Abstract
Case-control studies generally suggesting an inverse association between sun exposure and non-Hodgkin lymphoma (NHL) have led to speculation that vitamin D may protect against lymphomagenesis. To examine this hypothesis, the authors conducted a pooled investigation of circulating 25-hydroxyvitamin D (25(OH)D) and subsequent NHL risk within 10 cohorts participating in the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. The authors analyzed measurements from 1,353 cases and 1,778 controls using conditional logistic regression and other methods to estimate the association of 25(OH)D with NHL. No clear evidence of association between categories of 25(OH)D concentration and NHL was observed overall (Ptrend = 0.68) or by sex (men, Ptrend = 0.50; women, Ptrend = 0.16). Findings for other measures (continuous log(25(OH)D), categories of 25(OH)D using sex-/cohort-/season-specific quartiles as cutpoints, categories of season-adjusted residuals of predicted 25(OH)D using quartiles as cutpoints) were generally null, although some measures of increasing 25(OH)D were suggestive of an increased risk for women. Results from stratified analyses and investigations of histologic subtypes of NHL were also null. These findings do not support the hypothesis that elevated circulating 25(OH)D concentration is associated with a reduced risk of NHL. Future research investigating the biologic basis for the sunlight–NHL association should consider alternative mechanisms, such as immunologic effects.
Keywords: calcifediol; calcitrol; case-control studies; cohort studies; 25-hydroxyvitamin D 2; lymphoma, non-Hodgkin; prospective studies; vitamin D
Epidemiologic findings generally suggest that exposure to solar ultraviolet radiation may be associated with a reduced risk of non-Hodgkin lymphoma (NHL) (1). Increasing ambient ultraviolet radiation levels (or, as a proxy, decreasing latitude) have been associated with decreasing NHL incidence or mortality rates in the United States and some parts of Europe (2–5), although conflicting ecologic findings have also been reported (6–8). Several case-control studies from Australia, Europe, and the United States have also observed decreasing risks of NHL with increasing self-reported lifetime sun exposure (9–14), although an association with increased NHL risk was observed in a US population-based case-control study (15). In a pooled analysis involving 10 case-control studies participating in the International Lymphoma Epidemiology (InterLymph) Consortium, a reduction in NHL risk was observed for high recreational sun exposure at 18–40 years of age and in the 10 years before diagnosis for B-cell, but not T-cell, lymphomas (16). The pooled findings demonstrated a dose-response relation with amount of recreational ultraviolet radiation exposure; however, the magnitude of the association was weak, with a 25% reduction in risk for the highest quartile of exposure versus the lowest.
A plausible mechanism postulated for the observed protective association between ultraviolet radiation exposure and NHL risk is that of ultraviolet radiation–induced subcutaneous synthesis of vitamin D, a steroid prohormone with antineoplastic properties (17). The vitamin D receptor, through which vitamin D effects are mediated, is expressed in activated B- and T-lymphocytes (18, 19). Lymphocytes also express 1α-hydroxylase, the enzyme responsible for converting the major circulating form of vitamin D (25-hydroxyvitamin D (25(OH)D)) into its bioactive metabolite (1,25-dihydrovyvitamin D) (20). Vitamin D has been reported to inhibit proliferation and induce differentiation in lymphocytes (19) and lymphoma cell lines (21). In an early-phase clinical trial of the vitamin D analog alpha-calcidol, 24% of 34 treated patients with progressive low-grade lymphoma experienced tumor regression (22).
Prospective cohorts with biospecimen banks enable investigation of prediagnostic circulating 25(OH)D concentrations in people subsequently diagnosed with NHL relative to their peers. In the only such study published to date to our knowledge, serum concentrations of 25(OH)D were associated with a decreased risk of NHL diagnosed within the first 7 years of blood collection, but not thereafter, in a cohort of male Finnish smokers (23). In light of the study's small size and unique population, there is a clear need for additional epidemiologic studies to address the hypothesis that the vitamin D pathway inhibits lymphomagenesis. Ideally, such studies would be large enough to enable exploration of possible heterogeneity in 25(OH)D effects across NHL histologic subtypes and draw from populations spanning a wide range of latitudes to ensure broad variation in 25(OH)D levels.
With this goal in mind, the Vitamin D Pooling Project of Rarer Cancers (VDPP) was developed within the Cohort Consortium sponsored by the National Cancer Institute to examine the relation between circulating 25(OH)D levels and risk of NHL and other rarer malignancies (cancers of the endometrium, ovary, kidney, pancreas, and upper gastrointestinal tract). This article reports findings from the pooled investigation of NHL, which included 1,353 cases and 1,778 controls selected from 10 cohort studies conducted in the United States (n = 7), Finland (n = 1), and Shanghai, China (n = 2).
MATERIALS AND METHODS
Study design and population
A detailed description of the design of the VDPP is provided elsewhere in this issue of the Journal (24). Ten participating cohorts contributed plasma or serum toward the pooled investigation of NHL (Table 1). A nested case-control design was used, and a sample of subjects with stored serum or plasma specimens who were cancer free at the time of blood collection was selected from each cohort.
Table 1.
Characteristics of Participants, by Cohort, in the Investigation of Non-Hodgkin Lymphoma Within the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers
| Cohort | No. of Cases | No. of Controls | Median Years From Blood Collection to Cancer Diagnosis (Interquartile Range) | Median Circulating 25(OH)D, nmol/L (Interquartile Range) |
|
| Cases | Controls | ||||
| ATBC | 208 | 531 | 7.5 (4.2–10.9) | 46.1 (35.3–62.9) | 48.0 (35.3–64.5) |
| CLUE | 236 | 252 | 10.4 (6.0–14.5) | 60.2 (45.1–78.0) | 57.3 (43.8–73.7) |
| CPS-II | 135 | 141 | 2.5 (1.4–3.6) | 58.7 (46.6–76.4) | 60.3 (49.0–78.4) |
| HPFS | 133 | 147 | 4.4 (2.6–6.7) | 58.7 (44.8–70.3) | 58.6 (45.8–76.6) |
| MEC | 96 | 101 | 1.9 (0.7–3.3) | 55.4 (40.2–67.7) | 52.4 (40.3–66.0) |
| NHS | 145 | 145 | 5.8 (4.4–8.6) | 55.3 (38.5–72.2) | 56.3 (45.0–70.5) |
| NYU-WHS | 73 | 101 | 9.2 (5.9–12.6) | 49.5 (37.1–63.6) | 51.2 (34.9–65.4) |
| PLCO | 286 | 296 | 5.0 (2.8–7.0) | 56.0 (44.3–68.4) | 53.8 (40.1–65.6) |
| SMHS | 8 | 12 | 1.5 (0.7–2.3) | 46.8 (32.0–57.1) | 39.4 (22.5–65.7) |
| SWHS | 33 | 52 | 3.5 (1.0–6.7) | 32.2 (22.5–45.9) | 30.7 (23.0–49.2) |
| Total | 1,353 | 1,778 | 5.2 (2.5–8.7) | 55.7 (40.6–70.1) | 53.5 (38.8–68.8) |
Abbreviations: ATBC, Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; CPS-II, Cancer Prevention Study II Nutrition Cohort; HPFS, Health Professionals Follow-Up Study; MEC, The Multiethnic Cohort Study; NHS, Nurses’ Health Study; NYU-WHS, New York University Women's Health Study; 25(OH)D, 25-hydroxyvitamin D; PLCO, Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial; SMHS, Shanghai Men's Health Study; SWHS, Shanghai Women's Health Study.
