Abstract
Novel, bioerodible, thermosensitive poly(NIPAAm-co-dimethyl–γ-butyrolactone), with hydrolysis-dependent thermosensitivity, were synthesized by radical polymerization with varying dimethyl-γ-butyrolactone content and the properties of the copolymers were characterized using Differential Scanning Calorimetry (DSC), High Performance Liquid Chromatography (HPLC) in conjunction with Static Light Scattering, Fourier Transformed Infrared Spectroscopy (FTIR), Nuclear Magnetic Resonance (NMR) and acid titration. The lower critical solution temperature (LCST) of the copolymers decreased with increasing dimethyl-γ-butyrolactone content, but then increased after ring-opening hydrolysis of the dimethyl-γ-butyrolactone side group. FTIR and NMR spectra showed the copolymerization of these two monomers and the hydrolysis-dependent ring-opening of the dimethyl-γ-butyrolactone side group. It was also found that there are no low-molecular-weight by-products but rather dissolution of the polymer chains at 37°C during the time frame of application. Models of the kinetics suggest that the hydrolysis reaction is self-catalytic due to an increase in hydrophilicity, and thus accessible water concentration, caused by ring-opening of the dimethyl-γ-butyrolactone.
Keywords: thermosensitive, hydrolysis-dependent, bioerodible, lower critical solution temperature, injectable
Introduction
Temperature-sensitive polymer, poly(N-isopropylacrylamide) (poly-(NIPAAm)), and its copolymers have been of high research interest for biomedical applications, such as drug delivery, cell immobilization, in situ-gelling implantation, and tissue engineering. 1–3 Many thermosensitive polymer solutions have a lower critical solution temperature (LCST), a temperature at which these polymers in aqueous solution experience a phase change from a soluble state to an insoluble state. Poly(NIPAAm) exhibits a LCST at 32°C. 4, 5 It has been suggested that this temperature-induced phase transition of poly(NIPAAm) in aqueous solution is mainly driven by the thermal destruction of hydrogen bonds between water molecules and hydrophilic groups, such as -CO- and – NH- in the NIPAAm monomer and the increased interaction between the hydrophobic segments on the polymer with increased temperature. 6–10 The LCST of poly(NIPAAm) copolymers can be controlled by varying the monomer composition. Generally, the incorporation of hydrophobic comonomers leads to a lower LCST and that of hydrophilic comonomers leads to a higher LCST. Acrylic acid increases the LCST of poly(NIPAAm) at neutral pH; 11–13 2-Hydroxyethyl methacrylate-monolactate (HEMA-monolactate) lowers the LCST of poly(NIPAAm). 14–17
One challenge with using poly(NIPAAm) in biomedical applications such as controlled drug delivery is that poly(NIPAAm) is not degradable, limiting its application in temporary implantation applications. To overcome this challenge, several groups have been working to make biodegradable NIPAAm copolymers. Neradovic et. al. reported the synthesis of a new type of thermosensitive NIPAAm copolymer with hydrolysable lactate ester side groups and the LCST increases after the hydrolysis of the ester groups. 11, 14–17 In this system, a low molecular weight by-product of the degradation is lactic acid. Synthesis of NIPAAm with cyclic monomer, 2-methylene-1,3-dioxepane (MDO), and its biodegradable properties have been described by Sun, et. al. 18 Yoshida et. al. reported the synthesis of the NIPAAm copolymers cross-linked with biodegradable poly(amino acid). 19 Lee et. al. and Neradovic et. al. reported thermosensitive and bioerodible hydrogels with time-dependent LCST properties. 16, 20 In these studies, there are different types of small molecules produced after the degradation of the polymers. Although small molecules could be cleared by kidney, it could also be toxic and activates the immune response, such as phagocytosis or the local acid environment may kill the cells in the case of the lactic acid. In this work, we propose the synthesis of a new type of NIPAAm copolymer that contains both thermosensitive and bioerodible properties without low molecular weight by-products. This hypothesized copolymer would be useful in injectable, in situ-gelling controlled drug delivery systems. The LCST property of poly(NIPAAm) copolymer makes it an attractive candidate materials for injectable drug release system. Such a system could avoid surgical operation and ease the drug administration. Drugs or cells can be suspended in the polymer solution for therapeutic purposes when making a polymer solution. Upon injection of such a delivery system, the polymer solution will gel due to the temperature change from room temperature to body temperature. The suspended drugs will diffuse out of the gel. The copolymer in a physiological environment will result in the hydrolysis of dimethyl-γ-butyrolactone and this is expected to lead to an increase in the LCST with time. When the LCST increases above body temperature, the polymer becomes soluble again and will diffuse away 21. The proposed polymer is expected to have no low molecular weight by-product after degradation for the time frame of application which is desired for such a controlled drug delivery system to reduce cytotoxicity.
