Abstract
Following platelet activation, platelets undergo a dramatic shape change mediated by the actin cytoskeleton and accompanied by secretion of granule contents. While the actin cytoskeleton is thought to influence platelet granule secretion, the mechanism for this putative regulation is not known. We found that disruption of the actin cytoskeleton by latrunculin A inhibited α-granule secretion induced by several different platelet agonists without significantly affecting activation-induced platelet aggregation. In a cell-free secretory system, platelet cytosol was required for α-granule secretion. Inhibition of actin polymerization prevented α-granule secretion in this system and purified platelet actin could substitute for platelet cytosol to support α–granule secretion. To determine whether SNAREs physically associate with the actin cytoskeleton, we isolated the Triton X-100 insoluble actin cytoskeleton from platelets. VAMP-8 and syntaxin-2 associated only with actin cytoskeletons of activated platelets. Syntaxin-4 and SNAP-23 associated with cytoskeletons isolated from either resting or activated platelets. When syntaxin-4 and SNAP-23 were tested for actin binding in a purified protein system, only syntaxin-4 associated directly with polymerized platelet actin. These data show that the platelet cytoskeleton interacts with select SNAREs and that actin polymerization facilitates α-granule release.
The role of the actin cytoskeleton in granule exocytosis is enigmatic. It has been demonstrated to act both as a physical barrier that limits granule secretion and as a positive regulator of membrane fusion and cargo release. The ability of the resting actin cytoskeleton to serve as a barrier to granule secretion has been demonstrated in neutrophils, neurons, chromaffin cells, melanotrophs, pancreatic beta cells, and acinar cells (1-6). We have previously demonstrated that platelet granules are coated with actin and that the actin cytoskeleton impedes platelet dense granule and α-granule release (7). Partial disruption of this barrier results in augmented and more rapid release of granule contents from platelets. This actin cytoskeletal barrier may help prevent unregulated release of thrombogenic substances into the circulation (7).
Yet accumulating evidence indicates that actin polymerization can promote membrane fusion. Actin polymerization contributes to homotypic fusion of yeast vacuoles (8), fusion of phagosomes with endocytotic organelles (9) as well as secretion of granules from neuroendocrine cells (6, 10, 11), and mast cells (12). In some cells, actomyosin contraction and/or dynamic actin polymerization facilitates transport of secretory granules to the plasma membrane (13-15). In other systems, actin polymerization is required in a terminal step of membrane fusion, following priming and docking (8). Several studies have demonstrated that F-actin interacts directly with components of the secretory machinery (16-21). Yet the significance of these interactions remains uncertain.
One component of the secretory machinery with which F-actin has been demonstrated to interact is soluble NEM-sensitive attachment protein receptors (SNAREs)1. SNAREs are membrane-associated proteins that associate via hydrophobic coiled-coil domains and serve a central function in membrane fusion (22). SNAREs can be classified as vesicle-associated SNAREs (vSNAREs), which localize to the outer membrane of vesicles, and target-associated SNAREs (tSNAREs), which localize to the cytosolic leaflet of target membranes. Both vesicle-associated membrane proteins (VAMPs) 3 and 8 are SNAREs that have been demonstrated to function in platelet granule secretion (23-25); however, VAMP-8 has been demonstrated to be the dominant vSNARE in platelets (25, 26). Platelet tSNAREs with established function in platelet granule secretion include SNAP-23, syntaxin 2, and syntaxin 4 (23, 25, 27-30).
The dramatic morphologic change and granule centralization that occurs upon exposure to a strong agonist has lead to speculation that the cytoskeleton provides a contractile force that facilitates granule release (31-33). Yet this hypothesis remains unproven. An additional or alternative possibility is that platelet F-actin interacts directly with the secretory machinery in order to elicit granule release. To evaluate this possibility, we have assessed the ability of actin polymerization to support α-granule release in a cell-free secretory system. We have also determined whether platelet SNAREs associate with the actin cytoskeleton and have evaluated which platelet SNAREs directly bind actin. These studies demonstrate that actin polymerization facilitates platelet α–granule secretion, that VAMP-8 and syntaxin-2 associate with the actin cytoskeleton only following platelet activation while syntaxin-4 and SNAP-23 associate with the actin cytoskeleton in resting and activated platelets, and that purified platelet actin binds directly to syntaxin 4.
MATERIALS AND METHODS
Platelet preparation
Blood was obtained from healthy donors who had not ingested aspirin or NSAIDs in the 2 weeks prior to donation. Platelets were isolated by centrifugation followed by gel-filtration of platelet-rich plasma as previously described (34).
Recombinant SNARE proteins
Purified human platelet actin (>99% pure) conjugated to fluorescein was purchased from Cytoskeleton Inc. (Denver, CO). GST-tagged SNARE proteins were obtained by PCR from cDNAs containing human isoforms of syntaxin-4 (residues 1-272), SNAP-23, and VAMP-3 (residues 1-83) (generous gifts from Dr. Amira Klip, University of Toronto) and cloned into pGEX-2T from GE Life Sciences. All solutions were prepared using water purified by reverse-phase osmosis on a Millipore Milli-Q Purification Water System (Bedford, MA).
Antibodies
Rabbit anti-SNAP-23 antibody directed against the C terminus of SNAP-23 (DRIDIANARAKKLIDS) and anti-VAMP-3 antibody directed against a peptide consisting of the 12 N-terminal amino acids of VAMP-3 were developed in our laboratory (24, 34). A rabbit antibody directed against VAMP-8 antibody was purchased from Abcam (Cambridge, MA). Goat anti–syntaxin-2 antibody directed against a 19 amino acid portion toward the C-terminus of the cytoplasmic domain of syntaxin-1A was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse anti-syntaxin-4 antibody was obtained from BD Biosciences (San Jose, CA). Rabbit anti-human β–thromboglobulin antiserum was obtained from Nordic Immunology (Tilburg, The Netherlands). Phycoerythrin (PE)–conjugated mouse monoclonal anti–P-selectin antibody AC1.2 was purchased from BD Biosciences (San Jose, CA).
Differential Interference Contrast (DIC) microscopy
Platelets (0.5 × 108 to 1 × 108/mL) were treated with latrunculin A or buffer for 5 minutes. Platelets were subsequently plated on glass slides and visualized using an Olympus AX70 Provis microscope (Olympus, Melville, NY) with a 60×1.4 numerical aperture water-immersion lens. Digital images were captured using a Roper CoolSNAP HQ CCD camera (Roper Scientific, Ottobrun, Germany) and analyzed using Slidebook software (Intelligent Imaging Innovations, Denver, CO) in a 1392 × 1040 pixel format.
Evaluation of FITC-phalloidin binding
FITC-phalloidin binding to platelets was used to quantify F-actin content as previously described (35, 36). Gel-filtered platelets were incubated with DMSO or the indicated concentration of latrunculin A for 20 minutes and subsequently exposed to either buffer or 50 μM SFLLRN at room temperature. Following a 20 minute incubation, platelets were fixed with formaldehyde for 30 minutes at 37°C, permeabilized with 0.5% Triton X-100, and stained with 10 μM FITC-phalloidin for 1 hour at 25°C. Samples were subsequently analyzed for fluorescence using flow cytometry. Data are expressed as percentage of control of resting platelets that were incubated with vehicle alone. Data represent the mean±SEM of 3 experiments performed in triplicate.
Flow Cytometry
Flow cytometry was performed on gel-filtered platelet samples using a Becton Dickinson FACSCalibur flow cytometer (San Jose, CA). Fluorescent channels were set at logarithmic gain and 1 × 104 particles were acquired for each sample. A 585/42 band pass filter was used for FL-2 fluorescence to measure PE. For evaluation of FITC-phalloidin staining a 530/30 band pass filter was used to measure FL1 fluorescence. Data were analyzed using CellQuest software (BD Biosciences) on a MacIntosh PowerPC (Apple, Cupertino, CA).
Platelet Aggregation
Washed platelets were incubated with the indicated concentrations of latrunculin A at room temperature for 20 minutes and then exposed to SFLLRN or ADP. Aggregation was measured using a Chrono-Log 680 Aggregation System (Havertown, PA) as previously described (37).
Preparation of α-granule-enriched fraction
Preparation of the α–granule-enriched membrane fraction has been described previously (24). Briefly, platelet were obtained from healthy donors. Platelet counts were obtained using a HEMAVET Multispecies Hematology Analyzer (Drew Scientific, Oxford, Conn). For fractionation studies, 2 × 109 platelets in 10 ml were washed 3 times and disrupted by nitrogen cavitation. Uncavitated platelets were removed by centrifugation at 1,000g for 10 min and the supernatant demonstrated to be devoid of platelets by flow cytometry. The supernatant was then subjected to a second round of cavitation. The cavitate was then separated on an Optiprep (Sigma) step gradient by centrifugation at 40,000g for 2 h. An α-granule-enriched fraction was collected from a 25%/30% interface and dialyzed into buffer (25 mM PIPES, 2 mM EGTA, 137 mM KCl, 4 mM NaCl, pH 6.8) to a final volume of 5 ml. Marker studies indicated that this fraction was enriched in α-granules (38). This fraction also contained surface-connected membranes, which were identified by biotin labeling of platelet surface proteins as previously described (38). Platelet cytosol was prepared by subjecting nitrogen cavitate to centrifugation at 100,000g for 1 h and collecting the supernatant.