Incident cases included NHL (International Classification of Diseases for Oncology, Second Edition (ICD-O-2) codes 9591–9593, 9595, 9670–9677, 9680–9688, 9690–9698, 9700–9717, 9760–9762, 9764, 9821, 9823, 9825–9828, 9940, 9941; International Classification of Diseases for Oncology, Third Edition (ICD-O-3) codes 9591–9593, 9595, 9596, 9670, 9671, 9673, 9675, 9678–9680, 9684, 9687, 9689–9691, 9695, 9698–9702, 9705, 9708, 9709, 9714, 9716–9719, 9727–9729, 9761, 9762, 9823, 9825, 9827, 9831–9837, 9940, 9948) and other rare or unclassified lymphoid malignancies (ICD-O-2 codes 9590, 9594, 9731, 9820, 9822, 9824, 9850; ICD-O-3 codes 9590, 9594, 9731, 9734, 9820, 9970). Cases of Hodgkin lymphoma (ICD-O-2 and ICD-O-3 codes 9650–9667) were also included by the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study (ATBC) and cases of multiple myeloma (ICD-O-2 code 9732; ICD-O-3 codes 9732, 9733) by ATBC and the New York University Women's Health Study. To focus on NHL as an endpoint, non-NHL cases have been excluded from the final results presented here; however, their inclusion yielded virtually identical findings. The median time from blood collection to diagnosis of NHL cases was 5.2 years, with a range of <0.1–31.6 years.
Controls were individually matched to cases (1:1 ratio) from among individuals who were alive and cancer free at the time of case diagnosis; matching was performed on the basis of age at blood collection (±1 year), sex, race/ethnicity (white, black, Asian, other), and date of blood draw (±30 days). The Shanghai Women's Health Study additionally matched on menopausal status for women. Different control selection schemes were used by 2 cohorts whose study samples were drawn prior to VDPP. Within ATBC, 2 controls were matched to each case by birth year and month of baseline blood draw from among individuals who were lymphoma free at case diagnosis. In the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial, one control was matched to each case by age at baseline (5-year categories), sex, race, and date of baseline blood draw (3-month categories) from among individuals who were cancer free at case diagnosis.
Measurement of circulating 25(OH)D
Concentrations of 25(OH)D in 125-μL samples of serum or plasma were measured at Heartland Assays, Inc. (Ames, Iowa) using a direct, competitive chemiluminescence immunoassay (DiaSorin LIAISON 25 OH Vitamin D TOTAL assay; DiaSorin Inc., Stillwater, Minnesota) (25). Assays were performed in cohort-specific batches of as many as 100 samples, with several types of masked reference samples used to assess quality control. Each cohort was provided with samples of the vitamin D standard (Standard Reference Material 972 Vitamin D in Human Serum) from the National Institute of Standards and Technology (NIST) at both level 1 (∼60 nmol/L) and level 2 (∼35 nmol/L) to include with their samples, as described by Gallicchio et al. (24). In addition, 2 or 4 masked quality control samples from each cohort were included in each batch of their samples.
Coefficients of variation for duplicate serum/plasma aliquots included in all laboratory sample batches were calculated by using the variance components model of SAS software (PROC VARCOMP; SAS Institute, Inc., Cary, North Carolina) for the 3 types of quality control samples: NIST level 1, NIST level 2, and each cohort quality control. Interbatch and intrabatch coefficients of variation for NIST level 1 samples were 12.7% and 9.3%, respectively; interbatch and intrabatch coefficients of variation for NIST level 2 samples were 13.6% and 11.0%, respectively. The median interbatch coefficient of variation for the cohort quality control samples was 13.2% (range: 4.8–17.0); the median intrabatch coefficient of variation for the cohort quality control samples was 9.9% (range: 3.8–16.4). The majority of ATBC samples (189 of 208 cases, 506 of 531 controls) were assayed prior to VDPP by the same laboratory (interbatch coefficient of variation, 14.0%).
Statistical analysis
Statistical analyses were performed by using SAS software, versions 9.1.3 and 9.2 (SAS Institute, Inc.). The analytic strategy for VDPP is outlined in greater detail elsewhere (24). The distributions of several subject characteristics were compared between NHL cases and their matched controls by using Wald test statistics computed from simple conditional logistic regression models; this approach was used in favor of bivariate statistics (e.g., Pearson chi-square and Wilcoxon rank-sum tests) to account for the difference in case:control ratio between ATBC (1:2) and the other cohorts (1:1). All reported P values are 2-sided.
For the main analysis, 25(OH)D was analyzed as a categorical variable, with measurements divided into 6 categories by using clinically relevant cutpoints (<25, 25–<37.5, 37.5–<50, 50–<75, 75–<100, and ≥100 nmol/L) defined following a review of the literature (26–28). Analyses were also conducted by using the natural log of 25(OH)D as a continuous variable; the log-transformation was performed because of the right-skewed nature of the original 25(OH)D distribution. Two additional approaches for categorizing 25(OH)D were used to adjust for the known seasonal variation in 25(OH)D. In the first method, 25(OH)D measurements were categorized on the basis of their distribution within a particular stratum of cohort, sex, and season of blood collection, with stratum-specific quartiles among VDPP controls used as cutpoints. (A similar approach involving only sex- and season-specific strata yielded virtually identical results, which are not presented in this paper.) In the second method, the residuals were calculated from locally weighted scatter plot smoothing (loess) models regressing 25(OH)D onto week of blood draw stratified by gender and cohort (24). The residuals were categorized into quartiles based on the distributions within each sex- and cohort-specific stratum.
The association between 25(OH)D variables and NHL risk within the combined data set was evaluated by computing odds ratios and 95% confidence intervals with conditional logistic regression. Tests for trend of the categorical 25(OH)D variables were performed by using a Wald test statistic after assigning ordinal scores (0, 1, 2, 3, …) to the categories and modeling the variable as a continuous parameter. Alcohol consumption, education, body mass index, height, and hormone replacement therapy were assessed as potential confounding factors. Additional model adjustment for height (≤165, >165–171, >171–178, and >178 cm) was found to change odds ratio magnitudes for some 25(OH)D categories by more than 10%; consequently, height was included as a covariate in the reported results. Study-specific odds ratio estimates for the 25(OH)D categories of <25 nmol/L and ≥100 nmol/L (vs. 50–75 nmol/L) were summarized by using forest plots. Included in each forest plot was a summary odds ratio estimate calculated by meta-analysis using a random-effects model and a test of between-study odds ratio heterogeneity using the Q statistic. Forest plots and meta-analyses were conducted by using the R function MiMa (29). Sensitivity analyses were performed by excluding each study from the analysis to assess the extent to which the pooled results were influenced by each individual study.
