Abstract
Animal and human studies support a protective effect of Vitamin D sufficiency related to malignancy by uncovering paracrine and autocrine effects of extra-renal 25(OH)D activation including: regulation of cell cycle proliferation, apoptosis induction, and increased cell differentiation signaling. Recent epidemiologic studies demonstrate a reduction in non-Hodgkin lymphoma (NHL) risk with increased sunlight exposure. As sunlight is a major vitamin D source, it has been suggested that vitamin D status may mediate this observed association. This review provides a comprehensive discussion of the current epidemiologic evidence with regard to the investigation of an association between vitamin D status and NHL risk.
Keywords: Lymphoma, Vitamin D, Epidemiology, ultraviolet radiation, etiology
Introduction
Non-Hodgkin Lymphoma (NHL) is the 5th most common cancer overall in the United States, among both men and women, with an estimated incidence rate of 19.3 per 100,0001. While a large number of exogenous and endogenous factors have been examined, the etiology of most NHL subtypes remains largely unknown2. The best characterized risk factor for NHL is immunodeficiency, both primary and acquired2–6. Furthermore, personal history of several immune disorders, including rheumatoid arthritis, celiac disease, systemic lupus erythematosus, and Sjögren's syndrome, has been associated with an increased risk of lymphoma7. A number of infectious agents have been linked with, or suspected in, the pathogenesis of NHL in the HIV-negative population2, 6, 8–13. In addition, a first degree relative with NHL has been indicated as a risk factor for NHL development in both men and women5, 6, 14, 15, though a potential pattern for NHL heritability remains poorly understood5, 14, 16–19.
Most notably, there has been marked increase in incidence rates of NHL over the past 30 years, estimated as up to an 82% increase overall9, 20, 21, affecting almost all histologic categories9. This rate of increase seen in NHL is among the highest of all types of cancer, estimated as high as 3% per year in the US16, 22, although there is evidence that rise in NHL rate has been recently stabilizing7, 9. Many theories have been proposed as to why this increase may have occurred, including known risk factors, new diagnostic tools, changes in diagnostic criteria, and improved registry data. However, the increase in NHL attributable to AIDS, or the increase due to the other theories proposed, cannot account for the entire increase observed. The rate of increase in incidence is consistent with increased exposure to a ubiquitous environmental exposure, increased exposure to an aggregate of multiple weekly associated factors, or conversely, ubiquitous decreased exposure to factors protective against NHL risk.
Recent evaluation of the association between self-reported individual sun exposure and NHL risk, demonstrates a consistent inverse association between sunlight exposure and NHL risk in 623–28 of 923–31 published studies, and a review and discussion of this association has been previously published by Armstrong and Kricker in 200732. This evidence of an association between increased sun exposure and decreased NHL risk is particularly intriguing in light of the seemingly contradictory evidence from earlier ecological studies indicating, if anything, a possibly detrimental impact of sun exposure on NHL risk. The sun is the most important source of vitamin D, providing about 90% of the needed vitamin D for most people33. Vitamin D is also obtained through limited dietary means, including fatty fish, fortified foods, and supplements34. Since a link between solar radiation, vitamin D production, and decreased colon cancer mortality was established in a 1980 United States ecological study35, animal and human research has been ongoing to investigate the association between Vitamin D status and many cancers, including prostate, colon, lung, pancreatic, endometrial, breast and even skin cancer, and provide support for a protective effect of Vitamin D status related to malignancy36–38. In light of this research conducted in other cancers to date, one proposed explanation for this unexpected finding in the NHL literature is that the measures of sun exposure are actually proxy measurements of Vitamin D status, and that Vitamin D sufficiency is protective against lymphoma39.
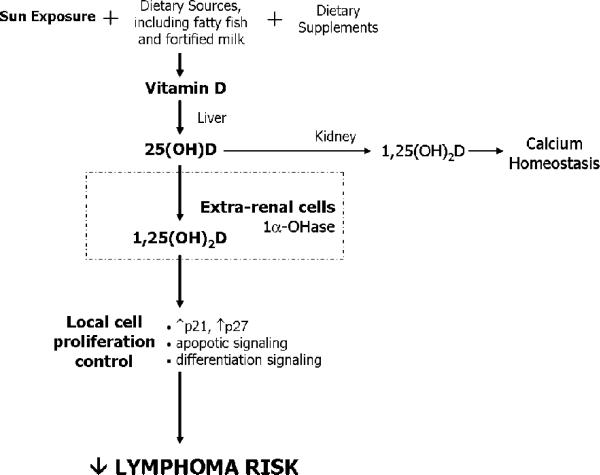
Vitamin D, once obtained though sun exposure, diet, and/or diet supplement intake, is metabolized in the liver to 25-hydroxyvitamin D (25(OH)D). 25(OH)D is further metabolized in the kidney to its active form, 1,25-dihydroxyvitamin D (1,25(OH)2D), which plays a major role in calcium homeostasis, through its ability to regulate intestinal calcium absorption and bone turnover in response to parathyroid hormone40. However, it is the extra-renal 1-α-hydroxylation of 25(OHD) to 1,25(OH)2D that appears to be central to chronic disease prevention, including cancer37, 41, 42. 1,25(OH)2D works through a nuclear vitamin D receptor (VDR), present in most cell types throughout the body including cells of the immune system41, 43, 44. The autocrine and paracrine effects of extra-renal 25(OH)D metabolism include maintaining regulation of cell proliferation, through increased transcription of p21 and p27 (both negative regulators of the cell cycle), apoptosis induction, and increased cell differentiation signals41, 44, 45 (Figure 1). Additionally, there is evidence of an immunomodulatory effect of 1,25(OH)2D on activated lymphocytes and dendritic cells, such that T cell responses are shifted away from inflammatory Th1 responses, and antigen presentation by dendritic cells is decreased33, 44, 46, 47. There is evidence of 1α-hydroxylase activity in cancer cells, and as such these malignant cells are able to convert 25(OH)D to active 1,25(OH)2D37. 1,25(OH)2D activity against metastasis has been demonstrated in various tumor models, including cancers of the lung, bone, colon, kidney, breast and prostate37. Furthermore, evidence of an effect of 1,25(OH)D on lymphoma cells in particular has been demonstrated both in the laboratory, with observed promotion of differentiation and antiproliferative effects on a variety of lymphoma cells line in vitro43, and in an early study demonstrating tumor response to alfacalcidol in 24% of 36 low grade follicular, small-cleaved cell type, lymphoma48.
Figure 1.
Proposed mechanism. It is hypothesized that extra-renal conversion of 25(OH)D to the active vitamin D metabolite (1,25(OH)2D) results in autocrine and paracrine signaling to control lymphocyte cell proliferation and decrease lymphoma risk.