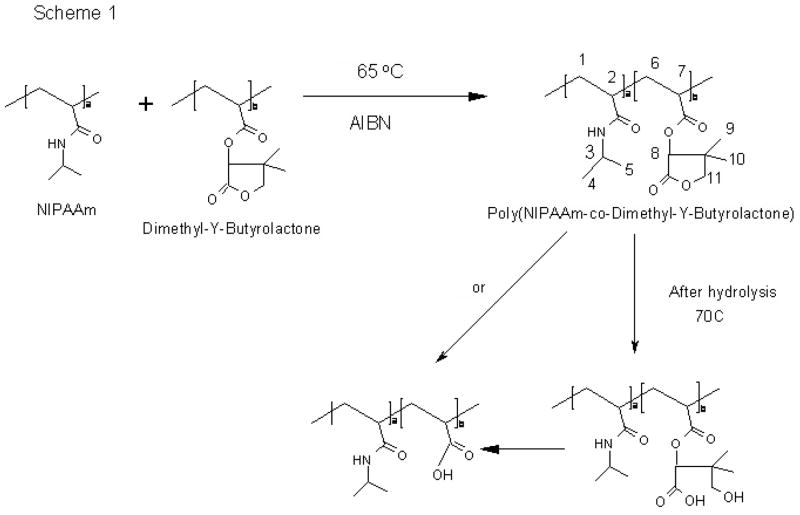
Fig. 1 shows the copolymerization scheme and the degradation of the proposed copolymer. There are two ester groups in dimethyl-γ-butyrolactone structure. One is connected to the polymer backbone and the other is in the ring structure. Due to these two ester groups, there are two possible reaction paths for the degradation of the dimethyl-γ-butyrolactone. If the ester group in the ring structure is broken sufficiently faster than the ester near the backbone, a thermosensitive and bioerodible copolymer will be obtained without low molecular weight byproduct, which is desirable and expected. If the reaction rates of hydrolysis of the esters are similar or the ester near the backbone degrades faster than the ring ester, there will be an undesired low molecular weight byproduct after hydrolysis during the time frame of the application. In this second mechanism, the ester group connected to the backbone is broken directly with or without ring opening and the ring structure falls off from the backbone. In either route of hydrolysis of the dimethyl-γ-butyrolactone, it is expected that this copolymer will have time-dependent thermosensitive properties. Therefore, the purpose of this work was to synthesize poly(NIPAAm-co-dimethyl-γ-butyrolactone) and evaluate its hydrolysis route and kinetics to determine feasibility of designing injectable drug delivery systems with the material.
Figure 1.
Synthesis and Hydrolysis of Copolymer Poly(NIPAAM-co-dimethyl-γ-butyrolactone)
Materials and Methods
Materials
All materials were reagent grade and obtained from Aldrich & Co. unless otherwise noted. NIPAAm was dissolved in hexanes (10g in 100mL at 40°C) and then re-crystallized at room temperature. (R)-(+)-α-Acryloyloxy-β,β-dimethyl-γ-butyrolactone was used as received. 2, 2′-Azobisisobutyronitrile (AIBN) was dissolved in methanol (1g/20mL) at room temperature and re-crystallized at −20°C. Anhydrous 1,4-Dioxane was used as the polymerization solvent and was treated by molecular sieve to remove the dissolved water before use. HPLC grade Tetrahydrofuran (THF) was used as the mobile phase for Static Light Scattering (MiniDawn, Wyatt Technology Corporation)/High Performance Liquid Chromatography (HPLC) (Shimadzu Corporation). Phosphate Buffered Solution (PBS) at pH 7.4 was used as the solvent for multi-cell Differential Scanning Calorimetry (DSC) (Calorimetry Science Corporation).
Synthesis of Poly(NIPAAm-co-Dimethyl-γ-Butyrolactone)
Poly(NIPAAm-co-dimethyl-γ-butyrolactone) [Poly(NDB)] was synthesized in 1,4-dioxane at 65°C for 16 hours by radical polymerization. The feed ratio of dimethyl-γ-butyrolactone was varied from 3 mol. % to 10 mol. % (Table 1). AIBN (7×10−3 mol AIBN/mol monomer) was used as the initiator for all reactions. The reaction was bubbled with Nitrogen (N2) for 15 min. before adding the initiator and then maintained under a nitrogen environment throughout the reaction to reduce oxygen. After polymerization, the solution was filtered and the solvent was evaporated. Then the copolymer was dissolved in acetone and collected by precipitation in 14- to 15-fold-excess of diethyl ether. Finally, the copolymer was vacuum-dried for one day.
Table 1.
Characterization Results: Feed Ratio, Chemical Composition, Molecular Weight and LCST Before and After Complete Hydrolysis
| Polymer | Dimethyl-γ-Butyrolactone Content (mol. %) |
MW(g/mol) | Pd(Mw/Mn) | LCST (oC) |
||
|---|---|---|---|---|---|---|
| Feed Ratio | Composition | Before | After | |||
| 1 | 3 | 2.9±0.2 | 1.6×105 | 1.94 | 25.8±0.1 | 36.8±0.1 |
| 2 | 5 | 5.5±0.5 | 1.5×105 | 1.94 | 23.3±0.1 | 41.8±0.2 |
| 3 | 7 | 6.0±0.2 | 1.3×105 | 2.00 | 20.8±0.2 | 43.1±0.4 |
| 4 | 10 | 7.2±0.2 | 8.6×104 | 1.99 | 16.3±0.1 | 50.0±0.2 |
Differential Scanning Calorimetry (DSC)
The lower critical solution temperature (LCST) of the synthesized copolymers was evaluated by differential scanning calorimetry (DSC). Scans were taken on 5 wt. % solutions in 0.1 M PBS (pH 7.4) at 1°C/min from 0°C to 80°C. A minimum of triplicates was performed for each copolymer before, after and at various time points during hydrolysis.
