toxicity; lowering this first BHT dose to 100 mg/kg reduces toxicity while allowing subsequent chronic inflammation to develop in response to repeated BHT treatment [17]. Five mice/treatment group were killed 6 and 30 days after the final BHT or vehicle injection. A/J mice were used for these experiments as a result of their robust inflammatory response after chronic administration of BHT compared with C57BL/6 mice, which are more resistant to chronic BHT exposure [17]. Additionally, A/J mice demonstrated lung-to-BM signaling in our previous lung cancer study [8].
BAL to obtain pulmonary macrophages
Mice were killed by lethal pentobarbital i.p. injection (0.9 mg/mouse), their tracheas cannulated, and lungs lavaged three times with 1 ml PBS containing 0.6 mM EDTA, as described previously [8]. Inflammatory cell infiltration was determined by pooling lavaged samples from each animal and counting cells using a hemocytometer. Twenty thousand BAL cells/sample were cytospun (Shandon Cytospin 3) and affixed to glass slides in −20°C methanol for 3 min. Cells were stained with Wright/Giemsa and classified differentially on the basis of nuclear morphology as monocytes/macrophages, lymphocytes, neutrophils, or eosinophils [8]. On average, >95% of BAL cells were monocyte/macrophages. As the volume of BALF lavaged from each mouse varies between animals and treatment groups, BAL data were expressed as cells/ml BALF.
BDMC preparation
After BAL was collected, one femur/mouse was removed and BM cells harvested by flushing 1 ml sterile PBS through the BM cavities with a 25 5/8-gauge syringe. Cell numbers were determined and 20,000 cells/sample cytospun onto slides; differential cell counts were performed as above [8]. On average, 2–4% of the extruded BM cells are monocytes.
ELISA determination of IFN-γ and IL-4 contents in BAL
BALF supernatant from the first of three lavages was collected from A/J mice 6 and 30 days after chronic treatment of BHT and 24 h after cytokine administration by microspray. IFN-γ and IL-4 contents were determined by ELISA following the manufacturer’s ELISA kit protocol (R&D Systems, Minneapolis, MN, USA).
Immunofluorescence
BAL cells and BM aspirates were exposed to the following antibodies: rat polyclonal F4/80 antibody (Caltag Labotatories, Carlsbad, CA, USA) at 1:100 for BAL macrophage identification, mouse polyclonal CD68 antibody (Dako, Glostrup, Denmark) for BDMC identification, goat polyclonal arginase I antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 1:100 as a marker of M2 polarization, and rabbit polyclonal iNOS antibody (BD PharMingen, Franklin Lakes, NJ, USA) at 1:100 dilution as a marker of M1 polarization. Samples were treated with biotin-conjugated IgG secondary antibody (Vector Laboratories, Burlingame, CA, USA), followed by fluorescent avidin conjugates (fluorescein, amino-methyl coumarin, and rhodamine), as described previously [8]. Three images were obtained per slide and a minimum of 200 cells analyzed to determine iNOS and arginase I expression. A digital deconvolution microscopy imaging system attached to a Zeiss Axioplan 2 epi-fluorescence microscope was used to image fluorescent staining. All fluorescent images are 630× final magnification.
iNOS, arginase 1, and mannose receptor IHC
Mouse lung, liver, and spleen macrophages were examined for activation status by IHC using antibodies against iNOS (lung, liver, and spleen) and arginase I (lung) or mannose receptor (CD206; liver and spleen), as described previously [8]. Sections were deparaffinized and rehydrated through a series of ethanol washes and antigens retrieved in boiling 100 mM citrate buffer. Samples were first blocked with serum and then exposed to goat polyclonal arginase I antibody (Santa Cruz Biotechnology) at 1:100 dilution, mouse monoclonal mannose receptor antibody (Serotec, UK) at 1:100 dilution, or rabbit polyclonal iNOS antibody (BD PharMingen) at 1:1000. Samples were treated with biotin-conjugated secondary antibodies (Vector Laboratories), followed by peroxidase-conjugated streptavidin complex (Vector Laboratories). Positively stained cells were detected with the peroxidase substrate 3,3-diaminobenzidine (Sigma Chemical Co.), and hematoxylin (Sigma Chemical Co.) was used as a counterstain.
Intrapulmonary administration of cytokines
This was performed as reported previously [25]. Briefly, after A/J mice were anesthetized by i.p. injection of 100 mg/kg body weight each of ketamine and xylazine, they were supported by their upper teeth on a 45° angled platform and the tongue pulled out gently. A laryngoscope guided the insertion of a microspray apparatus (Penn-Century, Philadelphia, PA, USA) into the trachea to deliver 2 μg IL-4 and/or 0.5 μg IFN-γ in 50 μl PBS (PeproTech, Rocky Hill, NJ, USA). Control mice received 50 μl PBS. After the microsprayer was withdrawn, the mouse was removed from the platform and monitored until full recovery from anesthesia. A/J mice were used for these experiments to demonstrate that exogenously administered cytokines could induce polarization changes in macrophages analogous to those observed following BHT administration.
TB model of chronic pulmonary inflammation
Pathogen-free C57BL/6 mice (females, 6–8 weeks of age), purchased from the Jackson Laboratory, were maintained in the Biosafety Level 3 Biohazard Facility at Colorado State University (Fort Collins, CO, USA) and given sterile water and rodent diet ad libitum. The Animal Care and Use Committee of Colorado State University approved the following experimental protocols. Mice were infected with a single low-dose aerosol exposure to M. tuberculosis strain Erdman, using a Glass-Col aerosol generator calibrated to deliver 50–100 bacteria into each lung as described previously [26]. The lungs, BALF, BAL cells, and BM from six mice/time-point were harvested 7, 21, 35, and 60 days after infection. One lobe from each animal was inflated and formalin-fixed for histopathological examination. BAL cells and BALF were obtained from the remaining four lobes. Female C57BL/6 mice were used for these experiments, as they are sensitive to M. tuberculosis infection and display a granulomatous reaction similar to the human disease but survive longer than other strains including A/J.
