Abstract
Astrocytes can be considered as signal integrators in central nervous system activity. These glial cells can respond to signals from the heterocellular milieu of the brain and subsequently release various molecules to signal to themselves and/or other neighboring neural cells. An important functional module that enables signal integration in astrocytes is exocytosis, a Ca2+-dependent process consisting of vesicular fusion to the plasma membrane. Astrocytes utilize regulated exocytosis to release various signaling molecules stored in the vesicular lumen. Here we review the properties of exocytotic release of three classes of gliotransmitters: i) amino acids, ii) nucleotides and iii) peptides. Vesicles may not only carry lumenal cargo, but also membrane associated molecules. Therefore, we also discuss exocytosis as a delivery mechanism for transporters and receptors to the plasma membrane, where these proteins are involved in astrocytic intercellular signaling.
Keywords: astrocytes, integrator, exocytosis, glutamate, D-serine, ATP, atrial natriuretic peptide, brain-derived neurotrophic factor
Introduction
The concept that astrocytes are multifunctional housekeeping cells subservient to neurons has largely been reconsidered. Besides maintaining the optimal environment for functioning of neurons (Nedergaard et al., 2003), evidence has emerged that astrocytes directly modulate processes such as synaptogenesis (Pfrieger and Barres, 1997), synaptic transmission (Araque et al., 1999; Newman, 2003), brain microcirculation (Anderson and Nedergaard, 2003; Zonta et al., 2003), short-term to long-term memory consolidation (Hertz and Gibbs, 2009), and immune responses (Fontana et al., 1984; Girvin et al., 2002). In this heterocellular signaling, astrocytes appear as signal integrators, since they generate outputs with variable timing in response to particular signals received from surrounding neural cells to affect neurons and/or other cellular components of the brain. A crucial element that facilitates the integrating functions of astrocytes is regulated exocytosis.
Astrocytes can release chemical messengers, gliotransmitters, into the extracellular space via Ca2+-dependent exocytosis (Parpura et al., 1994), which has emerged as the prevalent release mechanism in these glial cells. Additionally, astrocytes express a variety of channels, receptors and transporters on their surface that mediate release of gliotransmitters: (i) anion channels (Pasantes Morales and Schousboe, 1988); (ii) unpaired connexons/pannexons, commonly referred to as “hemichannels” (Cotrina et al., 1998; Iglesias et al., 2009); (iii) ionotropic purinergic receptors (Duan et al., 2003); and (iv) transporters (Szatkowski et al., 1990; Warr et al., 1999; Rosenberg et al., 1994).
Exocytosis is an evolutionary trait of eukaryotic cells characterized by the formation of an aqueous channel, the fusion pore, upon the merger of vesicular and plasma membranes. The majority of ~200 cell types present in the human body perform exocytosis. It is through this process that various cells release vesicular chemical content into the extracellular space. Exocytosis represents one of the fastest biological events known. Increases in cytosolic Ca2+ can trigger exocytosis within a subfraction of a millisecond. However, such an exquisite speed can be reached only when vesicles are already pre-filled with transmitter, primed and docked to the plasma membrane, waiting for the cytosolic Ca2+ signal. In general, this is the case with vesicles filled with amino acids and/or nucleotides. Here, the delay is mainly determined by the spatio-temporal characteristics of the Ca2+ signaling mechanisms. However, in the case of vesicles filled with peptides, the delay between the trigger and the response may be significantly longer, in part because of relatively distant positioning of such vesicles to their plasma membrane fusion sites. Moreover, the repetitive/prolonged stimulation of peptidergic transmission requires peptide synthesis, packaging and anterograde vesicular trafficking.
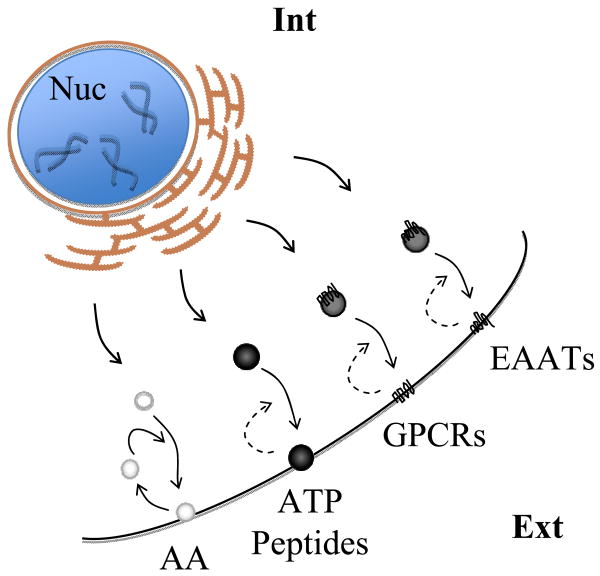
Naturally, the delivery of secretory vesicles and fusion to the plasma membrane in astrocytes has been demonstrated in a number of studies. Crippa et al. (2006) expressed a chimeric protein, where enhanced green fluorescent protein (EGFP) was fused to the C-terminus of the vesicule membrane associated protein synaptobrevin 2 (Sb2-EGFP), in astrocytes. Since the C-terminus of Sb2 is located in the vesicular lumen, EGFP was expressed intravesicularly. When astrocytes were stimulated with a Ca2+ ionophore to increase cytosolic Ca2+, many fluorescent Sb2-EGFP puncta vanished with a simultaneous enrichment in plasma membrane fluorescence, consistent with regulated exocytosis and fusion of labeled vesicles to the plasma membrane. Net addition of vesicular membrane to the plasma membrane can be directly measured by monitoring changes in membrane capacitance (Cm). Certainly, an agonist-induced rise in astrocytic intracellular calcium ion concentration ([Ca2+]i) increased Cm, while concomitant measurements recorded a release of the gliotransmitter glutamate (Zhang et al., 2004b). Further evidence for vesicular exocytosis from astrocytes was provided by total internal reflection fluorescence (TIRF) microscopy (Bezzi et al., 2004; Domercq et al., 2006; Bowser and Khakh, 2007; Pangrsic et al., 2007; Marchaland et al., 2008; Pryazhnikov and Khiroug, 2008), where individual vesicular fusions were reported. As a consequence of vesicular fusions, quantal events of transmitter release, representing an exocytotic hallmark (Del Castillo and Katz, 1954), have been recorded from astrocytes (Pasti et al., 2001; Chen et al., 2005; Pangrsic et al., 2007). In this review, therefore, we focus on the exocytotic mechanism(s) underlying the release of three classes of gliotransmitters: (i) amino acids, such as glutamate and D-serine (ii) nucleotides, like adenosine 5’-triphosphate (ATP), and (iii) peptides, such as atrial natriuretic peptide (ANP), neuropeptide Y (NPY), brain-derived neurotrophic factor (BDNF) and various cytokines/chemokines (Figure 1).
Figure 1.
Regulated exocytosis in astrocytes. Ca2+-dependent exocytosis is a mechanism underlying the release of three classes of gliotransmitters: amino acids (AA), nucleotides (e.g., ATP), and peptides from astrocytes. These chemical transmitters are stored in and released from at least two distinct classes of vesicles (clear and dense core) at the plasma membrane. Secretory vesicles can also carry membrane-associated molecules. Delivery of membrane signaling receptors, such as the G-protein coupled receptors (GPCRs) and transporters, such as the excitatory amino acid transporters (EAATs) to the plasma membrane is of special interest for astrocytic interactions with other neural cells. Arrows indicate direction of vesicle trafficking. Dashed arrow indicates the presumed recycling of ATP/peptide, GPCR, and EAAT containing vesicles. Ext, extracellular space; Int, intracellular space; Nuc, nucleus. Drawing is not to scale.
Secretory vesicles can also carry membrane-associated molecules. Delivery of membrane signaling proteins, receptors and transporters to the plasma membrane is of special interest for astrocytic interactions with other neural cells. Consequently, we also discuss intracellular vesicular traffic and/or exocytotic-mediated insertion of membrane proteins such as the excitatory amino acid transporter (EAAT) 2 and the G-protein coupled cannabinoid receptor 1 (CB1R) (Figure 1).
Exocytotic release of amino acids from astrocytes
Amino acid glutamate is synthesized within astrocytes as a by-product of the tricarboxylic acid (TCA) cycle. Glutamate is converted from the TCA cycle intermediate, α-ketoglutarate, usually via transamination of another amino acid, such as aspartate (Westergaard et al., 1996). Since astrocytes possess the enzyme pyruvate carboxylase, the synthesis of glutamate occurs de novo (Hertz et al., 1999). Through the action of serine racemase, an enzyme found predominately in astrocytes, D-serine is generated from L-serine, (Wolosker et al., 1999). These amino acid transmitters are stored intravesicularly. For their release into the extracellular space, they require increases in cytosolic Ca2+ concentrations, which can be caused by neuronal activity. Once released, they act upon neuronal receptors to modulate synaptic transmission (Ni et al., 2007; Oliet and Mothet, 2009).
