Abstract
Members of the Bcl-2 protein family modulate outer mitochondrial membrane permeability to control apoptosis1,2. However, these proteins also localize to the endoplasmic reticulum (ER), the functional significance of which is controversial3, 4. Here we provide evidence that anti-apoptotic Bcl-2 proteins regulate the inositol 1,4,5-trisphosphate receptor (InsP3R) ER Ca2+ release channel resulting in increased cellular apoptotic resistance and enhanced mitochondrial bioenergetics. Anti-apoptotic Bcl-XL interacts with the carboxyl terminus of the InsP3R and sensitizes single InsP3R channels in ER membranes to low [InsP3], enhancing Ca2+ and InsP3-dependent regulation of channel activity in vitro and in vivo, reducing ER Ca2+ content and stimulating mitochondrial energetics. The pro-apoptotic proteins Bax and tBid antagonize this effect by blocking the biochemical interaction of Bcl-XL with the InsP3R. These data support a novel model in which Bcl-XL is a direct effector of the InsP3R, increasing its sensitivity to InsP3 and enabling ER Ca2+ release to be more sensitively coupled to extracellular signals. As a consequence, cells are protected against apoptosis by a more sensitive and dynamic coupling of ER to mitochondria through Ca2+-dependent signal transduction that enhances cellular bioenergetics and preserves survival.
A central feature of molecular models of apoptosis is the control of outer mitochondrial membrane permeability by Bcl-2-related proteins. The pro-apoptotic Bcl-2-related proteins Bax and Bak are required to initiate cytochrome c release from mitochondria in response to diverse apoptotic stimuli1,5. Anti-apoptotic properties of Bcl-2 and Bcl-XL have been attributed to their ability to antagonize Bax/Bak by forming heterodimers that prevent their oligomerization and apoptosis initiation6. Pro- and anti-apoptotic Bcl-2 proteins also localize to the ER3,7, and it is now recognized that the ER has an important role in regulating apoptosis8,9. The ER is thought to contribute to apoptosis through its role as the principle Ca2+ storage organelle in cells8–11. At physiological levels, Ca2+ released from the ER during cell activation is taken up by mitochondria to stimulate oxidative phosphorylation and enhance ATP production12. However, sustained and complete release of ER Ca2+ can initiate Ca2+-dependent forms of apoptosis by activating calpain processing of caspase 12 (ref. 13) or by triggering the opening of the mitochondrial permeability transition pore14. It has been suggested that high levels of ER Ca2+ may sensitize cells to these pathways of apoptosis by providing a higher quantity of released Ca2+ (ref. 11).
Recent evidence suggests that Bcl-2-related proteins can localize to the ER and the nuclear envelope3 to regulate permeabilities of these membranes as well as those of mitochondria7. However, the effects of this localization have not led to a consistent set of observations that link the functions of these proteins, ER Ca2+ dynamics and apoptosis control4. Genetic studies have suggested that pro-apoptotic Bax and Bak can promote Ca2+ release from the ER and initiate a caspase 12-associated form of apoptosis7, whereas anti-apoptotic Bcl-2 overexpression also promotes Ca2+ release by causing an unregulated ER Ca2+ leak15. Physiological studies are also conflicting regarding the effects of Bcl-2 proteins on Ca2+ leakage4,11 and InsP3R activity16. The nature of Bcl-2-associated ER regulation and the effect that this regulation has on apoptosis are therefore uncertain, prompting us to utilize distinct approaches to investigate the ability of the anti-apoptotic Bcl-2 family member Bcl-XL to interact with the InsP3R and regulate Ca2+ release, ER [Ca2+] ([Ca2+]ER) and apoptosis.
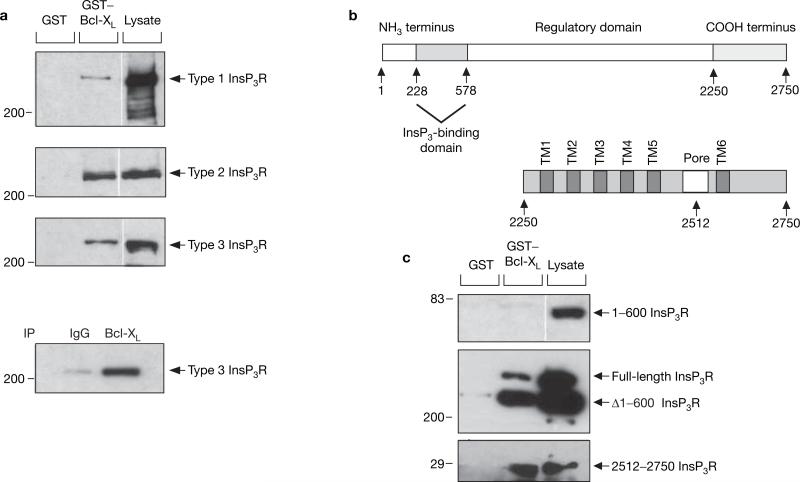
In in vitro pull-down assays, full-length human Bcl-XL interacted with all three mammalian InsP3R isoforms (Fig. 1a). Immunoprecipitation of Bcl-XL coprecipitated type 3 InsP3R from COS-7 cell lysates (Fig. 1a), suggesting that the endogenous proteins interact in vivo. Bcl-2 also binds to InsP3R (see Supplementary Information, Fig. S1), consistent with previous findings16. The interacting region in the InsP3R was localized to the C-terminal region of the channel (Fig. 1c).
Figure 1.
Interaction of Bcl-XL with InsP3R. (a) Bcl-XL binds to full-length types 1, 2 and 3 InsP3R. Lysates from DT40-InsP3R-KO cells stably expressing rat type 1 InsP3R and from COS-7 cells that endogenously express type 2 and type 3 InsP3R, were incubated with GST–Bcl-XL, and bound InsP3R was detected with isoform-specific antibodies (top three panels). Bottom panel: co-immunoprecipitation of endogenous Bcl-XL and type 3 InsP3R from COS-7 cells. (b) Domain structure of full-length InsP3R and of its C-terminal region. (c) Bcl-XL binds within the C terminus of InsP3R. GST–Bcl-XL failed to pull-down the V5-tagged 1–600 type 1 InsP3R fragment expressed in COS-7 cells (top panel). Expression of 1–600 InsP3R was verified by a western blot of cell lysates with V5-specific antibody (lane 3). Rat type 1 InsP3R lacking the first 600 residues (Δ1–600 InsP3R) expressed in COS-7 cells was successfully pulled-down along with endogenous InsP3R-1 (middle panel). GST–Bcl-XL effectively binds to the C-terminal 2512–2750 residues of type 1 InsP3R (bottom panel). All western blots depicted are representative of three independent experiments.