Additional indirect evidence exists to provide support for a hypothesized relationship between vitamin D status and NHL. B cell lymphomas that are associated with immunosuppressed states may be polyclonal, which may support the notion that these tumors arise from a lymphoproliferative state in the context of immune suppression, and thus decreased immune surveillance, particularly by T cells, of any DNA mutations that may result in the lymphoproliferative process49. Furthermore, as described previously, 1,25(OH)2D's effects are mediated through a nuclear hormone receptor, the vitamin D receptor (VDR), which directly binds DNA to modulate gene expression in different cell types50, 51. Evidence of increased VDR expression on cycling keratinocytes suggests that proliferating cells, such as expanding B cells as a result of chronic antigen stimulation, may be a target for 1,25(OH)2D activity51, providing indirect evidence of potential link between vitamin D status and not only lymphoma etiology but potentially lymphoma prognosis as well. In fact, survival benefit with higher vitamin D levels has been observed in a number of malignancies, and trials evaluating the use of vitamin D in the treatment of advanced prostate cancer are ongoing38. Similarly, in light of the effect of 25(OH)D metabolism on increasing p27 levels, a study published in 2001 by Bai, et al,52 reports that among 80 de novo diffuse large B cell cases examined, p27 expression was low/null in 73%, and low p27 status correlated with an increased expression of cyclin A, involved in G1 → S transition53. Not only do these results suggest impairment of cell-cycle control involving the cdk inhibitor p27 in enhanced lymphoma proliferation, they additionally lend support to the proposed hypothesis, and may also identify a potential therapeutic target for 1,25(OH)2D. Finally, while NHL incidence has been historically higher among men and whites as compared to women and blacks, respectively, the notable increase in NHL rates over the past 30 years, as previously discussed, has been disproportionately high among blacks and older women9. This observation is consistent with the hypothesized association between vitamin D status and NHL risk due to the reduced capacity for vitamin D production in response to sun exposure among those with dark skin and with increased age54.
These observations provide a biological framework for a potential mechanism by which Vitamin D sufficiency may protect against malignancy, including NHL. The purpose of this literature review is to summarize and discuss the current available epidemiologic evidence regarding the association between vitamin D status and NHL to date, and to propose potential future approaches for further evaluation of the relationship between solar ultraviolet radiation, vitamin D status and NHL risk.
Methods
We conducted a systematic review of the published literature to identify all studies investigating the association between vitamin D status, either dietary vitamin D intake or serum 25(OH)D levels, and NHL risk. The following syntax were used to search the NLCM PubMed index: ((“Lymphoma/epidemiology”[Mesh] OR “Lymphoma/etiology”[Mesh])) AND (“Vitamin D”[Mesh] OR “Vitamin D Deficiency”[Mesh]) / “Diet”[Mesh] AND (“Lymphoma/epidemiology”[Mesh] OR “Lymphoma/etiology”[Mesh]). References cited in candidate articles were manually searched for additional relevant publications. Our goal is not to conduct a formal meta-analysis, but rather to present a summary of the current evidence and a discussion of the methodologic approaches to evaluating the association between vitamin D status and NHL.
Results
Eight published studies have evaluated the association between NHL risk and vitamin D status29, 30, 55–60. Study characteristics are outlined in Table 1. Estimates of association between vitamin D status and NHL risk, with careful attention to both the dietary vitamin D intake and serum 25(OH)D levels captured in the exposure groups, are detailed in Table 2. Dietary vitamin D consumption in the exposed groups ranges from 77 IU/day to >296 IU/day. Likewise, the reference group limits within these studies ranges from 1–21 IU/day up to 176 IU/day. This table demonstrates the overlap of the categories of vitamin D consumption exposure between studies, which may potentially be masking some of the effect of vitamin D status.
Table 1.
Summary of published epidemiologic studies of Vitamin D and NHL risk
| Author, year | Study population study period | Study design | Study Population (sample size) | Vitamin D status measure | Outcome |
|---|---|---|---|---|---|
| Hartge et al., 200629^ |
United States 1998–2000 |
Case Control | Population based incident NHL cases and controls from 4 SEER registries (551 cases / 462 controls) |
Dietary consumption (usual adult eating habits >1 yr. prior to diagnosis) as measured on selfadministered, validated food frequency questionnaire | Incident NHL diagnosis |
| Chang et al. 200655 |
Sweden 2000–2002 |
Case Control | Incident NHL cases and population bases controls (591 cases / 460 controls) |
Dietary consumption (2 yrs. prior to diagnosis) as measured on selfadministered, validated semiquantitative food frequency questionnaire | Incident NHL (including CLL) diagnosis |
| Polesel, et al. 200656 |
Italy 1999–2002 |
Case Control | Incidence NHL cases and hospital-based controls (190 cases / 484 controls) |
Nutrients calculated from detailed food frequency questionnaires (intake 2 yrs. prior to diagnosis) using the Italian food composition database | Incident NHL diagnosis |
| Soni et al., 200730 |
Nebraska 1999–2002 |
Case Control | Incident NHL cases and population bases controls (387 cases / 535 controls) |
Dietary consumption (usual adult eating habits >1 yr. prior to diagnosis) as measured on selfadministered, validated semiquantitative food frequency questionnaire | Incident NHL diagnosis |
| Purdue, et al. 200757^ |
United States 1998–2000 |
Case Control | Population based incident NHL cases and controls from 4 SEER registries (551 cases / 462 controls) |
Self-administered survey to measure total dietary Vitamin D intake (usual adult eating habits >1 yr. prior to diagnosis); VDR genotyping from either blood or mouthwash buccal cell sample | Incident NHL diagnosis |
| Giovannucci, et al., 200658 |
United States 1986–2000 |
Cohort | Men in the Health Professionals Follow-up Study (N=47,800) |
25(OH)D measured in serum (1095 cohort members), and predictive model built. Model used to predict 25(OH)D for use in analysis (entire cohort) | Incident cancer or cancer death as reported on biennial follow-up surveys* (330 NHL cases identified) |
| Freedman et al., 200759 |
United States 1988–2000 |
Cohort | NHANES III participants (N=16,818) |
Baseline serum 25(OH)D measured by radioimmunoassay; samples collected 1988–1994. | Cancer mortality ascertained through National Death Index (40 NHL deaths identified) |
| Lim et al., 200960 |
Finland 1985–2002 |
Nested Case Control | Male smokers (age 50–69) in the Alpha-Tocopherol Beta-Carotene Cancer Prevention (ATBC) Trial (280 cases / 538 matched controls; N=29,133 men in original cohort) |
Baseline serum 25(OH)D measured with DiaSorin radioimmunoassay (Heartland Assay, Iowa); samples collected 1985 – 1988. | Incident lymphoid malignancy diagnosis (including NHL, MM, and HL) identified through Finish Cancer Registry |
90% confirmed by medical record, family member or death certificate
These two papers represent separate analyses of the same study.
N.B.: Bolded risk estimates indicate statistical significance; Confidence for all estimates provided when included in study results; NHL= non-Hodgkin's Lymphoma, CLL=chronic lymphocytic leukemia, VDR=vitamin D receptor, MM=multiple myeloma, HL=Hodgkin lymphoma
Table 2.
