Abstract
Aims
We evaluated whether changes over 10–11 years in weight, subcutaneous abdominal fat (SQAF), and intra-abdominal fat (IAF) differ by age and contrasted age-related adiposity changes by gender.
Methods
This is a prospective cohort study of non-diabetic, Japanese-American men and women aged 34–74 years. IAF and SQAF were measured by CT scan at baseline and 10–11 year follow-up visits.
Results
The youngest participants gained the most weight, SQAF and IAF over 10–11 years. Older age at baseline was associated with significantly less change in weight (β-coefficient: −0.19, 95% CI −0.22, −0.15), SQAF (β-coefficient −0.07: 95% CI −0.11, −0.02) and IAF (β-coefficient −0.74: 95% CI −1.03, −0.45) that persisted even after adjustments for sex, smoking, caloric intake, adiposity at baseline, and change in physical activity. Age was positively correlated with change in SQAF (β-coefficient 0.14: 95% CI 0.10, 0.18) and IAF (β-coefficient 0.51: 95% CI 0.21, 0.81) in separate models after further adjustment for weight change. Gender did not alter the associations between adiposity change and age.
Conclusions
Men and women gained the greatest absolute amount of weight, SQAF, and IAF at younger ages; however, older adults with comparable weight changes had relatively greater fat accumulation within IAF and SQAF depots.
Keywords: aging, intra-abdominal fat, adiposity, Japanese-American, longitudinal
1. Introduction
The physiological changes that occur throughout the aging process and confer risk for the development of cardiovascular disease and diabetes have not been fully studied. One potential contributor is an increase in adiposity, particularly visceral adiposity, which is strongly linked with an adverse cardiometabolic profile [1, 2]. Prior studies from the Japanese American Community Diabetes Study (JACDS) have determined intra-abdominal fat (IAF) to be a predictor of incident type 2 diabetes, impaired glucose tolerance, hypertension and coronary heart disease [3–6]. Given the multiple health risks associated with visceral adiposity, knowledge of the period in one’s lifespan when accumulation of IAF is greatest would help guide preventive measures.
Cross-sectional studies demonstrate a positive association between age and increased waist circumference, body mass index (BMI), total body fat and IAF [7, 8]. However, longitudinal studies show that weight gain and increases in BMI are highest in young adults while declines in weight and BMI are seen in adults over the age of 55 [9–11]. Since weight and BMI are measures which also include muscle mass and bone mass and are influenced by height; changes in these measurements may not accurately reflect changes in fat mass. To better understand the process of fat accumulation in different body depots, longitudinal studies of changes in visceral and subcutaneous fat depots with aging are needed.
Because of the metabolic risks associated with IAF and the poor ability of changes in weight and BMI to act as a surrogate for changes in regional adiposity, we investigated when the greatest increase in IAF and subcutaneous abdominal fat (SQAF) occur during the aging process and whether these associations vary by gender.
2. Methods
2.1 Study Subjects
Non-diabetic, Japanese-American men and women of 100% Japanese ancestry from the JACDS were included in this analysis. The recruitment and enrollment of this study population has been previously described [12]. Briefly, subjects were recruited from 1983–1988 and represented the age-distribution, parental immigration pattern, and demographic patterns of Japanese Americans in King County, Washington. The study protocol was reviewed and approved by the University of Washington Human Subjects Review Committee, subjects provided written informed consent, and study procedures were in compliance with ethical standards of the University of Washington Institutional Review Board.
A total of 658 Japanese-American men and non-pregnant women aged 34–74 years were enrolled and underwent a baseline exam. Subjects were excluded if they met the WHO criteria for type 2 diabetes at baseline or were on treatment for diabetes at any time during the study (n=148) because diabetes treatment may influence weight gain [13]. There was an 80% retention rate with only 103 participants lost to follow-up. Fourteen participants had missing data on outcome measurements. After these exclusions, our cohort included 393 participants comprised of 116 Nisei (second-generation) men, 97 Nisei women, 89 Sansei (third-generation) men and 91 Sansei women. No women became pregnant during the study.