Electron microscopy
To prepare the α–granule-enriched fraction for electron microscopy, material above the 25%/30% Optiprep interface was removed. Material at the 25%/30% interface was then fixed using 2 ml of 2.5% glutaraldehyde in 0.1 M cacodylate overnight. The fixed samples were dehydrated and embedded in Epon for ultrathin sectioning as previously described (39). Ultrathin sections were double-stained with uranyl acetate and lead citrate (Reynolds reagent) and visualized on a Hitachi 7500 transmission electron microscope (TEM).
Immunofluorescence Microscopy
Samples were fixed in 4% formaldehyde in PBS and centrifuged onto poly-L-lysine (1 μg / ml)-coated coverslips (500g, 2.5 min). Fractions were washed briefly in PBS and blocked at room temperature in blocking buffer (10% fetal bovine serum, 1% bovine serum albumin, and 0.05% sodium azide) then washed in triplicate with PBS. Samples were incubated at 1:100 anti-human CD62P antibody diluted in blocking buffer for 1 h at room temperature. Following triplicate washes, samples were incubated in secondary antibody conjugated to Alexa Fluor 488 at a dilution of 1:250 (room temperature, 1.5 h) followed by the addition of Alexa Fluor 568-phalloidin for 20 min. Following 3 washes in PBS, coverslips were mounted onto microscope slides using Aqua-Mount (Polysciences). Samples were visualized using an Olympus BX62 microscope with a 60×1.42 numerical aperature oil-immersion lens and captured with an Orca-ER cooled CCD camera (Hamamatsu). Image acquisitions were controlled by iVision-Mac (Biovision Technologies) and analysis was performed using ImageJ.
Cell Free Secretory System
To evaluate granule secretion in the α-granule-enriched membrane preparation, 19 μl α–granule-enriched membrane preparation was incubated in the presence of 2 μl of ATP (final concentration 5 mM) (7, 28, 40-42) and 19 μl platelet cytosol (final concentration 4 mg/ml) (9). Samples of α–granule-enriched membrane preparation incubated in the presence of buffer alone served as controls. Following a 10 minute incubation, 10 μl of reaction mixture was transferred into 5 μl of PE–conjugated anti–P-selectin antibody. Stained samples were evaluated by flow cytometry as described above. β-thromboglobulin release in α–granule-enriched membrane preparation was monitored by centrifugation of samples at 40,000g for 1 h to pellet α-granule-enriched membranes. Supernatants were analyzed for β-thromboglobulin by immunoblot analysis.
Isolation of platelet cytoskeleton
Platelet cytoskeleton was isolated as previously described by Fox et al. (43). Briefly, washed platelets were lysed at a 1:1 ratio in cold Triton X-100 lysis buffer (2% (v/v) Triton X-100, 10 μM leupeptin, 100 mM benzamidine, 100 mM Tris-HCl pH 7.4, and Complete Protease Inhibitors Cocktail Tablet, Roche Molecular Biochemicals) andd 10 mM EGTA. Cytoplasmic actin filaments were sedimented at 15,000g for 4 minutes. SNAREs associated with the cytoskeleton actin filaments were subsequently detected by immunoblot analysis.
Immunoblot analysis
Samples were diluted in sample buffer at 95°C for 5 minutes. Proteins were then separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Immunoblotting was performed using FITC-labeled secondary antibodies and visualized using fluorescence detection on a Typhoon 9400 Molecular Imager (GE Healthcare, Piscataway, N.J.).
Actin Cosedimentation Assay
Purified platelet actin (2 μM) was polymerized in 20 mM Tris pH 7.0, 0.2 mM DTT, 0.2 mM CaCl2, 100 mM KCl, 2 mM MgCl2 and 2 mM ATP for 30 minutes at room temperature. 2 μM recombinant GST-tagged SNARE proteins were incubated with F-actin for an additional 30 minutes. To sediment F-actin, samples were centrifuged at 100,000g for 30 min. Supernatants and pellets were then separated, and pellets resuspended in SDS loading buffer to reconstitute the volume. Equal volumes of pellet and supernatant were added to each lane of the gels and proteins were separated by SDS-PAGE.
RESULTS
Effects of inhibition of actin polymerization on platelet function
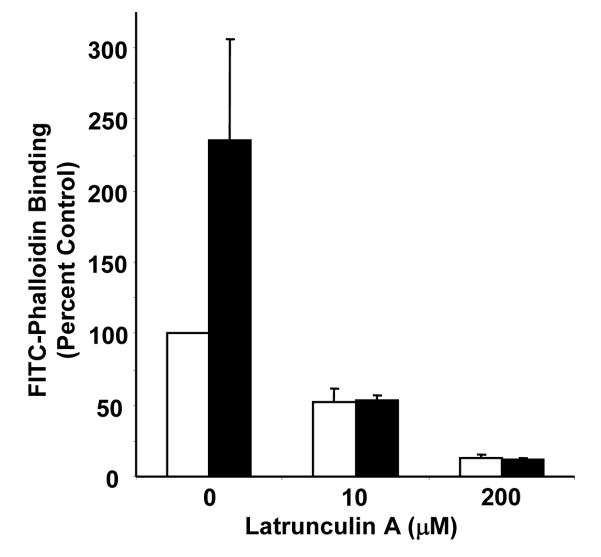
To evaluate the role of actin polymerization on platelet function, we used latrunculin A to disrupt the actin cytoskeleton. Latrunculin A binds monomeric actin, facilitating disassembly of the resting actin cytoskeleton (44). The specificity of latrunculin A is evidenced by the observation that a mutation in actin renders yeast resistant to >500 μM latrunculin A (45). Initial studies were performed to assess the effect of latrunculin A on F-actin content in resting and activated platelets using FITC-phalloidin to detect F-actin. As previously described (38), activation of platelets resulted in a substantial increase in F-actin formation (Fig. 1). F-actin content in both resting and activated platelets was markedly decreased following incubation with 10 μM latrunculin A. Incubation with 200 μM latrunculin A further decreased FITC-phalloidin binding and abrogated activation-dependent F-actin formation.
Figure 1. Effect of latrunculin A on F-actin content in resting and activated platelets.
Gel-filtered platelets were incubated with either vehicle alone, 10 μM latrunculin A, or 200 μM latrunculin A for 20 minutes and subsequently exposed to either buffer (white bars) or 50 μM SFLLRN (black bars). Following a 10 minute incubation, platelets were permeabilized with Triton X-100 and stained with 10 μM FITC-phalloidin. Samples were subsequently analyzed for fluorescence using flow cytometry. Data are expressed as percentage of control of resting platelets that were incubated with vehicle alone.
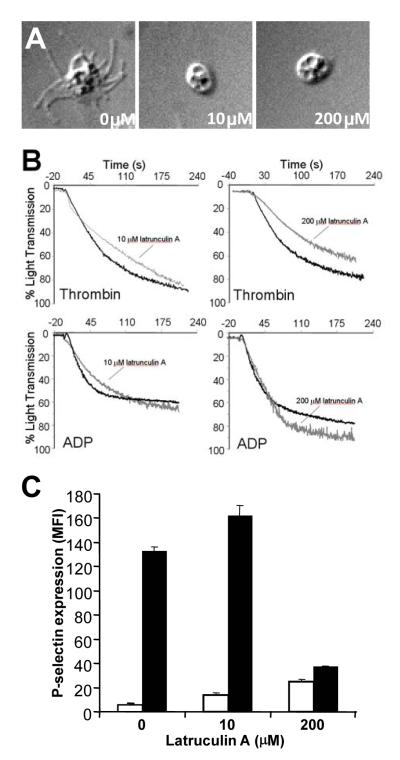
The effect of disruption of the actin cytoskeleton by latrunculin A on platelet shape change, platelet aggregation, and granule secretion was next evaluated to assess the role of actin in platelet function. To assess the effect of cytoskeletal disruption on platelet shape change, platelets were exposed to either buffer alone, 10 μM, or 200 μM latrunculin A and allowed to settle onto glass cover slips. Untreated platelets extended filopodia and had an average area of 39.5 ± 4 μm2 (Fig. 2A, left panel). Platelets exposed to 10 μM or 200 μM latrunculin A did not extend filopodia and had average areas of 11.3±1.2 μm2 and 12.4±0.8 μm2, respectively (Fig. 2A, middle and right panels).
Figure 2. Effect of disruption of the actin cytoskeleton on shape change, aggregation, and α-granule secretion.
A, gel-filtered platelets were incubated with vehicle alone (0 μM), 10 μM latrunculin A (10 μM), or 200 μM latrunculin A (200 μM). Platelets were subsequently plated on glass coverslips and imaged using DIC microscopy. B, platelet aggregation was monitored after incubation with either vehicle (black lines) or latrunculin A (gray lines) followed by stimulation with either 1 U/ml thrombin or 5 μM ADP as indicated. Data are representive tracings from 3 independent aggregation studies. C, gel-filtered platelets were incubated with either vehicle alone, 10 μM latrunculin A, or 200 μM latrunculin A as indicated and subsequently exposed to buffer (white bars) or 1 U/ml thrombin (black bars). P-selectin surface expression was monitored by flow cytometry. Data represent the mean ± S.D. of 3 experiments.