Vitamin D exposure level and association with NHL risk
| Study | Vitamin D Status |
Risk Estimate^ | Covariates | |
|---|---|---|---|---|
| Exposed Level | Reference Level | |||
| Hartge et al., 2006 | 77–203 IU/day+ (dietary intake) | 1–21 IU/day+ (dietary intake) | OR=1.1 (0.7–1.7) | Age, gender, ethnicity, center, metabolic equivalents per week of exercise (<30 vs ≥30), total energy |
| Chang et al., 2006 | >296 IU/day# (daily intake) | ≤176 IU/day# (daily intake) | OR=1.3 (0.8–2.1) | Age, sex, total energy intake (logarithm), retinol, calcium, phosphorus |
| Polesel, et al., 2006 | >131 IU/day#* (daily intake) | <92 IU/day#* (daily intake) | OR=0.6 (0.4–0.9) | Age, gender, center, education, place of birth, HCV test, total energy intake (Kcal) |
| Soni et al., 2007 | >213.6 IU/day | <114.3 IU/day | OR=0.9 (0.7–1.3) | Age, gender, family history of cancer |
| Purdue, et al., 2007 | 77–203 IU/day+ (dietary intake) | 1–21 IU/day+ (dietary intake) | OR=1.8 (0.7–4.5) TaqI TT** OR=1.9 (0.7–5.2) TaqI tt** |
Age, gender, site, ethnicity, education level, total caloric intake |
| Giovannucci, et al., 2006 | Difference between medians of highest and lowest decile = 27.8 nmol/L (serum 25(OH)D) | inverse association (not statistically significant) between 25 nmol/L interval 25(OH)D increase and NHL risk (no risk estimate provided) | Age, height, smoking history, intakes of total calories, alcohol, red meat, calcium, retinol, total fruits and vegetables (cohort was men only) | |
| Range of predicted 25(OH)D values = 68 nmol/L (22.8 – 90.8) | ||||
| Freedman et al., 2007 | ≥mol/L (serum 25(OH)D) | <62.5 nmol/L (serum 25(OH)D) | RR =1.3 (0.6–2.9) | Age, gender, race/ethnicity, smoking history |
| Lim et al., 2009 | 59.5–124.8 nmol/L (serum 25(OH)D) | 6.3–40.0 nmol/L (serum 25(OH)D) | OR = 0.82 (0.53–1.26) all cases | Age, month of blood collection |
| OR= 1.52 (0.82–2.80) diagnosis ≥7yrs after baseline; | ||||
| OR=0.43 (0.23–0.83) diagnosis <7yrs after baseline | ||||
Bolded risk estimates indicate statistical significance; Confidence for all estimates provided when included in study results
Average daily values estimated from reported weekly intake values
Values converted to IU from reported μg/day according to the following vitamin D specific conversion: 1μg = 40 IU
values for exposure levels estimated from standard normal distribution using the provided mean and standard deviation of daily vitamin D intake among the controls.
OR estimates of the association between vitamin D intake and NHL risk were estimated within genotype of the TaqI Vitamin D Receptor single nucleotide polymorphism.
HCV = Hepatitis C virus
Two cohort studies have examined the association between serum 25(OH)D and cancer incidence58 and mortality59. Neither study demonstrated a significant association with NHL risk. Giovannucci et al. did not provide specific relative risk and 95% confidence interval (CI) estimates, but the figure provided in their manuscript suggests an approximate 25% reduced relative risk of NHL with a 25 nmol/L increase in serum 25(OH)D (with a CI that spanned approximately 0.5 – 1.2)58. Based on 40 NHL deaths in their study, Freedman et al. estimated a 1.34 relative risk of NHL (i.e., a 34% increased risk is death) among those with ≥62.5 nmol/L 25(OH)D level at baseline as compared to those with <62.5 nmol./L death (95% CI: 0.62 – 2.91)59.
Six of the 8 studies to date are case control investigations29, 30, 55–57. Vitamin D status in 5 these studies is determined by self-report dietary consumption on food frequency questionnaires. While Chang et al. did not identify an association between estimated dietary vitamin D intake and NHL risk (OR=1.3, 95%CI: 0.8–2.1), comparing those with daily vitamin D intake >7.4 μg to those with daily intake ≤4.4 μg)55 in a Swedish population, a similar Italian study published by Polesel et al. demonstrated a 40% decrease in NHL risk among those in the 3rd tertile of vitamin D dietary intake (highest vitamin D intake) as compared to those in the 1st tertile (lowest vitamin D intake) (OR=0.6, 95%CI: 0.4–0.9). Purdue et al. investigated the role of vitamin D receptor polymorphisms in a potential association between both dietary vitamin D intake and sun exposure with NHL risk57. While these investigators demonstrated that their observed inverse association between sun exposure and NHL risk was modified by polymorphism in the TaqI VDR SNP, such that the risk of NHL with <7 hrs/day of sun was 90% higher among tt carriers than the risk of NHL with <7 hrs of sun exposure among TT carriers, no association between dietary vitamin D and NHL risk, overall or by genotype, was observed57. Furthermore, two case control studies designed to evaluate the association between individual self-reported UVR exposure and NHL risk also included analyses of dietary vitamin D intake and NHL risk29, 30, though neither study identified a significant association between dietary vitamin D intake and NHL risk29, 30.
The most recent published study of the association between vitamin D status and NHL risk was also a case control study, but different from the previously published studies in that it was nested within the cohort of the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study60. This nested case control design allowed for serum 25(OH)D evaluation of vitamin D status prior to NHL diagnosis. While the authors report no association between 25(OH)D level and NHL risk overall (OR=0.82; 95%CI: 0.53–1.26), they do demonstrate a difference in the estimated association by duration of follow-up between baseline 25(OH)D assessment and NHL diagnosis. Among patients with less than 7 years of follow-up, there a 57% reduced risk of lymphoma for those in the highest 25(OH)D tertile as compared to those in the lowest tertile (OR=0.43; 95% CI: 0.23–0.83), while no significant association is found among the subgroup of participants with greater than 7 years of follow-up (OR=1.52; 95%CI: 0.82–2.80)60.
Four of the studies reviewed investigated the association between vitamin D status and NHL by gender29, 30, 55, 56. While Hartge et al., Chang et al., and Soni et al. each report similar findings by gender29, 30, 55, Polesel et al. report a stronger protective effective of higher vitamin D intake on NHL risk among women as compared to men56 (results not shown). None of the 8 studies reviewed evaluated the association between vitamin D status and NHL risk by ethnicity, most likely due to limited minority sample size.