Evidence for Ca2+-dependent gliotransmitter release from astrocytes was initially demonstrated using high performance liquid chromatography to monitor glutamate release from cultured astrocytes (Parpura et al., 1994). Elevated [Ca2+]i caused by the Ca2+ ionophore ionomycin was sufficient and necessary to cause glutamate release from astrocytes. Consistent with this finding, other stimuli that can increase astrocytic [Ca2+]i including mechanical stimulation (Parpura et al., 1994; Araque et al., 1998b; Araque et al., 1998a; Innocenti et al., 2000; Hua et al., 2004; Montana et al., 2004), photostimulation (Parpura et al., 1994), photolysis of Ca2+ cages (Araque et al., 1998a; Parpura and Haydon, 2000; Zhang et al., 2004b), bradykinin (Parpura et al., 1994), prostaglandins (Bezzi et al., 1998) and ATP (Jeremic et al., 2001) all induce glutamate release. Furthermore, buffering of cytosolic Ca2+capacity in astrocytes with the Ca2+ chelator 1,2-bis(o-aminophenoxy)ethane-N,N,N’,N’-tetraacetic acid (BAPTA) reduces evoked glutamate release from astrocytes (Innocenti et al., 2000).
The majority of intracellular Ca2+ necessary for astrocytic glutamate release originates from endoplasmic reticulum (ER) stores (Figure 2). Depletion of internal Ca2+ stores with thapsigargin, a blocker of ER-specific Ca2+-ATPase, blocks glutamate release from astrocytes (Araque et al., 1998b; Bezzi et al., 1998; Innocenti et al., 2000; Hua et al., 2004; Montana et al., 2004). Alkalinization of the cytosol, as occurs in the presence of ammonia, stimulates Ca2+ release from the ER, raising [Ca2+]i levels, which induces glutamate release from astrocytes (Rose et al., 2005). Mechanical stimulation that causes increase of [Ca2+]i levels results in glutamate release (Hua et al., 2004). This mechanically-induced glutamate release from astrocytes can be blocked by diphenylboric acid 2-aminoethyl ester (2-APB) solution, a cell-permeant inositol 1,4,5-trisphosphate (IP3) receptor antagonist, implicating the role of IP3-sensitive internal stores in mediating Ca2+-dependent glutamate release from astrocytes. Ryanodine/caffeine-sensitive ER stores play a role as well, since the treatment of astrocytes with ryanodine (at concentrations that block release of Ca2+ from ryanodine/caffeine-sensitive stores) attenuates mechanically-induced glutamate release. Furthermore, the sustained presence of caffeine, which depletes ryanodine/caffeine stores, also reduces mechanically-induced glutamate release. Thus, Ca2+-dependent glutamate release from astrocytes involves both IP3- and ryanodine/caffeine-sensitive internal Ca2+ stores. (Hua et al., 2004). However, the functionality of ryanodine receptors in astrocytes is still debated since it has been reported that they lack activity in astrocytes in situ (Beck et al., 2004).
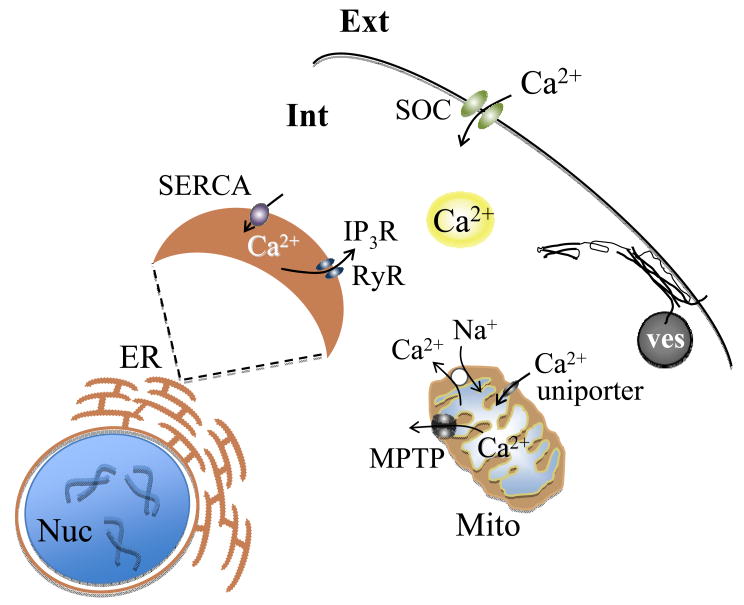
Figure 2.
Multiple sources of cytosolic Ca2+ that contribute to vesicular release from astrocytes. Vesicles (ves) fuse to the plasma membrane and release gliotransmitters. This process of regulated exocytosis is governed by the action of the ternary SNARE complex and is triggered by a preceding increase of cytosolic Ca2+. Cytosolic Ca2+ accumulation is predominately caused by the entry of Ca2+ from endoplasmic reticulum (ER) internal stores via ryanodine and inositol 1,4,5-trisphosphate receptors (RyR and IP3R, respectively). Store-specific Ca2+-ATPase (SERCA) fills these stores, which requires Ca2+ entry from the extracellular space (Ext) through store-operated Ca2+ channels (SOC) located at the plasma membrane. Mitochondria (Mito) represent a source/sink of cytosolic Ca2+; uptake is mediated by the uniporter, efflux occurs via the Na+/Ca2+ exchanger and the mitochondrial permeability transition pore (MPTP). Int, intracellular space; Nuc, nucleus. Drawing is not to scale.
Ca2+ entry from the extracellular space across the astrocytic plasma membrane is ultimately required for the (re)filling of ER Ca2+ stores (Figure 2). This occurs via store-operated Ca2+ (SOC) channels, which become activated when ER Ca2+ is depleted (Takemura and Putney, 1989; Golovina, 2005). The precise mechanism governing [Ca2+]i sensing by SOC channels remains elusive. However, astrocytes express canonical transient receptor potential (TRPC) channels implicated in SOC-mediated Ca2+ entry (Pizzo et al., 2001; Grimaldi et al., 2003; Golovina, 2005). Specifically, TRPC1 functionally contributes to Ca2+-dependent glutamate release from astrocytes (Malarkey et al., 2008). Immunological blockade of the TRPC1 pore region in astrocytes significantly decreases SOC entry and mechanically-induced glutamate release. Besides SOC channels, voltage-gated Ca2+ channels can mediate entry of Ca2+ from the extracellular space. These channels regulate exocytotic glutamate release from astrocytes of the ventrobasal thalamus (Parri et al., 2001). Finally, ionotropic transmitter receptors can also mediate entry of Ca2+ into astrocytes [reviewed in (Verkhratsky, 2009)], but understanding the role of these receptors in exocytotic glutamate release from astrocytes requires further investigation.
Mitochondria, as a source/sink of intracellular Ca2+, regulate exocytosis in astrocytes (Reyes and Parpura, 2008) (Figure 2). These organelles possess a Ca2+ uniporter that transports Ca2+ into the mitochondrial matrix when cytosolic [Ca2+] is greater than ~ 0.5 μM (Miyata et al., 1991; Simpson and Russell, 1998). Blocking this uniporter with ruthenium 360, increases mechanically-induced cytosolic Ca2+ accumulation and glutamate release in cortical astrocytes. Conversely, blocking the mitochondrial Na+/Ca2+ exchanger with 7-chloro-5-(2-chlorophenyl)-1,5-dihydro-4,1-benzothiazepin-2(3H)-one (CGP37157) decreases mitochondrial Ca2+ efflux and glutamate release in cortical astrocytes. Taken together, these data suggest that mitochondria have the capacity to modulate the magnitude of Ca2+-dependent glutamate release from astrocytes.
Ca2+-dependent exocytosis depends on the presence of exocytotic secretory machinery (Figure 2). Indeed, astrocytes express proteins of the soluble N-ethyl maleimide-sensitive fusion protein attachment protein receptor (SNARE) complex: Sb2, also called vesicle–associated membrane protein 2 (VAMP2), VAMP3 (also referred to as cellubrevin), syntaxins 1, 2 and 4, synaptosome-associated protein of 23 kDa (SNAP-23), as well as several ancillary proteins to this complex, including synaptotagmin 4 and mammalian UNCoordinated 18 (Munc18) [(Paco et al., 2009) and reviewed in (Montana et al., 2006)]. The use of Clostridial, tetanus, and various types of botulinum toxins, which cleave SNARE proteins, reduces Ca2+-dependent glutamate release in astrocytes [reviewed in (Montana et al., 2006)]. Additionally, the use of tetanus toxin, which cleaves astrocytic Sb2 and cellubrevin (Parpura et al., 1995), reduces exocytosis in astrocytes as reported by an attenuated increase in Cm (Kreft et al., 2004) and a reduction in the number of amperometric spikes (Chen et al., 2005). Accordingly, expression of the cytoplasmic domain of Sb2, which contains the SNARE domain but lacks the ability to anchor to the vesicular membrane, inhibits glutamate release from astrocytes (Zhang et al., 2004b). Similarly, expression of a mutated, dominant negative form of synaptotagmin 4 decreases Ca2+-dependent glutamate release from astrocytes (Zhang et al., 2004a).