2.2 Measurements
At baseline and 10–11 year follow-up visits, participants underwent physical exams and computed tomography imaging. BMI was calculated using weight in kilograms divided by height in meters squared. Blood pressure was measured with a mercury sphygmomanometer in triplicate on participants while supine and the latter two measurements were averaged and reported as millimeters of mercury. Measurements of IAF and SQAF areas in squared centimeters were derived from a single-slice CT of the abdomen at the level of the umbilicus because this level contains the greatest total fat/abdominal tissue ratio compared to neighboring slices [14]. Furthermore, this method of measuring IAF and SQAF areas correlates highly with IAF and SQAF volumes measured by helical CT [15].
2.3. Definitions
Smoking, menopausal status, diet, and physical activity history were obtained by questionnaire at baseline. Smoking was categorized as current versus former or no prior use of cigarettes. Menopausal history was derived from questions of menstrual history and estrogen use and classified into 8-categories to capture changes in menopausal status. The categories included stayed premenopausal, became perimenopausal, became postmenopausal, became postmenopausal on estrogen replacement, stayed postmenopausal, stayed postmenopausal but stopped estrogen replacement, stayed postmenopausal on estrogen replacement, and stayed postmenopausal but started estrogen replacement. Caloric intake in kilocalories per day was calculated from an 89-item food frequency questionnaire [16]. Physical activity in leisure time was obtained from a modified Paffenbarger questionnaire and calculated as weekly kilocalories of energy expenditure [17].
2.4. Statistical Analysis
Statistical analyses were performed using STATA SE10 (STATA Corp., College Station, TX). The average baseline measures for weight and SQAF were assessed for each gender. The ratio of SQAF/IAF was calculated for each study subject. Median values of IAF and SQAF/IAF ratio by gender were shown given the skewed distribution of these baseline measurements. Changes in weight, IAF, and SQAF were calculated as follow-up minus baseline measurements. Significant differences between baseline and follow-up measures were determined using Student’s t-test. The associations between the change in each measure of adiposity and baseline age as a continuous variable was evaluated using linear regression analysis in separate models. There was no evidence for non-linear associations between age and changes in adiposity based on addition of age2 or age2 plus age3 variables in the models. To evaluate for differences in adiposity change with age by gender, a first-order multiplicative interaction term between age and sex was examined in each linear regression model of the relationship between age and change in adiposity adjusted for sex. Variables considered potential confounders were included in models as covariates. In order to assess changes in SQAF or IAF independent of total weight change, we performed additional analyses adjusted for covariates and weight change. Statistical significance was determined by a two-sided p-value < 0.05.
3. Results
The average age of the cohort was 51.6 years. On average they were of normal weight at baseline with a mean BMI of 24.1 kg/m2. Men had greater mean weight, mean SQAF, median IAF and lower median SQAF/IAF at baseline compared to women: 71.1 Kg vs. 54.6 Kg, 88.0 cm2 vs. 83.3 cm2, 89.2 cm2 vs. 52.9 cm2, and 0.99 vs. 1.57, respectively. By age, the youngest individuals had the lowest amounts of SQAF and IAF and the highest physical activity (Table 1). Baseline, follow-up and the change in measures of adiposity are presented in Table 2. Baseline weight was lowest in adults over the age of 64 years; however this group had highest baseline visceral adiposity. While there was no significant change in weight and SQAF in adults aged ≥54 years, they still experienced a significant increase in IAF. The greatest gain in weight, SQAF and IAF over 10–11 years occurred in the youngest group aged 34–43 years.
Table 1.
Baseline characteristics by age group
| Age (years) | ||||
|---|---|---|---|---|
| 34–43 (n=139) |
44–53 (n=60) |
54–63 (n=122) |
≥64 (n=72) |
|
| Male | 50% | 55% | 55% | 50% |
| Smokers | 14% | 18% | 5% | 11% |
| Caloric intake (Kcal/day) | 1739 (598) | 1909 (549) | 1733 (564) | 1718 (578) |
| Physical activity (Kcal/week)* | 3023 (2237) | 2888 (2098) | 2522 (1754) | 2345 (1457) |
| Weight (Kg) | 63.7 (11.7) | 66.5 (14.5) | 63.0 (12.4) | 59.9 (9.9) |
| SQAF (cm2)* | 83 (8.9) | 87.8 (10.3) | 87 (8.0) | 87.1 (6.6) |
| IAF (cm2)* | 41.8 (23.4, 78.4) | 69.8 (40.4, 106.5) | 89.2 (61.7, 122.2) | 94.7 (69.3, 132.4) |
Data are means (s.d.), medians (interquartile range) or proportions.
p <0.05 for trend test
Table 2.