Those studies which included a stratified or subgroup analysis of the association between vitamin D status and NHL risk by NHL histological subtype are presented in Table 3. Neither of the cohort studies included adequate NHL outcomes to allow for analysis of the association of vitamin D status with specific NHL subtypes. Lim et al. did evaluate the association between serum 25(OH)D and NHL risk within lymphoma subtype subgroups, though no statistically significant associations were found60. Only Polesel et al., provide evidence of any statistically significant association between high self-reported dietary vitamin D and NHL by subtype56. These authors demonstrate that the association between high dietary intake of vitamin D and NHL may be limited to the follicular lymphoma subtype, demonstrating a significant 70% decrease in follicular lymphoma risk among those in the highest tertile of dietary vitamin D as compared to those in the lowest tertile.
Table 3.
Evaluation of the association between vitamin D and NHL risk by lymphoma histologic subtype
| Study | Sample Size | Exposure | B cell subtypes |
T cell lymphoma | |
|---|---|---|---|---|---|
| Diffuse large B cell lymphoma | Follicular lymphoma | ||||
| Hartge et al., 2006 | 551 cases / 462 controls | High self-reported, energy adjusted, dietary vitamin D (food sources and dietary supplements) | No Association* 189 cases | No Association* 145 cases | -- |
| Chang et al. 2006 | 591 cases / 461 controls | High self-reported, energy adjusted, dietary vitamin D (food sources only) | 1.0 (0.5, 1.9) 147 cases | 1.1 (0.5, 2.4) 118 cases | 5.0 (1.2, 19.9) 41 cases |
| Polesel, et al. 2006 | 190 cases / 484 controls | High self-reported, energy adjusted, dietary vitamin D (food sources only) | 0.7 (0.4 – 1.3) 93 cases | 0.3 (0.1 – 0.9) 31 cases | -- |
| Soni et al., 2007 | 387 cases / 535 controls | High self-reported dietary vitamin D (food and supplement sources) | 0.8 (0.5 – 1.5) 91 cases | 1.0 (0.6 – 1.6) 111 cases | 1.5 (0.5 – 5.0) 19 cases |
| Purdue, et al. 2007 | 551 cases / 462 controls | High self-reported, energy adjusted, dietary vitamin D (food sources and dietary supplements) | No Association* | 1.0 (0.2 – 5.0) TaqI TT 28 cases | -- |
| 4.8 (1.2 – 20.0) TaqI tt 19 cases | |||||
| Lim, et al. 2009 | 280 cases / 538 matched controls | High serum 25(OH)D measured at baseline | 0.85 (0.33 – 2.14) 41 cases | 1.21 (0.31 – 4.72) 23 cases | 0.73 (0.16 – 3.33) 22 cases |
Specific point estimates and confidence intervals were not published. N.B.: Bolded risk estimates indicate statistical significance; 95% confidence intervals for all estimates provided when included in study results;
Discussion
Overall, the evidence presented in the literature to date provides limited support for an association between vitamin D status and NHL. With the exception of the findings by Polesel et al.56, and Lim et al.60, the published estimates of association with dietary vitamin D intake or serum 25(OH)D and NHL risk are largely weak or null. Limitations inherent to both retrospective dietary vitamin D assessment and the epidemiologic investigation of NHL etiology may be obscuring a true influence of vitamin D status on NHL risk.
The majority of current evidence regarding the association between vitamin D status and NHL risk has been the result of case control analyses. Five of these case control studies have all used recall of dietary intake on a food frequency questionnaire for exposure assessment among cases, after a cancer diagnosis had been made, and controls. This method of retrospective exposure assessment is vulnerable to potential recall inaccuracy and bias, a common limitation of the case control design. Correlation between detailed dietary records and dietary recall 3–10 years later has been reported as high as 0.761. The five case control studies each assessed dietary vitamin D exposure by asking subjects to recall their usual diet only 1 to 2 years prior to study participation, indicating that recall in these studies is unlikely to be a major concern. Furthermore, the specifics of the hypothesized relationship between vitamin D status and NHL were not common knowledge within these populations, and therefore any misclassification of exposure was most likely to be non-differential between the cases and controls. Any misclassification in these studies would likely have resulted in an underestimate of the true association between vitamin D status and NHL62.
Aside from potential recall inaccuracy with self-report of diet, assessment of dietary status through measurement of dietary vitamin D intake is further limited by the variability of vitamin D content in both the naturally occurring and fortified sources. While salmon is one of the few sources of naturally occurring vitamin D, it has been reported that the vitamin D content varies largely according to whether it is wild (600–1000 IU) or farm-raised (100–250 IU)54, and whether it is baked (nearly all vitamin D maintained) or fried (approximately 50% vitamin D loss)63. Additionally, while many milk products are fortified with between 300 and 600 IU of vitamin D per quart, poor adherence to the labeled fortification level has been documented in the literature64–67. If there is in fact an association between vitamin D status and NHL risk, this variability in the actual vitamin D fortification of milk products could explain some of the inconsistency seen in the literature investigating the association between dairy and NHL risk68, 69.
The relevant etiologic period of exposure for lymphoma is difficult to define. As is the case with many chronic illnesses, complete natural history of NHL prior to onset of symptoms is still undefined, and as such, it is not possible to determine whether risk is affected by a short duration excessive exposure that occurred many years before diagnosis or a cumulative effect chronic exposure over many years. With particular regard to cancer, many steps are necessary for malignant transformation70, 71. An exposure might act at any `stage' duringcarcinogenesis, and the period of relevant exposure would be highly dependent on the point in the causal sequence during which it acted etiologically, i.e. whether the exposure was an initiator or promoter of the malignancy in question72. This critical period is particularly difficult to determine for exposures that are continuous or intermittent72. The period of relevant vitamin D exposure (dietary or serum 25(OH)D) has been explored in the literature demonstrating a reduced risk of lymphoma among those with high UVR exposure, from which the hypothesized association between vitamin D status and NHL was derived. An inverse association between sun exposure 10 years prior to survey completion and lymphoma risk was demonstrated by Smedby et al. in their 2005 study25. Furthermore, Hartge et al. reported in their 2006 study that, when comparing effect across 4 different life periods, high sun exposure 5–10 years prior to diagnosis was most strongly associated with NHL risk 29. Most recently, Lim et al. demonstrated a differential association between 25(OH)D and NHL risk by length of follow-up, with a statistically significant protective effective of higher serum 25(OH)D on NHL risk observed only in the subgroup of subjects with less than 7 years of follow-up60, a finding that is consistent with the evidence presented by Smedby and Hartge25, 29, and which emphasizes the importance of the timing of exposure assessment in investigations of NHL etiology. While the approach of estimating dietary intake 1–2 years prior to diagnosis is thought to possibly best measure `usual' adult dietary intake patterns while minimizing recall error, it should be noted that true exposure 5–10 years prior, potentially the more relevant period of exposure, may be misclassified by the exposure methods employed to date.