Proteins utilized for sequestering glutamate into vesicles have also been found in astrocytes. For example, astrocytes express vacuolar type H+ ATPase (V-ATPase), which drives protons into the vesicular lumen, thereby generating the proton concentration gradient necessary for glutamate transport into vesicles (Wilhelm et al., 2004). V-ATPase inhibition with bafilomycin A1 reduces glutamate release from astrocytes induced by various stimuli (Araque et al., 2000; Bezzi et al., 2001; Pasti et al., 2001; Montana et al., 2004; Crippa et al., 2006). Astrocytes also express the three known isoforms of VGLUTs: 1, 2 and 3, which use the proton gradient created by V-ATPases to package glutamate into vesicles (Fremeau et al., 2002; Bezzi et al., 2004; Kreft et al., 2004; Montana et al., 2004; Zhang et al., 2004b; Anlauf and Derouiche, 2005; Crippa et al., 2006). Introduction of Rose Bengal, an allosteric inhibitor of VGLUTs, greatly reduces glutamate release, illustrating that these transporters are functional within astrocytes (Montana et al., 2004). VGLUT3 and the cytosolic concentration of glutamate are key limiting factors in Ca2+-dependent release of glutamate from astrocytes (Ni and Parpura, 2009). Selective over-expression of individual VGLUT proteins in astrocytes showed that VGLUT3, but not VGLUT1 or VGLUT2, enhances mechanically-induced Ca 2+-dependent glutamate release. Similarly, inhibition of glutamine synthetase activity by L-methionine sulfoximine,which increases cytosolic glutamate concentrations, greatly potentiates mechanically-induced Ca 2+-dependent glutamate release from astrocytes without affecting intracellular Ca2+ dynamics (Ni and Parpura, 2009).
Secretory vesicles are the essential morphological elements needed for regulated Ca2+-dependent exocytosis. Immunoelectron microscopy (IEM) experiments demonstrate that Sb2 is located in the vicinity of electron-lucent (clear) vesicular structures (Maienschein et al., 1999). Sb2-containing vesicles isolated from cultured astrocytes were heterogeneous in size, ranging from 30 to over 100 nm (Crippa et al., 2006). VGLUTs 1 and 2 in astrocytes in situ were associated with small, clear vesicles with a mean diameter of ~ 30 nm (Bezzi et al., 2004). Recycling glutamatergic vesicles that capture the extracellular antibody against VGLUT1 in a Ca2+-dependent manner are electron-lucent and have a diameter of ~ 50 nm (Stenovec et al., 2007). Gliosomes are subcellular components of astrocyte processes isolated by fractionation and noted for their high rate of glutamate uptake (Nakamura et al., 1993). Vesicles found within gliosomes express Sb2 and VGLUT1. They are ~ 30 nm in diameter, and some are clathrin-coated (Stigliani et al., 2006). Larger vesicles, over 1 μm in diameter, have been observed to form within minutes of repeated stimulation with pharmacological dosages (5-50 mM) of glutamate (Kang et al., 2005; Xu et al., 2007). These vesicles can release glutamate, although they may represent a pharmacologically-induced phenomenon or may play a role in pathological processes (Bergersen and Gundersen, 2009). Overall, under physiologically relevant conditions Sb2 and VGLUTs are found in association with relatively small, clear vesicles in astrocytes.
Recycling of secretory vesicles at the plasma membrane has been demonstrated in astrocytes by several approaches. Application of ionomycin in the presence of extracellular Ca2+causes uptake of the membrane recycling dye, FM 4-64 (Krzan et al., 2003). Similarly, stimulation of membrane recycling and consequent trapping of styryl dyes (FM 1-43 or FM 2-10) shows a punctate pattern of FM fluorescence in astrocytes, indicating vesicular uptake (Chen et al., 2005). As already mentioned, another approach that demonstrated vesicular uptake in astrocytes used extracellular antibodies against VGLUT1 that bind the luminal/intravesicular epitope of this transporter. After increasing cytoplasmic Ca2+ levels in the presence of anti-VGLUT1 antibodies, there was an increase in the number and intensity of intracellular fluorescent puncta in cultured astrocytes (Stenovec et al., 2007) as well as in astrocytes that reside within slices (Potokar et al., 2009).
Astrocytes can also release the amino acid D-serine (Schell et al., 1995), a co-agonist of the glycine binding site of the N-methyl-D-aspartate (NMDA) receptor. Mothet at al. (2005) showed that astrocytes release D-serine following glutamate receptor stimulation in a Ca2+-dependent manner. This release was augmented by a Ca2+ ionophore and inhibited by application of thapsigargin. Furthermore, release of D-serine was reduced by concanamycin A, a V-ATPase inhibitor, and by tetanus toxin, implicating the involvement of a vesicular mechanism. Consistent with this notion, D-serine was found to co-localize with Sb2 based on immunocytochemistry and fluorescence microscopy. The mechanism underlying Ca2+-dependent release of D-serine from astrocytes was expanded in a subsequent study using confocal fluorescence microscopy (Martineau et al., 2008). Pharmacological inhibition of vesicular budding indicated that D-serine is packaged in astrocytes down stream of the Golgi apparatus. In these experiments, Sb2-containing vesicles were recruited to the plasma membrane with a concomitant disappearance of intracellular D-serine punctate staining. The molecular identity of the vesicular transporter for D-serine remains undetermined. However, these studies suggest that D-serine is secreted from astrocytes via a regulated exocytosis/vesicular pathway.
The delay between a stimulus and fusion of vesicles containing amino acids was studied using electrophysiology and flash photolysis. As mentioned above, the rise in astrocytic [Ca2+]i, which results in an increase of Cm, leads to release of glutamate (Zhang et al., 2004b). Using flash photolysis of caged Ca2+-compounds, Kreft et al. (Kreft et al., 2004) determined that at the [Ca2+]i that triggers the half-maximal response rate of Cm increase (proportional to the rate of exocytosis) the delay was > 100 ms, more than two orders of magnitude longer than the delay in synaptic transmission (Sabatini and Regehr, 1999). Since active zones have not been detected in astrocytes, it may be that the observed relatively slow exocytotic responsiveness reflects the lack of distinct molecular organization of the exocytotic apparatus.
Exocytotic release of ATP from astrocytes
ATP is produced via glycolysis and oxidative phosphorylation. Once released into the extracellular space, ATP can mediate intercellular signaling by acting directly onto purinergic receptors. Alternatively, upon its hydrolysis by membrane-bound ecto-nucleotidases, the extracellular degradation products, ADP and adenosine, can activate distinct plasma membrane receptors [reviewed in (Fields and Burnstock, 2006)]. These pathways can be utilized by astrocytes to signal to adjacent neurons (Zhang et al., 2003; Pascual et al., 2005; Halassa et al., 2009) and microglia (Bianco et al., 2005; Davalos et al., 2005). As already outlined, astrocytes possess the necessary secretory machinery required for exocytosis. In addition to the smaller, clear core vesicles, astrocytes also contain large, dense core granules with diameters of ~ 115 nm, as evidenced by EM. These vesicles contain the secretory peptide secretogranin II (Calegari et al., 1999) and ATP (Coco et al., 2003). Based on immunoblotting, subcellular fractions containing secretogranin II were mainly distinct from fractions containing Sb2 (Calegari et al., 1999). However, dense core vesicles represent ~ 2% of the total number of immuno-isolated Sb2-containing vesicles (Crippa et al., 2006). It was demonstrated by IEM that Sb2 associates with some dense core vesicular structures with diameters ranging from 100-700 nm (Maienschein et al., 1999). Following subcellular fractionation and immunoblotting, Sb2, syntaxin 1, cellubrevin and synaptotagmin 1 were found to co-localize with ATP-containing organelles (Maienschein et al., 1999). It appears that there is some variability in the association of Sb2 with dense-core ATP-containing vesicles. It should be noted, however, that the presence of synaptotagmin 1 was not detected in astrocytes by others (Parpura et al., 1994; Zhang et al., 2004a; Crippa et al., 2006). The protein responsible for ATP accumulation in secretory vesicles has recently been identified as the vesicular nucleotide transporter (VNUT) SLC17A9 by immunocytochemistry in astrocytes (Sawada et al., 2008).
Morphological and biochemical evidence suggests that ATP may be released from astrocytes by Ca2+-dependent exocytosis. In support of this idea, astrocytes exposed to nitric oxide demonstrated an increase in [Ca2+]i and in the release of ATP into the extracellular space (Bal-Price et al., 2002). Buffering of intracellular Ca2+ with BAPTA or preventing vesicular release with botulinum toxin C greatly reduced ATP release. Furthermore, Coco et al. (2003) demonstrated that mechanically stimulated astrocytes release ATP, which is inhibited by application of bafilomycin A1 or tetanus toxin. Interestingly, the reduction of ATP release by tetanus toxin was less pronounced than the reduction in glutamate release, indicating that ATP and glutamate release may be differentially regulated by distinct vesicular release machinery. Furthermore, expression of a dominant negative SNARE in astrocytes inhibits Ca2+-induced exocytosis of ATP-containing vesicles (Pangrsic et al., 2007).