Molecular Weight and Polydispersity Determination
The molecular weight and the polydispersity of the synthesized copolymers were determined by high performance liquid chromatography (HPLC) (Shimadzu VP) in conjunction with static light scattering using tetrahydrofuran (THF) as mobile phase. The molecular weight for each copolymer was approximated from the light scattering data (Wyatt MiniDawn). The sample was prepared by dissolving the copolymer in tetrahydrofuran (THF) with a concentration of 5 mg/ml.
Fourier Transformed Infrared Spectroscopy (FTIR)
FTIR (Thermoelectron Nexus 470) was used to determine the chemical composition of the synthesized copolymer. ATR-FTIR was conducted on copolymers before, after and during hydrolysis. Polymers were dissolved in THF, the solutions were put on the diamond, and then the THF was evaporated to cast a thin film for the experiment. A minimum of 64 scans was used for each sample.
1H Nuclear Magnetic Resonance (1H NMR)
1H NMR was used to determine the chemical composition of the synthesized copolymer.
1H NMR was conducted on copolymers before, after and during hydrolysis.
Copolymers of NIPAAm and dimethyl-γ-butyrolactone were synthesized as mentioned above. 1H NMR (CDCl3): δ = 5.3–5.5 (H8), 3.8–4.2 (H3, H11), 1.2–2.4 (H1, H2, H6, H7), 1.1 (H4, H5, H9, H10). 1H NMR (D2O): δ = 5.2–5.4 (H8), 3.5 (H3), 3.8(H11), 1.0–2.2 (H1, H2, H6, H7), 0.8 (H4, H5, H9, H10).
Hydrolysis
Both time-dependent hydrolysis and complete hydrolysis were evaluated at 70°C. Complete degradation of these materials is estimated to require nearly 1 year at normal physiological conditions, therefore accelerated degradation conditions of 70°C were used according to ISO 1099322.. Solutions of each copolymer were prepared in 0.1M PBS at 5 wt. %. For time-dependent hydrolysis, the pH of the polymer solution was adjusted to 7.4 daily. For complete hydrolysis experiments only, the pH was adjusted to 10.5 daily to further accelerate the hydrolysis process. The pH of the polymer solutions were adjusted everyday to maintain a nearly constant pH. Daily changes in pH were less than 0.5 pH units. All solutions were maintained at 70°C. Complete hydrolysis was assumed complete when the pH value of the polymer solutions remained the same for two consecutive days. FTIR, 1H NMR and DSC were conducted after hydrolysis for each polymer. The polymers were dialyzed against water and lyophilized before FTIR, 1H NMR and DSC tests.
Titration
The dimethyl-γ-butyrolactone content was determined by acid titration after complete hydrolysis. Titration was conducted manually for each copolymer using standard sodium hydroxide volumetric solution with the concentration of 0.01N. The polymer was dissolved in distilled water with the concentration of 0.15g/10mL. A minimum of triplicates was used.
Modeling
The change in LCST with time was modeled to further elucidate the hydrolytic reaction in the poly(NDB) gels. The reaction was assumed to be a self-catalytic reaction due to the increase in water associated with the polymer as the dimethyl-γ-butyrolactone hydrolyzed and became more hydrophilic. The change in the dimethyl-γ-butyrolactone content of the polymer with time due to hydrolysis was modeled as follows:
| Eq. 1 |
Where [A] is the molar content of dimethyl-γ-butyrolactone in the polymer in units of mol.%, t is time of hydrolysis, k1 is the reaction rate constant and [H2O] is the concentration of ‘accessible-water’ [water accessible to the dimethyl-γ-butyrolactone esters] in the gel. The concentration of ‘accessible-water’ was assumed to be related to The fraction of dimethyl-γ-butyrolactone hydrolyzed as follows:
| Eq. 2 |
Where k2 is a constant that defines the proportionality between the polymer content and the water content accessible to the dimethyl-γ-butyrolactone (assuming a linear relationship between monomer content and water content); [A]o is the initial dimethyl-γ-butyrolactone content of the polymer; [A] is the current dimethyl-γ-butyrolactone content of the polymer; and b is included to incorporate the initial accessible water before hydrolysis of any dimethyl-γ-butyrolactone content. Without this term, or the associated initial water content, there is no reaction. The solution to equation 1 with equation 2 substituted for the water content and k=k1 ×k2 is shown here:
| Eq. 3 |
[A]o was determined from the titration experiments and c is a constant of integration, which physically corresponds to the time until 50% hydrolysis. The LCST resulting from the monomer content of the polymer with time was modeled as follows:
| Eq. 4 |
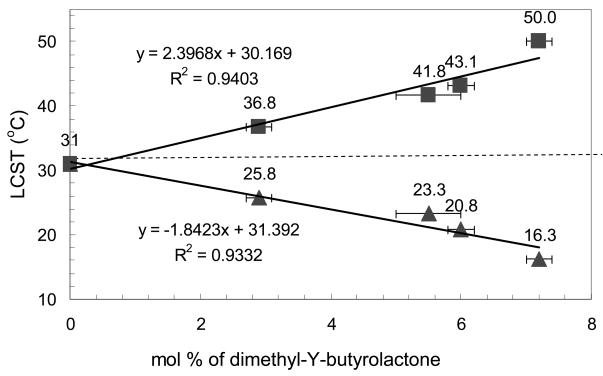
Where p1 is the slope determined from a linear fit to the LCST before hydrolysis versus dimethyl-γ-butyrolactone content found in DSC experiments seen in Fig. 4 and p2 is the slope from a linear fit to the LCST after complete hydrolysis versus dimethyl-γ-butyrolactone content seen in Fig. 4, and Tavg is approximately the LCST of NIPAAm determined as the average intercept for the same data in Fig. 4. The parameters k, c, and b were all determined by fitting the data using least squares in MathCAD 11®.