Even if dietary recall is unbiased and accurate, assessment of vitamin D status through measurement of dietary intake alone, as has been the case in the majority of the studies to date, may also lead to exposure misclassification. As mentioned earlier, sun exposure is the major source of vitamin D, and natural dietary sources of vitamin D are limited33. Circulating 25(OH)D is the preferred biomarker for determining Vitamin D sufficiency, and represents the combined contributions of both sun produced and dietary (D2 and D3) sources of vitamin D40, 73. The long half-life (approximately 2–4 weeks) of this metabolite makes 25(OH)D the major circulating form of vitamin D33, 74, 75. Within a particular season, there is not much intra-individual 25(OH)D variation34, 73, 75. While 2 prospective studies have reported associations between serum 25(OH)D and NHL risk but failed to reach statistical significance, the primary outcome in these studies was cancer incidence58 and cancer mortality59 in general, and they were both underpowered to draw definitive conclusions as to the relationship between vitamin D status and NHL in particular.
While the threshold for vitamin D sufficiency, particularly with regard to chronic disease prevention, is still a matter of much debate, it has been noted in the literature that supplemental intake of approximately 1,700 IU would be needed to raise serum 25(OH)D concentrations from 20 – 32 ng/mL76. Additionally, hydroxylation of vitamin D to 25(OH)D in the liver is inhibited by both vitamin D availability and serum 25(OH)D, resulting in a less robust response to increase in vitamin D with higher baseline 25(OH)D levels77. Furthermore, the true dose-response relationships between vitamin D (dietary or serum 25(OH)D) and both NHL specifically (if any) as well as other cancer types, is unknown78, 79. There is evidence in the literature to suggest that the threshold levels for an effect of 25(OH)D may vary by cancer type, and preventive effects may be limited to higher levels of 25(OH)D than anticipated79. For example, Garland et al. suggest that maintenance of 25(OH)D levels above 43 ng/mL is needed for prevention of breast cancer incidence79. As such, if such levels were also required for NHL prevention, all dietary intake levels that were examined in the epidemiologic studies to date would have been insufficient, and an association between vitamin D status and NHL would not be observed.
Despite the considerable clinical heterogeneity of the NHL subtypes9, 80–82, the studies to date that have evaluated the association between vitamin D status and NHL risk have been designed to evaluate this association with all NHL subtypes combined as the primary hypothesis. In light of the current inability to consistently demonstrate an association between vitamin D status and NHL risk, it is possible that any potential association between vitamin D status and individual subtypes could be muted when the subtypes are combined. As discussed recently by Evens and Chiu, evaluation of distinct etiologic processes within the NHL subtypes is one of the major and ongoing challenges in epidemiologic research83. Of the 8 studies which have investigated the association between vitamin D status and NHL, 6 did conduct secondary analyses by NHL subtype29, 30, 55–57, 60. While Chang et al. found no association between NHL and vitamin D status considering all subtypes, they did demonstrate an intriguing 5-fold (95% CI: 1.2 – 19.9) increase in T cell lymphoma among those in the highest quartile of dietary vitamin D intake (>7.4 μg/day) as compared to those with the lowest intake (≤4.4 μg/day)55. The inverse association between dietary vitamin D intake and NHL risk reported by Polesel et al. was strongest among the follicular lymphomas56. Similarly, the interaction between sun exposure and VDR genotype reported by Purdue et al. was also strongest among follicular lymphomas57.
Local antiproliferative effects of 25(OH)D depend on functional vitamin D binding proteins for transport to target tissue, expression of 1α-hydroxylase in the target tissue, and nuclear vitamin D receptors for transcription regulation84, 85. There is evidence in the vitamin D and cancer literature that genetic variations along this vitamin D pathway are associated with cancer risk. For example, in the breast cancer literature, recent evidence suggests that there is an association between Vitamin D binding protein genotype and breast cancer risk in postmenopausal women, independent of serum 25(OH)D status85, suggesting the importance of evaluating not only 25(OH)D status but the entire vitamin D pathway in assessing cancer risk. There is evidence to suggest that VDR polymorphisms may be associated with NHL risk in at least some subtypes57, 86. Purdue et al. investigated the role of vitamin D receptor polymorphisms in the potential association between both dietary vitamin D intake and sun exposure with NHL risk57. While this study failed to demonstrate an association between dietary vitamin D and NHL risk, either in general or by genotype, these investigators did demonstrate that their observed inverse association between sun exposure and NHL risk was modified by polymorphism in the TaqI VDR SNP, such that the risk of NHL with <7 hrs/day of sun was 90% higher among tt carriers than the risk of NHL with <7 hrs of sun exposure among TT carriers57. However, the low power to investigate interaction effects in this study, in combination with the high probability of chance findings in genetic association studies, should be recognized.
Conclusions
Irrespective of latitude, vitamin D insufficiency is becoming a global problem50, 77. In particular, it appears that the prevalence is on the rise, potentially due to a number of contributing factors, such as concerns over fat intake and lactose intolerance leading to lower intake of vitamin D fortified foods (particularly milk), increased use of sunblock and decreased exposure to sunlight, and increased prevalence and duration of breastfeeding given the minimal vitamin D content of breast milk87. In light of both the seasonal variation in available sunlight in many regions and the known risk of excessive chronic sun exposure39, investigation of the risks of vitamin D insufficiency and strategies for enhanced fortification of food sources of Vitamin D (namely dairy products) is warranted. The list of chronic conditions for which protective effects of vitamin D sufficiency is currently being assessed includes cancer, rickets, osteoporosis, diabetes, multiples sclerosis and rheumatoid arthritis, and is growing continually33, 36, 45, 88–90. If in fact vitamin D status is truly associated with a reduced risk of NHL, and many other chronic diseases, immediate public health measures should be taken to begin to increase 25(OH)D levels.
Finally, even if vitamin D status is ultimately not found to be associated with reduced risk of NHL, further evaluation of the recently discovered inverse association between NHL risk and increased sun exposure is necessary. Reports of such associations may lead to recommendations of sun exposure to increase serum 25(OH)D levels. However, the risk of increased skin cancer, along with uncertainty of the contributions of variables such as age, latitude, skin pigmentation, and sunscreen use, make this controversial and difficult to implement91. Therefore, identification of potential intermediate variables in the association between sun and NHL risk is of public health importance91.
The limited evidence of an association between vitamin D status and NHL risk to date may very well be due to methodological limitations, and further investigation of this potential association is warranted. Particular emphasis should be placed on measuring serum 25(OH)D to assess vitamin D status, within nested case control or cohort designs where possible, with careful attention to the timing of exposure assessment in relation to NHL diagnosis. Future research should incorporate investigation of genetic variations along the vitamin D pathway, evaluation within specific NHL subtypes, careful consideration of potential confounding factors and effect modifiers, and subgroup analysis by gender, race/ethnicity and age, in order to further elucidate the presence and magnitude of the association between vitamin D status and NHL risk.
Acknowledgements
This work was supported by a hematology training grant award from the National Institutes of Health, National Heart Lung and Blood Institute [T32 HL007152]; the Leukemia and Lymphoma Society Clinical Scholar Program; the National Institutes of Health, National Center for Research Resources [UL 1 RR024160 ]; and the National Institutes of Health, National Cancer Institute [P50 CA130805].