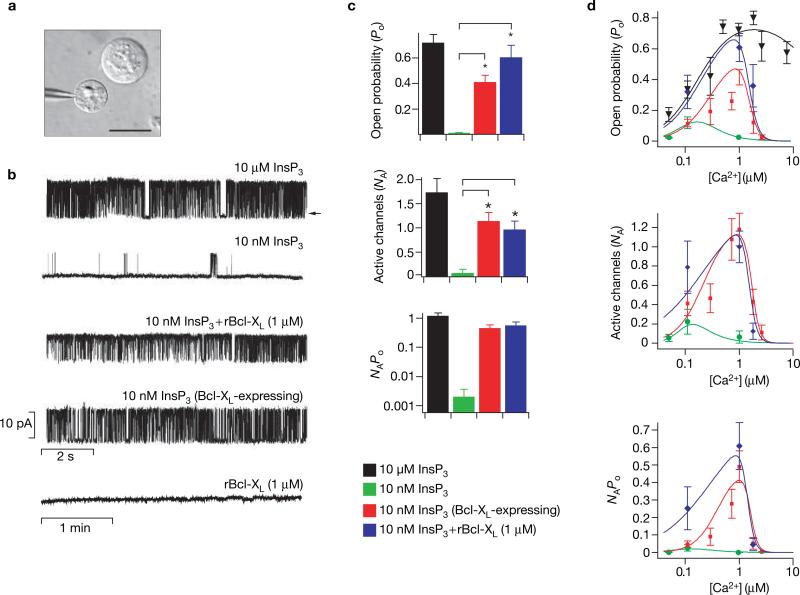
The functional consequences of the interaction of Bcl-XL with the InsP3R were explored by recording single InsP3R channels in native ER membranes, by patch-clamp electrophysiology of outer membranes of isolated nuclei17 (Fig. 2a). Robust InsP3R channel activity (open probability, Po ~0.7) was observed with pipette solutions facing the cytoplasmic aspect of the channel containing 10 μM InsP3 and 1 μM Ca2+, conditions optimal for channel activation (Ionescu, L., Mak, D.-O.D., C.W. & K.F., unpublished observations) (Fig. 2b). Channels were observed in 80% of patches, with the mean number of active channels per patch (NA) ~1.8 (Fig. 2c). When [InsP3] was reduced to 10 nM, channel activity was substantially decreased (Po ~0.02) (Fig. 2b), consistent with the [InsP3] dependence of channel gating17, and NA was only ~0.1 (Fig. 2c). Addition to the pipette solution of purified recombinant human Bcl-XL (rBcl-XL; 1 μM), with biological activity confirmed in cytochrome c release assays (see Supplementary Information, Fig. S1), did not affect the Po or NA of channels activated by 10 μM InsP3 (data not shown). In contrast, rBcl-XL markedly increased the number of channels (NA ~1.0) and gating (Po ~0.6) activated by 10 nM InsP3, to levels comparable to those observed with saturating [InsP3] (Fig. 2b, c). The product of Po and NA, a measure of total InsP3R-mediated ion flux across the ER membrane, was increased by over two orders of magnitude in the presence of Bcl-XL (Fig. 2c).
Figure 2.
Effects of Bcl-XL on InsP3R single-channel activity. (a) Isolated nuclear preparation from Sf9 cells, which endogenously express only type 1 InsP3R. Patch pipette approaching an isolated nucleus with an intact cell visible directly above; scale bar, 15 μM. (b) InsP3R channel activity. Typical InsP3R single-channel current recordings in the presence of saturating (10 μM) or low (10 nM) InsP3; in the absence or presence of recombinant Bcl-XL (rBcl-XL; 1 μM); or with 10 nM InsP3 in cells transiently transfected with Bcl-XL. Channel activity was not evoked by rBcl-XL (1 μM) alone. Pipette [Ca2+] was 1 μM, optimal for channel activity17; arrow indicates zero current level. (c) Summary of the effects of Bcl-XL on InsP3R channel activity. In pipettes containing 10 nM InsP3, the open probability (Po) increased from 0.022 ± 0.001 (n = 2) to 0.61 ± 0.09 (n = 10) with addition of 1 μM rBcl-XL (n = number of patches used in Po determination). Similarly, when Bcl-XL was overexpressed, Po increased to 0.42 ± 0.05 (n = 11). Bcl-XL also increased the number of activated channels (NA). In 10 nM InsP3, NA was increased from 0.10 ± 0.07 (n = 20) under control conditions to 1.00 ± 0.16 (n = 54) and 1.18 ± 0.17 (n = 61) in the presence of recombinant or expressed Bcl-XL, respectively. The total ER ion flux as indicated by the product NAPo was increased by Bcl-XL (note log scale). Asterisks indicate P < 0.001, unpaired t-test. Both His- and Flag-tagged rBcl-XL, generated using distinct purification protocols, were equally effective, whereas His-tagged NCS-1, which does not interact with InsP3R (ref. 32), had no effect either alone or in combination with 10 nM InsP3 (data not shown). (d) Dependence of InsP3R channel activity on [Ca2+] at the cytoplasmic face of the channel. Effect of [Ca2+]i on Po, NA and NAPo in the presence of 10 μM InsP3 (black inverted triangles), 10 nM InsP3 (green circles), 10 nM InsP3 + 1 μM rBcl-XL (blue diamonds) and 10 nM InsP3 + Bcl-XL expression (red squares).
InsP3R channel activity in nuclei isolated from cells transiently transfected with Bcl-XL showed enhanced NA and Po in 10 nM InsP3 in the absence of added recombinant protein (Fig. 2b, c), demonstrating an in vivo physical and functional membrane-delimited interaction between the InsP3R and Bcl-XL that was sufficiently strong to survive the nuclear isolation protocol. Bcl-XL-enhanced channel activities required InsP3 binding because they were not observed in the absence of added InsP3 (Fig. 2b), and the competitive InsP3 inhibitor heparin ablated the effects (data not shown). Gating of InsP3-liganded InsP3R channels is biphasically regulated by cytoplasmic [Ca2+] ([Ca2+]i) (ref. 17 and Ionescu et al. unpublished observations). Enhanced channel recruitment and gating conferred by either recombinant or expressed Bcl-XL was manifested between 100 nM and 1.8 μM Ca2+ (Fig. 2d). Thus, Bcl-XL-activated InsP3R channels retained biphasic [Ca2+]i regulation. Taken together, these data indicate that Bcl-XL binding to the C terminus of the InsP3R allosterically increases the sensitivity of the channel to very low levels of InsP3 that may exist in resting or minimally stimulated cells18 at physiological [Ca2+]. This suggests that the in vivo interaction of InsP3R with Bcl-XL does not induce an unregulated Ca2+ leak but rather increases the dynamic sensitivity of a highly regulated Ca2+ permeability of the ER membrane.