BM histopathology
Isolated femurs were decalcified in 8% formic acid for 3 h followed by formalin fixation, a series of ethanol rinses, and paraffin embedding. Sections (4 μm) were cut using an 820 Spencer microtome (American Optical Corp., Del Mar, CA, USA), rehydrated, stained in hematoxylin (Fisher Scientific, Pittsburgh, PA, USA) for 4 min, and rinsed in tap water. Sections were placed in 1% ammonium hydroxide for 5 min, fixed in 95% ethanol for 10 s, stained with Eosin Y (Fisher Scientific) for 10 s, and dehydrated in increasing concentrations of ethanol before a coverslip was applied. Sections were analyzed under an Olympus BX41 microscope equipped with a digital camera. All images are 400× final magnification.
Statistical analysis
The results are presented as means ± sem and represent n = 3–6 mice. Differences between groups were examined using Student’s unpaired t-test. One-way ANOVA compared more than two groups, and post-hoc Newman-Keuls tests identified differences between groups. P < 0.05 was considered to be significant.
Online supplemental material
To confirm M. tuberculosis infection, pulmonary bacterial counts (n=3) were performed at 7, 21, 35, and 60 days after infection by plating serial dilutions of lung homogenate onto nutrient 7H11 agar and counting CFU after a 3-week incubation at 37°C [26]. Lung localization of M. tuberculosis was determined by acid-fast staining. Formalin-fixed lungs were cut into 4 μm sections as described above. After depariffinization, slides were placed in 70°C carbol fuschin solution for 5 min, washed in deionized water, decolorized with 1.0% acid alcohol solution, and counterstained with methylene blue solution. Images are 1000× final magnification.
To confirm systemic effects in liver from M. tuberculosis-infected mice, livers were fixed in formalin, embedded, and cut into 4 μm sections, as described above. IHC analyses of macrophage arginase I, mannose receptor, and iNOS were performed as described above. Lymphocytic deposits were detected in H&E-stained slides.
RESULTS
Activation of alveolar macrophages and BDMCs after chronic administration of BHT
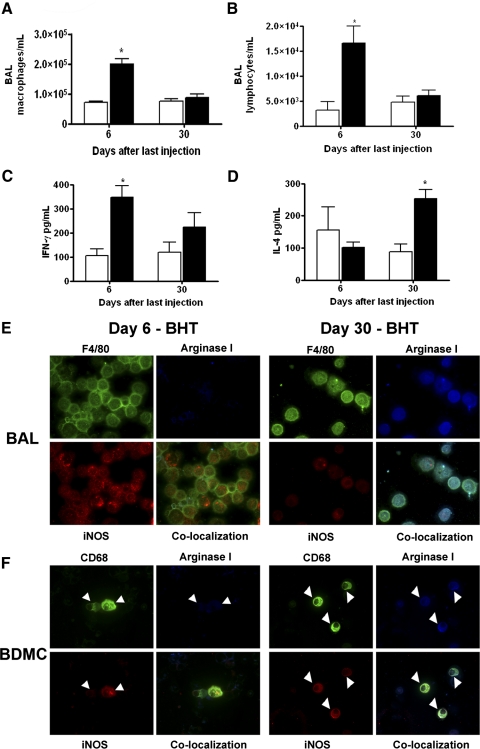
The inflammatory response that occurs after chronic BHT administration is characterized by a 2.8-fold increase in the number of lung BAL macrophages in A/J mice 6 days after the final BHT injection (Fig. 1A) and a fivefold increase in BAL lymphocytes (Fig. 1B). By 30 days after the last BHT injection, the numbers of BAL macrophages and lymphocytes return to control levels.
Figure 1.
Cellular infiltration and cytokine contents in pulmonary BAL change after BHT administration. (A) BAL macrophage content increases 6 days following the last of four weekly BHT injections. By 30 days after the last injection, population size has returned to basal levels. (B) Lymphocytes display similar population kinetics, rising at the 6-day time-point and returning to control levels by Day 30. (C) IFN-γ levels rose above control (open bars) 6 days after chronic BHT exposure and then declined by 30 days. (D) IL-4 levels 6 days after BHT are similar to corn oil controls but increase by Day 30. Data represent the mean ± sem. *, P < 0.001, compared with control; n = 5. Mice treated with corn oil control (open bars); mice treated with BHT (solid bars). (E) BAL macrophages (F4/80, green) express iNOS (red) but not arginase I (blue) 6 days after chronic BHT (upper row). (F) BDMCs (CD68, green, white arrowheads) also express iNOS but not arginase I 6 days after BHT (E and F). BAL macrophages and BDMCs (white arrowheads) from mice 30 days after chronic BHT administration are argIhigh-iNOShigh (lower rows). Final original magnification, ×630.
The BALF content of IFN-γ rose significantly within 6 days after the final BHT injection, as compared with vehicle controls, but by Day 30, these levels were decreasing (Fig. 1C). IL-4 content exhibited the opposite temporal trend, with amounts similar to control at the 6-day time-point but rising significantly by 30 days after the last BHT treatment (Fig. 1D). Although the number of macrophages decreased by Day 30 after treatment, IFN-γ and IL-4 were still produced, implying that increased production of these cytokines is not sufficient to sustain increased alveolar macrophage populations in this model.
Polarization of BAL macrophages and BDMCs, as defined by arginase I and iNOS expression, was examined at these same time-points. BAL macrophages and BDMCs from vehicle-treated control mice were argIlowiNOSlow. In contrast, 6 days after the last BHT injection, macrophages (Fig. 1E) and BDMCs (Fig. 1F) were argIlowiNOShigh, which in combination with the detection of IFN-γ in BALF at this time, indicates classical/M1 polarization. Thirty days after the final BHT administration, BAL macrophages and BDMCs expressed argImediNOSlow (iNOS levels were low but still detectable), corresponding to the low IFN-γ and increased IL-4 levels (Fig. 1, C and D) in BALF. This is shown by the colocalization of iNOS and arginase I in BAL macrophages and BDMCs (Fig. 1, E and F).