Stratified analyses were conducted for several factors: sex, age (<60 years, ≥60 years), season of blood collection (June 1–November 30, December 1–May 31), latitude (<35°N, 35°–42°N, >42°N), body mass index (<25, 25–<30, ≥30 kg/m2), use of calcium supplements (no current use, current use), physical activity (sedentary, light, moderate, vigorous), female hormone replacement therapy use at questionnaire administration (yes, no), and length of follow-up until case diagnosis (<5 years, ≥5 years). Analyses restricted to white subjects only and to US cohorts only were also performed. Conditional logistic regression models were used when the matched sets were retained after stratification (sex, length of follow-up until case diagnosis, restriction to white subjects, restriction to US cohorts). Unconditional regression models, additionally adjusted for cohort, age, sex, race, and season of blood collection, were used for the remaining stratified analyses because odds ratio estimates were essentially identical to those generated from conditional models, but 95% confidence intervals were narrower because more case-control matches were retained. Tests of interaction between 25(OH)D measures and each stratification factor were performed by using the likelihood ratio test.
Analyses for specific histologically defined NHL subtypes were also conducted (diffuse large B-cell lymphoma (DLBCL), follicular lymphoma, chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), other/not otherwise specified). Polytomous regression was used to explore possible heterogeneity in the effect of 25(OH)D on the common subtypes.
RESULTS
The distributions of selected characteristics among cases and controls are summarized in Table 2. The majority of subjects were white (88% of cases, 88% of controls), with a slight excess of men compared with women (men: 56% of cases, 63% of controls; the difference between cases and controls is due to the 2:1 matching ratio in ATBC). The median age at blood collection was 61 years (62 years for cases, 61 years for controls), with blood collection dates distributed evenly across winter, spring, summer, and fall. Cases and controls were generally comparable with respect to these and other characteristics, with the exception of height, for which a small, but statistically significant difference was reported (median: cases, 170.2 cm; controls, 171.0 cm; P = 0.03). Twenty-five percent of cases of disease were histologically classified as DLBCL, 15% as follicular lymphoma, 30% as CLL/SLL, and 30% as other or not otherwise specified histology.
Table 2.
Selected Characteristics of Cases and Controls in the Investigation of Non-Hodgkin Lymphoma Within the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers
| Characteristic | Cases (N = 1,353) |
Controls (N = 1,778)a |
P Valueb | ||||
| No. | % | Median (Interquartile Range) | No. | % | Median (Interquartile Range) | ||
| Age at blood collection, years | 62 (56–68) | 61 (55–67) | Matched | ||||
| Sex | Matched | ||||||
| Male | 762 | 56.3 | 1,127 | 63.4 | |||
| Female | 591 | 43.7 | 651 | 36.6 | |||
| Race | Matched | ||||||
| White | 1,191 | 88.0 | 1,572 | 88.4 | |||
| Black | 38 | 2.8 | 50 | 2.8 | |||
| Asian | 72 | 5.3 | 99 | 5.6 | |||
| Other | 42 | 3.1 | 41 | 2.3 | |||
| Missing | 10 | 0.7 | 16 | 0.9 | |||
| Season of blood collection | Matched | ||||||
| Winter | 289 | 21.4 | 441 | 24.8 | |||
| Spring | 327 | 24.2 | 420 | 23.6 | |||
| Summer | 384 | 28.4 | 452 | 25.4 | |||
| Fall | 353 | 26.1 | 465 | 26.2 | |||
| Height, cm | 170.2 (163.0–177.8) | 171.0 (165.0–177.8) | 0.03 | ||||
| Body mass index, kg/m2 | 0.29 | ||||||
| <25 | 523 | 38.7 | 699 | 39.3 | |||
| 25–<30 | 484 | 35.8 | 696 | 39.1 | |||
| ≥30 | 232 | 17.1 | 252 | 14.2 | |||
| Missing | 114 | 8.4 | 131 | 7.4 | |||
| Education | 0.29 | ||||||
| Less than high school | 215 | 15.9 | 359 | 20.2 | |||
| Completed high school | 221 | 16.3 | 267 | 15.0 | |||
| Vocational school | 134 | 9.9 | 276 | 15.5 | |||
| Some college | 317 | 23.4 | 400 | 22.5 | |||
| College graduate | 167 | 12.3 | 177 | 10.0 | |||
| Graduate studies | 282 | 20.8 | 284 | 16.0 | |||
| Missing | 17 | 1.3 | 15 | 0.8 | |||
| Alcohol consumption, g ethanol/day | 1.9 (0–11.7) | 2.8 (0–14.6) | 0.69 | ||||
| Dietary energy intake, kcal/day | 1,828 (1,441–2,436) | 1,992 (1,510–2,605) | 0.82 | ||||
| Energy-adjusted dietary vitamin D, IU/day | 188 (127–288) | 178 (125–263) | 0.43 | ||||
| Histologic subtype | |||||||
| DLBCL | 344 | 25.4 | |||||
| FL | 206 | 15.2 | |||||
| CLL/SLL | 401 | 29.6 | |||||
| Other/NOS | 402 | 29.7 | |||||
| Circulating 25(OH)D, nmol/L | 55.7 (40.6–70.1) | 53.5 (38.8–68.8) | 0.65 | ||||
Abbreviations: CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; NOS, not otherwise specified; 25(OH)D, 25-hydroxyvitamin D.
The number of controls is larger than the number of non-Hodgkin lymphoma cases because 1) 1 cohort (the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study (ATBC)) selected 2 controls per case, and 2) 2 cohorts (ATBC and the New York University Women's Health Study) included a small number of lymphoma cases other than non-Hodgkin lymphoma, which were later excluded from analysis.
Test of independence between variable and case-control status; performed by using the Wald test from conditional logistic regression.
The median concentrations of measured 25(OH)D among the cases and controls were 55.7 nmol/L and 53.5 nmol/L, respectively (P = 0.65). Table 3 summarizes findings from conditional models regressing case-control status on categorized circulating 25(OH)D, additionally adjusted for height. No association was observed between categories of 25(OH)D concentration and NHL risk (Ptrend = 0.68). Analyses of log(25(OH)D) as a continuous variable, 25(OH)D categories using sex-/cohort-/season-specific cutpoints, and categories of residuals of predicted 25(OH)D were similarly null for overall models (Table 4).
Table 3.
Odds Ratios and 95% Confidence Intervals for the Association Between Circulating 25(OH)D and Risk of Non-Hodgkin Lymphoma Within the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers: Results for Categories of 25(OH)D Using Clinically Relevant Cutpointsa
| Circulating 25(OH)D, nmol/L |
|||||||||||||||||||||||||
| <25 |
25–<37.5 |
37.5–<50 |
50–<75 |
75–<100 |
≥100 |
Ptrend | |||||||||||||||||||
| No. of Cases | No. of Controls | OR | 95% CI | No. of Cases | No. of Controls | OR | 95% CI | No. of Cases | No. of Controls | OR | 95% CI | No. of Cases | No. of Controls | OR | 95% CI | No. of Cases | No. of Controls | OR | 95% CI | No. of Cases | No. of Controls | OR | 95% CI | ||
| All subjectsb | 100 | 105 | 1.08 | 0.78, 1.50 | 154 | 203 | 0.92 | 0.71, 1.19 | 261 | 319 | 0.98 | 0.79, 1.21 | 505 | 567 | 1.0 | Referent | 204 | 198 | 1.15 | 0.91, 1.46 | 49 | 64 | 0.86 | 0.57, 1.27 | 0.68 |
| Menc | 50 | 55 | 1.21 | 0.78, 1.88 | 81 | 118 | 1.04 | 0.73, 1.47 | 151 | 207 | 0.97 | 0.74, 1.27 | 305 | 370 | 1.0 | Referent | 123 | 129 | 1.17 | 0.86, 1.59 | 23 | 44 | 0.65 | 0.38, 1.11 | 0.50 |
| Womenc | 50 | 50 | 0.92 | 0.56, 1.50 | 73 | 85 | 0.81 | 0.56, 1.18 | 110 | 112 | 0.98 | 0.70, 1.37 | 200 | 197 | 1.0 | Referent | 81 | 69 | 1.10 | 0.75, 1.61 | 26 | 20 | 1.25 | 0.67, 2.34 | 0.16 |
Abbreviations: CI, confidence interval; 25(OH)D, 25-hydroxyvitamin D; OR, odds ratio.