Footnotes
Declaration of Interest The authors declare no competing financial interests, corporate involvement or patent holdings.
References
- 1.Ries L, et al., editors. SEER Cancer Statistics Review, 1975–2004. National Cancer Institute; Bethesda, MD: 2005. http://seer.cancer.gov/csr/1973_2004/. Vol. based on November 2006 SEER data submission, posted to the SEER website, 2007. [Google Scholar]
- 2.Fisher SG, Fisher RI. The epidemiology of non-Hodgkin's lymphoma. Oncogene. 2004;23(38):6524–34. doi: 10.1038/sj.onc.1207843. [DOI] [PubMed] [Google Scholar]
- 3.Grulich AE, Vajdic CM, Cozen W. Altered immunity as a risk factor for non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2007;16(3):405–8. doi: 10.1158/1055-9965.EPI-06-1070. [DOI] [PubMed] [Google Scholar]
- 4.Engels EA, Goedert JJ. Human immunodeficiency virus/acquired immunodeficiency syndrome and cancer: past, present, and future. J Natl Cancer Inst. 2005;97(6):407–9. doi: 10.1093/jnci/dji085. [DOI] [PubMed] [Google Scholar]
- 5.Baris D, Zahm SH. Epidemiology of lymphomas. Curr Opin Oncol. 2000;12(5):383–94. doi: 10.1097/00001622-200009000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Tavani A, et al. Medical history and risk of Hodgkin's and non-Hodgkin's lymphomas. Eur J Cancer Prev. 2000;9(1):59–64. doi: 10.1097/00008469-200002000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Alexander DD, et al. The non-Hodgkin lymphomas: a review of the epidemiologic literature. Int J Cancer. 2007;120(Suppl 12):1–39. doi: 10.1002/ijc.22719. [DOI] [PubMed] [Google Scholar]
- 8.Engels EA. Infectious agents as causes of non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2007;16(3):401–4. doi: 10.1158/1055-9965.EPI-06-1056. [DOI] [PubMed] [Google Scholar]
- 9.Muller AM, et al. Epidemiology of non-Hodgkin's lymphoma (NHL): trends, geographic distribution, and etiology. Ann Hematol. 2005;84(1):1–12. doi: 10.1007/s00277-004-0939-7. [DOI] [PubMed] [Google Scholar]
- 10.Thomas RK, et al. Epidemiology and etiology of Hodgkin's lymphoma. Ann Oncol. 2002;13(Suppl 4):147–52. doi: 10.1093/annonc/mdf652. [DOI] [PubMed] [Google Scholar]
- 11.Adami HO, Hunter D, Trichopoulos D, editors. Textbook of Cancer Epidemiology. Oxford University Press, Inc.; New York: 2002. [Google Scholar]
- 12.Giordano TP, et al. Risk of non-Hodgkin lymphoma and lymphoproliferative precursor diseases in US veterans with hepatitis C virus. Jama. 2007;297(18):2010–7. doi: 10.1001/jama.297.18.2010. [DOI] [PubMed] [Google Scholar]
- 13.Fisher SG, Fisher RI. The emerging concept of antigen-driven lymphomas: epidemiology and treatment implications. Curr Opin Oncol. 2006;18(5):417–24. doi: 10.1097/01.cco.0000239878.31463.0b. [DOI] [PubMed] [Google Scholar]
- 14.Wang SS, et al. Family history of hematopoietic malignancies and risk of non-Hodgkin lymphoma (NHL): a pooled analysis of 10 211 cases and 11 905 controls from the International Lymphoma Epidemiology Consortium (InterLymph) Blood. 2007;109(8):3479–88. doi: 10.1182/blood-2006-06-031948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mensah FK, et al. Non-Hodgkin's lymphoma and family history of hematologic malignancy. Am J Epidemiol. 2007;165(2):126–33. doi: 10.1093/aje/kwj361. [DOI] [PubMed] [Google Scholar]
- 16.Longo DL. Lymphomas: complexity, not chaos. Curr Opin Oncol. 2000;12(5):379–82. doi: 10.1097/00001622-200009000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Banks P. Changes in diagnosis of non-Hodgkin's lymphomas over time. Cancer Res. 1992;52(19):5453s–5455. [PubMed] [Google Scholar]
- 18.Hartge P, Devesa SS. Quantification of the impact of known risk factors on time trends in non-Hodgkin's lymphoma incidence. Cancer Res. 1992;52(19Suppl):5566s–5569s. [PubMed] [Google Scholar]
- 19.Cerhan JR, et al. Anthropometric characteristics, physical activity, and risk of non-Hodgkin's lymphoma subtypes and B-cell chronic lymphocytic leukemia: a prospective study. Am J Epidemiol. 2002;156(6):527–35. doi: 10.1093/aje/kwf082. [DOI] [PubMed] [Google Scholar]
- 20.Devesa S, Fears T. Non-Hodgkin's lymphoma time trends: United States and international data. Cancer Res. 1992;52(19):5432s–5440. [PubMed] [Google Scholar]
- 21.Zheng T, et al. Epidemiology of non-Hodgkin lymphoma in Connecticut. 1935–1988. Cancer. 1992;70(4):840–9. doi: 10.1002/1097-0142(19920815)70:4<840::aid-cncr2820700420>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 22.Adami J, et al. Evidence of an association between non-Hodgkin's lymphoma and skin cancer. Bmj. 1995;310(6993):1491–5. doi: 10.1136/bmj.310.6993.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hughes AM, et al. Pigmentary characteristics, sun sensitivity and non-Hodgkin lymphoma. Int J Cancer. 2004;110(3):429–34. doi: 10.1002/ijc.20128. [DOI] [PubMed] [Google Scholar]
- 24.Hughes AM, et al. Sun exposure may protect against non-Hodgkin lymphoma: a case-control study. Int J Cancer. 2004;112(5):865–71. doi: 10.1002/ijc.20470. [DOI] [PubMed] [Google Scholar]
- 25.Smedby KE, et al. Ultraviolet radiation exposure and risk of malignant lymphomas. J Natl Cancer Inst. 2005;97(3):199–209. doi: 10.1093/jnci/dji022. [DOI] [PubMed] [Google Scholar]
- 26.Weihkopf T, et al. Sun exposure and malignant lymphoma: a population-based case-control study in Germany. Int J Cancer. 2007;120(11):2445–51. doi: 10.1002/ijc.22492. [DOI] [PubMed] [Google Scholar]
- 27.Kricker A, et al. Personal sun exposure and risk of non Hodgkin lymphoma: a pooled analysis from the Interlymph Consortium. Int J Cancer. 2008;122(1):144–54. doi: 10.1002/ijc.23003. [DOI] [PubMed] [Google Scholar]
- 28.Boffetta P, et al. Exposure to ultraviolet radiation and risk of malignant lymphoma and multiple myeloma--a multicentre European case-control study. Int J Epidemiol. 2008;37(5):1080–1094. doi: 10.1093/ije/dyn092. [DOI] [PubMed] [Google Scholar]