The effect of latrunculin A on platelet aggregation was evaluated to assess the role of disassembly of F-actin in platelet activation. Exposure of platelets to 10 μM latrunculin A demonstrated minimal effect on thrombin-induced platelet aggregation (Fig. 2B). Exposure to 200 μM latrunculin A resulted in less than 25% inhibition of thrombin-induced platelet activation. Platelet aggregation induced by 5 μM ADP in platelets exposed to 10 μM or 200 μM latrunculin A was comparable to aggregation in platelets exposed to buffer alone (Fig. 2B). These results indicate that even exposure to high concentrations of latrunculin A (i.e. 200 μM) does not result in substantial inhibition of platelet activation as measured by platelet aggregation.
In contrast, F-actin disassembly by latrunculin A had a significant effect on platelet α-granule secretion. P-selectin surface expression was analyzed to monitor α-granule secretion. In the absence of a secretagogue, exposure of platelets to increasing concentrations of latrunculin A resulted in a small increase in P-selectin surface expression (Fig. 2C). Thrombin-induced P-selectin expression was slightly augmented following exposure to 10 μM latrunculin A, to 117±13% of the thrombin alone sample. Exposure of platelets to 200 μM latrunculin A, however, inhibited thrombin-induced P-selectin expression to 10±4% of the thrombin alone control. This observation suggests that F-actin contributes to granule secretion.
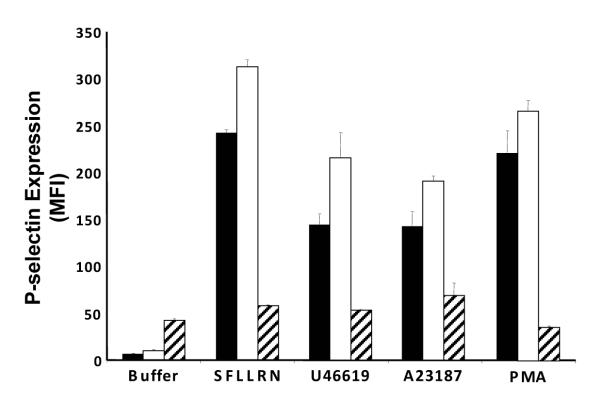
Evaluation of platelet α–granule secretion in response to several different agonists was performed to assess whether latrunculin A inhibited proximal mechanisms involved in α–granule secretion. Exposure of platelets to 10 μM latrunculin A consistently resulted in a slight augmentation of agonist-induced α-granule release (Fig. 3). At higher concentrations, however, latrunculin A inhibited α–granule release induced by SFLLRN through protease-activated receptor 1 or by U46619 through the thromboxane prostanoid receptor (Fig. 3). Latrunculin A also inhibited α–granule secretion induced by the Ca2+ ionophore A23187 and by PMA.
Figure 3. Disruption of the actin cytoskeleton inhibits α–granule release induced by distinct agonists.
Gel-filtered platelets were incubated with either vehicle alone (black bars), 10 μM latrunculin A (white bars), or 300 μM latrunculin A (hatched bars). Platelets were then exposed to buffer alone, 100 μM SFLLRN, 1 μM U46619, 1 μM A23187, or 0.2 μM PMA and analyzed for P-selectin surface expression by flow cytometry. Data represent the mean ± S.D. of 4-10 experiments.
Characterization of a cell-free platelet α-granule secretory system
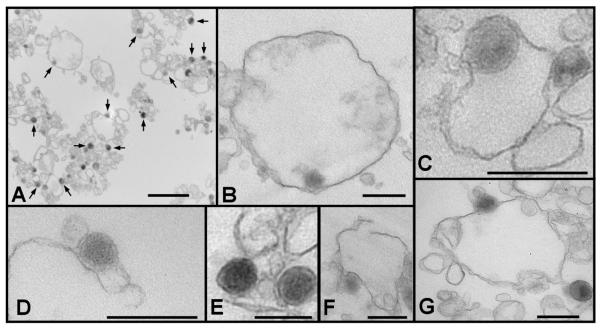
To evaluate requirements for α–granule secretion, we developed a cell-free platelet α–granule secretory system. An α-granule-enriched fraction was prepared from platelet cavitate as previously described (38). Evaluation of the α–granule-enriched cell-free membrane preparation by electron microscopy demonstrated α–granules immediately adjacent to membrane vesicles and membrane fragments (Fig. 4A). Occasional isolated α–granules were identified. However, most α–granules were associated with membrane vesicles or fragments. The preparation was devoid of intact platelets or dense granules. α–Granules in this preparation were localized both to the inside of vesicles (Fig. 4B-C) and outside of vesicles (Fig. 4F-G). α–Granules were also identified adjacent to membrane fragments (Fig. 4D-E) and disrupted membrane vesicles (Fig. 4F). This arrangement of granules and vesicles is similar to those observed in other granule-enriched fractions used to study granule secretion in cell-free systems (46-48).
Figure 4. Ultrastructure of the α–granule-enriched membrane fraction.
A, micrograph of the α–granule-enriched preparation demonstrated the presence of abundant α–granules along with other platelet membranes (scale bar, 2 μm). Representative α–granules are indicated by arrows. Nearly all α–granules were associated with membrane structures. α–Granules were found inside membrane vesicles (B,C), associated with membrane fragments (D,E) and disrupted vesicles (F), and identified outside membrane vesicles (G) (scale bars, 0.5 μm).
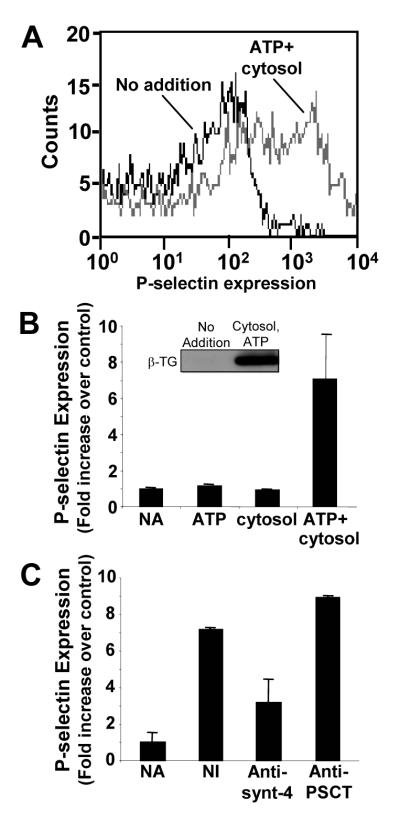
A cell-free platelet α–granule secretory system was modeled after previously described membrane fusion systems (9, 46, 49-53). Platelet cytosol (4 mg/ml) and ATP (5 mM) were incubated with the α–granule-enriched fraction. P-selectin was used as a marker to monitor membrane fusion. P-selectin expression increased severalfold following exposure of the membrane preparation to cytosol and ATP (Fig. 5). In contrast, no expression of P-selectin was observed when samples were exposed to either buffer, ATP, or cytosol alone (Fig. 5B). No significant increase in the binding of a non-immune antibody was observed following incubation with cytosol and ATP (data not shown). β-thromboglobulin, a soluble component of α–granules, was released from the α–granule-enriched preparation upon incubation with ATP and cytosol (Fig. 5B, insert). Antibody directed at syntaxin-4, which functions in α–granule secretion (28, 54), inhibited cytosol plus ATP-induced α–granule release by 61±19% (Fig. 5C). Antibody directed at the cytoplasmic tail of P-selectin had no significant effect on cytosol plus ATP-induced P-selectin expression. These results indicate that P-selectin exposure in the cell-free system does not result from non-specific permeabilization of α–granule membranes and that α-granule release in this cell-free system is dependent on cytosol and ATP.
Figure 5. P-selectin expression in an α-granule-enriched platelet membrane preparation is dependent on ATP and cytosol.
A, increased expression of P-selectin is observed following exposure of the α–granule enriched membrane fraction to 5 mM ATP plus 4 mg/ml platelet cytosol. B, P-selectin expression was monitored following incubation of the α–granule enriched membrane fraction with buffer alone (NA), with 5 mM ATP (ATP), with 4 mg/ml platelet cytosol (cytosol), or with ATP plus cytosol (ATP+cytosol). Data represent the mean ± S.D. of 3-4 experiments. Insert demonstrates release of β-thromboglobulin from α–granule-enriched fraction following exposure to 4 mg/ml platelet cytosol and 5 mM ATP. C, P-selectin expression was monitored following incubation of α–granule-enriched membrane fraction with buffer alone (NA), or in the presence of 5 mM ATP plus 4 mg/ml cytosol following addition of 25 μg/ml of either non-immune IgG (NI), anti-syntaxin 4 antibody (anti-synt-4) or anti-P-selectin cytoplasmic tail antibody (anti-PSCT). Following a 20 minute incubation, the sample was exposed to 5 mM ATP and 4 mg/ml platelet cytosol and analyzed for P-selectin expression by flow cytometry. Data represent the mean ± S.D. of 3 experiments.