Under particular experimental conditions, the exocytotic release of ATP stored in astrocytic lysosomes has also been detected (Zhang et al., 2007). Incubation with FM recycling dyes stained astrocytic lysosomes based on co-localization of FM with various lysosomal markers under fluorescence microscopy. Regulated exocytosis of ATP-containing lysosomes in astrocytes was observed under TIRF microscopy, and was blocked by BAPTA. These lysosomes readily took up the fluorescent ATP analogue MANT-ATP which was also released upon stimulation. Indeed, the astrocytic lysosomal fraction, isolated by density gradient centrifugation, contained abundant ATP. Thus, it appears that ATP in astrocytes could be stored in and exocytotically released from at least two distinct organelles, dense-core granules and lysosomes. It will be necessary to determine the factors that dictate recruitment of these two distinct pools of organelles. For example, do the same organelles deliver ATP for release under physiological and pathological conditions or are there specific organelles that operate under particular conditions? An unexplored but exciting area of future research in respect to gliotrasmisison in general concerns specificity of SNARE isoforms in mediating vesicular versus lysosomal versus non-secretory exocytosis (Sorensen et al., 2003). In other words, is there functional redundancy in different SNARE protein isoforms or do they confer specificity in the type of contents released and/or in the particular exocytotic mechanism in astrocytes? Further studies are needed to address these questions.
To examine the kinetics of vesicular ATP release, Pryaznikov and Khiroug (2008) monitored the delay between the increase in [Ca2+]i and vesicular release. To label ATP containing secretory vesicles, they incubated astrocytes with quinacrine. Using TIRF microscopy, quinacrine showed punctate staining. Increases in [Ca2+]i reduced quinacrine fluorescent puncta, an indirect measurement of ATP release. Their results show that ATP release is delayed by several minutes and is highly asynchronous with the rise in [Ca2+]i; i.e. some vesicles exhibit relatively more rapid vesicle cargo discharge than others. These results obtained by optical monitoring of single vesicles are consistent with earlier results obtained in whole astrocytes by electrophysiological measurements (Kreft et al., 2004). The kinetics associated with lysosomal-mediated ATP release remain to be determined.
Exocytosis of peptides from astrocytes
In contrast to amino acids and ATP, which are loaded into vesicles by membrane transporters, peptidergic gliotransmitters enter vesicles via the synthetic secretory pathway. Pro-peptides are made in the ER, concentrated in Golgi compartments, and sorted into organelles such as lysosomes and vesicles. They are then processed to their final form before plasma membrane incorporation or release (Dannies, 1999) (Figure 1). Vesicles carrying peptidergic transmitters exhibit electron dense cores by EM and are termed dense-core vesicles, large dense-core vesicles or secretory granules. In general, their diameter is somewhat larger (~100 nm) in comparison with the small synaptic-like, clear-core vesicles (30-50 nm) and contain secretogranins (Winkler and Fischer-Colbrie, 1992). Secretogranin II is released upon stimulation by different secretagogues including bradykinin, cyclic adenosine monophosphate (cAMP), ionomycin, and phorbol 12-myristate 13-acetate (PMA) in a Ca2+-dependent manner (Calegari et al., 1999). The specific SNARE isoforms involved in these processes in astrocytes have not been clearly defined.
ANP was one of the first peptides studied for exocytotic release from astrocytes, and may play a role in the regulation of cerebral blood flow (McKenzie et al., 2001). Transfection of astrocytes with a construct expressing pro-ANP fused with emerald green fluorescent protein (ANP.emd) results in accumulation of ANP.emd within secretogranin II-expressing secretory vesicles, represented as fluorescent puncta (Krzan et al., 2003; Paco et al., 2009). The number of puncta is reduced upon stimulation with ionomycin (Krzan et al., 2003). Concomitant with the Ca2+-dependent decrease in fluorescent ANP.emd puncta, the fluorescence intensity of the FM 4-64 dye, a reporter of cumulative exocytosis, also increases in a Ca2+-dependent manner. Together, these data strongly indicate that Ca2+-regulated exocytosis mediates the release of ANP from astrocytes. Furthermore, release of ANP from astrocytes is augmented in astrocytes that have been incubated in a cell-permeant form of cAMP, possibly due to increases in expression of vesicular release proteins such as synaptobrein 2, syntaxin 1 and Munc18a (Paco et al., 2009). The vesicular content of secretogranin II is also increased in astrocytes incubated in cAMP. Interestingly, vesicles containing ANP also contain ATP (Pangrsic et al., 2007), which is consistent with the report that ATP is stored in secretogranin II-containing vesicles (Coco et al., 2003).
In atrial myocytes, pro-ANP-containing vesicles are ~ 120-175 nm in diameter and the vesicle size appears to be determined by the aggregation of pro-ANP in vesicles (Baertschi et al., 2001). In astrocytes, the diameter of ANP-recycling vesicles was assessed by IEM using extracellular ANP antibodies and ranged between 30 to 100 nm with an average of 50 nm. In addition to being smaller, these vesicles were clearer than ANP-containing vesicles released from atrial myocytes (Potokar et al., 2008). Because the vesicular ANP content determines the physical characteristics of ANP-containing vesicles, these differences may reflect differences in ANP content in releasing versus recycling vesicles. The mobility of recycling ANP-containing vesicles was one order of magnitude smaller than that of ANP-containing vesicles trafficking to the plasma membrane (Potokar et al., 2005; Potokar et al., 2007). Whether these differences in mobility are related to vesicular size, physiological state of the cell, or to intrinsic properties of releasing versus recycling vesicles remains unclear.
Interestingly, the mobility of ANP antibody-capturing vesicles is dramatically reduced by cell stimulation (Potokar et al., 2008), which differs from stimulation-increased mobility of VGLUT1 antibody-capturing vesicles in astrocytes (Stenovec et al., 2007). The full functional significance of these observations is not clear, but the results clearly show that retrieving-vesicle mobility is governed by vesicle-specific properties and by the physiological state of the astrocyte (Potokar et al., 2008). It is possible to envision that arrested mobility of peptidergic retrieving vesicles can enhance vesicle cargo discharge by prolonging the interaction between the plasma membrane and the vesicle membrane. It was shown that the main mode of peptidergic vesicle exocytosis is transient fusion (kiss-and-run), and that stimulation increases the frequency in vesicle fusion events, as well as the dwell time of the established fusion pore and vesicle content discharge (Stenovec et al., 2004; Vardjan et al., 2007). However, delayed retrieval may also make re-uptake more efficient, which can be important in the case of peptides, which require de novo synthesis, processing and packaging. In contrast, in glutamatergic vesicles capturing the anti-VGLUT1 antibody, stimulation-induced enhanced post-fusion vesicle mobility may have a different function (Stenovec et al., 2007). In this case, where the diffusional mobility of glutamate is orders of magnitude higher than peptidergic hormones, reduced interaction time between the vesicle and the plasma membrane would provide a regulatory control on the total amount of glutamate released. At the same time however, stimulation increases the number of fusion events and the overall effect of increased events but decreased interaction times has not been assessed.
Expression of neuropeptide Y (NPY), a peptide involved in synaptic transmission, was detected in astrocytes in brain slices from 8 day-old mice by immunocytochemistry and was confirmed by mass spectrometry of purified cortical astrocytes (Ramamoorthy and Whim, 2008). NPY imunoreactivity colocalized with GM130, a marker of the cis-Golgi, and with carboxypeptidase E, an enzyme that processes prohormones, indicating that NPY is expressed in appropriate regions of the regulated secretory pathway. NPY-expressing vesicles were larger and physically distinct from VGLUT1-expressing vesicles. Glutamate induced NPY release from astrocytes, represented as a step-like decrease in fluorescence intensity of NPY-red fluorescent protein puncta. This effect was mediated via activation of group I metabotropic glutamate receptors and depended on release of Ca2+ from intracellular stores. Glutamate, but not ATP, bradykinin or histamine, induced NPY release from astrocytes. This finding is intriguing because all four of these secretogogues cause increases in [Ca2+]i, suggesting that regulation of peptide release requires additional mechanisms that are as yet undetermined.
The specialized endocytic compartments that mediate vesicle recycling in astrocytes may serve to facilitate bidirectional communication between neurons and glia. These vesicles can take up extracellular peptides and recycle them back into the extracellular space via the secretory pathway and regulated exocytosis. When studying activity-dependent secretion of BDNF and its extracellular availability, Bergami et al. (2008) demonstrated that BDNF, which is de novo synthesized in neurons, is secreted extracellularly after theta-burst stimulation in its pro-form into the extracellular medium. Pro-BDNF is then rapidly endocytosed via the the pan-neurotrophin receptor p75NTR in perineuronal astrocytes, thereby restricting the availability of this neurotrophin at neuron-astrocyte contacts. BDNF- yellow fluorescent protein (BDNF-YFP) labeled clear-core vesicles that were ~ 125 nm in diameter. Furthermore, they showed by IEM that internalized pro-BDNF is recycled and secreted from astrocytes. This secretion was enhanced by application of extracellular glutamate. The glutamate-evoked secretion of BDNF-YFP was inhibited if cells were pretreated with tetanus toxin. Co-localization between pro-BDNF and Sb2 confirmed that endocytosed pro-BDNF was routed into Sb2-containing vesicles. Taken together, this study shows that endocytic vesicles expressing p75NTR represent the main storage/recycling compartment for endocytosed pro-BDNF before routing it to the SNARE-dependent secretory pathway (Bergami et al., 2008).