Baseline, 10–11 year follow-up and change in measures of adiposity by age group
| Measurements |
Age Group |
Baseline |
Follow-up |
Difference |
% Change |
|---|---|---|---|---|---|
| Weight (Kg) | 34–43 | 63.7 (11.7) | 68.5 (12.4) | 4.8* | 7.5 |
| 44–53 | 66.5 (14.5) | 69.9 (15.4) | 3.4* | 5.1 | |
| 54–63 | 63.0 (12.4) | 63.2 (13.0) | 0.2 | 0.3 | |
| ≥64 | 59.9 (9.9) | 60.0 (10.2) | 0.1 | 0.2 | |
| SQAF (cm2) | 34–43 | 83.0 (8.9) | 84.8 (10.1) | 1.8* | 2.2 |
| 44–53 | 87.8 (10.4) | 89.6 (12.0) | 1.8† | 2.1 | |
| 54–63 | 87.0 (8.0) | 87.4 (9.9) | 0.4 | 0.5 | |
| ≥64 | 87.1 (6.6) | 87.1 (8.9) | 0.0 | 0.0 | |
| IAF (cm2) | 34–43 | 52.8 (33.8) | 80.3 (42.3) | 27.5* | 52.1 |
| 44–53 | 82.8 (53.9) | 105.9 (67.8) | 23.1* | 27.9 | |
| 54–63 | 95.6 (46.9) | 102.2 (51.7) | 6.6† | 7.0 | |
| ≥64 | 99.0 (48.3) | 110.1 (48.7) | 11.1† | 11.2 |
Data are means (s.d.)
p<0.001
p<0.05
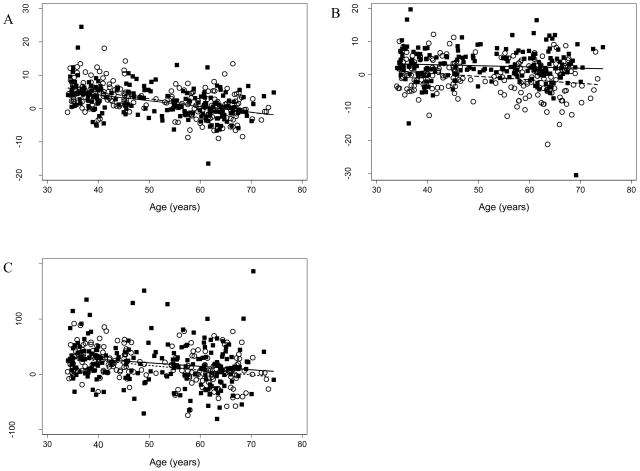
Univariate regression analyses revealed significantly less change over 10 years in weight, SQAF and IAF with increasing age at baseline, with β-coefficients for these slopes of −0.19, −0.07, and −0.74, respectively (Table 3, unadjusted models). The inverse relationship between age and change in weight over 10–11 years (Fig. 1, panel A, y= −0.17x +11.01 men, y= −0.20x+13.03 women) demonstrates that the youngest men and women gained the most weight. Based on the regression line, 34 year old men and women gained on average, 5.2 kg and 6.2 kg, respectively, whereas for men and women ≥ 65 years, 10–11 year weight change was zero or less (weight loss). The slopes between age and changes in SQAF were also negative for both genders (y= −0.04x + 4.54 men, y= −0.11x + 4.74 women), but were of smaller magnitude than the slopes seen for the associations between change in IAF and weight with age (Fig. 1, Panel B). The change in IAF over 10 years showed a similar relationship to weight with advancing age (Fig., Panel C, y= −0.63x+52.19 men and y= −0.86x+58.86 women). The greatest average predicted IAF change was at the youngest age of 34 years: increases of 29 cm2 for men and 26 cm2 for women. The negative slope of the line between age and change in IAF indicates less 10–11 year IAF gain among older compared to younger participants. Participants of all ages gained IAF on average during follow-up, as indicated by the fact that the regression line did not cross zero over the baseline age range of our population.
Table 3.