Conditional models were adjusted for height (≤165, >165–171, >171–178, >178 cm).
Does not include subjects with missing data for model covariate (height). Also does not include controls matched to lymphoma cases other than non-Hodgkin lymphoma, which were excluded from the analysis.
A test of interaction with sex was not statistically significant.
Table 4.
Odds Ratios and 95% Confidence Intervals for the Association Between Circulating 25(OH)D and Risk of Non-Hodgkin Lymphoma Within the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers: Results for Continuous 25(OH)D on a Log Scale, 25(OH)D Categories Using Stratum-specific Cutpoints, and Categories of Residuals From Modeled 25(OH)D
| Overall |
Mena |
Womena |
|||||||||||
| Measure | Quartile | No. of Cases | No. of Controlsb | ORc | 95% CI | No. of Cases | No. of Controls | OR | 95% CI | No. of Cases | No. of Controls | OR | 95% CI |
| Log(25(OH)D): per unit increase | 1.03 | 0.86, 1.24 | 0.88 | 0.69, 1.13 | 1.27 | 0.96, 1.68 | |||||||
| Sex-, cohort-, and season-specific cutpointsd | 1 | 289 | 364 | 1.00 | Referent | 170 | 225 | 1.00 | Referent | 119 | 139 | 1.00 | Referent |
| 2 | 301 | 357 | 1.11 | 0.89, 1.39 | 176 | 235 | 1.04 | 0.78, 1.38 | 125 | 122 | 1.23 | 0.87, 1.76 | |
| 3 | 335 | 367 | 1.19 | 0.95, 1.48 | 209 | 238 | 1.17 | 0.89, 1.56 | 126 | 129 | 1.20 | 0.84, 1.72 | |
| 4 | 348 | 368 | 1.21 | 0.97, 1.51 | 178 | 225 | 1.07 | 0.80, 1.43 | 170 | 143 | 1.43 | 1.01, 2.02 | |
| Ptrend | 0.08 | 0.49 | 0.06 | ||||||||||
| Residuals from modeled 25(OH)De | 1 | 295 | 370 | 1.00 | Referent | 172 | 232 | 1.00 | Referent | 123 | 138 | 1.00 | Referent |
| 2 | 306 | 375 | 1.06 | 0.85, 1.33 | 186 | 240 | 1.07 | 0.81, 1.41 | 120 | 135 | 1.06 | 0.74, 1.52 | |
| 3 | 332 | 342 | 1.24 | 1.00, 1.55 | 206 | 225 | 1.23 | 0.93, 1.63 | 126 | 117 | 1.27 | 0.88, 1.82 | |
| 4 | 340 | 369 | 1.16 | 0.93, 1.45 | 169 | 226 | 1.01 | 0.75, 1.35 | 171 | 143 | 1.41 | 1.00, 2.00 | |
| Ptrend | 0.09 | 0.66 | 0.03 | ||||||||||
Abbreviations: CI, confidence interval; 25(OH)D, 25-hydroxyvitamin D; OR, odds ratio.
Tests of interaction with sex were not statistically significant.
Does not include subjects with missing data for model covariate (height). Also does not include controls matched to lymphoma cases other than non-Hodgkin lymphoma, which were excluded from the analysis.
Conditional models were adjusted for height (≤165, >165–171, >171–178, >178 cm).
Cutpoints defined as quartiles among all controls within a given stratum based on sex, cohort, and season of blood collection.
Residuals from predicted circulating 25(OH)D computed by linear regression with adjustment for cohort, sex, and season of blood draw. Cutpoints were defined based on sex and cohort-specific quartiles among controls.
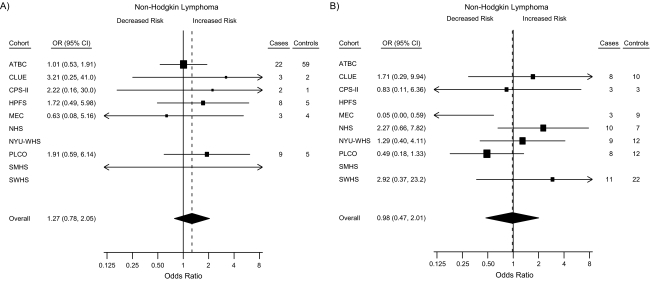
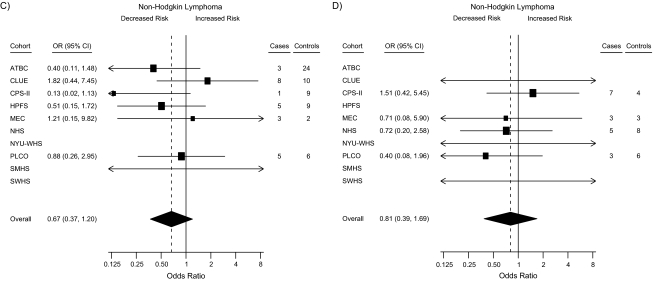
In analyses stratified by sex, categories of 25(OH)D concentration were not associated with NHL among men (Ptrend = 0.50), although the calculated odds ratio for men in the ≥100 nmol/L (vs. 50–<75 nmol/L) category was suggestive of an association with reduced risk (odds ratio = 0.65, 95% confidence interval: 0.38, 1.11; Table 3). When this category was further subdivided by using an a priori cutpoint of 120 nmol/L, the inverse association was stronger for the higher subcategory but was not statistically significant (100–<120 nmol/L: odds ratio = 0.74, 95% confidence interval: 0.40, 1.36; ≥120 nmol/L: odds ratio = 0.41, 95% confidence interval: 0.13, 1.29). However, no such findings were observed among women (100–<120 nmol/L: odds ratio = 1.25, 95% confidence interval: 0.60, 2.61; ≥120 nmol/L: odds ratio = 1.25, 95% confidence interval: 0.43, 3.66). In fact, there was suggestive evidence of a weak increased risk of NHL with higher 25(OH)D among women from analyses of continuous log(25(OH)), 25(OH)D categories using sex-/cohort-/season-specific cutpoints, and residuals of predicted 25(OH)D (Table 4).