Direct administration of cytokines to the lungs recruits and polarizes alveolar macrophages
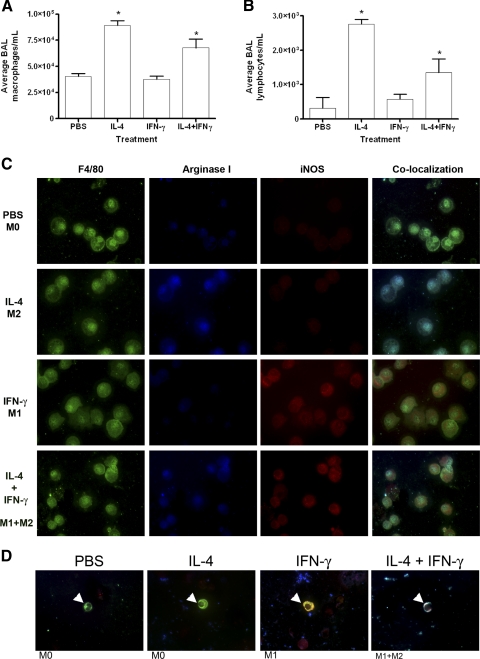
To assess directly the in vivo contribution of IFN-γ and IL-4 to macrophage polarization and recruitment, IL-4, IFN-γ, and a combination of IL-4 + IFN-γ were delivered by intrapulmonary microspray. Large increases in the BAL contents of the instilled cytokines were confirmed by ELISA assay 24 h after instillation (data not shown). The number of BAL macrophages and lymphocytes increased significantly above that of PBS-treated mice after IL-4 or IL-4 + IFN-γ was administered (Fig. 2, A and B). Instillation of IFN-γ did not affect leukocyte infiltration and reduced the number of lymphocytes that infiltrated as a result of IL-4 administration. BAL macrophages exposed to IL-4 alone were argIhighiNOSlow, and macrophages from IFN-γ-treated mice were argIlowiNOShigh (Fig. 2C). Alveolar macrophages exposed to a mixture of IL-4 and IFN-γ were argIhigh-iNOShigh, a mixed M1 + M2 phenotype. BDMC polarization in response to those exogenously administered cytokines mirrored that of BAL macrophages (Fig. 2D).
Figure 2.
Changes in BAL cellular composition and in the polarization of BAL macrophages and BDMCs upon exogenous pulmonary IL-4 and IFN-γ administration. Pulmonary macrophages (A) and lymphocytes (B) increase after IL-4 or IL-4 + IFN-γ administration by microspray into the trachea. Data represent the mean ± sem; n = 5; *, P < 0.001, compared with PBS- and IFN-γ-treated mice. (C) BAL macrophages (F4/80, green) were immunostained for arginase I and iNOS. Exposure to PBS resulted in an argIlowiNOSlow phenotype. Exposure to IL-4 induced arginase I (blue) but not iNOS, indicating M2 polarization. Exposure to IFN-γ induced iNOS (red) but not arginase I, indicating M1 polarization. Intrapulmonary administration of IL-4 and IFN-γ resulted in an argIhighiNOShigh phenotype. (D) Co-localized images of BDMCs (CD68, green, white arrowheads) express iNOS after pulmonary IFN-γ instillation, arginase I after IL-4 instillation, and iNOS and arginase I when instilled with both cytokines.
BAL and BM cellular composition after M. tuberculosis exposure
To determine whether lung macrophage and BDMC polarization occurred in an infection model exhibiting chronic macrophage involvement, we infected C57BL/6 mice with M. tuberculosis. Infection of M. tuberculosis in the lungs was confirmed by measuring CFU from homogenized lung tissue after each mouse harvest. The number of colonies increased and reached a plateau by Day 21 post-challenge (Supplemental Fig. 1A). The presence of M. tuberculosis within macrophages was detected by acid fast staining of the bacteria, which appear as pink rods (Supplemental Fig. 1B).
Aerosolized infection of mice with M. tuberculosis also induced systemic effects, as indicated by polarization of Kupfer cells and the presence of hepatic lymphocytic deposits. F4/80+/mannose receptorhigh macrophages were present in the liver 60 days after bacterial exposure (Supplemental Fig. 2, A and B), and lymphocytic deposits were detected in liver tissue within 60 days of M. tuberculosis exposure (Supplemental Fig. 2C).
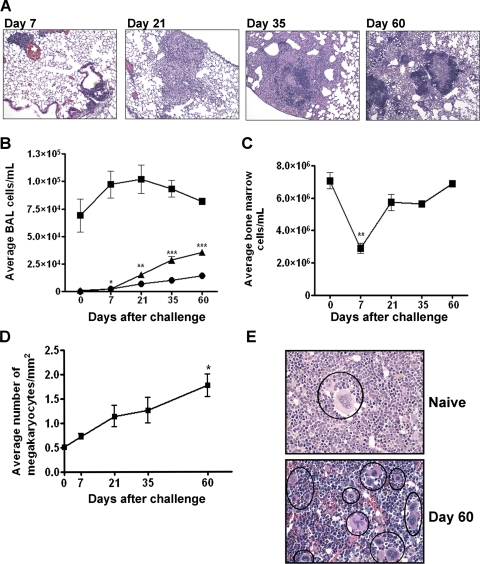
Over the course of M. tuberculosis infection, monocytes, lymphocytes, and neutrophils are recruited to the lungs. Infected macrophages help construct a developing granuloma by surrounding the infected site and containing the bacteria, and T cells supply immune activation signals [27, 28]. The number of pulmonary macrophages detected by histology increased with M. tuberculosis infection (Fig. 3A), but the number of macrophages in BAL did not (Fig. 3B). This is perhaps a result of tight adherence of macrophages within the granulomas. Infiltrating lymphocyte and neutrophil levels in BAL began to rise 7 days after M. tuberculosis exposure (Fig. 3B). There are virtually no neutrophils present in healthy lungs, but up to 1.1 × 104 neutrophils/ml were found 60 days after M. tuberculosis infection. Lymphocytes, present at 1.2 × 103 lymphocytes/ml in BAL from naïve lungs, increased 30-fold to 3.4 × 104 lymphocytes/ml after 60 days (Fig. 3B).