Actin polymerization in a cell-free α-granule secretory system
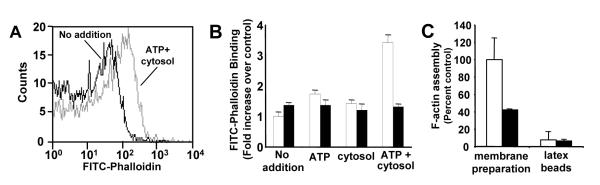
To evaluate the role of actin in platelet α–granule secretion, we determined whether actin polymerization occurred in our α–granule-enriched preparation following incubation with cytosol plus ATP. FITC-phalloidin binding to granules was monitored by flow cytometry. The combination of 4 mg/ml cytosol and 5 mM ATP enhanced the binding of FITC-phalloidin to the α–granule-enriched preparation by approximately 3-fold (Fig. 6). In contrast, neither buffer, ATP alone, nor cytosol alone elicited substantial FITC-phalloidin binding when incubated with the α–granule-enriched preparation. FITC-phalloidin binding observed in the presence of ATP plus cytosol was inhibited by incubation of the preparation with cytochalasin B (Fig. 6B).
Figure 6. Actin polymerization is induced in an α-granule-enriched platelet membrane preparation following incubation with either cytosol or platelet G-actin.
A, the α–granule enriched membrane fraction was incubated in the presence (gray) or absence (black) of 4 mg/ml platelet cytosol plus 5 mM ATP. Samples were then stained with FITC-phalloidin and evaluated by flow cytometry. B, the α-granule-enriched membrane preparation was incubated with buffer alone (No addition), 5 mM ATP (ATP), 4 mg/ml cytosol (cytosol), or 5 mM ATP plus 4 mg/ml cytosol (ATP+cytosol) in the presence (black bars) or absence (white bars) of 4 μM cytochalasin B. Samples were subsequently stained with FITC-phalloidin and analyzed by flow cytometry. Data represent the mean ± S.D. of 3-4 experiments. C, the α-granule-enriched membrane fraction or latex beads were incubated in the presence (black bars) or absence (white bars) of 4 μM cytochalasin E. Following a 15 minute exposure, samples were exposed to 2 μM fluorescein G-actin plus 5 mM ATP for 30 minutes. FL-1 fluorescence was subsequently analyzed by flow cytometry. Data represent the mean ± S.D. of 4 experiments.
Actin is highly concentrated in platelet cytosol (55) and is the most abundant protein in our cytosol preparation. Cytosol may provide a source of actin that enables actin polymerization in the cell-free system. To test this possibility, we determined whether purified actin could replace cytosol in mediating F-actin formation in the α-granule-enriched membrane preparation. For these experiments, the membrane preparation was incubated in the presence of 2 μM purified platelet G-actin covalently coupled to fluorescein. Actin polymerization in platelet granules was monitored by flow cytometry. Incubation of the granule preparation with fluorescein-labeled platelet actin plus 5 mM ATP resulted in actin polymerization (Fig. 6C). Actin polymerization was inhibited with cytochalasin E. In contrast, little polymerization was observed when latex beads were incubated in the presence of 2 μM G-actin plus 5 mM ATP in the absence of granules (Fig. 6C). Thus, α–granule-enriched membranes facilitates actin polymerization.
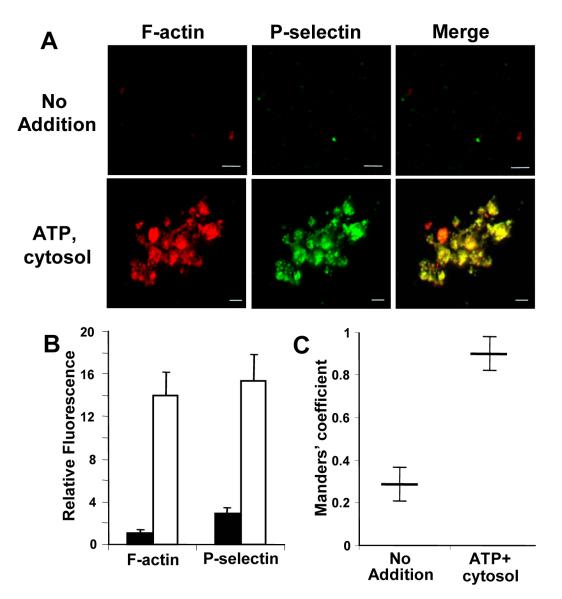
Immunofluorescence microscopy was performed to assess the relationship between actin polymerization and P-selectin expression. Two-color immunofluorescence of the untreated α-granule-enriched preparation demonstrated faint staining of both F-actin as detected by Alexa Fluor 568-phalloidin and P-selectin as detected with anti-P-selectin antibody (Fig. 7A). Following exposure to ATP plus cytosol, staining of both F-actin and P-selectin increased (Fig. 7A). P-selectin expression in samples incubated with ATP plus cytosol was primarily limited to areas of F-actin formation. Quantitative analyses of the images confirmed that both F-actin formation and P-selectin expression increased markedly following exposure to ATP plus cytosol (Fig. 7B). Analysis of colocalization of P-selectin and F-actin demonstrated markedly increased colocalization in samples exposed to ATP plus cytosol compared to untreated samples (Fig. 7C). The Manders’ coefficient of colocalization for F-actin and P-selectin in untreated samples was 0.28±0.07, indicating relative segregation of these markers. In the presence of ATP and cytosol, substantial colocalization of these markers was observed as indicated by the Manders’ coefficient of 0.90±0.08. These data demonstrate that F-actin in the α–granule-enriched membrane preparation is increased following incubation with ATP and cytosol and that P-selectin expression following exposure to ATP plus cytosol is localized to areas of F-actin formation.
Figure 7. Immunofluorescence microscopy of F-actin and P-selectin in an α-granule-enriched preparation.
A, two-color immunofluorescence was performed to evaluate F-actin using Alexa Flour 568-phalloidin (red), P-selectin using anti-P-selectin antibody (green), and colocalization (yellow). Representative images demonstrate increased staining and colocalization in samples exposed to ATP plus cytosol (ATP, cytosol) compared with untreated samples (No addition) Scale bar is 5 μm. B, relative fluorescence of Alexa Fluor 568-phalloidin and anti-P-selectin binding was analyzed in images of samples exposed to ATP plus cytosol (white bars) or untreated samples (black bars). Data represent the mean ± S.D. of 5-7 fields/slide. C, colocalization was evaluated using a Manders’ coefficient in samples exposed to ATP plus cytosol and untreated samples, as indicated. Data represent the mean ± S.D. of 5-7 fields/slide.
Purified G-actin can substitute for cytosol to facilitate α-granule secretion in a cell-free system
We next determined whether actin polymerization facilitates α–granule secretion in the cell-free secretory system. P-selectin expression observed in the presence of cytosol plus ATP was inhibited by cytochalasin B, indicating a role for actin polymerization in α–granule release (Fig. 8A). We then determined whether actin could replace cytosol in enabling α–granule secretion to occur in the cell-free system. Purified platelet G-actin (2 μM) in the presence of ATP was able to support the expression of P-selectin in the absence of cytosol (Fig. 8B). Actin-induced P-selectin expression was inhibited by cytochalasin E. In contrast, actin failed to affect binding of non-immune antibody to the granule-enriched population. These data demonstrate that platelet actin facilitates P-selectin expression in this cell-free system.
Figure 8. Assembly of F-actin mediates P-selectin expression in a cell-free secretory system.
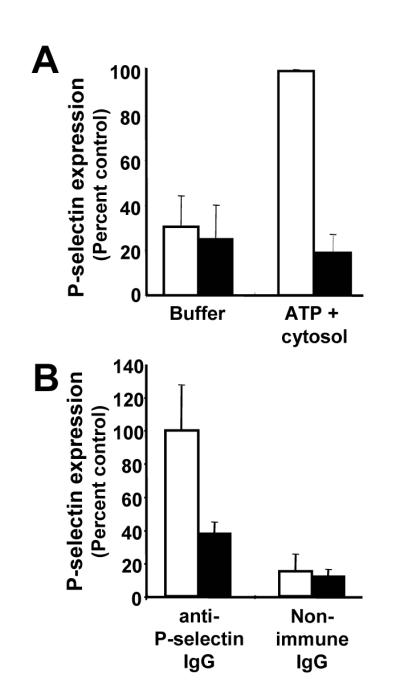
A, the platelet α-granule-enriched membrane preparation was incubated with buffer alone (buffer) or with 5 mM ATP plus 4 mg/ml cytosol (ATP+cytosol) in the presence (black bars) or absence (white bars) of 4 μM cytochalasin B and subsequently analyzed for P-selectin expression by flow cytometry. Data represent the mean ± S.D. of 3 experiments. B, α-granule-enriched membrane fractions were treated in the presence (black bars) or absence (white bars) of 4 μM cytochalasin E. Samples were subsequently incubated with 2 μM G-actin and 5 mM ATP and assayed for P-selectin expression by flow cytometry. Data represent the mean ± S.D. of 3 experiments.