Figure 4.
LCST with Different Dimethyl-γ-Butyrolactone Contents Before and After Complete Hydrolysis: solid triangle--before hydrolysis, solid square--after complete hydrolysis, n=3 (y-error bars smaller than data points.) (complete hydrolysis condition: pH=10.5 at 70°C)
Statistics
LCST data for each polymer is reported as the average temperature for three DSC runs per sample, the error is reported as standard deviation. The dimethyl-γ-butyrolactone content is reported as the mean ± standard deviation for 3 samples. The adjusted R2 is reported for the model fits 23.
Results and Discussion
The synthesis scheme for polymerization is shown in Figure 1. Feed ratios of polymerization, chemical composition, molecular weight, and LCST before and after complete hydrolysis are presented in Table 1.
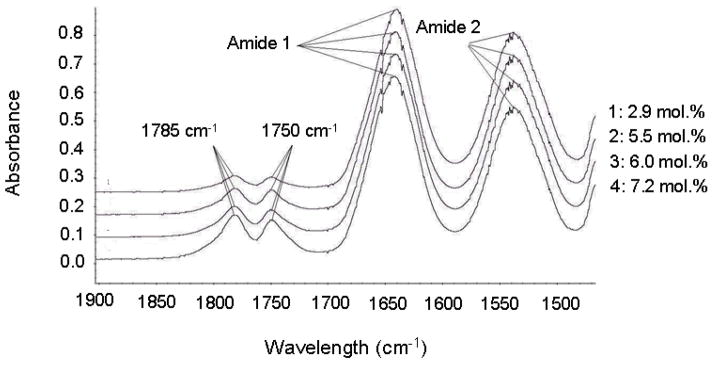
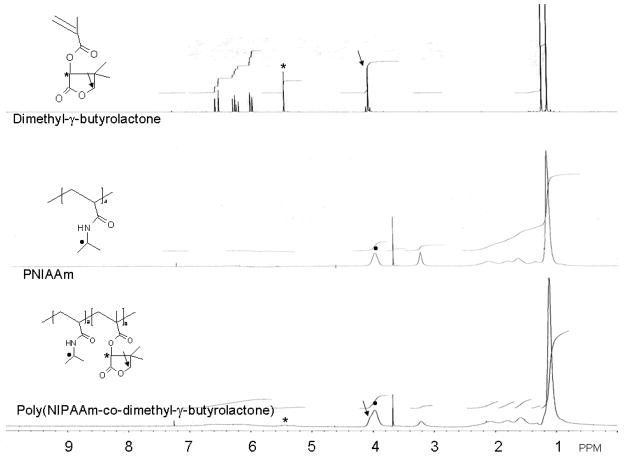
The molecular weights ranged from 86 kg/mol to 160 kg/mol. Polydispersities were from 1.94 to 2.0. The dimethyl-γ-butyrolactone contents of the copolymer were from 2.9 mol. % to 7.2 mol. %. FTIR and 1H NMR results show the copolymerization of the two monomers (Fig. 2 and Fig. 3). In the FTIR spectra, there are two peaks appearing at 1785cm−1 and 1750 cm−1 respectively. These two peaks are ascribed to the two carbonyl groups in dimethyl-γ-butyrolactone. The peak at 1785cm−1 is ascribed to the carbonyl group in the dimethyl-γ-butyrolactone ring structure and the peak at 1750cm−1 is the carbonyl group connected to the polymer backbone. Another two NIPAAm characteristic peaks around 1640cm−1 and 1550 cm−1 24 were used for quantitative comparison. By comparing the 1H NMR spectra of dimethyl-γ-butyrolactone monomer, PNIPAAm homopolymer and the copolymer synthesized, it was concluded that two peaks around 5.4 ppm are from dimethyl-γ-butyrolactone. The peak at 4.0 ppm is from NIPAAm and the shoulder on the left of this peak is from dimethyl-γ-butyrolactone. The reason for the double peaks around 5.4ppm (see the asterisk on the lower 1H-NMR of Figure 3) might be due to the heterogeneity of copolymerization resulting from different local environments for dimethyl-γ-butyrolactone along the polymer chain. Thus, both FTIR and 1H-NMR spectra show evidence of the copolymerization of these two monomers.
Figure 2.
FTIR Spectra of Poly(NIPAAm-co-Dimethyl-γ-Butyrolactone) with Different Dimethyl-γ-Butyrolactone Content before Hydrolysis: 1: 2.9 mol%, 2: 5.5 mol%, 3: 6.0 mol %, 4: 7.2 mol%
Figure 3.