Figure 3.
M. tuberculosis pathogenesis in mice. (A) Granuloma progression after M. tuberculosis infection. (B) BAL macrophage (▪) numbers rise and fall slightly, and lymphocytes (▴) and neutrophils (•) increase steadily. (C) The BM cell population decreases shortly after bacterial exposure but returns to normal levels by 60 days after infection. (D) The number of megakaryocytes increases during M. tuberculosis infection. (E) There are fewer megakaryocytes (black circles) in the marrow of naïve mice compared with BM from a mouse 60 days post-challenge. Data represent mean ± sem; n = 6; *, P < 0.05; **, P < 0.01; ***, P < 0.001, compared with control.
Seven days after bacterial challenge, the total number of BM cells decreased but then recovered and returned to control levels by Day 60 (Fig. 3, C and E). A significant elevation in the number of megakaryocytes was observed over 60 days in BM (Fig. 3D). This rise may be a result of elevated production of GM-CSF during the course of M. tuberculosis infection [25, 29]. These systemic effects on BM, along with the changes in hepatic macrophages and lymphocytes (Supplemental Fig. 2B) in this M. tuberculosis inhalation infection model are similar to that seen in animals where the bacteria is injected into the bloodstream [30].
Pulmonary macrophage polarization, but no BDMC polarization, after M. tuberculosis exposure
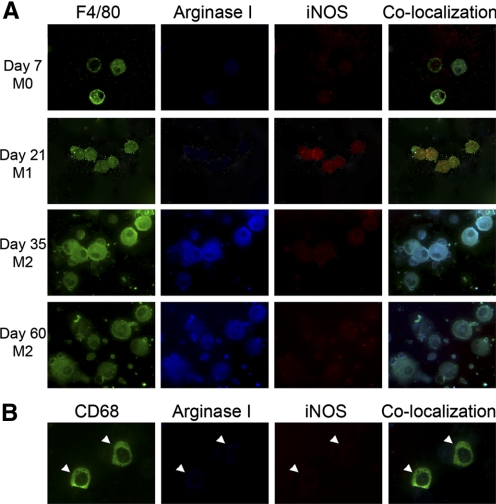
Pulmonary macrophages were isolated 7, 21, 35, and 60 days after M. tuberculosis infection. At 7 days, BAL macrophages were argIlowiNOSlow (Fig. 4A). By Day 21, BAL macrophages were argIlowiNOShigh (M1). BAL macrophages isolated 35–60 days after M. tuberculosis exposure were argIhighiNOSlow, indicating a switch from M1 to M2 polarization (Fig. 4A). These polarization changes correlated with reported levels of IFN-γ and IL-4 produced by CD4+ T cells isolated from lungs during M. tuberculosis infection [31, 32]. IFN-γ production by T cells increased significantly in infected lungs compared with control lungs, peaking at Day 10 after infection and remaining elevated until Day 21 [33]. A subsequent decline in IFN-γ content was accompanied by a steady increase in IL-4 production [33]. BDMCs were argIlowiNOSlow throughout TB progression, showing no polarization changes from those of naïve C57BL/6 mice (Fig. 4B).
Figure 4.
Pulmonary macrophages, but not BDMCs, are polarized during M. tuberculosis infection. (A) Macrophages (F4/80, green) express no arginase or iNOS at Day 7 following M. tuberculosis challenge. At Day 21, macrophages express iNOS (red) but not arginase (blue), an M1 polarization. At Days 35 and 60, macrophages lose iNOS expression and gain arginase I expression, an M2 phenotype. (B) BDMCs (CD68, green, white arrowheads) from mice after M. tuberculosis are argIlowiNOSlow. Final original magnification, ×630.
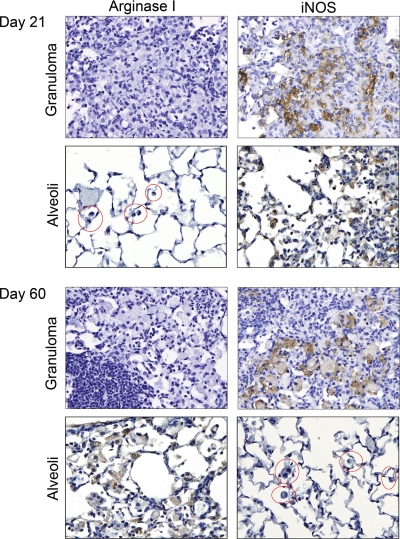
As macrophages in the granuloma core are not accessible by BAL, iNOS and arginase expression in these structures was examined by IHC (Fig. 5). Up to 21 days after bacterial exposure, alveolar and granuloma-associated macrophages were argIlowiNOShigh (Fig. 5). Granuloma-associated macrophages continued expressing this polarization phenotype throughout the 35- and 60-day time-points. In contrast, macrophages surrounding the granuloma corresponding with those examined in BAL (Fig. 4) became argIhighiNOSlow at later time-points (Fig. 5). The proximity of these two differentially polarized macrophage populations indicates that TH1- and TH2-dominated microenvironments can coexist.
Figure 5.
IHC staining of granulomas for iNOS and arginase I. IHC analysis of arginase I and iNOS in M. tuberculosis infected lungs 21 and 60 days after infection. Alveolar macrophages are circled in red. Granuloma-associated macrophages and alveolar macrophages exhibit iNOS staining starting 21 days after infection. By Day 60, alveolar macrophages express arginase I and not iNOS. Alveolar and granuloma macrophage IHC staining was photographed in the same lung at each time-point, and alveolar staining was examined near and distal to granulomas and found to be similarly stained for arginase I and iNOS regardless of distance from the granulomas. Final original magnification, ×400.