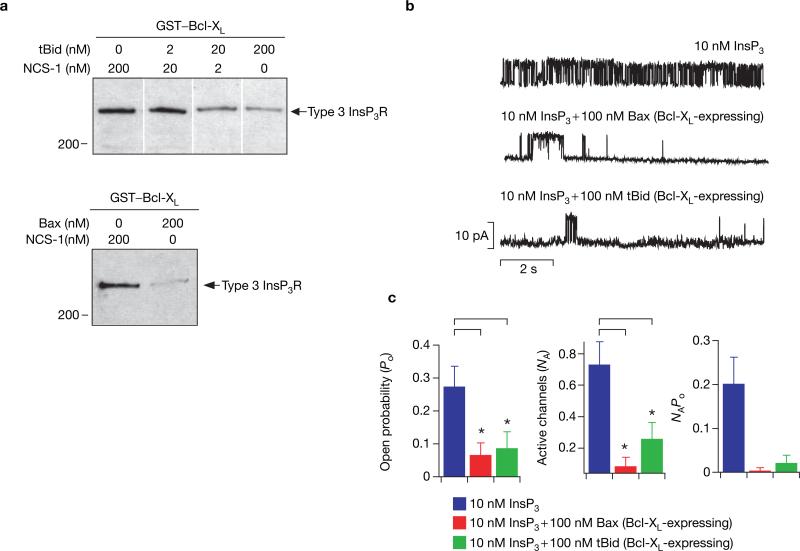
Purified recombinant Bax, a multi-domain family member, and tBid, a BH3-only member, each disrupted binding of InsP3R to GST–Bcl-XL (Fig. 3a) and abolished the functional effects of expressed Bcl-XL (Fig. 3b, c). In contrast, neither protein affected gating in the absence of Bcl-XL (see Supplementary Information, Fig. S2). It has been reported that Bcl-XL and Bax can form ion channels in artificial membrane systems19,20. However, novel conductances were never observed when either Bcl-XL, Bax, or tBid was present in the patch pipette solution, or when Bcl-XL was overexpressed, indicating that under the present experimental conditions these proteins do not form ion channels in native ER membranes.
Figure 3.
tBid and Bax antagonize the effects of Bcl-XL on InsP3R channel activity. (a) tBid and Bax inhibit binding of Bcl-XL to InsP3R. COS-7 cell lysates were incubated with GST–Bcl-XL in the absence or presence of recombinant tBid (2–200 nM; upper panel) or recombinant Bax (200 nM; lower panel), and bound InsP3R was detected with a type 3 antibody. Recombinant neuronal calcium sensor-1 (NCS-1) was used to control for total protein concentration in both experiments. Data are representative of three independent experiments. (b) tBid and Bax inhibit the electrophysiological effects of Bcl-XL. Typical current traces showing the effects of 100 nM recombinant tBid or Bax in the presence of 10 nM InsP3 in nuclei isolated from cells transiently transfected with Bcl-XL. Pipette [Ca2+] was 1 μM. (c) Addition of 100 nM tBid or Bax decreased Po from 0.28 ± 0.06 (n = 5) to 0.07 ± 0.03 (n = 3) and 0.09 ± 0.05 (n = 3), respectively. Similarly, NA was reduced from 0.74 ± 0.14 (n = 38) to 0.26 ± 0.10 (n = 33) in the presence of tBid and to 0.09 ± 0.05 (n = 33) in the presence of Bax. The product NAPo was also reduced. Asterisks indicate P < 0.001, unpaired t-test.
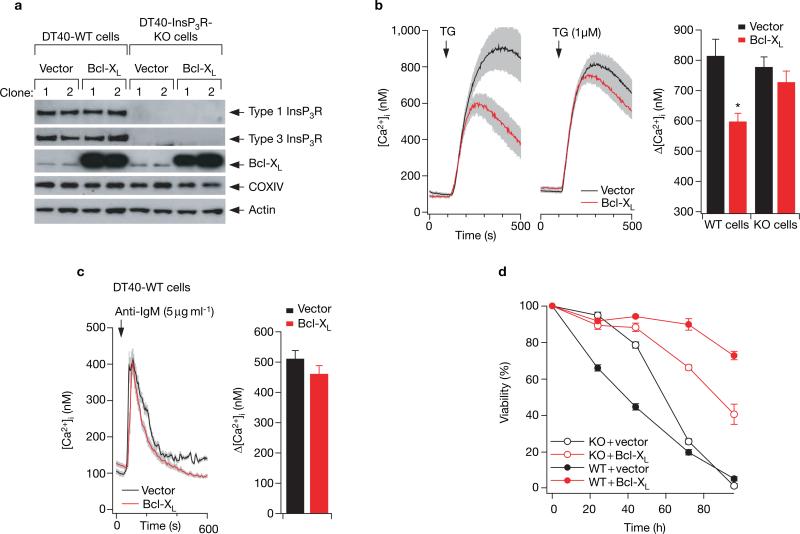
The cell physiological implications of the Bcl-XL–InsP3R interaction were evaluated using chicken DT40 B-cell lines expressing either Bcl-XL or vector alone (Fig. 4a). Stable expression of Bcl-XL in wild-type DT40 cells (DT40-WT), which express all three InsP3R isoforms21, reduced the Ca2+ content of the ER (Ca2+ER) by 25% (Fig. 4b), consistent with some previous observations4,11. In contrast, stable (Fig. 4b and see Supplementary Information, Fig. S3c) or transient (see Supplementary Information, Fig. S3a, b) Bcl-XL expression did not affect the Ca2+ER in a DT40 cell line that was genetically deficient in all InsP3R isoforms (DT40-InsP3R-KO)21. Thus, Bcl-XL-associated reduction of Ca2+ER depends critically on expression of the InsP3R, consistent with the hypothesis that Bcl-XL-mediated enhanced InsP3R ligand sensitivity is the molecular basis of the Bcl-2-enhanced ER Ca2+ permeability observed previously22–24.
Figure 4.
Interaction of Bcl-XL with InsP3R is essential for Bcl-XL effects on ER Ca2+ regulation and inhibition of apoptosis. (a) The empty vectors pIRES2-DsRed2 or pBcl-XL-IRES2-DsRed2 were stably expressed in DT40-WT and DT40-InsP3R-KO cells. Expression levels of types 1 and 3 InsP3R, Bcl-XL and OxPhos complex IV (COXIV; subunit 1) were examined by western blot. Expression of the mitochondrial complex IV protein was unchanged in the Bcl-XL-expressing clones. Depicted blots are representative of three independent experiments. (b) Effects of Bcl-XL expression on the Ca2+ content of the ER (Ca2+ER). Typical records depicting change in cytoplasmic [Ca2+] ([Ca2+]i) in response to application of 1 μM thapsigargin (TG) in DT40-WT and DT40-InsP3R-KO cells stably transfected with either Bcl-XL or vector alone. Ca2+ER was indirectly estimated by single-cell imaging of the [Ca2+]i responses to acute inhibition by thapsigargin of ER Ca2+ uptake. Each trace represents mean ± s.e.m. of at least six cells within the image field. Bar graph summarizes the effects of thapsigargin; data represent mean ± s.e.m. for at least 30 cells in multiple trials. Asterisk indicates P < 0.05, ANOVA. Mean values of resting [Ca2+]i ranged from 80 to 100 nM, with no significant differences among duplicate clones of the four cell lines (data not shown). (c) [Ca2+]i transients in response to 5 μg ml−1 anti-BCR antibody (anti-IgM) in DT40-WT cells stably transfected with either Bcl-XL or vector alone. Summary data represent the peak amplitude (mean ± s.e.m.) for at least 30 cells in multiple trials. (d) Cell viability after treatment with 20 μg ml−1 anti-BCR antibody (anti-IgM) (time 0) of DT40-WT (solid symbols) and DT40-InsP3R-KO (open symbols) cells stably expressing Bcl-XL (red) or vector alone (same clones as in b). Similar results were obtained using independent clones (see Supplementary Information, Fig. S3).