Astrocytes can also produce chemokines that directly modulate synaptic activity. Under neuroinflammatory conditions, activated astrocytes, as well as other immune cells in the CNS, can produce cytokines and chemokines such as interferon-α/β, ; interleukin(IL)-6, IL-8, IL-10, IL-12/IL-23, IL-17, IL-27, chemokine (C-C motif) ligand (CCL)2, CCL3, CCL4, CCL5, CCL10, chemokine CXC motif ligand (CXCL)10, CXCL12, insulin-like growth factor-1, transforming growth factor- β, tumor necrosis factor-α (TNF-α), granulocyte macrophage-colony stimulating factor and prostaglandin E2 (PGE2)(Montgomery, 1994; Constantinescu et al., 2005; Koizumi et al., 2005; Baker et al., 2009). Very few studies have addressed the potential role of cytokine and chemokine expression in modulating exocytosis and synaptic activity. However, one intriguing study demonstrated that activation of CXC receptor 4 (CXCR4), either by CXCL12 or by the human immunodeficiency virus peptide gp120, induces release of TNF-α from astrocytes and microglia (Bezzi et al., 2001). TNF-α, acting through TNF receptor 1, induced glutamate release from astrocytes, but not from microglia. In these studies, TNF-α induced production of PGE2, which increased [Ca2+]i and induced glutamate release from astrocytes. Excessive glutamate release can induce neuron cell death via excitotoxicity. Glutamate released from astrocytes can directly induce TNF-α production from microglia, providing a feed-forward mechanism of inflammation-induced excitotoxicity. Indeed, it was demonstrated that gp120-induced TNF-α production and subsequent glutamate release induced excitotoxic neuron death (Bezzi et al., 2001). TIRF studies demonstrated that CXCR4-mediated glutamate release occurs on the order of a few hundred milliseconds, which is longer than for synaptic vesicle release but faster than dense-core granule release from neurosecretory cells (Cali et al., 2008). CXCR4 is expressed on presynaptic terminals of neurons as well as on astrocytes (Banisadr et al., 2002; Tran et al., 2007). Additionally, when released from astrocytes, TNF-α can cause an increase in the surface expression of neuronal α-amino-3-hydroxy-5-methyl-isoxazole propionate (AMPA) receptors, and, thus, this factor plays a role in controlling synaptic strength (Beattie et al., 2002). Therefore, these results demonstrate how astrocytes may serve as integrators that recognize and respond to environmental signals, such as chemokines, and generate neuromodulatory responses, such as glutamate release and regulation of surface receptor expression.
Exocytosis as the mechanism for delivery of the plasma membrane signaling proteins
Vesicles may not only carry lumenal cargo, but also plasma membrane transporters (Figure 1) such as glutamate transporters (Robinson, 2002) which take up glutamate from the extracellular space [reviewed in (Danbolt, 2001)]. Mennerick and Zorumski (Mennerick and Zorumski, 1994) demonstrated that neurons can signal to astrocytes through the synaptic release of glutamate, which activates astrocytic glutamate uptake systems. In turn, astrocytic uptake of glutamate from the extracellular space modulates the kinetics of synaptic neurotransmission. It should be noted that pathophysiological events, such as ischemia, may favor transporters to operate in reverse, so that astrocytes can also utilize glutamate transporters as a mechanism of release (Szatkowski et al., 1990). Thus, there is a need for studying the traffic of EAAT in astrocytes. One study demonstrated that transfection of EAAT2 tagged with EGFP into astrocytes predominantly labels the plasma membrane (Stenovec et al., 2008). Ionomycin induces exocytosis in these cells, which correlates with local increases or decreases in plasma membrane fluorescence, indicating Ca2+-induced trafficking of EAAT2-EGFP. Furthermore, stimulation of astrocytes with PMA induces internalization of EAAT2-EGFP. This internalization is blocked by co-transfection of EAAT2-EGFP with a dominant negative form of dynamin, suggesting that internalization is likely clathrin-mediated. One can envision that similar mechanisms exist to regulate trafficking of transmembrane receptors as well.
Astrocytes possess a multitude of G-protein-coupled receptors (GPCR) [reviewed in e.g., (Verkhratsky, 2009)] that respond to signaling molecules released by neurons and themselves, including the CB1R (Rodriguez et al., 2001), which is thought to mediate neuron-astrocyte communication. Navarrete and Araque (Navarrete and Araque, 2008) demonstrated that in hippocampal slices depolarization of neurons leads to release of endocannbinoids that in turn signal to adjacent astrocytes by activating CB1Rs. This causes an increase in the astrocytic [Ca2+]i, triggering stimulated glutamate release, which then activates NMDA receptors on nearby neurons. The existence of this endocannabinoid-gluatamte signaling pathway underscores the importance of understanding the CB1R vesicular traffic in astrocytes. Protein trafficking plays an important role in regulating CB1R expression with receptors changing localization between the plasma membrane and intracellular compartments. In certain cell types at rest, CB1R is constitutively endocytosed leading to a predominantly intracellular localization (Leterrier et al., 2004; McDonald et al., 2007). Therefore, endocytic trafficking can be an important regulator of CB1R availability at the plasma membrane. The characteristics of constitutive CB1R trafficking within astrocytes have been recently investigated (Osborne et al., 2009) using recombinant fluorescent protein chimeras of the CB1R. Whether intracellular Ca2+ dynamics affect trafficking of CB1R and its delivery to the plasma mebrane remains to be determined. However, vesicular (intracellular) CB1 receptors can still engage cell signaling (Gomes et al., 2009) which may be activated by lipophillic (membrane-permeant) cannabinoid ligands or via innate, constitutive activity. Clearly, further studies are necessary to elucidate the mechanisms governing the trafficking of plasma membrane transporters and receptors.
Concluding remarks
Astrocytes can receive signals from neighboring neural cells and respond to these inputs with various delays. An important functional module that can contribute to the variable responsiveness is exocytosis, representing the fusion of vesicle membrane with the plasma membrane (Table 1 summarizes properties of vesicular release from astrocytes). Astrocytes can synthesize and store gliotransmitters, i.e., amino acids, ATP and peptides, in SNARE-associated vesicles. Moreover, exocytosis participates in the release of gliotransmitters as well as in the insertion of membrane signaling proteins into the plasma membrane.
Table 1.
Properties of vesicular release from astrocytes.
| Vesicle Cargo | Release Latency | Vesicle Appearance | Vesicle Size | Recycling |
|---|---|---|---|---|
| Amino Acids | milliseconds | clear core | ~ 30 - 50 nm | fast |
| Nucleotides | seconds | dense, not so dense, and clear core | ~ 100 nm | presumably fast |
| Peptides | seconds | dense and not so dense core | ~ 30-100 nm | slow |
Note: Vesicular size in astrocytes seems to substantially vary (see text for details)
Acknowledgments
The authors’ work is supported by grants from the National Institute of Mental Health (R01 MH 069791 to VP), National Science Foundation (CBET 0943343 to VP) and from the Slovenian Research Agency (P3 310 381; J3 -0133; J3-0031, J3-9417 to RZ). We dedicate this work to the late Glenn I. Hatton, whose energy and creativity inspired new views of astrocyte-neuronal interactions.