DISCUSSION
M1 and M2 macrophage polarization leads to the production of different arrays of TH1 and TH2 cytokines, and this balance shapes the progression of chronic inflammatory diseases. Alveolar macrophages become polarized when mouse lungs are inflamed, whether this inflammation is a result of the growth of a tumor [8], a response to an inciter of macrophage infiltration such as BHT, or a result of bacterial infection. The type of macrophage polarization depends on the nature of the cytokines in the pulmonary milieu. After systemic BHT administration and infection with M. tuberculosis by aerosol, there is an influx of alveolar macrophages, a rise then a fall in IFN-γ content, followed by a steady increase in IL-4 production. Alveolar macrophage phenotype changes initially from argIlowiNOSlow to argIlowiNOShigh and then progresses to argIhighiNOSlow (TB) or argIhighiNOShigh (BHT), depending on the presence of IL-4 and/or IFN-γ. Direct administration of IFN-γ and IL-4 into the lungs significantly impacted the polarization state of alveolar macrophages, recapitulating what was observed in BHT-treated mice. This pulmonary instillation of cytokines also resulted in BDMC activation that correlated with BAL macrophage activation.
Instilled IL-4 stimulated macrophage and lymphocyte infiltration into the lungs, and instilled IFN-γ did not. In fact, IFN-γ decreased the extent of lymphocyte infiltration when coadministered with IL-4. IL-4 thus signals inflammatory cells and epithelial cells to produce chemoattractants necessary for leukocyte migration [33]. IL-4 and other TH2 cytokines also increase T cell infiltration in Helicobacter pylori-induced gastric mucosa-associated lymphoid tissue lymphomas, and this lymphoma growth is TH2 cytokine-dependent [34, 35]. IL-4 stimulates leukocyte rolling, adhesion, and emigration in a rat asthma model [36].
Macrophages are essential to host defense against microbes. Classical polarization of macrophages by TH1 and TH2 cytokines affects lymphocyte proliferation and determines which cytokines are produced by the activated macrophages [3]. Classically/M1-polarized macrophages fuse their lysosomes efficiently with bacilli-containing phagosomes and up-regulate iNOS to expose bacteria to lethally high NO concentrations. IL-12 synthesized by M1 macrophages directs CD4+ T cells to differentiate into TH1 cells in a positive feedback loop. TB lung granuloma formation, consisting of a core of infected macrophages surrounded by lymphocytes, neutrophils, and fibroblasts, at least partially sequesters the bacteria to isolate it from the rest of the body to protect the organism and provide tolerance to bacterial infection [23]. It has been suggested recently, however, that pathogenic Mycobacteria exploit the granuloma by using infected macrophages to disseminate into newly recruited monocytes [37]. In either case, the cytokines present in the granuloma and the rest of the lungs during M. tuberculosis infection help differentiate and define macrophage responses in these compartments [38]. We observed differential polarization of macrophages within the granuloma as compared with those macrophages surrounding the granuloma in the alveolar space by 35 days after M. tuberculosis infection.
Two distinct inflammatory responses have been described during TB disease progression in mice [32], corresponding to M1 polarization followed by M2 polarization in pulmonary macrophages. At Day 7 after M. tuberculosis infection, macrophages expressed neither arginase I nor iNOS, but by 20–30 days after infection, a high amount of IFN-γ was produced by CD4+ T cells that subsided thereafter. IL-4 synthesis started at Day 20 and continued up to at least 60 days [32]. Expression of iNOS in granulomatous macrophages during chronic M. tuberculosis infection has been reported previously [39], and we confirmed this by IHC staining. However, we found that alveolar macrophages harvested in BALF were negative for iNOS but positive for arginase I at these later time-points. Cytokine mRNA analysis of microdissected granulomas in the guinea pig TB model demonstrated a similar time course. Initially (0–3 weeks postinfection), the disease is characterized by high IFN-γ and IL-12 production, but these cytokines diminished by 6 weeks. This may result from up-regulation of TGF-β and other immunosuppressive cytokines [40], which inhibit IFN-γ-driven iNOS production [41]. These decreases in IFN-γ and IL-12 correlate with a TH2 response; M2 macrophages produce low IL-12 and high IL-10, which prevents T cells from secreting IFN-γ to perpetuate TH2 cytokine pathways [42].
Other infectious diseases that induce chronic inflammation also require induction of TH2 cytokines for disease progression or maintenance. High levels of IL-4 are found in patients with idiopathic pulmonary fibrosis, and secretion of IL-4 and IL-13 by T cells is required for fibroblast migration and proliferation and their subsequent differentiation into myofibroblasts. IFN-γ, in contrast, attenuates the fibrotic response and induces collagen degradation [43]. Loss of M2 macrophages during Leishmania major infection delayed disease progression and decreased arginase activity, which improved macrophage cytotoxic functions; up-regulation of IFN-γ is associated with resistance [44]. During schistosomiasis, M2 macrophages protect the host against organ injury and inflammation. Genetic ablation of IL-4Rα in myeloid cells increases classical mediators, including TH1 cytokines and iNOS; these mutant mice die during Schistosoma mansoni infection [45]. BDMCs become M1 + M2-polarized, indicating that macrophage development is markedly affected by systemic effects of S. mansoni [46], similar to what we observed during BHT-induced chronic inflammation and lung tumor development.