Multivariable adjusted β-coefficients for age with Δ Weight, Δ SQAF and Δ IAF over 10–11 years as outcomes
| Outcome variables |
||||||||
|---|---|---|---|---|---|---|---|---|
| Δ Weight Model |
ΔSQAF Model |
ΔIAF Model |
||||||
| Unadjusted | Add Lifestyle Measures | Unadjusted | Add Lifestyle Measures | Add Δ Weight | Unadjusted | Add Lifestyle Measures | Add Δ Weight | |
| Age | −0.19* | −0.18* | −0.07† | −0.06† | 0.14* | −0.74* | −0.40† | 0.52* |
| Sex | 0.51 | −3.18* | −1.72† | −12.74† | −10.68† | |||
| Smoking | −0.76 | −0.59 | 0.13 | −7.97 | −4.26 | |||
| Caloric intake | −0.001 | 0.001 | 0.001 | −0.004 | −0.002 | |||
| Δ Activity | −0.0002 | −0.0003 | −0.0001 | −0.001 | 0.0002 | |||
| Weight | 0.02 | 0.16* | 0.21 | |||||
| Baseline SQAF | −0.04 | −0.22* | ||||||
| Baseline IAF | −0.19* | −0.20* | ||||||
| Δ Weight | 0.88* | 4.79* | ||||||
p ≤0.001,
p ≤0.01
Figure 1.
A: Linear regression of ΔWeight with Age, (r=−0.43, p<0.001 for males and r=−0.51, p<0.001 for females). B: Linear regression of ΔSQAF with Age, (r=−0.08, p=0.23 for males and r=−0.24, p=0.001 for females). C: Linear regression of ΔIAF with Age, (r=−0.18, p=0.008 for males and r=−0.34, p<0.001 for females).
Gender differences in the relationship between age and changes in adiposity were examined. The best fit regression line for men and women depicting the change in weight and IAF with age nearly overlapped and were similar for the association between change in SQAF and age (Fig. 1, Panels A–C). Insertion of an age × gender interaction term in adjusted regression models revealed no significant difference by gender in the relationship between age and change in weight or IAF in separate models (p-value for interaction terms = 0.35 and 0.42, respectively). Although the relationship between change in SQAF and age appeared to be less consistent between men and women compared to change in IAF or weight (Fig. 1, Panel C), we again found no significant difference by gender (p=0.14).
Multiple regression analyses were performed on the entire cohort given the absence of statistically significant gender differences to adjust for potential confounding variables. Adjustment for baseline smoking status, sex, caloric intake, change in physical activity, baseline measure of adiposity, and change in physical activity level over 10–11 years attenuated but did not significantly alter the relationship between baseline age and change in IAF; the association between baseline age and the changes in SQAF and weight were unaltered after these adjustments (Table 3, Models with Lifestyle Measures). Adjustment for the change in smoking status over 10–11 years did not significantly alter the relationships between age and changes in adiposity (data not shown). Multiple regression models were also used to determine if changes in adiposity with advancing age occurred independently of weight change during follow-up. A positive and significant association between age and change in SQAF and IAF emerged after adjustment for the change in weight (Table 3), indicating that for a given amount of weight change, the changes in SQAF and IAF increased with older age at baseline.
4. Discussion
We found a negative association between age and changes in adiposity over a 10–11 year period for Japanese-American men and women. These results revealed that the greatest absolute increases in body weight, SQAF and IAF occurred in the youngest men and women. Participants of all ages gained IAF, but the absolute amount decreased with older age. Interestingly, there was no significant difference in the relationship between age and changes in adiposity by gender. The finding that the amount of change in weight, SQAF and IAF was greatest in younger Japanese Americans suggests that preventive measures to control adverse changes in body fat accumulation and subsequent development of associated chronic diseases may yield greatest benefits when targeted at young adults.
Another important finding of this analysis was that for a given 10-yr weight change, there were greater increases in adipose tissue area in the intra-abdominal and subcutaneous abdominal fat depots among older compared with younger subjects (within the age range studied). This may seem contradictory on the surface with our finding of greater IAF accumulation at younger ages, but can be explained by the greater absolute weight and adipose tissue accumulations seen in younger subjects even though younger subjects accumulate a smaller proportion of adipose tissue in the SQAF and IAF depots for a given amount of weight gain. When older men and women gained weight, it appeared due to more abdominal fat mass (and by inference less lean mass) compared with younger adults who gained the same amount of weight.