Abbreviations
- ANP
atrial natriuretic peptide
- ATP
adenosine 5’-triphosphate
- BAPTA
1,2-bis(o-aminophenoxy)ethane-N,N,N’,N’-tetraacetic acid
- BBB
brain barrier
- BDNF
brain-derived neurotrophic factor
- [Ca2+]i
intracellular calcium ion concentration
- cAMP
cyclic adenosine monophosphate
- CB1R
cannabinoid receptor 1
- CCL
chemokine (C-C motif) ligand
- Cm
membrane capacitance
- CNS
central nervous system
- CXC
chemokine CXC motif
- CXCL
CXC ligand
- CXCR
CXC receptor
- EGFP
enhanced green fluorescent protein
- EAAT
excitatory amino acid transporter
- EM
electron microscopy
- ER
endoplasmic reticulum
- IP3
inositol 1,4,5-trisphosphate
- IEM
immuno EM
- IL
interleukin
- Munc
mammalian UNCoordinated
- NMDA
N-methyl-D-aspartate
- NPY
neuropeptide Y
- PGE2
prostaglandin E2
- PMA
phorbol 12-myristate 13-acetate
- Sb2
synaptobrevin 2
- SNAP-23
synaptosome-associated protein of 23 kDa
- SNARE
the soluble N-ethyl maleimide-sensitive fusion protein attachment protein receptor
- SOC
store-operated Ca2+
- TCA
tricarboxylic acid
- TIRF
total internal refection fluorescence
- TRPC
canonical transient receptor potential
- VGLUT
vesicular glutamate transporter
- V-ATPase
the vacuolar type of proton ATPase
- YFP
yellow fluorescent protein
- 2-APB
2-aminoethyl ester
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson CM, Nedergaard M. Astrocyte-mediated control of cerebral microcirculation. Trends Neurosci. 2003;26:340–344. doi: 10.1016/S0166-2236(03)00141-3. author reply 344-345. [DOI] [PubMed] [Google Scholar]
- Anlauf E, Derouiche A. Astrocytic exocytosis vesicles and glutamate: a high-resolution immunofluorescence study. Glia. 2005;49:96–106. doi: 10.1002/glia.20094. [DOI] [PubMed] [Google Scholar]
- Araque A, Sanzgiri RP, Parpura V, Haydon PG. Calcium elevation in astrocytes causes an NMDA receptor-dependent increase in the frequency of miniature synaptic currents in cultured hippocampal neurons. J Neurosci. 1998a;18:6822–6829. doi: 10.1523/JNEUROSCI.18-17-06822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur J Neurosci. 1998b;10:2129–2142. doi: 10.1046/j.1460-9568.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- Araque A, Li N, Doyle RT, Haydon PG. SNARE protein-dependent glutamate release from astrocytes. J Neurosci. 2000;20:666–673. doi: 10.1523/JNEUROSCI.20-02-00666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baertschi AJ, Monnier D, Schmidt U, Levitan ES, Fakan S, Roatti A. Acid prohormone sequence determines size, shape, and docking of secretory vesicles in atrial myocytes. Circ Res. 2001;89:E23–29. doi: 10.1161/hh1501.095715. [DOI] [PubMed] [Google Scholar]
- Baker BJ, Akhtar LN, Benveniste EN. SOCS1 and SOCS3 in the control of CNS immunity. Trends Immunol. 2009;30:392–400. doi: 10.1016/j.it.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal-Price A, Moneer Z, Brown GC. Nitric oxide induces rapid, calcium-dependent release of vesicular glutamate and ATP from cultured rat astrocytes. Glia. 2002;40:312–323. doi: 10.1002/glia.10124. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Fontanges P, Haour F, Kitabgi P, Rostene W, Melik Parsadaniantz S. Neuroanatomical distribution of CXCR4 in adult rat brain and its localization in cholinergic and dopaminergic neurons. Eur J Neurosci. 2002;16:1661–1671. doi: 10.1046/j.1460-9568.2002.02237.x. [DOI] [PubMed] [Google Scholar]
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- Beck A, Nieden RZ, Schneider HP, Deitmer JW. Calcium release from intracellular stores in rodent astrocytes and neurons in situ. Cell Calcium. 2004;35:47–58. doi: 10.1016/s0143-4160(03)00171-4. [DOI] [PubMed] [Google Scholar]
- Bergami M, Santi S, Formaggio E, Cagnoli C, Verderio C, Blum R, Berninger B, Matteoli M, Canossa M. Uptake and recycling of pro-BDNF for transmitter-induced secretion by cortical astrocytes. J Cell Biol. 2008;183:213–221. doi: 10.1083/jcb.200806137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergersen LH, Gundersen V. Morphological evidence for vesicular glutamate release from astrocytes. Neuroscience. 2009;158:260–265. doi: 10.1016/j.neuroscience.2008.03.074. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhauser C, Pilati E, Volterra A. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci. 2004;7:613–620. doi: 10.1038/nn1246. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, Volterra A. CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4:702–710. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- Bianco F, Pravettoni E, Colombo A, Schenk U, Moller T, Matteoli M, Verderio C. Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J Immunol. 2005;174:7268–7277. doi: 10.4049/jimmunol.174.11.7268. [DOI] [PubMed] [Google Scholar]
- Bowser DN, Khakh BS. Two forms of single-vesicle astrocyte exocytosis imaged with total internal reflection fluorescence microscopy. Proc Natl Acad Sci U S A. 2007;104:4212–4217. doi: 10.1073/pnas.0607625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calegari F, Coco S, Taverna E, Bassetti M, Verderio C, Corradi N, Matteoli M, Rosa P. A regulated secretory pathway in cultured hippocampal astrocytes. J Biol Chem. 1999;274:22539–22547. doi: 10.1074/jbc.274.32.22539. [DOI] [PubMed] [Google Scholar]
- Cali C, Marchaland J, Regazzi R, Bezzi P. SDF 1-alpha (CXCL12) triggers glutamate exocytosis from astrocytes on a millisecond time scale: imaging analysis at the single-vesicle level with TIRF microscopy. J Neuroimmunol. 2008;198:82–91. doi: 10.1016/j.jneuroim.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Chen X, Wang L, Zhou Y, Zheng LH, Zhou Z. “Kiss-and-run” glutamate secretion in cultured and freshly isolated rat hippocampal astrocytes. J Neurosci. 2005;25:9236–9243. doi: 10.1523/JNEUROSCI.1640-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coco S, Calegari F, Pravettoni E, Pozzi D, Taverna E, Rosa P, Matteoli M, Verderio C. Storage and release of ATP from astrocytes in culture. J Biol Chem. 2003;278:1354–1362. doi: 10.1074/jbc.M209454200. [DOI] [PubMed] [Google Scholar]
- Constantinescu CS, Tani M, Ransohoff RM, Wysocka M, Hilliard B, Fujioka T, Murphy S, Tighe PJ, Das Sarma J, Trinchieri G, Rostami A. Astrocytes as antigen-presenting cells: expression of IL-12/IL-23. J Neurochem. 2005;95:331–340. doi: 10.1111/j.1471-4159.2005.03368.x. [DOI] [PubMed] [Google Scholar]
- Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci U S A. 1998;95:15735–15740. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crippa D, Schenk U, Francolini M, Rosa P, Verderio C, Zonta M, Pozzan T, Matteoli M, Carmignoto G. Synaptobrevin2-expressing vesicles in rat astrocytes: insights into molecular characterization, dynamics and exocytosis. J Physiol. 2006;570:567–582. doi: 10.1113/jphysiol.2005.094052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Dannies PS. Protein hormone storage in secretory granules: mechanisms for concentration and sorting. Endocr Rev. 1999;20:3–21. doi: 10.1210/edrv.20.1.0354. [DOI] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Del Castillo J, Katz B. Quantal components of the end-plate potential. J Physiol. 1954;124:560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domercq M, Brambilla L, Pilati E, Marchaland J, Volterra A, Bezzi P. P2Y1 receptor-evoked glutamate exocytosis from astrocytes: control by tumor necrosis factor-alpha and prostaglandins. J Biol Chem. 2006;281:30684–30696. doi: 10.1074/jbc.M606429200. [DOI] [PubMed] [Google Scholar]
- Duan S, Anderson CM, Keung EC, Chen Y, Chen Y, Swanson RA. P2X7 receptor-mediated release of excitatory amino acids from astrocytes. J Neurosci. 2003;23:1320–1328. doi: 10.1523/JNEUROSCI.23-04-01320.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD, Burnstock G. Purinergic signalling in neuron-glia interactions. Nat Rev Neurosci. 2006;7:423–436. doi: 10.1038/nrn1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana A, Fierz W, Wekerle H. Astrocytes present myelin basic protein to encephalitogenic T-cell lines. Nature. 1984;307:273–276. doi: 10.1038/307273a0. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Burman J, Qureshi T, Tran CH, Proctor J, Johnson J, Zhang H, Sulzer D, Copenhagen DR, Storm-Mathisen J, Reimer RJ, Chaudhry FA, Edwards RH. The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proc Natl Acad Sci U S A. 2002;99:14488–14493. doi: 10.1073/pnas.222546799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girvin AM, Gordon KB, Welsh CJ, Clipstone NA, Miller SD. Differential abilities of central nervous system resident endothelial cells and astrocytes to serve as inducible antigen-presenting cells. Blood. 2002;99:3692–3701. doi: 10.1182/blood-2001-12-0229. [DOI] [PubMed] [Google Scholar]