Association of SNAREs with the platelet cytoskeleton
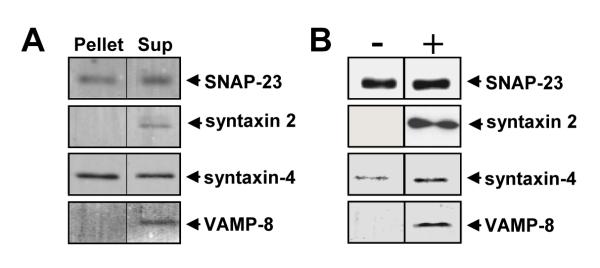
The primary SNAREs that mediate platelet α–granule release are VAMP-8, syntaxin-4, syntaxin-2, and SNAP-23 (23, 25, 28, 29, 54, 56). We next evaluated whether these SNAREs associated with the filamentous actin cytosolic network. Platelet cytoskeletons were prepared by lysis in 1% Triton X-100 in the presence of protease inhibitors and EGTA to preserve the cytoskeleton, followed by centrifugation at 15,000g (57, 58). VAMP-8, syntaxin-4, syntaxin-2, and SNAP-23 were all identified in supernatant (Fig. 9A). SNAP-23 and syntaxin-4, but not syntaxin-2 or VAMP-8, were identified in the pellet (Fig. 9A). The association of SNAREs with the filamentous actin cytosolic network was then evaluated before and after activation of platelets. VAMP-8, syntaxin-4, syntaxin-2, and SNAP-23 were identified within the Triton X-100-insoluble fraction sedimented at 15,000g following activation of platelets through protease-activated receptor-1 (Fig. 9B). To determine whether association of SNAREs with the cytoskeleton was dependent on platelet aggregation, experiments were repeated in the presence of 500 μM RGDS. RGDS had no effect on the association of SNAREs with cytoplasmic actin filaments (data not shown). These studies suggest that SNARE isoforms associate differentially with the Triton X-100-insoluble fraction.
Figure 9. Association of SNAREs with the platelet cytoskeleton.
A, platelets were lysed in 1.5 ml of Triton X-100 lysis buffer at 4°C. Samples were subject to centrifugation at 15,000g to isolate the platelet cytoskeletal actin network. The supernatant was removed and the pellet washed. Samples of the pellet (Pellet, 30% of total) and the supernatant (Sup, 1% of total) were solubilized in sample buffer and proteins within the samples separated by SDS-PAGE. SNAP-23, syntaxin-2, syntaxin-4, and VAMP-8 within the samples were detected by immunoblot analysis. B, platelets were incubated in the presence (+) or absence (−) of SFLLRN and lysed in Triton X-100 lysis buffer containing either EGTA. Lysates were then spun at 15,000g. Proteins in the pellet were separated by SDS-PAGE and analyzed for SNAP-23, syntaxin-2, syntaxin-4, and VAMP-8 by immunoblot analysis.
SNARE association with purified F-actin
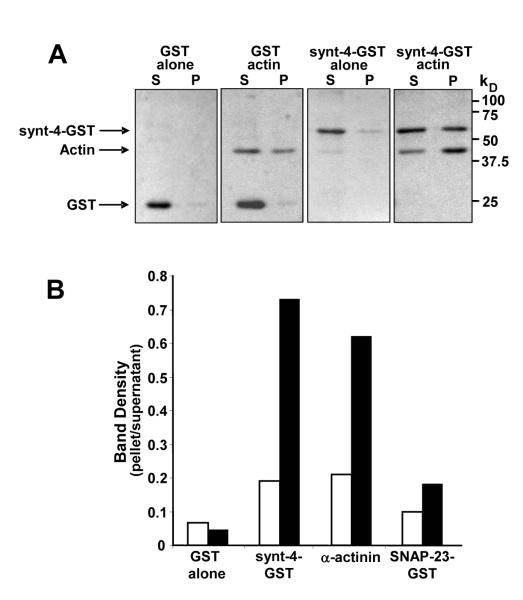
Association of SNAP-23 and syntaxin-4 with the Triton X-100-insoluble resting platelet cytoskeleton could result from direct binding of platelet actin to SNAREs. Alternatively, these SNAREs may associate with the actin cytoskeleton indirectly through proteins that bind actin. To distinguish between these two possibilities, we assessed the ability of recombinant SNAP-23 and syntaxin-4 to bind purified platelet actin. Purified platelet actin was polymerized in the presence of ATP. Platelet F-actin was then incubated with recombinant proteins. Samples were subsequently sedimented by ultracentrifugation and supernatants and pellets analyzed for proteins using Coomassie staining. GST alone did not cosediment with polymerized platelet actin as evidenced by the observation that >90% of GST remained in the supernatant following ultracentrifugation (Fig. 10). In contrast, GST-syntaxin-4 cosedimented with purified, polymerized platelet actin. The actin binding protein α-actinin behaved much like GST-syntaxin-4 in this system, demonstrating significant association with polymerized actin. In comparison, GST-SNAP-23 demonstrated little binding to polymerized platelet actin (Fig. 10B). These data indicate that syntaxin-4 binds directly to polymerized platelet actin.
Figure 10. A recombinant cytosolic domain of syntaxin-4 binds polymerized platelet actin.
A, proteins were incubated alone (GST alone; synt-4-GST alone) or in the presence of polymerized platelet actin (GST, actin; synt-4-GST, actin). Samples were pelleted by centrifugation. Proteins within supernatants and pellets were separated by SDS-PAGE and visualized by Coomassie blue staining. B, recombinant GST alone, syntaxin-4-GST, α-actinin, or SNAP-23-GST were incubated in the presence (black bars) or absence (white bars) of polymerized platelet actin. Samples were pelleted and proteins within the supernatants and pellets separated by SDS-PAGE. Bands corresponding to each of the proteins were quantified by densitometry. Data represent the ratio of band density in the pellet compared to that in the supernatant and are representative of 3-5 experiments.
DISCUSSION
These studies indicate that the platelet actin cytoskeleton facilitates granule secretion. Inhibition of actin polymerization with 200 μM latrunculin A did not significantly suppress upstream platelet activation mechanisms as evidenced by the fact that thrombin- or ADP-induced platelet aggregation remained intact. Yet such treatment nearly abolished α–granule release (Figs. 1 and 2). Moderate concentrations of latrunculin A (10 μM) augment α–granule release. The observation that an actin inhibitor can have opposite effects on granule secretion depending on its dose is consistent with results in other cell types that demonstrate augmentation of granule release following exposure to moderate doses of actin polymerization inhibitors or depolymerizing proteins, but inhibition of exocytosis following exposure to higher doses (5, 6). These observations suggest that disruption of the resting cytoskeleton barrier facilitates granule secretion, but that some degree of actin polymerization is required for secretion.
To evaluate the role of actin in promoting α-granule secretion, we used a cell-free secretory system (46, 59-61). Like other cell-free secretory systems, the platelet system was dependent on both cytosol and ATP. GTP was not a requirement of this secretory system. In this regard, membrane fusion in this system was more similar to that observed in latex phagosome system (62), which undergoes ATP-dependent membrane fusion in the absence of GTP, than to GTPase-mediated fusion systems. The phagosome membrane fusion system is dependent on de novo synthesis of PIP2 for actin assembly (9, 62). Previous studies from our laboratory demonstrate a role for PIP2 synthesis in platelet α-granule fusion (41, 63). Localized PIP2 synthesis in platelets may contribute to actin polymerization and thereby facilitate membrane fusion. A role for actin polymerization in cell-free membrane fusion has also been observed in a yeast vacuole system (8, 21). α-Granule release in the platelet cell-free system was blocked by inhibitors of actin polymerization and supported by actin in the absence of cytosol (Fig. 8). These data support the premise that actin polymerization facilitates platelet α–granule secretion and raise the question of how F-actin facilitates membrane fusion.
Actin polymerization can contribute to granule secretion by transport of granules to surface connected membranes (13-15, 64), and/or by participating in a terminal step of membrane fusion (6, 8-12). Actin has been demonstrated to associate with granules from many cell types, including platelets, pancreatic acinar cells, and chromaffin cells, phagosomes, yeast and others (7, 13, 65). In many instances, granule membranes serve as sites of assembly of F-actin (9, 13, 66-68). In platelets, actin surrounds isolated α–granules (7) and the α–granule-enriched membrane preparation stimulates actin polymerization in the presence of ATP (Fig. 4). In yeast and neuroendocrine cells, proteins with established importance in agonist-induced actin polymerization such as Rho proteins and Cdc42 associate with isolated granules and participate in membrane fusion (8, 10, 17, 21, 69). Actin associated with granules could distort or destabilize lipid bilayers as a prerequisite for fusion (8). Assembly of F-actin that occurs around fusing granules may serve to stabilize and compress granules (70-72). Alternatively, actin may control fusion pore dynamics (73). Actin binds proteins involved in membrane fusion (18, 19, 21) and actin remodeling may provide spatial constraints that restrict the secretory machinery to sites of membrane fusion (74).