Next we examined whether lowered Ca2+ER as a consequence of Bcl-XL expression represents a fundamental control point in the regulation of apoptosis, because it reduces the amount of Ca2+ released through the InsP3R by pro-apoptotic signals. DT40 pre-B cells undergo apoptosis following immunoglobulin receptor crosslinking in a process that recapitulates negative selection25. Bcl-XL blocks B-cell receptor (BCR)-mediated apoptosis26. Therefore, we examined antigen-receptor-mediated apoptosis in DT40-WT and DT40-InsP3R-KO cells stably expressing either Bcl-XL or empty vector. An anti-BCR antibody (anti-IgM) elicited comparable [Ca2+]i transients in Bcl-XL-expressing and control wild-type cells (Fig. 4c), whereas no [Ca2+]i transients were observed in the InsP3R-KO cells21 (data not shown). DT40-InsP3R-KO cells were resistant to apoptosis compared with DT40-WT cells during the initial 24 h (Fig. 4d), consistent with previous observations21. However, the resistance was transient, with apoptosis at later times being independent of InsP3R expression. Bcl-XL inhibited apoptosis in both wild-type and InsP3R-KO cells, as expected, because Bcl-XL has ER-independent (mitochondrial) mechanisms of action. We reasoned that if Bcl-XL was anti-apoptotic because it lowered Ca2+ER and therefore reduced the amount of Ca2+ that could be conveyed from the ER to mitochondria following InsP3R activation9,11, then InsP3R-KO cells expressing Bcl-XL should have maximal protection against apoptosis because they lack the mechanism (InsP3R) to convey Ca2+ from the ER to mitochondria. Surprisingly, the anti-apoptotic action of Bcl-XL was significantly greater in DT40-WT cells compared with InsP3R-KO cells (Fig. 4d and see Supplementary Information, Fig. S3c). These data demonstrate that the presence of InsP3R is required for Bcl-XL to be fully efficacious as an anti-apoptotic mediator.
The ER is an important control point for apoptotic responses to diverse stimuli8,9. Our results provide a molecular mechanism that links the ER, Ca2+ and Bcl-2 family proteins to apoptosis, involving the ubiquitous InsP3R ER Ca2+ release channel as a target of a direct effector function of anti-apoptotic Bcl-XL. This function of Bcl-XL is antagonized by proapoptotic family members, which conforms to a proposed rheostat model in which the balance of pro- to anti-apoptotic Bcl-2 members regulates apoptosis10, but differs from the prevailing concept that anti-apoptotic Bcl-2 proteins prevent apoptosis solely by antagonizing pro-apoptotic Bcl-2 family members2,6. Whereas this paradigm may account for the competing effects of pro- and anti-apoptotic members at mitochondria, the present results suggest that the converse is true at the ER. Here, the pro-apoptotic family member Bax can reverse the function of Bcl-XL without affecting ER permeability on its own.
Previous studies that observed reduced Ca2+ER in response to Bcl-2 expression or Bax/Bak deficiency concluded that the underlying mechanism is associated with an enhanced ER Ca2+ leak15,22–24. The current results establish the molecular identity of the enhanced leak to be the InsP3R. It was proposed that phosphorylation status of the type 1 InsP3R linked Bcl-2 expression to Ca2+ER (ref. 15). However, phosphorylation cannot account for the rapid effects of Bcl-XL on InsP3R gating observed here, as the experiments were performed with solutions that lacked Mg2+ — necessary for phosphorylation reactions — and treatment of nuclei with alkaline phosphatase did not affect the ability of Bcl-XL to activate channel gating (C.W. & K.F., unpublished observations). The effect of Bcl-XL expression in lowering Ca2+ER has not been consistently observed 4,11. The current observations demonstrate that this effect of Bcl-XL is InsP3R-dependent, with Bcl-XL-stimulated InsP3R channel activity remaining exquisitely regulated by its ligands InsP3 and Ca2+. Thus, cell-type differences in basal concentrations of these ligands and InsP3R expression can contribute to the magnitude of the effect of Bcl-2 expression on Ca2+ER, which might reconcile discrepant published observations.
Previously it was believed that reducing InsP3-mediated ER Ca2+ release would protect against Ca2+-induced mitochondrial permeability transition11,14. This model may possibly account for the early transient protection observed in the control InsP3R-KO cells (Fig. 4d), but it is insufficient to account for the InsP3R-dependent anti-apoptotic effects of Bcl-XL observed in DT40 cells. If the sole function of Bcl-XL at the ER was to lower [Ca2+]ER, as a mechanism to protect mitochondria from toxic effects of released Ca2+, then protection afforded by Bcl-XL should be maximal in the InsP3R-KO cells, where Bcl-XL can exert its mitochondrial effects1,2 and the ER is irrelevant because the absence of the release channels prevents delivery of Ca2+ to mitochondria. However, InsP3R expression conferred greater Bcl-XL-mediated protection. Thus, despite the correlation between reduced [Ca2+]ER and apoptosis resistance observed here and previously11, the InsP3R provides an anti-apoptotic signal that cannot be accounted for by reduced mitochondrial Ca2+ delivery through its effect on [Ca2+]ER.
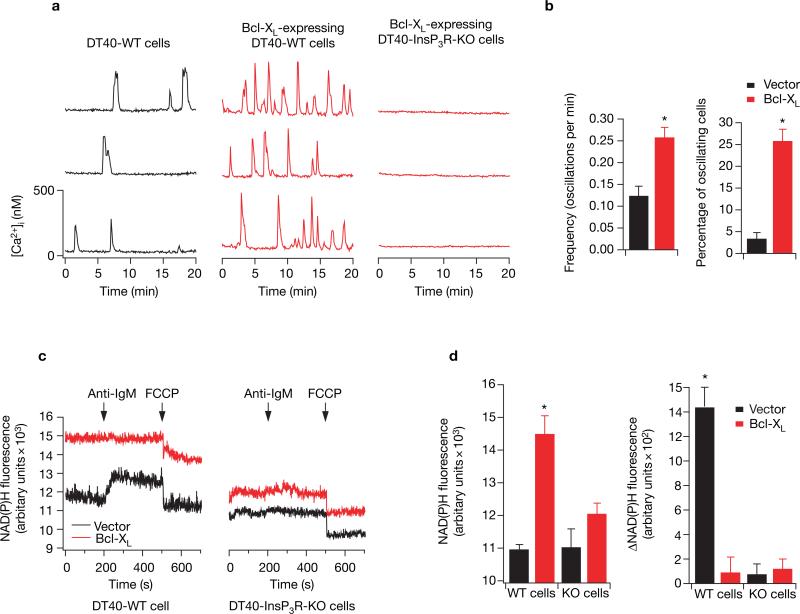
What is the nature of the anti-apoptotic signal that is conferred by InsP3R expression? We reasoned that Ca2+ released from the ER can enhance mitochondrial function by stimulating the citric-acid cycle and oxidative phosphorylation with consequent production of ATP and other important metabolic intermediates12. We speculated that maintenance of cellular metabolic homeostasis by Ca2+ activation of mitochondrial metabolism would enable cells to better withstand apoptotic insults. In such a model, Bcl-XL interaction with the InsP3R generates [Ca2+]i signals that enhance cellular bioenergetics that afford apoptosis resistance. In single DT40-WT cells perfused with serum-containing medium, infrequent, spontaneous, transient [Ca2+]i spikes were observed in fewer than 4% of cells (Fig. 5a, b). In contrast, spontaneous [Ca2+]i spikes were observed in over 25% of Bcl-XL-expressing DT40-WT cells and these spikes were consistently of higher frequency (Fig. 5a, b). Similarly, [Ca2+]i oscillations triggered in response to a low concentration of anti-IgM were observed in more Bcl-XL-expressing wild-type cells than in control wild-type cells (see Supplementary Information, Fig. S4), reminiscent of observed Bcl-2 enhancement of ligand-induced [Ca2+]i oscillations23. These spikes represent transient release from ER through InsP3R because they were absent in DT40-InsP3R-KO cells (Fig. 5a). Thus, Bcl-XL enhances low-level InsP3R-mediated [Ca2+]i signalling under resting cellular conditions and during low-level agonist stimulation.