Forest plots summarizing the statistically stable study-specific odds ratios for <25 nmol/L and ≥100 nmol/L for men and women are shown in Figure 1. In sensitivity analyses excluding individual studies, in turn, from the pooled analysis, the apparent association with 25(OH)D among women weakened after excluding CLUE and the Cancer Prevention Study II Nutrition Cohort and strengthened after excluding the Nurses’ Health Study (results not shown).
Figure 1.
Forest plots for the meta-analysis of the association between circulating 25-hydroxyvitamin D (25(OH)D) and the risk of non-Hodgkin lymphoma within the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Risk estimates, by cohort, for subjects with circulating 25(OH)D concentrations of <25 nmol/L and ≥100 nmol/L compared with the referent group (50–<75 nmol/L). Results for <25 nmol/L for A) men and B) women; results for ≥100 nmol/L for C) men and D) women. Odds ratios (ORs; boxes) and 95% confidence intervals (CIs; bars) were derived from conditional logistic regression models adjusted for height. The size of each box is inversely proportional to the variance of the log odds ratio estimate in each cohort. The overall estimates (diamonds) were derived from a meta-analysis using random-effects modeling. Data from some cohorts are missing from some forest plots—A): Nurses’ Health Study (NHS), New York University Women's Health Study (NYU-WHS), Shanghai Women's Health Study (SWHS); B): Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study (ATBC), Health Professionals Follow-up Study (HPFS), Shanghai Men's Health Study (SMHS); C): NHS, NYU-WHS, SWHS; D): ATBC, HPFS, SMHS—because they are sex-specific or have unstable risk estimates due to small numbers. CPS-II, Cancer Prevention Study II Nutrition Cohort; MEC, Multiethnic Cohort Study; PLCO, Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial.
Stratified analyses were performed to investigate whether the association between circulating 25(OH)D concentration and NHL risk differed across levels of other participant characteristics; selected results are summarized in Table 5. No association between 25(OH)D and NHL risk was observed within study subgroups defined by season of blood draw, age at baseline, latitude of residence, length of follow-up, hormone replacement therapy use, calcium supplementation, and physical activity (results for the last 3 stratified analyses not shown). Results of analyses restricted to white subjects and to US cohorts were similarly null. In an attempt to replicate the previously reported inverse association between 25(OH)D and lymphoma risk observed in ATBC, analyses were conducted restricted to men and to the first 5 years of follow-up. No associations were observed in these analyses (all studies, Ptrend = 0.26; excluding ATBC, Ptrend = 0.63).
Table 5.
Odds Ratios and 95% Confidence Intervals for the Association Between Circulating 25(OH)D and Risk of Non-Hodgkin Lymphoma Within the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers: Results From Analyses Stratified by Selected Study and Subject Characteristicsa
| Circulating 25(OH)D, nmol/L (Categorized by Using a Priori Cutpoints) |
|||||||||||||||
| Stratification Factor | No. of Cases | No. of Controls | <25 |
25–<37.5 |
37.5–<50 |
50–<75 |
75–<100 |
≥100 |
Ptrend | ||||||
| ORb | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | ||||
| Season of blood drawc | |||||||||||||||
| December–May | 573 | 836 | 0.97 | 0.66, 1.42 | 0.82 | 0.59, 1.13 | 0.90 | 0.67, 1.20 | 1.00 | Referent | 0.83 | 0.57, 1.23 | 0.79 | 0.38, 1.61 | 0.94 |
| June–November | 689 | 831 | 1.59 | 0.91, 2.76 | 0.96 | 0.66, 1.39 | 1.05 | 0.79, 1.40 | 1.00 | Referent | 1.41 | 1.06, 1.89 | 0.86 | 0.54, 1.37 | 0.90 |
| Age, yearsc | |||||||||||||||
| ≤60 | 508 | 767 | 1.28 | 0.81, 2.03 | 0.80 | 0.55, 1.17 | 0.95 | 0.69, 1.31 | 1.00 | Referent | 1.30 | 0.91, 1.85 | 0.96 | 0.52, 1.76 | 0.47 |
| >60 | 754 | 900 | 1.08 | 0.71, 1.63 | 0.98 | 0.71, 1.36 | 1.01 | 0.78, 1.32 | 1.00 | Referent | 1.06 | 0.78, 1.44 | 0.73 | 0.44, 1.20 | 0.61 |
| Restricted to Caucasians | 1,100 | 1,464 | 1.31 | 0.91, 1.89 | 0.93 | 0.71, 1.22 | 0.93 | 0.74, 1.16 | 1.00 | Referent | 1.19 | 0.93, 1.53 | 0.78 | 0.51, 1.19 | 0.93 |
| Restricted to US cohorts | 1,104 | 1,183 | 1.16 | 0.77, 1.74 | 0.83 | 0.61, 1.13 | 0.90 | 0.71, 1.15 | 1.00 | Referent | 1.21 | 0.93, 1.58 | 0.94 | 0.61, 1.45 | 0.27 |
| Latitude (°N)c | |||||||||||||||
| <35 | 225 | 262 | 0.60 | 0.29, 1.24 | 0.66 | 0.35, 1.24 | 1.15 | 0.68, 1.96 | 1.00 | Referent | 0.99 | 0.57, 1.73 | 1.22 | 0.52, 2.89 | 0.13 |
| 35–42 | 600 | 595 | 1.74 | 1.03, 2.94 | 1.10 | 0.74, 1.62 | 0.96 | 0.70, 1.32 | 1.00 | Referent | 1.50 | 1.08, 2.09 | 0.99 | 0.55, 1.77 | 0.95 |
| >42 | 437 | 811 | 1.06 | 0.66, 1.69 | 0.80 | 0.55, 1.15 | 0.91 | 0.67, 1.25 | 1.00 | Referent | 0.83 | 0.55, 1.24 | 0.52 | 0.25, 1.07 | 0.55 |
| Follow-up time to case diagnosis, yearsc | |||||||||||||||
| ≤5 | 634 | 767 | 1.04 | 0.66, 1.64 | 0.76 | 0.52, 1.10 | 1.03 | 0.76, 1.40 | 1.00 | Referent | 1.02 | 0.72, 1.42 | 0.86 | 0.51, 1.43 | 0.71 |
| >5 | 628 | 895 | 1.12 | 0.69, 1.80 | 1.09 | 0.77, 1.54 | 0.94 | 0.70, 1.26 | 1.00 | Referent | 1.32 | 0.94, 1.85 | 0.84 | 0.44, 1.59 | 0.82 |
| NHL subtype | |||||||||||||||
| DLBCL | 306 | 1,667 | 1.27 | 0.76, 2.12 | 0.87 | 0.57, 1.32 | 1.05 | 0.76, 1.47 | 1.00 | Referent | 0.94 | 0.64, 1.38 | 0.74 | 0.39, 1.42 | 0.40 |
| FL | 194 | 1,667 | 0.91 | 0.44, 1.92 | 0.72 | 0.41, 1.25 | 1.26 | 0.86, 1.86 | 1.00 | Referent | 1.18 | 0.76, 1.82 | 0.49 | 0.19, 1.26 | 0.98 |
| CLL/SLL | 379 | 1,667 | 1.11 | 0.70, 1.78 | 0.86 | 0.59, 1.25 | 0.76 | 0.55, 1.05 | 1.00 | Referent | 1.41 | 1.02, 1.95 | 0.56 | 0.28, 1.12 | 0.33 |
| Other/NOS | 383 | 1,667 | 1.28 | 0.82, 1.98 | 1.07 | 0.75, 1.54 | 1.04 | 0.76, 1.42 | 1.00 | Referent | 1.09 | 0.76, 1.56 | 1.39 | 0.83, 2.34 | 0.92 |
Abbreviations: CI, confidence interval; CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; NHL, non-Hodgkin lymphoma; NOS, not otherwise specified; 25(OH)D, 25-hydroxyvitamin D; OR, odds ratio.