Multiple changes in body composition are known to occur with aging. Our data confirm prior studies that men and women gain the most weight at younger ages, have progressively less weight gain with advancing age, and are relatively weight stable around the age of 70 [18, 19]. While it may seem contradictory for older adults to continue gaining IAF with no weight gain, this increase in visceral adiposity may occur concurrently with a loss in muscle mass or extremity fat. Cross-sectional studies evaluating body composition support this by showing that older men and women have higher BMI, total % body fat and fat mass, but decreased fat free mass compared with younger adults [8]. Furthermore, longitudinal changes in body composition in older adults demonstrate a loss in total, appendicular and leg fat-free mass with no significant change in fat mass [18]. Our findings are contrary to what may have been expected. Others have hypothesized a greater gain in fat mass and visceral adiposity in the elderly attributed to decreased physical activity as well as declines in sex steroid hormones and growth hormone with aging [20, 21].
Regulation of adiposity is multifactorial and may not be characterized simply by an imbalance between energy intake and energy expenditure. Lifestyle changes including increased caloric intake and decreased physical activity levels have been implicated in increases in adiposity. In our cohort, neither changes in activity level nor baseline caloric intake accounted for the greater changes in adiposity among younger participants; although we cannot exclude the possibility that measurement error resulted in incomplete adjustment for these confounders. Thus, it would appear that studying the period of young adulthood in greater detail could provide important information about factors that account for the major changes in adiposity occurring at this time.
Differences in adiposity change with age by gender were also evaluated and found to be absent. It is possible that the effect size or sample size was not great enough to detect a difference by gender in this association. Given that there was no interaction between age and sex, the relationship between age and changes in measures of adiposity were best estimated by the entire cohort.
Understanding changes associated with the aging process is best accomplished using longitudinal studies as opposed to comparing cross-sectional samples of different subjects across the age spectrum. However, longitudinal studies can be limited by low retention rates and high cost. This longitudinal study is unique in its use of repeat CT scans over a 10–11 year time period to measure IAF and SQAF. The high retention rate of 80% at 10 years is a major strength. The baseline characteristics of the participants lost to follow-up did not differ significantly from our analytic cohort. However, the participants lost to follow-up were on average slightly older, had lower caloric intake and a higher amount of IAF at baseline. Exclusion of participants who died likely did not bias our results in the direction of less weight gain with greater age, since weight loss rather than weight gain is associated with mortality in older individuals [22]. Limitations include the potential differences in generations and birth cohort that may have influenced adiposity changes. However, when the relationship between age and measures of adiposity was evaluated separately for Sansei and Nisei, similar slopes for the associations were found (data not shown). Given a study population that is 100% Japanese American, these results may not be applicable to other ethnic groups or different generations of Japanese Americans. For example, subjects in the INTERLIPID study had a higher average BMI compared to our cohort, but this study included 3rd and 4th generation Japanese Americans who may have experienced greater acculturation than subjects in our cohort [23]. Asians may have a greater amount of truncal fat at any given age than Caucasians and African-Americans [24]. While we cannot extrapolate these findings to other ethnic groups, our findings are supported by another study that examined CT-measured change in visceral fat area by age and ethnicity and showed that the greatest increase over 5 years occurred in the youngest subjects. While they found declines in fat accumulation by advancing age group, the trends were not significant; perhaps due to the shorter follow-up as compared to our study [25]. Our study population included participants between ages 34 and 74 at baseline with the youngest enrollees showing the greatest weight gain, IAF and SQAF accumulation. A report from the Netherlands that examined change in body mass by age showed that the greatest increase in BMI was seen for 20-year olds [26], suggesting that the greatest accumulation of IAF and SQAF may occur in very young adulthood. Studies including young adults and adolescents are also needed to evaluate when this surge of fat accumulation occurs, especially in the context of rising rates of obesity in younger adults. Although we found no differences in the associations between age and fat accumulation by gender, absence of this interaction may or may not be generalize to other populations.