Figure 5.
The Bcl-XL–InsP3R interaction modulates [Ca2+]i signalling and mitochondrial NADH levels. (a) Spontaneous [Ca2+]i oscillations in three representative DT40-WT cells stably expressing vector alone (left) or Bcl-XL (middle), and DT40-InsP3R-KO cells expressing Bcl-XL (right).(b) The difference in frequency and number of oscillating cells (mean ± s.e.m.) between vector only and Bcl-XL-expressing cells. (c) NAD(P)H fluorescence measurements from DT40-WT and DT40-InsP3R-KO cells expressing Bcl-XL or vector in response to 5 μg ml−1 anti-BCR antibody (anti-IgM) and FCCP (2 μM). The average (± s. e.m.) resting NAD(P)H fluorescence and the change in NAD(P)H fluorescence in response to anti-IgM stimulation for four independent experiments are plotted in d. Asterisk indicates P < 0.001, ANOVA. Similar results were obtained using independent clones (see Supplementary Information, Fig. S4).
Periodic Ca2+ release from the ER can elevate mitochondrial matrix [Ca2+] that stimulates Kreb's cycle dehydrogenases, elevating mitochondrial [NADH]12,27. Bcl-XL elevated NAD(P)H levels in both wild-type and InsP3R-KO cells, as observed in other cell types28, but the enhancement was greater in the cells expressing InsP3R (Fig. 5c, d). In wild-type cells, the elevated signal was in part due to increased mitochondrial NADH because the FCCP-induced fluorescence decrease from basal levels — although not different from those observed in InsP3R-KO cells — was greater in the Bcl-XL-expressing cells (P < 0.05; n = 7), a result that cannot be accounted for by increased numbers of mitochondria (Fig. 4a). Thus, spontaneous Bcl-XL -dependent InsP3R-mediated [Ca2+]i signals are correlated with enhanced NAD(P)H levels, consistent with these [Ca2+]i signals stimulating mitochondrial dehydrogenases27 under resting conditions. Although anti-IgM induced similar [Ca2+]i signals in wild-type cells in either the presence or absence of Bcl-XL (Fig. 4b), NAD(P)H fluorescence was unchanged in the Bcl-XL-transfected wild-type cells (Fig. 5c,d), whereas it was enhanced in the control wild-type cells (Fig. 5c) to levels closer to those achieved in the Bcl-XL-transfected cells (Fig. 5c), with kinetics correlated with the BCR-induced InsP3R-mediated [Ca2+]i signal (Fig. 4b). These results suggest either that mitochondrial dehydrogenases in the Bcl-XL-transfected wild-type cells were already maximally stimulated under resting conditions, or that Ca2+-stimulated NADH production was well compensated by increased oxidation with higher flux through the electron transport chain in these cells. In InsP3R-KO cells, anti-IgM elicited neither a [Ca2+]i response (data not shown) nor a stimulation of NAD(P)H fluorescence (Fig. 5c, d), demonstrating that the stimulation observed in wild-type cells was mediated by ER Ca2+ release. Similar results were obtained in different cell clones (see Supplementary Information, Fig. S4c). Thus, these results suggest that Bcl-XL enables mitochondria to cope with increased metabolic demand with high efficiency that preserves mitochondrial redox status, dependent upon the InsP3R.
Together the above results suggest that the effects of Bcl-XL at the ER are anti-apoptotic not only because they minimize the maximal delivery of Ca2+ to mitochondria as previously thought, but also because Bcl-XL facilitates InsP3R-mediated Ca2+ delivery to mitochondria that regulates mitochondrial bioenergetics. Such a model is consistent with a general scheme that links cellular resistance to apoptosis with the regulation of cellular metabolism29,30. Furthermore, it is consistent with the observation that Bcl-XL does not stimulate an unregulated leak — which would be energetically costly — but rather modulates the activity of an exquisitely regulated permeability. Nevertheless, because our results do not establish a causal link between enhanced bioenergetics and apoptosis susceptibility, other models are also possible. For example, it may be that the ER Ca2+ load per se, regulated by the interaction of Bcl-XL with the InsP3R, rather than Ca2+ release, directly determines apoptotic susceptibility. Bcl-XL interacts with the InsP3R at the ER membranes and increases its sensitivity to very low levels of InsP3, resulting in increased channel gating and cytoplasmic [Ca2+]i signals that enhance mitochondrial bioenergetics and increase apoptosis resistance. Anti-apoptotic Bcl-2 family members promote a more dynamic InsP3R response to achieve more sensitive coupling of Ca2+ release to extracellular signals and to increase cell survival. We suggest that Bcl-XL interaction with the InsP3R Ca2+ release channel is a critical cellular control point that couples the ER to apoptotic pathways. The interaction between Bcl-XL and InsP3R may provide a novel therapeutic target, with implications for processes involving apoptosis including cancer, neurodegeneration and cardiovascular disease.
METHODS
Cell culture and transfection
Spodoptera frugiperda (Sf9) cells were maintained in suspension culture at 27 °C in serum-free Sf-900 II SFM media (GIBCO/BLR, Gaithersburg, MD). A baculovirus expression system was used to transiently express Bcl-XL. COS-7 (Cercopithecus aethiops kidney) cells were grown in DMEM/high glucose medium containing 10% (v/v) fetal bovine serum (FBS) (GIBCO/BLR) in a humidified 95/5% air/CO2 atmosphere. DT40 cells were maintained in suspension culture at 37 °C (95/5% air/CO2) in RPMI 1640 media (GIBCO/BLR) supplemented with 10% (v/v) FBS, 1% chicken serum, 2 mM glutamine, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin. Full-length human Bcl-XL cDNA was cloned into pIRES2-DsRed2 vector (Clontech, Palo Alto, CA). Cells were transfected with either empty vector pIRES2-DsRed2 or pBcl-XL-IRES2-DsRed2 using a Nucleofector Device (Amaxa, Gaithersburg, MD). For selection of stable clones, transfected cells were cultured for 2 weeks in the presence of 2 mg ml−1 G418. Transfected cells were identified on the basis of DsRed fluorescence and were further sub-cloned using fluorescence-activated cell sorting (Becton Dickinson, San Jose, CA) into individual wells of a 96-well plate. Bcl-XL expression was confirmed by western blot and quantified using infrared imaging Odyssey (LI-COR).