1H NMR Spectra of Dimethyl-γ-Butyrolactone, PNIPAAm and Poly(NIPAAm-co-Dimethyl-γ-Butyrolactone)
The relationship between dimethyl-γ-butyrolactone content and the LCST before and after complete hydrolysis is shown in Figure 4. This data indicates that for these four copolymers, an increase of dimethyl-γ-butyrolactone content from 2.9 mol % to 7.2 mol % results in a decrease in the LCST from 25.8 to 16.3 °C. This trend could be explained by the hydrophobicity of dimethyl-γ-butyrolactone. With more dimethyl-γ-butyrolactone copolymerized with NIPAAm, the copolymer becomes more hydrophobic and the LCST decreases. With the lowest dimethyl-γ-butyrolactone content of 2.9 mol %, the LCST is around room temperature, 25.8°C. As the content of dimethyl-γ-butyrolactone increases, the hydrophobicity of polymer increases and this increased hydrophobicity further lowers the LCST of the polymer. During incubation of aqueous solutions of theses polymers, the ester groups in dimethyl-γ-butyrolactone are hydrolyzed and this process resulted in the increase in hydrophilicity of the polymer. Due to increased hydrophilicity, the LCST of the polymer also increases. For the lowest dimethyl-γ-butyrolactone content of 2.9 mol %, the LCST increased to 36.8°C after complete hydrolysis. With the dimethyl-γ-butyrolactone content increased to 7.2 mol. %, accordingly, the copolymer becomes more hydrophilic and the LCST increases to about 50°C after complete hydrolysis. Fig. 4 also indicated that except for the copolymer with 2.9 mol % dimethyl-γ-butyrolactone, all other copolymers have a LCST above body temperature after complete hydrolysis. The complete hydrolysis was investigated at pH 10.5 and 70°C. Under this condition, hydrolysis was completed after 15, 18, 20, 22 days for poly(NDB) with 2.9 mol.%, 5.5 mol.%, 6.0 mol.% and 7.2 mol.% dimethyl-γ-butyrolactone contents respectively. This indicates that this copolymer, if used in controlled drug delivery system, will become soluble again after complete hydrolysis and diffuse away. This evidence suggests that the thermosensitive copolymer, poly(NIPAAm-co-dimethyl-γ-butyrolactone) is bioerodible and is a potential candidate materials for controlled drug delivery and tissue engineering applications.
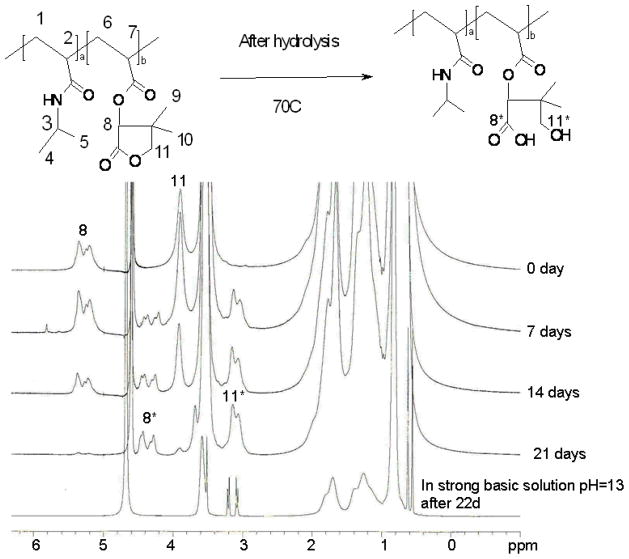
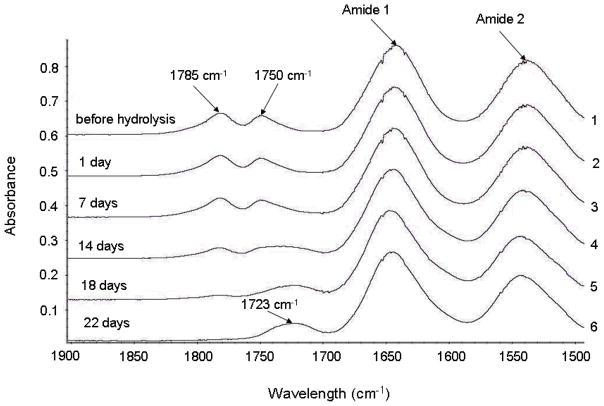
A time-dependent hydrolysis study was also conducted at 70°C. 1H NMR, FTIR and LCST tests were conducted after time-dependent hydrolysis. Fig. 5 and Fig. 6 show 1H NMR and FTIR spectra of the copolymer with 7.2 mol. % dimethyl-γ-butyrolactone. D2O was used as the solvent for 1H NMR after degradation. In 1H NMR spectra, it was noticed that the two peaks around 5.2–5.4ppm, peak assignments are seen in Fig. 5, before hydrolysis decreased at 7 days and 14days, at 22 days these peaks were almost gone. Meanwhile, there are another two peaks that show up around 4.4ppm and increase with time. It was also observed that the peak at 3.8ppm decreased with time and that two more peaks appeared around 3.1 ppm and grew with time. All the materials after degradation were dialyzed against water and lyophilized before 1H NMR and FTIR test. So, combining all these evidence, it was believed that after hydrolysis, the ester bond in the ring structure was broken and thus, the ring in dimethyl-γ-butyrolactone was open. To further confirm this degradation mechanism, NaOH was added into the polymer solution in D2O after 22 days’ degradation to make a strong basic solution (pH=13) and this solution was put in 37°C water bath for 1 day. After that, 1H NMR was conducted on this polymer in D2O. It was found that two peaks around 3.1 ppm became very sharp. There is also one sharp peak showing up around 3.5 ppm and other two sharp peaks appearing around 0.6ppm. The appearance of these sharp peaks indicates that there are small molecules in the polymer solution and it was believed that these small molecules are the result of hydrolysis of the backbone ester leading to the ring falling off of the polymer backbone after opening. This evidence suggests that the ring structure in dimethyl-γ-butyrolactone will fall off of the polymer backbone eventually after opening. But for the time frame of application for controlled drug delivery, there should be no low molecular weight byproducts after ring-opening hydrolysis. In the FTIR spectra, the peak at 1785 cm−-1 gradually decreased until it was almost flat at 22 days. The peak at 1750cm−1 shifted to the right and finally reached 1723cm−1. This also indicated that the ring structure in dimethyl-γ-butyrolactone gradually opened resulting in the decrease of the peak at 1785 cm−1 until it was gone and the peak at 1750cm−1 shifted to 1723cm−1 because the change in local environment due to ring opening. The 1H NMR and FTIR spectra of the copolymers with other chemical compositions showed the same trend. (Data not shown) The 1H NMR and FTIR spectra before, during, and after time-dependent hydrolysis indicates, as hydrolysis progresses, the dimethyl-γ-butyrolactone ring structure was opened due to hydrolysis of the ester in the ring structure. This resulted in the change in both 1H NMR and FTIR spectra. These results also show that there are no low molecular weight byproducts from this polymer during hydrolysis for the time frame of application. This suggests that this polymer should be bioerodible without low molecular weight byproducts.