Does not include subjects with missing data for model covariate (height). Includes controls matched to lymphoma cases other than non-Hodgkin lymphoma, which were excluded from the analysis.
Odds ratios for subject strata were computed by using unconditional logistic regression adjusting for height (≤165, >165–171, >171–178, >178 cm). Odds ratios for restricted analyses (Caucasians, US cohorts) and follow-up time to diagnosis strata were computed by using conditional logistic regression adjusting for height. Odds ratios for histologic subtypes were computed by using polytomous regression adjusting for height.
Tests of interaction with each stratification factor were not statistically significant.
No clear evidence of association between common histologic subtypes of NHL and 25(OH)D was observed (Table 5). Odds ratio estimates for follicular lymphoma and CLL/SLL in individuals with a concentration of ≥100 nmol/L (vs. 50–<75 nmol/L) were 0.49 and 0.56, respectively, although the corresponding 95% confidence intervals included the null value and trend tests were negative. When this category was subdivided by using the a priori cutpoint of 120 nmol/L, the apparent inverse association with follicular lymphoma became stronger with increasing 25(OH)D but was not statistically significant (100–<120 nmol/L: odds ratio = 0.55, 95% confidence interval: 0.19, 1.57; ≥120 nmol/L: odds ratio = 0.35, 95% confidence interval: 0.05, 2.67). There were no cases of CLL/SLL with a 25(OH)D concentration of ≥120 nmol/L. The odds ratios for DLBCL were observed to change with increasing 25(OH)D in a pattern generally consistent with a dose-response relation, although the test for trend was not statistically significant (P = 0.40).
DISCUSSION
On the basis of several case-control findings suggesting an inverse association between sun exposure and NHL, it was hypothesized that the vitamin D pathway may be protective against lymphomagenesis. However, in this pooled analysis involving serum or plasma specimens from 10 cohort studies, there was no clear evidence of an association between circulating concentrations of the vitamin D metabolite 25(OH)D and risk of NHL.
The only previous study known to have directly investigated circulating 25(OH)D and NHL, which was conducted within the ATBC cohort of male Finnish smokers, also found no association overall (23). However, the investigators noted a statistically significant inverse association with risk within the first 7 years of follow-up. Among cases diagnosed after 7 years of follow-up, a non-statistically-significant increased risk with increasing serum 25(OH)D was observed. In this pooled analysis, which included the ATBC data, we observed no overall evidence of association among men. A non-statistically-significant association with reduced risk was observed among men at very high levels of 25(OH)D; however, no such finding was observed among women or among measures of 25(OH)D that used alternate methods to deal with the influence of season of blood collection. Results of analyses of men restricted to the first 5 years of follow-up, both including and excluding the ATBC subjects, were similarly null. The totality of the evidence from this pooled analysis thus does not support an association with 25(OH)D among men or within an earlier period of follow-up from blood collection.
The findings among women, suggestive of a weak increased risk of NHL with increasing 25(OH)D, were unexpected. In an analysis investigating correlates of 25(OH)D among VDPP controls, women were observed to have significantly lower levels than men did; the median 25(OH)D concentrations in men and women in the United States were 58.4 nmol/L and 51.7 nmol/L, and those in China were 38.0 nmol/L and 33.1 nmol/L, respectively (30). Interestingly, one population-based case-control study of NHL conducted among women in Connecticut observed a statistically significant increased risk with increasing sun exposure (15). However, analyses of recreational sun exposure and NHL risk restricted to women in the International Lymphoma Epidemiology (InterLymph) Consortium pooled analysis suggested a weak inverse association with increasing recreational sun exposure and no association with total ultraviolet radiation exposure. We are unaware of any biologic rationale for vitamin D to have an adverse effect on NHL risk for women, but not men. It is possible that these findings may have arisen because of chance.
Analyses by the common histologic subtypes of NHL generally did not show an association with 25(OH)D. Although individuals with the highest concentrations of 25(OH)D were at lower risk of DLBCL, follicular lymphoma, and CLL/SLL, the individual estimates and tests for trends were not statistically significant. The published epidemiologic evidence regarding vitamin D and NHL subtypes is extremely limited. In a previously published investigation within ATBC, the investigators observed an inverse association between 25(OH)D concentration and CLL/SLL risk, whereas findings for follicular lymphoma or diffuse lymphoma were null (23). Recent NHL case-control studies that have reported inverse associations with sun exposure have generally not discovered different findings across B-cell subtypes (16). However, in one study, the estimated association between sun exposure and follicular lymphoma was found to vary across genotypes of the vitamin D receptor gene polymorphism TaqI (31). Given the evidence in hand and the limited study power to detect subtype-specific associations, the findings from this study for follicular lymphoma, DLBCL, and CLL/SLL are inconclusive.
An important strength of this study is its sample size; with more than 1,300 cases, it is one of the largest prospective, biospecimen-based investigations of NHL conducted to date. Another strength is the broad variation in measured 25(OH)D concentrations, at least partly due to the broad range in latitude covered by the participating cohorts. The study also benefited from having specimens from all 10 of the cohorts measured at the same laboratory and, excepting ATBC, at the same time.
A limitation of this study, shared by virtually all prospective investigations of vitamin D metabolites and cancer risk, is its reliance upon a single measurement of 25(OH)D as a surrogate for long-term vitamin D status. The long-term intraindividual variation in circulating 25(OH)D is unclear, although, in one study of 144 men with 2 blood specimens collected 3–4 years apart, the correlation between the 2 plasma 25(OH)D measurements was fairly high, with a Pearson correlation coefficient of 0.70 (32). Nonetheless, the possibility remains that these single measurements did not adequately capture long-term 25(OH)D levels, and a real association with vitamin D may have been missed. Lastly, although the numbers of follicular lymphoma, DLBCL, and CLL/SLL cases in this study were relatively high compared with other studies of NHL, statistical power to detect subtype-specific associations of moderate magnitude was limited.
Notwithstanding these limitations, the totality of the evidence from this pooled analysis does not support the hypothesis that circulating 25(OH)D concentration is associated with reduced NHL risk. In view of these findings, it seems unlikely that the weak inverse association between sun exposure and NHL risk reported by recent case-control studies is mediated by the vitamin D pathway alone. Assuming that the association between sun exposure and NHL is real and not simply the product of confounding from other lifestyle factors, other possible mechanisms for this association must be considered. One such possibility is ultraviolet radiation–induced immune modulation. Exposure to ultraviolet radiation depresses T-helper 1–mediated immune responses and enhances T-helper 2–mediated immune activity, both locally and systemically (33–35). Several studies have reported lower NHL risks for people with allergic conditions (proposed markers of a dominant T-helper 2 immune response; summarized by Grulich et al. (36)) and have led to the suggestion that a T-helper 2–dominated immune response may be associated with a reduced risk of NHL (36). Additional research investigating ultraviolet radiation–induced immunologic effects and other hypotheses addressing the biologic basis for the sunlight–NHL association is needed.