- 29.Hartge P, et al. Ultraviolet radiation, dietary vitamin D, and risk of non-Hodgkin lymphoma (United States) Cancer Causes Control. 2006;17(8):1045–52. doi: 10.1007/s10552-006-0040-8. [DOI] [PubMed] [Google Scholar]
- 30.Soni LK, et al. Sun exposure and non-Hodgkin lymphoma: A population-based, case-control study. Eur J Cancer. 2007;43(16):2388–95. doi: 10.1016/j.ejca.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, et al. Ultraviolet Radiation Exposure and Risk of Non-Hodgkin's Lymphoma. Am J Epidemiol. 2007;165(11):1255–64. doi: 10.1093/aje/kwm020. [DOI] [PubMed] [Google Scholar]
- 32.Armstrong BK, Kricker A. Sun Exposure and Non-Hodgkin Lymphoma. Cancer Epidemiol Biomarkers Prev. 2007;16(3):396–400. doi: 10.1158/1055-9965.EPI-06-1068. [DOI] [PubMed] [Google Scholar]
- 33.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80(6 Suppl):1678S–88S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 34.Calvo MS, Whiting SJ, Barton CN. Vitamin D fortification in the United States and Canada: current status and data needs. Am J Clin Nutr. 2004;80(6 Suppl):1710S–6S. doi: 10.1093/ajcn/80.6.1710S. [DOI] [PubMed] [Google Scholar]
- 35.Grant WB. Ecologic studies of solar UV-B radiation and cancer mortality rates. Recent Results Cancer Res. 2003;164:371–7. doi: 10.1007/978-3-642-55580-0_27. [DOI] [PubMed] [Google Scholar]
- 36.Grant WB. Health benefits of solar UV-B radiation through the production of vitamin D. Comment and response. Photochem Photobiol Sci. 2003;2(12):1307–8. doi: 10.1039/b305583c. discussion 1308–10. [DOI] [PubMed] [Google Scholar]
- 37.Giovannucci E. The epidemiology of vitamin D and cancer incidence and mortality: A review (United States) Cancer Causes Control. 2005;16(2):83–95. doi: 10.1007/s10552-004-1661-4. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz GG, Skinner HG. Vitamin D status and cancer: new insights. Curr Opin Clin Nutr Metab Care. 2007;10(1):6–11. doi: 10.1097/MCO.0b013e328011aa60. [DOI] [PubMed] [Google Scholar]
- 39.Egan KM, Sosman JA, Blot WJ. Sunlight and reduced risk of cancer: is the real story vitamin D? J Natl Cancer Inst. 2005;97(3):161–3. doi: 10.1093/jnci/dji047. [DOI] [PubMed] [Google Scholar]
- 40.Holick MF. Vitamin D: A millenium perspective. J Cell Biochem. 2003;88(2):296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 41.Banerjee P, Chatterjee M. Antiproliferative role of vitamin D and its analogs--a brief overview. Mol Cell Biochem. 2003;253(1–2):247–54. doi: 10.1023/a:1026072118217. [DOI] [PubMed] [Google Scholar]
- 42.Whiting SJ, Calvo MS. Vitamin D and cancer symposium: Dietary recommendations to meet both endocrine and autocrine needs of Vitamin D. J Steroid Biochem Mol Biol. 2005;97(1–2):7–12. doi: 10.1016/j.jsbmb.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 43.Hickish T, et al. The effect of 1,25-dihydroxyvitamin D3 on lymphoma cell lines and expression of vitamin D receptor in lymphoma. Br J Cancer. 1993;68(4):668–72. doi: 10.1038/bjc.1993.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80(6 Suppl):1689S–96S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 45.Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79(3):362–71. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 46.Mathieu C, et al. Vitamin D and 1,25-dihydroxyvitamin D3 as modulators in the immune system. J Steroid Biochem Mol Biol. 2004;89–90(1–5):449–52. doi: 10.1016/j.jsbmb.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 47.van Etten E, Decallonne B, Mathieu C. 1,25-dihydroxycholecalciferol: endocrinology meets the immune system. Proc Nutr Soc. 2002;61(3):375–80. doi: 10.1079/PNS2002170. [DOI] [PubMed] [Google Scholar]
- 48.Raina V, et al. Alfacalcidol is a nontoxic, effective treatment of follicular small-cleaved cell lymphoma. Br J Cancer. 1991;63(3):463–5. doi: 10.1038/bjc.1991.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Potter M. Pathogenetic mechanisms in B-cell non-Hodgkin's lymphomas in humans. Cancer Res. 1992;52(19 Suppl):5522s–5528s. [PubMed] [Google Scholar]
- 50.Reginster JY. The high prevalence of inadequate serum vitamin D levels and implications for bone health. Curr Med Res Opin. 2005;21(4):579–86. doi: 10.1185/030079905X41435. [DOI] [PubMed] [Google Scholar]
- 51.Bohnsack BL, Hirschi KK. Nutrient regulation of cell cycle progression. Annu Rev Nutr. 2004;24:433–53. doi: 10.1146/annurev.nutr.23.011702.073203. [DOI] [PubMed] [Google Scholar]
- 52.Bai M, et al. Low expression of p27 protein combined with altered p53 and Rb/p16 expression status is associated with increased expression of cyclin A and cyclin B1 in diffuse large B-cell lymphomas. Mod Pathol. 2001;14(11):1105–13. doi: 10.1038/modpathol.3880444. [DOI] [PubMed] [Google Scholar]
- 53.Sherr CJ. Cancer cell cycles. Science. 1996;274(5293):1672–7. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 54.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 55.Chang ET, et al. Nutrient intake and risk of non-Hodgkin's lymphoma. Am J Epidemiol. 2006;164(12):1222–32. doi: 10.1093/aje/kwj330. [DOI] [PubMed] [Google Scholar]
- 56.Polesel J, et al. Linoleic acid, vitamin D and other nutrient intakes in the risk of non-Hodgkin lymphoma: an Italian case-control study. Ann Oncol. 2006;17(4):713–8. doi: 10.1093/annonc/mdl054. [DOI] [PubMed] [Google Scholar]
- 57.Purdue MP, et al. Sun exposure, vitamin D receptor gene polymorphisms and risk of non-Hodgkin lymphoma. Cancer Causes Control. 2007;18(9):989–99. doi: 10.1007/s10552-007-9039-z. [DOI] [PubMed] [Google Scholar]
- 58.Giovannucci E, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98(7):451–9. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 59.Freedman DM, et al. Prospective study of serum vitamin D and cancer mortality in the United States. J Natl Cancer Inst. 2007;99(21):1594–602. doi: 10.1093/jnci/djm204. [DOI] [PubMed] [Google Scholar]