Figure 5.
Time-dependent 1H NMR Spectra of Poly(NIPAAm-co-Dimethyl-γ-Butyrolactone) with 7.2 mol.% Dimethyl-γ-Butyrolactone Content in D2O.
Figure 6.
Time-dependent FTIR Spectra of Poly(NIPAAm-co-Dimethyl-γ-Butyrolactone) 7.2 mol. % Dimethyl-γ-Butyrolactone Content. 1: Before Hydrolysis, 2: 1 day Hydrolysis, 3: 7 days Hydrolysis, 4: 14 days Hydrolysis, 5: 18 days Hydrolysis, 6: 22 days Hydrolysis
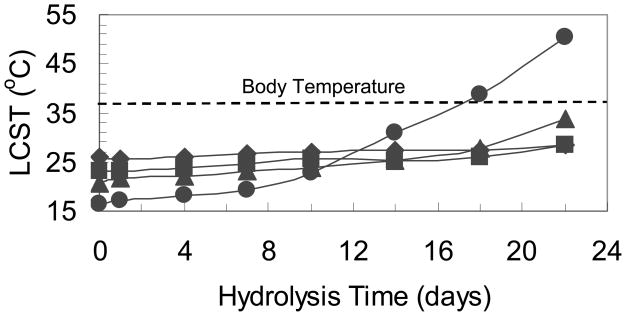
The time-dependent LCST was investigated at pH 7.4 under 70°C and the results are plotted in Fig. 7. It is seen that the LCST increased over the hydrolysis time. Also, with the increase in dimethyl-γ-butyrolactone content, the LCST increased faster. After 22 days of hydrolysis, for the copolymer with 2.9 mol.% dimethyl-γ-butyrolactone, the LCST increases from 25.8°C to 28.6°C. For the copolymer with 7.2 mol.% dimethyl-γ-butyrolactone, the LCST increases from 16.3°C to 50.33°C. The increased rate of LCST change is due to the carboxylic acid group resulting from ring-opening hydrolysis of the dimethyl-γ-butyrolactone. The polymer thus becomes more hydrophilic and becomes charged, increasing the accessibility of water to the polymer for increased hydrolysis rates. When the copolymer has a higher dimethyl-γ-butyrolactone content, this would accelerate the hydrolysis as more of the dimethyl-γ-butyrolactone groups were opened. For the copolymer with 7.2 mol.% dimethyl-γ-butyrolactone content, the LCST increased above body temperature after 18 days of hydrolysis at the accelerated condition of 70°C. Arrhenius kinetics suggests an approximately 10-fold acceleration under these conditions. This indicated that the material would become soluble after hydrolysis and that the polymer will diffuse away when used as a controlled drug delivery system. For copolymers with lower dimethyl-γ-butyrolactone content, the hydrolysis rate is slower. The time-dependent LCST result is another evidence showing that this new copolymer, poly(NIPAAm-co-dimethyl-γ-butyrolactone) should be bioerodible. Future work will also be focused on optimizing the original LCST, accelerating the hydrolysis process for drug delivery and tissue engineering applications, evaluating polymers with appropriate size and shape for kidney clearance for in vivo application,25 and investigating the in vitro and in vivo cytotoxicity/biocompatibility of these materials.
Figure 7.
Time-dependent LCST of Poly(NIPAAm-co-Dimethyl-γ-Butyrolactone) copolymer with different Dimethyl-γ-Butyrolactone content: diamond--2.9 mol.%, square-- 5.5 mol.%, triangle--6.0 mol.%, circle--7.2 mol.%. (time-dependent hydrolysis condition: pH=7.4 at 70°C)
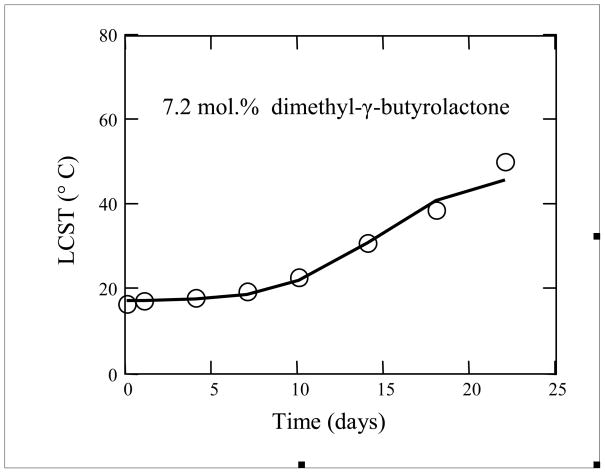
Figure 8 presents the LCST model fit for the polymer with 7.2 mol.% dimethyl-γ-butyrolactone. In this fit, by the least squares method, k = 0.049/(day x mol. %), b=0.176 mol.%, and c = 14.6 days. This fit had an adjusted R2 of 0.927.