The observation that SNAREs interact with the platelet cytoskeleton suggests a means by which the actin cytoskeleton could impart spatial constraints on the secretory machinery. SNAREs have previously been demonstrated to bind either directly or indirectly to actin. SNAP-23, syntaxin-4, and VAMP-2 colocalize to actin-rich membrane ruffles following insulin stimulation of muscle cells (16). VAMP-7 colocalizes with actin at sites of outgrowth in neuronal growth cones (17). In purified yeast vacuoles, actin depolymerization promotes SNARE interactions and subsequent actin polymerization is required following trans SNARE complex formation (8, 75). This observation suggests that actin is required in a terminal event leading to membrane fusion (8, 75). Certain SNAREs bind directly to F-actin. Syntaxin 4 binds directly to both F-actin (18, 20) and the membrane skeletal protein, fodrin (76, 77). The yeast R-SNAREs Ykt6p1 and Nyvp1, which both contain longin domains, bind F-actin (21).
We demonstrate an association of platelet SNAREs with the F-actin cytosolic network. A limitation of our methodology is that we cannot determine with certainty the concentration of SNAREs associated with the platelet cytoskeleton, since we do not know that it has been isolated in its entirety. These results indicate, however, that SNAP-23, VAMP-8, syntaxin 2, and syntaxin 4 associate with the platelet cytoskeleton (Fig. 9). Of these SNAREs, however, only syntaxin 4 binds directly to platelet actin (Fig. 10). Syntaxin-4 associates with the cytoskeleton both before and after activation since it binds directly to F-actin. SNAP-23 interacts with syntaxin-4 in resting platelets (34, 78) and may associate with the resting filamentous actin cytosolic network indirectly via this interaction. VAMP-8 and syntaxin-2 only associate with the filamentous actin cytosolic network following platelet activation. The association of VAMP-8 and syntaxin-2 may be indirect and result in part from activation induced by binding to syntaxin-4 and SNAP-23, respectively.
We have previously demonstrated that the platelet cytoskeleton can function as a barrier to granule release (7). How might actin act both as a negative and positive regulator of platelet granule release? One possibility is that in the resting state, granules are in close apposition to surface-connected membranes, but fusion is prevented by an intact membrane skeleton, consisting largely of α-adducin, spectrin, and short actin filaments (79, 80) as well as by the resting cytosolic actin cytoskeleton. Upon platelet activation, remodeling of the cytoskeleton including destabilization of the membrane skeleton and de novo actin polymerization could ensue. Newly formed actin filaments originating from the granules may tether granule and surface-connected membranes. The association of SNAREs with actin tethers could facilitate SNARE complex formation and subsequent membrane fusion.
ACKNOWLEDGMENT
Supported by NIH grants HL63250 and HL87203 (R.F.) and T32 HL07917 (K.W., L.D., P.S.B., C.G.P.). R.F. is a recipient of an Established Investigator Award from the American Heart Association and a Special Project Award from Bayer Healthcare.
Footnotes
Supported by NIH grants HL63250 and HL87203 (R.F.) and T32 HL07917 (K.W., L.D., P.S.B., C.G.P.). R.F. is a recipient of an Established Investigator Award from the American Heart Association and a Special Project Award from Bayer Healthcare.
- PMA
- phorbol 12-myristate 13-acetate
- SNARE, SNAP
- (soluble N-ethylmaleimide-sensitive factor attachment protein) Receptor
- tSNARE
- target membrane SNARE
- VAMP
- vesicle-associated membrane protein
- vSNARE
- vesicular SNARE
REFERENCES
- 1.Morales M, Colicos MA, Goda Y. Actin-dependent regulation of neurotransmitter release at central synapses. Neuron. 2000;27:539–550. doi: 10.1016/s0896-6273(00)00064-7. [DOI] [PubMed] [Google Scholar]
- 2.Vitale ML, Rodriguez Del Castillo A, Tchakarov L, Trifaro JM. Cortical filamentous actin disassembly and scinderin redistribution during chromaffin cell stimulation precede exocytosis, a phenomenon not exhibited by gelsolin. J Cell Biol. 1991;113:1057–1067. doi: 10.1083/jcb.113.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chowdhury HH, Kreft M, Zorec R. Distinct effect of actin cytoskeleton disassembly on exo- and endocytic events in a membrane patch of rat melanotrophs. J Physiol. 2002;545:879–886. doi: 10.1113/jphysiol.2002.028043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jog NR, Rane MJ, Lominadze G, Luerman GC, Ward RA, McLeish KR. The actin cytoskeleton regulates exocytosis of all neutrophil granule subsets. Am J Physiol Cell Physiol. 2007;292:C1690–1700. doi: 10.1152/ajpcell.00384.2006. [DOI] [PubMed] [Google Scholar]
- 5.Orci L, Gabbay KH, Malaisse WJ. Pancreatic beta-cell web: its possible role in insulin secretion. Science. 1972;175:1128–1130. doi: 10.1126/science.175.4026.1128. [DOI] [PubMed] [Google Scholar]
- 6.Muallem S, Kwiatkowska K, Xu X, Yin HL. Actin filament disassembly is a sufficient final trigger for exocytosis in nonexcitable cells. J Cell Biol. 1995;128:589–598. doi: 10.1083/jcb.128.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flaumenhaft R, Dilks JR, Rozenvayn N, Monahan-Earley RA, Feng D, Dvorak AM. The actin cytoskeleton differentially regulates platelet alpha-granule and dense-granule secretion. Blood. 2005;105:3879–3887. doi: 10.1182/blood-2004-04-1392. [DOI] [PubMed] [Google Scholar]
- 8.Eitzen G, Wang L, Thorngren N, Wickner W. Remodeling of organelle-bound actin is required for yeast vacuole fusion. J Cell Biol. 2002;158:669–679. doi: 10.1083/jcb.200204089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jahraus A, Egeberg M, Hinner B, Habermann A, Sackman E, Pralle A, Faulstich H, Rybin V, Defacque H, Griffiths G. ATP-dependent membrane assembly of F-actin facilitates membrane fusion. Mol Biol Cell. 2001;12:155–170. doi: 10.1091/mbc.12.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gasman S, Chasserot-Golaz S, Malacombe M, Way M, Bader MF. Regulated exocytosis in neuroendocrine cells: a role for subplasmalemmal Cdc42/N-WASP-induced actin filaments. Mol Biol Cell. 2004;15:520–531. doi: 10.1091/mbc.E03-06-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malacombe M, Ceridono M, Calco V, Chasserot-Golaz S, McPherson PS, Bader MF, Gasman S. Intersectin-1L nucleotide exchange factor regulates secretory granule exocytosis by activating Cdc42. Embo J. 2006;25:3494–3503. doi: 10.1038/sj.emboj.7601247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pendleton A, Koffer A. Effects of latrunculin reveal requirements for the actin cytoskeleton during secretion from mast cells. Cell Motil Cytoskeleton. 2001;48:37–51. doi: 10.1002/1097-0169(200101)48:1<37::AID-CM4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 13.Valentijn JA, Valentijn K, Pastore LM, Jamieson JD. Actin coating of secretory granules during regulated exocytosis correlates with the release of rab3D. Proc Natl Acad Sci U S A. 2000;97:1091–1095. doi: 10.1073/pnas.97.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varadi A, Tsuboi T, Rutter GA. Myosin Va transports dense core secretory vesicles in pancreatic MIN6 beta-cells. Mol Biol Cell. 2005;16:2670–2680. doi: 10.1091/mbc.E04-11-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudolf R, Kogel T, Kuznetsov SA, Salm T, Schlicker O, Hellwig A, Hammer JA, 3rd, Gerdes HH. Myosin Va facilitates the distribution of secretory granules in the F-actin rich cortex of PC12 cells. J Cell Sci. 2003;116:1339–1348. doi: 10.1242/jcs.00317. [DOI] [PubMed] [Google Scholar]