- 60.Lim U, et al. A prospective investigation of serum 25-hydroxyvitamin D and risk of lymphoid cancers. Int J Cancer. 2009;124(4):979–86. doi: 10.1002/ijc.23984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Willett WC. Nutritional Epidemiology. 2nd ed. Oxford University Press; New York and Oxford: 1998. [Google Scholar]
- 62.Gordis L. Epidemiology. 4th ed. W.B. Saunders Company; Philadelphia: 2009. [Google Scholar]
- 63.Lu Z, et al. An evaluation of the vitamin D3 content in fish: Is the vitamin D content adequate to satisfy the dietary requirement for vitamin D? J Steroid Biochem Mol Biol. 2007;103(3–5):642–4. doi: 10.1016/j.jsbmb.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tanner JT, et al. Survey of vitamin content of fortified milk. J Assoc Off Anal Chem. 1988;71(3):607–10. [PubMed] [Google Scholar]
- 65.Holick MF, et al. The vitamin D content of fortified milk and infant formula. N Engl J Med. 1992;326(18):1178–81. doi: 10.1056/NEJM199204303261802. [DOI] [PubMed] [Google Scholar]
- 66.Faulkner H, et al. A survey of vitamin A and D contents of fortified fluid milk in Ontario. J Dairy Sci. 2000;83(6):1210–6. doi: 10.3168/jds.S0022-0302(00)74986-1. [DOI] [PubMed] [Google Scholar]
- 67.Murphy SC, et al. Fluid milk vitamin fortification compliance in New York State. J Dairy Sci. 2001;84(12):2813–20. doi: 10.3168/jds.S0022-0302(01)74737-6. [DOI] [PubMed] [Google Scholar]
- 68.Skibola CF. Obesity, diet and risk of non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2007;16(3):392–5. doi: 10.1158/1055-9965.EPI-06-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cross AJ, Lim U. The role of dietary factors in the epidemiology of non-Hodgkin's lymphoma. Leuk Lymphoma. 2006;47(12):2477–87. doi: 10.1080/10428190600932927. [DOI] [PubMed] [Google Scholar]
- 70.Rangarajan A, et al. Species- and cell type-specific requirements for cellular transformation. Cancer Cell. 2004;6(2):171–83. doi: 10.1016/j.ccr.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 71.Weiss RA. Multistage carcinogenesis. Br J Cancer. 2004;91(12):1981–2. doi: 10.1038/sj.bjc.6602318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rothman KJ, Poole C. Causation and causal inference. In: Fraumeni S.a., editor. Cancer Epidemiology and Prevention. Oxford University Press; New York: 1996. [Google Scholar]
- 73.Holick MF. Evolution and function of vitamin D. Recent Results Cancer Res. 2003;164:3–28. doi: 10.1007/978-3-642-55580-0_1. [DOI] [PubMed] [Google Scholar]
- 74.Holick MF. Vitamin D Status: Measurement, Interpretation, and Clinical Application. Ann Epidemiol. 2008;19(2):73–8. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wild CP, et al. A critical evaluation of the application of biomarkers in epidemiological studies on diet and health. Br J Nutr. 2001;86(Suppl 1):S37–53. doi: 10.1079/bjn2001338. [DOI] [PubMed] [Google Scholar]
- 76.Vieth R, et al. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr. 2007;85(3):649–50. doi: 10.1093/ajcn/85.3.649. [DOI] [PubMed] [Google Scholar]
- 77.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87(4):1080S–6S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 78.Giovannucci E. Strengths and limitations of current epidemiologic studies: vitamin D as a modifier of colon and prostate cancer risk. Nutr Rev. 2007;65(8 Pt 2):S77–9. doi: 10.1111/j.1753-4887.2007.tb00345.x. [DOI] [PubMed] [Google Scholar]
- 79.Garland CF, et al. What is the dose-response relationship between vitamin D and cancer risk? Nutr Rev. 2007;65(8 Pt 2):S91–5. doi: 10.1111/j.1753-4887.2007.tb00349.x. [DOI] [PubMed] [Google Scholar]
- 80.Ansell SM, Armitage J. Non-Hodgkin lymphoma: diagnosis and treatment. Mayo Clin Proc. 2005;80(8):1087–97. doi: 10.4065/80.8.1087. [DOI] [PubMed] [Google Scholar]
- 81.Cheson BD. What is new in lymphoma? CA Cancer J Clin. 2004;54(5):260–72. doi: 10.3322/canjclin.54.5.260. [DOI] [PubMed] [Google Scholar]
- 82.Fisher RI. Overview of non-Hodgkin's lymphoma: biology, staging, and treatment. Semin Oncol. 2003;30(2 Suppl 4):3–9. doi: 10.1053/sonc.2003.23797. [DOI] [PubMed] [Google Scholar]
- 83.Evens AM, Chiu BC. The Challenges of Epidemiologic Research in Non-Hodgkin Lymphoma. JAMA. 2008;300(17):2059–2061. doi: 10.1001/jama.2008.589. [DOI] [PubMed] [Google Scholar]
- 84.Townsend K, et al. Biological actions of extra-renal 25-hydroxyvitamin D-1alpha-hydroxylase and implications for chemoprevention and treatment. J Steroid Biochem Mol Biol. 2005;97(1–2):103–9. doi: 10.1016/j.jsbmb.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 85.Abbas S, et al. The Gc2 allele of the vitamin D binding protein is associated with a decreased postmenopausal breast cancer risk, independent of the vitamin d status. Cancer Epidemiol Biomarkers Prev. 2008;17(6):1339–43. doi: 10.1158/1055-9965.EPI-08-0162. [DOI] [PubMed] [Google Scholar]
- 86.Purdue MP, et al. Vitamin D receptor gene polymorphisms and risk of non-Hodgkin's lymphoma. Haematologica. 2007;92(8):1145–6. doi: 10.3324/haematol.11053. [DOI] [PubMed] [Google Scholar]
- 87.Raiten DJ, Picciano MF. Vitamin D and health in the 21st century: bone and beyond. Executive summary. Am J Clin Nutr. 2004;80(6 Suppl):1673S–7S. doi: 10.1093/ajcn/80.6.1673S. [DOI] [PubMed] [Google Scholar]
- 88.Cantorna MT. Vitamin D and autoimmunity: is vitamin D status an environmental factor affecting autoimmune disease prevalence? Proc Soc Exp Biol Med. 2000;223(3):230–3. doi: 10.1046/j.1525-1373.2000.22333.x. [DOI] [PubMed] [Google Scholar]
- 89.Cantorna MT, Mahon BD. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med (Maywood) 2004;229(11):1136–42. doi: 10.1177/153537020422901108. [DOI] [PubMed] [Google Scholar]
- 90.Munger KL, et al. Serum 25-Hydroxyvitamin D Levels and Risk of Multiple Sclerosis. JAMA. 2006;296(23):2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 91.Harris DM, Go VL. Vitamin D and colon carcinogenesis. J Nutr. 2004;134(12 Suppl):3463S–3471S. doi: 10.1093/jn/134.12.3463S. [DOI] [PubMed] [Google Scholar]