Figure 8.
LCST versus Time model fitting for poly(NIPAAm-co-dimethyl-γ-butyrolactone) with 7.2 mol.% dimethyl-γ-butyrolactone (n=3 for data points): circle--, data; solid line--model.
The model supports the hypothesis that the hydrolysis is self-catalytic. The model explains this as due to the increase in H2O. The increased water content may result from an increase in polymer hydrophilicity and charge after hydrolysis. An alternate explanation might be pH effects as seen in lactic acid polymers 26, 27. However, in this case, the pH was adjusted frequently to keep the pH nearly constant. Thus the acceleration effect must be due to other factors. The physical significance of the variable, c, in the model corresponds to the time to degradation of half the dimethyl-γ-butyrolactone. Table 2 presents the model fits and correlation coefficients for all of the polymers. Particularly noteworthy in the data found in Table 2 and Figure 7, is the integration constant, c, physically equivalent to the half-life of the dimethyl-γ-butyrolactone ring-opening. This further shows an increased rate of degradation with increased dimethyl-γ-butyrolactone content.
Table 2.
Model Parameters
| Model Parameters | ||||
|---|---|---|---|---|
| Polymer | k (1/(mol.% × day)) | c (days) | b (mol %) | R2 |
| 1 | 0.034 | 34.16 | 0.001 | 0.862 |
| 2 | 0.011 | 35.21 | 0.001 | 0.904 |
| 3 | 0.025 | 21.84 | 0.0003 | 0.969 |
| 4 | 0.049 | 14.60 | 0.176 | 0.927 |
It is expected from the hydrophobicity of the monomer that increased monomer content would reduce the water content of the gel, thus protecting the dimethyl-γ-butyrolactone monomer from hydrolysis. However, the data shows that the charge resulting from the degradation of the butyrolactone ring increases the water accessible to the polymer chain enough to counter balance this effect and cause acceleration in degradation. In poly(NIPAAm-co-Hydroxyethyl methacrylate [HEMA]-lactate), an increase in the HEMA-lactate monomer increases the time of degradation. 11 In that case, the degradation product, HEMA, left on the polymer chain does not produce a charge on the polymer and does not significantly affect the hydrophilicity.
Conclusion
A novel thermosensitive, bioerodible copolymer, poly(NIPAAm-co-dimethyl-γ-butyrolactone) was synthesized and characterized. It shows a decrease of LCST from 25.8 to 16.3°C, depending on the content of dimethyl-γ-butyrolactone, before hydrolysis and an increase in LCST from 36.8 to 50°C after complete hydrolysis. FTIR and 1H NMR spectra verified the copolymerization of these two monomers. FTIR and 1H NMR spectra and LCST results after time-dependent hydrolysis indicate that incorporation of dimethyl-γ-butyrolactone hydrolytic cyclic group provides the degradability of the copolymer which makes this new copolymer a candidate material for controlled drug delivery and tissue engineering applications. The ring structure in dimethyl-γ-butyrolactone was open due to hydrolysis without breaking the ester nearer the backbone avoiding low molecular weight byproducts on applicable time scales which is desirable for biomedical applications. An understanding of the kinetics of the degradation for these polymers will enable rationale design of drug delivery materials with planned properties.
Acknowledgments
The authors acknowledge the National Institutes of Health (NIH) for financial support, NIH grant number GM065917. The authors also gratefully acknowledge the use of facilities within the Center for Solid State Science at Arizona State University
References
- 1.Li F, Griffith M, Li Z, Tanodekaew S, Sheardown H, Hakim M, Carlsson DJ. Recruitment of multiple cell lines by collagen-synthetic copolymer matrices in corneal regeneration. Biomaterials. 2005;26(16):3093–3104. doi: 10.1016/j.biomaterials.2004.07.063. [DOI] [PubMed] [Google Scholar]
- 2.Liu SQ, Tong YW, Yang YY. Incorporation and in vitro release of doxorubicin in thermally sensitive micelles made from poly(N-isopropylacrylamide-co-N, N-dimethylacrylamide)-b-poly(D, L-lactide-co-glycolide) with varying compositions. Biomaterials. 2005;26(24):5064–5074. doi: 10.1016/j.biomaterials.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 3.Park K-h, Yun K. J Biosci Bioeng. 2004;97:374–377. doi: 10.1016/S1389-1723(04)70221-2. [DOI] [PubMed] [Google Scholar]
- 4.Fujishige S, Kubota K, Ando I. Phase-Transition of Aqueous-Solutions of Poly(N-Isopropylacrylamide) and Poly(N-Isopropylmethacrylamide) Journal of Physical Chemistry. 1989;93(8):3311–3313. [Google Scholar]
- 5.Heskin M, Guillet JE. Solution properties of poly(N-isopropylacrylamide) J Macromol Sci-Chem. 1968;A2(8):1441–1455. [Google Scholar]