- 16.Tong P, Khayat ZA, Huang C, Patel N, Ueyama A, Klip A. Insulin-induced cortical actin remodeling promotes GLUT4 insertion at muscle cell membrane ruffles. J Clin Invest. 2001;108:371–381. doi: 10.1172/JCI12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alberts P, Rudge R, Irinopoulou T, Danglot L, Gauthier-Rouviere C, Galli T. Cdc42 and actin control polarized expression of TI-VAMP vesicles to neuronal growth cones and their fusion with the plasma membrane. Mol Biol Cell. 2006;17:1194–1203. doi: 10.1091/mbc.E05-07-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Band AM, Ali H, Vartiainen MK, Welti S, Lappalainen P, Olkkonen VM, Kuismanen E. Endogenous plasma membrane t-SNARE syntaxin 4 is present in rab11 positive endosomal membranes and associates with cortical actin cytoskeleton. FEBS Lett. 2002;531:513–519. doi: 10.1016/s0014-5793(02)03605-0. [DOI] [PubMed] [Google Scholar]
- 19.Thurmond DC, Gonelle-Gispert C, Furukawa M, Halban PA, Pessin JE. Glucose-Stimulated Insulin Secretion Is Coupled to the Interaction of Actin with the t-SNARE (Target Membrane Soluble N-Ethylmaleimide-Sensitive Factor Attachment Protein Receptor Protein) Complex. Mol Endocrinol. 2003;17:732–742. doi: 10.1210/me.2002-0333. [DOI] [PubMed] [Google Scholar]
- 20.Jewell JL, Luo W, Oh E, Wang Z, Thurmond DC. Filamentous Actin Regulates Insulin Exocytosis through Direct Interaction with Syntaxin 4. J Biol Chem. 2008;283:10716–10726. doi: 10.1074/jbc.M709876200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isgandarova S, Jones L, Forsberg D, Loncar A, Dawson J, Tedrick K, Eitzen G. Stimulation of actin polymerization by vacuoles via Cdc42p-dependent signaling. J Biol Chem. 2007;282:30466–30475. doi: 10.1074/jbc.M704117200. [DOI] [PubMed] [Google Scholar]
- 22.Jahn R, Scheller RH. SNAREs--engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 23.Polgar J, Chung SH, Reed GL. Vesicle-associated membrane protein 3 (VAMP-3) and VAMP-8 are present in human platelets and are required for granule secretion. Blood. 2002;100:1081–1083. doi: 10.1182/blood.v100.3.1081. [DOI] [PubMed] [Google Scholar]
- 24.Feng D, Crane K, Rozenvayn N, Dvorak AM, Flaumenhaft R. Subcellular distribution of 3 functional SNARE proteins: Human cellubrevin, SNAP-23, and Syntaxin 2. Blood. 2002;99:4006–4014. doi: 10.1182/blood.v99.11.4006. [DOI] [PubMed] [Google Scholar]
- 25.Ren Q, Barber HK, Crawford GL, Karim ZA, Zhao C, Choi W, Wang CC, Hong W, Whiteheart SW. Endobrevin/VAMP-8 is the primary v-SNARE for the platelet release reaction. Mol Biol Cell. 2007;18:24–33. doi: 10.1091/mbc.E06-09-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graham GJ, Ren Q, Dilks JR, Blair P, Whiteheart SW, Flaumenhaft R. Endobrevin/VAMP-8-dependent dense granule release mediates thrombus formation in vivo. Blood. 2009;114:1083–1090. doi: 10.1182/blood-2009-03-210211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lemons PP, Chen D, Bernstein AM, Bennett MK, Whiteheart SW. Regulated secretion in platelets: identification of elements of the platelet exocytosis machinery. Blood. 1997;90:1490–1500. [PubMed] [Google Scholar]
- 28.Flaumenhaft R, Croce K, Chen E, Furie B, Furie BC. Proteins of the exocytotic core complex mediate platelet alpha-granule secretion. Roles of vesicle-associated membrane protein, SNAP-23, and syntaxin 4. J Biol Chem. 1999;274:2492–2501. doi: 10.1074/jbc.274.4.2492. [DOI] [PubMed] [Google Scholar]
- 29.Polgar J, Lane WS, Chung SH, Houng AK, Reed GL. Phosphorylation of SNAP-23 in activated human platelets. J Biol Chem. 2003;278:44369–44376. doi: 10.1074/jbc.M307864200. [DOI] [PubMed] [Google Scholar]
- 30.Chen D, Bernstein AM, Lemons PP, Whiteheart SW. Molecular mechanisms of platelet exocytosis: role of SNAP-23 and syntaxin 2 in dense core granule release. Blood. 2000 Feb 1;95:921–929. [PubMed] [Google Scholar]
- 31.Painter RG, Ginsberg MH. Centripetal myosin redistribution in thrombin-stimulated platelets. Relationship to platelet Factor 4 secretion. Exp Cell Res. 1984;155:198–212. doi: 10.1016/0014-4827(84)90781-x. [DOI] [PubMed] [Google Scholar]
- 32.Gerrard JM, Israels SJ, Friesen LL. Protein phosphorylation and platelet secretion. Nouv Rev Fr Hematol. 1985;27:267–273. [PubMed] [Google Scholar]
- 33.White JG, Gerrard JM. Recent advances in platelet structural physiology. Suppl Thromb Haemost. 1978;63:49–60. [PubMed] [Google Scholar]
- 34.Flaumenhaft R, Rozenvayn N, Feng D, Dvorak AM. SNAP-23 and syntaxin-2 localize to the extracellular surface of the platelet plasma membrane. Blood. 2007;110:1492–1501. doi: 10.1182/blood-2006-11-055772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hartwig JH, Kung S, Kovacsovics T, Janmey PA, Cantley LC, Stossel TP, Toker A. D3 phosphoinositides and outside-in integrin signaling by glycoprotein IIb-IIIa mediate platelet actin assembly and filopodial extension induced by phorbol 12-myristate 13-acetate. J Biol Chem. 1996;271:32986–32993. doi: 10.1074/jbc.271.51.32986. [DOI] [PubMed] [Google Scholar]
- 36.Kovacsovics TJ, Bachelot C, Toker A, Vlahos CJ, Duckworth B, Cantley LC, Hartwig JH. Phosphoinositides 3-kinase inhibition spares actin assembly in activating platelets but reverses platelet aggregation. J Biol Chem. 1995;270:11358–11366. doi: 10.1074/jbc.270.19.11358. [DOI] [PubMed] [Google Scholar]
- 37.Sim DS, Dilks JR, Flaumenhaft R. Platelets Possess and Require an Active Protein Palmitoylation Pathway for Agonist-Mediated Activation and In Vivo Thrombus Formation. Arterioscler Thromb Vasc Biol. 2007;27:1478–1485. doi: 10.1161/ATVBAHA.106.139287. [DOI] [PubMed] [Google Scholar]
- 38.Feng D, Crane K, Rozenvayn N, Dvorak AM, Flaumenhaft R. Subcellular distribution of 3 functional platelet SNARE proteins: human cellubrevin, SNAP-23, and syntaxin 2. Blood. 2002;99:4006–4014. doi: 10.1182/blood.v99.11.4006. [DOI] [PubMed] [Google Scholar]
- 39.Kieseier BC, Wisniewski KE, Goebel HH. The monocyte-macrophage system is affected in lysosomal storage diseases: an immunoelectron microscopic study. Acta Neuropathol. 1997;94:359–362. doi: 10.1007/s004010050719. [DOI] [PubMed] [Google Scholar]
- 40.Flaumenhaft R, Furie B, Furie BC. Alpha-granule secretion from alpha-toxin permeabilized, MgATP-exposed platelets is induced independently by H+ and Ca2+ J Cell Physiol. 1999;179:1–10. doi: 10.1002/(SICI)1097-4652(199904)179:1<1::AID-JCP1>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 41.Rozenvayn N, Flaumenhaft R. Phosphatidylinositol 4,5-Bisphosphate Mediates Ca2+-induced Platelet alpha -Granule Secretion. Evidence for type II phosphatidylinositol 5-phosphate 4-kinase function. J Biol Chem. 2001;276:22410–22419. doi: 10.1074/jbc.M008184200. [DOI] [PubMed] [Google Scholar]
- 42.Lai KC, Flaumenhaft R. SNARE protein degradation upon platelet activation: Calpain cleaves SNAP-23. J Cell Physiol. 2003;194:206–214. doi: 10.1002/jcp.10222. [DOI] [PubMed] [Google Scholar]
- 43.Fox JE, Lipfert L, Clark EA, Reynolds CC, Austin CD, Brugge JS. On the role of the platelet membrane skeleton in mediating signal transduction. Association of GP IIb-IIIa, pp60c-src, pp62c-yes, and the p21ras GTPase-activating protein with the membrane skeleton. J Biol Chem. 1993;268:25973–25984. [PubMed] [Google Scholar]
- 44.Spector I, Braet F, Shochet NR, Bubb MR. New anti-actin drugs in the study of the organization and function of the actin cytoskeleton. Microsc Res Tech. 1999;47:18–37. doi: 10.1002/(SICI)1097-0029(19991001)47:1<18::AID-JEMT3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 45.Ayscough KR, Stryker J, Pokala N, Sanders M, Crews P, Drubin DG. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J Cell Biol. 1997;137:399–416. doi: 10.1083/jcb.137.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin TF, Kowalchyk JA. Docked secretory vesicles undergo Ca2+-activated exocytosis in a cell-free system. J Biol Chem. 1997;272:14447–14453. doi: 10.1074/jbc.272.22.14447. [DOI] [PubMed] [Google Scholar]
- 47.Burgoyne RD. Mechanisms of secretion from adrenal chromaffin cells. Biochim Biophys Acta. 1984;779:201–216. doi: 10.1016/0304-4157(84)90009-1. [DOI] [PubMed] [Google Scholar]
- 48.de Curtis I, Simons K. Isolation of exocytic carrier vesicles from BHK cells. Cell. 1989;58:719–727. doi: 10.1016/0092-8674(89)90106-2. [DOI] [PubMed] [Google Scholar]