Acknowledgments
Author affiliations: Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, Maryland (Mark P. Purdue, D. Michal Freedman, Nathaniel Rothman, Demetrius Albanes, Stephanie J. Weinstein, Kai Yu, Patricia Hartge); Epidemiology Research Program, American Cancer Society, Atlanta, Georgia (Susan M. Gapstur, Victoria L. Stevens); Department of Epidemiology, The Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland (Kathy J. Helzlsouer, Judith Hoffman-Bolton); The Prevention and Research Center, The Weinberg Center for Women's Health and Medicine, Mercy Medical Center, Baltimore, Maryland (Kathy J. Helzlsouer); Channing Laboratory, Department of Medicine, Brigham and Women's Hospital, Boston, Massachusetts (Francine Laden, Walter Willett); Department of Environmental Health, Harvard School of Public Health, Boston, Massachusetts (Francine Laden); Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts (Francine Laden, Kimberly Bertrand, Edward L. Giovannucci, Walter Willett); Department of Nutrition, Harvard School of Public Health, Boston, Massachusetts (Edward L. Giovannucci, Walter Willett); Cancer Research Center, University of Hawaii, Honolulu, Hawaii (Unhee Lim, Gertraud Maskarinec, Laurence N. Kolonel); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, Tennessee (Xiao-Ou Shu, Wei Zheng); Department of Environmental Medicine and Cancer Institute, New York University School of Medicine, New York, New York (Anne Zeleniuch-Jacquotte, Alan A. Arlsan, Richard B. Hayes); Information Management Services, Inc., Silver Spring, Maryland (Lonn Irish, Kirk Snyder); Heartland Assays, Inc., Ames, Iowa (Ronald L. Horst); and Shanghai Cancer Institute, Shanghai, China (Yong-Bing Xiang).
This work was supported by the Extramural Research Program of the National Institutes of Health, Division of Cancer Control and Population Sciences, National Cancer Institute (NCI) (Bethesda, Maryland) and the Intramural Research Program of the National Institutes of Health, Division of Cancer Epidemiology and Genetics, NCI. The New York University Women's Health Study was supported by the NCI (grant R01 CA098661). The Health Professionals Follow-up Study and the Nurses’ Health Study were supported by the NCI (grants P01 CA055075, P01 CA87969, R01 CA49449, and R01 CA082838). The Multiethnic Cohort Study was supported by the NCI (grants R37 CA54281, P01 CA33619, R01 CA063464, and N01-PC35137). The Shanghai Men's Health Study was supported by the NCI (grant R01 CA82729). The Shanghai Women's Health Study was supported by the NCI (grants R37 CA70867 and N02-CP-11010-66). The Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial was supported by contracts from the NCI to the University of Colorado, Denver, Colorado (grant N01-CN-25514); Georgetown University, Washington, DC (grant N01-CN-25522); the Pacific Health Research Institute (grant N01-CN-25515); the Henry Ford Health System (grant N01-CN-25512); the University of Minnesota, Minneapolis, Minnesota (grant N01-CN-25513); Washington University, St. Louis, Missouri (grant NO1-CN-25516); the University of Pittsburgh, Pittsburgh, Pennsylvania (grant N01-CN-25511); the University of Utah, Salt Lake City, Utah (grant N01-CN-25524); the Marshfield Clinic Research Foundation, Marshfield, Wisconsin (grant N01-CN-25518); the University of Alabama, Birmingham, Alabama (grant NO1-CN-75022); Westat, Inc., Rockville, Maryland (grant N01-CN-25476); and the University of California, Los Angeles, Los Angeles, California (grant NO1-CN-25404). The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study was supported by funding provided by the Intramural Research Program of the NCI and US Public Health Service contracts (N01-CN-45165, N01-RC-45035, N01-RC-37004). CLUE was supported by the National Institute on Aging (grant U01 AG018033) and the NCI (grants R01 CA105069, K07 CA73790). The participation of CLUE investigators was also supported by an NCI contract awarded to Mercy Medical Center through the University of Hawaii (Honolulu, Hawaii). The Cancer Prevention Study II Nutrition Cohort was supported by the American Cancer Society (Atlanta, Georgia).
The authors thank Dr. Karen Phinney of the National Institute of Standards and Technology for providing the SRM 972 Vitamin D in Human Serum used in this work.
Members of the VDPP NHL Writing Committee: Mark P. Purdue, D. Michal Freedman, Susan M. Gapstur, Kathy J. Helzlsouer, Francine Laden, Unhee Lim, Gertraud Maskarinec, Nathaniel Rothman, Xiao-Ou Shu, Victoria L. Stevens, Anne Zeleniuch-Jacquotte, Kimberly Bertrand, Stephanie J. Weinstein, and Patricia Hartge.
This report is based at least in part on information provided by the Maryland Cancer Registry, Maryland Department of Health and Mental Hygiene.
Dr. Ronald L. Horst is the President and Chief Executive Officer of Heartland Assays, Inc.