In conclusion, in this longitudinal analysis, the greatest absolute increase in weight, SQAF and IAF occurred at younger ages. When weight change during follow-up was held constant through statistical adjustment, older age was associated with higher SQAF and IAF change. Although greater weight and adiposity gain occurred at younger ages, a smaller degree of weight gain at older ages may result in similar adverse metabolic consequences due to an expansion of the IAF depot. These results would argue that efforts aimed at preventing weight gain be directed to younger age groups in order to reduce the overall amount of fat mass, and in particular visceral fat mass, gained in a lifespan.
Acknowledgments
This work was supported by National Institutes of Health Grants DK-31170, HL-49293, and DK-02654; by facilities and services provided by the Diabetes and Endocrinology Research Center (DK-17047), Clinical Nutrition Research Unit (DK-35816), and the General Clinical Research Center (RR-00037) at the University of Washington, and by the Medical Research Service of the Department of Veterans Affairs.
All authors have been involved in one or more of the following study activities: design, data collection, data analysis, and manuscript preparation. We are grateful to the King County Japanese-American Community for support and cooperation.
Footnotes
These data have been presented at the following meetings:
American Federation for Medical Research Western Regional Meeting. Carmel, CA. January 2009.
11th Symposium of the International Diabetes Epidemiology Group. Montreal, CAN. October 2009.
Disclosure: The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 2.Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116:1234–1241. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 3.Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L. Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes Care. 2000;23:465–471. doi: 10.2337/diacare.23.4.465. [DOI] [PubMed] [Google Scholar]
- 4.Hayashi T, Boyko EJ, Leonetti DL, McNeely MJ, Newell-Morris L, Kahn SE, et al. Visceral adiposity is an independent predictor of incident hypertension in Japanese Americans. Ann Intern Med. 2004;140:992–1000. doi: 10.7326/0003-4819-140-12-200406150-00008. [DOI] [PubMed] [Google Scholar]
- 5.Fujimoto WY, Bergstrom RW, Boyko EJ, Chen KW, Leonetti DL, Newell-Morris L, et al. Visceral adiposity and incident coronary heart disease in Japanese-American men. The 10-year follow-up results of the Seattle Japanese-American Community Diabetes Study. Diabetes Care. 1999;22:1808–1812. doi: 10.2337/diacare.22.11.1808. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi T, Boyko EJ, Leonetti DL, McNeely MJ, Newell-Morris L, Kahn SE, et al. Visceral adiposity and the risk of impaired glucose tolerance: a prospective study among Japanese Americans. Diabetes Care. 2003;26:650–655. doi: 10.2337/diacare.26.3.650. [DOI] [PubMed] [Google Scholar]
- 7.Cefalu WT, Wang ZQ, Werbel S, Bell-Farrow A, Crouse JR, 3rd, Hinson WH, et al. Contribution of visceral fat mass to the insulin resistance of aging. Metabolism. 1995;44:954–959. doi: 10.1016/0026-0495(95)90251-1. [DOI] [PubMed] [Google Scholar]
- 8.Guo SS, Zeller C, Chumlea WC, Siervogel RM. Aging, body composition, and lifestyle: the Fels Longitudinal Study. The American journal of clinical nutrition. 1999;70:405–411. doi: 10.1093/ajcn/70.3.405. [DOI] [PubMed] [Google Scholar]
- 9.Rissanen A, Heliovaara M, Aromaa A. Overweight and anthropometric changes in adulthood: a prospective study of 17,000 Finns. Int J Obes. 1988;12:391–401. [PubMed] [Google Scholar]
- 10.Williamson DF, Kahn HS, Remington PL, Anda RF. The 10-year incidence of overweight and major weight gain in US adults. Arch Intern Med. 1990;150:665–672. [PubMed] [Google Scholar]
- 11.Droyvold WB, Lund Nilsen TI, Lydersen S, Midthjell K, Nilsson PM, Nilsson JA, et al. Weight change and mortality: the Nord-Trondelag Health Study. J Intern Med. 2005;257:338–345. doi: 10.1111/j.1365-2796.2005.01458.x. [DOI] [PubMed] [Google Scholar]