- 49.Jahraus A, Tjelle TE, Berg T, Habermann A, Storrie B, Ullrich O, Griffiths G. In vitro fusion of phagosomes with different endocytic organelles from J774 macrophages. J Biol Chem. 1998;273:30379–30390. doi: 10.1074/jbc.273.46.30379. [DOI] [PubMed] [Google Scholar]
- 50.Gu F, Aniento F, Parton RG, Gruenberg J. Functional dissection of COP-I subunits in the biogenesis of multivesicular endosomes. J Cell Biol. 1997;139:1183–1195. doi: 10.1083/jcb.139.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lenhard JM, Kahn RA, Stahl PD. Evidence for ADP-ribosylation factor (ARF) as a regulator of in vitro endosome-endosome fusion. J Biol Chem. 1992;267:13047–13052. [PubMed] [Google Scholar]
- 52.Kinuta M, Yamada H, Abe T, Watanabe M, Li SA, Kamitani A, Yasuda T, Matsukawa T, Kumon H, Takei K. Phosphatidylinositol 4,5-bisphosphate stimulates vesicle formation from liposomes by brain cytosol. Proc Natl Acad Sci U S A. 2002;99:2842–2847. doi: 10.1073/pnas.261715599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Avery J, Ellis DJ, Lang T, Holroyd P, Riedel D, Henderson RM, Edwardson JM, Jahn R. A cell-free system for regulated exocytosis in PC12 cells. J Cell Biol. 2000;148:317–324. doi: 10.1083/jcb.148.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lemons PP, Chen D, Whiteheart SW. Molecular mechanisms of platelet exocytosis: requirements for alpha-granule release. Biochem Biophys Res Commun. 2000;267:875–880. doi: 10.1006/bbrc.1999.2039. [DOI] [PubMed] [Google Scholar]
- 55.Blikstad I, Markey F, Carlsson L, Persson T, Lindberg U. Selective assay of monomeric and filamentous actin in cell extracts, using inhibition of deoxyribonuclease I. Cell. 1978;15:935–943. doi: 10.1016/0092-8674(78)90277-5. [DOI] [PubMed] [Google Scholar]
- 56.Bernstein AM, Whiteheart SW. Identification of a cellubrevin/vesicle associated membrane protein 3 homologue in human platelets. Blood. 1999;93:571–579. [PubMed] [Google Scholar]
- 57.Fox JE, Boyles JK, Berndt MC, Steffen PK, Anderson LK. Identification of a membrane skeleton in platelets. J Cell Biol. 1988;106:1525–1538. doi: 10.1083/jcb.106.5.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fox JE, Boyles JK, Reynolds CC, Phillips DR. Actin filament content and organization in unstimulated platelets. J Cell Biol. 1984;98:1985–1991. doi: 10.1083/jcb.98.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Avery J, Jahn R, Edwardson JM. Reconstitution of regulated exocytosis in cell-free systems: a critical appraisal. Annu Rev Physiol. 1999;61:777–807. doi: 10.1146/annurev.physiol.61.1.777. [DOI] [PubMed] [Google Scholar]
- 60.Nadin CY, Rogers J, Tomlinson S, Edwardson JM. A specific interaction in vitro between pancreatic zymogen granules and plasma membranes: stimulation by G-protein activators but not by Ca2+ J Cell Biol. 1989;109:2801–2808. doi: 10.1083/jcb.109.6.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Almeida MT, Ramalho-Santos J, Oliveira CR, Pedroso de Lima MC. Evidence that synaptobrevin is involved in fusion between synaptic vesicles and synaptic plasma membrane vesicles. Biochem Biophys Res Commun. 1997;236:184–188. doi: 10.1006/bbrc.1997.6928. [DOI] [PubMed] [Google Scholar]
- 62.Defacque H, Bos E, Garvalov B, Barret C, Roy C, Mangeat P, Shin HW, Rybin V, Griffiths G. Phosphoinositides regulate membrane-dependent actin assembly by latex bead phagosomes. Mol Biol Cell. 2002;13:1190–1202. doi: 10.1091/mbc.01-06-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rozenvayn N, Flaumenhaft R. Protein kinase C mediates translocation of type II phosphatidylinositol 5-phosphate 4-kinase required for platelet alpha-granule secretion. J Biol Chem. 2003;278:8126–8134. doi: 10.1074/jbc.M206493200. [DOI] [PubMed] [Google Scholar]
- 64.Lang T, Wacker I, Wunderlich I, Rohrbach A, Giese G, Soldati T, Almers W. Role of actin cortex in the subplasmalemmal transport of secretory granules in PC-12 cells. Biophys J. 2000;78:2863–2877. doi: 10.1016/S0006-3495(00)76828-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fowler VM, Pollard HB. In vitro reconstitution of chromaffin granule-cytoskeleton interactions: ionic factors influencing the association of F-actin with purified chromaffin granule membranes. J Cell Biochem. 1982;18:295–311. doi: 10.1002/jcb.1982.240180305. [DOI] [PubMed] [Google Scholar]
- 66.Wilkins JA, Lin S. Association of actin with chromaffin granule membranes and the effect of cytochalasin B on the polarity of actin filament elongation. Biochim Biophys Acta. 1981;642:55–66. doi: 10.1016/0005-2736(81)90137-1. [DOI] [PubMed] [Google Scholar]
- 67.Defacque H, Egeberg M, Habermann A, Diakonova M, Roy C, Mangeat P, Voelter W, Marriott G, Pfannstiel J, Faulstich H, Griffiths G. Involvement of ezrin/moesin in de novo actin assembly on phagosomal membranes. Embo J. 2000;19:199–212. doi: 10.1093/emboj/19.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Defacque H, Egeberg M, Antzberger A, Ansorge W, Way M, Griffiths G. Actin assembly induced by polylysine beads or purified phagosomes: quantitation by a new flow cytometry assay. Cytometry. 2000;41:46–54. [PubMed] [Google Scholar]
- 69.Bader MF, Doussau F, Chasserot-Golaz S, Vitale N, Gasman S. Coupling actin and membrane dynamics during calcium-regulated exocytosis: a role for Rho and ARF GTPases. Biochim Biophys Acta. 2004;1742:37–49. doi: 10.1016/j.bbamcr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 70.Sokac AM, Co C, Taunton J, Bement W. Cdc42-dependent actin polymerization during compensatory endocytosis in Xenopus eggs. Nat Cell Biol. 2003;5:727–732. doi: 10.1038/ncb1025. [DOI] [PubMed] [Google Scholar]
- 71.Yu HY, Bement WM. Control of local actin assembly by membrane fusion-dependent compartment mixing. Nat Cell Biol. 2007;9:149–159. doi: 10.1038/ncb1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nemoto T, Kojima T, Oshima A, Bito H, Kasai H. Stabilization of exocytosis by dynamic F-actin coating of zymogen granules in pancreatic acini. J Biol Chem. 2004;279:37544–37550. doi: 10.1074/jbc.M403976200. [DOI] [PubMed] [Google Scholar]
- 73.Larina O, Bhat P, Pickett JA, Launikonis BS, Shah A, Kruger WA, Edwardson JM, Thorn P. Dynamic regulation of the large exocytotic fusion pore in pancreatic acinar cells. Mol Biol Cell. 2007;18:3502–3511. doi: 10.1091/mbc.E07-01-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eitzen G. Actin remodeling to facilitate membrane fusion. Biochim Biophys Acta. 2003;1641:175–181. doi: 10.1016/s0167-4889(03)00087-9. [DOI] [PubMed] [Google Scholar]
- 75.Wang L, Merz AJ, Collins KM, Wickner W. Hierarchy of protein assembly at the vertex ring domain for yeast vacuole docking and fusion. J Cell Biol. 2003;160:365–374. doi: 10.1083/jcb.200209095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nakano M, Nogami S, Sato S, Terano A, Shirataki H. Interaction of syntaxin with alpha-fodrin, a major component of the submembranous cytoskeleton. Biochem Biophys Res Commun. 2001;288:468–475. doi: 10.1006/bbrc.2001.5795. [DOI] [PubMed] [Google Scholar]
- 77.Liu L, Jedrychowski MP, Gygi SP, Pilch PF. Role of insulin-dependent cortical fodrin/spectrin remodeling in glucose transporter 4 translocation in rat adipocytes. Mol Biol Cell. 2006;17:4249–4256. doi: 10.1091/mbc.E06-04-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chung SH, Polgar J, Reed GL. Protein kinase C phosphorylation of syntaxin 4 in thrombin-activated human platelets. J Biol Chem. 2000;275:25286–25291. doi: 10.1074/jbc.M004204200. [DOI] [PubMed] [Google Scholar]
- 79.Gilligan DM, Sarid R, Weese J. Adducin in platelets: activation-induced phosphorylation by PKC and proteolysis by calpain. Blood. 2002;99:2418–2426. doi: 10.1182/blood.v99.7.2418. [DOI] [PubMed] [Google Scholar]
- 80.Barkalow KL, Italiano JE, Jr., Chou DE, Matsuoka Y, Bennett V, Hartwig JH. {alpha}-Adducin dissociates from F-actin and spectrin during platelet activation. J Cell Biol. 2003;161:557–570. doi: 10.1083/jcb.200211122. [DOI] [PMC free article] [PubMed] [Google Scholar]