When cytokines were applied directly to the lung via microspray, BDMC activation mirrored that of the lung macrophages, indicating that cytokine signals in the lung are disseminated rapidly to the BM. BDMCs also adopt the same biochemical phenotype as lung macrophages in a mouse model of lung tumorigenesis, where BAL macrophage polarization precedes BDMC polarization by 1 week [8], and in the BHT model of chronic inflammation described herein. Six days after chronic BHT treatment, BDMCs were argIlowiNOShigh, but by Day 30, BDMCs became argIhighiNOShigh. We cannot rule out the possibility that systemic exposure to BHT results in the dual polarization of BAL macrophages and BDMCs; however, we have demonstrated previously that alveolar macrophages and BDMCs display similar polarization phenotypes in response to lung tumor formation [8], and BDMCs also become polarized in response to prostate tumor formation and tumor xenografts [47]. In contrast, BDMCs in M. tuberculosis-infected mice remain argIlowiNOSlow at all time-points examined, so that the alveolar macrophage response to TH1 and TH2 cytokines is strictly local and not systemic in the TB model. As BM cellularity and megakaryocyte levels changed in response to M. tuberculosis infection, systemic effects of infection occurred in other BM cell populations. In the TB model, lung macrophages may not signal BDMCs, as infected macrophages may be sequestered in the lung and nearby lymph nodes and cannot migrate to the BM to signal polarization changes. As alveolar macrophages in the tumor and BHT models are not infected with bacteria, they may be free to migrate throughout the body. Another possibility is that M. tuberculosis itself produces a signal that blocks lung-to-marrow signaling. Also, in contrast to BHT-induced chronic inflammation, where BHT metabolites are cleared rapidly from the body, and the animals recover eventually, inflammation persists in TB-infected mice. As this is a chronic condition, unlike direct instillation of cytokines and even the slow but eventual resolution of BHT-induced inflammation, the signaling from lung to marrow may be dampened as a result of the persistent infection. Finally, although a similar IFN-γ-to-IL-4 sequence of cytokine production occurs with BHT and M. tuberculosis infection, the profile of TH1 and TH2 cytokines produced in response to each agent may differ and differentially affect lung-to-BM signaling.
BDMC monocytes differentiate into macrophage precursors, which become resident tissue macrophages or inflammatory macrophages [48]. Our data suggest that polarization of these precursor cells may influence monocyte differentiation or only occur in certain monocyte precursor populations. As spleen and liver macrophage polarization does not correspond to that of lung macrophages, BDMC cells predestined to become inflammatory instead of resident macrophages may be polarized prior to migrating to the lung and maturing into macrophages. Whether mature macrophages derived from these precursor cells can shift between activation states in vivo is not known but is an intriguing question.
Although BDMCs were not polarized in the TB model, M. tuberculosis infection in humans is associated with hematological abnormalities, including anemia, leukopenia, and thrombocytopenia [49]. Our histological examination of BM showed a significant decrease in all leukocytes 7 days after bacterial challenge and recovery by Day 21. Megakaryocytes in the BM, however, increased throughout disease progression. Increased numbers and sizes of megakaryocytes are associated with thrombocytopenia purpura in TB patients with a compensatory response to the premature destruction of platelets by immune targeting [49, 50]. Analyses of BM changes in experimental models of TB have been conducted, and a mechanism involving GM-CSF as mediating the increased production of megakaryocytes has been postulated [25]. Systemic effects on macrophage polarization were observed in the liver of M. tuberculosis-infected mice but not in BDMCs, indicating that although infection can elicit changes in pulmonary macrophage/monocyte polarization and BM cellularity, BDMC polarization did not occur in this model.
In summary, activated BDMCs circulate during lung cancer progression and following chronic dosing with BHT but not when M. tuberculosis infection has caused granulomatous disease. The presence of these polarized macrophages in the circulation is not therefore diagnostic of all chronic inflammatory pulmonary conditions. When they are present systemically, identification of those signals or cells mediating communication between the lungs and BM may indicate novel therapeutic approaches aimed at blocking their production or signaling actions.
AUTHORSHIP
Elizabeth F. Redente: first author, designed and performed BHT and TB experiments; David M. Higgins: designed and performed TB experiments; Lori D. Dwyer-Nield: corresponding author, performed BHT experiments, data interpretation, and manuscript preparation; Ian M. Orme: principal investigator of TB lab; Mercedes Gonzalez-Juarrero: design of TB experiments, data interpretation, and manuscript preparation; and Alvin M. Malkinson: principal investigator.
ACKNOWLEDGMENT
This work was supported by National Institutes of Health grants CA33497, CA132552, and AI44072.
Supplementary Material
Footnotes
Abbreviations: BALF=bronchoalveolar lavage fluid, BDMC=bone marrow-derived monocyte, BHT=butylated hydroxytoluene, BM=bone marrow, IHC=immunohistochemistry, iNOS=inducible NO synthase, TB=tuberculosis
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
References
- Kobzik L, Bredt D S, Lowenstein C J, Drazen J, Gaston B, Sugarbaker D, Stamler J S. Nitric oxide synthase in human and rat lung: immunocytochemical and histochemical localization. Am J Respir Cell Mol Biol. 1993;9:371–377. doi: 10.1165/ajrcmb/9.4.371. [DOI] [PubMed] [Google Scholar]