Biochemistry
Human Bcl-XL or Bcl-2 cDNA in pGEX6P-1 (Amersham Pharmacia, Piscataway, NJ) were expressed as GST-fusion proteins in Escherichia coli (BL-21; Stratagene, La Jolla, CA) and immobilized on glutathione–Sepharose 4B (Amersham Pharmacia). InsP3R (type 1) fragments were cloned into pcDNA3.1 or pcDNA3.1/V5 vectors (Invitrogen, Carlsbad, CA). COS-7 cells were transfected (Lipofectamine 2000), and 48–60 h later washed twice with PBS and harvested into 1 ml PBS containing 2% glycerol, 0.05% Triton and protease inhibitor cocktail (Sigma, St Louis, MO). After brief sonication (10 s) and centrifugation, total protein concentration in the lysate was adjusted to 5 mg ml−1 and incubated with GST-fusion protein (1 h, 4 °C). Beads were centrifuged, washed three times and prepared for western blot. Co-immunoprecipitation and western blot analysis were performed according to standard protocols.
Baculoviral generation and protein purification
Baculoviruses were generated by first sub-cloning full-length human Bcl-XL into pVL1393 vector (BD PharMingen, Bedford, MA) with specific tags. pVL1393-flag-Bcl-XL and pVL1393-his-Bcl-XL were transfected into Sf9 cells and viral supernatants collected. Viral supernatants were used to infect Sf9 cells until recombinant Bcl-XL expression was maximized. Flag-tagged Bcl-XL was purified by anti-FlagM2-agarose affinity beads (Sigma) and eluted by incubation with buffer 0.1 μg ml−1 Flag peptide. Bcl-XL cDNA for His-tagged recombinant Bcl-XL was cloned into pET-16b (Novagen, Madison, WI) and expressed in BL-21, and the resulting His-tagged Bcl-XL was purified on a Ni-NTA column (Qiagen, Valencia, CA). The plasmid pGEX-4T-1-tBid-his (gift from D. Newmeyer) was transformed into BL-21 and the cell lysate applied to glutathione-Sepharose, digested with thrombin (Novagen; 4 U ml−1, 25 °C, 5 h ) and purified using Ni-NTA chromatography. PTYB1-mBax was obtained by inserting mouse Bax cDNA into pTYB1 vector (New England Biolabs, Beverly, MA). E. coli ER2566 (New England Biolabs) bearing the plasmid pTYB1-mBax were cultured and the fusion protein of Bax–chitin-binding protein was purified using a chitin-affinity column (New England Biolabs). Full-length Bax protein was eluted by incubating with 50 mM DTT at 16 °C for 40 h. All purified recombinant proteins were dialysed in buffer with 20 mM HEPES pH 7.5 and 20% glycerol, and stored at –80 °C. Protein samples for patch-clamping were first dialysed against buffer containing 10 mM HEPES pH 7.3, 140 mM KCl and 10% glycerol, and concentrated using Microcon (Millipore, Bedford, MA) to concentrations higher than 50 μM.
Electrophysiology
Sf9 cells were washed twice with PBS and suspended in a nuclear isolation solution containing: 150 mM KCl, 250 mM sucrose, 1.5 mM β-mercapoethanol, 10 mM Tris-HCl, 0.05 mM phenylmethylsulphonyl fluoride, protease inhibitor cocktail (Complete, Roche Diagnostics, Indianapolis, IN), pH 7.5. Nuclei were isolated using a Dounce glass homogenizer and plated onto a 1-ml glass-bottomed dish containing standard bath solution: 140 mM KCl, 10 mM HEPES and 0.5 mM BAPTA (free [Ca2+] = 300 nM), pH 7.1. The pipette solution contained 140 mM KCl, 0.5 mM ATP, 10 mM HEPES, pH 7.1. The free [Ca2+] in all solutions was adjusted to the desired level by the addition of an appropriate Ca2+ chelator, as described previously17. Experiments were performed at room temperature. Data were acquired using an Axopatch-1D amplifier (Axon Instruments, Union City, CA) and single-channel analysis performed using QuB software (University of Buffalo).
Calcium measurement and apoptosis assay
DT40 cells were plated onto a glass-bottomed perfusion chamber mounted on the stage of an inverted microscope (Nikon Eclipse TE2000) and incubated with Fura-2 AM (Molecular Probes, Eugene, OR; 2 μM) for 30 min at room temperature in normal culture media. Cells were then continuously perfused with Hanks’ balanced salt solution (Sigma), containing 1.8 mM CaCl2 and 0.8 mM MgCl2, pH 7.4. In experiments in which calcium oscillations were measured, cells were perfused with complete culture media without phenol. Fura-2 was alternately excited at 340 and 380 nm, and the emitted fluorescence filtered at 510 nm was collected and recorded using a CCD-based imaging system running Ultraview software (Perkin-Elmer, Norwalk, CT). Thapsigargin (Sigma) was applied in Ca2+-free Hanks’ solution containing 0.5 mM LaCl3 to block Ca2+ extrusion across the plasma membrane31. Dye calibration was achieved by applying experimentally determined constants to the standard equation: [Ca2+] = Kd β(R−Rmin)/(Rmax−R). Cell viability was determined after application of anti-BCR antibody (IgM; SouthernBiotech, Birmingham, AL) using DAPI staining (1 μg ml−1) and assays were performed on an LSR flow cytometer (Beckton Dickinson).
NAD(P)H measurements
DT40 cells (10 × 106 cells per ml) were suspended in Hanks’ balanced salt solution (Sigma). Autofluorescence of NAD(P)H was monitored at 350/460 nm (excitation/emission) using a multi-wavelength excitation, dual-wavelength emission fluorimeter (Delta RAM, PTI , Birmingham, NJ). Experiments were performed at 37 °C.
Analysis and statistics
Data were summarized as the mean ± s.e.m. and the statistical significance of differences between means was assessed using unpaired t-tests or analysis of variance (ANOVA) for repeated measures, using Fisher's protected least-significant difference (PLSD) test. Differences between means were accepted as statistically significant at the 95% level (P < 0.05).
BIND identifiers
Four BIND identifiers (www.bind.ca) are associated with this manuscript: 331015, 331016, 331017 and 331018.
Supplementary Material
Figure S1 Interaction of Bcl-2 with type-1 InsP3R and Inhibition of cytochrome C release from mitochondria under in vitro apoptotic conditions is inhibited by recombinant Bcl-XL. a, Lysates from DT40-InsP3R-KO cells stably expressing rat type-1 InsP3R were incubated with GST-Bcl-2 and bound InsP3R was detected with isoform-specific antibody. b,. His-hBcl-XL and Flag-hBcl-XL proteins were purified from bacterial cells or insect cells, respectively, and their purity examined by Coomassie Blue staining. c, Bcl-XL inhibits tBid-mediated cytochrome C release. Mitochondria were isolated from a Bak-/-Bax-/- hematopoietic cell line in which Bax was re-expressed. In the absence of tBid cytochrome C was detected exclusively in the mitochondrial fraction (M). After incubation with tBid cytochrome C was released from mitochondria and detected in the supernatant (S). Addition of Bcl-XL blocked the release of cytochrome C in the presence of tBid.