- 6.Ben-Neim A. Hydrophobic Interaction. Plenum; New York: 1980. [Google Scholar]
- 7.Hirotsu S. Phase-Transition of a Polymer Gel in Pure and Mixed-Solvent Media. Journal of the Physical Society of Japan. 1987;56(1):233–242. [Google Scholar]
- 8.Otake K, Inomata H, Konno M, Saito S. A New Model for the Thermally Induced Volume Phase-Transition of Gels. Journal of Chemical Physics. 1989;91(2):1345–1350. [Google Scholar]
- 9.Otake K, Inomata H, Konno M, Saito S. Thermal-Analysis of the Volume Phase-Transition with N-Isopropylacrylamide Gels. Macromolecules. 1990;23(1):283–289. [Google Scholar]
- 10.Solis F, Weiss-Malik R, Vernon B. Local monomer activation model for phase behavior and calorimetric properties of LCST gel-forming polymers. Macromol. 2005;38:4456–4464. [Google Scholar]
- 11.Lee BH, Vernon B. Copolymers of N-isopropylacrylamide, HEMA-lactate and acrylic acid with time-dependent lower critical solution temperature as a bioresorbable carrier. Polymer International. 2005;54(2):418–422. [Google Scholar]
- 12.Stile RA, Burghardt WR, Healy KE. Synthesis and characterization of injectable poly(N-isopropylacrylamide)-based hydrogels that support tissue formation in vitro. Macromolecules. 1999;32(22):7370–7379. [Google Scholar]
- 13.Vernon B, Kim SW, Bae YH. Thermoreversible copolymer gels for extracellular matrix. J Biomed Mater Res. 2000;51(1):69–79. doi: 10.1002/(sici)1097-4636(200007)51:1<69::aid-jbm10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 14.Neradovic D, Hinrichs WLJ, Kettenes-van den Bosch JJ, Hennink WE. Poly(N-isopropylacrylamide) with hydrolyzable lactic acid ester side groups: a new type of thermosensitive polymer. Macromolecular Rapid Communications. 1999;20(11):577–581. [Google Scholar]
- 15.Neradovic D, Hinrichs WLJ, Kettenes-van den Bosch JJ, van Nostrum CF, Hennink WE. Poly(N-isopropylacrylamide) with hydrolyzable lactic acid ester side groups: A new type of thermosensitive polymer suitable for drug delivery purposes. Journal of Controlled Release. 2001;72(1–3):252–253. [Google Scholar]
- 16.Neradovic D, van Nostrum CF, Hennink WE. Thermoresponsive polymeric micelles with controlled instability based on hydrolytically sensitive N-isopropylacrylamide copolymers. Macromolecules. 2001;34(22):7589–7591. [Google Scholar]
- 17.Neradovic D, van Steenbergen MJ, Vansteelant L, Meijer YJ, van Nostrum CF, Hennink WE. Degradation mechanism and kinetics of thermosensitive polyacrylamides containing lactic acid side chains. Macromolecules. 2003;36(20):7491–7498. [Google Scholar]
- 18.Sun LF, Zhuo RX, Liu ZL. Studies on the synthesis and properties of temperature responsive and biodegradable hydrogels. Macromolecular Bioscience. 2003;3(12):725–728. [Google Scholar]
- 19.Yoshida T, Aoyagi T, Kokufuta E, Okano T. Newly designed hydrogel with both sensitive thermoresponse and biodegradability. Journal of Polymer Science Part a-Polymer Chemistry. 2003;41(6):779–787. [Google Scholar]
- 20.Lee BH, Lee YM, Sohn YS, Song SC. Thermosensitive and hydrolysis-sensitive poly(organophosphazenes) Polymer International. 2002;51(7):658–660. [Google Scholar]
- 21.Lee B, Vernon BL. In Situ-Gelling, Erodible N-isopropylacrylamide Copolymers. Macromolecular Biosciences. 2005;5(7):629–635. doi: 10.1002/mabi.200500029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Standardization, I. O. f. ISO10993–13: Biological evaluation of medical devices. Identification and quantification of degradation products from polymeric medical devices. 1998.
- 23.Montgomery DC. Design and Analysis of Experiments. John Wiley & Sons, Inc.; New York: 2001. p. 684. [Google Scholar]
- 24.Pei Y, Chen J, Yang LM, Shi LL, Tao Q, Hui BJ, Li R. The effect of pH on the LCST of poly(N-isopropylacrylamide) and poly(N-isopropylacrylamide-co-acrylic acid) Journal of Biomaterials Science-Polymer Edition. 2004;15(5):585–594. doi: 10.1163/156856204323046852. [DOI] [PubMed] [Google Scholar]
- 25.Ohlson Effects of filtration rate on the glomerular barrier and clearance of four differently shaped molecules. American Journal of Physiology: Renal Physiology. 2001;(281):103–113. doi: 10.1152/ajprenal.2001.281.1.F103. [DOI] [PubMed] [Google Scholar]
- 26.Li SM. Hydrolytic degradation characteristics of aliphatic polyesters derived from lactic and glycolic acids. Journal of Biomedical Materials Research. 1999;48(3):342–353. doi: 10.1002/(sici)1097-4636(1999)48:3<342::aid-jbm20>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 27.Park TG, Lu WQ, Crotts G. Importance Of In-Vitro Experimental Conditions On Protein Release Kinetics, Stability And Polymer Degradation In Protein Encapsulated Poly(D, L-Lactic Acid-Co-Glycolic Acid) Microspheres. JOURNAL OF CONTROLLED RELEASE. 1995;33(2):211–222. [Google Scholar]