- 12.Fujimoto WY, Leonetti DL, Kinyoun JL, Newell-Morris L, Shuman WP, Stolov WC, et al. Prevalence of diabetes mellitus and impaired glucose tolerance among second-generation Japanese-American men. Diabetes. 1987;36:721–729. doi: 10.2337/diab.36.6.721. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Part 1: Diagnosis and Classification fo Diabetes Mellitus. Geneva: 1999. Definition, diagnosis and classification of diabetes mellitus and its complications: Report of a WHO Consultation. [Google Scholar]
- 14.Shuman WP, Morris LL, Leonetti DL, Wahl PW, Moceri VM, Moss AA, et al. Abnormal body fat distribution detected by computed tomography in diabetic men. Invest Radiol. 1986;21:483–487. doi: 10.1097/00004424-198606000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi J, Tadokoro N, Watanabe M, Shinomiya M. A novel method of measuring intra-abdominal fat volume using helical computed tomography. Int J Obes Relat Metab Disord. 2002;26:398–402. doi: 10.1038/sj.ijo.0801921. [DOI] [PubMed] [Google Scholar]
- 16.Tsunehara CH, Leonetti DL, Fujimoto WY. Diet of second-generation Japanese-American men with and without non-insulin-dependent diabetes. The American journal of clinical nutrition. 1990;52:731–738. doi: 10.1093/ajcn/52.4.731. [DOI] [PubMed] [Google Scholar]
- 17.Leonetti DL, Tsunehara CH, Wahl PW, Fujimoto WY. Educational attainment and the risk of non-insulin-dependent diabetes or coronary heart disease in Japanese-American men. Ethn Dis. 1992;2:326–336. [PubMed] [Google Scholar]
- 18.Fantin F, Di Francesco V, Fontana G, Zivelonghi A, Bissoli L, Zoico E, et al. Longitudinal body composition changes in old men and women: interrelationships with worsening disability. The journals of gerontology. 2007;62:1375–1381. doi: 10.1093/gerona/62.12.1375. [DOI] [PubMed] [Google Scholar]
- 19.Droyvold WB, Nilsen TI, Kruger O, Holmen TL, Krokstad S, Midthjell K, et al. Change in height, weight and body mass index: Longitudinal data from the HUNT Study in Norway. Int J Obes (Lond) 2006;30:935–939. doi: 10.1038/sj.ijo.0803178. [DOI] [PubMed] [Google Scholar]
- 20.Bjorntorp P. Aging and body composition. Nutrition. 1997;13:572–573. doi: 10.1016/s0899-9007(97)00122-6. [DOI] [PubMed] [Google Scholar]
- 21.Beaufrere B, Morio B. Fat and protein redistribution with aging: metabolic considerations. Eur J Clin Nutr. 2000;54(Suppl 3):S48–53. doi: 10.1038/sj.ejcn.1601025. [DOI] [PubMed] [Google Scholar]
- 22.Arnold AM, Newman AB, Cushman M, Ding J, Kritchevsky S. Body weight dynamics and their association with physical function and mortality in older adults: the Cardiovascular Health Study. The journals of gerontology. 65:63–70. doi: 10.1093/gerona/glp050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ueshima H, Okayama A, Saitoh S, Nakagawa H, Rodriguez B, Sakata K, et al. Differences in cardiovascular disease risk factors between Japanese in Japan and Japanese-Americans in Hawaii: the INTERLIPID study. Journal of human hypertension. 2003;17:631–639. doi: 10.1038/sj.jhh.1001606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu CH, Heshka S, Wang J, Pierson RN, Jr, Heymsfield SB, Laferrere B, et al. Truncal fat in relation to total body fat: influences of age, sex, ethnicity and fatness. Int J Obes (Lond) 2007;31:1384–1391. doi: 10.1038/sj.ijo.0803624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hairston KG, Scherzinger A, Foy C, Hanley AJ, McCorkle O, Haffner S, et al. Five-year change in visceral adipose tissue quantity in a minority cohort: the Insulin Resistance Atherosclerosis Study (IRAS) family study. Diabetes care. 2009;32:1553–1555. doi: 10.2337/dc09-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nooyens AC, Visscher TL, Verschuren WM, Schuit AJ, Boshuizen HC, van Mechelen W, et al. Age, period and cohort effects on body weight and body mass index in adults: The Doetinchem Cohort Study. Public Health Nutr. 2008:1–9. doi: 10.1017/S1368980008003091. [DOI] [PubMed] [Google Scholar]