- Golovina VA. Visualization of localized store-operated calcium entry in mouse astrocytes. Close proximity to the endoplasmic reticulum. J Physiol. 2005;564:737–749. doi: 10.1113/jphysiol.2005.085035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Grushko JS, Golebiewska U, Hoogendoorn S, Gupta A, Heimann AS, Ferro ES, Scarlata S, Fricker LD, Devi LA. Novel endogenous peptide agonists of cannabinoid receptors. Faseb J. 2009 Apr 20; doi: 10.1096/fj.09-132142. On-Line. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi M, Maratos M, Verma A. Transient receptor potential channel activation causes a novel form of [Ca2+]I oscillations and is not involved in capacitative Ca2+ entry in glial cells. J Neurosci. 2003;23:4737–4745. doi: 10.1523/JNEUROSCI.23-11-04737.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Haydon PG. Tripartite synapses: roles for astrocytic purines in the control of synaptic physiology and behavior. Neuropharmacology. 2009;57:343–346. doi: 10.1016/j.neuropharm.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L, Gibbs ME. What learning in day-old chickens can teach a neurochemist: focus on astrocyte metabolism. J Neurochem. 2009;109(Suppl 1):10–16. doi: 10.1111/j.1471-4159.2009.05939.x. [DOI] [PubMed] [Google Scholar]
- Hertz L, Dringen R, Schousboe A, Robinson SR. Astrocytes: glutamate producers for neurons. J Neurosci Res. 1999;57:417–428. [PubMed] [Google Scholar]
- Hua X, Malarkey EB, Sunjara V, Rosenwald SE, Li WH, Parpura V. Ca2+-dependent glutamate release involves two classes of endoplasmic reticulum Ca2+ stores in astrocytes. J Neurosci Res. 2004;76:86–97. doi: 10.1002/jnr.20061. [DOI] [PubMed] [Google Scholar]
- Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E. Pannexin 1: the molecular substrate of astrocyte “hemichannels”. J Neurosci. 2009;29:7092–7097. doi: 10.1523/JNEUROSCI.6062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocenti B, Parpura V, Haydon PG. Imaging extracellular waves of glutamate during calcium signaling in cultured astrocytes. J Neurosci. 2000;20:1800–1808. doi: 10.1523/JNEUROSCI.20-05-01800.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeremic A, Jeftinija K, Stevanovic J, Glavaski A, Jeftinija S. ATP stimulates calcium-dependent glutamate release from cultured astrocytes. J Neurochem. 2001;77:664–675. doi: 10.1046/j.1471-4159.2001.00272.x. [DOI] [PubMed] [Google Scholar]
- Kang N, Xu J, Xu Q, Nedergaard M, Kang J. Astrocytic glutamate release-induced transient depolarization and epileptiform discharges in hippocampal CA1 pyramidal neurons. J Neurophysiol. 2005;94:4121–4130. doi: 10.1152/jn.00448.2005. [DOI] [PubMed] [Google Scholar]
- Koizumi S, Fujishita K, Inoue K. Regulation of cell-to-cell communication mediated by astrocytic ATP in the CNS. Purinergic Signal. 2005;1:211–217. doi: 10.1007/s11302-005-6321-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreft M, Stenovec M, Rupnik M, Grilc S, Krzan M, Potokar M, Pangrsic T, Haydon PG, Zorec R. Properties of Ca2+-dependent exocytosis in cultured astrocytes. Glia. 2004;46:437–445. doi: 10.1002/glia.20018. [DOI] [PubMed] [Google Scholar]
- Krzan M, Stenovec M, Kreft M, Pangrsic T, Grilc S, Haydon PG, Zorec R. Calcium-dependent exocytosis of atrial natriuretic peptide from astrocytes. J Neurosci. 2003;23:1580–1583. doi: 10.1523/JNEUROSCI.23-05-01580.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leterrier C, Bonnard D, Carrel D, Rossier J, Lenkei Z. Constitutive endocytic cycle of the CB1 cannabinoid receptor. J Biol Chem. 2004;279:36013–36021. doi: 10.1074/jbc.M403990200. [DOI] [PubMed] [Google Scholar]
- Maienschein V, Marxen M, Volknandt W, Zimmermann H. A plethora of presynaptic proteins associated with ATP-storing organelles in cultured astrocytes. Glia. 1999;26:233–244. doi: 10.1002/(sici)1098-1136(199905)26:3<233::aid-glia5>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Malarkey EB, Ni Y, Parpura V. Ca2+ entry through TRPC1 channels contributes to intracellular Ca2+ dynamics and consequent glutamate release from rat astrocytes. Glia. 2008 doi: 10.1002/glia.20656. [DOI] [PubMed] [Google Scholar]
- Marchaland J, Cali C, Voglmaier SM, Li H, Regazzi R, Edwards RH, Bezzi P. Fast subplasma membrane Ca2+ transients control exo-endocytosis of synaptic-like microvesicles in astrocytes. J Neurosci. 2008;28:9122–9132. doi: 10.1523/JNEUROSCI.0040-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau M, Galli T, Baux G, Mothet JP. Confocal imaging and tracking of the exocytotic routes for D-serine-mediated gliotransmission. Glia. 2008;56:1271–1284. doi: 10.1002/glia.20696. [DOI] [PubMed] [Google Scholar]
- McDonald NA, Henstridge CM, Connolly CN, Irving AJ. An essential role for constitutive endocytosis, but not activity, in the axonal targeting of the CB1 cannabinoid receptor. Mol Pharmacol. 2007;71:976–984. doi: 10.1124/mol.106.029348. [DOI] [PubMed] [Google Scholar]
- McKenzie JC, Juan YW, Thomas CR, Berman NE, Klein RM. Atrial natriuretic peptide-like immunoreactivity in neurons and astrocytes of human cerebellum and inferior olivary complex. J Histochem Cytochem. 2001;49:1453–1467. doi: 10.1177/002215540104901113. [DOI] [PubMed] [Google Scholar]
- Mennerick S, Zorumski CF. Glial contributions to excitatory neurotransmission in cultured hippocampal cells. Nature. 1994;368:59–62. doi: 10.1038/368059a0. [DOI] [PubMed] [Google Scholar]
- Miyata H, Silverman HS, Sollott SJ, Lakatta EG, Stern MD, Hansford RG. Measurement of mitochondrial free Ca2+ concentration in living single rat cardiac myocytes. Am J Physiol. 1991;261:H1123–1134. doi: 10.1152/ajpheart.1991.261.4.H1123. [DOI] [PubMed] [Google Scholar]
- Montana V, Ni Y, Sunjara V, Hua X, Parpura V. Vesicular glutamate transporter-dependent glutamate release from astrocytes. J Neurosci. 2004;24:2633–2642. doi: 10.1523/JNEUROSCI.3770-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montana V, Malarkey EB, Verderio C, Matteoli M, Parpura V. Vesicular transmitter release from astrocytes. Glia. 2006;54:700–715. doi: 10.1002/glia.20367. [DOI] [PubMed] [Google Scholar]
- Montgomery DL. Astrocytes: form, functions, and roles in disease. Vet Pathol. 1994;31:145–167. doi: 10.1177/030098589403100201. [DOI] [PubMed] [Google Scholar]
- Mothet JP, Pollegioni L, Ouanounou G, Martineau M, Fossier P, Baux G. Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter D-serine. Proc Natl Acad Sci U S A. 2005;102:5606–5611. doi: 10.1073/pnas.0408483102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Iga K, Shibata T, Shudo M, Kataoka K. Glial plasmalemmal vesicles: a subcellular fraction from rat hippocampal homogenate distinct from synaptosomes. Glia. 1993;9:48–56. doi: 10.1002/glia.440090107. [DOI] [PubMed] [Google Scholar]
- Navarrete M, Araque A. Endocannabinoids mediate neuron-astrocyte communication. Neuron. 2008;57:883–893. doi: 10.1016/j.neuron.2008.01.029. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Newman EA. New roles for astrocytes: regulation of synaptic transmission. Trends Neurosci. 2003;26:536–542. doi: 10.1016/S0166-2236(03)00237-6. [DOI] [PubMed] [Google Scholar]
- Ni Y, Parpura V. Dual regulation of Ca2+-dependent glutamate release from astrocytes: Vesicular glutamate transporters and cytosolic glutamate levels. Glia. 2009;57:1296–1305. doi: 10.1002/glia.20849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Y, Malarkey EB, Parpura V. Vesicular release of glutamate mediates bidirectional signaling between astrocytes and neurons. J Neurochem. 2007;103:1273–1284. doi: 10.1111/j.1471-4159.2007.04864.x. [DOI] [PubMed] [Google Scholar]
- Oliet SH, Mothet JP. Regulation of N-methyl-D-aspartate receptors by astrocytic D-serine. Neuroscience. 2009;158:275–283. doi: 10.1016/j.neuroscience.2008.01.071. [DOI] [PubMed] [Google Scholar]
- Osborne KD, Lee W, Malarkey EB, Irving AJ, Parpura V. Dynamic imaging of cannabinoid receptor 1 vesicular trafficking in cultured astrocytes. ASN Neuro. 2009 doi: 10.1042/AN20090040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paco S, Margeli MA, Olkkonen VM, Imai A, Blasi J, Fischer-Colbrie R, Aguado F. Regulation of exocytotic protein expression and Ca2+-dependent peptide secretion in astrocytes. J Neurochem. 2009;110:143–156. doi: 10.1111/j.1471-4159.2009.06116.x. [DOI] [PubMed] [Google Scholar]
- Pangrsic T, Potokar M, Stenovec M, Kreft M, Fabbretti E, Nistri A, Pryazhnikov E, Khiroug L, Giniatullin R, Zorec R. Exocytotic release of ATP from cultured astrocytes. J Biol Chem. 2007;282:28749–28758. doi: 10.1074/jbc.M700290200. [DOI] [PubMed] [Google Scholar]
- Parpura V, Haydon PG. Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proc Natl Acad Sci U S A. 2000;97:8629–8634. doi: 10.1073/pnas.97.15.8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Fang Y, Basarsky T, Jahn R, Haydon PG. Expression of synaptobrevin II, cellubrevin and syntaxin but not SNAP-25 in cultured astrocytes. FEBS Lett. 1995;377:489–492. doi: 10.1016/0014-5793(95)01401-2. [DOI] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Pasantes Morales H, Schousboe A. Volume regulation in astrocytes: a role for taurine as an osmoeffector. J Neurosci Res. 1988;20:503–509. doi: 10.1002/jnr.490200415. [DOI] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Pasti L, Zonta M, Pozzan T, Vicini S, Carmignoto G. Cytosolic calcium oscillations in astrocytes may regulate exocytotic release of glutamate. J Neurosci. 2001;21:477–484. doi: 10.1523/JNEUROSCI.21-02-00477.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfrieger FW, Barres BA. Synaptic efficacy enhanced by glial cells in vitro. Science. 1997;277:1684–1687. doi: 10.1126/science.277.5332.1684. [DOI] [PubMed] [Google Scholar]