- American Lung Association Lung Disease Data2006. 2006:1–97. lungusa.org. [Google Scholar]
- Mills C D, Kincaid K, Alt J M, Heilman M J, Hill A M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Stout R D, Suttles J. Immunosenescence and macrophage functional plasticity: dysregulation of macrophage function by age-associated microenvironmental changes. Immunol Rev. 2005;205:60–71. doi: 10.1111/j.0105-2896.2005.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski N. Microarray analysis of idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2003;29:S32–S36. [PubMed] [Google Scholar]
- Kunkel S L, Chensue S W, Lukacs N, Hogaboam C. Cytokine phenotypes serve as a paradigms for experimental immune-mediated lung diseases and remodeling. Am J Respir Cell Mol Biol. 2003;29:S63–S66. [PubMed] [Google Scholar]
- Redente E F, Orlicky D J, Bouchard R J, Malkinson A M. Tumor signaling to the bone marrow changes the phenotype of monocytes and pulmonary macrophages during urethane-induced primary lung tumorigenesis in A/J mice. Am J Pathol. 2007;170:693–708. doi: 10.2353/ajpath.2007.060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Stout R D, Jiang C, Matta B, Tietzel I, Watkins S K, Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol. 2005;175:342–349. doi: 10.4049/jimmunol.175.1.342. [DOI] [PubMed] [Google Scholar]
- Goerdt S, Politz O, Schledzewski K, Birk R, Gratchev A, Guillot P, Hakiy N, Klemke C D, Dippel E, Kodelja V, Orfanos C E. Alternative versus classical activation of macrophages. Pathobiology. 1999;67:222–226. doi: 10.1159/000028096. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sozzani S, Vecchi A, Locati M, Sica A. Chemokines in the recruitment and shaping of the leukocyte infiltrate of tumors. Semin Cancer Biol. 2004;14:155–160. doi: 10.1016/j.semcancer.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Marino A A, Mitchell J T. Lung damage in mice following intraperitoneal injection of butylated hydroxytoluene. Proc Soc Exp Biol Med. 1972;140:122–125. doi: 10.3181/00379727-140-36407. [DOI] [PubMed] [Google Scholar]
- Adamson I Y, Bowden D H, Cote M G, Witschi H. Lung injury induced by butylated hydroxytoluene: cytodynamic and biochemical studies in mice. Lab Invest. 1977;36:26–32. [PubMed] [Google Scholar]
- Malkinson A M. Prevention of butylated hydroxytoluene-induced lung damage in mice by cedar terpene administration. Toxicol Appl Pharmacol. 1979;49:551–560. doi: 10.1016/0041-008x(79)90457-5. [DOI] [PubMed] [Google Scholar]
- Witschi H, Malkinson A M, Thompson J A. Metabolism and pulmonary toxicity of butylated hydroxytoluene. Gram T E, editor. Tarrytown, NY, USA: Pergamon; Metabolic Activation and Toxicity of Chemical Agents to Lung Tissue and Cells. 1993:185–212. [Google Scholar]
- Bauer A K, Dwyer-Nield L D, Keil K, Koski K, Malkinson A M. Butylated hydroxytoluene (BHT) induction of pulmonary inflammation: a role in tumor promotion. Exp Lung Res. 2001;27:197–216. doi: 10.1080/019021401300053948. [DOI] [PubMed] [Google Scholar]
- Miller A C, Dwyer L D, Auerbach C E, Miley F B, Dinsdale D, Malkinson A M. Strain-related differences in the pneumotoxic effects of chronically administered butylated hydroxytoluene on protein kinase C and calpain. Toxicology. 1994;90:141–159. doi: 10.1016/0300-483x(94)90212-7. [DOI] [PubMed] [Google Scholar]
- Thompson J A, Schullek K M, Fernandez C A, Malkinson A M. A metabolite of butylated hydroxytoluene with potent tumor-promoting activity in mouse lung. Carcinogenesis. 1989;10:773–775. doi: 10.1093/carcin/10.4.773. [DOI] [PubMed] [Google Scholar]
- Bauer A K, Dwyer-Nield L D, Hankin J A, Murphy R C, Malkinson A M. The lung tumor promoter, butylated hydroxytoluene (BHT), causes chronic inflammation in promotion-sensitive BALB/cByJ mice but not in promotion-resistant CXB4 mice. Toxicology. 2001;169:1–15. doi: 10.1016/s0300-483x(01)00475-9. [DOI] [PubMed] [Google Scholar]
- Dharmadhikari A S, Nardell E A. What animal models teach humans about tuberculosis. Am J Respir Cell Mol Biol. 2008;39:503–508. doi: 10.1165/rcmb.2008-0154TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluger N W, Rom W N. The host immune response to tuberculosis. Am J Respir Crit Care Med. 1998;157:679–691. doi: 10.1164/ajrccm.157.3.9708002. [DOI] [PubMed] [Google Scholar]
- Dannenberg A M. Pathophysiology: basic aspects. Schlossberg D, editor. Philadelphia, PA, USA: W.B. Saunders Co.; Tuberculosis and Nontuberculosis Mycobacterial Infections. 1992:17–47. [Google Scholar]
- Cox J S, Chen B, McNeil M, Jacobs W R., Jr Complex lipid determines tissue-specific replication of Mycobacterium tuberculosis in mice. Nature. 1999;402:79–83. doi: 10.1038/47042. [DOI] [PubMed] [Google Scholar]
- Higgins D M, Sanchez-Campillo J, Rosas-Taraco A G, Higgins J R, Lee E J, Orme I M, Gonzalez-Juarrero M. Relative levels of M-CSF and GM-CSF influence the specific generation of macrophage populations during infection with Mycobacterium tuberculosis. J Immunol. 2008;180:4892–4900. doi: 10.4049/jimmunol.180.7.4892. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Juarrero M, Hattle J M, Izzo A, Junqueira-Kipnis A P, Shim T S, Trapnell B C, Cooper A M, Orme I M. Disruption of granulocyte macrophage-colony stimulating factor production in the lungs severely affects the ability of mice to control Mycobacterium tuberculosis infection. J Leukoc Biol. 2005;77:914–922. doi: 10.1189/jlb.1204723. [DOI] [PubMed] [Google Scholar]
- Rhoades E R, Frank A A, Orme I M. Progression of chronic pulmonary tuberculosis in mice aerogenically infected with virulent Mycobacterium tuberculosis. Tuber Lung Dis. 1997;78:57–66. doi: 10.1016/s0962-8479(97)90016-2. [DOI] [PubMed] [Google Scholar]