Figure S2 InsP3R activity in the absence of Bcl-XL and presence of Bax or tBid. a, Typical InsP3R current traces in the presence of 100 nM InsP3 in Sf9 nuclei, pipette [Ca2+] was 1 μM. Addition of 100 nM tBid or Bax along with 100 nM InsP3 to the pipette solution had no effect on Po, NA or NAPo, (P>0.05, unpaired t-test). b, Similarly, in the presence of 10 nM InsP3, pipette [Ca2+] 0.85 μM, addition of 100 nM Bax or tBid had no effect on channel Po, NA or NAPo, (P>0.05, unpaired t-test).
Figure S3 Interaction of Bcl-XL with InsP3R is essential for Bcl-XL effects on ER Ca2+ regulation and apoptosis. a, Effects of Bcl-XL transient expression on Ca2+ER. Typical records depicting change in cytoplasmic [Ca2+] ([Ca2+]i) in response to application of 1 μM thapsigargin in DT40-WT and DT40-InsP3R-KO cells transiently transfected with either Bcl-XL or vector alone. Each trace represents the mean ± SEM of at least six individual cells within the image field. b, Summary of the effects of Bcl-XL transient expression on Ca2+ER. Thapsigargin-induced increases in [Ca2+]i in vector alone and Bcl-XL-expressing cell lines. Data represent mean ± SEM for at least 30 individual cells in two independent transfections. Asterisk indicates P<0.05, ANOVA.c, Effects of Bcl-XL expression on ER Ca2+ regulation in independent clones. Summary of the effects of stable Bcl-XL expression on Ca2+ER. Thapsigargin-induced increases in [Ca2+]i in vector alone and Bcl-XL-expressing cell lines. Data represent mean ± SEM for at least 30 individual cells in multiple trials. Asterisk indicates P<0.05, ANOVA. d, Cell viability after treatment with 20 μg/ml α-IgM (time 0) of DT40-WT (solid symbols) and DT40-InsP3R-KO (open symbols) cells stably expressing Bcl-XL (Blue) or vector alone (Same clones as in c).
Figure S4 Receptor stimulation is more sensitively coupled to calcium signalling in Bcl-XL expressing cells and modulates mitochondrial NADH levels. a, [Ca2+]i measurements in DT40-WT cells stably expressing Bcl-XL. Receptor stimulation with low concentration of α-IgM evokes Ca2+ oscillations in many cells that were initially quiescent, examples of three responsive cells are shown. b, The percentage of cells that respond to α-IgM stimulation in this way, and the oscillation frequency is summarised as the mean ± SEM for at least 80 individual cells in no less than two independent trials. Asterisk indicates P<0.0001, unpaired t-test. c, The Bcl-XL - InsP3R interaction modulates mitochondrial NADH levels. The average (± SEM) resting NAD(P)H fluorescence and the change in NAD(P)H fluorescence in response to α-IgM stimulation for three replicate experiments are plotted.Asterisk indicates P<0.001, ANOVA.
ACKNOWLEDGEMENTS
We are grateful to D. Newmeyer for the tBid expression plasmid, and S. Joseph and A. Tanimura for InsP3R antibodies. C.W. and C.L. contributed equally to the major intellectual and technical aspects of the studies. J.Y. and N.B.P. contributed molecular biological and electrophysiology support, respectively. M.M. performed the NADH assays. C.B.T. and J.K.F. contributed ideas and assisted in the preparation of the text. This work was supported by NIH grants (C.B.T. and J.K.F.), an NIH Training Grant (C.L.), an American Heart Association Fellowship (C.W.) and the Abramson Family Cancer Research Institute.
Footnotes
Note: Supplementary Information is available on the Nature Cell Biology website.
COMPETING FINANCIAL INTERESTS
The authors declare that they have no competing financial interests.
LETTERS
- 1.Wei MC, et al. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vander Heiden MG, Thompson CB. Bcl-2 proteins: regulators of apoptosis or of mitochondrial homeostasis? Nature Cell Biol. 1999;1:E209–E216. doi: 10.1038/70237. [DOI] [PubMed] [Google Scholar]
- 3.Krajewski S, et al. Investigation of the subcellular distribution of the Bcl-2 oncoprotein - residence in the nuclear envelope, endoplasmic reticulum, and outer mitochondrial membranes. Cancer Res. 1993;53:4701–4714. [PubMed] [Google Scholar]
- 4.Distelhorst CW, Shore GC. Bcl-2 and calcium: controversy beneath the surface. Oncogene. 2004;23:2875–2880. doi: 10.1038/sj.onc.1207519. [DOI] [PubMed] [Google Scholar]
- 5.Jurgensmeier JM, et al. Bax directly induces release of cytochrome c from isolated mitochondria. Proc. Natl Acad. Sci. USA. 1998;95:4997–5002. doi: 10.1073/pnas.95.9.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antonsson B, et al. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997;277:370–372. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- 7.Zong WX, et al. Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. J. Cell Biol. 2003;162:59–69. doi: 10.1083/jcb.200302084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breckenridge DG, Germain M, Mathai JP, Nguyen M, Shore GC. Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene. 2003;22:8608–8618. doi: 10.1038/sj.onc.1207108. [DOI] [PubMed] [Google Scholar]
- 9.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nature Rev. Mol. Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 10.Scorrano L, et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: A control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 11.Rizzuto R, et al. Calcium and apoptosis: facts and hypotheses. Oncogene. 2003;22:8619–8627. doi: 10.1038/sj.onc.1207105. [DOI] [PubMed] [Google Scholar]
- 12.Duchen MR. Mitochondria and calcium: from cell signalling to cell death. J. Physiol. 2000;529:57–68. doi: 10.1111/j.1469-7793.2000.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakagawa T, Yuan J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J. Cell Biol. 2000;150:887–894. doi: 10.1083/jcb.150.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szalai G, Krishnamurthy R, Hajnoczky G. Apoptosis driven by IP3-linked mitochondrial calcium signals. EMBO J. 1999;18:6349–6361. doi: 10.1093/emboj/18.22.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oakes SA, et al. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc. Natl Acad. Sci. USA. 2005;102:105–110. doi: 10.1073/pnas.0408352102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen R, et al. Bcl-2 functionally interacts with inositol 1,4,5-trisphosphate receptors to regulate calcium release from the ER in response to inositol 1,4,5-trisphosphate. J. Cell Biol. 2004;166:193–203. doi: 10.1083/jcb.200309146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mak DO, McBride S, Foskett JK. Inositol 1,4,5-trisphosphate activation of inositol trisphosphate receptor Ca2+ channel by ligand tuning of Ca2+ inhibition. Proc. Natl Acad. Sci. USA. 1998;95:15821–15825. doi: 10.1073/pnas.95.26.15821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luzzi V, Sims CE, Soughayer JS, Allbritton NL. The physiologic concentration of inositol 1,4,5-trisphosphate in the oocytes of Xenopus laevis. J. Biol. Chem. 1998;273:28657–28662. doi: 10.1074/jbc.273.44.28657. [DOI] [PubMed] [Google Scholar]