- Pizzo P, Burgo A, Pozzan T, Fasolato C. Role of capacitative calcium entry on glutamate-induced calcium influx in type-I rat cortical astrocytes. J Neurochem. 2001;79:98–109. doi: 10.1046/j.1471-4159.2001.00539.x. [DOI] [PubMed] [Google Scholar]
- Potokar M, Kreft M, Pangrsic T, Zorec R. Vesicle mobility studied in cultured astrocytes. Biochem Biophys Res Commun. 2005;329:678–683. doi: 10.1016/j.bbrc.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Potokar M, Stenovec M, Kreft M, Kreft ME, Zorec R. Stimulation inhibits the mobility of recycling peptidergic vesicles in astrocytes. Glia. 2008;56:135–144. doi: 10.1002/glia.20597. [DOI] [PubMed] [Google Scholar]
- Potokar M, Kreft M, Lee SY, Takano H, Haydon PG, Zorec R. Trafficking of astrocytic vesicles in hippocampal slices. Biochem Biophys Res Commun. 2009 doi: 10.1016/j.bbrc.2009.10.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potokar M, Kreft M, Li L, Daniel Andersson J, Pangrsic T, Chowdhury HH, Pekny M, Zorec R. Cytoskeleton and vesicle mobility in astrocytes. Traffic. 2007;8:12–20. doi: 10.1111/j.1600-0854.2006.00509.x. [DOI] [PubMed] [Google Scholar]
- Pryazhnikov E, Khiroug L. Sub-micromolar increase in [Ca2+]i triggers delayed exocytosis of ATP in cultured astrocytes. Glia. 2008;56:38–49. doi: 10.1002/glia.20590. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy P, Whim MD. Trafficking and fusion of neuropeptide Y-containing dense-core granules in astrocytes. J Neurosci. 2008;28:13815–13827. doi: 10.1523/JNEUROSCI.5361-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes RC, Parpura V. Mitochondria modulate Ca2+-dependent glutamate release from rat cortical astrocytes. J Neurosci. 2008;28:9682–9691. doi: 10.1523/JNEUROSCI.3484-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MB. Regulated trafficking of neurotransmitter transporters: common notes but different melodies. J Neurochem. 2002;80:1–11. doi: 10.1046/j.0022-3042.2001.00698.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez JJ, Mackie K, Pickel VM. Ultrastructural localization of the CB1 cannabinoid receptor in mu-opioid receptor patches of the rat Caudate putamen nucleus. J Neurosci. 2001;21:823–833. doi: 10.1523/JNEUROSCI.21-03-00823.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose C, Kresse W, Kettenmann H. Acute insult of ammonia leads to calcium-dependent glutamate release from cultured astrocytes, an effect of pH. J Biol Chem. 2005;280:20937–20944. doi: 10.1074/jbc.M412448200. [DOI] [PubMed] [Google Scholar]
- Rosenberg PA, Knowles R, Knowles KP, Li Y. Beta-adrenergic receptor-mediated regulation of extracellular adenosine in cerebral cortex in culture. J Neurosci. 1994;14:2953–2965. doi: 10.1523/JNEUROSCI.14-05-02953.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini BL, Regehr WG. Timing of synaptic transmission. Annu Rev Physiol. 1999;61:521–542. doi: 10.1146/annurev.physiol.61.1.521. [DOI] [PubMed] [Google Scholar]
- Sawada K, Echigo N, Juge N, Miyaji T, Otsuka M, Omote H, Yamamoto A, Moriyama Y. Identification of a vesicular nucleotide transporter. Proc Natl Acad Sci U S A. 2008;105:5683–5686. doi: 10.1073/pnas.0800141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell MJ, Molliver ME, Snyder SH. D-serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci U S A. 1995;92:3948–3952. doi: 10.1073/pnas.92.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson PB, Russell JT. Role of mitochondrial Ca2+ regulation in neuronal and glial cell signalling. Brain Res Brain Res Rev. 1998;26:72–81. doi: 10.1016/s0165-0173(97)00056-8. [DOI] [PubMed] [Google Scholar]
- Sorensen JB, Nagy G, Varoqueaux F, Nehring RB, Brose N, Wilson MC, Neher E. Differential control of the releasable vesicle pools by SNAP-25 splice variants and SNAP-23. Cell. 2003;114:75–86. doi: 10.1016/s0092-8674(03)00477-x. [DOI] [PubMed] [Google Scholar]
- Stenovec M, Kreft M, Poberaj I, Betz WJ, Zorec R. Slow spontaneous secretion from single large dense-core vesicles monitored in neuroendocrine cells. Faseb J. 2004;18:1270–1272. doi: 10.1096/fj.03-1397fje. [DOI] [PubMed] [Google Scholar]
- Stenovec M, Kreft M, Grilc S, Pangrsic T, Zorec R. EAAT2 density at the astrocyte plasma membrane and Ca2+-regulated exocytosis. Mol Membr Biol. 2008;25:203–215. doi: 10.1080/09687680701790925. [DOI] [PubMed] [Google Scholar]
- Stenovec M, Kreft M, Grilc S, Potokar M, Kreft ME, Pangrsic T, Zorec R. Ca2+-dependent mobility of vesicles capturing anti-VGLUT1 antibodies. Exp Cell Res. 2007;313:3809–3818. doi: 10.1016/j.yexcr.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Stigliani S, Zappettini S, Raiteri L, Passalacqua M, Melloni E, Venturi C, Tacchetti C, Diaspro A, Usai C, Bonanno G. Glia re-sealed particles freshly prepared from adult rat brain are competent for exocytotic release of glutamate. J Neurochem. 2006;96:656–668. doi: 10.1111/j.1471-4159.2005.03631.x. [DOI] [PubMed] [Google Scholar]
- Szatkowski M, Barbour B, Attwell D. Non-vesicular release of glutamate from glial cells by reversed electrogenic glutamate uptake. Nature. 1990;348:443–446. doi: 10.1038/348443a0. [DOI] [PubMed] [Google Scholar]
- Takemura H, Putney JW., Jr Capacitative calcium entry in parotid acinar cells. Biochem J. 1989;258:409–412. doi: 10.1042/bj2580409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PB, Banisadr G, Ren D, Chenn A, Miller RJ. Chemokine receptor expression by neural progenitor cells in neurogenic regions of mouse brain. J Comp Neurol. 2007;500:1007–1033. doi: 10.1002/cne.21229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardjan N, Stenovec M, Jorgacevski J, Kreft M, Zorec R. Subnanometer fusion pores in spontaneous exocytosis of peptidergic vesicles. J Neurosci. 2007;27:4737–4746. doi: 10.1523/JNEUROSCI.0351-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A. Neurotransmitter receptors in astrocytes. In: Parpura V, Haydon PG, editors. Astrocytes in (patho)physiology of the nervous system. Boston, MA: Springer; 2009. pp. 50–67. [Google Scholar]
- Warr O, Takahashi M, Attwell D. Modulation of extracellular glutamate concentration in rat brain slices by cystine-glutamate exchange. J Physiol. 1999;514(Pt 3):783–793. doi: 10.1111/j.1469-7793.1999.783ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergaard N, Drejer J, Schousboe A, Sonnewald U. Evaluation of the importance of transamination versus deamination in astrocytic metabolism of [U-13C]glutamate. Glia. 1996;17:160–168. doi: 10.1002/(SICI)1098-1136(199606)17:2<160::AID-GLIA7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Wilhelm A, Volknandt W, Langer D, Nolte C, Kettenmann H, Zimmermann H. Localization of SNARE proteins and secretory organelle proteins in astrocytes in vitro and in situ. Neurosci Res. 2004;48:249–257. doi: 10.1016/j.neures.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Winkler H, Fischer-Colbrie R. The chromogranins A and B: the first 25 years and future perspectives. Neuroscience. 1992;49:497–528. doi: 10.1016/0306-4522(92)90222-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosker H, Sheth KN, Takahashi M, Mothet JP, Brady RO, Jr, Ferris CD, Snyder SH. Purification of serine racemase: biosynthesis of the neuromodulator D-serine. Proc Natl Acad Sci U S A. 1999;96:721–725. doi: 10.1073/pnas.96.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Peng H, Kang N, Zhao Z, Lin JH, Stanton PK, Kang J. Glutamate-induced exocytosis of glutamate from astrocytes. J Biol Chem. 2007;282:24185–24197. doi: 10.1074/jbc.M700452200. [DOI] [PubMed] [Google Scholar]
- Zhang JM, Wang HK, Ye CQ, Ge W, Chen Y, Jiang ZL, Wu CP, Poo MM, Duan S. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron. 2003;40:971–982. doi: 10.1016/s0896-6273(03)00717-7. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Fukuda M, Van Bockstaele E, Pascual O, Haydon PG. Synaptotagmin IV regulates glial glutamate release. Proc Natl Acad Sci U S A. 2004a;101:9441–9446. doi: 10.1073/pnas.0401960101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Pangrsic T, Kreft M, Krzan M, Li N, Sul JY, Halassa M, Van Bockstaele E, Zorec R, Haydon PG. Fusion-related release of glutamate from astrocytes. J Biol Chem. 2004b;279:12724–12733. doi: 10.1074/jbc.M312845200. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Chen G, Zhou W, Song A, Xu T, Luo Q, Wang W, Gu XS, Duan S. Regulated ATP release from astrocytes through lysosome exocytosis. Nat Cell Biol. 2007;9:945–953. doi: 10.1038/ncb1620. [DOI] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]