- Orme I M. The immunopathogenesis of tuberculosis: a new working hypothesis. Trends Microbiol. 1998;6:94–97. doi: 10.1016/s0966-842x(98)01209-8. [DOI] [PubMed] [Google Scholar]
- Turgeon M L. Philadelphia, PA, USA: Lippincott Williams and Wilkins; Hematopoiesis. Clinical Hematology: Theory and Procedures. 1999:49–57. [Google Scholar]
- Adams L B, Mason C M, Kolls J K, Scollard D, Krahenbuhl J L, Nelson S. Exacerbation of acute and chronic murine tuberculosis by administration of a tumor necrosis factor receptor-expressing adenovirus. J Infect Dis. 1995;171:400–405. doi: 10.1093/infdis/171.2.400. [DOI] [PubMed] [Google Scholar]
- Orme I M, Collins F M. Mouse model of tuberculosis. Bloom B R, editor. Washington, DC, USA: ASM; TuberculosisPathogenesis, Protection, and Control. 1994:113–133. [Google Scholar]
- Orme I M, Roberts A D, Griffin J P, Abrams J S. Cytokine secretion by CD4 T lymphocytes acquired in response to Mycobacterium tuberculosis infection. J Immunol. 1993;151:518–525. [PubMed] [Google Scholar]
- D'Ambrosio D, Mariani M, Panina-Bordignon P, Sinigaglia F. Chemokines and their receptors guiding T lymphocyte recruitment in lung inflammation. Am J Respir Crit Care Med. 2001;164:1266–1275. doi: 10.1164/ajrccm.164.7.2103011. [DOI] [PubMed] [Google Scholar]
- Hussell T, Isaacson P G, Crabtree J E, Spencer J. Helicobacter pylori-specific tumor-infiltrating T cells provide contact dependent help for the growth of malignant B cells in low-grade gastric lymphoma of mucosa-associated lymphoid tissue. J Pathol. 1996;178:122–127. doi: 10.1002/(SICI)1096-9896(199602)178:2<122::AID-PATH486>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Greiner A, Knorr C, Qin Y, Schultz A, Marx A, Kroczek R A, Muller-Hermelink H K. CD40 ligand and autoantigen are involved in the pathogenesis of low-grade B-cell lymphomas of mucosa-associated lymphoid tissue. Dev Immunol. 1998;6:187–195. doi: 10.1155/1998/18679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortijo J, Sanz M J, Iranzo A, Montesinos J L, Nabah Y N, Alfon J, Gomez L A, Merlos M, Morcillo E J. A small molecule, orally active, α4β1/α4β7 dual antagonist reduces leukocyte infiltration and airway hyper-responsiveness in an experimental model of allergic asthma in Brown Norway rats. Br J Pharmacol. 2006;147:661–670. doi: 10.1038/sj.bjp.0706658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J M, Ramakrishnan L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell. 2009;136:37–49. doi: 10.1016/j.cell.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordway D, Harton M, Henao-Tamayo M, Montoya R, Orme I M, Gonzalez-Juarrero M. Enhanced macrophage activity in granulomatous lesions of immune mice challenged with Mycobacterium tuberculosis. J Immunol. 2006;176:4931–4939. doi: 10.4049/jimmunol.176.8.4931. [DOI] [PubMed] [Google Scholar]
- D'Souza C D, Cooper A M, Frank A A, Ehlers S, Turner J, Bendelac A, Orme I M. A novel nonclassic β2-microglobulin-restricted mechanism influencing early lymphocyte accumulation and subsequent resistance to tuberculosis in the lung. Am J Respir Cell Mol Biol. 2000;23:188–193. doi: 10.1165/ajrcmb.23.2.4063. [DOI] [PubMed] [Google Scholar]
- Ly L H, Russell M I, McMurray D N. Microdissection of the cytokine milieu of pulmonary granulomas from tuberculous guinea pigs. Cell Microbiol. 2007;9:1127–1136. doi: 10.1111/j.1462-5822.2006.00854.x. [DOI] [PubMed] [Google Scholar]
- Hernández-Pando R, Orozco-Esteves H, Maldonado H A, Aguilar-León D, Vilchis-Landeros M M, Mata-Espinosa D A, Mendoza V, López-Casillas F. A combination of a transforming growth factor-β antagonist and an inhibitor of cyclooxygenase is an effective treatment for murine pulmonary tuberculosis. Clin Exp Immunol. 2006;144:264–272. doi: 10.1111/j.1365-2249.2006.03049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi M, Alard P, Streilein J W. TGF-β promotes immune deviation by altering accessory signals of antigen-presenting cells. J Immunol. 1998;160:1589–1597. [PubMed] [Google Scholar]
- Wynn T A. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4:583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holscher C, Arendse B, Schwegmann A, Myburgh E, Brombacher F. Impairment of alternative macrophage activation delays cutaneous leishmaniasis in nonhealing BALB/c mice. J Immunol. 2006;176:1115–1121. doi: 10.4049/jimmunol.176.2.1115. [DOI] [PubMed] [Google Scholar]
- Herbert D R, Holscher C, Mohrs M, Arendse B, Schwegmann A, Radwanska M, Leeto M, Kirsch R, Hall P, Mossmann H, Claussen B, Forster I, Brombacher F. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 2004;20:623–635. doi: 10.1016/s1074-7613(04)00107-4. [DOI] [PubMed] [Google Scholar]
- Joshi A D, Raymond T, Coelho A L, Kunkel S L, Hogaboam C M. A systemic granulomatous response to Schistosoma mansoni eggs alters responsiveness of bone-marrow-derived macrophages to Toll-like receptor agonists. J Leukoc Biol. 2008;83:314–324. doi: 10.1189/jlb.1007689. [DOI] [PubMed] [Google Scholar]
- Redente E F, Dwyer-Nield L D, Merrick D T, Raina K, Agarwal R, Pao W, Rice P L, Shroyer K R, Malkinson A M. Tumor progression stage and anatomical site regulate tumor-associated macrophage and bone marrow-derived monocyte polarization. Am J Pathol. 2010 doi: 10.2353/ajpath.2010.090879. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C, Sieweke M H, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy S, Yadav S. Immune thrombocytopenic purpura as a presentation of childhood tuberculosis. Indian J Pediatr. 2007;74:853–855. doi: 10.1007/s12098-007-0152-5. [DOI] [PubMed] [Google Scholar]
- Talbot S, Dowling A, Dowling J P, Fuller A, Schwarz M. Mediastinal nodal tuberculosis presenting as immune thrombocytopenia. Aust N Z J Med. 1998;28:465–466. doi: 10.1111/j.1445-5994.1998.tb02084.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.