Glossary
Abbreviations
- ATBC
Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study
- CLL/SLL
chronic lymphocytic leukemia/small lymphocytic lymphoma
- DLBCL
diffuse large B-cell lymphoma
- ICD-O-2/3
International Classification of Diseases for Oncology, Second/Third Edition
- NHL
non-Hodgkin lymphoma
- NIST
National Institute of Standards and Technology
- 25(OH)D
25-hydroxyvitamin D
- VDPP
Cohort Consortium Vitamin D Pooling Project of Rarer Cancers
References
- 1.Armstrong BK, Kricker A. Sun exposure and non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2007;16(3):396–400. doi: 10.1158/1055-9965.EPI-06-1068. [DOI] [PubMed] [Google Scholar]
- 2.Hartge P, Devesa SS, Grauman D, et al. Non-Hodgkin's lymphoma and sunlight. J Natl Cancer Inst. 1996;88(5):298–300. doi: 10.1093/jnci/88.5.298. [DOI] [PubMed] [Google Scholar]
- 3.Freedman DM, Zahm SH, Dosemeci M. Residential and occupational exposure to sunlight and mortality from non-Hodgkin's lymphoma: composite (threefold) case-control study. BMJ. 1997;314(7092):1451–1455. doi: 10.1136/bmj.314.7092.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grant WB. Ecologic studies of solar UV-B radiation and cancer mortality rates. Recent Results Cancer Res. 2003;164:371–377. doi: 10.1007/978-3-642-55580-0_27. [DOI] [PubMed] [Google Scholar]
- 5.Hu S, Ma F, Collado-Mesa F, et al. Ultraviolet radiation and incidence of non-Hodgkin's lymphoma among Hispanics in the United States. Cancer Epidemiol Biomarkers Prev. 2004;13(1):59–64. doi: 10.1158/1055-9965.epi-03-0187. [DOI] [PubMed] [Google Scholar]
- 6.McMichael AJ, Giles GG. Have increases in solar ultraviolet exposure contributed to the rise in incidence of non-Hodgkin's lymphoma? Br J Cancer. 1996;73(7):945–950. doi: 10.1038/bjc.1996.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bentham G. Association between incidence of non-Hodgkin's lymphoma and solar ultraviolet radiation in England and Wales. BMJ. 1996;312(7039):1128–1131. doi: 10.1136/bmj.312.7039.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langford IH, Bentham G, McDonald AL. Mortality from non-Hodgkin lymphoma and UV exposure in the European Community. Health Place. 1998;4(4):355–364. doi: 10.1016/s1353-8292(98)00026-4. [DOI] [PubMed] [Google Scholar]
- 9.Hughes AM, Armstrong BK, Vajdic CM, et al. Sun exposure may protect against non-Hodgkin lymphoma: a case-control study. Int J Cancer. 2004;112(5):865–871. doi: 10.1002/ijc.20470. [DOI] [PubMed] [Google Scholar]
- 10.Smedby KE, Hjalgrim H, Melbye M, et al. Ultraviolet radiation exposure and risk of malignant lymphomas. J Natl Cancer Inst. 2005;97(3):199–209. doi: 10.1093/jnci/dji022. [DOI] [PubMed] [Google Scholar]
- 11.Hartge P, Lim U, Freedman DM, et al. Ultraviolet radiation, dietary vitamin D, and risk of non-Hodgkin lymphoma (United States) Cancer Causes Control. 2006;17(8):1045–1052. doi: 10.1007/s10552-006-0040-8. [DOI] [PubMed] [Google Scholar]
- 12.Weihkopf T, Becker N, Nieters A, et al. Sun exposure and malignant lymphoma: a population-based case-control study in Germany. Int J Cancer. 2007;120(11):2445–2451. doi: 10.1002/ijc.22492. [DOI] [PubMed] [Google Scholar]
- 13.Petridou ET, Dikalioti SK, Skalkidou A, et al. Sun exposure, birth weight, and childhood lymphomas: a case control study in Greece. Cancer Causes Control. 2007;18(9):1031–1037. doi: 10.1007/s10552-007-9044-2. [DOI] [PubMed] [Google Scholar]
- 14.Soni LK, Hou L, Gapstur SM, et al. Sun exposure and non-Hodgkin lymphoma: a population-based, case-control study. Eur J Cancer. 2007;43(16):2388–2395. doi: 10.1016/j.ejca.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Holford TR, Leaderer B, et al. Ultraviolet radiation exposure and risk of non-Hodgkin's lymphoma. Am J Epidemiol. 2007;165(11):1255–1264. doi: 10.1093/aje/kwm020. [DOI] [PubMed] [Google Scholar]
- 16.Kricker A, Armstrong BK, Hughes AM, et al. Personal sun exposure and risk of non Hodgkin lymphoma: a pooled analysis from the InterLymph Consortium. Int J Cancer. 2008;122(1):144–154. doi: 10.1002/ijc.23003. [DOI] [PubMed] [Google Scholar]
- 17.Kricker A, Armstrong B. Does sunlight have a beneficial influence on certain cancers? Prog Biophys Mol Biol. 2006;92(1):132–139. doi: 10.1016/j.pbiomolbio.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Bhalla AK, Amento EP, Clemens TL, et al. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation. J Clin Endocrinol Metab. 1983;57(6):1308–1310. doi: 10.1210/jcem-57-6-1308. [DOI] [PubMed] [Google Scholar]
- 19.Provvedini DM, Tsoukas CD, Deftos LJ, et al. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221(4616):1181–1183. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- 20.Chen S, Sims GP, Chen XX, et al. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007;179(3):1634–1647. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 21.Hickish T, Cunningham D, Colston K, et al. The effect of 1,25-dihydroxyvitamin D3 on lymphoma cell lines and expression of vitamin D receptor in lymphoma. Br J Cancer. 1993;68(4):668–672. doi: 10.1038/bjc.1993.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raina V, Cunningham D, Gilchrist N, et al. Alfacalcidol is a nontoxic, effective treatment of follicular small-cleaved cell lymphoma. Br J Cancer. 1991;63(3):463–465. doi: 10.1038/bjc.1991.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim U, Freedman DM, Hollis BW, et al. A prospective investigation of serum 25-hydroxyvitamin D and risk of lymphoid cancers. Int J Cancer. 2009;124(4):979–986. doi: 10.1002/ijc.23984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gallicchio L, Helzlsouer KJ, Chow WH, et al. Circulating 25-hydroxyvitamin D and the risk of rarer cancers: design and methods of the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010;172(1):10–20. doi: 10.1093/aje/kwq116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagner D, Hanwell HE, Vieth R. An evaluation of automated methods for measurement of serum 25-hydroxyvitamin D. Clin Biochem. 2009;42(15):1549–1556. doi: 10.1016/j.clinbiochem.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 26.Looker AC, Dawson-Hughes B, Calvo MS, et al. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30(5):771–777. doi: 10.1016/s8756-3282(02)00692-0. [DOI] [PubMed] [Google Scholar]
- 27.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 28.Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19(2):73–78. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viechtbauer W. MiMa: an S-Plus/R function to fit meta-analytic mixed-, random-, and fixed-effects models [computer software and manual] 2006 ( http://www.wvbauer.com/downloads.html). (Accessed February 6, 2009) [Google Scholar]
- 30.McCullough ML, Weinstein SJ, Freedman DM, et al. Correlates of circulating 25-hydroxyvitamin D: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010;172(1):21–35. doi: 10.1093/aje/kwq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Purdue MP, Hartge P, Davis S, et al. Sun exposure, vitamin D receptor gene polymorphisms and risk of non-Hodgkin lymphoma. Cancer Causes Control. 2007;18(9):989–999. doi: 10.1007/s10552-007-9039-z. [DOI] [PubMed] [Google Scholar]
- 32.Platz EA, Leitzmann MF, Hollis BW, et al. Plasma 1,25-dihydroxy- and 25-hydroxyvitamin D and subsequent risk of prostate cancer. Cancer Causes Control. 2004;15(3):255–265. doi: 10.1023/B:CACO.0000024245.24880.8a. [DOI] [PubMed] [Google Scholar]
- 33.Ullrich SE. Does exposure to UV radiation induce a shift to a Th-2-like immune reaction? Photochem Photobiol. 1996;64(2):254–258. doi: 10.1111/j.1751-1097.1996.tb02454.x. [DOI] [PubMed] [Google Scholar]
- 34.Duthie MS, Kimber I, Norval M. The effects of ultraviolet radiation on the human immune system. Br J Dermatol. 1999;140(6):995–1009. doi: 10.1046/j.1365-2133.1999.02898.x. [DOI] [PubMed] [Google Scholar]
- 35.Clydesdale GJ, Dandie GW, Muller HK. Ultraviolet light induced injury: immunological and inflammatory effects. Immunol Cell Biol. 2001;79(6):547–568. doi: 10.1046/j.1440-1711.2001.01047.x. [DOI] [PubMed] [Google Scholar]
- 36.Grulich AE, Vajdic CM, Kaldor JM, et al. Birth order, atopy, and risk of non-Hodgkin lymphoma. J Natl Cancer Inst. 2005;97(8):587–594. doi: 10.1093/jnci/dji098. [DOI] [PubMed] [Google Scholar]