- 19.Korsmeyer SJ, et al. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 2000;7:1166–1173. doi: 10.1038/sj.cdd.4400783. [DOI] [PubMed] [Google Scholar]
- 20.Minn AJ, et al. Bcl-xL forms an ion channel in synthetic lipid membranes. Nature. 1997;385:353–357. doi: 10.1038/385353a0. [DOI] [PubMed] [Google Scholar]
- 21.Sugawara H, Kurosaki M, Takata M, Kurosaki T. Genetic evidence for involvement of type 1, type 2 and type 3 inositol 1,4,5-trisphosphate receptors in signal transduction through the B-cell antigen receptor. EMBO J. 1997;16:3078–3088. doi: 10.1093/emboj/16.11.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foyouzi-Youssefi R, et al. Bcl-2 decreases the free Ca2+ concentration within the endoplasmic reticulum. Proc. Natl Acad. Sci. USA. 2000;97:5723–5728. doi: 10.1073/pnas.97.11.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmer AE, Jin C, Reed JC, Tsien RY. Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc. Natl Acad. Sci. USA. 2004;101:17404–17409. doi: 10.1073/pnas.0408030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinton P, et al. Reduced loading of intracellular Ca2+ stores and downregulation of capacitative Ca2+ influx in Bcl-2-overexpressing cells. J. Cell Biol. 2000;148:857–862. doi: 10.1083/jcb.148.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niiro H, Clark EA. Regulation of B-cell fate by antigen-receptor signals. Nature Rev. Immunol. 2002;2:945–956. doi: 10.1038/nri955. [DOI] [PubMed] [Google Scholar]
- 26.Doi T, Motoyama N, Tokunaga A, Watanabe T. Death signals from the B cell antigen receptor target mitochondria, activating necrotic and apoptotic death cascades in a murine B cell line, WEHI-231. Int. Immunol. 1999;11:933–941. doi: 10.1093/intimm/11.6.933. [DOI] [PubMed] [Google Scholar]
- 27.Hajnoczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995;82:415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- 28.Kowaltowski AJ, Fiskum G. Redox mechanisms of cytoprotection by Bcl-2. Antioxid. Redox. Signal. 2005;7:508–514. doi: 10.1089/ars.2005.7.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hammerman PS, Fox CJ, Thompson CB. Beginnings of a signal-transduction pathway for bioenergetic control of cell survival. Trends Biochem. Sci. 2004;29:586–592. doi: 10.1016/j.tibs.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Plas DR, Thompson CB. Cell metabolism in the regulation of programmed cell death. Trends Endocrinol. Metab. 2002;13:75–78. doi: 10.1016/s1043-2760(01)00528-8. [DOI] [PubMed] [Google Scholar]
- 31.Shimizu H, Borin ML, Blaustein MP. Use of La to distinguish activity of the plasmalemmal Ca2+ pump from Na+/Ca2+ exchange in arterial myocytes. Cell Calcium. 1997;21:31–41. doi: 10.1016/s0143-4160(97)90094-4. [DOI] [PubMed] [Google Scholar]
- 32.Haynes LP, Tepikin AV, Burgoyne RD. Calcium-binding protein 1 is an inhibitor of agonist-evoked, inositol 1,4,5-trisphosphate-mediated calcium signaling. J. Biol. Chem. 2004;279:547–555. doi: 10.1074/jbc.M309617200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Interaction of Bcl-2 with type-1 InsP3R and Inhibition of cytochrome C release from mitochondria under in vitro apoptotic conditions is inhibited by recombinant Bcl-XL. a, Lysates from DT40-InsP3R-KO cells stably expressing rat type-1 InsP3R were incubated with GST-Bcl-2 and bound InsP3R was detected with isoform-specific antibody. b,. His-hBcl-XL and Flag-hBcl-XL proteins were purified from bacterial cells or insect cells, respectively, and their purity examined by Coomassie Blue staining. c, Bcl-XL inhibits tBid-mediated cytochrome C release. Mitochondria were isolated from a Bak-/-Bax-/- hematopoietic cell line in which Bax was re-expressed. In the absence of tBid cytochrome C was detected exclusively in the mitochondrial fraction (M). After incubation with tBid cytochrome C was released from mitochondria and detected in the supernatant (S). Addition of Bcl-XL blocked the release of cytochrome C in the presence of tBid.
Figure S2 InsP3R activity in the absence of Bcl-XL and presence of Bax or tBid. a, Typical InsP3R current traces in the presence of 100 nM InsP3 in Sf9 nuclei, pipette [Ca2+] was 1 μM. Addition of 100 nM tBid or Bax along with 100 nM InsP3 to the pipette solution had no effect on Po, NA or NAPo, (P>0.05, unpaired t-test). b, Similarly, in the presence of 10 nM InsP3, pipette [Ca2+] 0.85 μM, addition of 100 nM Bax or tBid had no effect on channel Po, NA or NAPo, (P>0.05, unpaired t-test).
Figure S3 Interaction of Bcl-XL with InsP3R is essential for Bcl-XL effects on ER Ca2+ regulation and apoptosis. a, Effects of Bcl-XL transient expression on Ca2+ER. Typical records depicting change in cytoplasmic [Ca2+] ([Ca2+]i) in response to application of 1 μM thapsigargin in DT40-WT and DT40-InsP3R-KO cells transiently transfected with either Bcl-XL or vector alone. Each trace represents the mean ± SEM of at least six individual cells within the image field. b, Summary of the effects of Bcl-XL transient expression on Ca2+ER. Thapsigargin-induced increases in [Ca2+]i in vector alone and Bcl-XL-expressing cell lines. Data represent mean ± SEM for at least 30 individual cells in two independent transfections. Asterisk indicates P<0.05, ANOVA.c, Effects of Bcl-XL expression on ER Ca2+ regulation in independent clones. Summary of the effects of stable Bcl-XL expression on Ca2+ER. Thapsigargin-induced increases in [Ca2+]i in vector alone and Bcl-XL-expressing cell lines. Data represent mean ± SEM for at least 30 individual cells in multiple trials. Asterisk indicates P<0.05, ANOVA. d, Cell viability after treatment with 20 μg/ml α-IgM (time 0) of DT40-WT (solid symbols) and DT40-InsP3R-KO (open symbols) cells stably expressing Bcl-XL (Blue) or vector alone (Same clones as in c).
Figure S4 Receptor stimulation is more sensitively coupled to calcium signalling in Bcl-XL expressing cells and modulates mitochondrial NADH levels. a, [Ca2+]i measurements in DT40-WT cells stably expressing Bcl-XL. Receptor stimulation with low concentration of α-IgM evokes Ca2+ oscillations in many cells that were initially quiescent, examples of three responsive cells are shown. b, The percentage of cells that respond to α-IgM stimulation in this way, and the oscillation frequency is summarised as the mean ± SEM for at least 80 individual cells in no less than two independent trials. Asterisk indicates P<0.0001, unpaired t-test. c, The Bcl-XL - InsP3R interaction modulates mitochondrial NADH levels. The average (± SEM) resting NAD(P)H fluorescence and the change in NAD(P)H fluorescence in response to α-IgM stimulation for three replicate experiments are plotted.Asterisk indicates P<0.001, ANOVA.